Background: LewisX is a glycan moiety expressed by neural stem cells.
Results: LRP1 is a LewisX carrier protein in the mouse CNS; its deletion reduces oligodendrogenesis in the neurosphere model in vitro.
Conclusion: Mouse neural stem cell lineage progression requires LRP1.
Significance: This is the first study investigating LRP1 in the context of CNS development and neural stem cell biology.
Keywords: Carbohydrate, Development, Differentiation, Extracellular Matrix Proteins, Glycoprotein, Lipoprotein-like Receptor (LRP), Neural Stem Cell
Abstract
In the developing and adult CNS multipotent neural stem cells reside in distinct niches. Specific carbohydrates and glycoproteins are expressed in these niche microenvironments which are important regulators of stem cell maintenance and differentiation fate. LewisX (LeX), also known as stage-specific embryonic antigen-1 or CD15, is a defined carbohydrate moiety expressed in niche microenvironments of the developing and adult CNS. LeX-glycans are involved in stem cell proliferation, migration, and stemness. A few LeX carrier proteins are known, but a systematic analysis of the targets of LeX glycosylation in vivo has not been performed so far. Using LeX glycosylation as a biomarker we aimed to discover new glycoproteins with a potential functional relevance for CNS development. By immunoaffinity chromatography we enriched LeX glycoproteins from embryonic and postnatal mouse brains and used one-dimensional nLC-ESI-MS/MS for their identification. We could validate phosphacan, tenascin-C, and L1-CAM as major LeX carrier proteins present in vivo. Furthermore, we identified LRP1, a member of the LDL receptor family, as a new LeX carrier protein expressed by mouse neural stem cells. Surprisingly, little is known about LRP1 function for neural stem cells. Thus, we generated Lrp1 knock-out neural stem cells by Cre-mediated recombination and investigated their properties. Here, we provide first evidence that LRP1 is necessary for the differentiation of neural stem cells toward oligodendrocytes. However, this function is independent of LeX glycosylation.
Introduction
Glycosylation is a common modification of proteins. In the CNS, glycans act mainly as modulators of protein function. They support or inhibit the activation of membrane receptors and control cell-cell or cell-matrix interactions. Moreover, due to their distinct spatiotemporal expression, glycans serve as excellent biomarkers. They allow the identification of subpopulations of cells at defined differentiation stages (1, 2).
A glycan biomarker, utilized to identify and isolate neural stem/progenitor cells (NSPCs),2 is LewisX (3). LewisX (LeX), also known as CD15 or stage-specific embryonic antigen-1, is a glycan motif associated with glycoproteins and glycolipids. Throughout neurogenesis, LewisX is present on radial glia which represent the neural stem cell population in the developing embryonic cortex (4, 5). Anti-LeX antibodies are routinely used to isolate NSPCs from embryonic and adult mouse brains as well as for the purification of neural stem cells after differentiation of human ES cells (2, 4, 6). However, the functional relevance of LeX-glycans in vivo is not well investigated. In vitro data propose that LeX-glycans are involved in migration, proliferation, and maintenance of stemness (7–9).
Glycosylation varies dramatically depending on the tissue, cell type, or time point of investigation. Predictions of which proteins are glycosylated in vivo based on in vitro data are difficult. Using various protein sources, a number of LeX carrier proteins have been identified, including phosphacan, the secreted splice variant of the protein-tyrosine phosphatase receptor-type ζ (Ptprz1), the extracellular matrix protein tenascin-C, L1 cell adhesion molecule (L1-CAM), β1-integrin, lysosomal-associated membrane protein-1, CD24, and Thy-1 (tabularization in Ref. 3). However, a systematic analysis of LeX glycoproteins present during CNS development has not been performed so far. LeX carriers in vivo need to be specified which would allow studying LeX function in a protein-dependent context.
In this study, we used anti-LeX antibodies to isolate glycoproteins from mouse CNS tissue at neurogenic and gliogenic developmental stages. First, this allowed us to further specify the LeX-glycosylated proteins expressed in vivo. Second, the identification of LeX carrier proteins present during critical developmental periods was aimed at detecting glycoproteins that are important for neural stem cell biology and CNS development. In this context, we present LRP1 as a novel LeX-glycosylated protein in the early CNS. Finally, we reveal the importance of LRP1 for neural stem cell differentiation by demonstrating that Lrp1 knock-out NSPCs are impaired in their capacity to generate oligodendrocytes.
EXPERIMENTAL PROCEDURES
Antibodies and Primers
Antibodies are listed in supplemental Table S2, and primer sequences are shown in supplemental Table S3.
Animals
Lrp1flox/flox mice (10) were obtained from the Jackson Laboratories (B6;129S7-Lrp1tm2Her/J) and kept on a CL57/B6 (Charles River) background. Except for Lrp1 knock-out studies, mice of the NMRI strain (Charles River) were used. All animals were housed under standard conditions on a 12-h light/dark cycle with access to water and food ad libitum. The day of the vaginal plug was considered as embryonic day 0.5 (E0.5).
Cultivation of NSPCs
Acutely dissociated cells were obtained from cortices of E14.5 embryos as described previously (5). For the cultivation of NSPCs as neurospheres, 100,000 cells/ml were plated in NSPC medium (DMEM/F12 (1:1), 0.2 mg/ml l-glutamine (all Sigma), 2% (v/v) B27, 100 units/ml penicillin, 100 μg/ml streptomycin (all Invitrogen)) supplemented with 20 ng/ml EGF, 20 ng/ml FGF2 (all Peprotech), and 0.5 unit/ml heparin (Sigma).
Cre Protein Transduction
Recombinant HTN-Cre fusion protein (11) was expressed from pTriEx-HTNC and purified as described previously (12). For transduction, neurospheres from Lrp1flox/flox mice or their wild-type littermates were dissociated using 0.05% (v/v) trypsin-EDTA (Invitrogen), and 50,000 cells were plated on polyornithine- (15 μg/ml; Sigma) and laminin- (2 μg/ml; BD Bioscience) coated 16-mm dishes (Nunc) in NSPC medium supplemented with 10 ng/ml EGF and FGF2 overnight. The next day, the cells were incubated in NSPC medium containing 20 ng/ml growth factors and 0.5 μm Cre recombinase for 8–15 h (12). 24 h after Cre treatment the cells were removed from the dish by trypsinization and cultivated as free-floating neurospheres.
Neural Stem Cell Differentiation Assay
Cre-treated NSPCs were plated on polyornithine- and laminin- (5 μg/ml) coated dishes at a density of 30,000 cells/cm2 in NSPC medium supplemented with 1% (v/v) FCS (Invitrogen) for 7 days. For some experiments single cells derived from second- or third-passage NMRI mice neurosphere were differentiated in the presence of 50 μg/ml immunopurified mAb 487LeX, 75 μm receptor-associated protein (RAP), or 75 μm GST for 7 days. Medium and additives were replaced every 2nd day.
Membrane Preparation
The separation of membrane and soluble proteins from mouse tissue was performed as described previously (13). The membrane pellet was washed with 0.1 m Na2CO3, pH 11, for 30 min, and centrifugation at 100,000 × g was repeated. The membrane pellet was lysed in buffer C (20 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA) supplemented with 1% (v/v) Triton X-100 overnight. Insoluble material was removed at 2000 × g for 20 min. Before chromatography, the lysate was diluted to 0.5% (v/v) Triton X-100.
Immunoaffinity Chromatography
Rat IgMs were purified from hybridoma supernatants as described (14) and coupled to cyanogen bromide-activated Sepharose 4B according to the manufacturer's instructions (Amersham Biosciences). Cleared membrane lysates were circulated over an isotype-matched control column (4860, rat IgM against a glycan-epitope associated exclusively with lipids, not with proteins (15)), followed by anti-LeX mAbs 5750LeX (5) and 487LeX (16) affinity columns for at least 48 h with a flow rate of 0.5 ml/min. Each column was washed with 30 volumes of buffer C + 0.1% (v/v) Triton X-100, 10 volumes of buffer C + 0.1% (v/v) Triton X-100 + 0.5 m NaCl, and 10 volumes of buffer C + 0.1% (v/v) Triton X-100 at 2 ml/min. Before elution, 2 volumes of buffer C + 3 mm n-dodecyl-β-d-maltoside were passed over the column. The bound protein was eluted with 2 volumes of elution buffer, pH 11.5 (100 mm NaCl, 100 mm diethylamine, 1 mm EDTA, 1 mm EGTA, 3 mm n-dodecyl-β-d-maltoside). All washing buffers contained protease inhibitors (1 mm PMSF, 100 μm iodoacetic acid, 10 mm N-ethylmaleimide, 2 mm benzamidine, 1 mm α-aminocaproic acid). The eluate was neutralized with 1 m HCl, adjusted to 20 mm Tris, and concentrated in Centricon centrifugal filter units (Millipore) with a 75-kDa molecular mass cutoff.
One-dimensional nLC-ESI-MS/MS
For in-solution digestion, 200 μg of protein was TCA-precipitated, reconstituted in 8 m urea/100 mm Tris, pH 8, reduced and alkylated sequentially using 5 mm tris(2-carboxyethyl)phosphine and 10 mm iodoacetic acid for 20 min. Proteins were digested with 0.01 μg/μl trypsin (Promega) in 20 μl 2 m urea/100 mm Tris, pH 8, overnight at 37 °C. Samples were desalted using C18 solid phase extraction tips (Varian). For in-gel digestions (17), gel pieces were excised, destained using 25 mm NH4HCO3, 50% (v/v) acetonitrile and digested with 12.5 ng/μl trypsin in 25 mm NH4HCO3, followed by sonication in 50% acetonitrile, 0.5% (v/v) TCA. Protein detection via one-dimensional nLC-ESI-MS/MS on an LTQ Orbitrap was performed as described previously (18). Raw data files were searched against the Mus musculus NCBI database using SEQUEST algorithm embedded in Bioworks 3.3.1 SP1 (Thermo Fisher Scientific). Mass accuracy was set to 10 ppm for precursor ions and 1 atomic mass unit for fragment ions. Only tryptic peptides with at most two missed cleavage sites were accepted. Oxidation of methionine and alkylation (carbamidomethylation) of cysteine were admitted as peptide modifications; glycosylation modifications were not taken into account. Results were filtered according to peptide and protein probability (<0.001), requiring at least two different peptides per protein.
Immunoprecipitation
For precipitation of LeX glycoproteins 20 μl of protein A/G-agarose slurry (Santa Cruz Biotechnology) was incubated with 2.5 μg of unconjugated goat anti-rat IgM for 4 h on a rotating wheel in PBS, followed by incubation with 5750LeX rat IgM antibody or isotype control for 2 h in 1 ml of PBS + 0.1% (w/v) BSA. The beads were then incubated overnight with 500 μg of protein lysate in 50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm EDTA, 5 mm EGTA, 1% (v/v) Triton X-100, 0.1% (v/v) sodium deoxycholate, 0.1% (v/v) SDS, and washed five times. All buffers contained 1 mm PMSF and 2 μg/μl aprotinin. SDS-PAGE samples were boiled in loading buffer (60 mm Tris-HCl, pH 6.8, 2.5% (v/v) SDS, 10% (v/v) glycerol, 5% (v/v) β-mercaptoethanol, 0.01% (w/v) bromphenol blue). For co-precipitation of LRP1 α and β chain, 4 μl of anti-LRP1 rabbit IgG and co-immunoprecipitation buffer (30 mm Tris-HCl, pH 7.4, 150 mm NaCl, 2 mm MgCl2, 2 mm CaCl2, 1% (v/v) Triton X-100) were used. N-Glycanase F (EC 3.5.1.52) digestion was performed as described previously (5). For the precipitation of LRP1 using the RAP as ligand, full-length mouse RAP was cloned into pGEX4T1 (Amersham Biosciences). The RAP-GST fusion protein was expressed in Escherichia coli and purified via its GST tag according to standard protocols. 50 μg of RAP-GST or GST alone was coupled to 20 μl of glutathione-Sepharose 4B (Amersham Biosciences) and incubated with protein lysate in GST buffer (50 mm Tris, pH 7.4, 100 mm NaCl, 2 mm MgCl2, 1% (v/v) Nonidet P-40, 10% (v/v) glycerol).
Western Blot Analysis
Western blotting was performed according to standard protocols as outlined in Ref. 5.
Immunochemistry
Immunohistochemistry was performed as described previously (5) with the following modifications. Cryosections were rehydrated and blocked in PBS containing 10% (v/v) FCS and 0.2% (v/v) Triton X-100. For the detection of Ctip2, antigen retrieval was performed by boiling the sections for 5 min in citrate buffer, pH 6. For immunocytochemistry, refer to Ref. 15.
Documentation and Data Analysis
Images were taken with the Axioplan2 Axiovision Software (Zeiss). For the quantification of O4-positive cells at least 1500 cells were counted per condition in at least four independent experiments. Statistical significance was assessed using the paired two-sample t test.
RESULTS
LeX Carrier Proteins Change from Embryonic toward Postnatal CNS Development
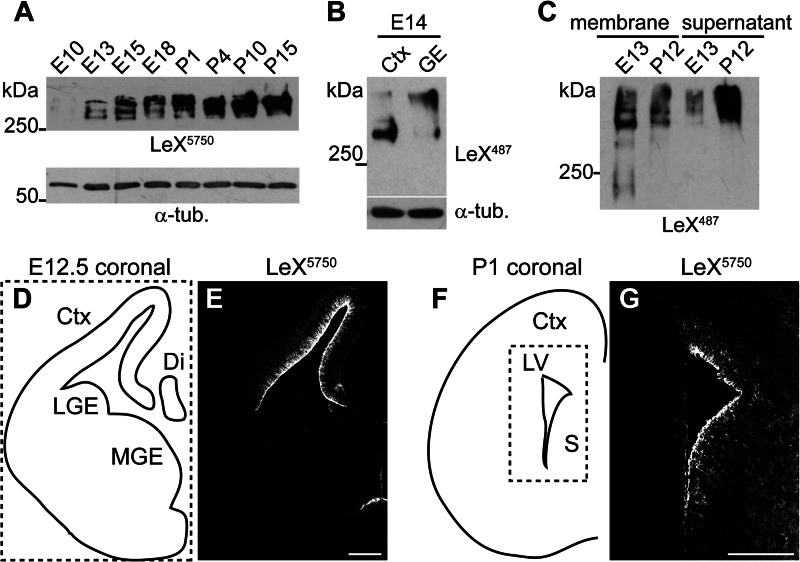
To investigate the expression of LeX glycoproteins in the course of CNS development, we first performed a Western blot analysis of embryonic (E10, E13, E15, and E18) and postnatal (P1, P4, P10, and P15) mouse brains. When we examined LeX in brain tissue lysates using Western blots, we detected three distinct protein bands at E13 and E15, whereas only one was detectable at P15 (Fig. 1A). Next we compared tissue from E14 cortex and ganglionic eminence. The main LeX carrier protein detected in cortical tissue had a lower molecular mass than the major carrier protein in ganglionic eminence (Fig. 1B). This suggested that depending on the time point and tissue investigated, different sets of proteins are LeX-glycosylated. To test whether indeed different LeX carrier proteins can be distinguished, we separated membrane proteins from the membrane-independent fraction containing extracellular matrix and cytosolic proteins. In lysates from E13 mouse brain, the LeX-glycan was detected primarily on membrane proteins. In contrast, at P12, LeX-positive proteins predominated in the membrane-free fraction (Fig. 1C). This suggests that the LeX-glycan distribution shifts from an association with membrane proteins during embryonic development toward an attachment to secreted extracellular matrix proteins at later developmental stages. Furthermore, we investigated LeX expression by immunohistochemistry. Immunohistochemical stainings of coronal forebrain sections at E12.5 showed that LeX expression was very prominent within the embryonic cortex (Fig. 1, D and E), which is in accordance with previous publications (4, 5). In extension of these reports we noted that the staining pattern for LeX changed as development proceeded. Immunostaining of a P1 coronal forebrain section revealed a more diffuse LeX expression in the cortex and the anterior septum. Prominent LeX immunoreactivity at later stages remained detectable exclusively in the stem cell niche microenvironment at the lateral ventricle, in particular the medial wall of the septum and in the subcallosal zone (Fig. 1, F and G).
FIGURE 1.
LeX carrier proteins change during mouse CNS development. A, Western blot with anti-LeX mAb 5750LeX of brain lysates at the indicated developmental stages. Note that the detected LeX-positive proteins shift from embryonic to postnatal stages. B, Western blot against LeX of E14 cortex (Ctx) and ganglionic eminence (GE). C, at E13 most LeX-positive proteins are membrane-associated, whereas at P12 LeX proteins accumulate in the membrane-free (supernatant) fraction after differential centrifugation. D–G, LeX immunostainings of E12.5 (E) or P1 (G) coronal forebrain sections depicted in D and F. L/MGE, lateral/medial GE; Di, diencephalon; LV, lateral ventricle; S, septum, α-tub., α-tubulin. Scale bars, 200 μm
Thus, in contrast to other stem cell marker molecules, LeX expression did not vanish during postnatal development. Instead, it shifted from membrane toward soluble proteins of the extracellular matrix. This observation prompted us to further specify the LeX carrier proteins expressed in vivo.
LeX-glycans Are Associated with Ptrpz1, L1-CAM, Tenascin-C, and LRP1
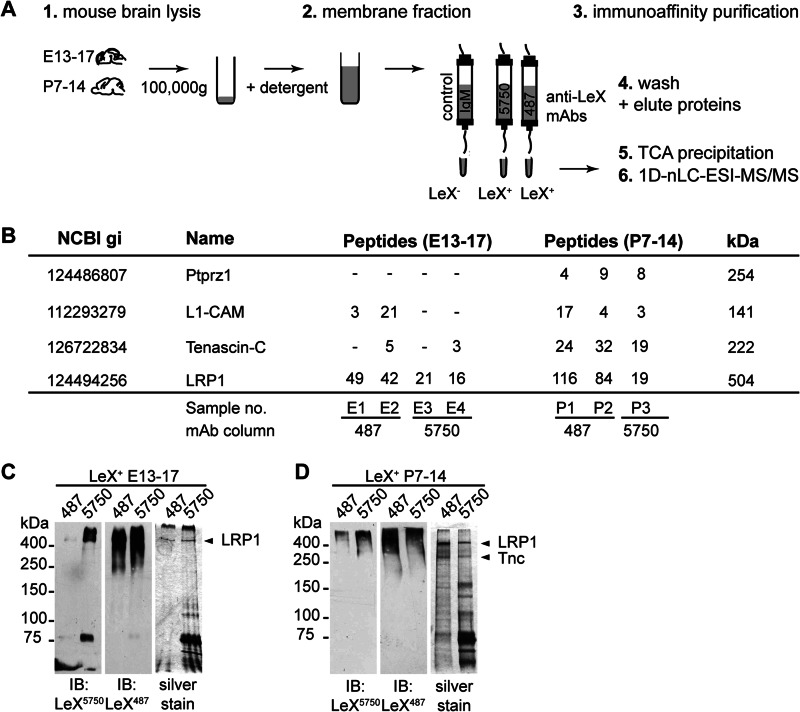
To identify LeX carrier proteins expressed during critical developmental periods, we isolated LeX-positive proteins by immunoaffinity chromatography from embryonic (E13–16) and postnatal (P7–14) mouse brains. These time points correspond to neurogenic and gliogenic developmental phases, respectively. We previously reported that individual anti-LeX mAbs exhibit differences in their affinity toward LeX-containing glycans (5). Therefore, here we used two different antibody clones for our analysis, mAbs 5750LeX and 487LeX, with the intention to capture a broad spectrum of LeX-containing glycans. The detailed LeX structures detected by these mAbs have been characterized previously by glycan array (5).
Because many glycosylated proteins do not focus in distinct bands following SDS-PAGE, we performed a gel-free analysis of the immunopurified protein samples (Fig. 2B). In total, four independent samples purified from embryonic brain and three samples from postnatal brain were analyzed. To verify that LeX proteins were truly enriched via our immunoaffinity purification strategy, we first looked at three LeX-glycosylated proteins that have been previously identified as carriers of LeX-glycans: phosphacan, an isoform of the Ptprz1 gene, tenascin-C, and L1-CAM. Ptprz1, tenascin-C, and L1-CAM peptides could be detected in all samples from postnatal brain. However, Ptprz1 was not detected in embryonic samples, and tenascin-C and L1-CAM were detected only in two of four samples from embryonic brain (Fig. 2B). This correlated with the expression of these glycoproteins which was higher during postnatal development (data not shown). Peptides corresponding to the Ptprz1 gene locus were only found in samples from postnatal brain. A distinction between the different Ptprz1 isoforms was not possible because the detected peptides were localized within the first 400 amino acids of the protein, which are common to all four isoforms of the Ptprz1 gene including membrane-bound and secreted splice variants. In the isotype control sample, tenascin-C, L1-CAM, and Ptprz1 were not detected, demonstrating that LeX-positive proteins could be enriched successfully.
FIGURE 2.
Mass spectrometric identification of LRP1 as a novel LeX glycoprotein. A, experimental setup for the purification of LeX-positive proteins from mouse brains by immunoaffinity chromatography and subsequent identification via one-dimensional nLC-ESI-MS/MS. B, tabular summary of known LeX carrier proteins and LRP1 identified in embryonic and postnatal tissue preparations, listing the peptide counts in each of the seven independently analyzed protein samples. C and D, LeX-positive proteins immunopurified from embryonic or postnatal brains using either anti-LeX mAbs 487LeX or 5750LeX were separated by SDS-PAGE and analyzed by Western blotting or silver staining. Prominent protein bands were excised from the gel and analyzed via one-dimensional nLC-ESI-MS/MS. Note that some LeX carrier proteins such as Ptprz1 expose glycosaminoglycan chains and do not focus into distinct bands in SDS-PAGE. However, these constituents appear as high molecular smear on immunoblots.
Next, we screened the list of identified proteins for membrane associated proteins with a molecular mass of >100 kDa, which corresponds to the molecular range where we detected the strongest LeX signals in the Western blot. We found that LRP1 was enriched in both embryonic and postnatal protein samples. LRP1 is a glycosylated membrane protein with an apparent molecular mass of >600 kDa. In supplemental Table 1 the LRP1 peptides detected in a sample prepared from embryonic brain are listed.
In addition to the gel-free analysis, samples were also analyzed after one-dimensional SDS-PAGE (Fig. 2, C and D). With this approach, two distinct protein bands could be assigned to LRP1 and tenascin-C.
So far, LRP1 expression has been described for various cell types including fibroblasts, Schwann cells, neurons, and glia (19–21). However, NSPCs are the main source of LeX immunoreactivity in the early developing CNS (4, 5). Therefore, we reasoned that LeX glycoforms of LRP1 could be expressed by NSPCs. Further experiments were performed to confirm this hypothesis.
LeX Glycoforms of LRP1 Are Expressed by NSPCs
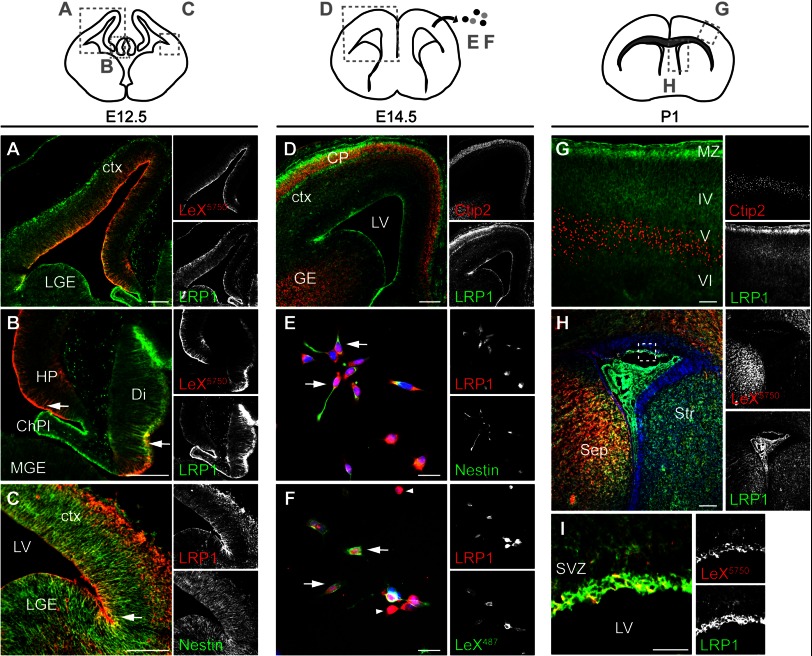
To the best of our knowledge, Lrp1 expression in the developing cortex has not been investigated in detail so far. Lrp1 gene knock-out, however, is lethal at the stage of embryo implantation, leaving unanswered whether Lrp1 is of functional relevance for CNS development (22). Thus, we first studied LRP1 expression in the developing CNS. Immunohistochemical stainings against LRP1 on cryosections of mouse embryos showed that LRP1 immunoreactivity was detectable in the ventricular zone at E12.5. Double labeling with LeX revealed an overlap of LRP1 on cells near the ventricle, as well as in the hippocampal anlage and the diencephalon (Fig. 3, A and B), presumably radial glia. LRP1 expression partially overlapped also with Nestin which suggests that LRP1 is indeed expressed by undifferentiated NSPCs (Fig. 3C). At E14.5 LRP1 expression was up-regulated in the cortical plate, where Ctip2-positive deep layer neurons accumulate but remained also present in the ventricular and subventricular zone (Fig. 3D). Next, we stained acutely dissociated cells obtained from E14.5 cortices against LRP1 and the stem cell markers LeX and Nestin (Fig. 3, E and F). Double stainings revealed that LRP1 was expressed by LeX- and Nestin-positive cells, however, not exclusively. Toward the end of neurogenesis, LRP1 was detectable throughout the cortex. Expression was pronounced in the marginal zone near the pial surface as well as adjacent to the lateral and dorsal wall of the lateral ventricles and the choroid plexus (Fig. 3, G and H). At the dorsal and medial wall of the lateral ventricle, LeX immunoreactivity overlapped with prominent LRP1 expression (Fig. 3I).
FIGURE 3.
LRP1 is expressed by neural stem cells in the embryonic cortex. A–C, E12.5, coronal plane, and cortical sections immunostained against LRP1 (green) and LeX (red) (A and B), or LRP1 (red) and Nestin (green) (C). Note that LRP1 co-localizes with stem cell markers on a subpopulation of radial cells (arrow). D, coronal forebrain section at E14.5 stained for LRP1 (green) and Ctip2 (red) labeling layer VI/V neurons. E and F, acutely dissociated E14.5 cortical cells plated on laminin for 2 h and stained against the indicated markers. Note that some cells with pronounced LRP1 expression do not express stem cell markers (arrowhead). G and H, LRP1 staining (green) at P1 in cortex (G) and lateral ventricle (H). For orientation, sections were co-stained against Ctip2 (G) or LeX (H). Note the up-regulation of LRP1 protein in the marginal zone (G) and adjacent to the lateral ventricle (H). I, magnification of the boxed area in H depicting LRP1- and LeX-double positive cells at the lateral ventricle. In E, F, and H, nuclei are stained with Hoechst (blue). ChPl, choroid plexus; ctx, cortex; M/LGE, medial/lateral ganglionic eminence; HP, hippocampal anlage; Di, diencephalon; SVZ, subventricular zone; LV, lateral ventricle. Scale bars, 100 μm (A–D, G, and H); 25 μm (E, F, and I).
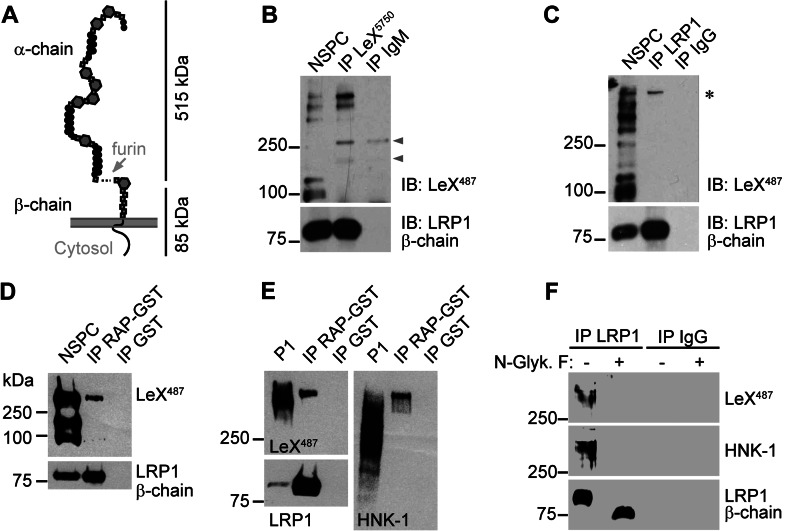
To investigate whether LeX and LRP1 are merely co-expressed on NSPCs or if LRP1 is indeed LeX-glycosylated, we performed immunoprecipitations. We analyzed protein lysates from NSPCs cultivated as neurospheres. In contrast to blots of postnatal whole brain tissue where LeX immunoreactivity ran as a broad smear (Fig. 1, A–C, and Fig. 4E), LeX carrier proteins from NSPC lysates gave rise to multiple more distinct signals. This resembled more closely the Western blot signals seen in embryonic brain lysates (Fig. 1A). To confirm that NSPCs express LeX-carrying glycoforms of LRP1, an immunoprecipitation with anti-LeX mAb 5750LeX was performed (Fig. 4B). The Western blot with anti-LRP1 β-chain-specific antibody confirmed that LRP1 was successfully precipitated from NSPC lysates along with other LeX carrier proteins. In the reverse experiment anti-LRP1 β-chain antibody was used to co-precipitate LRP1 α- and β-chain from NSPC protein lysates (Fig. 4C). A single LeX-positive protein band above 500 kDa was detected (Fig. 4C). Its size correlated with the expected size of the α-chain of LRP1, indicating that mainly the LRP1 α-chain carried the LeX-glycans. (Note that the mature LRP1 protein consists of two noncovalently linked subunits, a 515-kDa α-chain and a 85-kDa β-chain, which run as separate protein bands in the Western blot (Fig. 4A).) Next, we directly precipitated LRP1 α-chain from NSPC and P1 protein lysates using RAP as a ligand. RAP binds to extracellular domains of LDL receptor family members, including LRP1 and LRP2. When using RAP for precipitation, Western blotting again demonstrated the presence of LeX in NSPC and P1 mouse brain lysates (Fig. 4, D and E). Probing against the human natural killer antigen-1 (HNK-1) revealed that other functionally relevant glycan motifs are attached to the precipitated protein backbone in addition to LeX (Fig. 4E). To specify further whether LeX and HNK-1 glycans are N-linked to LRP1, we cleaved N-linked sugars from the protein core by using protein N-glycosidase F. This resulted in complete removal of LeX and HNK-1 immunoreactivity (Fig. 4F). Thus, we conclude that LeX and HNK-1 glycans are N-linked to LRP1 α-chain.
FIGURE 4.
NSPCs express LeX glycoforms of LRP1. A, LRP1 domain structure. Note that LRP1 α- and β-chain are noncovalently linked and run as separate subunits in SDS-PAGE. B, immunoprecipitation (IP) of LeX from NSPCs cultivated as neurospheres. The Western blot against LeX reveals multiple LeX-positive proteins in NSPC input protein lysates. LRP1 precipitates with LeX-positive proteins shown by the detection of LRP1 β-chain. Arrowheads label unspecific antibody signals. C, LRP1 immunoprecipitation from NSPCs yielding a single LeX-positive protein with a size correlating to the expected size of the LRP1 α-chain (asterisk). LRP1 precipitation was confirmed by detection of LRP1 β-chain. D and E, RAP-mediated affinity purification of LRP1 from NSPC (D) or P1 brain (E) lysates. The RAP ligand is positive for LeX and HNK-1. F, Western blots after antibody-mediated immunoprecipitation of LRP1 and subsequent N-glycanase F (N-Glyk. F) treatment. Note that LeX and HNK-1 immunoreactivity on LRP1 α-chain are lost after enzymatic removal of N-linked glycans.
LRP1 Promotes the Generation of Oligodendrocytes
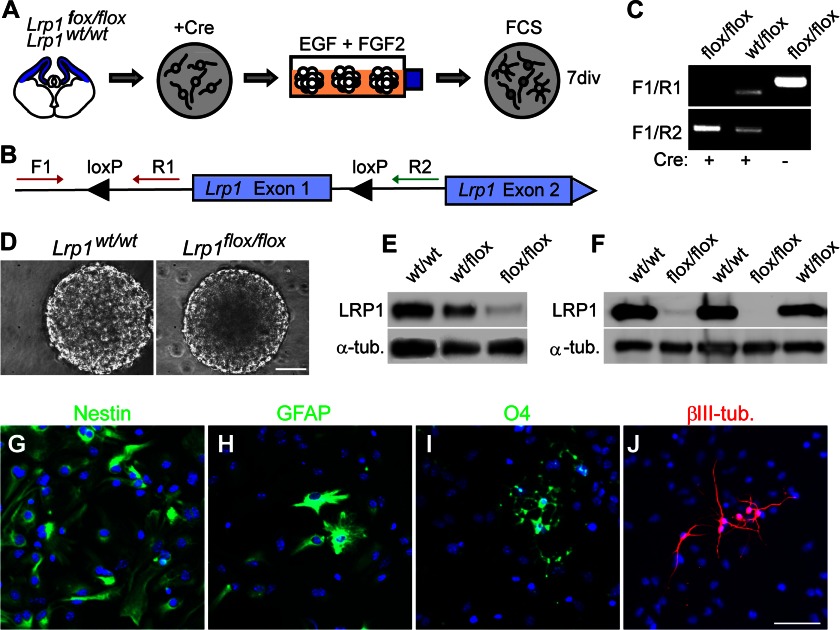
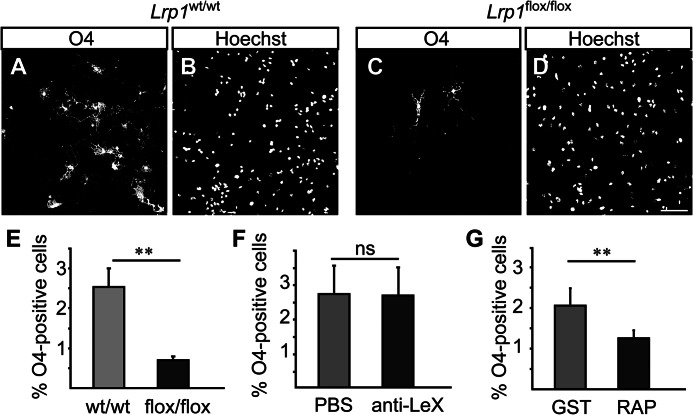
Because LRP1 expression seemed to be regulated in a spatiotemporal manner, we were interested, whether LRP1 is also involved in the regulation of CNS development. To investigate the function of LRP1 we generated Lrp1 knock-out NSPCs from Lrp1flox/flox mice. We adapted previously published protocols based on cell-permeant Cre recombinase that result in recombination efficiencies of >90% (12). The protocol is outlined in Fig. 5A, NSPCs were derived from cortical tissue of Lrp1flox/flox mice and Lrp1wt/wt littermates and treated with Cre recombinase to induce recombination leading to deletion of exon 1 of the Lrp1 gene (10). Recombination could be verified by PCR (Fig. 5, B and C). Lrp1 knock-out NSPCs proliferated and could be maintained in EGF and FGF2 as neurospheres (Fig. 5D). LRP1 protein was barely detectable in Lrp1 knock-out NSPC cultures, also after several weeks of cultivation (Fig. 5, E and F). This indicated that incompletely recombined cells did not overgrow the culture upon passaging. Lrp1 knock-out NSPCs retained the capacity to differentiate into all major neural cell types (Fig. 5, G–J). In general, Lrp1 does not seem to be necessary for stem cell proliferation and maintenance of stemness in the neurosphere model. However, upon differentiation, Lrp1 knock-out NSPCs generated three times less oligodendrocyte precursor cells, as identified by O4 staining, compared with Lrp1wt/wt cells (Lrp1flox/flox 0.69% ± 0.10%; Lrp1wt/wt 2.53% ± 0.46%, p = 0.004, Fig. 6, A–E). This suggests that Lrp1 supports differentiation toward an oligodendroglial cell fate.
FIGURE 5.
Lrp1 knock-out NSPCs proliferate and differentiate in vitro. A, schematic diagram outlining the generation of Lrp1 knock-out NSPCs. NSPCs obtained from Lrp1flox/flox mice or Lrp1wt/wt littermates were treated with cell-permeant Cre recombinase, expanded as neurospheres and differentiated. B, scheme of the Lrp1 gene locus illustrating the location of the loxP sites and the primers used to assess recombination. C, exemplary PCR verifying successful recombination in Cre-treated cells. D, phase contrast image of neurospheres derived from Cre-treated Lrp1flox/flox or Lrp1wt/wt cells. E, Western blot demonstrating the reduction of LRP1 protein in NSPCs after recombination. F, LRP1 expression permanently eliminated in Cre-treated Lrp1flox/flox NSPCs even when passaged over a time period of 6 weeks. G–J, immunostainings against cell type-specific biomarkers after differentiation of Lrp1 knock-out. Nestin (neural stem precursor cells (NSPCs)); GFAP, glial fibrillary acidic protein (astrocytes); O4 (oligodendrocytes); βIII-tubulin, (young neurons). Scale bar, 50 μm.
FIGURE 6.
Lrp1 knock-out inhibits oligodendroglial differentiation. A–D, immunolabeling of oligodendrocytes by O4 (A and C) in Lrp1 wild-type (Lrp1wt/wt), or knock-out (Lrp1flox/flox) NSPC cultures, differentiated for 7 days. The corresponding Hoechst stained nuclei are shown in B and D. E, quantification of O4-positive cells in wild-type (wt/wt) or knock-out (flox/flox) cultures. F, differentiation of wild-type NSPCs in the presence of LeX-epitope blocking mAb 487LeX. The anti-Lex treatment does not influence the percentage of O4-positive cells compared with PBS-treated control. G, differentiation of wild-type NSPCs in the presence of RAP reducing the amount of O4-positive cells compared with GST-treated control. Scale bar, 75 μm; ns, not significant. Data are expressed as mean ± S.D. (error bars), n = 4; **, p ≤ 0.01.
To address whether LRP1 function is dependent on LeX glycosylation, we blocked LeX-glycans by adding mAb 487LeX to the cell culture medium. This approach has previously been shown to affect cell migration and process formation (7, 23). However, the number of O4-positive cells was not significantly altered upon differentiation in the presence of mAb 487LeX (487LeX, 2.79% ± 0.82%; control, 2.74% ± 1.03%; p = 0.70, Fig. 6F). In contrast, blocking the extracellular part of LRP1 by adding RAP to the cell culture medium resulted in a reduction of O4-positive cells. The difference, however, was not as dramatic as after complete Lrp1 knock-out (RAP, 1.23% ± 0.19%; control, 2.03% ± 0.42%, Fig. 6G). In conclusion, Lrp1 function in oligodendroglial lineage progression appears to be independent of LeX glycosylation.
In summary, using LeX-glycans as a biomarker we identified LRP1 as a novel LeX glycosylation target expressed by NSPCs. Our investigations provide the first evidence of a functional role of LRP1 in neural stem cell differentiation.
DISCUSSION
Studies based on in vitro models demonstrate that LeX-glycans are involved in NSPC proliferation, migration, and maintenance of stemness (7–9). However, proof underlining the significance of LeX glycosylation in vivo is lacking. The identification of LeX carrier proteins present in the mouse CNS provides a first step toward understanding the role of LeX glycosylation in vivo. Our results propose that the glycoproteins Ptprz1, L1-CAM, and tenascin-C are major LeX glycosylation targets during postnatal development. Moreover, we characterized LRP1 as a new membrane-associated LeX carrier protein on NSPCs which is involved in oligodendrogenesis.
To date, LRP1 function is well investigated in the context of myelin phagocytosis and Alzheimer disease (21, 24). Our data now propose that LRP1 is additionally involved in CNS development and neural stem cell biology. However, LRP1 may not be necessary for stem cell maintenance per se, as Lrp1 knock-out NSPCs can be expanded in vitro and retain their ability to differentiate. Nevertheless, oligodendrocyte lineage progression is severely impaired.
Because LRP1 is capable of interacting with >30 ligands (20), there exist many scenarios of how LRP1 could modify NSPC differentiation. LRP1 mediates endocytosis of various growth factors such as PDGF and TGFβ that are essential regulators of cell proliferation and differentiation in the CNS (25). Recently, Gan et al. reported that apoE, an LDL receptor family ligand, stimulates the formation of oligodendrocytes (26). However, this study did not further specify the apoE receptor involved. Our data now strongly suggest LRP1 as the receptor mediating this effect. Moreover, in addition to its role as apoE receptor, it has been suggested that LRP1 can modulate Wnt signaling by binding to the Wnt co-receptor Frizzled (27). Wnt signaling is an important regulator of the timing of oligodendrocyte precursor differentiation (28). Additionally, LRP1 together with thrombospondin-2 has been shown to stimulate Notch activity by transendocytosis (29). Notch signaling is responsible for stem cell maintenance and gliogenic versus neurogenic fate choices (30). Last but not least, LRP1 is also involved in lipid uptake, which could also affect cell differentiation upon Lrp1 deletion (20). Which of the before-mentioned pathways is responsible for LRP1 function during oligodendrocyte differentiation remains to be investigated in the future.
Interestingly, LeX-glycans are also involved in two of these pathways: Wnt and Notch signaling (4, 9). The general abrogation of LeX-glycans by siRNA-mediated knockdown of fucosyltransferase 9 leads to a reduced expression of Numb and Hes5, downstream molecules in the Notch signaling pathway (9). However, fucosyltransferase 9 knockdown experiments performed so far failed to demonstrate direct effects on cell specification. Also, by antibody-mediated LeX epitope blocking we could not observe significant modifications of oligodendrocyte numbers in this study. Therefore, it remains unclear whether, apart from modulating cell proliferation (8, 9), LeX-glycans also influence cell fate choices. The data rather suggest that the role of LRP1 during oligodendrogenesis is independent of LeX glycosylation.
Interestingly, we found only a subfraction of LRP1 to be LeX-glycosylated. Glycosylation is a complex process whereby the expression of a specific set of glycosyltransferases and the availability of the individual sugar molecules result in the synthesis of a glycan chain. Hence, it is not surprising to detect different glycoforms of a protein depending on the developmental time point, tissue, and species where the protein is investigated. Remarkably, although glycosylation is highly variable, the LeX-glycan motif is conserved on neural stem cells also in other species. In humans, however, LeX-glycans appear exclusively upon neural differentiation. In mice, embryonic stem cells express LeX already prior to neural lineage specification (31). In conclusion, our screen for LeX carrier proteins, which led to the identification of LRP1, sheds new light on a well known glycoprotein by suggesting a new role during CNS development.
Acknowledgment
We thank Tanja Bojarzyn for assisting in the MS measurements.
This work was supported by the German Research Foundation Deutsche Forschungsgemeinschaft Grants Fa 159/16-1 (to A. F.) and ED79/1-2 (to F. E.) and by the Federal Ministry of Education and Research Grant BMBF 01GN0503 (to A. F.).

This article contains supplemental Tables S1–S3.
- NSPC
- neural stem/progenitor cell
- En
- embryonic day n
- ESI
- electrospray ionization
- HNK-1
- human natural killer antigen-1
- L1-CAM
- L1 cell adhesion molecule
- LeX
- LewisX
- LRP1
- low density lipoprotein receptor-related protein 1
- MS/MS
- tandem MS
- Pn
- postnatal day n
- Ptprz1
- protein-tyrosine phosphatase receptor type ζ
- RAP
- receptor-associated protein.
REFERENCES
- 1. Yanagisawa M., Yu R. K. (2007) The expression and functions of glycoconjugates in neural stem cells. Glycobiology 17, 57R–74R [DOI] [PubMed] [Google Scholar]
- 2. Pruszak J., Sonntag K. C., Aung M. H., Sanchez-Pernaute R., Isacson O. (2007) Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem Cells 25, 2257–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennen E., Faissner A. (2012) LewisX: a neural stem cell-specific glycan? Int. J. Biochem. Cell Biol. 44, 830–833 [DOI] [PubMed] [Google Scholar]
- 4. Capela A., Temple S. (2006) LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment, and binds Wnt-1. Dev. Biol. 291, 300–313 [DOI] [PubMed] [Google Scholar]
- 5. Hennen E., Czopka T., Faissner A. (2011) Structurally distinct LewisX glycans distinguish subpopulations of neural stem/progenitor cells. J. Biol. Chem. 286, 16321–16331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Capela A., Temple S. (2002) LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35, 865–875 [DOI] [PubMed] [Google Scholar]
- 7. Yanagisawa M., Taga T., Nakamura K., Ariga T., Yu R. K. (2005) Characterization of glycoconjugate antigens in mouse embryonic neural precursor cells. J. Neurochem. 95, 1311–1320 [DOI] [PubMed] [Google Scholar]
- 8. Li Y. L., Wu G. Z., Zeng L., Dawe G. S., Sun L., Loers G., Tilling T., Cui S. S., Schachner M., Xiao Z. C. (2009) Cell surface sialylation and fucosylation are regulated by the cell recognition molecule L1 via PLCγ and cooperate to modulate embryonic stem cell survival and proliferation. FEBS Lett. 583, 703–710 [DOI] [PubMed] [Google Scholar]
- 9. Yagi H., Saito T., Yanagisawa M., Yu R. K., Kato K. (2012) Lewis X-carrying N-glycans regulate the proliferation of mouse embryonic neural stem cells via the Notch signaling pathway. J. Biol. Chem. 287, 24356–24364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rohlmann A., Gotthardt M., Willnow T. E., Hammer R. E., Herz J. (1996) Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol. 14, 1562–1565 [DOI] [PubMed] [Google Scholar]
- 11. Peitz M., Pfannkuche K., Rajewsky K., Edenhofer F. (2002) Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc. Natl. Acad. Sci. U.S.A. 99, 4489–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nolden L., Edenhofer F., Haupt S., Koch P., Wunderlich F. T., Siemen H., Brüstle O. (2006) Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nat. Methods 3, 461–467 [DOI] [PubMed] [Google Scholar]
- 13. Faissner A., Kruse J. (1990) J1/tenascin is a repulsive substrate for central nervous system neurons. Neuron 5, 627–637 [DOI] [PubMed] [Google Scholar]
- 14. Bazin H., Xhurdebise L. M., Burtonboy G., Lebacq A. M., De Clercq L., Cormont F. (1984) Rat monoclonal antibodies. I. Rapid purification from in vitro culture supernatants. J. Immunol. Methods 66, 261–269 [DOI] [PubMed] [Google Scholar]
- 15. Czopka T., Hennen E., von Holst A., Faissner A. (2009) Novel conserved oligodendrocyte surface epitope identified by monoclonal antibody 4860. Cell Tissue Res. 338, 161–170 [DOI] [PubMed] [Google Scholar]
- 16. Streit A., Yuen C. T., Loveless R. W., Lawson A. M., Finne J., Schmitz B., Feizi T., Stern C. D. (1996) The Lex carbohydrate sequence is recognized by antibody to L5, a functional antigen in early neural development. J. Neurochem. 66, 834–844 [DOI] [PubMed] [Google Scholar]
- 17. Shevchenko A., Wilm M., Vorm O., Mann M. (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 18. Haussmann U., Poetsch A. (2012) Global proteome survey of protocatechuate- and glucose-grown Corynebacterium glutamicum reveals multiple physiological differences. J. Proteomics 75, 2649–2659 [DOI] [PubMed] [Google Scholar]
- 19. Bu G., Maksymovitch E. A., Nerbonne J. M., Schwartz A. L. (1994) Expression and function of the low density lipoprotein receptor-related protein (LRP) in mammalian central neurons. J. Biol. Chem. 269, 18521–18528 [PubMed] [Google Scholar]
- 20. Lillis A. P., Mikhailenko I., Strickland D. K. (2005) Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J. Thromb. Haemost. 3, 1884–1893 [DOI] [PubMed] [Google Scholar]
- 21. Gaultier A., Wu X., Le Moan N., Takimoto S., Mukandala G., Akassoglou K., Campana W. M., Gonias S. L. (2009) Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis. J. Cell Sci. 122, 1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herz J., Clouthier D. E., Hammer R. E. (1992) LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71, 411–421 [DOI] [PubMed] [Google Scholar]
- 23. Streit A., Nolte C., Rásony T., Schachner M. (1993) Interaction of astrochondrin with extracellular matrix components and its involvement in astrocyte process formation and cerebellar granule cell migration. J. Cell Biol. 120, 799–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q., Trotter J., Zhang J., Peters M. M., Cheng H., Bao J., Han X., Weeber E. J., Bu G. (2010) Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age-dependent synapse loss and neurodegeneration. J. Neurosci. 30, 17068–17078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lillis A. P., Van Duyn L. B., Murphy-Ullrich J. E., Strickland D. K. (2008) LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 88, 887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gan H. T., Tham M., Hariharan S., Ramasamy S., Yu Y. H., Ahmed S. (2011) Identification of apoE as an autocrine/paracrine factor that stimulates neural stem cell survival via MAPK/ERK signaling pathway. J. Neurochem. 117, 565–578 [DOI] [PubMed] [Google Scholar]
- 27. Zilberberg A., Yaniv A., Gazit A. (2004) The low density lipoprotein receptor-1, LRP1, interacts with the human frizzled-1 (HFz1) and down-regulates the canonical Wnt signaling pathway. J. Biol. Chem. 279, 17535–17542 [DOI] [PubMed] [Google Scholar]
- 28. Fancy S. P., Baranzini S. E., Zhao C., Yuk D. I., Irvine K. A., Kaing S., Sanai N., Franklin R. J., Rowitch D. H. (2009) Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meng H., Zhang X., Lee S. J., Strickland D. K., Lawrence D. A., Wang M. M. (2010) Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. J. Biol. Chem. 285, 23047–23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Louvi A., Artavanis-Tsakonas S. (2006) Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 [DOI] [PubMed] [Google Scholar]
- 31. Satomaa T., Heiskanen A., Mikkola M., Olsson C., Blomqvist M., Tiittanen M., Jaatinen T., Aitio O., Olonen A., Helin J., Hiltunen J., Natunen J., Tuuri T., Otonkoski T., Saarinen J., Laine J. (2009) The N-glycome of human embryonic stem cells. BMC Cell Biol. 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]