Background: Extracellular matrices regulate megakaryocyte function in the bone marrow environment.
Results: Collagen receptor discoidin domain receptor 1 (DDR1) regulates human megakaryocyte migration by the modulation of phosphatase SHP1 activity.
Conclusion: DDR1 is a new regulator of megakaryocyte function.
Significance: This is the first evidence that the new receptor DDR1 is involved in the complex modulation of megakaryocyte-collagen interactions.
Keywords: Bone Marrow, Extracellular Matrix, Hematopoiesis, Phosphatase, Receptors, Discoidin Domain Receptor 1, Megakaryocytes
Abstract
Growing evidence demonstrates that extracellular matrices regulate many aspects of megakaryocyte (MK) development; however, among the different extracellular matrix receptors, integrin α2β1 and glycoprotein VI are the only collagen receptors studied in platelets and MKs. In this study, we demonstrate the expression of the novel collagen receptor discoidin domain receptor 1 (DDR1) by human MKs at both mRNA and protein levels and provide evidence of DDR1 involvement in the regulation of MK motility on type I collagen through a mechanism based on the activity of SHP1 phosphatase and spleen tyrosine kinase (Syk). Specifically, we demonstrated that inhibition of DDR1 binding to type I collagen, preserving the engagement of the other collagen receptors, glycoprotein VI, α2β1, and LAIR-1, determines a decrease in MK migration due to the reduction in SHP1 phosphatase activity and consequent increase in the phosphorylation level of its main substrate Syk. Consistently, inhibition of Syk activity restored MK migration on type I collagen. In conclusion, we report the expression and function of a novel collagen receptor on human MKs, and we point out that an increasing level of complexity is necessary to better understand MK-collagen interactions in the bone marrow environment.
Introduction
Megakaryocyte (MK)2 migration from the osteoblastic to the vascular niche is required to complete maturation, extend proplatelets, and release newly generated platelets into the bloodstream (1–3). Therefore, dynamic regulation of MK motility, within the bone marrow environment, is critical for functional platelet production. A variety of extracellular matrix (ECM) components, differently located in the bone marrow niches, may be involved in regulating mature MK migration toward the vascular niche (4, 5). Among these, type I collagen is the most abundant ECM of the osteoblastic niche and has been reported to regulate different phases of MK development (6). To this regard, we have recently demonstrated that the tensile strength of fibrils in type I collagen structure also represents a major determinant in the regulation of MK migration and platelet formation (7–9). Integrin α2β1 and GPVI were extensively studied as regulators of MK function in the bone marrow matrix environment, whereas the expression and function of other receptors on human MKs are unknown (10–14). Discoidin domain receptors (DDR1 and DDR2) are tyrosine-kinase collagen receptors (receptor tyrosine kinases) that are stimulated by fibrillar and basement membrane collagens and mediate cell adhesion and migration in different tissues (15, 16). Both DDR1 and DDR2 bind to several collagen subtypes, but the receptors require an intact triple helical domain for signaling, as denatured collagen, or gelatin, does not induce signaling through DDRs. Although receptor tyrosine kinases reach maximum activation within minutes after ligand binding, DDR1 has a particular kinetics of activation, slow and sustained, suggesting that this receptor may regulate longer term signals (17). After its activation, subsequent to the autophosphorylation, the receptor interacts in a phosphotyrosine dependent manner with a series of Src homology 2/3 (SH2/3) and protein tyrosine binding (PTB) domain-containing proteins, triggering DDR1 downstream responses (18).
Spleen tyrosine kinase (Syk) is a 72-KDa cytoplasmic nonreceptor tyrosine kinase, widely expressed in hemopoietic cells, characterized by a C-terminal kinase domain, an adjacent linker region, and an N-terminal tandem SH2 domain. Numerous studies demonstrated that Syk participates not only in regulating cell division and proliferation, but also cell invasiveness and metastatic capacity, in particular being down-regulated in metastatic cancer (19–21). Interestingly, it has been suggested that interaction of DDR1 and Syk may regulate epithelial cell motility (22).
The phosphorylation status of receptor tyrosine kinases is tightly regulated by the concerted activity of kinases and phosphatases (23). DDR1 was reported to recruit the non-transmembrane phosphatase SHP2 to suppress integrin signaling (24). Interestingly, although SHP2 is expressed ubiquitously, the expression of SHP1, another member of the family, is restricted to the hemopoietic lineages. Further, Huang et al. (25) demonstrated that DDR1 interaction with myosin IIA regulates cell spreading and migration on type I collagen in epithelial cells.
Altogether, these data point out that DDR1 may represent an important new regulator of MK function. Thus, in the present study, we show, for the first time, that human MKs express DDR1 that is activated upon MK adhesion on fibrillar type I collagen. Further, we demonstrate that DDR1 regulates MK Syk-mediated migration through activation of the tyrosine phosphatase SHP1. These experiments provide evidence that new receptors are involved in the elaborate regulation of MK function in the ECM component environment.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Type I collagen was purified as described previously (26). The DDR1-Fc recombinant protein was from R&D systems (Minneapolis, MN). Protein A-Sepharose from Staphylococcus aureus was from Sigma-Aldrich. Sodium stibogluconate from Calbiochem. R406 was from Axon Medchem (Groningen, The Netherlands). The following antibodies were used: rabbit polyclonal anti-DDR1 (C-20) (Santa Cruz Biotechnology; Santa Cruz, CA); mouse monoclonal anti-CD61 (clone SZ21) (Immunotech; Marseille, France); Alexa Fluor-conjugated antibodies (Invitrogen); rabbit polyclonal anti-non-muscle myosin IIA (clone BT-567) (BTI; Oklahoma City, OK); rabbit polyclonal anti-phospho-MLC2 (Ser-19) (Cell Signaling; Danvers, MA); mouse monoclonal anti-phosphotyrosine (clone 4G10) (Millipore; Billerica, MA); rabbit monoclonal anti-phospho-Syk (Tyr-525/526) (clone C87C1) (Cell Signaling); rabbit polyclonal anti-Syk (N-19) (sc-1077) (Santa Cruz Biotechnology); rabbit monoclonal anti-phospho-MAPK 1/2 (ERK 1/2) (Thr-185/Tyr-187) (clone AW39) (Millipore); mouse monoclonal anti-MAPK 1/2 (ERK 1/2) (clone 3A7) (Cell Signaling); rabbit polyclonal anti-SHP1 (sc-287) (Santa Cruz Biotechnology); mouse monoclonal anti-SHP2 (sc-7384) (Santa Cruz Biotechnology); rabbit polyclonal anti-phospho-SHP1 (Tyr-536) (Abcam; Cambridge, UK); mouse monoclonal anti-β-actin (Sigma-Aldrich); mouse monoclonal anti-activated β1 integrin (HUTS-4) (Millipore); and mouse monoclonal anti-LAIR-1 (sc-59281) (Santa Cruz Biotechnology); the anti-GPVI antibody (clone 9E18) was kindly provided by M. Jandrot-Perrus (INSERM, U698, Hôpital Bichat, Paris, France). The anti-DDR1 blocking peptide was from Santa Cruz Biotechnology; Fc receptor (FcR) blocking solution was from Miltenyi Biotec (Bergisch Gladbach, Germany). DMEM and FBS were from Invitrogen (Invitrogen). Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse secondary antibodies were from Bio-Rad (Milan, Italy). Precision Plus protein standard was from Bio-Rad.
Cell Culture
Human umbilical cord blood was collected following normal pregnancies and deliveries upon informed consent of the parents, in accordance with the ethical committee of the IRCCS Policlinico San Matteo Foundation and the principles of the Declaration of Helsinki. CD34+ cells were separated and cultured as described previously (27). Skin human fibroblasts were cultured in DMEM supplemented with 10% FBS.
RT-PCR and Quantitative RT-PCR
CD61+ MKs at day 13 of culture were separated, using the immunomagnetic beads technique (Miltenyi Biotec), and total cellular RNA was extracted using the mammalian GeneElute total RNA kit (Sigma-Aldrich) and reverse-transcribed to cDNA using MuLV reverse transcriptase (Applera; Monza, Italy) following the manufacturer's instructions. For DDR1 and β2-microglobulin expression, one-tenth of the resulting cDNA was amplified using the following primers: DDR1 5′-TCTATGCTGGGGACTATTACCG-3′ and 5′-GTCACTCGCAGTCGTGAACTT-3′, β2 microglobulin 5′-CCCCCACTGAAAAAGATGAGT-3′ and 5′-TGATGCTGCTTACATGTCTCG-3′. 20 μl of the PCR products was loaded on an ethidium bromide-stained 1% agarose gel. For quantitative expression analysis of DDR1, real-time PCR was performed using the Rotor-Gene 6000 (Eurogentec, San Diego, CA), and one-twentieth of the resulting cDNA was amplified in triplicate using the MESA GREEN quantitative PCR MasterMix Plus for SYBR assay, No ROX sample (Eurogentec). Rotor-Gene 6000 series software Version 1.7 was used for the comparative concentration analysis (8).
Immunofluorescence, Confocal, and Atomic Force Microscopy Analysis
To evaluate DDR1 distribution on the MK surface, cells were fixed and stained with anti-DDR1 and anti-CD61 antibodies both diluted 1:100 as described previously (27). Analyses by conventional fluorescence and confocal microscopy were performed with an Olympus BX51 microscope using a 20×/0.5 UPlanF1 objective (Olympus; Watford, UK) and a TCS SPII confocal laser scanning microscopy system equipped with a DM IRBE inverted microscope using a 40× oil NA 1.25 objective (Leica; Bensheim, Germany), as described previously (27, 28). To analyze matrix structures in different conditions, glass coverslips were coated with type I collagen, as described previously (27), in the presence or absence of DDR1-Fc, for 16 h at 37 °C in PBS and then observed by tapping-mode atomic force microscopy on a Digital Instruments multimode NanoScope III/a SPM (Digital Instruments, Santa Barbara, CA) with Olympus OTR 8 oxide-sharpened silicon nitride probes.
Binding Assays
96-well microtiter plates were coated overnight at 4 °C with the solutions of collagenous samples in NaCl/Pi (200 μl/well). Control wells were coated with 200 μl containing 5 μg of BSA in NaCl/Pi. All analyses were done at least in triplicate. After rinsing with 0.15 m NaCl, 0.05% (v/v) Tween 20, the wells were incubated with 200 μl of 1% (w/v) BSA in NaCl/Pi for 1 h at room temperature. After rinsing as described above, the coated wells were incubated for 2 h at room temperature with 20 μg/ml DDR1-Fc blocking molecule in 200 μl of NaCl/Pi, 0.05% (v/v) Tween 20. For every solid phase experiment, control for dose-dependent, nonspecific binding to coated BSA wells was performed, under identical conditions. Bound DDR1-Fc were incubated for 2 h with HRP-conjugated anti-human IgG1 antibodies for detection. Horseradish peroxidase was diluted 1:1000 in 2 mg/ml BSA solution followed by a rinse and by the substrate solution (0.04% o-phenylenediamine dihydrochloride and 0.04% (v/v) hydrogen peroxide in a buffer containing 514 mm disodium hydrogen phosphate, 24.3 mm citric acid, pH 5). Color development was stopped by adding 100 μl of 3 m HCl, and the absorbance was measured at 490–655 nm using a microplate reader.
Preparation of Platelet Lysates from Washed Human Platelets for Western Immunoblotting Analysis
Peripheral blood from healthy subjects was collected in citric acid/citrate/dextrose (ACD) as anticoagulant (blood:ACD, 6:1, v/v) in the presence of 0.2 units/ml apyrase and 1 μm PGE1. Platelet-rich plasma was prepared by centrifuging blood samples at 200 × g for 10 min. The platelet-rich plasma was then aspirated, centrifuged at 1200 × g for 10 min, and washed with PIPES buffer (20 mm PIPES and 136 mm NaCl, pH 6.5). Washed platelets were then lysed in sodium dodecyl sulfate (SDS) buffer (0.5 m Tris, pH 6.8, 2% SDS, 10% glycerol), in the presence of a 2% protease inhibitor mixture (Sigma), on ice for 30 min. Lysates were clarified by centrifugation at 15,700 × g at 4 °C for 15 min.
Immunoprecipitation and Western Blotting
MKs, 1 × 106 cells/condition, were seeded on 25 μg/ml type I collagen-coated 6-well plates or on plastic. Where indicated, type I collagen was incubated with 20 μg/ml DDR1-Fc for 4 h at 4 °C, as described previously (29, 30). Cells were left to adhere for 16 h at 37 °C and 5% CO2 and then lysed in Hepes-glycerol lysis buffer (50 mm Hepes, 150 mm NaCl, 10% glycerol, 1% Triton X-100, 1.5 mm MgCl2, 1 mm EGTA, 100 mm NaF, 1 mm PMSF, 1 mm Na3VO4, 1 μg/ml leupeptin, 1 μg/ml aprotinin). Lysates were centrifuged at 14,000 × g at 4 °C for 15 min. For immunoprecipitation, cellular lysates were precleared by incubation with protein A-Sepharose. Precleared lysates were incubated with 2 μg of anti-DDR1 or anti-LAIR-1 or anti-SHP1 (Santa Cruz Biotechnology) for 4 h at 4 °C on a rotatory shaker followed by adding 100 μl of 50 mg/ml protein A-Sepharose and incubation overnight at 4 °C on a rotatory shaker. Beads were washed three times in lysis buffer. Protein were eluted with Laemmli buffer at 90 °C for 5 min and separated by nonreducing SDS-PAGE for activated β1 integrin and for dimeric GPVI, or reducing SDS-PAGE for other proteins, and transferred to a polyvinylidene fluoride (PVDF) membrane. Western blots were developed with Western Immobilon (Millipore). Densitometric analyses were performed after ECL chemiluminescence detection using quantity one software (Bio-Rad).
Evaluation of Cell Adhesion
To analyze MK adhesion onto type I collagen, 12-mm glass coverslips were coated with 25 μg/ml type I collagen as described previously (27). Where indicated, type I collagen was incubated with 20 μg/ml DDR1-Fc for 4 h at 4 °C (29, 30) or with an unrelated IgG as control. Cells at day 13 of culture were harvested and allowed to adhere for 16 h (1 × 105 cells/well) at 37 °C and 5% CO2. Where indicated, cells were preincubated with FcR blocking solution for 15 min, according to the manufacturer's instructions, before being seeded. Adhering cells were washed with PBS, and after being fixed and stained with anti-CD61 antibody and Hoechst 33258, they were counted by fluorescence microscopy (27).
Migration Assays
MK migration and invasion assay were performed in Transwell migration chambers (8 μm, Millipore) coated with 25 μg/ml type I collagen containing or not containing 20 μg/ml DDR1-Fc or an unrelated IgG as control. Briefly, 50 μl of collagen solution was overlaid on polycarbonate insert overnight at 4 °C. MKs (25 × 103 in 100 μl) were seeded on the upper well in 100 μl of StemSpan and incubated at 37 °C and 5% CO2; in some experiments, 5 μm R406 or 13.4 μm sodium stibogluconate was added to the medium. Where indicated, cells were preincubated with FcR blocking solution for 15 min before being seeded. After 16 h, MKs that had passed through the Transwell to the other side of the filters and in the outer wells, which contained StemSpan medium with 100 ng/ml SDF1-α (PeproTech, London, UK), were recovered and counted by inverted microscope (9). Thereafter, the upper side of the filters was carefully washed with cold PBS, and cells remaining on the upper face of the filters were removed with a cotton wool swab. Transwell filters were fixed in 3% paraformaldehyde for 20 min at room temperature, stained using a monoclonal antibody against CD61 and with Hoechst 33258, cut out with a scalpel, and mounted onto glass slides, putting the lower face on the top (31, 32). The total number of cells that had migrated was counted, and images were acquired using a Olympus BX51 using 20×/0.5 UPlanF1 objective. Each experiment was performed in triplicate. Data are expressed as numbers of total migrated cells per insert or as percentages of cells related to that of the control.
Phosphatase Assay
Tyrosine phosphatase assay was performed using p-nitrophenyl phosphate as substrate. Cells were lysed with Hepes-glycerol lysis buffer without sodium orthovanadate and sodium fluoride (33). Cell lysate centrifugation and SHP1 immunoprecipitation were performed as described under “Immunoprecipitation and Western Blotting.” Sample volumes were adjusted to analyze equal protein concentrations. For total phosphatase activity, phosphatase assay buffer was added to have a final volume of 600 μl and a final concentration of 25 μm p-nitrophenyl phosphate. Samples were incubated for 150 min at 37 °C under shaking. The optical density was measured at 405 nm.
For SHP1 phosphatase activity, the immune complexes were washed twice with Hepes-glycerol buffer without phosphatase inhibitors and twice with phosphatase assay buffer (10 mm HEPES; 0.2 mm EDTA; 0.5% BSA; 1 mm dithiothreitol; pH 7.5). Immune complexes were incubated in 600 μl of phosphatase assay buffer with a final concentration of 25 μm p-nitrophenyl phosphate for 150 min at 37 °C under shaking. After centrifugation (14,000 × g, 4 °C) for 1 min, the optical density of supernatants was measured at 405 nm. The corresponding immune complexes, bound to protein A-Sepharose, were submitted to Western blotting to confirm the equal loading.
Statistics
Data are presented as means ± S.D. of at least 3 independent experiments. Differences between means were evaluated by either a Student's t test or a one-way analysis of variance and considered statistically significant at a p value of < 0.05. Statistical analyses were performed with Prism Version 5 (GraphPad).
RESULTS
Human MKs Express and Synthesize DDR1
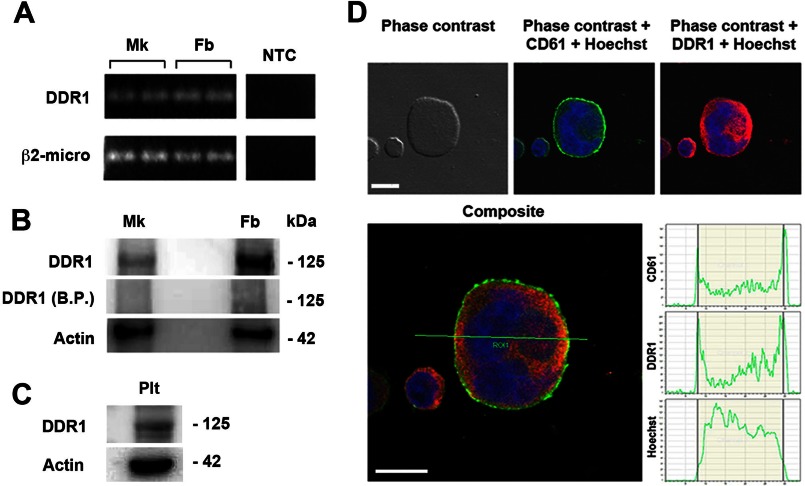
Human MKs were derived from umbilical cord blood progenitor cells, and the expression of DDR1 was analyzed by RT-PCR and Western blot. As shown in Fig. 1, A and B, DDR1 expression was detected in mature MKs at day 13 of culture, and in human fibroblasts as positive control, at both mRNA and protein levels. Interestingly, by Western blot, we also detected DDR1 in peripheral blood platelet lysates (Fig. 1C). Finally, by confocal microscopy, DDR1 was shown to be expressed mostly on MK cellular membrane (Fig. 1D).
FIGURE 1.
Human MKs express and synthesize DDR1 tyrosine kinase. A, total cellular RNA was extracted from MKs and fibroblasts (Fb) as positive control. β2-microglobulin was used as housekeeping gene. NTC indicates “no template” controls in the reverse transcriptase and PCR steps. RT-PCR products were loaded in duplicates for each cell type. B, MK and fibroblast lysates were subjected to Western blot analysis using an anti-DDR1 antibody. The anti-DDR1 blocking peptide (B.P.) was used to confirm the specificity of the antibody. Actin was probed to show equal loading. C, DDR1 expression was demonstrated in peripheral blood platelet lysate (Plt) by Western blot. Shown here are representative Western blots out of three independent experiments. D, MKs were cytospun on polylysine-coated glass coverslips, fixed, and stained with an anti-DDR1 antibody (red) and an anti-CD61 antibody (green). The graphs report the intensity of the fluorescence signal along the x axis for each fluorochrome on the optical section. (Immunofluorescence staining, DM IRBE inverted microscope, magnification 40×.) Scale bars are 25 μm. Nuclei were counterstained with Hoechst 33288 (blue).
Type I Collagen-dependent DDR1 Activation
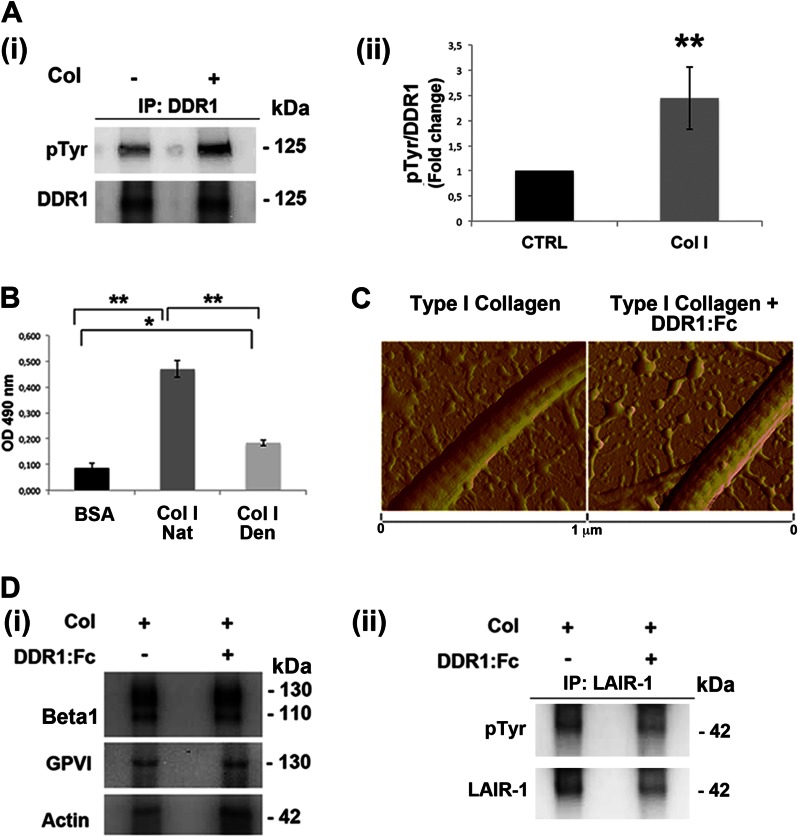
The catalytic activity of DDR1 upon type I collagen binding was demonstrated by the phosphotyrosine probing of DDR1 immunoprecipitates (Fig. 2A, panels i and ii). To evaluate the role of DDR1 activation in modulating MK function on type I collagen, we took advantage of a recombinant chimera protein (DDR1-Fc), which had been shown to block DDR1-collagen interactions (34, 35). By solid phase binding assay, we demonstrated the DDR1-Fc ability to bind to our type I collagen (Fig. 2B). Further, by atomic force microscopy, we verified that the pretreatment of type I collagen with DDR1-Fc did not modify the structural properties and the normal banding of the extracellular protein (Fig. 2C). Finally, we asked whether the DDR1 blocking molecule could impact the function of the other collagen receptor expressed by human MK, α2β1 integrin, and GPVI. By Western blot analysis, we demonstrated that the DDR1-Fc molecule works by specifically blocking the activation of DDR1, whereas the activation of β1 integrin and of GPVI is unaffected (Fig. 2D, panel i). The levels of activated integrin were determined using a β1 integrin antibody (clone HUTS-4) recognizing epitopes in the 355–425 region (hybrid domain), whose expression parallels the activity of β1 integrin (36). It is known that GPVI may form a dimer that has functional significance. The activation of GPVI was determined using an antibody that binds to the dimeric form of the receptor with an affinity 200-fold higher than to the monomeric form (37). Further, the activation of the collagen receptor LAIR-1, expression of which was recently demonstrated in mature MKs (38), appears unaffected by the treatment of megakaryocyte with DDR1-Fc (Fig. 2D, panel ii).
FIGURE 2.
Type I collagen-dependent DDR1 activation. A, panel i, lysates of MKs, plated on tissue culture plastic or on type I collagen (Col) for 16 h, were immunoprecipitated (IP) with an anti-DDR1 antibody and subjected to Western blotting. Membranes were stained with a monoclonal antibody against phosphotyrosine and with anti-DDR1 antibody. Panel ii, densitometry analysis of the Western blots of 125-KDa phospho-Tyr (pTyr) band on DDR1 immunoprecipitates. B, binding assay to evaluate the DDR1-Fc binding affinity on native type I collagen (Nat Col I) with respect to denatured type I collagen (Den Col I). BSA was used as negative control. Optical density (OD) was measured at 490 nm. C, atomic force microscopy images of dehydrated collagen-coated coverslips in the presence or absence of DDR1-Fc showing similar banded fibrils. D, panel i, MKs were plated, for 2 h, on type I collagen, in the presence or absence of DDR1-Fc. Lysates were subjected to Western blot analysis of active β1 integrin (HUTS-4) and dimeric GPVI. Actin was probed to show equal loading. Panel ii, lysates of megakaryocyte treated as above described were immunoprecipitated with anti-LAIR-1 antibody and subjected to Western blot. Membranes were stained with antibodies anti-phospho-Tyr (pTyr) and anti-LAIR-1. Shown here are representative Western blots out of three independent experiments. Data are presented as means ± S.D. (n = 5 and 4 independent experiments). *, p < 0.05. **, p < 0.01.
DDR1-dependent Regulation of MK Migration
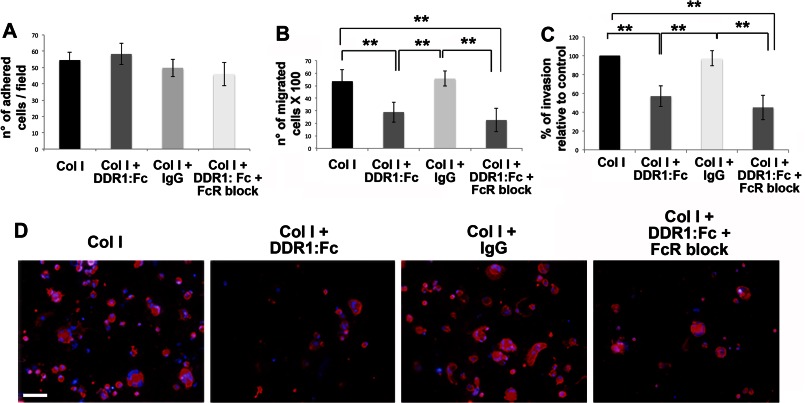
Mature MKs were plated on type I collagen-coated coverslips, containing or not containing the DDR1 blocking molecule (DDR1-Fc) or an IgG as control. In addition, to avoid a possible binding of the FcR with the chimera (DDR1-Fc), where indicated, we pretreated cells with an FcR blocking solution. After 16 h, cells were stained with anti-CD61 antibody, and then adherent cells were counted by fluorescence microscopy with a 20×/0.5 UPlanF1 objective. A comparable number of CD61+ cells were counted per field in all conditions, revealing that MK adhesion on type I collagen was not affected by DDR1 activation (Fig. 3A). Moreover, to investigate whether DDR1 activation could promote MK migration on type I collagen, MKs were seeded in the upper well of a Transwell plate, upon coating the Transwell filter with type I collagen mixed with DDR1-Fc or with an IgG as control. Pretreatment of cells with the FcR blocking solution was used as control of possible binding of the FcR with the chimera (DDR1-Fc). MKs were allowed to migrate for 16 h and then cells that had passed through the filter in the lower well were counted. The number of migrated MKs was reduced by about 40% in the presence of DDR1-Fc as compared with control samples (Fig. 3B). Additionally, blocking the FcR did not alter the number of migrated cells on type I collagen mixed to DDR1-Fc. These effects were further validated by staining the lower side of the coated Transwell filters with an anti-CD61 antibody to detect MKs that had invaded the type I collagen-coated filters (Fig. 3, C and D).
FIGURE 3.
DDR1-dependent regulation of MK migration. A, MKs were plated on type I collagen (Col I)-coated coverslips, in the presence of the DDR1-Fc blocking molecule or an IgG as control. Where indicated, MKs were pretreated with FcR blocking solution. After 16 h, adherent MKs were fixed, stained with anti-CD61 antibody, and then counted by fluorescence microscopy. B, MKs were left to migrate in a Transwell plate, upon coating the Transwell filter with type I collagen mixed with the DDR1-Fc blocking molecule or an IgG as control. Where indicated, MKs were pretreated with FcR blocking solution. After 16 h, MKs that had passed in the lower chamber were collected and counted by phase contrast microscopy. C, MKs adhering on the lower side of the Transwell filter were fixed and stained with anti-CD61 antibody and then counted by fluorescence microscopy. D, representative images of MK invasion of type I collagen. Cells adhering to the lower side of the Transwell coated filter, were fixed and stained with anti-CD61 antibody (red) antibody. (Immunofluorescence staining, Olympus BX51 microscope, magnifications 20×.) Scale bars are 100 μm. Nuclei were stained with Hoechst 33288 (blue). Reported results are the means ± S.D. (n = 6 independent experiments). **, p < 0.01.
Syk Is Involved in DDR1-dependent MK Migration
To explore the mechanisms underlying the reduction of MK migration rate on type I collagen following DDR1 inhibition, we investigated the involvement and phosphorylation-dependent activation of the tyrosine kinase Syk, the MAPK ERK, and the myosin light chain (MLC2), known to be involved in DDR1 signaling as well as in DDR1-dependent regulation of cell migration processes (22, 25, 39). First, we demonstrated that DDR1 interacts with Syk and with myosin IIA dependently on MK-type I collagen interaction (Fig. 4A, panels i and ii). Thereafter, we showed that inhibition of DDR1 by DDR1-Fc determines an increase in the phosphorylation levels of Syk kinase and, to a lesser extent, of MLC2 in type I collagen adhering-MKs after 16 h, whereas ERK activation was concomitantly reduced (Fig. 4B, panels i and ii). Interestingly, after 3 h of adhesion, we did not observe significant differences in protein phosphorylation, probably due to the slow kinetics of DDR1 activation (data not shown). Overall these results highlighted a marked increase of phosphorylated Syk upon DDR1 inhibition, suggesting a DDR1-dependent negative modulation of Syk activation. To explore the role of Syk in reducing MK migration upon DDR1 inhibition, migration assays using the specific Syk inhibitor R406 were performed. Fig. 4C shows that Syk inhibition restored MK migration to a level comparable with untreated control, overcoming DDR1 inhibition. These effects were further validated by staining the lower side of the coated Transwell filters with an anti-CD61 antibody to detect MKs that had invaded the type I collagen-coated filters (Fig. 4, D and E).
FIGURE 4.
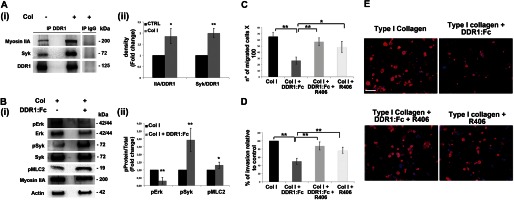
Syk kinase is involved in DDR1-mediated MK migration on type I collagen. A, panel i, DDR1 was immunoprecipitated (IP) in cell lysates of MKs plated for 16 h on type I collagen (Col) or on tissue culture plastic. A control sample was immunoprecipitated with an unrelated antibody (IgG). Membranes were probed with anti-Syk and anti-myosin IIA antibodies to show DDR1-interacting protein and reprobed with anti-DDR1 antibody to show equal loading. Panel ii, densitometry analysis of the Western blots of Myosin IIA and Syk co-immunoprecipitated with DDR1. B, total cellular lysates of MKs plated for 16 h on type I collagen, inhibiting or not inhibiting DDR1 activation, were subjected to Western blot analysis. Membranes were probed with the indicated antibody, with p indicating the phosphorylated form. Actin was probed to show equal loading. Panel ii, densitometry analysis of the Western blots of phospho-ERK (pERK), phospho-Syk (pSyk), and phospho-MLC2 (pMLC2). C, Transwell migration assay of mature MKs through type I collagen, in the presence of the DDR1-Fc blocking molecule and of Syk specific inhibitor compound R406 (5 μm) either mixed or used singularly. After 16 h, MKs that had passed in the lower chamber were counted by phase contrast microscopy. D, MKs adhering to the lower side of the Transwell filter were fixed and stained with anti-CD61 antibody and then counted by fluorescence microscopy. E, representative images of MK invasion of type I collagen. Cells adhering to the lower side of the Transwell coated filter were fixed and stained with anti-CD61 antibody (red). (Immunofluorescence staining, Olympus BX51 microscope, magnifications 20×.) Scale bars are 100 μm. Nuclei were stained with Hoechst 33288 (blue). Data are presented as means ± S.D.(n = 5, 5, and 5 independent experiment). *, p < 0.05. **, p < 0.01.
SHP1 Modulates Syk Activation upon Type I Collagen-DDR1 Binding in MKs
We further investigated the negative regulatory mechanism mediated by DDR1 on Syk phosphorylation, focusing on a possible involvement of phosphatase enzymes. We performed phosphatase activity assays on cell lysates of MKs plated on type I collagen for 16 h, in the presence or absence of the DDR1-Fc blocking molecule. The total tyrosine phosphatase activity appeared significantly decreased upon inhibition of DDR1 activation (73.3 ± 6.6% relative to controls). Subsequently, we analyzed the interaction between DDR1 and two SH2 domain-containing tyrosine phosphatases, already known as active regulators of Syk dephosphorylation, SHP1 and SHP2 (40, 41). Thus, by Western blot, we analyzed the co-immunoprecipitation of DDR1 with both phosphatases in MKs plated on type I collagen, or plated on tissue culture plastic as a negative control of DDR1 activation. The adhesion of cells on type I collagen demonstrated a strong increase of the interaction of DDR1 with SHP1 (Fig. 5A, panels i and ii), whereas SHP2, whose interaction with DDR1 was previously demonstrated (24), surprisingly was not detected in all tested conditions. To verify whether the kinase activity of DDR1 was implicated in modulating the phosphorylation status of SHP1, which reflects its enzymatic activity, we performed the SHP1 phospho-specific probing of Western blot analysis of MK lysates, cultured in the presence or absence of the DDR1-Fc blocking molecule. A significant decrease in SHP1 phosphorylation was observed when DDR1 activity was blocked (Fig. 5B, panels i and ii). The reduction in SHP1 activity was further confirmed by phosphatase activity assays of SHP1 immunoprecipitates. As shown in Fig. 5C (panel i), the phosphatase assay revealed a reduction of about 20% in SHP1 enzymatic activity when DDR1 activation was prevented; equal loading was analyzed by submitting to Western blot the corresponding immune complexes, bound to protein A-Sepharose (Fig. 5C, panel ii).
FIGURE 5.
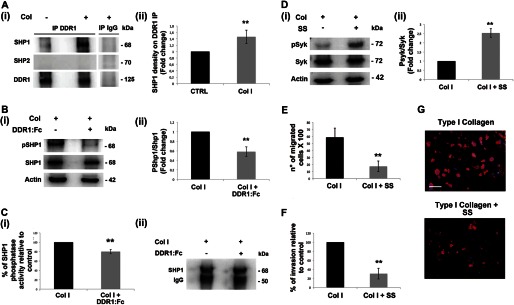
SHP1 modulates Syk activation upon type I collagen-DDR1 binding in MKs. A, panel i, DDR1 immunoprecipitates (IP) from MKs plated for 16 h on type I collagen (Col) or on plastic were analyzed by Western blot. A control sample was immunoprecipitated with an unrelated antibody (IgG). Membranes were probed with anti-SHP1 and anti-SHP2 antibodies to show the co-immunoprecipitation and with anti-DDR1 antibody to show equal loading. Panel ii densitometry analysis of the Western blots of SHP1 protein co-immunoprecipitated with DDR1. B, panel i, Western blot analysis of cell lysates of MKs plated for 16 h on type I collagen mixed with or without DDR1-Fc blocking molecule. Membranes were probed with the indicated antibodies with p indicating the phosphorylated form. Actin was probed to show equal loading. Panel ii, densitometry analysis of the Western blots of pSHP1. CTRL, control. C, panel i, SHP1 phosphatase activity measured in a phosphatase assay on SHP1 immunoprecipitates from MK lysates, using p-nitrophenyl phosphate as substrate. MKs were plated for 16 h on type I collagen mixed with or without DDR1-Fc blocking molecule. Panel ii, Western blot analysis of SHP1 immunoprecipitates used for SHP1 phosphatase activity assay. SHP1 phosphatase activity was related to the same concentration of SHP1. Shown here is a representative Western blot out of four independent experiments. D, panel i, MKs were treated with the SHP1 specific inhibitor sodium stibogluconate (SS) (13.4 μm) and plated for 3 h on type I collagen. Cell lysates were subjected to Western blot analysis. Membranes were probed with anti-phospho Syk (Tyr-525/526) antibody and anti-Syk and anti-actin antibodies to show equal loading. Panel ii, densitometry analysis of the Western blots of phospho-Syk (pSyk). E, Transwell migration assay of mature MKs through type I collagen, in the presence of the SHP1 specific inhibitor SS (13.4 μm). After 16 h, MKs that had passed in the lower chamber were counted by phase contrast microscopy. F, MKs adhering to the lower side of the Transwell filter were fixed and stained with anti-CD61 antibody and then counted by fluorescence microscopy. G, representative images of MK invasion of type I collagen. Cells adhering to the lower side of the Transwell coated filter were fixed and stained with anti-CD61 antibody (red) antibody. (Immunofluorescence staining, Olympus BX51 microscope, magnifications 20×.) Scale bars are 100 μm. Nuclei were stained with Hoechst 33288 (blue). Data are presented as means ± S.D. (n = 5, 4, 4, 4, 3, and 3 independent experiments). *, p < 0.05 **, p < 0.01.
To strengthen these results, we tested the existence of interdependence between Syk activation versus SHP1 phosphatase activity reduction. MKs were treated with the SHP1 specific inhibitor sodium stibogluconate, and consistently, Western blot analysis revealed an increase in Syk phosphorylation in treated MKs relative to control, reflecting the DDR1 blocking condition (Fig. 5D). Further, treatment of MKs with sodium stibogluconate resulted in a decrease of cell migration (Fig. 5, E–G). Overall these data suggested that DDR1 activation promotes MK migration by increasing SHP1 activity that, in turn, leads to Syk dephosphorylation, preventing the Syk-mediated inhibition of MK migration on type I collagen.
DISCUSSION
To release platelets, mature MKs migrate toward the vascular sinusoids crawling on the different matrices that fill the bone marrow environment (1). Thus, a fine regulation of MK interaction with the bone marrow matrix environment is necessary to drive MKs to their final maturation site. Type I collagen is one of the most abundant ECM components in the bone marrow (4, 5), and it is known to be mostly located in the endosteal niche where it inhibits MK maturation (6, 7). On the contrary, other ECM components, proposed to fill the vascular district, support MK maturation and platelet production (27, 42, 43). On this basis, it appears that modulating MK-type I collagen interaction is a fundamental step in MK development. In this regard, previous works pointed out the role of collagen receptors α2β1 and GPVI in regulating MK function and platelet production on type I collagen; however, the expression and function of other matrix receptors on MKs are still unknown (11–14). Discoidin domain receptors (DDR1 and DDR2) are tyrosine-kinase collagen receptors that are stimulated by fibrillar and basement membrane collagens and mediate cell adhesion and migration in different tissues. DDR1 is more likely to be expressed by epithelial cells and leukocytes, whereas DDR2 is expressed by cells of mesenchymal origin (10). In this work, we demonstrate, for the first time, that human MKs express DDR1, and we propose a new mechanism underlying MK crawling on type I collagen. Our hypothesis is that activation of different receptors, besides α2β1 and GPVI, intervenes to guide MK movements from the type I collagen-rich osteoblastic niche. Interestingly, it is known that DDR1 blocks Syk-mediated inhibition of cell migration (22) and that depletion of DDR1 determines a decrease of collective cancer cell invasion and metastasis (44). In this context, this work reports that DDR1 promotes MK migration on type I collagen by inhibiting Syk phosphorylation. It is known that cell function is mediated by biochemical signaling that results from a balance of kinase and phosphatase activation. The SH2 domain-containing phosphatases SHP1 and SHP2 are the best studied of the classical nonreceptor tyrosine phosphatases (45), and they are known to bind DDR1 and then modulate activity of downstream molecules (24). Importantly, here we show that Syk dephosphorylation was dependent on activation of SHP1 by DDR1. Altogether, these results point out DDR1 as a new regulatory checkpoint of MK migration on type I collagen. Recently, Mazharian et al. (46) described a mechanism by which αIIbβ3 promotes MK migration on fibronectin through Syk activation. Together with these results, our data demonstrate how MKs differently regulate their function to migrate on a sequence of different matrices in the bone marrow environment. Different works have been performed to unravel the role of Syk and SHP1 in regulating MK and platelet function. The Syk-deficient mouse model (Syk−/−) presented high morbidity, whereas deletion of Syk in the hematopoietic system using Vav-iCre led to the formation of blood-filled vessels in the skin of embryos (47). Further, chimeric mice were generated by injecting Syk−/− fetal liver cells in BALB/c female mice upon irradiation (48). Platelet counts were normal in the Syk−/− and in the chimeric models, whereas the platelet counts were not performed in the Sykfl/flVav-iCre model. Given the intrinsic complexity of these models, it may be difficult to have a comprehensive understanding of the role of Syk in regulating MK function in adult mice in vivo. Finally, motheaten viable mice (mev/mev), which contain a mutation in the shp1 gene that results in nearly complete loss of catalytic activity, are severely thrombocytopenic (49).
To further demonstrate DDR1 involvement in the regulation of MK function, we have preliminary results showing a tendency of MK numbers to decrease in the bone marrow of DDR1−/− mice with respect to controls (67.9 ± 15.2% relative to controls). Altogether, these data represent a clear indication that further investigations are worthwhile to unravel the specific alterations in these mice. Overall it appears that the regulation of MK development and platelet production is regulated by a multistep mechanism. This may explain compensation effects when receptor deficiencies or partial mechanism failures occur as in the case of integrin IIb-knock-out and integrin α2-deficient mice that present normal peripheral blood platelet count (43, 50).
In conclusion, this study extends the list of collagen receptors that regulate MK function in the bone marrow environment, opening new perspectives in the study of megakaryopoiesis in both physiological and pathological scenarios.
Acknowledgments
We thank M. Jandrot-Perrus (INSERM, U698, Hôpital Bichat, Paris) for the anti-GPVI antibody, P. Vaghi (“Centro Grandi Strumenti,” University of Pavia, Italy) for technical assistance in confocal microscopy studies, C. Perotti (Immunohematology and Transfusion Service, Apheresis and Cell Therapy Unit, IRCCS San Matteo Foundation, Pavia, Italy) for providing cord blood samples, P. Rebuzzini (Department of Biology and Biotechnology, University of Pavia, Italy) for helping with RT-PCR analysis, M. Raspanti (Department of Human Morphology, University of Insubria, Varese, Italy) for Atomic Force Microscopy analysis, C. Balduini and M. E. Tira for critical discussion of the manuscript, and G. Wulff (Department of Hematology and Oncology, University Medical Center Goettingen, Germany) for helping with DDR1−/− mouse bone marrow analysis.
This work was supported by the Cariplo Foundation (Grant 2010-0807), Regione Lombardia – Project SAL-45, Almamater Foundation (Pavia), and Regione Lombardia Progetto di cooperazione scientifica e tecnologica internazionale and by National Institutes of Health Grant 1R01 EB016041-01 (to A. B.).
- MK
- megakaryocyte
- ECM
- extracellular matrix
- GPVI
- glycoprotein VI
- DDR
- discoidin domain receptor
- Syk
- spleen tyrosine kinase
- FcR
- Fc receptor
- SH
- Src homology.
REFERENCES
- 1. Avecilla S. T., Hattori K., Heissig B., Tejada R., Liao F., Shido K., Jin D. K., Dias S., Zhang F., Hartman T. E., Hackett N. R., Crystal R. G., Witte L., Hicklin D. J., Bohlen P., Eaton D., Lyden D., de Sauvage F., Rafii S. (2004) Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 10, 64–71 [DOI] [PubMed] [Google Scholar]
- 2. Patel S. R., Hartwig J. H., Italiano J. E., Jr. (2005) The biogenesis of platelets from megakaryocyte proplatelets. J. Clin. Invest. 115, 3348–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Junt T., Schulze H., Chen Z., Massberg S., Goerge T., Krueger A., Wagner D. D., Graf T., Italiano J. E., Jr., Shivdasani R. A., von Andrian U. H. (2007) Dynamic visualization of thrombopoiesis within bone marrow. Science 317, 1767–1770 [DOI] [PubMed] [Google Scholar]
- 4. Nilsson S. K., Debatis M. E., Dooner M. S., Madri J. A., Quesenberry P. J., Becker P. S. (1998) Immunofluorescence characterization of key extracellular matrix proteins in murine bone marrow in situ. J. Histochem. Cytochem. 46, 371–377 [DOI] [PubMed] [Google Scholar]
- 5. Takaku T., Malide D., Chen J, Calado R. T., Kajigaya S., Young N. S. (2010) Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood 116, e41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang Y., Auradé F., Larbret F., Zhang Y., Le Couedic J. P., Momeux L., Larghero J., Bertoglio J., Louache F., Cramer E., Vainchenker W., Debili N. (2007) Proplatelet formation is regulated by the Rho/ROCK pathway. Blood 109, 4229–4236 [DOI] [PubMed] [Google Scholar]
- 7. Chen Z., Naveiras O., Balduini A., Mammoto A., Conti M. A., Adelstein R. S., Ingber D., Daley G. Q., Shivdasani R. A. (2007) The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood 110, 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malara A., Gruppi C., Rebuzzini P., Visai L., Perotti C., Moratti R., Balduini C., Tira M. E., Balduini A. (2011) Megakaryocyte-matrix interaction within bone marrow: new roles for fibronectin and factor XIII-A. Blood 117, 2476–2483 [DOI] [PubMed] [Google Scholar]
- 9. Malara A., Gruppi C., Pallotta I., Spedden E., Tenni R., Raspanti M., Kaplan D., Tira M. E., Staii C., Balduini A. (2011) Extracellular matrix structure and nano-mechanics determine megakaryocyte function. Blood 118, 4449–4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leitinger B., Hohenester E. (2007) Mammalian collagen receptors. Matrix Biol 26, 146–155 [DOI] [PubMed] [Google Scholar]
- 11. Zou Z., Schmaier A. A., Cheng L., Mericko P., Dickeson S. K., Stricker T. P., Santoro S. A., Kahn M. L. (2009) Negative regulation of activated α-2 integrins during thrombopoiesis. Blood 113, 6428–6439 [DOI] [PubMed] [Google Scholar]
- 12. Lagrue-Lak-Hal A. H., Debili N., Kingbury G., Lecut C., Le Couedic J. P., Villeval J. L., Jandrot-Perrus M., Vainchenker W. (2001) Expression and function of the collagen receptor GPVI during megakaryocyte maturation. J. Biol. Chem. 276, 15316–15325 [DOI] [PubMed] [Google Scholar]
- 13. Sabri S., Jandrot-Perrus M., Bertoglio J., Farndale R. W., Mas V. M., Debili N., Vainchenker W. (2004) Differential regulation of actin stress fiber assembly and proplatelet formation by α2β1 integrin and GPVI in human megakaryocytes. Blood 104, 3117–3125 [DOI] [PubMed] [Google Scholar]
- 14. Mossuz P., Schweitzer A., Molla A., Berthier R. (1997) Expression and function of receptors for extracellular matrix molecules in the differentiations of human megakariocytes in vitro. Br. J. Haematol. 98, 819–827 [DOI] [PubMed] [Google Scholar]
- 15. Vogel W. (1999) Discoidin domain receptors: structural relations and functional implications. FASEB J. 13, (suppl.) S77–S82 [DOI] [PubMed] [Google Scholar]
- 16. Lu K. K., Trcka D., Bendeck M. P. (2011) Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovasc. Pathol. 20, 71–76 [DOI] [PubMed] [Google Scholar]
- 17. Vogel W. F., Abdulhussein R., Ford C. E. (2006) Sensing extracellular matrix: An update on discoidin domain receptor function. Cell. Signal. 18, 1108–1116 [DOI] [PubMed] [Google Scholar]
- 18. Lemeer S., Bluwstein A., Wu Z., Leberfinger J., Müller K., Kramer K., Kuster B. (2012) Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J. Proteomics 75, 3465–3477 [DOI] [PubMed] [Google Scholar]
- 19. Coopman P. J., Do M. T., Barth M., Bowden E. T., Hayes A. J., Basyuk E., Blancato J. K., Vezza P. R., McLeskey S. W., Mangeat P. H., Mueller S. C. (2000) The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature 406, 742–747 [DOI] [PubMed] [Google Scholar]
- 20. Li J., Sidell N. (2005) Growth-related oncogene produced in human breast cancer cells and regulated by Syk protein-tyrosine kinase. Int. J. Cancer 117, 14–20 [DOI] [PubMed] [Google Scholar]
- 21. Wang L., Devarajan E., He J., Reddy S. P., Dai J. L. (2005) Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Cancer Res. 65, 10289–10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neuhaus B., Bühren S., Böck B., Alves F., Vogel W. F., Kiefer F. (2011) Migration inhibition of mammary epithelial cells by Syk is blocked in the presence of DDR1 receptors. Cell. Mol. Life Sci. 68, 3757–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neel B. G., Gu H., Pao L. (2003) The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28, 284–293 [DOI] [PubMed] [Google Scholar]
- 24. Wang C. Z., Su H. W., Hsu Y. C., Shen M. R., Tang M. J. (2006) A discoidin domain receptor 1/SHP-2 signaling complex inhibits α2β1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol. Biol. Cell 17, 2839–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Y., Arora P., McCulloch C. A., Vogel W. F. (2009) The collagen receptor DDR1 regulates cell spreading and motility by associating with myosin IIA. J. Cell Sci. 122, 1637–1646 [DOI] [PubMed] [Google Scholar]
- 26. Tenni R., Sonaggere M., Viola M., Bartolini B., Tira M. E., Rossi A., Orsini E., Ruggeri A., Ottani V. (2006) Self-aggregation of fibrillar collagens I and II involves lysine side chains. Micron 37, 640–647 [DOI] [PubMed] [Google Scholar]
- 27. Balduini A., Pallotta I., Malara A., Lova P., Pecci A., Viarengo G., Balduini C. L., Torti M. (2008) Adhesive receptors, extracellular proteins, and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J. Thromb. Haemost. 6, 1900–1907 [DOI] [PubMed] [Google Scholar]
- 28. Balduini A., Pecci A., Lova P., Arezzi N., Marseglia C., Bellora F., Perotti C., Balduini C., Balduini C. L., Torti M. (2004) Expression, activation, and subcellular localization of the Rap1 GTPase in cord blood-derived human megakaryocytes. Exp. Cell. Res. 300, 84–93 [DOI] [PubMed] [Google Scholar]
- 29. Hachehouche L. N., Chetoui N., Aoudjit F. (2010) Implication of discoidin domain receptor 1 in T cell migration in three-dimensional collagen. Mol. Immunol. 47, 1866–1869 [DOI] [PubMed] [Google Scholar]
- 30. Chetoui N., El Azreq M. A., Boisvert M., Bergeron M. È., Aoudjit F. (2011) Discoidin domain receptor 1 expression in activated T cells is regulated by the ERK MAP kinase signaling pathway. J. Cell. Biochem. 112, 3666–3674 [DOI] [PubMed] [Google Scholar]
- 31. Ponte A. L., Marais E., Gallay N., Langonné A., Delorme B., Hérault O., Charbord P., Domenech J. (2007) The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25, 1737–1745 [DOI] [PubMed] [Google Scholar]
- 32. Oberoi T. K., Dogan T., Hocking J. C., Scholz R. P., Mooz J., Anderson C. L., Karreman C., Meyer zu Heringdorf D., Schmidt G., Ruonala M., Namikawa K., Harms G. S., Carpy A., Macek B., Köster R. W., Rajalingam K. (2012) IAPs regulate the plasticity of cell migration by directly targeting Rac1 for degradation. EMBO J. 31, 14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Honorat J. F., Ragab A., Lamant L., Delsol G., Ragab-Thomas J. (2006) Shp1 tyrosine phosphatase negatively regulates NPM-ALK tyrosine kinase signaling. Blood 107, 4130–4138 [DOI] [PubMed] [Google Scholar]
- 34. Bhatt R. S., Tomoda T., Fang Y., Hatten M. E. (2000) Discoidin domain receptor 1 functions in axon extension of cerebellar granule neurons. Genes Dev. 14, 2216–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S. H., Lee S., Suk K., Bark H., Jun C. D., Kim D. K., Choi C. H., Yoshimura T. (2007) Discoidin domain receptor 1 mediates collagen-induced nitric oxide production in J774A.1 murine macrophages. Free Radic. Biol. Med. 42, 343–352 [DOI] [PubMed] [Google Scholar]
- 36. Du J., Chen X., Liang X., Zhang G., Xu J., He L., Zhan Q., Feng X. Q., Chien S., Yang C. (2011) Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. U.S.A. 108, 9466–9471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loyau S., Dumont B., Ollivier V., Boulaftali Y., Feldman L., Ajzenberg N., Jandrot-Perrus M. (2012) Platelet glycoprotein VI dimerization, an active process inducing receptor competence, is an indicator of platelet reactivity. Arterioscler. Thromb. Vasc. Biol. 32, 778–785 [DOI] [PubMed] [Google Scholar]
- 38. Xue J., Zhang X., Zhao H., Fu Q., Cao Y., Wang Y. (2011) Leukocyte-associated immunoglobulin-like receptor-1 is expressed on human megakaryocytes and negatively regulates the maturation of primary megakaryocyte progenitors and cell line. Biochem. Biophys. Res Commun. 405, 128–133 [DOI] [PubMed] [Google Scholar]
- 39. Mazharian A., Watson S. P., Séverin S. (2009) Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp. Hematol. 37, 1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dustin L. B., Plas D. R., Wong J., Hu Y. T., Soto C., Chan A. C., Thomas M. L. (1999) Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J. Immunol. 162, 2717–2724 [PubMed] [Google Scholar]
- 41. Okazaki T., Maeda A., Nishimura H., Kurosaki T., Honjo T. (2001) PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc. Natl. Acad. Sci. U.S.A. 98, 13866–13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaushansky K. (2009) Determinants of platelet number and regulation of thrombopoiesis. Hematology Am. Soc. Hematol. Educ. Program. 147–152 [DOI] [PubMed] [Google Scholar]
- 43. Larson M. K., Watson S. P. (2006) Regulation of proplatelet formation and platelet release by integrin αIIbβ3. Blood 108, 1509–1514 [DOI] [PubMed] [Google Scholar]
- 44. Hidalgo-Carcedo C., Hooper S., Chaudhry S. I., Williamson P., Harrington K., Leitinger B., Sahai E. (2011) Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat. Cell Biol. 13, 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poole A. W., Jones M. L. (2005) A SHPing tale: perspective on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell. Signal. 17, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 46. Mazharian A., Thomas S. G., Dhanjal T. S., Buckley C. D., Watson S. P. (2010) Critical role of Src-Syk-PLCγ2 signalling in megakaryocyte migration and thrombopoiesis. Blood 116, 793–800 [DOI] [PubMed] [Google Scholar]
- 47. Finney B. A., Schweighoffer E., Navarro-Núñez L., Bénézech C., Barone F., Hughes C. E., Langan S. A., Lowe K. L, Pollitt A. Y., Mourao-Sa D., Sheardown S., Nash G. B., Smithers N., Reis e Sousa C., Tybulewicz V. L., Watson S. P. (2012) CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood 119, 1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Poole A., Gibbins J. M., Turner M., van Vugt M. J., van de Winkel J. G. J., Saito T., Tybulewicz V. L. J., Watson S. P. (1997) The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 16, 2333–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pasquet J. M., Quek L., Pasquet S., Poole A., Matthews J. R., Lowell C., Watson S. P. (2000) Evidence of a role for SHP-1 in platelet activation by the collagen receptor Glycoprotein VI. J. Biol. Chem. 275, 28526–28531 [DOI] [PubMed] [Google Scholar]
- 50. He L., Pappan L. K., Grenache D. G., Li Z., Tollefsen D. M., Santoro S. A., Zutter M. M. (2003) The contributions of the α2β1 integrin to vascular thrombosis in vivo. Blood 102, 3652–3657 [DOI] [PubMed] [Google Scholar]