Background: The secreted protease inhibitor cystatin C is internalized into cancer cells.
Results: Cystatin variants with altered uptake characteristics were identified, and shown to differently inhibit intracellular cathepsin B and legumain activities.
Conclusion: The internalization process, and hence intracellular enzyme activity, can be modulated by selected cystatin variants.
Significance: The cystatin C uptake system may be targeted to control cancer-promoting activities of tumor cells.
Keywords: Breast Cancer, Cysteine Protease, Endocytosis, Protease Inhibitor, Protein Chemistry, Cathepsin B, Legumain
Abstract
To elucidate the molecular requirements for cancer cell internalization of the extracellular cysteine protease inhibitor cystatin C, 12 variants of the protein were produced and used for uptake experiments in MCF-7 cells. Variants with alterations in the cysteine cathepsin binding region ((Δ1–10)-, K5A-, R8G-, (R8G,L9G,V10G)-, (R8G,L9G,V10G,W106G)-, and W106G-cystatin C) were internalized to a very low extent compared with the wild-type inhibitor. Substitutions of N39 in the legumain binding region (N39K- and N39A-cystatin C) decreased the internalization and (R24A,R25A)-cystatin C, with substitutions of charged residues not involved in enzyme inhibition, was not taken up at all. Two variants, W106F- and K75A-cystatin C, showed that the internalization can be positively affected by engineering of the cystatin molecule. Microscopy revealed vesicular co-localization of internalized cystatin C with the lysosomal marker proteins cathepsin D and legumain. Activities of both cysteine cathepsins and legumain, possible target enzymes associated with cancer cell invasion and metastasis, were down-regulated in cell homogenates following cystatin C uptake. A positive effect on regulation of intracellular enzyme activity by a cystatin variant selected from uptake properties was illustrated by incubating cells with W106F-cystatin C. This resulted in more efficient down-regulation of intracellular legumain activity than when cells were incubated with wild-type cystatin C. Uptake experiments in prostate cancer cells corroborated that the cystatin C internalization is generally relevant and confirmed an increased uptake of W106F-cystatin C, in PC3 cells. Thus, intracellular cysteine proteases involved in cancer-promoting processes might be controled by cystatin uptake.
Introduction
Cystatins are natural protein inhibitors regulating the activities of papain-like cysteine cathepsins and legumain-like enzymes belonging to protease families C1 and C13, respectively (1). The human cystatins are divided into three types. Type 1 includes the small intracellular inhibitors cystatin A and B (stefin A and B), expressed in most cells. Cystatin C, D, E/M, F, G, S, SA, and SN are cystatins of type 2, which as the type 1 cystatins are single-domain proteins but are synthesized with a signal peptide, hence being secreted and consequently found in body fluids. Among the type 2 cystatins, cystatins E/M and C are special with their ability to physiologically regulate both papain-like enzymes and legumain (2, 3). The inhibitors of type 3 (kininogens) are multi-domain cystatins and the main cysteine protease inhibitors in blood plasma and synovial fluid.
Regulation of proteases is of profound importance under normal physiological conditions. Besides participating in the lysosomal protein turnover some of the cysteine cathepsins and legumain are involved in the process of cancer invasion (4), growth, and metastasis (5–7).
Breast cancer is of particular interest for studies of the role of cysteine proteases, as it was early noted that cystatin E/M was down-regulated in metastasis of breast cancer compared with primary tumors (8). More recent studies strongly indicate that epigenetic silencing of the cystatin E/M gene is frequent in breast cancer, as well as in gastric carcinoma (9), prostate cancer (10), and glioma (11). Expression of cystatin E/M suppresses legumain activity and thereby invasion of human melanoma cells in Matrigel (12).
Uptake of cystatin C in amounts sufficient to affect the activities of intracellular cysteine proteases was recently described in five human cancer cell lines (13). The purpose of the present study was to investigate the structural requirements of the cystatin C molecule leading to efficient uptake and increased intracellular protease inhibition, to elucidate the initial steps of the molecular pathway leading to internalization. We have focused on MCF-7 (human breast adenocarcinoma) cells as it was shown that growth of these cells could be controlled by a cysteine protease inhibitor (14). This cell line has previously been used to study the altered intracellular activities of proteases and their inhibitors typical of cancer generally (15). The expression of both cathepsin B and L is also high in this cell line (16).
EXPERIMENTAL PROCEDURES
Proteins
To produce variants of cystatin C site-directed mutagenesis (QuickChange II-E site-directed mutagenesis kit, Stratagene, La Jolla, CA) was used according to the manufacturer's recommendations. In short the pHD313 expression plasmid (17) was used as template for thermal cycling with primers encoding the different mutations. The non-mutated plasmids were digested while the mutated plasmids were purified and electroporated into XL1-Blue electrocompetent cells. Bacteria containing plasmids were selected on agar plates, clones were picked, and subsequently grown in LB medium. The bacterial suspensions were used for isolation of plasmids followed by DNA sequencing (AB3010 Genetic Analyzer, BM-labbet, Lund, Sweden). Plasmids which contained correct mutations were electroporated into Escherichia coli MC1061 bacteria for protein expression as described elsewhere (17, 18). N-terminally truncated cystatin C was obtained by incubating wild-type cystatin C with leukocyte elastase at the molar ratio 100:1 in 37 °C for 4 h.
Protein Purification and Characterization
Purification of the recombinant proteins obtained by E. coli expression was achieved by Butyl-S SepharoseTM (GE Healthcare Life Sciences AB, Uppsala, Sweden) in 20 mm Tris buffer, pH 7.4, containing 1 mm benzamidinium chloride, or Q-SepharoseTM (GE Healthcare) in 20 mm ethanolamine buffer, pH 9.0, depending on the properties of the protein. (R24A,R25A)-cystatin C was further purified by size exclusion chromatography (SephadexTM 75; GE Healthcare).
The purity and size of isolated proteins were analyzed by agarose gel electrophoresis at pH 8.6 (19), SDS-PAGE (NuPAGE, 4–12% Bis-Tris, Invitrogen Life Science, Grand Island, NY) and Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker-Daltonics Ultraflex time-of-flight, Institut de Biotecnologia i Biomedicina, Universitat Autónoma de Barcelona, Spain). Protein concentration was determined by Coomassie Protein Assay Reagent (Thermo Fisher Scientific Inc, Rockford, IL) according to the manufacturer's recommendations and by measurement of A280 (NanoDrop 2000, Thermo Fisher Scientific Inc.). The empirically determined extinction coefficient 0.83 for wild-type cystatin C was used for calculations (20). For other variants of cystatin C the extinction coefficients were calculated by ProtParam. As both these methods to determinate protein concentration have limitations we in addition measured the concentration by ELISA (see below).
To ensure that the mutated cystatin C molecules were properly folded and functional as inhibitors an activity assay was performed. Buffer, enzyme, inhibitor, and a fluorescent substrate were mixed in a 96-well microtiter plate. Buffer used for the cathepsin activity assay was 0.1 m sodium phosphate buffer, pH 6.0, with 1 mm EDTA, and 4 mm DTT, and for the legumain activity assay 0.1 m Na2HPO4 buffer, pH 5.8, with 0.1% CHAPS, 1 mm EDTA, and 4 mm DTT. Z-Phe-Arg-NMec was used as substrate for cathepsin B and Z-Ala-Ala-Asn-NMec (Bachem; Bubendorf, Switzerland) for legumain. The fluorescence was monitored every 30 s 60 times in a Fluoroscan Ascent plate reader (Labsystems, Stockholm, Sweden) at excitation/emission wavelengths of 355/460.
Protein Labeling
Pure cystatin C was incubated with biotin ester (Invitrogen, Carlsbad, CA) or Alexa Fluor 488 (Molecular Probes Europe BV, Leiden, The Netherlands) according to the manufacturers' advice. The biotinylated cystatin C was according to papain titration functionally indistinguishable from non-labeled cystatin C (data not shown).
Cells and Reagents
One human breast adenocarcinoma cell line, MCF-7, and three human prostate cancer cell lines, PC3, DU145, and LNCaP (ATCC, Rockville, MD), were used.
The cells were cultured in Dulbecco's Modified Eagle's Medium with 4500 mg/liter glucose, GlutaMAX-I, and pyruvate (Invitrogen) supplemented with 100 IU/ml penicillin/streptomycin (Invitrogen) and 10% fetal calf serum (FCS, Invitrogen). Cells were detached using 0.05% Trypsin-EDTA (Invitrogen). Trypsin was inactivated by addition of complete culturing medium to the detached cells. After centrifugation, the cell pellet was washed once with PBS without calcium or magnesium (Invitrogen) before lysis in 0.2% Triton X-100 (Sigma-Aldrich Chemie, Steinheim, Germany) in PBS. A protease inhibitor mixture was added to all cell homogenates to a final concentration of 5 mm benzamidinium hydrochloride, 15 mm NaN3, and 10 mm EDTA.
Invasion and Migration Studies
MCF-7 cells (50,000) were starved for 4 h in basal medium with 0.1% bovine serum albumin (BSA, Sigma-Aldrich Chemie). The cells were then seeded in starving medium in Matrigel invasion chambers or control inserts (both BD Biosciences, Bedford, MA), without or with addition of either 1 μm wild-type cystatin C or 1 μm of the cell permeable cysteine protease inhibitor E64d (Sigma-Aldrich Chemie). As chemoattractant, in the lower wells, BSA was replaced by 10% FCS. After 20 h incubation (37 °C, 5% CO2) the medium in the insert was aspirated and the Matrigel with non-invaded cells wiped off. The invasive cells on the lower surface of the membrane were stained with 0.25% crystal violet, rinsed with water, and then dried. The stained cells were dissolved in 10% acetic acid. The absorbance of the solution was then measured at 560 nm in an ELISA plate reader. The results from the control cells were set to 100% in each experiment.
Cell Experiments
All experiments were basically performed in the same way: 400,000 cells were seeded in 24-well culture plates (Fischer Scientific Inc., Gothenburg, Sweden) and allowed to settle for 24 h. The cells were then washed with PBS once and incubated in 500 μl of fresh medium without or with 1 μm of the different cystatin C variants including wild-type. At harvest the cells were incubated with 300 μl trypsin in 37 °C for 10 min. Cell pellets were lysed in 250 μl of lysis buffer and incubated overnight in 4 °C. Finally the cystatin C and total protein content of the lysates were measured. The experiments were performed in triplicate at three different days. Control cells incubated in medium without cystatin C were included in each uptake experiment. The level of cystatin C in these cells represented the cells' (genitive) own production and was thus subtracted from the measured cystatin C level in cells exposed to different cystatin C variants. 100% uptake was defined as the cystatin C content in cells incubated in medium containing 1 μm of the wild-type, after correction for endogenous cystatin C (as described above).
In time-response experiments MCF-7 cells were incubated with wild-type cystatin C or biotin labeled wild-type cystatin C for different periods of time: 5 min, 30 min, 2 h, 6 h, and 24 h before harvest. The endogenous cystatin C was represented by control cells incubated in standard medium for 24 h. In control experiments no biotinylated cystatin C could be measured.
To study the half-life of internalized cystatin C 200,000 MCF-7 cells were incubated with biotinylated wild-type cystatin C for 24 h. The cystatin C containing medium was then removed and the cells were washed twice with PBS before addition of fresh standard medium. Cells were harvested 0 min, 30 min, 2 h, 6 h, and 24 h after medium change.
The enzyme activity experiments were performed in cell culture microtiter plates (Nunc A/S, Roskilde, Denmark). A total of 80,000–100,000 cells were seeded and incubated for 6 h in 100 μl of medium, without or with addition of 1 or 5 μm inhibitor. The medium was removed, and the cells were washed with PBS prior to addition of 40 μl of lysis buffer to the wells followed by 30 min of incubation on a shaking plate.
To study uptake of cystatin C in cell lines other than MCF-7, prostate cells (LNCaP, DU145, and PC3) were cultured and incubated with 1 μm biotinylated wild-type cystatin C, or 1 μm unlabeled wild-type or W106F-cystatin C, for 6–24 h, as described for the MCF-7 cells above.
Quantification of Cystatin C in Cells and Media
Cystatin C in cell lysates was quantified by a double-sandwich ELISA specific for human cystatin C described elsewhere (21). Briefly, wells in a 96-well microtiter plate were coated with a polyclonal rabbit anti-(human cystatin C) antibody (antiserum 8206) for capture of the antigen. A secondary biotinylated monoclonal mouse anti-(human cystatin C) antibody was then added followed by horseradish peroxidase-conjugated streptavidin and a substrate for detection.
To be able to specifically measure the internalized inhibitor we used biotin-labeled cystatin C for incubation with the cells and a modified ELISA method for detection. The capturing antibody was identical, but as the cystatin C was biotinylated the secondary antibody could be omitted. The microtiter plate with captured cystatin C was therefore incubated directly with horseradish-peroxidase conjugated streptavidin before addition of the substrate (22). Cystatin C levels in the lysates were correlated to total protein content measured by Coomassie Protein Assay.
Enzyme Activity Assays
Cells were lysed directly in the wells of a 96-well plate for activity assays performed in the same way as described above. The substrate Z-Phe-Arg-NMec is a general substrate for degradation by cathepsins which is an advantage when cell lysates containing a flora of different cathepsins are studied. To analyze the specific activities of cathepsin B and legumain we included the substrates Z-Arg-Arg-NMec and Z-Ala-Ala-Asn-NMec, respectively. The fluorescence was monitored at ex 355/em 460 in a Fluoroscan Ascent plate reader for 2.5–5 h. To calculate the rate of the enzyme activity in relation to cell protein content 2 μl of each cell lysate was transferred to a new tube for Coomassie Protein Assay.
Confocal Laser Scanning Microscopy (CLSM)
Cells were seeded in a 8-well iBidi μ-slide chamber (iBidi GmbH, Martinsried, Germany) or on cover slips (Knittel Glasbearbeitung GmbH, Braunschweig, Germany) placed in 6-well culture plates (Fisher Scientific) and incubated for 6 h with 5 μm Alexa Fluor 488-labeled cystatin C. Then the cells were fixed with 2% paraformaldehyde for 20 min in the cold.
For immunocytochemistry cells were first permeabilized with 0.1% Triton X-100 for 4 min followed by blocking of unspecific binding sites with 1% bovine serum albumin. Cells were then incubated with primary antibodies either against cathepsin B (Santa Cruz Biotechnology, Santa Cruz, CA), legumain (R&D Systems, Minneapolis, MN) or cathepsin D (Abcam, Cambridge, UK) for 60 min before incubation with Alexa Fluor 568-labeled antibodies (Molecular Probes) for detection. The antibodies used were either donkey anti-goat IgG (H+L) or goat anti-rabbit IgG (H+L) depending on the primary antibodies. Specificity of the second antibodies was analyzed by incubating cells without the primary antibody solution. Cells were incubated with Alexa Fluor 568-labeled transferrin (Molecular Probes) for the last hour of the 6 h of cystatin C incubation to study the co-internalization of the molecules.
To study the co-localization of the internalized cystatin C and acidic organelles Lysotracker (Molecular Probes) was added to the cell cultures when 2.5 h of the 6-h incubation with cystatin C remained. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride; Molecular Probes).
Cells on cover slips were mounted on microscope slides with Vectashield mounting medium (Vector Laboratories, Cambridge, UK). When iBidi-slides were used no mounting was needed.
Fluorescence was detected by confocal laser scanning microscopy (CLSM)2 using a Zeiss LSM 510 meta microscope. The settings were optimized for each fluorophore and images were separately grabbed and then merged with the overlay function.
RESULTS
Cystatin C Variants for Elucidation of Structural Requirements for Cellular Uptake
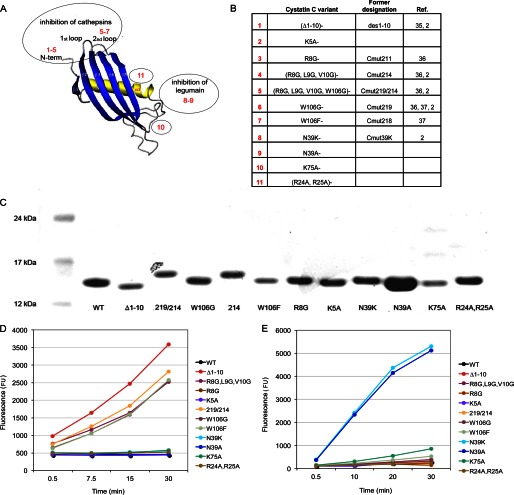
The cystatin C molecule is a natural double-headed inhibitor with capacity to simultaneously bind and inhibit one cysteine cathepsin and one legumain molecule (2). The cysteine cathepsin binding site includes amino acids at positions 8–11 in the N-terminal segment (Arg-Leu-Val-Gly), at positions 55–59 in the first hairpin loop (Gln-Ile-Val-Ala-Gly) and the amino acids at positions 105–106 in the second hairpin loop (Pro-Trp). To investigate whether the cysteine cathepsin binding site overlaps with a site required for uptake, the first set of cystatin variants produced included variants with substitutions of these residues. The variants R8G-, (R8G,L9G,V10G)-, W106G- and W106F-cystatin C were expressed in E. coli as well as a variant with combined alterations in both the N-terminal segment and the second hairpin loop (R8G,L9G,V10G,W106G)-cystatin C. Truncated cystatin C with the first ten amino acids of the N-terminal segment cleaved off was also included in the set, thereby eliminating the residues known to be important for cathepsin binding but also elimination of charged residues, which might influence uptake or binding. To complete the series we expressed and isolated a cystatin C variant with K5A as the only substitution (Fig. 1, A and B).
FIGURE 1.
Characteristics of cystatin C variants. A, schematic cystatin molecule (adopted from Ref. 34). Cystatin C variants 1–7 have amino acid substitutions in parts of the molecule involved in cysteine cathepsin inhibition. In variants 8 and 9, the amino acid residue in position 39 is substituted, which influences legumain inhibition. Variants 10 and 11 have substitutions of amino acids not considered to participate in enzyme inhibition. B, description of the amino acid substitutions of the cystatin C variants used in the study. C, analysis of cystatin C variants by SDS-PAGE after reduction in a 4–12% Bis-Tris polyacrylamide gel. Lane 1: size marker. Lane 2: wild-type cystatin C. Lanes 3–13: cystatin C variants after final purification. D, inhibition of cathepsin B activity by cystatin C variants monitored by the fluorescent substrate Z-Phe-Arg-NMec. Variants 1, 4, 5, 6, and 7 (curves for 6 and 7 are overlapping) did not inhibit cathepsin B, as they contained serial amino acid substitutions in the cysteine cathepsin binding site. E, inhibition of legumain by cystatin C variants assayed using the fluorescent substrate Z-Ala-Ala-Asn-NMec. Variants 8 and 9 did not inhibit legumain, as the side-chain of residue N39 is important for legumain inhibition.
To study the importance of the opposite side of the cystatin C molecule for uptake kinetics the key amino acid for legumain inhibition, Asn-39, was altered either to a lysine or an alanine residue (N39K- and N39A-cystatin C; Fig. 1, A and B).
Finally, we expressed and isolated two variants of cystatin C with substitutions of charged amino acids, supposedly located on the surface, in parts of the molecule not known to participate in enzyme inhibition, K75A- and (R24A,R25A)-cystatin C (Fig. 1, A and B).
Purity and size of the E. coli expressed cystatin C variants were analyzed by SDS-PAGE, which revealed a single protein band of size ∼13 kDa in all cases (Fig. 1C). The expected mass values after amino acid substitutions were confirmed by MALDI-TOF MS, and the expected charge differences were verified by native agarose gel electrophoresis (data not shown). The purity of the expressed proteins was >95% according to all analysis methods.
The expression yields were relatively good for all variants compared with that for the wild-type protein, indicating no major defects in folding or stability due to the substitutions introduced. The inhibitory properties of the purified cystatin C variants were furthermore investigated, to provide final evidence that the scaffold protein domain was not seriously affected by the amino acid substitution. To this end, assays with fluorogenic substrates for cathepsin B and legumain were used to independently monitor the variants' intactness in the cysteine cathepsin- and legumain-binding sites.
The cystatin C variants with dramatic changes of amino acid residues at the cathepsin binding site ((R8G,L9G,V10G)-, (R8G,L9G,V10G,W106G)-, W106G-, W106F-cystatin C, and the N-terminally truncated (Δ1–10)- variant) were all poor inhibitors of cathepsin B. However the R8G- and K5A-cystatin C variants with minor changes at the same site were still functional as inhibitors (Fig. 1, A and D). Legumain was inhibited by all cystatin C variants except N39A- and N39K-cystatin C, which was in agreement with expectations (Fig. 1, A and E).
K75A- and (R24A,R25A)-cystatin C, the two variants with amino acid substitutions in parts of the molecule not associated with enzyme inhibition were as efficient inhibitors of both enzymes as wild-type cystatin C (Fig. 1, A, C, and D). The characterization of the cystatin C variants thus suggested that all retained a correct cystatin fold and capacity as inhibitors as expected.
Basic Uptake Kinetics for Cystatin C
The MCF-7 cell line was selected for detailed studies on molecular requirements for cystatin C uptake (see Introduction), partly because it has been shown that both cell permeable and non-cell-permeable low-molecular-weight cysteine protease inhibitors have effects on its migration and invasion (23, 24). To test whether cystatin C can affect such biologically relevant properties of these cells, we performed Matrigel experiments comparing cells incubated with and without cystatin C (1 μm) added to the medium. The results demonstrated an effect essentially as large as that of the cell permeable inhibitor E64d on MCF-7 cell invasion, but also on its migration properties (Fig. 2).
FIGURE 2.
Effects on invasion and migration of MCF-7 cells in vitro. Cells were starved for 4 h and then seeded and incubated for 20 h without or with addition of either 1 μm wild-type cystatin C or 1 μm E64d. Cells attached to the lower surface of the insert membrane were stained and dissolved in acetic acid. The absorbance of the solution was then measured. In each experiment the absorbance of the control cells was set to 100%. A, invasion in Matrigel invasion chambers. B, migration in control inserts.
The MCF-7 cells were incubated in standard culturing medium or medium containing 1 μm cystatin C for 5 min, 30 min, 2 h, 6 h, or 24 h before lysis. The content of cystatin C in the cell extracts was analyzed with an ELISA specific for human cystatin C. The increase of cystatin C was rapid and pronounced, and continued throughout the 24 h experiment in agreement with previous results (13) (data not shown).
The ordinary ELISA cannot distinguish between cystatin C produced by the cells and cystatin C that has been taken up. To detect the internalized inhibitor exclusively we used biotin-labeled cystatin C for cell incubation and thus modified the ELISA method used for detection. This method showed that the uptake began immediately and continued during 24 h in a linear way (Fig. 3A).
FIGURE 3.
Internalization of biotinylated cystatin C. A, cells were incubated with 1 μm biotinylated cystatin C for different periods of time. The biotinylated cystatin C content of cell lysates was measured by ELISA and correlated to total protein levels. Closed triangles represent mean values of triplicate wells in three independent experiments. Closed circles represent median values of all three experiments, and the dotted line represents the overall trend. Time point 0 refers to control cells incubated without biotinylated cystatin C. B, turnover of internalized biotinylated cystatin C. Cells were seeded and incubated with medium containing 1 μm biotinylated cystatin C. After 24 h the medium was exchanged for standard medium, and cells were harvested at different time points. Cystatin C at time 0 represents the intracellular amount at 24 h. In lysates of control cells incubated with standard medium no signal could be detected. The figure shows two independent experiments, and each symbol represents the mean value of triplicate wells.
To study the molecular fate of the internalized cystatin C, a pulse-chase experiment was conducted. Following incubation for 24 h in medium containing 1 μm biotinylated cystatin C, the cells were washed with PBS before the medium was replaced by standard medium. Cystatin C quantification in cell lysates showed that the internalized cystatin C could be detected up to 24 h after the medium was changed. In lysates of control cells incubated in standard medium without addition of biotinylated cystatin C no signal could be detected (Fig. 3B). We consequently conclude that cystatin C is internalized in MCF-7 cells in a linear fashion, that the internalization continues up to 24 h and that the turnover of the internalized cystatin C is slow.
Effect on Uptake of Amino Acid Substitutions in the Binding Site for Papain-like Cysteine Cathepsins
The cystatin C variants with substitutions of amino acids in the papain-binding site of the molecule are K5A-, R8G-, (R8G,L9G,V10G)-, W106G-, W106F-, and (R8G,L9G,V10G,W106G)-cystatin C. We also used a truncated form of cystatin C where the ten first amino acids of the N-terminal segment had been cleaved off (Fig. 1, A and B).
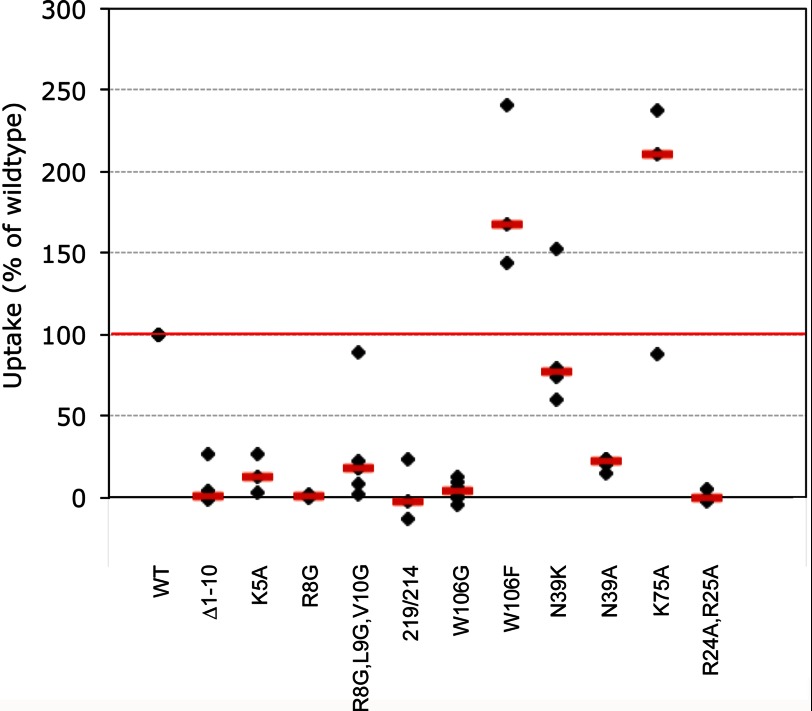
Cleavage of the N-terminal segment of the cystatin C molecule appeared to give rise to severe effects on uptake, as the median value was only 1% of the uptake of the wild-type inhibitor (Fig. 4). Among the first ten amino acid residues four are either charged or hydrophobic, properties that could affect the efficiency of the uptake. The median uptake (compared with the wild-type) of the other variants with substitutions of amino acids exclusively in the N-terminal part of the molecule, K5A-, R8G-, and (R8G,L9G,V10G)-cystatin C, were 12, 1, and 18%, respectively (Fig. 4).
FIGURE 4.
Uptake of cystatin C variants. Cells were incubated for 6 h with 1 μm recombinant human cystatin C. The total cystatin C content in lysates was measured by ELISA and correlated to the total protein content. Cystatin C content (ng/mg protein) in lysates of cells incubated in standard medium was subtracted. The resulting values were compared with the uptake of wild-type cystatin C in each experiment, which was set to 100%. Black symbols represent mean values of triplicate wells in a single experiment. Red bars represent median values (n = 3–6). For description of variants and the designation 219/214, see Fig. 1.
It was revealed that the second hair-pin loop with the conserved amino acid residue W106 is an important region of the molecule for uptake when the variants W106G-, W106F-, and (R8G,L9G,V10G,W106G)-cystatin C were used. The impact of W106 turned out to be very strong as the uptake of (R8G,L9G,V10G,W106G)- and W106G-cystatin C was essentially not detectable (Fig. 4). However, when the residue at position 106 was replaced by a phenylalanine the internalization was positively affected. The median value was 168% of the uptake of wild-type cystatin C (Fig. 4). Thus, our results indicate that the uptake of cystatin C in MCF-7 cells is dependent on both charged amino acids of the N-terminal segment and on a hydrophobic amino acid at position 106, domains which are involved in the inhibition of cysteine cathepsins.
Effect on Uptake of Amino Acid Substitutions in the Binding Site for Legumain
The inhibitory site for legumain is situated on the opposite side of the cystatin C molecule compared with that responsible for cathepsin binding and appears to be centered around a critical asparagine residue at position 39. Consequently the uptake properties of two variants of cystatin C with amino acid substitutions of N39 were investigated (Fig. 1, A and B). Compared with the uptake of the wild-type inhibitor the median values for internalization of N39K- and N39A-cystatin C were 77 and 22%, respectively (Fig. 4).
Effect on Uptake of Substitutions of Charged Amino Acids Not Associated with Enzyme Inhibition
Two more variants of cystatin C were included in the uptake analyses, K75A- and (R24A,R25A)-cystatin C. Neither amino acid residues K75, R24, nor R25 are considered to take part in enzyme inhibition, but their charged properties at the surface of the molecule could possibly affect a cellular recognition and uptake process (Fig. 1, A and B).
Uptake of the double mutant (R24A,R25A)-cystatin C was not detectable, while K75A-cystatin C was taken up most efficiently with a median value 211% of that for wild-type cystatin C (Fig. 4).
Subcellular Localization of Internalized Cystatin C
Cells were incubated for 6 h with fluorescently labeled cystatin C before fixation and immunostaining with antibodies directed against possible target enzymes and subcellular markers. CLSM showed that the internalized Alexa Fluor 488-cystatin C aggregated in vesicular acidic compartments, resembling lysosomes, which is in agreement with previous results (13) (data not shown).
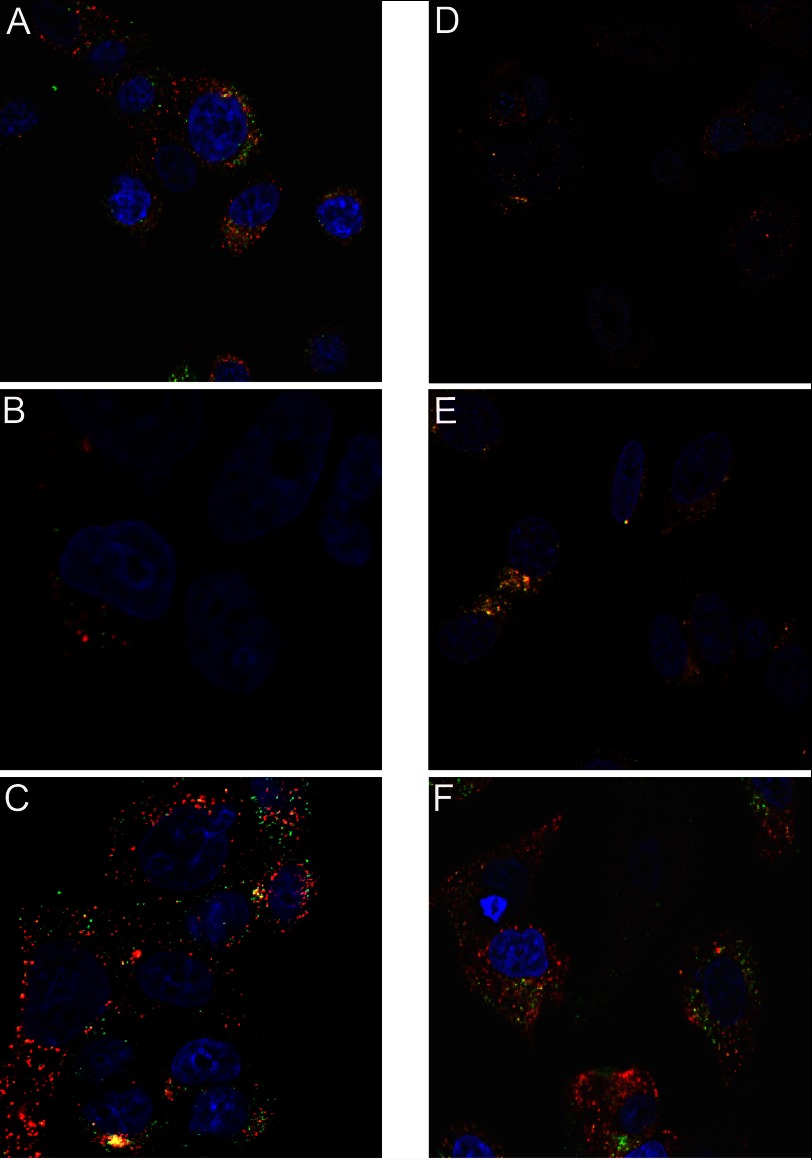
To investigate whether endo-lysosomal enzymes and cystatin C co-localize, additional immunostaining for endogenously produced lysosomal enzymes (9, 25) was performed on cells previously incubated with Alexa Fluor 488-cystatin C. Antibodies directed against legumain (Fig. 5A), cathepsin B (Fig. 5B) or cathepsin D (Fig. 5C) were used. Vesicular co-localization of Alexa Fluor 488-cystatin C and the lysosomal enzymes cathepsin D and legumain was seen, but no obvious co-localization of Alexa Fluor 488-cystatin C and cathepsin B could be found under the conditions of our experiment. Control experiments showed that endogenously produced legumain was co-located with both cathepsin D and B in intracellular compartments (Fig. 5, D and E).
FIGURE 5.
Subcellular localization of internalized cystatin C showed by confocal laser scanning microscopy. A–C, cells were seeded and incubated with 5 μm Alexa Fluor 488-cystatin C (green) for 6 h before immunostaining with antibodies directed against either legumain (A, red), cathepsin B (B, red) or cathepsin D (C, red). D–E, immunostaining showing co-localization of endogenous lysosomal enzymes. D, legumain, green; cathepsin B, red, E, legumain, green; cathepsin D, red. F, Alexa Fluor 568-transferrin (red) was used to show co-internalization with Alexa Fluor 488-cystatin C (green). Nuclei were stained by DAPI (blue) in all images. Yellow, merged.
Alexa Fluor 568-labeled transferrin (Fig. 5F) was used for simultaneous incubation with Alexa Fluor 488-labeled cystatin C. This resulted in parallel uptake and co-localization in endosomes. These results indicate that after endocytosis the internalized cystatin C follows the lysosomally directed pathway to eventually end up in association to the target enzymes in the lysosome.
Effects of Cystatin C Uptake on Intracellular Protease Activity
The cathepsin and legumain activity in cell lysates was measured after cystatin C uptake and compared with the activity in control cells. Cells were seeded in 96-well plates, incubated for 6 h with or without cystatin C and then lysed directly in the wells. Legumain activity was monitored by the cleavage of the fluorescent substrate Z-Ala-Ala-Asn-NMec. The substrate Z-Phe-Arg-NMec was used for analysis of cysteine cathepsin activity in general and Z-Arg-Arg-NMec was used for more specific analysis of cathepsin B activity (Fig. 6).
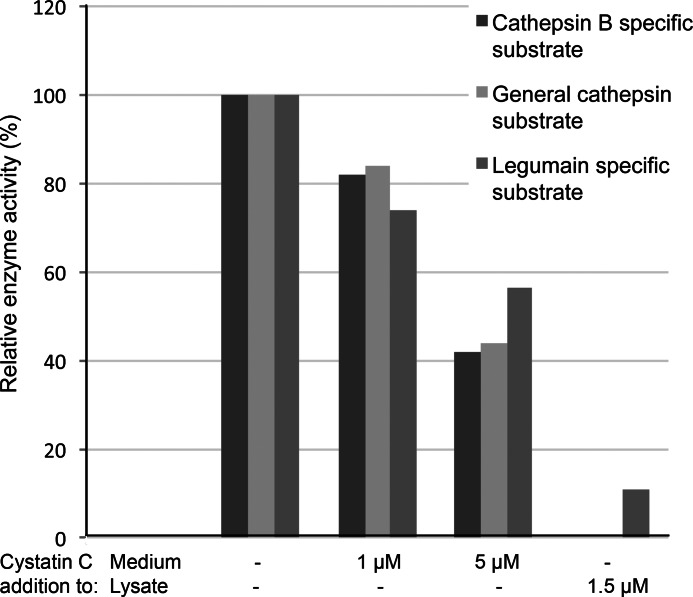
FIGURE 6.
Dose-dependent inhibition of intracellular enzyme activity after incubation with cystatin C. Cells were seeded in 96-well plates and incubated with standard medium or medium containing either 1 or 5 μm cystatin C for 6 h. After cell lysis a fluorescent substrate, specific for the different enzymes, was added. An end point value of the fluorescence was measured after 2.5 h. Bars represent mean values (n = 3–9) and are correlated to time and total protein content of the homogenate (fluorescence units/min/mg protein). The enzyme activity in lysates from cells incubated in medium without cystatin C addition was set to 100% in each experiment. As a control cells where incubated in standard medium and lysed before addition of cystatin C.
The inhibition of enzyme activity was found to be dose dependent when cells incubated with either 1 or 5 μm cystatin C were analyzed. The enzyme activity was measured as increase of fluorescence (FU) per time unit (min) and then correlated to total protein content of the cell homogenate. To make sure that the decrease of fluorescence was due to cystatin C inhibition of the enzymes we added the inhibitor at a final concentration of 1.5 μm to homogenates of cells incubated in standard medium (Fig. 6).
In all activity assays mentioned above the enzyme activity was inhibited in cells that had been incubated with cystatin C, suggesting that the internalized cystatin C is undegraded and functional as a cysteine protease inhibitor. Furthermore, it is clear that the amount of internalized cystatin C is substantial and significant as the total enzyme activity of the cells is influenced.
Regulation of Intracellular Legumain
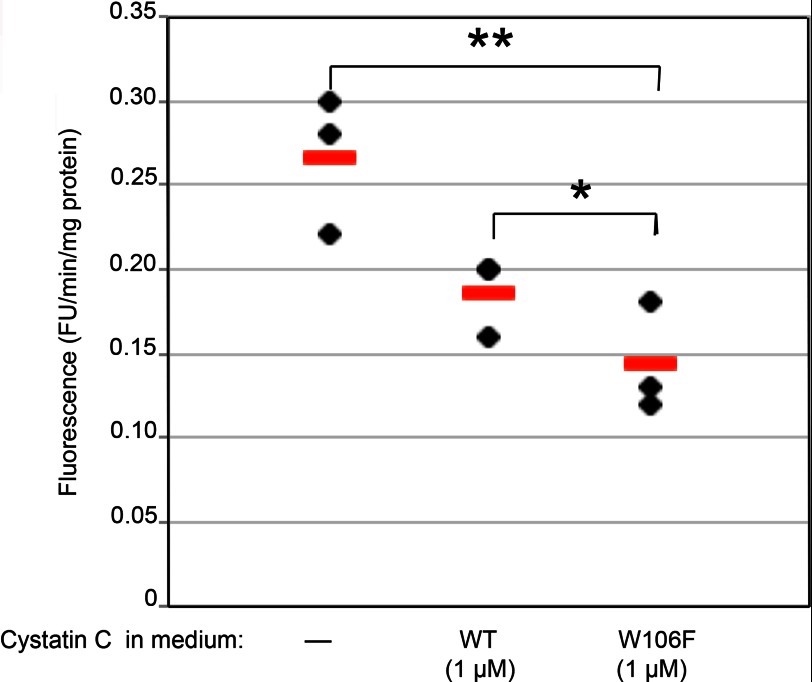
The initial uptake experiments showed that the two cystatin C variants W106F- and K75A-cystatin C were internalized more efficiently than the wild-type inhibitor. This leads us to examine if the improved uptake would matter for intracellular target enzyme activity. Since the legumain inhibiting part is intact in these cystatin C variants, we used the legumain-specific substrate Z-Ala-Ala-Asn-NMec to monitor intracellular protease activity.
Cells were seeded in 96-well microtiter plates and incubated with either wild-type or W106F-cystatin C (1 μm) before lysis. Homogenates of cells incubated in standard medium without any cystatin C addition were used as controls, representing 100% legumain activity.
Legumain activity was even more reduced in lysates of cells after incubation with W106F-cystatin C compared with lysates from cells incubated with wild-type cystatin C (Fig. 7), which is in accordance with its relatively more efficient internalization.
FIGURE 7.
Regulation of intracellular legumain. Cells were seeded in 96-well plates and incubated in standard medium or medium with addition of either 1 μm wild-type cystatin C or W106F-cystatin C. After cell lysis a fluorescent substrate for legumain (Z-Ala-Ala-Asn-NMec) was added. The increase in fluorescence per minute was correlated to the total protein content in each sample. Closed symbols represent mean values of triplicate wells in one experiment, and bars represent median values. The non-parametric Mann-Whitney U test was used to compare the groups.
Cystatin C Uptake in Prostate Cell Lines
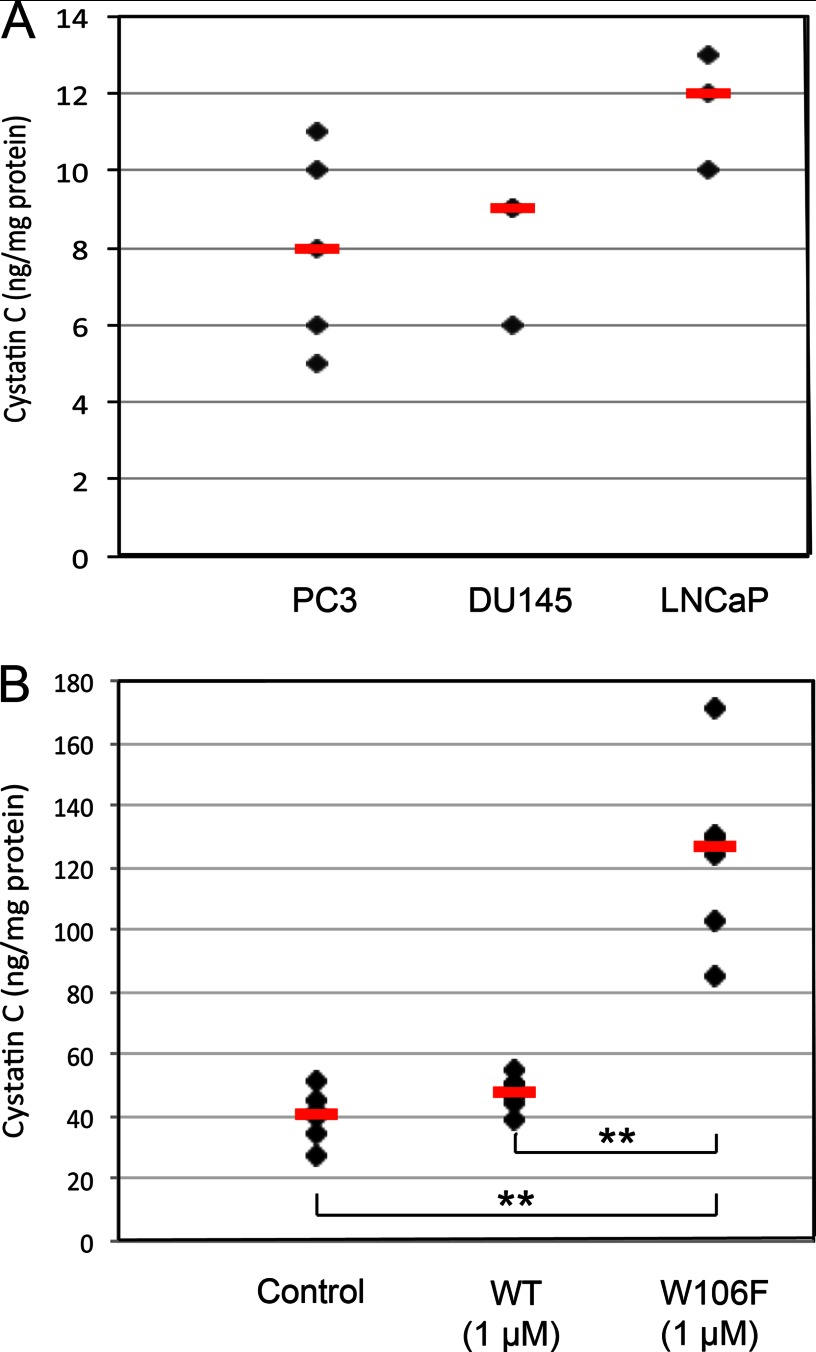
Three prostate cancer cell lines were studied to confirm that the uptake seen in MCF-7 cells is general for cancer cells. Uptake experiments in PC3, DU145, and LNCaP cells verified that internalization of 1 μm biotin-labeled cystatin C is prominent and comparable to the uptake in MCF-7 breast cancer cells (Fig. 8A).
FIGURE 8.
Cystatin C uptake in prostate cancer cell lines. A, PC3, DU145, and LNCaP cells were incubated with 1 μm biotinylated cystatin C for 24 h. The biotinylated cystatin C content of cell lysates was measured by ELISA and correlated to total protein levels. B, cells were incubated for 6 h with 1 μm unlabeled wild-type or W106F-cystatin C. The total cystatin C content in lysates was measured by ELISA and correlated to the total protein content. Black symbols represent mean values of triplicate wells in a single experiment. Red bars represent median values (n = 3–6). The non-parametric Mann-Whitney U test was used to compare groups.
Cellular uptake of W106F-cystatin C was furthermore studied in PC3 cells, prompted by the improved uptake properties seen for this variant in MCF-7 cells. In PC3 cells, the mean cystatin C content after incubation with 1 μm wild-type cystatin C was increased to about 125% of the basic level (Fig. 8B). After incubation with W106F-cystatin C, the content was about three times higher than the basic level, which indicates an even more pronounced uptake in PC3 than in MCF-7 cells.
DISCUSSION
Protease activity is generally elevated in cancer and contributes to cancer metastasis, growth, invasion and angiogenesis. Cystatin C is an excellent naturally occurring inhibitor of cysteine cathepsins associated to cancer, such as cathepsin B and L, and one of three inhibitors capable to also inhibit legumain (2). We have previously presented data for several human cell lines from different tissues, demonstrating that cystatin C uptake appears to be a general phenomenon for cancer cells of different types (13, 22). In this study we have in addition shown a pronounced uptake in carcinoma cell lines (PC3, DU145, and LNCaP) from human prostate, verifying this notion. The possibility that the extracellular inhibitor cystatin C has impact on intracellular activities of cancer-associated enzymes should thus be considered.
The human breast adenocarcinoma cell line MCF-7 was chosen for further analysis as it was previously used in studies of the effect of protease inhibition on cancer cell metabolism (23, 24). The focus of this work was to delineate the first part of the internalization pathway by analyzing the importance of charge and hydrophobicity for uptake, as these properties have been shown to contribute to cellular internalization (26–28) to study the subcellular localization of the internalized inhibitor and the intracellular effects of the uptake.
With the aim to elucidate how the structure influences the uptake we expressed 12 variants of cystatin C (including wild-type) with substitutions of selected amino acids. The selection was based on charge and hydrophobicity of surface-located residues, and most of the substitutions were introduced among the amino acids that form part of the enzyme-inhibiting regions.
All variants with charged or hydrophobic amino acid residues replaced by glycines in the cysteine cathepsin-binding region were scarcely internalized (median values: 1 to 18% of wild-type) and in some cases even below the detection limit intracellularly. A hydrophobic amino acid residue at position 106 appeared to be a crucial feature as the uptake of W106G- and (R8G,L9G,V10G,W106G)-cystatin C was nearly abolished (median value 4% and not detectable, respectively) compared with the uptake of wild-type cystatin C. Moreover, W106F-cystatin C was internalized more efficiently than wild-type cystatin C (median value, 168% of wild-type) in MCF-7 cells. This result could be verified in PC3 cells, which suggests a specific uptake mechanism in part relying on an appropriate hydrophobic residue located on the cystatin C surface.
The amino acid residue N39 at the opposite side of the cystain C molecule is considered important for legumain inhibition. To study this residue's impact on uptake, it was replaced either by a lysine or an alanine residue (N39K- and N39A-cystatin C). Both variants displayed reduced internalization, but N39K-cystatin C appeared to have the best uptake characteristics of the two (median 77% of wild-type cystatin C compared with 22% for N39A-cystatin C). This could mean that an Asn residue in position 39 of the cystatin contributes to initial cellular binding leading to internalization normally, but that loss of this contribution is partly compensated for by introduction of a positive charge. Such a positive effect of surface charge can also be deduced from our results with (R24A,R25A)-cystatin C, which showed internalization below the detection limit, indicating that the natural Arg residues in positions 24 and 25 are of importance for the uptake process.
K75A-cystatin C showed more than a doubled degree of uptake despite a lost surface charge (Fig. 1A). This could possibly mean that the structure of the cystatin C molecule is affected by the amino acid substitution although all the cystatin C variants used in this project were shown to still be functional inhibitors. Alternatively, the exchange of lysine in position 75 to alanine might allow the amino acid residue at position 39 to easier interact with an assumed receptor and thereby facilitate internalization, or will diminish unproductive binding of this side of the molecule to the cellular surface. The latter possibility would agree with productive binding to an uptake receptor by the side harboring R24 and R25 rather than the loop where K75 resides in the wild-type inhibitor (see Fig. 1A).
The modified ELISA we used in the present study is an important tool to ensure that the difference in uptake is mechanistic and not dependent on differences in the binding capacity of the monoclonal antibody used for standard detection of cystatin C. But biotinylation of the different cystatin C variants may introduce other problems, such as potentially different degrees of biotinylation of different protein variants. Biotin is attached to lysine residues which in some molecular variants were substituted. The wild-type cystatin C molecule contains 7 lysines of which typically 3 (range, 1–5) are biotinylated according to agarose gel electrophoresis and MALDI-TOF MS (data not shown). Modifications of the molecule by amino acid substitutions could possibly mildly affect the structure, and thereby expose normally hidden parts and influence the binding of biotin molecules. To avoid this we used unlabeled cystatin C variants for the uptake screening.
Both cysteine cathepsins and legumain are lysosomal enzymes participating in the normal protein turnover. Up-regulated expression of the enzymes as well as increased activity has been seen in many tumors, including those of the breast, colon, ovary, and prostate. Association of the enzymes to the plasma membrane and secretion to the extracellular matrix (ECM) occurs and leads to increased tumor cell invasion. Consequently cystatin C has the capacity to regulate the proteolysis in several locations, in addition to intracellularly also extracellularly as the ECM-forming proteins laminin, fibronectin, and collagen IV are substrates for cathepsin B and legumain. Cathepsin B could also indirectly affect the proteolytic cascade by activating other proteolytic enzymes including urokinase-type-proplasminogen (uPA) and matrix metalloproteases (MMPs) (7, 29–32), which regardless of whether it occurs intra- or extracellularly could be counteracted by cystatin C according to our present results.
In all our experiments cells were incubated with 1 μm cystatin C, which is a physiological concentration (33) and sufficient to influence invasion and migration of the MCF-7 cells under study (Fig. 2). We demonstrated that the internalized cystatin C in cell homogenates influences the total enzyme inhibiting capacity of the cell. This implicates that it is possible to regulate the intracellular enzymatic activity as the inhibition was dose-dependent and depending on the uptake of the cystatin C variant. We also showed that cystatin C after uptake follows the lysosomally directed pathway and ends up in endo-lysosomal structures where it co-localizes with the target enzymes, which could enable an even more pronounced local in vivo effect than we could measure in total cell homogenates.
In cell homogenates we showed that both the cathepsin B and legumain inhibiting capacity, as well as total cysteine cathepsin inhibiting capacity, was suppressed when cells had been incubated with cystatin C. However, it is not clear from the present study if this effect is physiological regarding cathepsin B, since no obvious co-localization could be seen of labeled cystatin C with immunodetected cathepsin B by confocal microscopy. By contrast, legumain and the aspartic protease cathepsin D showed such co-localization with cystatin C. In the case of legumain, being a target for cystatin C binding and inhibition in vitro (2), the co-localization strongly indicates that the enzyme and its inhibitor indeed meet and, thus, that cystatin C uptake influences the activity of this cancer-related enzyme in distinct subcellular compartments. Immunocytochemical control experiments showed that cathepsin D and legumain were found in the same intracellular compartments at double staining, as well as legumain and cathepsin B. It is possible that the two cathepsins sometimes are located in the same compartment and sometimes in different, and that cystatin C only ends up in the compartment with no cathepsin B. Further in-depth studies are clearly merited to prove or disprove that internalized cystatin C really meets and inhibits cathepsin B intracellularly. Results supporting that cathepsin B and cystatin C indeed form a physiological enzyme-inhibitor pair with relevance for cell function have been published, demonstrating reduced penetration of Matrigel by squamous carcinoma SCC-VII cells when the cells overexpressed cystatin C and increased invasion when cathepsin B was up-regulated (4).
The differences between tissues and established cell lines and the variation in cell culture conditions make it difficult to understand the exact function of cystatin C in cancer cell processes. However, it is clear that cystatin C and possibly other cystatins play a role in regulation and fine-tuning of the participating proteases, both intracellularly and in the cell microenvironment. It will be important to address determinants for cystatin C uptake on the cellular surface in future work, to e.g. define receptors involved, to elucidate the entire pathway leading to cystatin internalization. The present study aimed to define structural determinants on the internalized inhibitor required for efficient uptake, to clarify the initial steps leading to internalization, and demonstrated that selected amino acid substitutions can promote uptake leading to up-regulated intracellular protease inhibition. This indicates that targeting of intracellular cancer-promoting proteolysis via the cystatin pathway should be possible and may prove fruitful.
Acknowledgments
We thank Anne-Cathrine Carlberg-Löfström for technical assistance, Ida Lindgren for work with mutagenesis of expression plasmid constructs and Dr. Bo Holmqvist (ImaGene-iT, Lund, Sweden) for help with confocal microscopy experiments.
This study was supported by the Faculty of Medicine at Lund University and BioCARE, and in part by grants from the Swedish Research Council (no. 05196) and the A. Österberg Foundation.
- CLSM
- confocal laser scanning microscopy
- DAPI
- 4′,6-diamidino-2-phenylindole
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid.
REFERENCES
- 1. Rawlings N. D., Barrett A. J., Bateman A. (2010) MEROPS: the peptidase database. Nucleic Acids Res. 38, D227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez-Fernandez M., Barrett A. J., Gerhartz B., Dando P. M., Ni J., Abrahamson M. (1999) Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J. Biol. Chem. 274, 19195–19203 [DOI] [PubMed] [Google Scholar]
- 3. Cheng T., Hitomi K., van Vlijmen-Willems I. M., de Jongh G. J., Yamamoto K., Nishi K., Watts C., Reinheckel T., Schalkwijk J., Zeeuven P. L. (2006) Cystatin M/E is a high affinity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain-binding site. A novel clue for the role of cystatin M/E in epidermal cornification. J. Biol. Chem. 281, 15893–15899 [DOI] [PubMed] [Google Scholar]
- 4. Coulibaly S., Schwihla H., Abrahamson M., Albini A., Cerni C., Clark J. L., Ng K. M., Katunuma N., Schlappack O., Glössl J., Mach L. (1999) Modulation of invasive properties of murine squamous carcinoma cells by heterologous expression of cathepsin B and cystatin C. Int. J. Cancer 83, 526–531 [DOI] [PubMed] [Google Scholar]
- 5. Vasiljeva O., Papazoglou A., Krüger A., Brodoefel H., Korovin M., Deussing J., Augustin N., Nielsen B. S., Almholt K., Bogyo M., Peters C., Reinheckel T. (2006) Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 66, 5242–5250 [DOI] [PubMed] [Google Scholar]
- 6. Vasiljeva O., Turk B. (2008) Dual contrasting roles of cysteine cathepsins in cancer progression: apoptosis versus tumour invasion. Biochimie 90, 380–386 [DOI] [PubMed] [Google Scholar]
- 7. Koblinski J. E., Ahram M., Sloane B. F. (2000) Unraveling the role of proteases in cancer. Clin. Chim. Acta 291, 113–135 [DOI] [PubMed] [Google Scholar]
- 8. Sotiropoulou G., Anisowicz A., Sager R. (1997) Identification, cloning, and characterization of cystatin M, a novel cysteine proteinase inhibitor, down-regulated in breast cancer. J. Biol. Chem. 272, 903–910 [DOI] [PubMed] [Google Scholar]
- 9. Chen X., Cao X., Dong W., Xia M., Luo S., Fan Q., Xie J. (2010) Cystatin M expression is reduced in gastric carcinoma and is associated with promoter hypermethylation. Biochem. Biophys. Res. Commun. 391, 1070–1074 [DOI] [PubMed] [Google Scholar]
- 10. Pulukuri S. M., Gorantla B., Knost J. A., Rao J. S. (2009) Frequent loss of cystatin E/M expression implicated in the progression of prostate cancer. Oncogene 28, 2829–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu J., Ai L., Ramachandran C., Yao B., Gopalakrishnan S., Fields C. R., Delmas A. L., Dyer L. M., Melnick S. J., Yachnis A. T., Schwartz P. H., Fine H. A., Brown K. D., Robertson K. D. (2008) Invasion suppressor cystatin E/M (CST6): high-level cell type-specific expression in normal brain and epigenetic silencing in gliomas. Lab. Invest. 88, 910–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Briggs J. J., Haugen M. H., Johansen H. T., Riker A. I., Abrahamson M., Fodstad Ø., Maelandsmo G. M., Solberg R. (2010) Cystatin E/M suppresses legumain activity and invasion of human melanoma. BMC Cancer 10, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekström U., Wallin H., Lorenzo J., Holmqvist B., Abrahamson M., Avilés F. X. (2008) Internalization of cystatin C in human cell lines. FEBS J. 275, 4571–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xing R., Mason R. W. (1998) Design of a transferrin-proteinase inhibitor conjugate to probe for active cysteine proteinases in endosomes. Biochem. J. 336, 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scaddan P. B., Dufresne M. J. (1993) Characterization of cysteine proteases and their endogenous inhibitors in MCF-7 and adriamycin-resistant MCF-7 human breast cancer cells. Invasion Metastasis 13, 301–313 [PubMed] [Google Scholar]
- 16. Ishibashi O., Mori Y., Kurokawa T., Kumegawa M. (1999) Breast cancer cells express cathepsins B and L but not cathepsins K or H. Cancer Biochem. Biophys. 17, 69–78 [PubMed] [Google Scholar]
- 17. Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. (1988) Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 236, 14–18 [DOI] [PubMed] [Google Scholar]
- 18. Dalbøge H., Jensen E. B., Tøttrup H., Grubb A., Abrahamson M., Olafsson I., Carlsen S. (1989) High-level expression of active human cystatin C in Escherichia coli. Gene 79, 325–332 [DOI] [PubMed] [Google Scholar]
- 19. Jeppson J. O., Laurell C.-B., Franzén B. (1979) Agarose gel electrophoresis. Clin. Chem. 25, 629–638 [PubMed] [Google Scholar]
- 20. Lindahl P., Abrahamson M., Björk I. (1992) Interaction of recombinant human cystatin C with the cysteine proteinases papain and actinidin. Biochem. J. 281, 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olafsson I., Löfberg H., Abrahamson M., Grubb A. (1988) Production, characterization and use of monoclonal antibodies against the major extracellular human cysteine proteinase inhibitors cystatin C and kininogen. Scand J. Clin. Lab. Invest. 48, 573–582 [DOI] [PubMed] [Google Scholar]
- 22. Wallin H., Bjarnadottir M., Vogel L. K., Wassélius J., Ekström U., Abrahamson M. (2010) Cystatins – Extra- and intracellular cysteine protease inhibitors: High-level secretion and uptake of cystatin C in human neuroblastoma cells. Biochimie 92, 1625–1634 [DOI] [PubMed] [Google Scholar]
- 23. Xing R., Wu F., Mason R. W. (1998) Control of breast tumor cell growth using a targeted cysteine protease inhibitor. Cancer Res. 58, 904–909 [PubMed] [Google Scholar]
- 24. Kolkhorst V., Stürzebecher J., Wiederanders B. (1998) Inhibition of tumour cell invasion by protease inhibitors: correlation with the protease profile. J. Cancer Res. Clin. Oncol. 124, 598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bastholm L., Nielsen M. H., De Mey J., Danø K., Brünner N., Høyer-Hansen G., Rønne E., Elling F. (1994) Confocal fluorescence microscopy of urokinase plasminogen activator receptor and cathepsin D in human MDA-MB-231 breast cancer cells migrating in reconstituted basement membrane. Biotech. Histochem. 69, 61–67 [DOI] [PubMed] [Google Scholar]
- 26. Schiffer M., Chang C. H., Stevens F. J. (1992) The functions of tryptophan residues in membrane proteins. Protein Eng. 5, 213–214 [DOI] [PubMed] [Google Scholar]
- 27. Derossi D., Calvet S., Trembleau A., Brunissen A., Chassaing G., Prochiantz A. (1996) Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271, 18188–18193 [DOI] [PubMed] [Google Scholar]
- 28. Lalazar A., Weisgraber K. H., Rall S. C., Jr., Giladi H., Innerarity T. L., Levanon A. Z., Boyles J. K., Amit B., Gorecki M., Mahley R. W., Vogel T. (1988) Site-specific mutagenesis of human apolipoprotein E. Receptor binding activity of variants with single amino acid substitutions. J. Biol. Chem. 263, 3542–3545 [PubMed] [Google Scholar]
- 29. Chauhan S. S., Goldstein L. J., Gottesman M. M. (1991) Expression of cathepsin L in human tumors. Cancer Res. 51, 1478–1481 [PubMed] [Google Scholar]
- 30. Kobayashi H., Schmitt M., Goretzki L., Chucholowski N., Calvete J., Kramer M., Günzler W. A., Jänicke F., Graeff H. (1991) Cathepsin B efficiently activates the soluble and the tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (Pro-uPA). J. Biol. Chem. 266, 5147–5152 [PubMed] [Google Scholar]
- 31. Buck M. R., Karustis D. G., Day N. A., Honn K. V., Sloane B. F. (1992) Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 282, 273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morita Y., Araki H., Sugimoto T., Takeuchi K., Yamane T., Maeda T., Yamamoto Y., Nishi K., Asano M., Shirahama-Noda K., Nishimura M., Uzu T., Hara-Nishimura I., Koya D., Kashiwagi A., Ohkubo I. (2007) Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett. 581, 1417–1424 [DOI] [PubMed] [Google Scholar]
- 33. Abrahamson M., Barrett A. J., Salvesen G., Grubb A. (1986) Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J. Biol. Chem. 261, 11282–11289 [PubMed] [Google Scholar]
- 34. Alvarez-Fernandez M., Liang Y. H., Abrahamson M., Su X. D. (2005) Crystal structure of human cystatin D, a cysteine peptidase inhibitor with restricted inhibition profile. J. Biol. Chem. 280, 18221–18228 [DOI] [PubMed] [Google Scholar]
- 35. Abrahamson M., Mason R. W., Hansson H., Buttle D. J., Grubb A., Ohlsson K. (1991) Human cystatin C: Role of the N-terminal segment in the inhibition of human cysteine proteinases and in its inactivation by leucocyte elastase. Biochem. J. 273, 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall A., Håkansson K., Mason R. W., Grubb A., Abrahamson M. (1995) Structural basis for the biological specificity of cystatin C. Identification of leucine 9 in the N-terminal binding region as a selectivity-conferring residue in the inhibition of mammalian cysteine peptidases. J. Biol. Chem. 270, 5115–5121 [DOI] [PubMed] [Google Scholar]
- 37. Björk I., Brieditis I., Raub-Segall E., Pol E., Håkansson K., Abrahamson M. (1996) The importance of the second hairpin loop of cystatin C for proteinase binding. Characterization of the interaction of Trp-106 variants of the inhibitor with cysteine proteinases. Biochemistry 35, 10720–10726 [DOI] [PubMed] [Google Scholar]