Background: Axonal transport deficits are part of Alzheimer disease (AD) pathobiology.
Results: β-Amyloid (Aβ) impairs BDNF-dependent retrograde signaling, which is rescued by increasing cellular UCH-L1 levels.
Conclusion: In AD, Aβ impairs neurotrophin-mediated retrograde signaling by disrupting ubiquitin homeostasis.
Significance: Elucidating the mechanism by which Aβ causes transport deficits that compromise synaptic plasticity and neuronal survival is crucial for discovering novel therapeutics to reverse cognitive deficits in AD.
Keywords: Alzheimer Disease, Amyloid, BDNF, Trafficking, Ubiquitin, Microfluidic Device
Abstract
We previously found that BDNF-dependent retrograde trafficking is impaired in AD transgenic mouse neurons. Utilizing a novel microfluidic culture chamber, we demonstrate that Aβ oligomers compromise BDNF-mediated retrograde transport by impairing endosomal vesicle velocities, resulting in impaired downstream signaling driven by BDNF/TrkB, including ERK5 activation, and CREB-dependent gene regulation. Our data suggest that a key mechanism mediating the deficit involves ubiquitin C-terminal hydrolase L1 (UCH-L1), a deubiquitinating enzyme that functions to regulate cellular ubiquitin. Aβ-induced deficits in BDNF trafficking and signaling are mimicked by LDN (an inhibitor of UCH-L1) and can be reversed by increasing cellular UCH-L1 levels, demonstrated here using a transducible TAT-UCH-L1 strategy. Finally, our data reveal that UCH-L1 mRNA levels are decreased in the hippocampi of AD brains. Taken together, our data implicate that UCH-L1 is important for regulating neurotrophin receptor sorting to signaling endosomes and supporting retrograde transport. Further, our results support the idea that in AD, Aβ may down-regulate UCH-L1 in the AD brain, which in turn impairs BDNF/TrkB-mediated retrograde signaling, compromising synaptic plasticity and neuronal survival.
Introduction
Alzheimer disease (AD)3 is defined pathologically by the accumulation of extracellular Aβ plaques and intracellular neurofibrillary tangles, accompanying synaptic and neuronal loss in the AD brain. Although Aβ plaque accumulation is a clear risk factor associated with AD, cognitive decline precedes plaque pathology (1). Studies now suggest that soluble and/or oligomeric Aβ that accumulates early in the disease causes synaptic deficits and correlates more closely with cognitive dysfunction than Aβ plaque load (2–4). Consistent with these data, cerebral infusion of soluble Aβ oligomers impairs hippocampal long term potentiation, a form of synaptic plasticity associated with memory formation, and disrupts hippocampal-dependent learning (5, 6). Also, AD transgenic mice that accumulate soluble oligomers exhibit impaired hippocampal long term potentiation and hippocampal-dependent learning along with synaptic loss, prior to frank plaque deposition (7–10).
BDNF/TrkB signaling plays a major role in synaptic plasticity, learning, and memory (11). Similar to the deficits induced by oligomeric Aβ, reduced BDNF signaling also causes AD-like synaptic plasticity deficits (12–19). The parallels have given rise to the hypothesis that a potential mechanism underlying Aβ-mediated synaptic dysfunction involves disrupted BDNF signaling (20–22). Indeed, down-regulation of BDNF signaling may be an early and possibly primary event in AD, based on the finding that in early stages of AD (i.e., mild cognitive impairment), BDNF levels are decreased and correlate with cognitive decline (23).
Consistent with the hypothesis that Aβ-mediated synaptic dysfunction involves disrupted BDNF signaling, we have found that soluble Aβ impairs retrograde axonal trafficking of the BDNF receptor, TrkB (22). Retrograde axonal transport of the BDNF-TrkB complex to the soma drives downstream signaling events important for neuronal health, survival, and plasticity, including CREB-dependent gene transcription (24). Retrograde axonal trafficking of the TrkB receptor involves multiple steps, including 1) TrkB internalization from the cell surface, 2) sorting/processing of TrkB to late endosomes/multivesicular bodies (MVBs), and 3) transport from the axon to the soma, mediated by dynein motors (24–29).
An important sorting signal that marks tyrosine kinase receptors for entry into the MVB pathway is ligand-induced ubiquitination, particularly monoubiquitination (30–33). Although the contribution of ubiquitin in TrkB retrograde trafficking has not been elucidated in detail, TrkB is multimonoubiquitinated in response to BDNF (34), suggesting that ubiquitin may be important for TrkB signaling. Taken together, with the recent finding that Aβ accumulation in neurons impairs the MVB sorting pathway in part by inhibiting the activities of deubiquitinating enzymes (35), one mechanism by which Aβ impairs TrkB retrograde trafficking may be via interfering with ubiquitin homeostasis.
Here we build on our previous finding that oligomeric Aβ results in a net decrease in TrkB retrograde transport and have identified a potential mechanism underlying this deficit. Oligomeric Aβ does not affect TrkB receptor internalization but impairs endosomal retrograde trafficking/signaling. Also, we demonstrate that oligomeric Aβ interferes with BDNF/TrkB signaling by impairing ubiquitin homeostasis. Specifically, Aβ-mediated trafficking/signaling deficits are mimicked by an inhibitor of the deubiquitinating enzyme ubiquitin C-terminal hydrolase L1 (UCH-L1). Furthermore, Aβ-mediated impairments are rescued by elevating intracellular UCH-L1 levels. UCH-L1 functions to maintain cellular ubiquitin homeostasis, and by manipulating this pathway, we show that the ubiquitin recycling pathway plays a role in neurotrophin-mediated retrograde signaling. These results suggest that in AD, soluble and/or oligomeric forms of β-amyloid disrupt BDNF-mediated retrograde signaling by altering ubiquitin homeostasis. This leads to deficits in neurotrophin-dependent gene expression that compromise synaptic plasticity and neuronal survival.
EXPERIMENTAL PROCEDURES
Synthesis of Aβ Oligomers
Oligomers were prepared as described previously (36). Briefly, Aβ that was lyophilized as a hexafluoroisopropanol film (EMD Millipore) was dissolved in neat, sterile Me2SO (5 mm) and diluted in PBS, pH 7.4, to 100 μm and aged overnight (4 °C). Aβ oligomer preparations were centrifuged (14,000 × g, 10 min, 4 °C); the supernatants were transferred to fresh Eppendorf tubes and stored at 4 °C until use. Confirmation of Aβ oligomers was carried out by Western analysis as described previously (22).
Purification of BDNF-GFP
Endotoxin-free BDNF-GFP plasmid (generous gift from Dr. Masami Kojima) was introduced by nucleofection (Lonza) into HEK cells followed by selection in DMEM containing 10% FBS and G418 (1 mg/ml, plasmid contains a neomycin cassette). BDNF-GFP was isolated from stably transfected pre-pro-BDNF-GFP HEK293 cells as follows. After cells reached confluency, secreted pro-BDNF-GFP from the medium was removed and concentrated with Amicon YM-30 centrifugal filters (5000 × g, 2 h) (30,000 molecular weight cutoff, Millipore). Pro-BDNF was converted to mature BDNF-GFP by treatment with plasmin (Sigma) as described previously (37). Mature BDNF-GFP was further purified by size exclusion chromatography (Amicon YM-50) where the flow-through contained the protein of interest. BDNF-GFP is indistinguishable from BDNF both biochemically and biologically (38, 39), and we previously confirmed that our purified BDNF-GFP was biologically active (22). The BDNF-GFP concentration was determined by BDNF ELISA (Promega).
Assembly of Microfluidic Culture Chambers
The chamber was fabricated in PDMS using rapid prototyping and soft lithography similar to previously published procedures (40). Briefly, glass coverslips (24 × 40 mm, No. 1, Corning Inc.) sonicated in 95% EtOH (30 min) and dried in a sterile hood were immersed in sterile aqueous solution (0.5 mg/ml poly-l-lysine (Sigma)) in PBS (24 h, 5% CO2, 37 °C incubator), rinsed, and allowed to air dry in a sterile hood. The chambers are noncovalently assembled by conformal contact. The chambers consist of two parallel microfluidic compartments, connected by inlet and outlet wells. The two compartments are separated by a solid barrier region with microgrooves embedded in the bottom of the connecting barrier. A slight volume difference between the two compartments (40 μl) is used to generate a fluidic resistance within the microgrooves, facilitating the isolation of BDNF to axons.
Primary Neuronal Cell Cultures
All of the procedures were performed under an Institutional Animal Care and Use Committee-approved protocol. Primary hippocampal or cortical neuron cultures were derived from rat embryo (embryonic day 18) as described previously (41). Briefly, dissected tissue was dissociated with trypsin, triturated, and either plated on poly-l-lysine-coated 6-well plates or plated in microfluidic chambers fitted with poly-l-lysine-coated glass coverslips in serum-free Neurobasal supplemented with B27 (Invitrogen). The cells were plated at a density of 5 × 106 cells/ml (for microfluidic chambers) and 5 × 105 cells/ml (for 6-well plates). Neuronal purity was assessed by immunostaining with a mouse monoclonal β-III-tubulin (1:1000; EMD Millipore) and rabbit polyclonal glial fibrillary acidic protein (1:4000, DAKO). Glial contamination was <5% (n = 6). Aβ oligomer treatments (1 μm) and the transduction with TAT-HA-UCH-L1 (transduction domain of HIV-transactivator protein and hemagglutinin fused to UCH-L1) (20 nm) were carried out at 7 DIV. The expression and purification of TAT-UCH-L1 were carried out as described previously (42). LDN was added for 24 h at a final concentration of 5 μm.
Cell Surface Biotinylation Assays
To assess TrkB internalization, (7 DIV) primary neurons were either treated with or without BDNF (50 ng/ml, 30 min) and then placed on ice to prevent further TrkB internalization. The remaining cell surface TrkB receptors were biotinylated with Sulfo-NHS-LC-Biotin (100 mg/ml, 30 min; Thermo Scientific) and then washed with 0.1 m Tris-HCl (pH 7.5), three times. The cells were lysed with radioimmunoprecipitation assay buffer containing a protease inhibitor mixture (Roche Applied Science), and biotinylated TrkB was immunoprecipitated with streptavidin-agarose beads that had been pre-equilibrated in radioimmunoprecipitation assay buffer. Immunoprecipitated proteins were incubated in sample buffer and processed for Western blot analysis using rabbit polyclonal TrkB (EMD Millipore).
Measuring the Velocity of BDNF-containing Endosomes
Time lapse microscopy was utilized to measure the rates of BDNF-GFP-containing endosomes within the microfluidic devices. Rat primary neurons (7 DIV) were imaged using an inverted Bio-Rad Radiance 2100 confocal microscope and a 60× oil emersion objective. Regions of interest from five axon segments from each chamber were randomly selected for time lapse imaging. The images were acquired every 5 s for a total of 60 images (5 min). To determine velocity of BDNF-GFP particles within axons, kymographs were generated from the image stack of each time lapse experiment. The velocities of BDNF-GFP containing endosomes were determined in each kymograph, and statistical comparisons were performed using a Student's paired t test.
Quantification of Overall Retrograde Trafficking (BDNF-GFP) or Signaling (pERK5 and CRE-GFP) within Microfluidic Chambers
Cell culture medium (40 μl) was removed from each axonal well prior to the addition of BDNF-GFP. The resultant volume difference restricts BDNF-GFP to only the axonal compartment. After 2 h, somal compartments were analyzed for either net BDNF-GFP transport or p-ERK5 activation by immunocytochemical analysis as described previously (43). In brief, the microfluidic devices were removed, and the coverslips were rinsed with PBS, paraformaldehyde-fixed (4%), permeabilized in 0.25% Triton X-100 in PBS, (pH 7.4), and blocked with 5% goat serum. The cells were incubated in appropriate primary antibody overnight at 4 °C. GFP was stained with rabbit anti-GFP (Invitrogen) followed by anti-rabbit Alexa 488 secondary antibodies. p-ERK5 was stained with anti-p-ERK5 (1:1000; Cell Signaling) followed by anti-mouse Alexa 568. The cells were washed and then immunolabeled with TOTO-3 (Invitrogen) to identify nuclei. CREB-mediated gene expression was assessed in neurons that had been transfected with CRE-GFP (Stratagene) using Amaxa nucleofection (according to their protocol) prior to plating in the microfluidic devices. BDNF (50 ng/ml) was added to the axonal compartment following a similar protocol to the one for BDNF-GFP above.
Images were captured on a Bio-Rad Radiance 2100 confocal system using lambda strobing mode to avoid nonspecific cross-excitation or cross-detection of fluorophores. For each chamber device, three regions of interest were taken using the same settings, for each region of interest, five random areas were chosen and quantitated using ImageJ software (National Institutes of Health). The mean pixel intensity for each area was determined and normalized to ΤΟΤΟ-3.
p-ERK5 translocation was also assessed by Western blot analysis. The lysates were prepared from each treatment group by aspirating the media from each well, and then removing the chamber from the coverslips. Next radioimmunoprecipitation assay buffer (100 μl) was added to the area of the coverslips, which contained neurons from the somal side, and then collected. Protein levels were determined by BCA, and equal protein amounts were separated by SDS-PAGE (10%) and processed for Western analysis with either p-ERK5 or total ERK5 antibodies (1:1000; Cell Signaling). Following secondary antibodies, the blots were developed with SuperSignal West Femto chemiluminescent substrate (ThermoFisher).
Microarray Methods
UCHL-1 gene expression changes in AD brain were assessed using a microarray database consisting of brain tissue from AD cases (n = 26; range, 74–95 years; mean age, 85.7 ± 6.5 years) and age-matched controls (n = 33; range, 69–99 years; mean age, 84.2 ± 8.9 years). The criteria for the selection of cases was described previously (44). RNA expression profiles were obtained from 40 hippocampal samples (AD, n = 17; controls, n = 23) and 43 superior frontal gyrus samples (AD, n = 20; controls, n = 23), using 83 Affymetrix HgU133 plus 2.0 arrays, based on the method described previously (44). Two probe sets were identified on the HgU133 plus 2.0 array corresponding to UCHL-1 (Unigene Hs.518731), both of which had Present flags in all microarrays, indicating high expression reliability of the probes. Expression values were averaged across the probe sets to obtain an overall value for each case, followed by t test comparisons for each region and significance set at p < 0.05.
Preparation of Protein Samples from Brain Tissue
Transgenic mouse brain specimens were obtained from the University of California, Irvine Alzheimer Disease Research Center Tissue Repository. Wild-type and Tg2576 (an AD transgenic mouse line) mouse hippocampus or cortex (aged 15 months) was mechanically homogenized with a 1-ml syringe fitted with a 28 1/2-gauge needle (BD Biosciences) by repeated uptake in 200 μl of radioimmunoprecipitation assay buffer containing protease inhibitors (Roche Applied Science). The lysates were centrifuged (80,000 × g for 1 h), the protein concentration of the supernatant was determined by BCA, and samples were stored at −20 °C until analyzed.
RESULTS
Aβ Oligomers Directly Disrupt BDNF/TrkB Axonal Retrograde Trafficking by Impairing Vesicle Velocities
Recent evidence suggests that an aspect of neurodegenerative pathology is impaired neurotrophin-dependent retrograde transport (45, 46). In the case of AD, Trk retrograde trafficking deficits are likely Aβ-mediated (22, 47). To define the mechanism underlying the net decrease in BDNF/TrkB retrograde trafficking in the presence of Aβ oligomers (22), we investigated whether soluble Aβ interferes with 1) TrkB internalization at the membrane surface and/or 2) translocation/transport of the BDNF/TrkB-containing endosome from the axon to the soma.
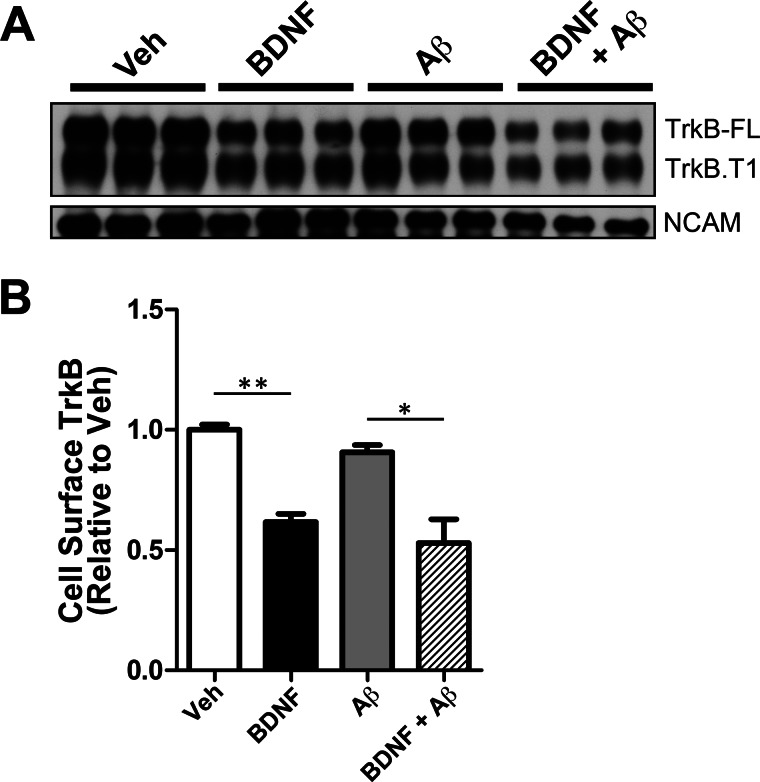
Cell surface biotinylation assays were employed to determine whether Aβ affected TrkB internalization. We found that Aβ did not impair TrkB receptor internalization in cultured rat primary neurons (7 DIV) (Fig. 1). In the absence of Aβ, BDNF treatment drove internalization of 38.3 ± 3.40% (**, p < 0.001) of cell surface TrkB. Similarly, in the presence of Aβ oligomers, BDNF treatment led to the internalization of 41.5 ± 9.8% (*, p < 0.05) of cell surface TrkB relative to Aβ-only treatment. No significant reduction in cell surface TrkB was observed with Aβ preincubation alone, in the absence of BDNF. The TrkB antibody detects both full-length TrkB and a truncated form of TrkB (48), enabling us to determine that soluble Aβ does not affect internalization of either full-length or truncated TrkB (Fig. 1). In addition, TrkB internalization was BDNF-specific because neural cell adhesion molecule was not internalized by BDNF treatment. Although previous studies have demonstrated that Aβ can alter cell surface receptor internalization (for example, of AMPA and NMDA receptors) (49, 50), our data demonstrate that Aβ does not affect the internalization of TrkB and suggest that Aβ-mediated trafficking deficits are downstream of TrkB internalization.
FIGURE 1.
Aβ oligomers do not affect the internalization of BDNF receptors. A, cell surface biotinylation was employed to measure TrkB levels at the cell surface following BDNF treatment (50 ng/ml, 30 min) as described under “Experimental Procedures.” BDNF addition led to a decrease in cell surface levels of full-length TrkB (TrkB-FL) and truncated TrkB (TrkB.T1). Preincubation with Aβ oligomers (24 h) does not impair TrkB-FL or TrkB-T1 internalization. BDNF specifically caused internalization of BDNF receptors, but not the internalization of neural cell adhesion molecule (NCAM). B, cell surface TrkB-FL was quantitated using ImageJ (National Institutes of Health). The mean ± S.E. represents TrkB-FL levels normalized to vehicle-treated neurons (n = 3). Following BDNF treatment, we found that 38.3 ± 3.40% of TrkB-FL was internalized (black bar) when compared with vehicle (white bar) (**, p < 0.001). In the presence of Aβ, BDNF led to 41.5 ± 9.80% of TrkB-FL internalized (hatched bar) when compared with Aβ-only (gray bar) (*, p < 0.05). Veh, vehicle.
Next, we investigated whether Aβ oligomers impair retrograde trafficking by directly affecting the velocities of BDNF-containing endosomes. Vesicle velocities were measured using a novel microfluidics device developed in our laboratory that was described previously (51). This device allows axons to grow along a patterned surface and forces the separation of axons and soma within compartments, enabling the isolated manipulation of axons, soma, or both. Thus, the device can be used to assess events occurring in the soma following axonal treatment and is ideal to study axonal retrograde transport and downstream events.
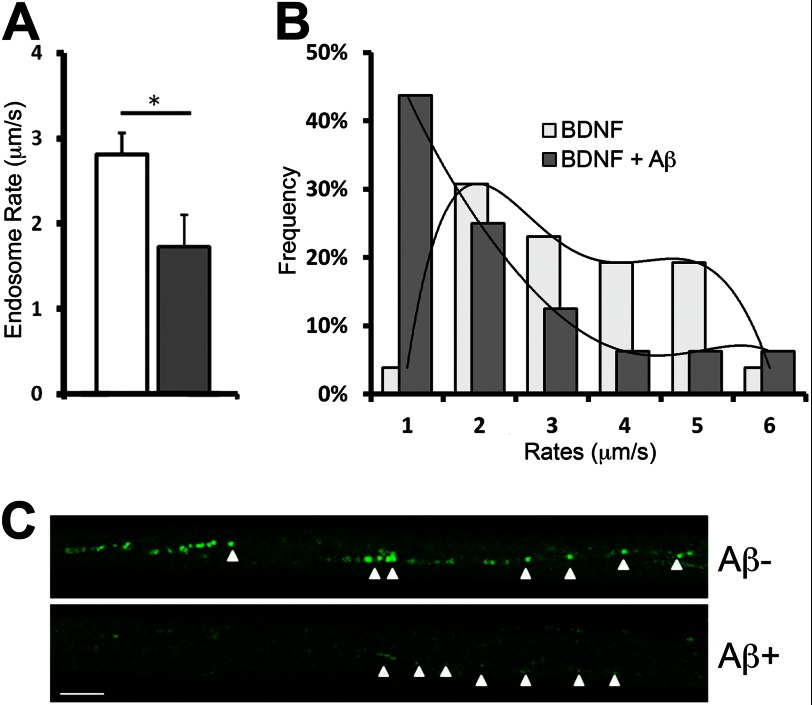
BDNF-GFP was added to the axonal compartment to allow for the visualization of axonal retrograde trafficking within neurons. We found that Aβ oligomers reduce BDNF-GFP-containing vesicle velocities within axons by 38.4 ± 13.4% (*, p < 0.01) relative to vehicle-treated neurons (Fig. 2A). The average vesicle velocity was 2.81 ± 0.253 μm/s in vehicle-treated axons, whereas the average vesicle velocity in Aβ-treated axons was 1.73 ± 0.378 μm/s. This is in agreement with our previous study that demonstrated that the velocities of BDNF-containing endosomes were markedly reduced in Tg2576 neurons when compared with wild-type neurons (22).
FIGURE 2.
Aβ oligomers impair the trafficking of BDNF-GFP endosomes. A, in the presence of Aβ, the average velocity of BDNF-GFP-containing endosomes was 1.73 ± 0.378 μm/s. This represented a 38.4 ± 13.4% (*, p < 0.01) decrease when compared with the average velocity of endosomes in the absence of velocities of BDNF-GFP-positive endosomes determined as described previously (22). B, distribution plot of the vesicle velocities reveal that in the presence of Aβ oligomers, the percentage of vesicle velocities >2 mm/s was greatly reduced (gray bars), and the majority of the vesicle velocity was <1 μm/s. C, representative time lapse image of BDNF-GFP containing endosomes demonstrates the amount of BDNF-TrkB complex that undergoes retrograde transport of the (from right to left). Scale bar, 10 μm.
Examination of the vesicle velocity distribution revealed that the presence of Aβ oligomers significantly decreased the percentage of endosomes with velocities >2 μm/s, with the majority of the endosome velocities being <1 μm/s (Fig. 2B). Additionally, representative time lapse images reveal that in the presence of Aβ, the BDNF-GFP signal that can be visualized within trafficking vesicles is greatly reduced (Fig. 2C). These results suggest that Aβ oligomers can disrupt retrograde trafficking by affecting both the vesicle velocities of BDNF-containing endosomes and the amount of TrkB that is contained within the transported endosomes.
β-Amyloid Impairs BDNF-dependent Retrograde Signaling
The signaling endosome hypothesis implies that if the retrograde trafficking of BDNF-GFP-positive endosomes is impaired, then the propagation of BDNF retrograde signaling will also be impaired. To test this hypothesis, BDNF-GFP was added to the axonal compartment of the microfluidic chambers, followed by assessment of ERK activation. ERK activation was determined by measuring the phosphorylation of ERK5 (p-ERK5) in the soma of neurons. ERK5 is the main ERK that is activated in response to axonally derived BDNF (28).
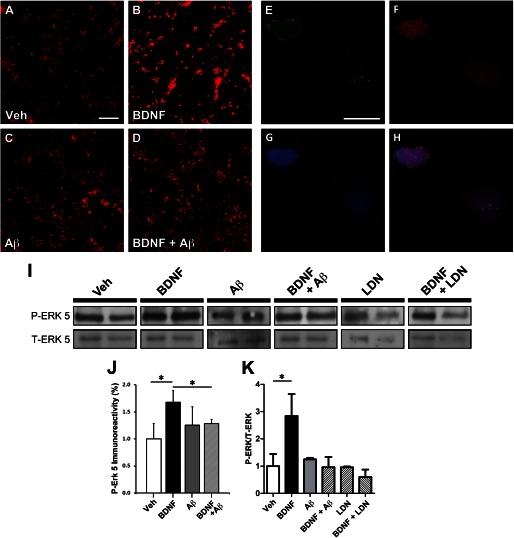
Representative images (Fig. 3) revealed that BDNF-GFP led to robust p-ERK5 activation within neuronal cell bodies located in the somal compartment. This is indicated by increased p-ERK5 labeling following BDNF-GFP, when compared with vehicle-only neurons (Fig. 3, A and B). However, p-ERK5 activation in response to BDNF-GFP is not readily apparent in neurons preincubated with Aβ (Fig. 3, C and D, respectively). Quantification of p-ERK5 immunoreactivity revealed that BDNF treatment increased p-ERK5 by 68.1 ± 8.4% (*, p < 0.05) when compared with vehicle only, whereas p-ERK5 levels were not significantly increased by BDNF treatment in neurons preincubated with Aβ oligomers, relative to Aβ-only treatment (Fig. 3J). These results are consistent with a retrograde signaling deficit caused by impaired BDNF/TrkB retrograde transport.
FIGURE 3.
Aβ oligomers impair the trafficking of the signaling endosome complex including p-ERK5. Microfluidic devices were employed to measure the retrograde transport dependent ERK5 activation. A–D, representative images demonstrating p-ERK5 levels (red) in the somal compartment of microfluidic chamber of vehicle-treated neurons (A) and following BDNF treatment (B), in the presence of Aβ oligomers only (C), and after BDNF treatment (D). Scale bar, 200 μm. E–H, representative images of a neuron demonstrating the co-localization of BDNF-GFP (green) on the outer surface of the nucleus (blue). Also, p-ERK5 (red) co-localized with the nucleus, suggesting that the BDNF-mediated retrograde signal, i.e., the signaling endosome, undergoes retrograde transport from the axonal compartment to the soma and specifically the nucleus. Scale bar, 20 μm. I, Western blot analysis of p-ERK5 and T-ERK5 isolated from the somal compartment of microfluidics devices as described under “Experimental Procedures.” J, somal p-ERK5 was quantitated as described under “Experimental Procedures.” BDNF leads to a 68.1 ± 8.4% (*, p < 0.05) increase in p-ERK5. However, in neurons preincubated with Aβ oligomers, p-ERK5 levels are not increased following axonal BDNF treatment. K, quantification of p-ERK5 relative to total ERK5 levels in the somal compartment following BDNF treatment. BDNF leads to a 284% increase in p-ERK5. However, in the presence of Aβ oligomers, BDNF does not lead to increased p-ERK5. Also, a UCH-L1 inhibitor (LDN-57444) was used to inhibit deubiquitinating activity and revealed that it could mimic the effect of Aβ oligomers in impairing BDNF-mediated retrograde signaling. Veh, vehicle; LDN, LDN-57444.
At higher magnification, p-ERK5 (Fig. 3F) appeared to co-localize with the nuclear marker TOTO-3 (Fig. 3G) in a representative neuron, which suggests that p-ERK5 is translocated to the nucleus and is consistent with the literature (28). Additionally, BDNF-GFP immunoreactivity decorated the outer surface of the nucleus, indicating that axonally applied BDNF-GFP trafficked back to the soma (Fig. 3E). This observation supports the “signaling endosome” hypothesis for neurotrophin signaling, because it demonstrates that ligand-receptor complexes that originate from the axonal compartment undergo retrograde transport to the soma.
Next, we measured the extent of p-ERK5 translocation to the somal compartment by Western blot analysis. It revealed that somal p-ERK5 levels (normalized to total ERK5) increase almost 3-fold (*, p = 0.05) in response to axonally applied BDNF (Fig. 3, I and K). However, in cultures preincubated with Aβ, p-ERK5 translocation was not observed. Although p-ERK5 levels were lower following BDNF treatment in neurons preincubated with Aβ, it was not significant. The assessment of p-ERK5 translocation by Western blot analysis is consistent with our immunocytochemical results and together supports the notion that Aβ impairs BDNF-dependent retrograde signaling.
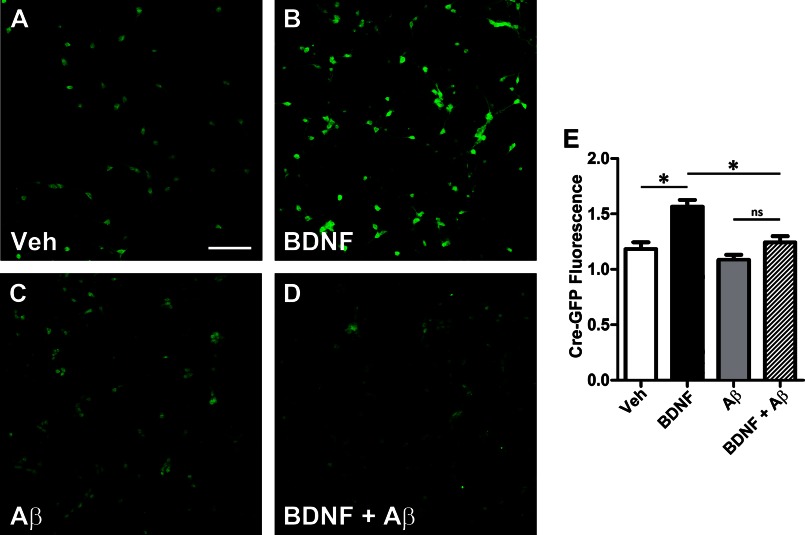
Next, CREB-dependent gene transcription was assessed to further validate the hypothesis that soluble Aβ impairs BDNF-dependent retrograde signaling. CREB-mediated gene transcription was measured by quantifying GFP within neurons transfected with a CRE-GFP reporter plasmid (Stratagene). CRE-GFP is a cAMP response element (CRE) fused to GFP that is used to monitor downstream cAMP/PKA signaling. In neurons transfected with CRE-GFP, axonal BDNF treatment led to a robust increase in GFP immunoreactivity within soma (35.9 ± 4.73%; *, p < 0.01) when compared with vehicle only (Fig. 4, A, B, and E). GFP immunoreactivity was normalized with the neuronal marker, βIII-tubulin. In contrast, in neurons preincubated with Αβ oligomers, no significant increase in GFP immunoreactivity was observed following BDNF (Fig. 4, C–E). Thus, Aβ oligomers also impair axonal BDNF-mediated CREB-dependent gene activation. This impairment was also observed in APP (Tg2576) neurons (data not shown). Taken together, these results demonstrate that Aβ oligomers cause deficits in BDNF/TrkB retrograde signaling by affecting the trafficking of BDNF-GFP-containing endosomes, which in turn results in the decreased retrograde transport and activation of ERK5 and CREB-dependent transcription that is necessary to maintain proper synaptic function and neuronal survival.
FIGURE 4.
Aβ oligomers lead to decreased CREB-dependent gene expression. Microfluidic devices were used to assess CREB-mediated gene expression. Rat primary neurons (embryonic day 18) were transfected with a CRE-GFP reporter construct to assess CREB-mediated gene activation. At 7 DIV, the axonal compartment was treated with BDNF (50 ng/ml, 2 h), and the chambers were processed for immunochemical analysis as described under “Experimental Procedures” using polyclonal anti-GFP (Invitrogen) to measure CRE-GFP levels and were normalized to the neuronal marker, BIII-tubulin (red). A, representative image of CRE-GFP levels (green) within the somal compartment and co-imaged with the neuronal marker, BIII-tubulin (red) in vehicle-treated neurons, and in neurons treated with BDNF. B and C, in the presence of Aβ oligomers, base-line levels of CRE-GFP are not significantly reduced when compared with vehicle. D, in the presence of Aβ oligomers, the increase in the amount of CRE-GFP is greatly reduced. E, CRE-GFP levels were quantified, and the means ± S.E. represent n = 4 and demonstrate that BDNF treatment to the axonal compartment led to a 35.9 ± 4.73 (*, p < 0.01) increase in somal CRE-GFP immunoreactivity when compared with vehicle (white bar). However, in the presence of Aβ oligomers, CRE-GFP was only increased by 14.6 ± 5.23%, when compared with Aβ oligomer only cells, but this was not significant (p = 0.076). Therefore, in the presence of Aβ, axonal BDNF leads to reduced CRE-GFP immunoreactivity (hatched bar) when compared with vehicle treated neurons (black bar) (*, p < 0.01). Scale bar, 200 μm. Veh, vehicle.
Impaired Deubiquitination Mimics the Effects of Amyloid on BDNF Retrograde Signaling
Previous studies suggested that Aβ impairs proteasome function and deubiquitinating enzyme activity, which in turn can impair receptor sorting to MVBs and neurotrophin receptor trafficking (35, 52). Because MVBs represent the endosomal compartment that mediates sustained neurotrophin signaling from axon terminals to the soma (25, 53), it suggests that the BDNF/TrkB trafficking deficits may be caused by Aβ impairing deubiquitinating activity. To test this hypothesis, we assessed whether inhibiting deubiquitinating activity could mimic the effect of Aβ oligomers on retrograde transport. Deubiquitinating activity was inhibited with LDN (LDN-57444, EMD), a cell-permeable UCH-L1-specific inhibitor. As predicted, inhibiting deubiquitination with LDN impaired BDNF retrograde signaling as assessed by measuring p-ERK5 activation (Fig. 5, A and B). BDNF treatment led to a 45.7 ± 10.3% (*, p < 0.05) increase in nuclear p-ERK5 levels (Fig. 5B). However, in neurons pretreated with LDN, the addition of BDNF did not lead to an increase in p-ERK5 levels. Interestingly, basal p-ERK5 levels were lower when UCH-L1 was inhibited. We attribute this decrease to the importance of ubiquitin turnover to synaptic function. Nonetheless, these results support the hypothesis that Αβ oligomers may affect TrkB retrograde signaling by impairing UCH-L1 activity.
FIGURE 5.
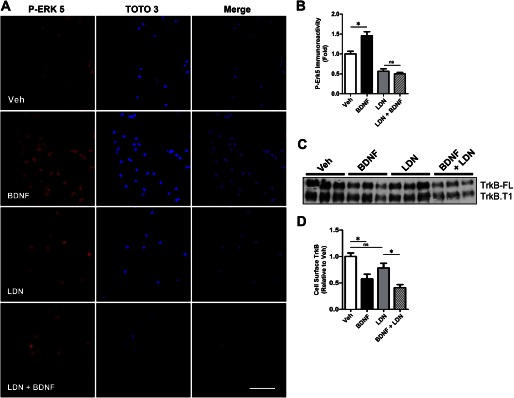
The UCH-L1 inhibitor LDN mimics the effect of Aβ oligomers on BDNF-dependent retrograde signaling. To assess the effect of LDN on BDNF-dependent retrograde signaling, we measured p-ERK5 activation in the presence of LDN. A, representative images that demonstrate that BDNF led to an increase in somal p-ERK5, but the UCH-L1 inhibitor, LDN, led to decreased basal somal p-ERK5, and in the presence of BDNF, the nuclear translocation of p-ERK5 is not detected. p-ERK5 immunoreactivity was normalized to the nuclear counterstain, TOTO-3. B, quantification of somal p-ERK5 levels demonstrate that although BDNF treatment (black bar) leads to a 45.7 ± 10.2% (*, p < 0.05) increase in p-ERK5 when compared with vehicle (white bar), in neurons preincubated with LDN (gray bar), we do not observe an increase in somal p-ERK5 following BDNF (hatched bar). C, cell surface biotinylation assays were employed to determine the effect of LDN on TrkB internalization and demonstrate that LDN does not affect the internalization of full-length TrkB (TrkB-FL) or truncated TrkB (TrkB.T1). D, quantification of cell surface TrkB-FL demonstrates that in vehicle-treated neurons (white bar), the addition of BDNF (black bar) led to a 45.7 ± 10.2% (*, p < 0.05) decrease in cell surface TrkB levels, whereas in the presence of LDN (gray bar), BDNF led to a 44.3 ± 8.56 (*, p < 0.05) decrease in TrkB-FL. The mean ± S.E. represents n = 3. Scale bar, 20 μm. Veh, vehicle; LDN, LDN-57444.
Similarly, LDN pretreatment impaired the translocation of p-ERK5 to the soma following BDNF as assessed by Western blot analysis (Fig. 3, I and K). Following BDNF treatment p-ERK5 translocation, the soma was not observed. These results were similar to Aβ-treated neurons and support the hypothesis that Aβ affects BDNF/TrkB signaling by impairing deubiquitinating activity.
Additionally, like Aβ, LDN did not impair TrkB endocytosis. Using cell surface biotinylation assays, we show that the addition of BDNF led to a 45.7 ± 10.2% (*, p < 0.05) decrease in cell surface TrkB levels when compared with vehicle (Fig. 5, C and D). In neurons preincubated with LDN, we observed a similar decrease in TrkB (44.3 ± 8.56%; *, p < 0.05). Taken together, these results indicate that LDN mimics Aβ oligomers not by inhibiting TrkB internalization, but by impairing downstream BDNF retrograde signaling.
UCH-L1 Rescues TrkB Retrograde Trafficking Deficits Caused by Aβ
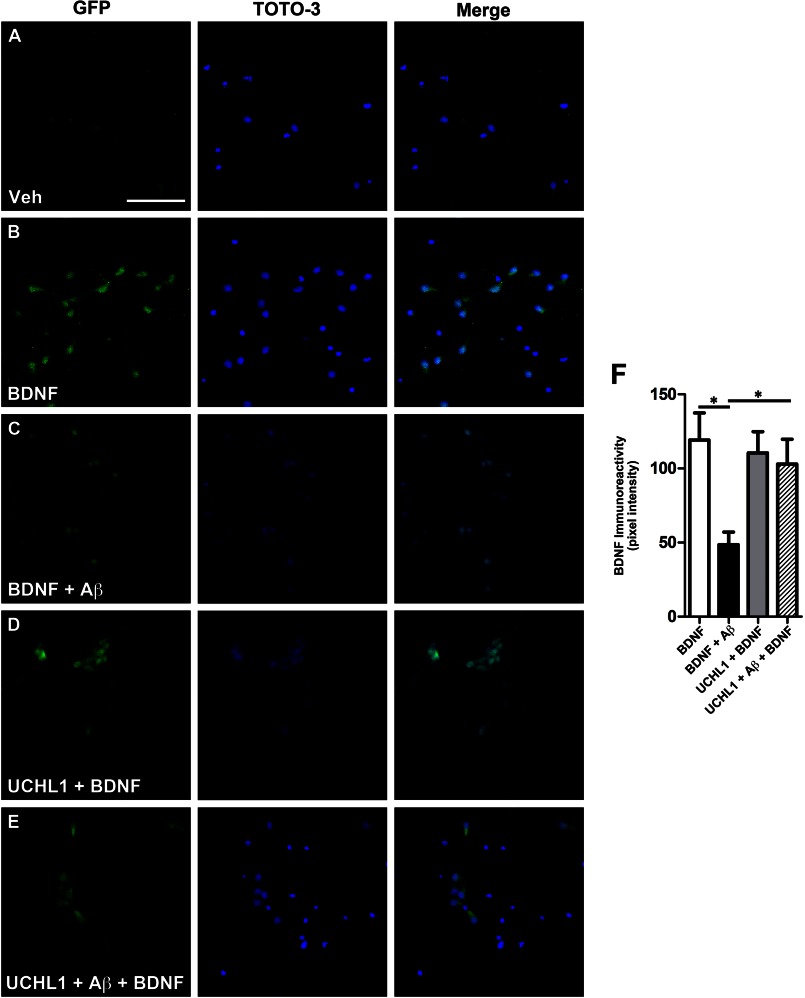
Next, we investigated whether BDNF-mediated transport deficits induced by Aβ could be rescued by increasing UCH-L1 levels using a TAT-HA-UCH-L1 construct as described previously (42). UCH-L1 treatment rescued BDNF-GFP retrograde trafficking deficits caused by Aβ (Fig. 6). In the presence of Aβ oligomers, BDNF-GFP levels in the soma were 60.3 ± 7.1% (*, p = 0.003), lower than vehicle-treated neurons. However, in the presence of UCH-L1 alone, BDNF-GFP levels were 92.6 ± 12.0% of vehicle only. UCH-L1 rescued the Aβ-induced deficit in BDNF-GFP levels in the soma to 86.3 ± 14.1% of the vehicle-treated neurons (*, p = 0.04).
FIGURE 6.
Transduction of UCH-L1 rescued Aβ-mediated retrograde transport deficits. To assess whether increasing UCH-L1 could rescue Aβ-mediated transport deficits, we measured the extent of BDNF-GFP trafficking in neurons transduced with UCH-L1. A and B, representative images demonstrate that somal levels of BDNF-GFP are increased in neurons following BDNF-GFP treatment. GFP immunoreactivity was normalized to the nuclear marker, TOTO-3. C, pretreatment with Aβ led to decrease in BDNF-GFP when compared with vehicle-treated neurons. D, the addition of UCH-L1 alone led to increased somal BDNF-GFP immunoreactivity. E, UCH-L1 rescues the deficit in BDNF-GFP trafficking caused by Aβ. F, quantification of somal BDNF levels normalized to cell number (TOTO-3-positive nuclei) reveals that Aβ causes a 60.3 ± 7.1% (*, p = 0.003) decrease in the retrograde transport of BDNF-GFP back to soma compared with vehicle-treated neurons. In the presence of UCH-L1, BDNF-GFP levels were 92.6 ± 12.0% of vehicle plus BDNF. Importantly, UCH-L1 rescued the deficit in BDNF-GFP trafficking caused by Aβ. BDNF-GFP levels were 86.3 ± 14.1% of vehicle treated and revealed that UCH-L1 restored trafficking deficits caused by Aβ oligomers (*, p = 0.04). Scale bar, 200 μm. Veh, vehicle.
Thus, we demonstrate that Αβ-mediated deficits in retrograde transport can be rescued by UCH-L1. Taken together with our LDN data (Fig. 5), these results demonstrate that modulating ubiquitin homeostasis via UCH-L1 impacts BDNF-mediated retrograde trafficking.
UCH-L1 Is Decreased in the Hippocampus in APP-Tg2576 Mice and in AD
Next, we determined whether UCH-L1 levels are also affected in an AD transgenic mouse model. We measured UCH-L1 protein levels in the hippocampus or cortex of 15-month-old wild-type or Tg2576 mice. We found that hippocampal but not cortical UCH-L1 levels were significantly decreased in Tg2576 mice relative to age-matched wild-type mice (Fig. 7, A and B) (*, p < 0.03).
FIGURE 7.
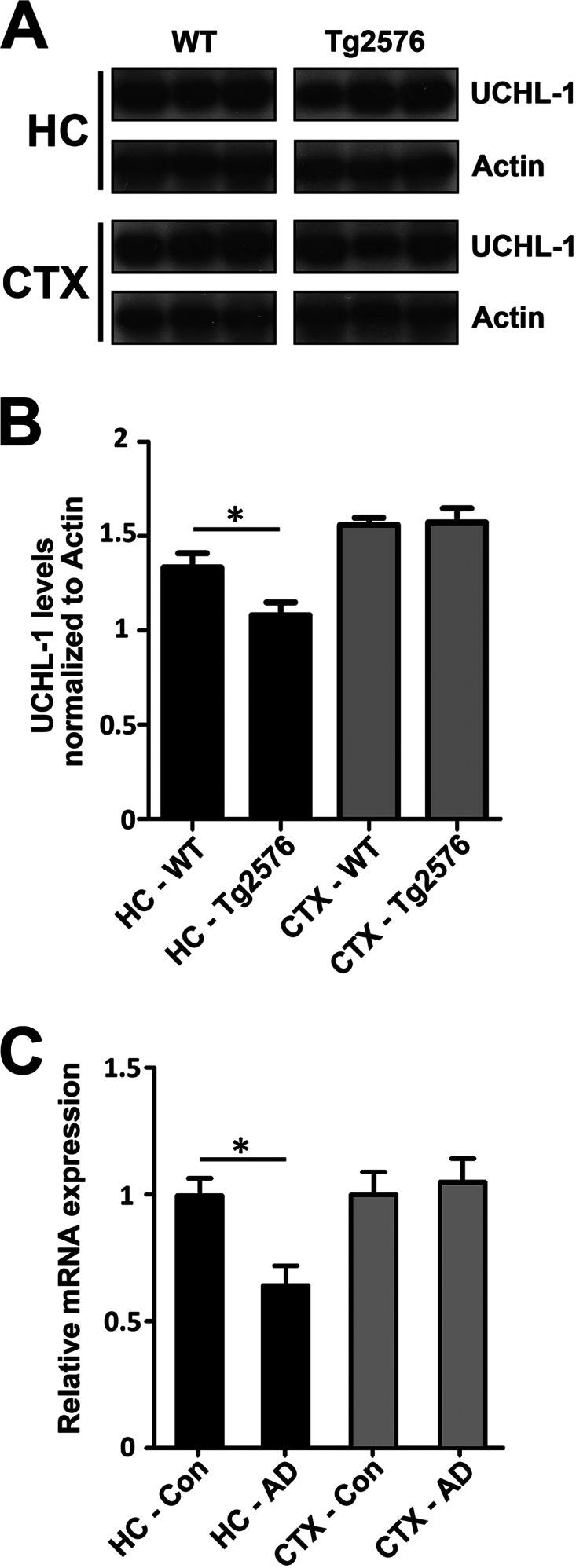
UCH-L1 is decreased in Tg2576 mice and in the Alzheimer disease brain. A, levels of UCH-L1 are decreased in APP-Tg2576 hippocampus. Hippocampal lysates were prepared as described under “Experimental Procedures.” Protein was separated on SDS-PAGE, and Western blot analysis was carried out to determine the amount of UCH-L1 protein in wild-type and Tg2576 mouse brain. B, UCH-L1 protein levels are decreased within the hippocampus but not the cortex of 15-month-old Tg2576 mice (*, p < 0.03). UCH-L1 levels were quantitated and normalized to actin protein as described under “Experimental Procedures.” C, UCHL-1 gene expression is lower in AD brain. Expression profiles were obtained from a microarray database consisting of brain tissue from AD cases (n = 26; range, 74–95 years; mean age, 85.7 ± 6.5 years) and age-matched controls (n = 33; range, 69–99 years; mean age, 84.2 ± 8.9 years) and were generated using Affymetrix HgU133 plus 2.0 arrays as described previously (44). Two probe sets corresponding to UCHL-1 (Unigene Hs.518731) were identified on the HgU133 plus 2.0 array, both of which had Present flags in all microarrays, indicating high expression reliability of the probes. Expression values were averaged across the probe sets to obtain an overall value for each case, followed by t test comparisons for each region and significance set at p < 0.05 (*).
Lastly, we investigated whether the decrease in UCH-L1 translates to the in vivo condition in humans. We compared UCH-L1 gene expression levels in the hippocampus and superior frontal cortex (BA9/46) in AD brain versus age-matched cognitively intact controls. UCHL-1 gene expression data were obtained from a microarray database as described previously (44) and revealed that UCH-L1 gene expression was significantly reduced in the hippocampal regions of AD cases versus aged-matched controls (Fig. 7B; p < 0.05). The decrease in UCH-L1 expression levels was hippocampus-specific, because we did not detect a difference in UCH-L1 expression in the frontal cortex.
DISCUSSION
Overall, our results demonstrate that soluble Aβ impairs BDNF/TrkB retrograde axonal trafficking and signaling. Building on our previous findings that Aβ oligomers cause a net decrease in the amount of BDNF/TrkB trafficked back to soma (22), this is not due to Aβ oligomers affecting the internalization of TrkB. Although previous studies have demonstrated that Aβ can alter cell surface receptor internalization (for example, of AMPA and NMDA receptors) (49, 50), our data demonstrate that Aβ does not affect the internalization of TrkB and suggested that Aβ-mediated trafficking deficits were downstream of receptor internalization.
Using a novel microfluidic chamber that facilitates the study of axonal transport, we found that Aβ oligomers cause a decrease in the amount of BDNF-GFP that is found within endosomes that undergo retrograde axonal transport. In addition, we found that the average velocity of BDNF-GFP-containing endosomes was decreased in the presence of Aβ, with the distribution of the vesicle velocities shifted to ones with lower velocities. These data suggest that soluble Aβ impairs the sorting of BDNF/TrkB to MVBs, the endosomal compartment that mediates TrkB retrograde axonal trafficking, and also reduces the velocity of trafficking MVBs. Together, these deficits contribute to impaired BDNF/TrkB retrograde transport. It is of note that early in the progression of AD and prior to Aβ deposition, when Aβ oligomers are likely present, enlarged endosomal structures can be detected in neurons (54). It is tempting to speculate that the decrease in vesicle velocity is attributed to enlarged MVBs. Because proteins sorted to MVBs are also degraded in a lysosome-dependent process (33), our data are also consistent with the finding that Aβ impairs lysosome-mediated degradation of TrkB (35).
We also demonstrated that BDNF/TrkB axonal trafficking deficits induced by Aβ were accompanied by impaired downstream BDNF/TrkB signaling, notably impaired nuclear translocation of p-ERK5, and down-regulated CREB-mediated signaling events. These results reveal that the presence of soluble Aβ impairs retrograde trafficking, resulting in diminished signaling between axons and cell bodies, supporting the signaling endosome hypothesis that describes how cellular signals that are initiated at axon terminals undergo retrograde transport and are propagated back to the soma (55). Furthermore, the disruption by Aβ may be due to a generalized impairment in trafficking because the trafficking of other organelles/cargos is also impaired by Aβ (47, 56–59).
Our data reveal that a mechanism underlying the retrograde trafficking deficits in the presence of Aβ involves altered ubiquitin homeostasis. Ubiquitin is central for proteasome-dependent protein turnover as well as for intracellular trafficking of cargoes including many receptors (i.e., glutamate and neurotrophins) that are fundamental for synaptic remodeling and plasticity (60). Although ubiquitin can be synthesized de novo, the bulk of the cellular ubiquitin pool is derived from ubiquitin that is recovered from deubiquitinating enzymes (61). Thus, ubiquitin levels can be regulated by modulating deubiquitinating enzyme levels or activity of deubiquitinating enzymes such as UCH-L1 (42, 62). Remarkably, UCH-L1 represents 1–2% of the total protein within the brain and thus is an important regulator of brain ubiquitin levels (62–64).
Ubiquitination regulates many important processes including the targeting and delivery of receptors to MVBs, including trophic factor receptors (65–67). For example, ubiquitination mediates the delivery of the well studied EGF receptor to MVBs for its degradation within lysosomes (33). In the case of Trk receptors, ubiquitination may regulate its endocytic trafficking to MVBs for sustained retrograde signaling (34, 53, 68).
Therefore, we tested whether disrupting the ubiquitin recycling pathway, which regulates cellular ubiquitin levels, can lead to BDNF-dependent retrograde transport deficits. Indeed, we found that BDNF/TrkB retrograde trafficking and signaling could be affected by manipulating deubiquitinating activity by either inhibiting or increasing UCH-L1. Specifically, inhibiting UCH-L1 with LDN resulted in retrograde trafficking deficits parallel to those found induced by soluble Aβ, and retrograde trafficking deficits caused by Aβ could be rescued by increasing cellular UCH-L1 levels.
Although ubiquitination mediates the internalization of numerous receptors, we found that neither Aβ nor impairing ubiquitin recycling with LDN affected TrkB internalization. Our data support the finding that TrkB ubiquitination is not a prerequisite for its internalization (34) and may only regulate its endocytic fate (69, 70). Taken together, these results suggest that Aβ impairs the retrograde trafficking of TrkB by affecting ubiquitin homeostasis via UCH-L1 at a step that is downstream from receptor internalization.
Further, our data suggest that ubiquitin homeostasis may be impaired in the hippocampus in AD, in both Tg2576 mouse model of AD and in the human brain. We demonstrate that in Tg2576 mice, hippocampal but not cortical UCH-L1 protein levels are reduced compared with wild-type littermates, similar to the findings in the brain of APP/presenilin 1 mice at 4–6 months of age, suggesting that the decrease in UCH-L1 follows the development of pathology (42). In parallel, we demonstrate that hippocampal but not cortical UCH-L1 gene expression is decreased in the AD brain relative to age-matched cognitively intact cases. The reduced availability of UCH-L1 in the AD brain likely impairs neurotrophin signaling in vivo, based on our in vitro data, which found that inhibiting UCH-L1 caused deficits in TrkB/BDNF retrograde trafficking and signaling. In support of our hypothesis that Aβ itself directly affects UCH-L1 levels, a decrease in monomeric ubiquitin levels caused by Aβ is reversed by increasing UCH-L1 levels in hippocampal slices (42).
These data add to the growing evidence that disrupted ubiquitin homeostasis is an important aspect of AD pathobiology, with previous studies demonstrating that impaired deubiquitination alters synaptic protein distribution and spine morphology and causes neurodegeneration (62, 71), which are salient features in AD. Altered ubiquitin homeostasis may contribute to generalized axonal transport deficits observed in AD. Inducing lysosome dysfunction impairs axonal retrograde transport of late endosomes and lysosomes and leads to AD-like axonal pathology (72). Because the sorting of proteins to lysosomes is ubiquitin-dependent (73), it suggests that by altering ubiquitin homeostasis, Aβ can trigger lysosome dysfunction and the observed transport deficits. Furthermore, we and others have found that Aβ directly affects mitochondrial transport and may be due to defective fission/fusion (58, 75), which is regulated by ubiquitination (76). Thus, it suggests that ubiquitination/deubiquitination plays a vital role in regulating axonal transport. Lastly, balancing ubiquitination/deubiquitination may also affect Aβ production because APP ubiquitination inhibits APP endocytosis and promotes the nonamyloidogenic processing (77).
Our data plus a growing body of evidence suggest that in AD there may be a general defect in intracellular trafficking. Our results describe a novel mechanism by which Aβ can impair ubiquitin homeostasis that leads to endosomal axonal retrograde transport deficits, impairs neurotrophin signaling, and contributes to impaired synaptic plasticity. As Aβ accumulates, one of the consequences may be impaired intracellular trafficking of cellular components that depend on ubiquitin conjugation for signal transduction and protein sorting and degradation. Defective trafficking in the etiology of AD is supported by the recent identification of GWAS-AD-linked polymorphisms that encode proteins linked to endosome function, e.g. PICALM and BIN1 (74). Therefore, therapeutics aimed at modulating ubiquitin homeostasis may rescue intracellular trafficking deficits found in AD and improve cognition.
Acknowledgment
We thank Meredith Chabrier for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants AG016573 and AG000538 (to C. W. C) and AG00096 (to A. J. C.).
- AD
- Alzheimer disease
- APP
- amyloid precursor protein
- Aβ
- β-amyloid
- CRE
- cAMP response element
- CREB
- cAMP-responsive element-binding protein
- CRE-GFP
- CREB-responsive element fused to GFP
- DIV
- days in vitro
- LDN
- LDN-57444
- MVB
- multivesicular body
- TrkB
- tropomyosin-receptor kinase B
- UCH-L1
- ubiquitin C-terminal hydrolase L1.
REFERENCES
- 1. Jack C. R., Jr., Knopman D. S., Jagust W. J., Shaw L. M., Aisen P. S., Weiner M. W., Petersen R. C., Trojanowski J. Q. (2010) Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 9, 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Näslund J., Haroutunian V., Mohs R., Davis K. L., Davies P., Greengard P., Buxbaum J. D. (2000) Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 283, 1571–1577 [DOI] [PubMed] [Google Scholar]
- 3. Lue L. F., Kuo Y. M., Roher A. E., Brachova L., Shen Y., Sue L., Beach T., Kurth J. H., Rydel R. E., Rogers J. (1999) Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am. J. Pathol. 155, 853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., Bush A. I., Masters C. L. (1999) Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann. Neurol. 46, 860–866 [DOI] [PubMed] [Google Scholar]
- 5. Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 [DOI] [PubMed] [Google Scholar]
- 6. Cleary J. P., Walsh D. M., Hofmeister J. J., Shankar G. M., Kuskowski M. A., Selkoe D. J., Ashe K. H. (2005) Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84 [DOI] [PubMed] [Google Scholar]
- 7. Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 [DOI] [PubMed] [Google Scholar]
- 8. Hsia A. Y., Masliah E., McConlogue L., Yu G. Q., Tatsuno G., Hu K., Kholodenko D., Malenka R. C., Nicoll R. A., Mucke L. (1999) Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc. Natl. Acad. Sci. U.S.A. 96, 3228–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. (2000) High-level neuronal expression of Aβ 1–42 in wild-type human amyloid protein precursor transgenic mice. Synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 11. Tyler W. J., Alonso M., Bramham C. R., Pozzo-Miller L. D. (2002) From acquisition to consolidation. On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 9, 224–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez A., Alcántara S., Borrell V., Del Río J. A., Blasi J., Otal R., Campos N., Boronat A., Barbacid M., Silos-Santiago I., Soriano E. (1998) TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J. Neurosci. 18, 7336–7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Minichiello L., Korte M., Wolfer D., Kühn R., Unsicker K., Cestari V., Rossi-Arnaud C., Lipp H. P., Bonhoeffer T., Klein R. (1999) Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 24, 401–414 [DOI] [PubMed] [Google Scholar]
- 14. Xu B., Zang K., Ruff N. L., Zhang Y. A., McConnell S. K., Stryker M. P., Reichardt L. F. (2000) Cortical degeneration in the absence of neurotrophin signaling. Dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron 26, 233–245 [DOI] [PubMed] [Google Scholar]
- 15. Patterson S. L., Abel T., Deuel T. A., Martin K. C., Rose J. C., Kandel E. R. (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16, 1137–1145 [DOI] [PubMed] [Google Scholar]
- 16. Korte M., Carroll P., Wolf E., Brem G., Thoenen H., Bonhoeffer T. (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 92, 8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pozzo-Miller L. D., Gottschalk W., Zhang L., McDermott K., Du J., Gopalakrishnan R., Oho C., Sheng Z. H., Lu B. (1999) Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J. Neurosci. 19, 4972–4983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Genoud C., Knott G. W., Sakata K., Lu B., Welker E. (2004) Altered synapse formation in the adult somatosensory cortex of brain-derived neurotrophic factor heterozygote mice. J. Neurosci. 24, 2394–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Causing C. G., Gloster A., Aloyz R., Bamji S. X., Chang E., Fawcett J., Kuchel G., Miller F. D. (1997) Synaptic innervation density is regulated by neuron-derived BDNF. Neuron 18, 257–267 [DOI] [PubMed] [Google Scholar]
- 20. Tong L., Balazs R., Thornton P. L., Cotman C. W. (2004) β-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J. Neurosci. 24, 6799–6809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garzon D. J., Fahnestock M. (2007) Oligomeric amyloid decreases basal levels of brain-derived neurotrophic factor (BDNF) mRNA via specific downregulation of BDNF transcripts IV and V in differentiated human neuroblastoma cells. J. Neurosci. 27, 2628–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poon W. W., Blurton-Jones M., Tu C. H., Feinberg L. M., Chabrier M. A., Harris J. W., Jeon N. L., Cotman C. W. (2011) β-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol. Aging 32, 821–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peng S., Wuu J., Mufson E. J., Fahnestock M. (2005) Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J. Neurochem. 93, 1412–1421 [DOI] [PubMed] [Google Scholar]
- 24. Watson F. L., Heerssen H. M., Moheban D. B., Lin M. Z., Sauvageot C. M., Bhattacharyya A., Pomeroy S. L., Segal R. A. (1999) Rapid nuclear responses to target-derived neurotrophins require retrograde transport of ligand-receptor complex. J. Neurosci. 19, 7889–7900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valdez G., Akmentin W., Philippidou P., Kuruvilla R., Ginty D. D., Halegoua S. (2005) Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J. Neurosci. 25, 5236–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimes M. L., Zhou J., Beattie E. C., Yuen E. C., Hall D. E., Valletta J. S., Topp K. S., LaVail J. H., Bunnett N. W., Mobley W. C. (1996) Endocytosis of activated TrkA. Evidence that nerve growth factor induces formation of signaling endosomes. J. Neurosci. 16, 7950–7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delcroix J. D., Valletta J. S., Wu C., Hunt S. J., Kowal A. S., Mobley W. C. (2003) NGF signaling in sensory neurons. Evidence that early endosomes carry NGF retrograde signals. Neuron 39, 69–84 [DOI] [PubMed] [Google Scholar]
- 28. Watson F. L., Heerssen H. M., Bhattacharyya A., Klesse L., Lin M. Z., Segal R. A. (2001) Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat. Neurosci. 4, 981–988 [DOI] [PubMed] [Google Scholar]
- 29. Heerssen H. M., Pazyra M. F., Segal R. A. (2004) Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat. Neurosci. 7, 596–604 [DOI] [PubMed] [Google Scholar]
- 30. Haglund K., Di Fiore P. P., Dikic I. (2003) Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28, 598–603 [DOI] [PubMed] [Google Scholar]
- 31. Thien C. B., Langdon W. Y. (2001) Cbl. Many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2, 294–307 [DOI] [PubMed] [Google Scholar]
- 32. Miyake S., Lupher M. L., Jr., Druker B., Band H. (1998) The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha. Proc. Natl. Acad. Sci. U.S.A. 95, 7927–7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levkowitz G., Waterman H., Zamir E., Kam Z., Oved S., Langdon W. Y., Beguinot L., Geiger B., Yarden Y. (1998) c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 12, 3663–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arévalo J. C., Waite J., Rajagopal R., Beyna M., Chen Z. Y., Lee F. S., Chao M. V. (2006) Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron 50, 549–559 [DOI] [PubMed] [Google Scholar]
- 35. Almeida C. G., Takahashi R. H., Gouras G. K. (2006) β-Amyloid accumulation impairs multivesicular body sorting by inhibiting the ubiquitin-proteasome system. J. Neurosci. 26, 4277–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Felice F. G., Wu D., Lambert M. P., Fernandez S. J., Velasco P. T., Lacor P. N., Bigio E. H., Jerecic J., Acton P. J., Shughrue P. J., Chen-Dodson E., Kinney G. G., Klein W. L. (2008) Alzheimer's disease-type neuronal Tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging 29, 1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pang P. T., Teng H. K., Zaitsev E., Woo N. T., Sakata K., Zhen S., Teng K. K., Yung W. H., Hempstead B. L., Lu B. (2004) Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306, 487–491 [DOI] [PubMed] [Google Scholar]
- 38. Kohara K., Kitamura A., Morishima M., Tsumoto T. (2001) Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science 291, 2419–2423 [DOI] [PubMed] [Google Scholar]
- 39. Hartmann M., Heumann R., Lessmann V. (2001) Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 20, 5887–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor A. M., Rhee S. W., Tu C. H., Cribbs D. H., Cotman C. W., Jeon N. L. (2003) Microfluidic multicompartment device for neuroscience research. Langmuir 19, 1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cribbs D. H., Kreng V. M., Anderson A. J., Cotman C. W. (1996) Cross-linking of concanavalin A receptors on cortical neurons induces programmed cell death. Neuroscience 75, 173–185 [DOI] [PubMed] [Google Scholar]
- 42. Gong B., Cao Z., Zheng P., Vitolo O. V., Liu S., Staniszewski A., Moolman D., Zhang H., Shelanski M., Arancio O. (2006) Ubiquitin hydrolase UCH-L1 rescues β-amyloid-induced decreases in synaptic function and contextual memory. Cell 126, 775–788 [DOI] [PubMed] [Google Scholar]
- 43. Blurton-Jones M., Kuan P. N., Tuszynski M. H. (2004) Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression. J. Comp. Neurol. 468, 347–360 [DOI] [PubMed] [Google Scholar]
- 44. Berchtold N. C., Cribbs D. H., Coleman P. D., Rogers J., Head E., Kim R., Beach T., Miller C., Troncoso J., Trojanowski J. Q., Zielke H. R., Cotman C. W. (2008) Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. U.S.A. 105, 15605–15610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salehi A., Delcroix J. D., Belichenko P. V., Zhan K., Wu C., Valletta J. S., Takimoto-Kimura R., Kleschevnikov A. M., Sambamurti K., Chung P. P., Xia W., Villar A., Campbell W. A., Kulnane L. S., Nixon R. A., Lamb B. T., Epstein C. J., Stokin G. B., Goldstein L. S., Mobley W. C. (2006) Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51, 29–42 [DOI] [PubMed] [Google Scholar]
- 46. Her L. S., Goldstein L. S. (2008) Enhanced sensitivity of striatal neurons to axonal transport defects induced by mutant huntingtin. J. Neurosci. 28, 13662–13672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vossel K. A., Zhang K., Brodbeck J., Daub A. C., Sharma P., Finkbeiner S., Cui B., Mucke L. (2010) Tau reduction prevents Aβ-induced defects in axonal transport. Science 330, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Escandón E., Soppet D., Rosenthal A., Mendoza-Ramírez J. L., Szönyi E., Burton L. E., Henderson C. E., Parada L. F., Nikolics K. (1994) Regulation of neurotrophin receptor expression during embryonic and postnatal development. J. Neurosci. 14, 2054–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., Greengard P. (2005) Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 8, 1051–1058 [DOI] [PubMed] [Google Scholar]
- 50. Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. (2006) AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron 52, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., Jeon N. L. (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moises T., Wüller S., Saxena S., Senderek J., Weis J., Krüttgen A. (2009) Proteasomal inhibition alters the trafficking of the neurotrophin receptor TrkA. Biochem. Biophys. Res. Commun. 387, 360–364 [DOI] [PubMed] [Google Scholar]
- 53. Philippidou P., Valdez G., Akmentin W., Bowers W. J., Federoff H. J., Halegoua S. (2011) Trk retrograde signaling requires persistent, Pincher-directed endosomes. Proc. Natl. Acad. Sci. U.S.A. 108, 852–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cataldo A. M., Petanceska S., Terio N. B., Peterhoff C. M., Durham R., Mercken M., Mehta P. D., Buxbaum J., Haroutunian V., Nixon R. A. (2004) Aβ localization in abnormal endosomes. Association with earliest Aβ elevations in AD and Down syndrome. Neurobiol. Aging 25, 1263–1272 [DOI] [PubMed] [Google Scholar]
- 55. Howe C. L., Mobley W. C. (2004) Signaling endosome hypothesis. A cellular mechanism for long distance communication. J. Neurobiol. 58, 207–216 [DOI] [PubMed] [Google Scholar]
- 56. Decker H., Lo K. Y., Unger S. M., Ferreira S. T., Silverman M. A. (2010) Amyloid-β peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3β in primary cultured hippocampal neurons. J. Neurosci. 30, 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hiruma H., Katakura T., Takahashi S., Ichikawa T., Kawakami T. (2003) Glutamate and amyloid β-protein rapidly inhibit fast axonal transport in cultured rat hippocampal neurons by different mechanisms. J. Neurosci. 23, 8967–8977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim H. J., Park J. W., Byun J. H., Poon W. W., Cotman C. W., Fowlkes C. C., Jeon N. L. (2012) Quantitative analysis of axonal transport by using compartmentalized and surface micropatterned culture of neurons. ACS Chem. Neurosci. 3, 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rui Y., Tiwari P., Xie Z., Zheng J. Q. (2006) Acute impairment of mitochondrial trafficking by β-amyloid peptides in hippocampal neurons. J. Neurosci. 26, 10480–10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yi J. J., Ehlers M. D. (2007) Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol. Rev. 59, 14–39 [DOI] [PubMed] [Google Scholar]
- 61. Wilkinson K. D. (2000) Ubiquitination and deubiquitination. Targeting of proteins for degradation by the proteasome. Semin Cell Dev. Biol. 11, 141–148 [DOI] [PubMed] [Google Scholar]
- 62. Cartier A. E., Djakovic S. N., Salehi A., Wilson S. M., Masliah E., Patrick G. N. (2009) Regulation of synaptic structure by ubiquitin C-terminal hydrolase L1. J. Neurosci. 29, 7857–7868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wilkinson K. D., Lee K. M., Deshpande S., Duerksen-Hughes P., Boss J. M., Pohl J. (1989) The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science 246, 670–673 [DOI] [PubMed] [Google Scholar]
- 64. Osaka H., Wang Y. L., Takada K., Takizawa S., Setsuie R., Li H., Sato Y., Nishikawa K., Sun Y. J., Sakurai M., Harada T., Hara Y., Kimura I., Chiba S., Namikawa K., Kiyama H., Noda M., Aoki S., Wada K. (2003) Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum. Mol. Genet. 12, 1945–1958 [DOI] [PubMed] [Google Scholar]
- 65. Alwan H. A., van Zoelen E. J., van Leeuwen J. E. (2003) Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 278, 35781–35790 [DOI] [PubMed] [Google Scholar]
- 66. Devon R. S., Orban P. C., Gerrow K., Barbieri M. A., Schwab C., Cao L. P., Helm J. R., Bissada N., Cruz-Aguado R., Davidson T. L., Witmer J., Metzler M., Lam C. K., Tetzlaff W., Simpson E. M., McCaffery J. M., El-Husseini A. E., Leavitt B. R., Hayden M. R. (2006) Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc. Natl. Acad. Sci. U.S.A. 103, 9595–9600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Longva K. E., Blystad F. D., Stang E., Larsen A. M., Johannessen L. E., Madshus I. H. (2002) Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell Biol. 156, 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lauwers E., Jacob C., André B. (2009) K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J. Cell Biol. 185, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Georgieva M. V., de Pablo Y., Sanchis D., Comella J. X., Llovera M. (2011) Ubiquitination of TrkA by Nedd4–2 regulates receptor lysosomal targeting and mediates receptor signaling. J. Neurochem. 117, 479–493 [DOI] [PubMed] [Google Scholar]
- 70. Geetha T., Wooten M. W. (2008) TrkA receptor endolysosomal degradation is both ubiquitin and proteasome dependent. Traffic 9, 1146–1156 [DOI] [PubMed] [Google Scholar]
- 71. Ryu K. Y., Garza J. C., Lu X. Y., Barsh G. S., Kopito R. R. (2008) Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proc. Natl. Acad. Sci. U.S.A. 105, 4016–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee S., Sato Y., Nixon R. A. (2011) Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy. J. Neurosci. 31, 7817–7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Piper R. C., Lehner P. J. (2011) Endosomal transport via ubiquitination. Trends Cell Biol. 21, 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guerreiro R. J., Hardy J. (2011) Alzheimer's disease genetics. Lessons to improve disease modelling. Biochem. Soc. Trans. 39, 910–916 [DOI] [PubMed] [Google Scholar]
- 75. Wang X., Perry G., Smith M. A., Zhu X. (2010) Amyloid-β-derived diffusible ligands cause impaired axonal transport of mitochondria in neurons. Neurodegener. Dis. 7, 56–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Park Y. Y., Lee S., Karbowski M., Neutzner A., Youle R. J., Cho H. (2010) Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J. Cell Sci. 123, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Watanabe T., Hikichi Y., Willuweit A., Shintani Y., Horiguchi T. (2012) FBL2 regulates amyloid precursor protein (APP) metabolism by promoting ubiquitination-dependent APP degradation and inhibition of APP endocytosis. J. Neurosci. 32, 3352–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]