Background: PCa stem/progenitor cells develop higher chemoresistance.
Results: High TR4 levels in PCa stem/progenitor cells were shown to be critical in conferring chemoresistance to these cells.
Conclusion: TR4-Oct4-IL1Ra signaling is important in conferring chemoresistance to PCa stem/progenitor cells.
Significance: This finding suggests that targeting TR4 and its downstream molecules may be a better therapeutic approach to battle PCa stem/progenitor cell-originated chemoresistance.
Keywords: Cancer Stem Cells, Chemoresistance, Drug Resistance, Nuclear Receptors, Prostate Cancer, Oct4, Testicular Orphan Nuclear Receptor 4
Abstract
Prostate cancer (PCa) stem/progenitor cells are known to have higher chemoresistance than non-stem/progenitor cells, but the underlying molecular mechanism remains unclear. We found the expression of testicular nuclear receptor 4 (TR4) is significantly higher in PCa CD133+ stem/progenitor cells compared with CD133− non-stem/progenitor cells. Knockdown of TR4 levels in the established PCa stem/progenitor cells and the CD133+ population of the C4-2 PCa cell line with lentiviral TR4 siRNA led to increased drug sensitivity to the two commonly used chemotherapeutic drugs, docetaxel and etoposide, judging from significantly reduced IC50 values and increased apoptosis in the TR4 knockdown cells. Mechanism dissection studies found that suppression of TR4 in these stem/progenitor cells led to down-regulation of Oct4 expression, which, in turn, down-regulated the IL-1 receptor antagonist (IL1Ra) expression. Neutralization experiments via adding these molecules into the TR4 knockdown PCa stem/progenitor cells reversed the chemoresistance, suggesting that the TR4-Oct4-IL1Ra axis may play a critical role in the development of chemoresistance in the PCa stem/progenitor cells. Together, these studies suggest that targeting TR4 may alter chemoresistance of PCa stem/progenitor cells, and this finding provides the possibility of targeting TR4 as a new and better approach to overcome the chemoresistance problem in PCa therapeutics.
Introduction
Prostate cancer (PCa)4 is the most commonly diagnosed malignancy and the second leading cause of cancer-related death in men in the western world. In the United States, it is estimated as the most prevalent cancer among males (43%) (1). Patients with advanced and metastatic PCa initially respond well to androgen deprivation therapy. However, in most cases, patients with PCa inevitably suffer a relapse, and their tumors develop into castration-resistant prostate cancer (CRPC) and further advance into metastatic CRPC (mCRPC) (2).
The results of chemotherapy using docetaxel (a member of the taxane family) are successful in some patients with PCa (3), but others develop chemoresistance problems, and most of these patients receive a second line chemotherapy. However, many clinical trials of second line chemotherapy have been disappointing, as Colloca et al. (4) summarize in their review of taxane-based and non-taxane-based chemotherapy for docetaxel-resistant CRPC patients. The taxane-based chemotherapy includes carboplatin plus docetaxel or estramustine plus docetaxel (5–8), and non-taxane-based chemotherapy includes calcitriol plus docetaxel (9) or replacing docetaxel with mitoxantrone (10, 11) and prednisone (12). However, none of them showed satisfactory results. For example, combined use of abiraterone acetate with docetaxel in a Phase III trial extended the survival of mCRPC patients (13), but recent clinical studies have suggested that the activity of docetaxel post-abiraterone appeared lower than anticipated, and no responses to docetaxel were observed in abiraterone-refractory patients (14), and some side effects were also reported (15). Even in the patients who responded to docetaxel without toxicity developed chemoresistance when rechallenged with docetaxel as a second line treatment (16). Thus, development of better chemotherapy strategies is urgently needed.
Increasing evidence has indicated that PCa stem/progenitor cells, which are characterized with high expression of CD133, CD44, and Oct4 (17, 18), are resistant to chemotherapeutic drugs (19, 20), and early reports suggested that chemoresistance in cancer stem cells could be due to their high expression of drug-resistant related genes, including ABCB1/MDR1, ABCG2/BCRP, and ABCC1/MRP1 (21, 22).
The testicular nuclear receptor 4 (TR4) belongs to the nuclear receptor superfamily and was first cloned from human prostate and testis cDNA libraries (23). It has been known to modulate many signaling pathways by interacting with the thyroid receptor, androgen receptor, retinoic acid receptor/retinoid X receptor, and estrogen receptor (24–26). TR4 knock-out mouse studies have shown that TR4 knockout results in defects in development and abnormalities in spermatogenesis and reproductive systems in both genders, which indicates that TR4 might play important roles in stem/progenitor cell differentiation (24). On the other hand, TR4 was also shown to play protective roles against oxidative stress- and ionizing radiation-induced damage (27). These results prompted us to investigate whether TR4 has a role in the development of chemoresistance in PCa stem/progenitor cells.
MATERIALS AND METHODS
Reagents and Cell Culture
The C4-2 human PCa cells and prostate cancer stem cells (PCSCs, Celprogen (San Pedro, CA)) were cultured in the recommended media (Celprogen) and maintained at 37 °C in a humidified incubator at 5% CO2. The chemotherapeutic agents docetaxel and etoposide (LC Laboratories, Woburn, MA) were dissolved in 100% DMSO and stored at −20 °C until use. pCDNA3.3-OCT4 was purchased from Addgene (Cambridge, MA), purified, and used in transfection experiments.
Magnetic Bead Isolation of CD133+ Stem/Progenitor Cells
Cells (2 × 107) were detached with 5 mm EDTA and incubated with streptavidin magnetic beads (Invitrogen) that had been conjugated with biotinylated CD133 antibody (Miltenyi Biotec, Cambridge, MA). The bead-bound cells were separated by placing tubes in a magnetic field. The stem/progenitor marker expressions in the isolated CD133-positive (CD133+) stem/progenitor cells were confirmed by qPCR or immunofluorescence staining. The isolated CD133+ stem/progenitor cells were cultured in keratinocyte serum-free media (Invitrogen) with 2% FBS and 0.1% leukemia inhibitor factor (Sigma) as described (17), and cells within two passages were used in the experiments.
Plasmids and Cell Infection
TR4 siRNA was cloned in pLKO plasmid. For incorporation of TR4 siRNA or scrambled control plasmids into PCa cells, lentivirus carrying either control (pLKO-vector) or TR4 siRNA (pLKO-TR4 siRNA) was transfected into 293T cells with a mixture of pLKO-TR4 siRNA, psPAX2 (virus-packaging plasmid), and pMD2G (envelope plasmid) (4:3:2 ratio) using Lipofectamine 2000 (Invitrogen). After the prostate cancer cells were infected with target virus for 6 h, the culture media containing the virus were replaced with normal culture media, and cells were maintained under the cell culture conditions. After the cells were subcultured, the stable clone cells were selected by adding 2 μg/ml puromycin (Sigma) and then maintained in media containing 1.0 μg/ml puromycin.
RNA Extraction, cDNA Synthesis, and Quantitative RT-PCR
RNAs were extracted using TRIzol reagent (Invitrogen) based on the manufacturer's instructions. RNAs (1 μg) were then subjected to reverse transcription using the iscriptTM cDNA synthesis kit (Bio-Rad), and the obtained cDNAs were used for qPCR analysis in a Bio-Rad CFX96 system. The primer sequences for TR4, stem cell markers (CD133, Oct4, Nanog, Notch, and Sox2), drug resistance genes (ABCB1/MDR1, ABCG2/BCRP, and ABCC1/MRP1), and Oct4 downstream genes are listed in supplemental Table 1. GAPDH was used as a control, and all reactions were run at least in triplicate.
Western Blot Analysis
Cells were harvested and washed with cold PBS and lysed in radioimmune precipitation assay buffer supplemented with protease inhibitor mixture tablets. The protein concentration was estimated using the Bio-Rad protein assay (Bio-Rad). Samples (20–40 μg of protein) were separated on a 10–12% SDS-polyacrylamide gel, and transferred to PVDF membranes (Millipore, Billerica, MA), and nonspecific binding was blocked using 5% milk in TBST. Membranes were incubated with primary antibodies overnight at 4 °C, washed in TBST solution, and incubated with HRP-conjugated second antibody, and the protein bands were visualized with an enhanced chemiluminescence detection system (Bio-Rad).
Cytotoxicity Test Using Docetaxel and Etoposide
Cytotoxicity of docetaxel and etoposide was tested using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) assay at 5 mg/ml. Target cells were seeded on 24-well plates (2 × 104 cells/well) and treated with various concentrations of docetaxel and etoposide. At the end of a 48-h incubation, MTT tests were performed, and absorbance at 570 nm was measured. Cell viability was calculated using the formula, OD sample/OD blank control × 100. Triplicate experiments were performed, and average values with mean ± S.E. are presented. The IC50 value was calculated using GraphPad Prism version 5.0 software.
TUNEL Assay
PCSC-sc and PCSC-siTR4 cells were treated with docetaxel (2 nm) or etoposide (2 μg/ml), and a TUNEL assay was performed according to the manufacturer's protocol (Roche Applied Science).
Statistical Analysis
GraphPad Prism version 5.0 was used for data analysis. Values are expressed as mean ± S.E., and statistical analysis was performed using one-way analysis of variance and Student's t test. The values were considered as statistically significant if the p value was less than 0.05.
RESULTS
TR4 and Drug Resistance-associated Genes Were More Highly Expressed in PCa CD133+ Stem/Progenitor Cells than CD133− Non-stem/Progenitor Cells
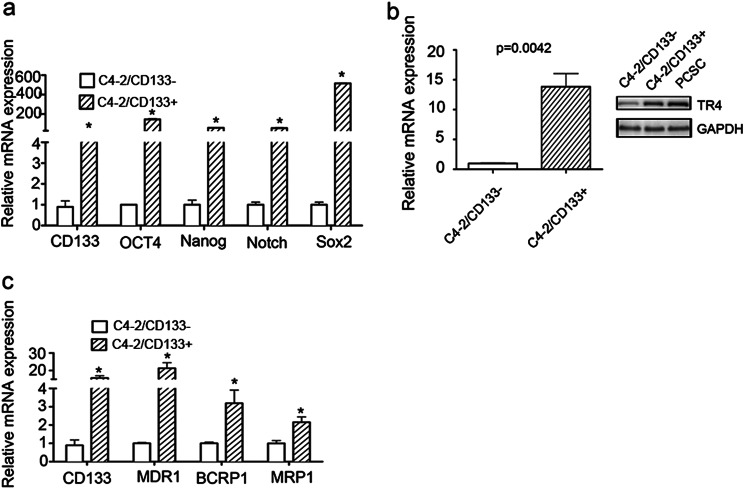
Early reports suggested that PCa stem/progenitor cells showed higher chemoresistance compared with non-stem/progenitor cells (19, 21, 28, 29). We isolated CD133+ stem/progenitor and CD133− non-stem/progenitor cells of the PCa C4-2 cell line by a magnetic sorting method using CD133 antibody and found that the C4-2 CD133+ cells showed high stem cell marker expression (Fig. 1a). We then investigated the TR4 level in those two populations of cells and found that TR4 was significantly more highly expressed in C4-2/CD133+ stem/progenitor cells compared with the non-stem/progenitor cells (Fig. 1b, left, mRNA level; right, protein level). We also found that TR4 was highly expressed in PCSCs, the established PCa stem cell line (Fig. 1b, right, Western blot data). The PCSC cell line was originally obtained from a human PCa patient and immortalized by Celprogen (San Pedro, CA) (30–32). These cells were shown to be homogenous and positive for stem cell markers such as Oct4 and Nanog (data not shown).
FIGURE 1.
Higher expressions of TR4 and chemoresistance associated gene expressions in PCa CD133+ stem/progenitor cells compared with CD133- non-stem/progenitor cells. a, stem cell markers expressions in C4-2/CD133+ PCa stem/progenitor and CD133− non-stem/progenitor cells. b, TR4 expression in C4-2/CD133+ PCa stem/progenitor and CD133− non-stem/progenitor cells. mRNA levels are shown on the left, and protein levels are shown on the right. c, expression of several taxel-related drug resistance genes, including ABCB1(MDR1), ABCG2(BCRP), and ABCC1(MRP1), in C4-2/CD133+ PCa stem/progenitor and CD133− non-stem/progenitor cells. Error bars, S.E.
Because some drug resistance-associated genes, such as ABCB1/MDR1, ABCG2/BCRP, and ABCC1/MRP1, are known to contribute to the taxel-related chemoresistance in cancer stem cells (21, 22) and in liver cancer cells (33), we investigated their expression in C4-2/CD133+ stem/progenitor and CD133− non-stem/progenitor cells. We found that these genes were more highly expressed in the C4-2/CD133+ stem/progenitor cells compared with the CD133− non-stem/progenitor cells (Fig. 1c). These results imply that high levels of TR4 in PCa stem/progenitor cells might be important in conferring chemoresistance properties to these cells.
TR4 Knockdown Led to Enhanced Chemosensitivity of PCa Stem/Progenitor Cells
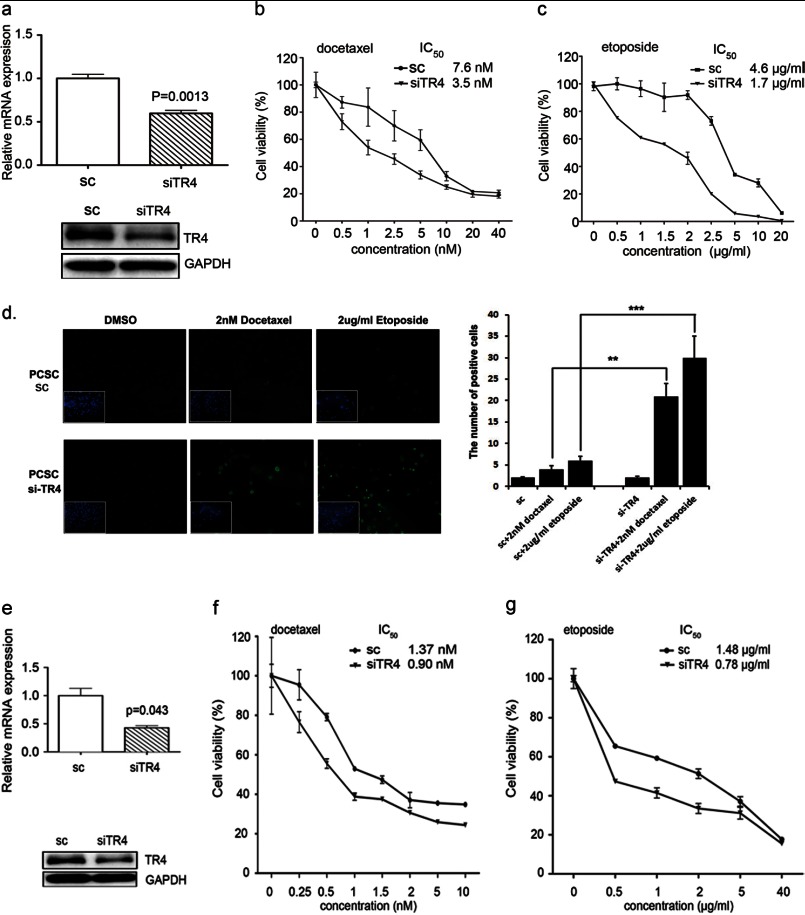
To investigate the linkage between the high level of TR4 and chemoresistance genes in PCa stem/progenitor cells, we performed in vitro manipulations of TR4 expression in the two sources of stem/progenitor cells, PCSCs and C4-2 CD133+. Successful knockdown of TR4 in PCSCs was shown in Fig. 2a (top, mRNA level; bottom, protein level). We then treated these cells with docetaxel and used MTT assay to analyze the cytotoxicity of these cells to docetaxel.
FIGURE 2.
Higher expression of TR4 led to higher chemoresistance in PCa stem/progenitor cells. a, qPCR (top) and Western blot (bottom) analysis results showing successful TR4 knockdown in PCSCs. PCSCs were infected with lentivirus carrying either si-TR4 or scrambled (sc) control sequence, and TR4 mRNA and protein levels were analyzed by qPCR and Western blot analysis, respectively. GAPDH served as a control in analyses. b and c, drug sensitivity test for docetaxel (b) and etoposide (c) in PCSC-siTR4 and PCSC-sc cells. Cells were treated with various indicated concentrations of drugs for 48 h, and cell viability upon drug treatment was analyzed by an MTT assay. The IC50 value was calculated using GraphPad Prism version 5.0 software. Triplicate experiments were performed, and mean values ± S.E. (error bars) are presented. d, TUNEL assay result. PCSCs were treated with the indicated concentrations of docetaxel and etoposide, and a TUNEL assay was performed after a 24-h incubation according to the manufacturer's instructions. Quantitation is shown on the right. e, qPCR (top) and Western blot (bottom) analysis results showing successful TR4 knockdown in C4-2/CD133+ cells. Cells were infected with lentivirus carrying either si-TR4 or scrambled control sequence, and TR4 levels were analyzed as in a. f and g, drug sensitivity test for docetaxel (f) and etoposide (g) in C4-2siTR4-CD133+ and C4-2sc-CD133+ cells. Cells were treated with the various indicated concentrations of drugs for 48 h, and cell viability upon drug treatment was analyzed by an MTT assay as in b and c. **, p < 0.01; ***, p < 0.001.
We found that PCSCs were more sensitive to docetaxel treatment in the TR4 knockdown (PCSC-siTR4) cells compared with the scrambled control (PCSC-sc) cells with IC50 of 3.5 nm versus IC50 of 7.6 nm, respectively (Fig. 2b). We used another commonly used clinical drug, etoposide, and compared the cytotoxicity of PCSC-siTR4 and PCSC-sc cells to this drug. We obtained an IC50 value of 4.6 μg/ml for PCSC-sc cells versus 1.7 μg/ml for PCSC-siTR4 cells (Fig. 2c), also implying that TR4 knockdown increased drug sensitivity. We also performed a TUNEL assay to investigate apoptotic death differences in the PCSC-siTR4 and PCSC-sc cells and obtained similar results showing higher apoptotic death in PCSC-siTR4 cells upon docetaxel and etoposide treatments (Fig. 2d).
We performed similar experiments using C4-2/CD133+ stem/progenitor cells, but lower concentrations of the two drugs were applied because the parental C4-2 cells showed higher sensitivity to these two drugs compared with the PCSCs (data not shown). We infected C4-2/CD133+ stem/progenitor cells with lentivirus carrying siTR4 or scrambled control plasmid, and Fig. 2e shows successful knockdown of TR4 in C4-2/CD133+ cells (top, mRNA level; bottom, protein level). We tested the cytotoxicity of these cells to docetaxel and found that the TR4 knockdown C4-2/CD133+ stem/progenitor (C4-2siTR4-CD133+) cells showed increased drug sensitivity to docetaxel compared with scrambled control (C4-2sc-CD133+) cells (IC50 of 1.37 nm in C4-2sc-CD133+ cells versus 0.90 nm in C4-2siTR4-CD133+ cells; Fig. 2f). Similarly, an IC50 value of 1.48 μg/ml in C4-2sc-CD133+ cells versus 0.78 μg/ml in C4-2siTR4-CD133+ cells was obtained in cytotoxicity tests against etoposide (Fig. 2g). Together, the results from Fig. 2 suggest that TR4 plays a critical role in conferring chemoresistance to PCa stem/progenitor cells.
TR4 Conferred Chemoresistance to PCa Stem/Progenitor Cells through Up-regulation of Oct4 and IL1Ra
Several previous reports have indicated that Oct4 contributes to drug resistance. Linn et al. (18) reported that drug-resistant PCa cells express high levels of Oct4, and knockdown of this molecule attenuated growth of the drug-resistant cells. Significant up-regulation of Oct4 was also observed in cisplatin-resistant patients with oral squamous cell carcinomas (34). In addition, knockdown of Oct4 in drug-resistant colorectal cancer cells showed increased cell apoptosis and decreased expression of stem cell markers and weakened tumorigenicity (35). Furthermore, TR4 regulation of Oct4 in embryonic stem cells at the transcriptional level was also demonstrated (36). Therefore, we investigated the potential linkage between TR4 and Oct4 in altering chemoresistance in PCa stem/progenitor cells and found that expression of Oct4 was significantly higher in C4-2/CD133+ stem/progenitor cells compared with CD133- non-stem/progenitor cells (Fig. 3, mRNA level (a) and protein level (b)). We used TR4 knockdown PCa stem/progenitor and scrambled control cells and examined mRNA expressions of Oct4 and several Oct4 downstream genes associated with drug resistance and stem cells (18, 33, 37), including GATA6, GDF6, KLF5, and IL1RN, and found that IL1RN gene expression was most significantly reduced in the TR4 knockdown PCSCs (Fig. 3c, left, mRNA level; right, protein level) and C4-2/CD133+ stem/progenitor cells (Fig. 3d, left, mRNA level; right, protein level).
FIGURE 3.
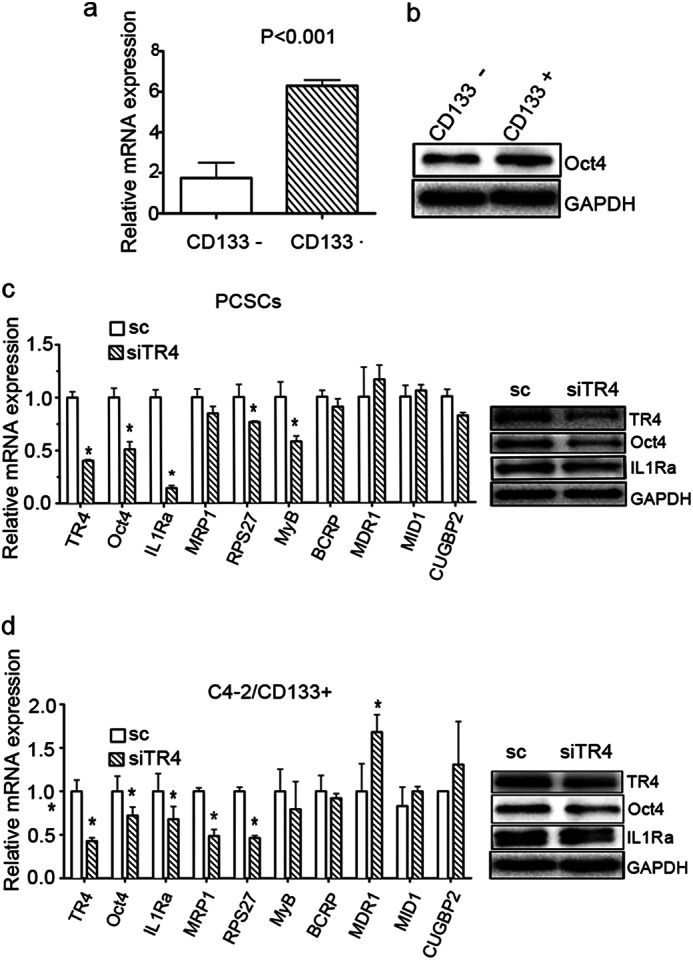
TR4 contributes to the chemoresistance in PCa stem/progenitor cells through up-regulation of Oct4 and IL1Ra expression. qPCR (a) and Western blot (b) analysis results show high expression of Oct4 in C4-2/CD133+ stem/progenitor cells. c, expression of TR4, Oct4, IL-1Ra, and several taxel-related chemoresistance genes in PCSC-siTR4 and PCSC-sc cells. qPCR (left) and Western blot analysis (right) are shown. d, qPCR (left) and Western blot (right) results showing expression of Oct4, IL-1Ra, and several taxel-related drug resistance genes in TR4 knockdown C4-2/CD133+ PCa stem/progenitor cells. *, p < 0.05. Error bars, S.E.
We further investigated which drug resistance-associated downstream genes are modulated by the TR4-Oct4-IL1Ra axis and found that expression of some genes, such as RSP27, MyB, and MRP1, was down-regulated, but expression of other genes, including BCRP1, MDR1, and MID1, was not modulated significantly (Fig. 3d, left).
We then applied neutralization/interruption approaches to confirm if TR4 is required for the Oct4-IL1Ra signaling to modulate the chemoresistance against docetaxel in these cells. Incorporating Oct4 into the PCSC siRNA cells (Fig. 4a shows high Oct level in these cells) reversed the TR4 knockdown-mediated drug sensitivity increase (Fig. 4b), and the addition of the recombinant IL1Ra to the PCSC-siTR4 and C4-2siTR4-CD133+ cell culture also reversed the TR4 knockdown effect in enhancing drug sensitivity (Fig. 4, c and d), implying that IL1Ra is implicated in conferring drug resistance to PCa stem/progenitor cells. Together, the results from Figs. 3 and 4 suggest that TR4 may modulate chemoresistance of PCa stem/progenitor cells via up-regulation of the TR4-Oct4-IL1Ra signaling.
FIGURE 4.
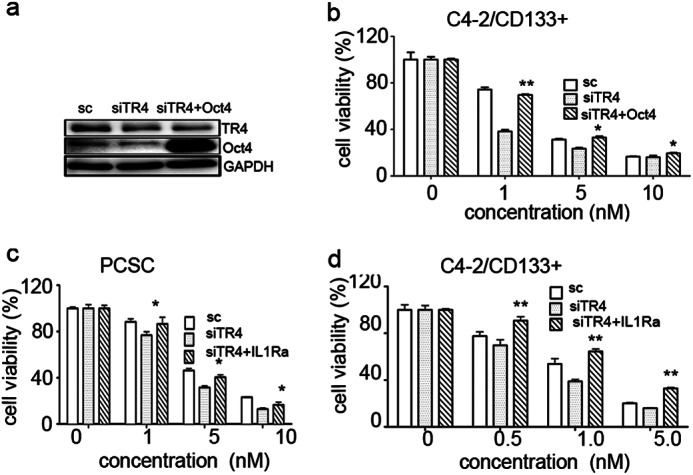
Rescue experiments showing chemosensitivity reversed by incorporating Oct4 and IL1Ra into PCSCs and C4-2/CD133+ PCa stem/progenitor cells. a, Western blot analysis of TR4 and Oct4 protein levels in PCSCs-siTR4 cells, infected with either Oct4 or vector. b, effect of Oct4 incorporation in rescuing reduced PCSCs viability induced by TR4 knockdown. Cells were infected by lentivirus carrying Oct4 and then treated with various concentrations of docetaxel for 48 h. Cell viability was analyzed by MTT assays. c and d, effect of recombinant IL1Ra addition in rescuing reduced PCSC (c) and C4-2/CD133+ PCa stem/progenitor cell (d) viability induced by TR4 knockdown. Cells were treated with 2 ng/ml recombinant IL1Ra and then treated with docetaxel for 48 h, and cell viability was analyzed by MTT assays. Triplicate experiments were performed, and mean values ± S.E. (error bars) are presented. *, p < 0.05; **, p < 0.01.
DISCUSSION
Androgen deprivation therapy is the standard treatment strategy for locally advanced and metastatic PCa. Although there are some options for the treatment of CRPC, such as intermittent androgen blockade or second line androgen deprivation, these treatments can only partially postpone the progression to mCRPC.
Based on the TAX 327 study results, in which docetaxel showed extended survival times when compared with mitoxantrone in treating mCRPC patients (38), the National Institutes for Health and Clinical Excellence and American Urological Association recommended docetaxel-based chemotherapy as the first line chemotherapeutic strategy for mCRPC. Subsequently, the effect of the combination therapy of luteinizing hormone-releasing hormone agonist with docetaxel or abiraterone has been tested (STAMPEDE study) (39). Etoposide was also used in Phase II studies of the combination therapy targeting mCRPC (40). In addition, docetaxel plus estramustine (anti-microtubule agent) chemotherapy represents an active and well tolerated treatment in mCRPC patients in Japan (41). However, not all mCRPC patients respond well to chemotherapy (40–43) at this stage, and the only expectancy is to allow mCRPC patients to live longer or have a better quality of life. Many attempts have been made to overcome this drug resistance problem (e.g. application of the new generation of taxel-derived medicines, such as cabazitaxel), but whether this new agent has a better effect remains inconclusive (43, 44).
Earlier reports suggested that PCa stem/progenitor cells showed higher chemoresistance compared with non-stem/progenitor cells (20, 21, 23). For example, the CD133+ stem/progenitor cells isolated from the highly invasive WPE1-NB26 PCa cell line were shown to be more resistant to docetaxel than the CD133− non-stem/progenitor cell (45). Similarly, the CD133+/CD117high/ABCG2high/nestin+ PCa cell subpopulation, isolated from the CWR22RV1 PCa cell line, was more resistant to the commonly used chemotherapeutic drugs, such as cisplatin, paclitaxel, and methotrexate, than the CD133−/ABCG2low cells (46). Early reports showed that the CD133+/CD44+ PCa cells isolated from a non-adherent suspension of PC-3 cells are more resistant to cisplatin (47, 48). Recently, Zhang et al. (47) suggested that tumor sphere-forming PCa cells, which are characteristic of stem/progenitor cells, displayed higher chemoresistance when compared with adherent cells. It was also shown that normal and malignant epithelial cells with stemlike properties have an extended G2 cell cycle phase that is associated with apoptotic resistance (49). All of these results indicate that the CD133+ stem/progenitor cells have more chemoresistance than CD133− cells, although Yan et al. (50) reported a contradictory finding that the drug-tolerant PCa cells showed reduced tumor-initiating capacity due to the loss of stem cell characteristics.
In this study, we investigated the role of TR4 in PCa stem/progenitor cells in drug resistance using two sources of PCa stem/progenitor cells, the isolated CD133+ cell population of the C4-2 PCa cell line and the established PCSC cell line, and the two common clinically used drugs, docetaxel and etoposide, and showed a positive role of TR4 in affecting chemoresistance. This positive role of TR4 is consistent with the previous reports showing a protective role of TR4 in oxidative stress- and ionizing radiation-induced damage (24–27).
There have been many attempts to investigate the underlying mechanism of chemoresistance in CRPC. For example, it was reported that calcitonin induces apoptosis resistance in PCa cell lines against cytotoxic drugs via the Akt/survivin pathway (51). Methylseleninic acid therapy (52) and targeting p38/p53/p21 signaling (53) have also been suggested to battle chemoresistance. In this study, we investigated the mechanism conferring chemoresistance to PCa stem/progenitor population cells and provided one critical target molecule, TR4.
Targeting TR4 directly may be a problem because there is no known specific inhibitor of TR4 on the market, although metaformin, an activator of AMPK (54) that could suppress TR4 signaling indirectly (55), could be used. Therefore, we attempted to reveal downstream signal molecules of TR4. We found that TR4 may exert its action through Oct4-IL1Ra signaling. The role of Oct4 in drug-resistant PCa cells has been reported (18), and TR4 regulation of Oct4 has also been suggested (36). Our results showed TR4 modulation of Oct4 in PCa stem/progenitor cells and revealed its downstream molecule, IL1Ra. It has been suggested that an IL1Ra could be used for the treatment of cancer (56), which supports our findings. Therefore, we believe that the TR4-Oct4-IL1Ra signal axis may contribute to chemoresistance in PCa stem/progenitor cells. However, in vivo studies should be done to confirm this.
In summary, in this study, we clearly demonstrated a positive role of TR4 in rendering drug chemoresistance in PCa stem/progenitor cells. Furthermore, we provide a possibility of using its downstream signaling axis, Oct4-IL1Ra, as a potential target to battle chemoresistance originating from the PCa stem/progenitor cells.
Acknowledgment
We thank Karen Wolf for help in the preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants CA122840 and CA156700. This work was also supported by the George Whipple Professorship Endowment, a Taiwan Department of Health Clinical Trial, Research Center of Excellence Grant DOH99-TD-B-111-004 (to China Medical University), National Basic Research Program of China Grant 2012CB518304, and Pao Yu-Kong Chair Professorship 201006.

This article contains supplemental Table 1.
- PCa
- prostate cancer
- CRPC
- castration-resistant prostate cancer
- mCRPC
- metastatic CRPC
- PCSC
- prostate cancer stem cell
- qPCR
- quantitative PCR
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
REFERENCES
- 1. Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 [DOI] [PubMed] [Google Scholar]
- 2. Seruga B., Tannock I. F. (2011) Chemotherapy-based treatment for castration-resistant prostate cancer. J. Clin. Oncol. 29, 3686–3694 [DOI] [PubMed] [Google Scholar]
- 3. Hwang C. (2012) Overcoming docetaxel resistance in prostate cancer. A perspective review. Ther. Adv. Med. Oncol. 4, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colloca G., Venturino A., Checcaglini F. (2012) Second-line chemotherapy in metastatic docetaxel-resistant prostate cancer. A review. Med. Oncol. 29, 776–785 [DOI] [PubMed] [Google Scholar]
- 5. Ross R. W., Beer T. M., Jacobus S., Bubley G. J., Taplin M. E., Ryan C. W., Huang J., Oh W. K. (2008) A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer 112, 521–526 [DOI] [PubMed] [Google Scholar]
- 6. Nakabayashi M., Sartor O., Jacobus S., Regan M. M., McKearn D., Ross R. W., Kantoff P. W., Taplin M. E., Oh W. K. (2008) Response to docetaxel/carboplatin-based chemotherapy as first- and second-line therapy in patients with metastatic hormone-refractory prostate cancer. BJU Int. 101, 308–312 [DOI] [PubMed] [Google Scholar]
- 7. Reuter C. W., Morgan M. A., Ivanyi P., Fenner M., Ganser A., Grünwald V. (2010) Carboplatin plus weekly docetaxel as salvage chemotherapy in docetaxel-resistant and castration-resistant prostate cancer. World J. Urol. 28, 391–398 [DOI] [PubMed] [Google Scholar]
- 8. Caffo O., Sava T., Comploj E., Giampaolo M. A., Segati R., Valduga F., Cetto G., Galligioni E. (2010) Estramustine plus docetaxel as second-line therapy in patients with hormone-refractory prostate cancer resistant to docetaxel alone. Urol. Oncol. 28, 152–156 [DOI] [PubMed] [Google Scholar]
- 9. Beer T. M., Ryan C. W., Venner P. M., Petrylak D. P., Chatta G. S., Ruether J. D., Chi K. N., Young J., Henner W. D. (2008) Intermittent chemotherapy in patients with metastatic androgen-independent prostate cancer. Results from ASCENT, a double-blinded, randomized comparison of high-dose calcitriol plus docetaxel with placebo plus docetaxel. Cancer 112, 326–330 [DOI] [PubMed] [Google Scholar]
- 10. Rosenberg J. E., Ryan C. J., Weinberg V. K., Smith D. C., Hussain M., Beer T. M., Ryan C. W., Mathew P., Pagliaro L. C., Harzstark A. L., Sharib J., Small E. J. (2009) Phase I study of ixabepilone, mitoxantrone, and prednisone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel-based therapy. A study of the Department of Defense prostate cancer clinical trials consortium. J. Clin. Oncol. 27, 2772–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michels J., Montemurro T., Murray N., Kollmannsberger C., Nguyen Chi K. (2006) First- and second-line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer. Does sequence matter? Cancer 106, 1041–1046 [DOI] [PubMed] [Google Scholar]
- 12. Heidenreich A., Rawal S. K., Szkarlat K., Bogdanova N., Dirix L., Stenzl A., Welslau M., Wang G., Dawkins F., de Boer C. J., Schrijvers D. (2013) A randomized, double-blind, multicenter, phase 2 study of a human monoclonal antibody to human αν integrins (intetumumab) in combination with docetaxel and prednisone for the first-line treatment of patients with metastatic castration-resistant prostate cancer. Ann. Oncol. 24, 329–336 [DOI] [PubMed] [Google Scholar]
- 13. Ryan C. J., Smith M. R., de Bono J. S., Molina A., Logothetis C. J., de Souza P., Fizazi K., Mainwaring P., Piulats J. M., Ng S., Carles J., Mulders P. F., Basch E., Small E. J., Saad F., Schrijvers D., Van Poppel H., Mukherjee S. D., Suttmann H., Gerritsen W. R., Flaig T. W., George D. J., Yu E. Y., Efstathiou E., Pantuck A., Winquist E., Higano C. S., Taplin M. E., Park Y., Kheoh T., Griffin T., Scher H. I., Rathkopf D. E. (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mezynski J., Pezaro C., Bianchini D., Zivi A., Sandhu S., Thompson E., Hunt J., Sheridan E., Baikady B., Sarvadikar A., Maier G., Reid A. H., Mulick Cassidy A., Olmos D., Attard G., de Bono J. (2012) Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone. Clinical evidence for cross-resistance? Ann. Oncol. 23, 2943–2947 [DOI] [PubMed] [Google Scholar]
- 15. Sternberg C. N., Molina A., North S., Mainwaring P., Fizazi K., Hao Y., Rothman M., Gagnon D. D., Kheoh T., Haqq C. M., Cleeland C., de Bono J. S., Scher H. I. (2013) Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann. Oncol. 24, 1017–1025 [DOI] [PubMed] [Google Scholar]
- 16. Loriot Y., Massard C., Gross-Goupil M., Di Palma M., Escudier B., Bossi A., Chauchereau A., Fizazi K. (2010) The interval from the last cycle of docetaxel-based chemotherapy to progression is associated with the efficacy of subsequent docetaxel in patients with prostate cancer. Eur. J. Cancer 46, 1770–1772 [DOI] [PubMed] [Google Scholar]
- 17. Collins A. T., Berry P. A., Hyde C., Stower M. J., Maitland N. J. (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951 [DOI] [PubMed] [Google Scholar]
- 18. Linn D. E., Yang X., Sun F., Xie Y., Chen H., Jiang R., Chen H., Chumsri S., Burger A. M., Qiu Y. (2010) A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer 1, 908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maitland N. J., Collins A. (2005) A tumour stem cell hypothesis for the origins of prostate cancer. BJU Int. 96, 1219–1223 [DOI] [PubMed] [Google Scholar]
- 20. Crea F., Danesi R., Farrar W. L. (2009) Cancer stem cell epigenetics and chemoresistance. Epigenomics 1, 63–79 [DOI] [PubMed] [Google Scholar]
- 21. Dean M., Fojo T., Bates S. (2005) Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–284 [DOI] [PubMed] [Google Scholar]
- 22. Frame F. M., Maitland N. J. (2011) Cancer stem cells, models of study and implications of therapy resistance mechanisms. Adv. Exp. Med. Biol. 720, 105–118 [DOI] [PubMed] [Google Scholar]
- 23. Chang C., Da Silva S. L., Ideta R., Lee Y., Yeh S., Burbach J. P. (1994) Human and rat TR4 orphan receptors specify a subclass of the steroid receptor superfamily. Proc. Natl. Acad. Sci. U.S.A. 91, 6040–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan S. J., Lee Y. F., Ting H. J., Liu N. C., Liu S., Lin S. J., Yeh S. D., Li G., Chang C. (2012) Deficiency in TR4 nuclear receptor abrogates Gadd45a expression and increases cytotoxicity induced by ionizing radiation. Cell. Mol. Biol. Lett. 17, 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim E., Ma W. L., Lin D. L., Inui S., Chen Y. L., Chang C. (2007) TR4 orphan nuclear receptor functions as an apoptosis modulator via regulation of Bcl-2 gene expression. Biochem. Biophys. Res. Commun. 361, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu S., Yan S. J., Lee Y. F., Liu N. C., Ting H. J., Li G., Wu Q., Chen L. M., Chang C. (2011) Testicular nuclear receptor 4 (TR4) regulates UV light-induced responses via Cockayne syndrome B protein-mediated transcription-coupled DNA repair. J. Biol. Chem. 286, 38103–38108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee Y. F., Liu S., Liu N. C., Wang R. S., Chen L. M., Lin W. J., Ting H. J., Ho H. C., Li G., Puzas E. J., Wu Q., Chang C. (2011) Premature aging with impaired oxidative stress defense in mice lacking TR4. Am. J. Physiol. Endocrinol. Metab. 301, E91–E98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malik B., Nie D. (2012) Cancer stem cells and resistance to chemo and radio therapy. Front. Biosci. (Elite Ed.) 4, 2142–2149 [DOI] [PubMed] [Google Scholar]
- 29. Morrison R., Schleicher S. M., Sun Y., Niermann K. J., Kim S., Spratt D. E., Chung C. H., Lu B. (2011) Targeting the mechanisms of resistance to chemotherapy and radiotherapy with the cancer stem cell hypothesis. J. Oncol. 2011, 941876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klarmann G. J., Hurt E. M., Mathews L. A., Zhang X., Duhagon M. A., Mistree T., Thomas S. B., Farrar W. L. (2009) Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis 26, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawasaki B. T., Hurt E. M., Kalathur M., Duhagon M. A., Milner J. A., Kim Y. S., Farrar W. L. (2009) Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells. An integrated molecular profiling approach. Prostate 69, 827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duhagon M. A., Hurt E. M., Sotelo-Silveira J. R., Zhang X., Farrar W. L. (2010) Genomic profiling of tumor initiating prostatospheres. BMC Genomics 11, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X. Q., Ongkeko W. M., Chen L., Yang Z. F., Lu P., Chen K. K., Lopez J. P., Poon R. T., Fan S. T. (2010) Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology 52, 528–539 [DOI] [PubMed] [Google Scholar]
- 34. Tsai L. L., Yu C. C., Chang Y. C., Yu C. H., Chou M. Y. (2011) Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J. Oral Pathol. Med. 40, 621–628 [DOI] [PubMed] [Google Scholar]
- 35. Wen K., Fu Z., Wu X., Feng J., Chen W., Qian J. (2013) Oct-4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells. Effects associated with STAT3/Survivin. Cancer Lett. 333, 56–65 [DOI] [PubMed] [Google Scholar]
- 36. Shyr C. R., Kang H. Y., Tsai M. Y., Liu N. C., Ku P. Y., Huang K. E., Chang C. (2009) Roles of testicular orphan nuclear receptors 2 and 4 in early embryonic development and embryonic stem cells. Endocrinology 150, 2454–2462 [DOI] [PubMed] [Google Scholar]
- 37. Greco S. J., Liu K., Rameshwar P. (2007) Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells 25, 3143–3154 [DOI] [PubMed] [Google Scholar]
- 38. Berthold D. R., Pond G. R., Soban F., de Wit R., Eisenberger M., Tannock I. F. (2008) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. Updated survival in the TAX 327 study. J. Clin. Oncol. 26, 242–245 [DOI] [PubMed] [Google Scholar]
- 39. James N. D., Sydes M. R., Mason M. D., Clarke N. W., Anderson J., Dearnaley D. P., Dwyer J., Jovic G., Ritchie A. W., Russell J. M., Sanders K., Thalmann G. N., Bertelli G., Birtle A. J., O'Sullivan J. M., Protheroe A., Sheehan D., Srihari N., Parmar M. K. (2012) Celecoxib plus hormone therapy versus hormone therapy alone for hormone-sensitive prostate cancer. First results from the STAMPEDE multiarm, multistage, randomised controlled trial. Lancet Oncol. 13, 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith D. C., Tangen C. M., Van Veldhuizen P. J., Jr., Harrer G. W., Golshayan A., Mills G. M., Vogelzang N. J., Thompson I. M., Hussain M. H. (2011) Phase II evaluation of early oral estramustine, oral etoposide, and intravenous paclitaxel combined with hormonal therapy in patients with high-risk metastatic prostate adenocarcinoma. Southwest Oncology Group S0032. Urology 77, 1172–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matsumoto A., Inoue A., Yokoi S., Nozumi K., Miyazaki K., Hosoki S., Nagata M., Yamaguchi K. (2009) Evaluation of docetaxel plus estramustine in the treatment of patients with hormone-refractory prostate cancer. Int. J. Urol. 16, 687–691 [DOI] [PubMed] [Google Scholar]
- 42. Tannock I. F., de Wit R., Berry W. R., Horti J., Pluzanska A., Chi K. N., Oudard S., Théodore C., James N. D., Turesson I., Rosenthal M. A., Eisenberger M. A., and TAX 327 Investigators (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 [DOI] [PubMed] [Google Scholar]
- 43. Yap T. A., Pezaro C. J., de Bono J. S. (2012) Cabazitaxel in metastatic castration-resistant prostate cancer. Expert Rev. Anticancer Ther. 12, 1129–1136 [DOI] [PubMed] [Google Scholar]
- 44. Heidenreich A., Scholz H. J., Rogenhofer S., Arsov C., Retz M., Müller S. C., Albers P., Gschwend J., Wirth M., Steiner U., Miller K., Heinrich E., Trojan L., Volkmer B., Honecker F., Bokemeyer C., Keck B., Otremba B., Ecstein-Fraisse E., Pfister D. (2013) Cabazitaxel plus prednisone for metastatic castration-resistant prostate cancer progressing after docetaxel. Results from the German compassionate-use programme. Eur. Urol. 63, 977–982 [DOI] [PubMed] [Google Scholar]
- 45. Mimeault M., Johansson S. L., Batra S. K. (2012) Pathobiological implications of the expression of EGFR, pAkt, NF-κB and MIC-1 in prostate cancer stem cells and their progenies. PloS One 7, e31919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu T., Xu F., Du X., Lai D., Liu T., Zhao Y., Huang Q., Jiang L., Huang W., Cheng W., Liu Z. (2010) Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol. Cell. Biochem. 340, 265–273 [DOI] [PubMed] [Google Scholar]
- 47. Zhang L., Jiao M., Li L., Wu D., Wu K., Li X., Zhu G., Dang Q., Wang X., Hsieh J. T., He D. (2012) Tumorspheres derived from prostate cancer cells possess chemoresistant and cancer stem cell properties. J. Cancer Res. Clin. Oncol. 138, 675–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fan X., Liu S., Su F., Pan Q., Lin T. (2012) Effective enrichment of prostate cancer stem cells from spheres in a suspension culture system. Urol. Oncol. 30, 314–318 [DOI] [PubMed] [Google Scholar]
- 49. Harper L. J., Costea D. E., Gammon L., Fazil B., Biddle A., Mackenzie I. C. (2010) Normal and malignant epithelial cells with stem-like properties have an extended G2 cell cycle phase that is associated with apoptotic resistance. BMC Cancer 10, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yan H., Chen X., Zhang Q., Qin J., Li H., Liu C., Calhoun-Davis T., Coletta L. D., Klostergaard J., Fokt I., Skora S., Priebe W., Bi Y., Tang D. G. (2011) Drug-tolerant cancer cells show reduced tumor-initiating capacity. Depletion of CD44 cells and evidence for epigenetic mechanisms. PloS One 6, e24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thomas S., Shah G. (2005) Calcitonin induces apoptosis resistance in prostate cancer cell lines against cytotoxic drugs via the Akt/survivin pathway. Cancer Biol. Ther. 4, 1226–1233 [DOI] [PubMed] [Google Scholar]
- 52. Yin S., Dong Y., Li J., Fan L., Wang L., Lu J., Vang O., Hu H. (2012) Methylseleninic acid potentiates multiple types of cancer cells to ABT-737-induced apoptosis by targeting Mcl-1 and Bad. Apoptosis 17, 388–399 [DOI] [PubMed] [Google Scholar]
- 53. Gan L., Wang J., Xu H., Yang X. (2011) Resistance to docetaxel-induced apoptosis in prostate cancer cells by p38/p53/p21 signaling. Prostate 71, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 54. Hawley S. A., Gadalla A. E., Olsen G. S., Hardie D. G. (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51, 2420–2425 [DOI] [PubMed] [Google Scholar]
- 55. Kim E., Liu N. C., Yu I. C., Lin H. Y., Lee Y. F., Sparks J. D., Chen L. M., Chang C. (2011) Metformin inhibits nuclear receptor TR4-mediated hepatic stearoyl-CoA desaturase 1 gene expression with altered insulin sensitivity. Diabetes 60, 1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dinarello C. A. (2010) Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 29, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]