Abstract
Salmon lice, Lepeophtheirus salmonis, are naturally occurring parasites of salmon in sea water. Intensive salmon farming provides better conditions for parasite growth and transmission compared with natural conditions, creating problems for both the salmon farming industry and, under certain conditions, wild salmonids. Salmon lice originating from farms negatively impact wild stocks of salmonids, although the extent of the impact is a matter of debate. Estimates from Ireland and Norway indicate an odds ratio of 1.1:1-1.2:1 for sea lice treated Atlantic salmon smolt to survive sea migration compared to untreated smolts. This is considered to have a moderate population regulatory effect. The development of resistance against drugs most commonly used to treat salmon lice is a serious concern for both wild and farmed fish. Several large initiatives have been taken to encourage the development of new strategies, such as vaccines and novel drugs, for the treatment or removal of salmon lice from farmed fish. The newly sequenced salmon louse genome will be an important tool in this work. The use of cleaner fish has emerged as a robust method for controlling salmon lice, and aquaculture production of wrasse is important towards this aim. Salmon lice have large economic consequences for the salmon industry, both as direct costs for the prevention and treatment, but also indirectly through negative public opinion.
Keywords: aquaculture, Atlantic salmon, Lepeophtheirus salmonis, management, Pacific salmon, socio-economic impact
Introduction
The Danish–Norwegian bishop, Erik L. Pontoppidan (1698–1764), was probably the first to describe the salmon louse in print by his description of ‘great schools of salmon moving from the sea into fresh water, partly to refresh themselves, and partly to rid themselves by rubbing and washing in the swift currents and waterfalls, of a kind of greenish vermin called ‘Laxe-Luus,’ attached between the fins, plaguing it in the heat of spring’ (Berland & Margolis 1983). Bishop Pontoppidan's report suggests that salmon lice were abundant on Atlantic salmon around 1750 in sufficient quantities to induce signs of discomfort or wounds and that ‘salmon louse’ was a commonly used name for the parasite. A report in 1940 from the Moser River (Nova Scotia, Canada) describes severe salmon louse infections and associated deaths: ‘fish, which were apparently freshly ascended from the estuary, carried hundreds of lice … some of the grilse had an almost complete layer of lice extending from the posterior edge of the eyes to the caudal peduncle on the dorsal part of the body with also a few lice around the anal and pelvic fins’ (White 1940). These accounts suggest a substantial annual variation in salmon louse infection rates. Salmon lice on Atlantic salmon caught in rivers were once considered a sign of prime quality as this indicated that the fish only recently entered the river and had not yet suffered the decline in quality associated with sexual maturation. The initial scientific interest in salmon lice was low, however, with the publication of only a few reports until salmon lice began to cause problems for the aquaculture production of Atlantic salmon (Brandal, Egidius & Romslo 1976; Brandal & Egidius 1977; Johannessen 1978).
Aquaculture production of Atlantic salmon, Salmo salar L., reached approximately 1.5 million tons in 2009, with Norway being the largest producer, followed by Chile, the United Kingdom and Canada (Torrissen et al. 2011). Wild stocks of Atlantic salmon declined during the same period (Anon 2011; NASCO 2011), and the nominal catch in 2010 was 1589 tons or approximately 0.1% of the total landings of wild and cultured Atlantic salmon (Fig. 1). In general, sea pen culture of salmon has greatly increased our knowledge of marine pathogens (Bakke & Harris 1998). The salmon louse has been a serious problem for the Atlantic salmon farming industry since the 1970s (Brandal et al. 1976; Brandal & Egidius 1977), and the salmon louse has a greater economic impact than any other parasite (Costello et al. 2004). The year-round high density of hosts provides the ideal conditions for salmon lice. Not surprisingly, within a few years of the onset of intensive salmon aquaculture, salmon farms were proposed to be the primary sources of salmon louse epizootics on wild sea trout in Ireland (Tully & Whelan 1993).
Figure 1.
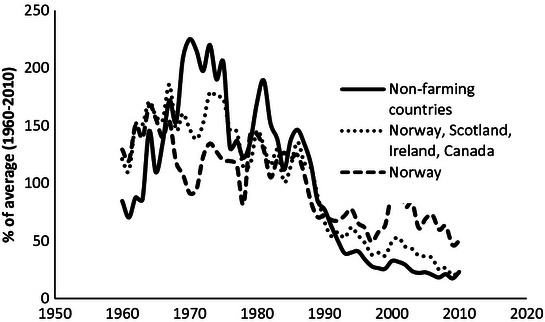
Relative nominal catch of Atlantic salmon from 1960 to 2010 in ‘non-farming countries’ (USA, Russia, Iceland, Sweden, Denmark, The Faeroe Islands, Greenland the UK – except Scotland, France and Spain), ‘Norway, Scotland, Ireland and Canada’, and ‘Norway’(NASCO 2011). The Faroe Islands is included among ‘non-farming’ as their salmon fishery is a marine fishery.
The apparent inverse relationship between the Atlantic salmon aquaculture production and the catch or abundance of wild salmon has led to discussions and conflicts between the salmon farming industry and society, often represented by different non-governmental organizations (NGO). The core of the conflicts has been disagreement on the scale of the impact of salmon lice or their therapeutants on the decline of wild salmon populations or non-targeted species (http://www.worldwildlife.org; http://www.puresalmon.org; http://www.mangroveactionproject.org; http://www.farmedanddangerous.org). In this respect, there is a common failure to recognize that a correlation between the two sets of data does not necessarily indicate a cause–effect relationship (McVicar 2004). The current controversy arises partly from a lack of good data, leading to over-interpretation and possibly misinterpretation of the available information (McVicar 2004).
Aquaculture production of salmonids in open-cage systems will probably always be challenged by salmon lice and, as with many other diseases in farmed animals and humans, the management of salmon lice infestations will remain an ongoing battle. In this battle, the farming industry will pursue multiple strategies to control salmon lice infestation rates to acceptable levels and the parasite will demonstrate a capacity to adapt to these efforts. The 9th International Symposium on Sea Lice was held in Bergen Norway in May 2012. The intention of this article is to summarize the current knowledge of salmon louse biology, including the epidemiology, host interaction and impact on wild fish, as well as advances in the treatment, control and management of salmon lice. We also discuss salmon lice from a social and economic perspective.
Socio-economic considerations
The main social impact of salmon is to create jobs and livelihood. Aquaculture is the largest activity in this respect, but the recreational and commercial wild fisheries are also substantial. Negative impacts on wild salmon stocks from farming activities are perceived to reduce wild catches for anglers and decrease the demand for fishing licences (Olaussen & Liu 2011). While fish farms may displace fishermen from their traditional harvesting grounds, farming may also lead to local increases in catches as wild fish forage on waste feed available near farms. Salmon aquaculture also provides an indirect livelihood for a number of people, such as suppliers, administrators and processors. Olavsen et al. (2011) estimate that the number of people employed in aquaculture-related jobs is twice that of people directly involved in the aquaculture core operation. The recent loss of more than 20 000 jobs in salmon farming and processing as a result of infectious salmon anaemia and salmon lice in Chile provides a dramatic example of the importance of disease and parasite management (Alvial et al. 2011).
The salmon lice issue receives attention partly because of the potential spread of lice from farmed to wild salmon and partly because of interactions between farms. It is necessary to distinguish between the costs to individual farms associated with salmon louse infestation and the social costs of salmon lice discharged from those farms. Costs to individual farms include those associated with lost production due to disease or fallowing and with treatment. Costs associated with salmon lice that impinge other farms or the broader ecosystem include higher production costs for other farms due to elevated infestations or reduced catches of wild salmon due to increased mortality of wild salmon stocks (Asche, Guttormsen & Tveterås 1999).
Farm-specific costs due to salmon lice are relatively well understood. A farm will normally treat against sea lice when production costs are increased due to a reduced growth rate, increased feed conversion rate and reduced marketability due to skin injuries at a levels exceeding the treatment costs. For the 2006 global production of Atlantic salmon (1.6 million tons), it is estimated that lice treatment cost the industry approximately 305 million € (Costello 2009).
The influence of salmon lice on production costs at one farm tends to influence several farms in a region (Tveterås 2002). For the industry in the region, this provides a justification for regulations to limit this negative externality.
Criticism of the salmon farming industry for how they have handled the salmon louse problem has influenced the design of regulations and licences to operate. For example in Norway, concerns with respect to salmon lice led to a postponed implementation and possibly abandonment of an increase in the maximum allowable biomass (MAB) (Asche & Bjørndal 2011). The strong negative publicity on the sea lice issue may also influence the public reputation of salmon and the salmon industry, reducing the demand and consequently the price for salmon. As there is a global market for salmon (Asche et al. 2005), however, this effect is most likely limited.
A thorough bioeconomic model that accounts for the externalities caused by neighbourhood farms and regulations is required to fully determine the cost of salmon lice. Limited knowledge is available regarding the level of this cost, but it is likely to vary substantially between farms and regions. For the economic sustainability of salmon farming, it is important that regulatory measures are evaluated with respect to the economic as well as environmental impact. In general, regulatory design and the compatibility of regulatory measures with fish farmers′ incentives will significantly influence costs incurred by the regulations as well as the effectiveness of the regulations. Regional bioeconomic models may best evaluate the economic impacts of different regulatory measures.
On the basis of production growth and employment, salmon aquaculture is a success story. Innovations that enhance productivity and improve competitiveness are the main factors behind the growth (Guttormsen 2002; Asche 2008). However, salmon aquaculture has the potential to cause negative externalities both by excessive use of resources in the surrounding ecosystem and through interactions with wild stocks as exemplified by salmon lice. Hence, regulations and good governance are necessary to establish a sustainable industry (Smith et al. 2010). Regulations, however, directly influence the competitiveness of an industry, and thus, regulatory design is very important for the economic and societal sustainability of the industry. For example, regulations have eroded the competitiveness of the salmon production industries in Canada and Scotland (Asche & Bjørndal 2011). Hence, if salmon aquaculture is to be sustainable, the lice challenge must be addressed with cost-efficient measures that allow firms and societies to continue to thrive with the industry.
General biology
The salmon louse, Lepeophtheirus salmonis, has a circumpolar distribution in the Northern Hemisphere (Kabata 1979) and is principally a parasite of salmonids in the genera Salmo, Oncorhynchus and Salvelinus. Atlantic salmon post-smolts leave coastal waters quickly after migration from the rivers and are unlikely to represent a significant source of salmon louse larvae in coastal waters (Butler 2002). Prior to salmon aquaculture operations, the year-round presence of sea trout, Salmo trutta L., in coastal waters probably supported a local over-wintering population of salmon lice (Butler 2002; Rikardsen 2004). Returning Atlantic salmon are a significant source of lice with a nearly 100% prevalence, and the louse population comprises predominantly ovigerous females (Copley et al. 2005; Jackson et al. 2012), particularly in areas with few salmon farms.
The salmon louse is a stenohaline copepod whose survival and development are optimal in high-salinity sea water. In this context, it is informative to consider the life history of L. salmonis as a series of behavioural and reproductive strategies to cope with an obvious dilemma: survival in an environment that ranges from low host density and high salinity to one of high host density and ultimately fresh water. For populations of L. salmonis that occur on wild salmon, reproduction and transmission of the copepod are coordinated with the two intervals in the life history of the salmon in which host density and salinity are optimized: in coastal ecosystems during the spawning migration of adults and following the return of juvenile salmon to the ocean. Alternatively, on captive salmon populations that reside in net pens in high-salinity coastal environments for 12–24 months, the opportunities for reproduction and transmission are theoretically continuously high, with increased opportunity for more intense and therefore harmful infections and for shedding larvae at higher densities into the surrounding water column over prolonged periods. Much of the current scientific interest in L. salmonis results from a new host–parasite system in which wild salmonids interact with populations of captive salmon in these high-salinity coastal ecosystems.
The life cycle of L. salmonis comprises non-feeding planktonic larvae (nauplii), infective planktonic copepodites, immature chalimi embedded on the host skin and mobile pre-adults and adults that move freely over the host skin (Hayward, Andrews & Nowak 2011). The infectious larval copepodid subsists entirely on endogenous lipid reserves and therefore devotes its time entirely to host-finding and attachment via a suite of adaptive behavioural traits. These traits include positive phototaxis, positive semiotaxis and positive rheotaxis, which confer to the larval copepod the ability to display diurnal vertical migrations, respond to waterborne gradients of host-derived chemicals and move towards vibrations of host origin, respectively (Heuch, Parsons & Boxaspen 1995; Heuch & Karlsen 1997; Aarseth & Schram 1999; Bailey et al. 2006). In addition, attachment following settlement is mediated by chemoreceptors associated with the copepodid antennules (Bron, Sommerville & Rae 1993). At compatible temperatures and salinities, the free-swimming copepodid survives up to 7 days (Stucchi et al. 2011), although energy content and attachment to the host decline between 3 and 7 days (Tucker, Sommerville & Wootten 2000).
Although L. salmonis occurs throughout the Northern Hemisphere, genetically distinct variants occur in the Atlantic and Pacific Oceans. Microsatellite data from six loci revealed significant variations between populations from the Pacific Ocean and Atlantic Ocean (Todd et al. 2004). Similarly, Tjensvoll, Glover & Nylund (2006) reported differences in the mitochondrial genome between a population of L. salmonis from Japan and the Atlantic Ocean. Subsequently, based on samples obtained from nine populations throughout the Pacific Ocean, it was found that nuclear genes differ, on average, by 3.2% and the mitochondrial genome by 7.1% between Pacific and Atlantic forms of the parasite (Yazawa et al. 2008). This finding is consistent with the geographic isolation and divergence of salmon belonging to Oncorhynchus and Salmo 11–24 million years ago (McKay, Devlin & Smith 1996). A weak but statistically significant genetic differentiation was detected among salmon lice sampled in the North Atlantic, suggesting that salmon lice display a subtle population structure throughout this range. A positive relationship between geographic and genetic distance has also been reported (Glover et al. 2011). A related study failed to detect a population structure in L. salmonis from the Pacific Ocean (Messmer et al. 2011). Gene flow among populations in both oceans therefore appears high and most likely results from the association of the parasite with highly migratory hosts.
Lice–host interactions
The host suffers significant physiological and pathological consequences due to its interactions with L. salmonis that are largely dependent on the number and developmental stage of the copepod. In Atlantic salmon, while physiological changes are evident during infection by the chalimus stages, the feeding behaviour of the pre-adult and adult copepods combined with their unrestricted mobility on the host is responsible for the most severe pathophysiological consequences (Finstad et al. 2000). Heavy infections lead to erosion of the epidermis with exposure of the dermis and, in severe cases, skeletal muscle. Morbidity and mortality resulting from infection with L. salmonis are rare among wild salmon (Johnson et al. 1996). Prior to the systematic application of efficacious treatments, severe infections were common among netpen-reared salmon (Johnson et al. 2004). More common, and therefore of greater significance, are the subclinical physiological consequences of infections, including stress, changes in blood glucose or electrolytes, reduced haematocrits and reduced swimming performance (Wagner, Fast & Johnson 2008). These effects are strongly influenced by the number of copepods and their stage of development. In contrast, the effects of L. salmonis vary among species of Pacific salmon (Oncorhynchus spp.) because of differences in natural susceptibility to the parasite. Salmon lice are rejected more rapidly by coho, Oncorhynchus kisutch (Walbaum), and pink, O. gorbuscha (Walbaum), salmon than by Chinook, O. tshawytscha (Walbaum), and chum, O. keta (Walbaum), salmon, and, in contrast to chum salmon, juvenile coho and pink salmon avoid the clinical consequences of infections (Johnson & Albright 1992; Jones et al. 2007). Pink salmon first enter the ocean at a mean weight of approximately 0.3 g and may be exposed to L. salmonis at this time, thus providing a unique opportunity to assess the ontogeny of innate resistance to L. salmonis. Mortality is significantly elevated among salmon weighing 0.3 g, but not among larger size classes following laboratory exposure, suggesting that the onset of resistance to L. salmonis occurs in pink salmon between 0.3 and 0.7 g (Jones, Kim & Bennett 2008a). Subsequent research confirmed this initial finding and, based on body ion and maximum swimming velocity tests, defined a ‘no-effect’ threshold of 0.5 g for L. salmonis infections in juvenile pink salmon (Nendick et al. 2011; Sackville et al. 2011). Further, these studies showed that in the smallest salmon, salmon lice induce changes to the skin that result in the loss of ionoregulatory homoeostasis.
A more thorough understanding of the mechanisms of innate and acquired defence responses of salmonids to L. salmonis may form the basis of novel management strategies. Differences in susceptibility to L. salmonis among salmon species were initially associated with histological evidence of local inflammatory processes at the infection site (Johnson & Albright 1992). Subsequent studies indicated a relationship between susceptibility and the reduced or delayed expression of a relatively small number of proinflammatory genes (Fast et al. 2006; Jones et al. 2007; Jones, Fast & Johnson 2008b). Coincidently, L. salmonis feeding behaviour consists not only of mechanical abrasion and consumption of host tissues, but also the production of parasite excretory/secretory products (SEPs) (Fast et al. 2003). The L. salmonis SEPs contain prostaglandin E2 (Fast et al. 2004), and in vitro studies showed that the SEPs trigger a significant dysregulation of immune-related genes in either primary or immortalized Atlantic salmon head kidney leucocytes (Fast, Ross & Johnson 2005; Fast et al. 2007). Further research on the differential immunomodulatory capacities of L. salmonis SEPs from Atlantic- and Pacific-type L. salmonis (Yazawa et al. 2008) is necessary to better define the resistance characteristics among salmon species.
Management of L. salmonis will also benefit from improved salmon breeding programmes and the application of genomic technologies. In Atlantic salmon, intraspecific heterogeneity in resistance to L. salmonis is observed among spawning stocks and full-sib families (Glover et al. 2004, 2005; Kolstad et al. 2005; Gjerde, Odegard & Thorland, 2011). Although the heritability of louse counts ranges from 0.07 to 0.33, indicating a genetic basis for differences among families, there is disagreement regarding the identity of acquired immunological markers of resistance to the parasite (Glover et al. 2007; Gharbi et al. 2009). An improved understanding of innate markers may be necessary to explain resistance to L. salmonis (Gharbi et al. 2009). Global gene expression studies have begun to elucidate the pathways of innate and acquired salmonid defence responses to L. salmonis infections (Skugor et al. 2008; Sutherland et al. 2011; Tadiso et al. 2011). There is currently very little evidence that Atlantic salmon mount a protective immune response to either L. salmonis infection or immunization with parasite antigens (Grayson et al. 1991; Reilly & Mulcahy 1993; Roper et al. 1995). Furthermore, Atlantic salmon remain susceptible to reinfection following recovery from L. salmonis (Raynard et al. 2002). Thus, the development of a vaccine against L. salmonis in Atlantic salmon remains a long-term goal and may depend on the selection of suitable salmon strains in which natural resistance is already high.
However, salmon lice will also be under the same evolutionary mechanisms as other animals. Intensive farming will alter the selection criteria such as life-history traits and virulence where frequent use of antiparasite drugs and increased host density may select for faster production of parasite transmission stages via earlier reproduction and increased early fecundity (Mennerat et al. 2010, 2012). They also show a clear link between early reproduction, increased fecundity and increased virulence.
Salmon louse population dynamics
Wild salmonids
Within Europe, significant anthropogenic influences have affected the historical native range of Atlantic salmon; in many rivers, salmon can only breed with human intervention. Many populations are exposed to pollution, changed temperature regimes and low or managed water flow. The effects of diseases and parasites on the salmon populations are particularly difficult to determine (Bakke & Harris 1998). Salmon lice are endemic on wild Atlantic salmon, sea trout and Arctic charr, Salvelinus alpinus (L.), in the North Atlantic. In Ireland, a 20-year monitoring programme indicates that the prevalence of L. salmonis is consistently in excess of 90% (Jackson & Minchin 1992; Costelloe et al. 1998; Copley et al. 2005; Jackson et al. 2012). The reported levels are consistent with data from Scotland (Todd et al. 2000) and Norway (Berland 1993). The near-shore population structure of lice infesting wild Atlantic salmon is quite different from that of populations observed in the offshore or returning adults. Samples of Atlantic salmon (n = 547) captured in an interceptory offshore drift net fishery over a 3-year period showed a prevalence approaching 100% for L. salmonis, and the population comprised largely of adult lice. Atlantic salmon captured in an inshore and estuarine draft net fishery (n = 381) over a 2-year period had a much higher proportion of juvenile lice and over 30% of samples contained fish with chalimus stages (Jackson et al. 2012, this issue). The presence of chalimus stages indicates recent successful infestation and points to re-infestation of returning adult wild populations in inshore waters with a potential for amplification of louse levels that are not evident in offshore stocks.
Salmon louse populations on wild Atlantic salmon show a mean abundance of 6–33 per fish (D. Jackson, unpubl. data, Copley et al. 2005; Costelloe et al. 1998; Jackson et al. 2012). Adult female mean abundance varies from 0 to 17 per fish. Female L. salmonis from wild salmon are larger than those recorded on farmed fish (Jackson & Minchin 1992; Copley et al. 2005) and have higher fecundity (Jackson & Minchin 1992). There is good evidence for a pulse of infestation pressure in the spring as water temperatures rise and the returning adult wild salmon arrive off the coast (Jackson et al. 1997). This spring pulse of infectivity coincides with a maximum in adult female somatic size, which is linked to increased fecundity (Jackson et al. 2000). Several studies have identified evidence for increased infestation pressure on farmed salmon related to infective stages from wild salmonids in Ireland (Jackson et al. 1997), in association with spring salmon runs in Killary Harbour and grilse runs in Clifden Bay, and in Scotland (Marshall 2003), in association with sea trout in Laxford Bay, Sutherland. Unpublished data from Beartragh Buí Bay, a hydrographically isolated bay where the farm had been fallow for a number of years, shows a sustained level of infestation pressure on autumn smolts stocked in late 2008 through the winter months. The most likely host reservoir to serve as a source of these infestations is resident wild sea trout from the river systems entering into the bay. These river systems support significant populations of sea trout and two of the systems operate as commercial recreational fisheries for sea trout. A sustained level of infestation pressure was recorded from December 2008 through May 2009 with juveniles detected in each month rising to a maximum in May of 4.6 juveniles per fish in the absence of any adult ovigerous females on the farmed fish (D. Jackson, pers. comm.).
In the mid-North Pacific Ocean and Bering Sea, L. salmonis was consistently observed on salmon belonging to six Oncorhynchus spp. surveyed between 1991 and 1997 (Nagasawa 2001). The parasite occurred on 93.8% of all (n = 1267) pink salmon examined, with a mean intensity of 5.9 lice per fish. On chum salmon, the prevalence was 36.4% with a mean intensity of 2.1. Among Chinook, coho and sockeye, O. nerka (Walbaum). salmon, and steelhead, O. mykiss (Walbaum), trout, the prevalence ranged from 7.8% (sockeye) to 91.5% (steelhead) and the intensity ranged from 1.1 lice (sockeye) to 6.07 lice (steelhead). The consistently high abundance of salmon lice on chum salmon in all years and of pink salmon in alternating years indicates that these species support the vast majority of the L. salmonis population in the North Pacific Ocean, supporting earlier work in which pink and chum salmon accounted for 90% of all salmon lice (Nagasawa 1987). The Pacific coast of North America is unique in supporting large populations of anadromous salmonids coincident with the production of Atlantic salmon in seawater netpens (Noakes & Beamish 2011). Surveys conducted along the Pacific coast of North America confirmed the high prevalence of salmon lice observed on the high seas and showed that the proportion of gravid L. salmonis ranged from 14% to 36% on chinook, coho, sockeye, pink and chum salmon (Beamish et al. 2005). In another study, it was shown that in coastal waters of western Canada and the USA, larger salmon were more heavily infested with L. salmonis than smaller salmon (<1 year at sea) (Trudel et al. 2007), in agreement with observations by Nagasawa (1987).
Farmed salmonids
The concentration of infective copepodites is important in the population dynamics of salmon lice. Within an area, the copepodite concentration is dependent on the number of mature females, their fecundity and survival of the nauplii. Water temperature affects female fecundity, development times through all life-cycle stages and probably also survival through all life-cycle stages of salmon lice (Stien et al. 2005). This intrinsic temperature dependency is assumed to be a basic driving force for the population dynamics of salmon lice, which has been characterized by annual oscillations in parasite abundance (Lees, Gettinby & Revie 2008b; Jansen et al. 2012). Temperature probably affects all life processes in the multi-stage life cycle of salmon lice, but the relationship between temperature and salmon louse population dynamics is not simple. Annual peaks and troughs in the abundance of mobile salmon lice may appear delayed compared with maximum and minimum annual temperature (Jansen et al. 2012). Additionally, factors other than temperature may promote cyclic population dynamics. In Pacific Canada, for example, the number of salmon lice peaked in spring and fell to the minimum in late summer. The increase in louse abundance during the autumn and winter periods was suggested to be associated with the spread of infection from wild salmon returning to spawn (Marty, Saksida & Quinn 2010). Factors such as the abundance of plankton-consuming organisms and of pelagic fish serving as unsuitable targets for the copepodites could also influence the survival of the planktonic stages of salmon lice.
Experimentally, salmon louse survival is compromised at salinity levels below 29 parts per thousand (ppt) (Bricknell et al. 2006), and nauplii do not develop into infective copepodites at salinities below 25 ppt (Johnson & Albright 1991). Assessing the effects of varying salinity levels on salmon louse infections, however, is complicated by the fact that layers of water with varying salinity levels tend to stratify in the water column, with low-salinity layers on top. Hence, salmon lice may actively avoid low-salinity waters by vertical movement (Heuch 1995). Nevertheless, farms that are exposed to freshwater runoff from rivers are negatively associated with salmon lice abundance (Heuch et al. 2009).
Salmon louse nauplii and pre-infection copepodites disperse as plankton in the water currents. Hence, it is expected that water current characteristics will influence salmon louse infections. Hydrodynamic modelling as a basis for tracking salmon louse particles in water currents over time is increasingly being used to study the spread of salmon louse infection (Amundrud & Murray 2009). Such models are in need of broader validation through analyses of salmon louse data. The integration of hydrodynamic modelling and population modelling of salmon lice is a growing field of research where future advances may be expected.
Fish size is associated with salmon louse abundance in farmed fish, such that large fish carry high intensities of infection (Lees et al. 2008b; Heuch et al. 2009; Jansen et al. 2012). This phenomenon can be due to an increased contact rate between infective parasites and large hosts (Tucker, Sommerville & Wootten 2002), and/or the fact that the increased exposure time of large hosts leads to accumulated intensities of infection (Jackson & Minchin 1992). Regardless of the mechanism, the tendency for large salmonid hosts to carry many salmon lice implies that high concentrations of large-sized farmed salmon potentially support large parasite populations. This may be a concern for pest management strategies that involve synchronized production in some areas, that is, towards the late part of the production cycle when all farmed fish within an area have reached a large size.
A key concept in theoretical epidemiology is that increasing host density should promote the population growth of a parasite because the chances of a host contact increase as host density increases, that is, increased transmission increases the parasite reproductive rate (Anderson & May 1991). Salmonid farming in many areas, for example, in Norway, results in host densities that massively increase the abundance of salmon lice (Johansen et al. 2011), raising the expectation that transmission and population size of the parasite will also increase. This host density effect has been documented at both the level of individual salmon farms and on larger scales. At the farm level, the rate of salmon louse infections on juvenile wild salmon increases during migration past salmon farms, suggesting that salmon louse transmission is associated with farm-produced infectious stages of lice (Krkosek, Lewis & Volpe 2005). Furthermore, a close relationship between estimated numbers of salmon lice on farmed fish and the prevalence of salmon lice on juvenile migrating pink salmon has been demonstrated (Marty et al. 2010).
A long-term study (2002–2007) from a Scottish loch suggested that spatial and temporal densities of salmon lice planktonic stages depend on the location of salmon farms (Penston et al. 2008). Significant correlations between copepodites in the water column and estimated numbers of gravid female lice on farms were reported, and generally farms with the greatest number of salmon were suggested to contribute more to the densities of copepodites in the water column than farms with fewer fish (Penston & Davies 2009).
In a large-scale study covering all cohorts of farmed salmonids in Norway over the years 2002–2010, local densities of farmed fish were found to affect parasite numbers as well as efforts to control infections. Farms situated in high-density clusters reported generally higher sea louse abundance, increased frequency of chemotherapy treatments and more frequent use of cleaner fish to control infections. Adding to the effect of local densities of farmed fish was a strong temporal correlation with farm-level reports on salmon louse abundance (Jansen et al. 2012). This latter effect is due in part to the close proximity of a high number of susceptible hosts leading to auto-infections.
These studies convincingly demonstrate that the increased host density associated with salmon farming promotes transmission and population growth of the salmon louse. The implication of the host density effect is that management should aim to focus on salmon louse infection pressure, that is, accounting for host and parasite densities (Penston & Davies 2009; Jansen et al. 2012). These data also suggest that effective countermeasures to sea louse infections must take into consideration host and farm densities in the context of local oceanographic and other environmental conditions.
Interactions between farmed and wild salmonids
Salmon farms no doubt have profound effects on the local abundance of some parasites (Bakke & Harris 1998), but the quantitative impact of their effect on wild population sizes of salmon and trout remains controversial. A 99% collapse in the pink salmon population and population extinction in only 3.9 generations were predicted due to the impact of salmon lice originating from Atlantic salmon farms in the Broughton Archipelago, BC, Canada (Krkosek et al. 2007). These authors estimated an 80% (range 16–97%) louse-induced mortality for pink salmon juveniles. Nevertheless, the population of pink salmon has steadily increased over the last few years (Brooks & Jones 2008), and the latter authors suggested misinterpretation of data was the basis for the prediction of extinction. The number of pink salmon returning to spawn in the fall predicts the number of female salmon lice on farm fish in the following spring. This accounts for 98% of the annual variability in the prevalence of salmon lice on out-migrating wild juvenile salmon (Marty et al. 2010). The latter study concluded that productivity of wild salmon is not negatively associated with either farm louse number or farm fish production, although this conclusion is not without controversy (Krkosek et al. 2011).
Nominal catches of wild Atlantic salmon in the North Atlantic (NASCO 2011) have declined since the turn of the twentieth century (Hesthagen & Hansen 1991), indicating a corresponding reduction in stock size. While this says nothing about the quantitative impact of salmon lice on populations or individual stocks, it indicates that non-aquaculture factors contribute to the overall variation in seawater survival of the wild salmon population in the North Atlantic. The size of salmonid stocks in both freshwater and marine environments results from an interaction of many anthropogenic and natural biological and physical factors. The wide array of factors that cause variations in the size of salmon and trout stocks is frequently underestimated or overlooked in attempts to find simple explanations. However, the complexity makes it extremely difficult to separate and quantify the effect on each parameter. Figure 1 shows the relative nominal catch of Atlantic salmon from 1960 until today in countries without or with limited salmon aquaculture (USA, Russia, Iceland, Sweden, Denmark, The Faroe Islands, Greenland, UK [except Scotland], France and Spain), major aquaculture producers of Atlantic salmon in countries with spawning wild Atlantic salmon (Norway, Ireland, Scotland and Atlantic Canada) and Norway as the dominating producer. Nominal catches have significantly declined in all regions, and the pattern of decline is similar among regions. Since 1990, the aquaculture production in Norway has increased 6.5-fold to 1 million tons in 2011, and a recent meta-analysis suggests the decline in population sizes tends to be higher in areas with high density of salmon farms (Otero et al. 2011). Despite the decline, the International Council for the Exploration of the Seas (ICES) has estimated that the North-East Atlantic salmon stock complex remains at full reproductive capacity (Committee 2011; ICES 2011).
In Norway, several approaches have been used to investigate infection rates on wild Atlantic salmon and sea trout stocks. Direct measurements of the salmon louse infection rate of wild smolts have been obtained by surface trawling of wild migrating smolts (Holm, Holst & Hansen 2000; Holst et al. 2003; Heuch et al. 2005). The number of lice on sentinel smolts held in small cages in a fjord during the time of natural smolt migration is also used to estimate infestation rates in addition to catches of sea trout and salmon in gillnets and traps. All methods have limitations, and there are obvious risks for getting skewness in the data (Bjørn et al. 2011). Only fish that survived the infestation will be caught, behaviour and catchability will depend on infestation rate, fishing gear is size- and species sensitive, and place and time may not be representative for the overall situation.
Early seawater mortality and performance have also been estimated by treating smolts with anti-lice drugs prior to their release and comparing their recapture rates as adults with untreated control groups. The efficacy of the drugs is limited to weeks or in some cases months (Stone et al. 2000a; Hvidsten et al. 2007; Skilbrei et al. 2008), and it is assumed that differences in ocean survival are caused by infestation of control fish with salmon lice during the early stage of smolt migration. The drug emamectin benzoate is usually administered to the fish, either orally (Slice®) (Skilbrei & Wennevik 2006; Jackson et al. 2011a,b; Gargan et al. 2012; Skilbrei et al. 2012) or by intraperitoneal injection to increase the mean dosage and reduce the variability between individuals (Glover et al. 2010; Skilbrei et al. 2012). Substance EX (Pharmaq) has also been used (Hvidsten et al. 2007; Skilbrei et al. 2012). Following the release of hatchery-reared smolts of the Orkla River stock in Trondheimsfjord in mid-Norway from 1996 to 1998 (Hvidsten et al. 2007), significantly more treated smolts survived in 1998, coincident with a high rate of salmon louse infection in the wild smolts. Hatchery-reared smolts from Dale River stock in western Norway from 1997 to 1999, and from 2001 to 2009, were also released in an area that houses one of the largest concentrations of fish farms in Norway. Significant differences were detected in 3 of the 35 groups released at different times and sites: the only release in 1997, one in 2002 and one in 2007 (Skilbrei & Wennevik 2006; Skilbrei et al. 2012), but there were no tendency in the majority of the release groups suggesting differences in survival between the treated and control groups. Overall, the probability of recapturing a treated compared with an untreated smolt was estimated to have an odds ratio of 1.17:1.
Similar treat-and-release studies have been conducted in Ireland. Releases of Burrishoole grilse stock smolt from western Ireland from 2001 to 2008 resulted in a clear trend in which treated fish returned in higher numbers in 9 of 10 years (Jackson et al. 2011b), and the differences were statistically significant in 4 of 10. The magnitude of the differences was not large, however, and no differences were observed between the mean returns of treated and untreated groups (analysis of variance n = 20). The authors concluded that the level of infestation pressure by salmon lice experienced by the outwardly migrating smolts was not a consistently significant source of additional marine mortality. Similar results were obtained with three other river stocks in 2002 and 2006 (Jackson et al. 2011b). In a separate study, releases of smolts over 3 years in two rivers and 2 years in a third river in Ireland from 2004 to 2006 showed a similar treatment effect (Gargan et al. 2012). The recapture of treated fish was significantly higher in three of eight releases and also for the combined data. They concluded that treated smolts were, in general, 1.8 times more likely to return and that salmon louse-induced mortality in adult returns in Ireland can be significant. Significant differences were found both where adjacent farms had no adult female burden (one case) and where there were adjacent farms with adult female lice (two cases).
The results based on these assessments of early sea mortality due to salmon louse infection vary considerably in relation to location, release date and from year to year. The Norwegian investigations, however, indicated a binominal tendency: the numbers of recaptures of treated and control fish were either clearly equal or significantly different. This suggests that salmon lice nauplii are not uniformly distributed in the sea, but rather have a patchy distribution in space and time. This type of variability represents one of the methodological challenges when using release/recapture experiments to produce estimates of early sea mortality due to salmon lice on migrating smolts, especially if there is only one release per location per year. Other potential biases are the large annual variability in ocean survival resulting in low recaptures (Jackson et al. 2011b; Skilbrei et al. 2012) and uncertainties regarding duration of efficacy against salmon lice afforded by the treatment (Skilbrei et al. 2008, 2009; Glover et al. 2010). The latter point recognizes the absence of efficacy of emamectin benzoate against salmon lice in several regions. At present, the available information suggests that the majority of the released smolt groups were not at all or only moderately affected by salmon lice, but that some groups were clearly affected. There are also clear local and year-to-year differences in the risk of being too heavily infested with salmon lice. A more up-to-date study, comprising data already published by Jackson et al. (2011a,b), together with results published by Gargan et al. (2012) and previously unpublished data, including a second time series from a catchment on Ireland's west coast (Jackson, et al., 2012), has confirmed these findings. The data, comprising over 350 000 fish from eight locations across nine release dates, show a similar trend in survival between treated and control groups over time when fitted to regression lines (Fig. 2). Analysis based on modelling the percentage return as a binomial response variable, adjusted for location and release-year effects, estimates the probability of returning as 1.14:1 in favour of the treated group, or an absolute difference in sea water returns of approximately 1%. Analysis based on modelling the percentage as a continuous response variable indicates that location (i.e. river; P < 0.001) and release date (P = 0.001) were both more highly significant than treatment (P = 0.034) and an approximately 1% difference between treated and control groups. Thus, estimates from Ireland and Norway indicate an odds ratio of 1.1:1-1.2:1 for sea lice treated Atlantic salmon smolt to survive sea migration compared to untreated smolts. According to the opinion of a Norwegian expert group (Taranger et al. 2012), an estimated salmon lice-induced mortality above 10% is expected to have a moderate population regulatory impact, whereas higher mortalities, close to 50%, would have a far greater impact on the affected salmon population.
Figure 2.
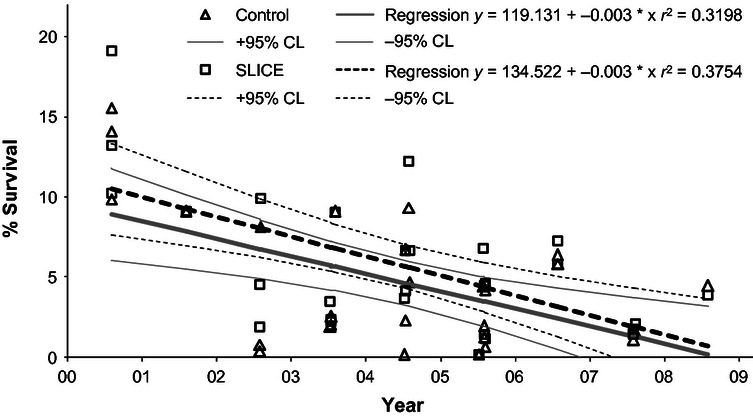
Percentage survival of smolts in Ireland from 2001 to 2009 with 95% confidence limits (CL) fitted. Sea lice infestation was not implicated in the observed decline in survival common to both groups (Jackson et al. 2011a). The treated groups were given a prophylactic SLICE treatment giving protection against sea lice infestation for approximately 9 weeks (Jackson et al. 2011b).
Control: farm monitoring and management thresholds
Lice levels on farmed fish have been monitored in Ireland since 1991, and a comprehensive monitoring programme has been in place since 1993 (O'Donohoe et al. 2011). Lice levels on farmed salmon increase with time at sea (Jackson et al. 2000) with two sea-winter fish carrying the heaviest burden. Fallowing and separation of generations of farmed fish reduce the effect of sea age on the lice burden (Jackson et al. 1997; O'Donohoe et al. 2011) of farmed fish. Studies of wild salmon at sea reveal a similar increased abundance of lice with sea age in salmon obtained from north of the Faroe Islands (Jacobsen & Gaard 1997). Salmon lice monitoring and control measures were modified in 2000 and formed the basis of an integrated management protocol for salmon lice in farmed salmon in Ireland (Jackson, Hassett & Copley 2002). Crucial elements of this strategy were identified as separation of generations, annual fallowing of sites and strategic applications of treatments, good fish health management and close cooperation between farms. The monitoring and inspection programme results revealed the benefits of this approach on levels of control achieved from 2000 through 2004 (Fig. 3). From 2005 to 2007, there was a progressive increase in the mean levels of infestation of farmed fish. The increased infestation was identified as resulting from a range of factors, including changes in production practices (Jackson 2011). To address these issues, the Irish authorities issued new guidelines as a Strategy for Improved Pest Control on Irish Salmon Farms in 2008. These guidelines were implemented over the succeeding 2 years and have led to a progressive reduction in the mean levels of infestation (Fig. 3).
Figure 3.
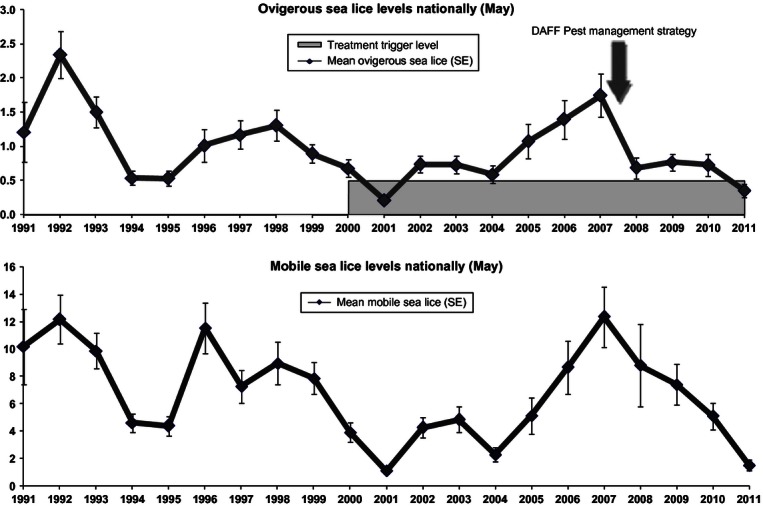
Annual trend (May mean) (SE) of Lepeophtheirus salmonis on one-sea-winter salmon.
In British Columbia, Canada, the autumn rise in L. salmonis counts in farmed Atlantic salmon is a consequence of transmission from the large population of returning wild Pacific salmon (Saksida et al. 2007a,b). Despite this annual occurrence, routine monitoring of louse numbers on farmed salmon in British Columbia began only in 2003, in response to concerns that infections on juvenile pink salmon were the result of transmission from farmed salmon (Morton et al. 2004). A management plan, established in 2003, defined seasonally adjusted salmon louse monitoring and reporting of data and the establishment of action thresholds (Saksida et al. 2007a). Since 2007, coordinated treatment with emamectin benzoate applied to farmed salmon 1 or 2 months prior to the migration of juvenile pink and chum salmon into the ocean in the Broughton Archipelago has coincided with a significant and persistent decline in L. salmonis abundance on the wild salmon (Jones & Hargreaves 2009; Jones & Beamish 2011). Furthermore, in British Columbia, there is no evidence that the efficacy of emamectin benzoate against L. salmonis has changed between 2003 and 2008 (Saksida, Morrison & Revie 2010). In this region, there are ongoing efforts to adequately define salmon lice interactions between wild and farmed salmon using mathematical models (Marty et al. 2010; Krkosek et al. 2011).
The Salmon Lice Directive (FOR-2009-08-18-1095 2009) provides a framework for the Norwegian surveillance programme for salmon lice in farms. This directive requires that each farm has a general plan for prevention and treatment of salmon lice which as a minimum should contain plans for counting lice in the farm, routines and methods for treatment, including coordinated treatment within the region and documentation of ability to complete treatments within deadlines, routines and methods for evaluation of effect of treatment, routines for use of cleaner fish, routines for fallowing, which other farms are included in the coordinated treatment plan and how the farm seeks to protect wild salmon and trout from negative impacts. An annual update of the plan is required by the Norwegian Food Safety Authority.
This directive imposes a requirement for recording and reporting of data on water temperature, salinity, date and cages counted (minimum 50% of all cages, all if farm has <3), number of sessile lice, number of mobile lice, number of mature females, dates for treatment, drugs used in treatments and possible drug resistance. The key data are published on a weekly basis on http://www.lusedata.no.
Action levels in Norway are 0.5 mature female or three mobile lice on average during the period 1 January through 31 August and one mature female or five mobile during the rest of the year.
The Norwegian Food Safety Authority also has the authority to impose specific and stricter regulations in exposed areas, including reduction in biomass and coordinated treatment and fallowing (FOR-2009-08-18-1095 2009).
Control: chemotherapeutants interventions
Over the years, a variety of treatments have been tried against salmon louse infestations. Initially, formaldehyde baths were tested, but proved to have questionable effects (Johannessen 1974). Organophosphates were then introduced, the first being metrifonate as an oral treatment (Brandal & Egidius 1977). However, the low safety margin of oral delivery in salmon led to the introduction of bath applications (Brandal & Egidius 1979). In Scotland, dichlorvos, a related organophosphate, was introduced in 1979 (Rae 1979) and subsequently became the treatment of choice in most salmon-producing countries until the early 1990s when resistance problems became evident (Jones, Sommerville & Wootten 1992; Denholm et al. 2002). Alternative bath treatments were launched: first, natural pyrethrins, which were administered as a top dressing in oil at the surface of the pen (Jakobsen & Holm 1990). The administration method, however, was rather impractical. Despite a narrow safety margin, bath treatments with hydrogen peroxide were later introduced, especially in areas where lice had increased tolerance to dichlorvos (Thomassen 1993). In the late 1980s, oral treatments with other compounds were also tried, the first being the macrocyclic lactone ivermectin, which demonstrated a good and long-lasting effect, but also a low margin of safety (Palmer et al. 1987). The chitin synthesis inhibitor diflubenzuron was tested in the early 1990s (Horsberg & Hoy 1991); later, another similar compound, teflubenzuron (Branson, Ronsberg & Ritchie 2000), appeared. These compounds had a very broad safety margin, but only targeted the early developmental stages and not adult parasites. In the mid-1990s, azamethiphos, an organophosphate posing less of an occupational hazard than dichlorvos, was launched (Roth et al. 1996), after which the synthetic pyrethroids cypermethrin (Hart et al. 1997) and deltamethrin (Roth 2000) were introduced. The pyrethroids had a reasonably good safety margin and a good effect on all developmental stages. Finally, in 1999, the macrocyclic lactone emamectin benzoate came to the market (Stone et al. 2000b). The safety margin for this orally delivered compound was substantially better than that for ivermectin, and the compound was effective against all developmental stages, lasting up to 10 weeks. After 1999, no new therapeutic agents against salmon lice have been launched. In Norway, the utilization of the different products has been recorded since the early 1980s, and Table 1 illustrates their rise and fall over time.
Table 1.
Consumption (kilograms active substance) of different agents against salmon lice in Norway from 1981 to 2011 (Grave, Engelstad & Søli 1991; Grave et al. 2004; The Norwegian Institute of Public Health 2012)
| Metrifonate (Neguvon) | Dichlorvos (Nuvan) | Azamethiphos (Salmosan) | Hydrogen peroxide | Diflubenzuron (Lepsidon, Releeze) | Teflubenzuron (Ektobann) | Pyrethrins (Py-Sal) | Cypermethrin (Excis, Betamax) | Deltamethrin (Alpha max) | Emamectin (Slice) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1981 | 4920 | |||||||||
| 1982 | 6300 | |||||||||
| 1983 | 9810 | |||||||||
| 1984 | 14 820 | |||||||||
| 1985 | 28 260 | |||||||||
| 1986 | 24 860 | 250 | ||||||||
| 1987 | 7390 | 1310 | ||||||||
| 1988 | 3190 | 3200 | ||||||||
| 1989 | 3300 | 3488 | ||||||||
| 1990 | 2408 | 3416 | ||||||||
| 1991 | 2144 | 3588 | ||||||||
| 1992 | 1946 | 3115 | ||||||||
| 1993 | 1779 | 2470 | 710 000 | |||||||
| 1994 | 1227 | 1147 | 389 | 290 000 | 32 | |||||
| 1995 | 395 | 738 | 340 000 | 26 | ||||||
| 1996 | 161 | 606 | 160 000 | 160 | 610 | 9 | 23 | |||
| 1997 | 36 | 315 | 20 000 | 361 | 1510 | 18 | 28 | |||
| 1998 | 182 | 437 | 1334 | 3 | 19 | |||||
| 1999 | 14 | 50 | 231 | 19 | 11 | 4 | ||||
| 2000 | 12 | 62 | 73 | 23 | 30 | |||||
| 2001 | 28 | 69 | 19 | 12 | ||||||
| 2002 | 62 | 23 | 20 | |||||||
| 2003 | 59 | 16 | 23 | |||||||
| 2004 | 55 | 17 | 32 | |||||||
| 2005 | 45 | 16 | 39 | |||||||
| 2006 | 49 | 23 | 60 | |||||||
| 2007 | 30 | 29 | 73 | |||||||
| 2008 | 66 | 32 | 39 | 81 | ||||||
| 2009 | 1884 | 308 000 | 1413 | 2028 | 88 | 62 | 41 | |||
| 2010 | 3346 | 3 071 000 | 1839 | 1080 | 107 | 61 | 22 | |||
| 2011 | 2437 | 3 144 000 | 704 | 26 | 48 | 54 | 105 |
Most anti-salmon lice agents act by disrupting neuronal signalling (Roberts & Hutson 1999). Organophosphates inhibit the enzyme acetylcholine esterase, which is responsible for catalysing the hydrolysis of the neurotransmitter acetylcholine at the post-synaptic membrane. Failure to degrade acetylcholine to choline and acetic acid results in continuous neuronal firing, subsequently followed by paralysis and death. The effect is best on pre-adult and adult parasites. Organophosphates (azamethiphos) have a rapid effect that can be recorded after a few hours. The pyrethroids (cypermethrin and deltamethrin) interfere with nerve impulses by modulating the opening and closing of voltage-gated sodium channels in axons, leading to repetitive synaptic discharge, followed by paralysis and death. Pyrethroids are effective against all developmental stages, but the full effect can only be determined after 1–2 weeks, depending on the temperature. Avermectins (emamectin benzoate) modulate specific glutamate- and gamma-aminobutyric acid-gated anion channels. The influx of chloride ions results in hyperpolarization, leading to disruption of nerve impulses, paralysis and death. They are effective against all developmental stages, but the full effect can only be determined after 2–3 weeks. In arthropods, they act through ingestion as stomach poisons, and emamectin benzoate has therefore been developed as a premix for medicated feed. Chitin biosynthesis inhibitors (diflubenzuron and teflubenzuron) are also used as in-feed compounds. They do not interfere with neuronal signalling, instead they inhibit key processes in the chitin synthesis of the parasite, which results in a thin and fragile exoskeleton after moulting. Hydrogen peroxide is a potent oxidizing compound that disrupts membranes. The effect is rapid and most efficacious on pre-adult and adult parasites.
The development of resistance in a parasite population renders an antiparasitic treatment ineffective as was evident in several regions in Norway, where organophosphates in the early and mid-1990s totally lost their effect against salmon lice (Denholm et al. 2002). Later, evidence of treatment failures with pyrethroids was reported in Norway, Scotland and Ireland (Sevatdal & Horsberg 2003; Sevatdal et al. 2005a).
Treatment failures have also been reported for emamectin benzoate. Initially, these incidents were isolated cases and could frequently be attributed to erroneous calculations of biomass, concurrent diseases and other factors. Appetite varies considerably between individual fish, causing huge variations in the obtained tissue concentrations of orally administered agents (Berg & Horsberg 2009), and this can be misinterpreted as resistance. In 2006, however, several reports from Chile indicated a systematic failure of efficacy by emamectin benzoate towards Caligus rogercresseyi (Bravo, Sevatdal & Horsberg 2008). Bioassay tests were established, and the parasites demonstrated significantly reduced sensitivity. In addition, there were reports of reduced efficacy of emamectin against L. salmonis in Ireland and Scotland. A Scottish epidemiological survey of salmon lice burdens linked to emamectin treatments between 2002 and 2006 demonstrated a trend towards gradually reduced efficacy. Although salmon lice infestations were reduced following treatments, not all treatments were effective (Lees et al. 2008a). In Norway, no comprehensive data have been published, but a survey demonstrated reduced sensitivity towards emamectin benzoate in more than 50% of the salmon lice strains examined (Horsberg 2012). Reduced sensitivity to emamectin benzoate has so far not been recorded on the Pacific coast of Canada (Saksida et al. 2011).
Resistance is documented and quantified through efficacy monitoring (parasite counts before and after treatments) and bioassays (toxicological tests of the susceptibility of parasites towards increasing concentrations of the agent in question). Bioassays are labour-intensive and require 50–100 pre-adult parasites per agent (Sevatdal & Horsberg 2003; Sevatdal et al. 2005a; Westcott et al. 2008). A simplified version, however, is currently under development (Helgesen & Horsberg 2012). Rapid, high-throughput in vitro methods, for example, quantitative polymerase chain reaction-based assays, would be preferable but are dependent on knowledge about the specific resistance mechanisms. These have only partly been elucidated in salmon lice and include target site alterations (Fallang et al. 2004, 2005), enhanced metabolism (Sevatdal et al. 2005b) and possibly enhanced elimination mediated by P-glycoprotein efflux pumps (Tribble, Burka & Kibenge 2007; Heumann et al. 2012). As several different mechanisms may cause resistance problems, a panel of in vitro tests is needed.
The development of resistance in salmon lice, especially L. salmonis, is a serious situation. In Norway, Scotland, Ireland and eastern Canada, the number of farmed salmon is far greater than the number of wild salmon. Thus, the main source for re-infestation comes from the farms themselves where regular parasite treatments put a constant selection pressure on resistance development. In these countries, the influx of naive parasites from wild fish hosts is limited. Thus, the problem will not disappear by itself. New chemicals may only be valuable for a limited time period. Management practices with a variety of non-chemical control methods, preservation of sensitive parasites, coordinated production zones, synchronized treatments and synchronized fallowing of sites in larger areas seem to be the most promising strategy to handle the problem.
Control: cleaner fish
The use of wrass as cleaner fish for salmon lice control was developed in the late 1980s (Bjørdal 1988a,b, 1990). The aquaculture industry has depended on wild catches for their supply, and several species are used, for example, goldsinny wrasse, Ctenolabrus rupestris, ballan wrasse, Labrus bergylta Ascanius, corkwing wrasse, Symphodus melops (L.), rock cook, Centrolabrus exoleus (L.), cuckoo wrasse, Labrus bimaculatus L., and scale-rayed wrasse, Acantholabrus palloni (Risso) (Espeland et al. 2010). Goldsinny wrasse, ballan wrasse and corkwing wrasse are the species used most frequently in Norway (Blom 2010). Stocking density of approximately 4 wrass per 100 salmon is common, slightly more for small wrasses as goldsinny and less for larger wrasses. Ballan wrasse are efficient and are often used at rates of 1 per 100 salmon. The successful use of cleaner fish depends on healthy fish and clean cages. Wrass require shelter for well-being and readily seek alternative feed sources if the nets are overgrown. Recent experiments have shown that lumpfish, Cyclopterus lumpus L., can be used as cleaner fish and that their aquaculture production is possible (Anon 2012).
The ballan wrasse is the largest of the European wrass, attaining a maximum size of approximately 60 cm (Quignard & Pras 1986), and is therefore a suitable size for being kept in cages with large salmon (3–6 kg) (Muncaster 2008). Based on their wide geographical distribution, they tolerate a wide range of environmental conditions and have been shown to survive in cages over winter in Norway (Bjelland et al. 1996). Over the last few years, there has been a growing interest in farming wrass, with a focus on the ballan wrasse (I. Opstad, P.G. Kvenseth, P. Jensen and A.B. Skiftesvik, unpubl. data). Although weaning the ballan wrasse from live food to a formulated diet is challenging, I. Opstad, P.G. Kvenseth, P. Jensen and A.B. Skiftesvik (unpubl. data) developed a successful weaning diet that supports survival up to 88%.
The rapid increase in wrasse fisheries has raised concerns (Fig. 4), as their biology, ecology and population dynamics are poorly known. Many species change sex during their lifetime, and some have longevity of 20–25 years and are territorial. These species may therefore be vulnerable to overexploitation (Espeland et al. 2010).
Figure 4.
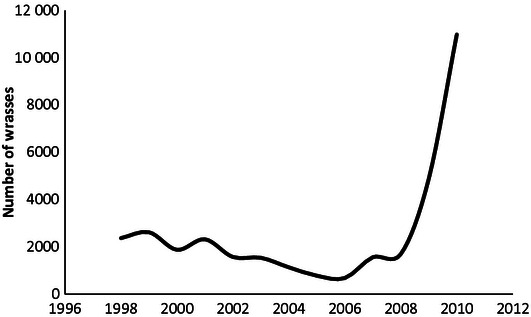
Catches of wrass in Norway for use as cleaner fish in salmon cages (Fiskeridirektoratet 2012).
Control: vaccination
There are two published studies in which vaccine candidate antigens were tested against salmon lice. Grayson et al. (1995) extracted proteins from adult L. salmonis and used these antigens to immunize Atlantic salmon. They found a significant reduction in gravid female lice on the vaccinated fish, and the lice also produced fewer eggs. More recently, Carpio et al. (2011) made a recombinant antigen from the novel my32 gene obtained from C. rogercresseyi. The my32 from C. rogercresseyi is similar to a protective antigen from ticks (Almazan et al. 2005). Carpio et al. (2011) found that immunization with recombinant my32 resulted in a significant reduction in the number of C. rogercresseyi 24 h post-infection and a delayed developmental rate of immunised fish. Further analysis of the results showed that the vaccine effect was due to the reduced settlement of larvae produced by lice on the immunized fish. The overall effect of the vaccine in that experiment was 57% inhibition of infestation. A homologue to the my32 antigen from C. rogercresseyi is also present in L. salmonis. Using this as a vaccine antigen in Atlantic salmon, however, did not significantly reduce the number of lice or lice fitness (F. Nilsen, R. Skern-Mauritzen, C. Eichner and S. Dalvin, unpubl. data). Despite these differences, studies of C. rogercresseyi point to the possibility of a future commercial salmon lice vaccine.
Recent developments in sequencing technology have led to a decrease in the sequencing costs and large increase in sequencing throughput. The salmon louse genome has been sequenced and assembled, and final annotation is in progress (Skern-Mauritzen et al. 2012). This means that all potential treatment targets will be available, and an approach utilizing all this information is possible. A preliminary count indicates about 22 000 genes occur in the salmon louse, although only a small fraction of these would be useful as vaccine antigens. A key challenge is therefore to identify the best candidates that could be used in a future commercial vaccine. Recently, Maritz-Olivier, Van Zyl & Stutzer (2012) proposed a functional genomics approach to identify vaccine candidates in cattle ticks. Their approach is based on several steps of in silico evaluation of candidates prior to experimental verification of antigenicity and testing of recombinant antigens in trial vaccines. Although the current knowledge of efficient protective antigens against ectoparasites is limited, using all the genomic information in the initial analysis will be very helpful towards identifying a number of vaccine antigen candidates. During the last years, a set of tools and resources were established that facilitate the development of new control tools for salmon lice. Efficient and accurate experimental facilities are crucial for the development of new treatment methods for salmon lice. Hamre, Glover & Nilsen (2009) established specific laboratory strains of L. salmonis along with procedures for maintaining and breeding salmon lice. A refined and more accurate set-up for conducting experiments with salmon lice was also recently developed (Hamre & Nilsen 2011). In addition, a set of molecular methods was established that will facilitate research leading to new treatment methods. Examples are lice-specific microarrays (Eichner et al. 2008; Sutherland et al. 2012) and systemic RNA interference methods (Dalvin et al. 2009), which will be very useful in the future.
Classical bacterial and virus vaccines enhance the resistance to infection by limiting the capacity for pathogen replication within the host. For parasites like ticks and salmon lice that do not proliferate on or in the host, the situation is quite different, and for these parasites, a vaccine that reduces the number of offspring has a direct effect on new infections. For the commercially available cattle tick vaccine, the overall effect of the vaccine is about a 90% reduction in the tick reproduction capacity. This includes increased tick mortality and a reduction in the number of eggs produced per female. The obvious question is whether a vaccine effect similar to that observed for cattle ticks is sufficient to make a difference for salmon lice. For example, a salmon louse vaccine that reduces the number of eggs/female by 50% will be comparable with a 50% reduction in the number of female lice/fish. A vaccine like the cattle tick vaccine or a vaccine against salmon lice with a similar level of effect will not be a stand-alone tool in parasite control. Together with other antiparasitic measures, however, the effect will be large. The reliance on chemotherapeutants will be reduced, and the lifetime for valuable medicine will be extended. If all farmed fish are vaccinated, the vaccine would also be effective on escapees and hence contribute further to lice control.
Conclusions
Salmon lice are natural parasites on salmonids in the sea water with a circumpolar distribution in the northern Hemisphere. The populations in the Atlantic and Pacific oceans are genetically distinct. Intensive salmon farming has improved the conditions for the growth and transmission of the parasites compared with natural conditions. Gene flow among populations appears high and most likely results from association with highly migratory hosts. There are distinct differences in the susceptibility to salmon lice infections among salmonid fish species.
Salmon recreational fishery, commercial fishery (sea fishery) and aquaculture have different stakeholders, practices, traditions and management objectives and strategies (Liu, Olaussen & Skonhoft 2011). Sea lice have clearly impacted wild salmon and trout fisheries without compensating for the imposed negative external costs. The quantitative estimates of these impacts show large variations. Further research is needed in order to understand the mechanisms and processes. The density of farms in an area has a clear effect on the levels of sea lice at individual farms within that area.
Since the start of large-scale salmon farming in the 1970s, control of salmon lice has been based mainly on chemotherapy. This has been effective and simple to use, but also creates unwanted environmental effects, occupational hazards and drug resistance problems. During the last few years, there has been a trend towards a more integrated management approach with synchronized treatments, biological control (cleaner fish), immunological interference (immunostimulants), mechanical de-lousing systems, selective breeding for louse-resistant salmon and regulatory approaches (zones with synchronized production and fallowing).
Sea lice resistance to chemotherapeutants is a serious concern. In Norway, Scotland, Ireland and eastern Canada, the number of salmon in farms greatly exceeds the number of wild salmon. Thus, the main sources of re-infestation are the farms themselves, where regular parasite treatments place constant selection pressure on resistance development. New chemicals may only be valuable for a limited period of time. Management practices with a variety of methods will be necessary to keep the sea lice under control in salmon farms.
Two published studies tested vaccine candidate antigens against salmon lice, which resulted in a reduced infection rate (Grayson et al. 1995; Carpio et al. 2011). For parasites like salmon lice that do not proliferate on or in the host, a vaccine will primarily reduce infection pressure. Salmon lice create problems for both the salmon farming industry and, under certain conditions, wild salmonids. A vaccine will probably not be adequate as a stand-alone treatment, but it would be a valuable element in the hierarchy of salmon lice prevention methods.
For the foreseeable future, salmon lice will continue to be a serious problem for the salmon farming industry and a threat to their environmental credibility. Salmon farmers invest in expensive sea lice monitoring and treatment programmes. The key to a sustainable production is to integrate several management practices. This will require a substantial increase in research in areas such as new pharmaceuticals, mechanical lice removal, vaccines and immunostimulants, selective breeding for increased resistance, effective aquaculture production and use of cleaner fish, and the development of coastal hydrographic models to estimate transmission dynamics and to support farm siting decisions and coordinated management.
Acknowledgments
This article was initiated and supported by the Norwegian Centre of Expertise – Aquaculture. Financial support was also received from the Norwegian Research Council and the Norwegian Seafood Research Fund through the research project PrevenT.
References
- Aarseth KA, Schram TA. Wavelength-specific behaviour in Lepeophtheirus salmonis and Calanus finmarchicus to ultraviolet and visible light in laboratory experiments (Crustacea: Copepoda) Marine Ecology-Progress Series. 1999;186:211–217. [Google Scholar]
- Almazan C, Blas-Machado U, Kocan KM, Yoshioka JH, Blouin EF, Mangold AJ, De Fuente La J. Characterization of three Ixodes scapularis cDNAs protective against tick infestations. Vaccine. 2005;23:4403–4416. doi: 10.1016/j.vaccine.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Alvial A, Forster J, Kibenge FSB, Burgos JM, Ibarra R. 2011. The recovery of the Chilean salmon industry. Presentation at: Global Outlook for Aquaculture Leadership, Santiago, Chile, Nov. 1-5, http://www.gaalliance.org/cmsAdmin/uploads/GAA_ISA-Report.pdf. [Google Scholar]
- Amundrud TL, Murray AG. Modelling sea lice dispersion under varying environmental forcing in a Scottish sea loch. Journal of Fish Diseases. 2009;32:27–44. doi: 10.1111/j.1365-2761.2008.00980.x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press; 1991. [Google Scholar]
- Anon. Status for norske laksebestander (The Status for Norwegian Salmon Populations) Trondheim, Norway: Vitenskapelig råd for lakseforvaltning; 2011. p. 285. [Google Scholar]
- Anon. Nytt fra Havbruk. Oslo, Norway: Forskningsrådet (Norwegian Research Council); 2012. Lovende resultat med rognkjeks (Promising results with lumpfish) p. 1. [Google Scholar]
- Asche F, Guttormsen AG, Sebulonsen T, Sissener EH. Competition between farmed and wild salmon: the Japanese salmon market. Agricultural Economics. 2005;33:333–340. [Google Scholar]
- Asche F. Farming the Sea. Marine Resource Economics. 2008;23:527–547. [Google Scholar]
- Asche F, Bjørndal T. The Economics of Salmon Aquaculture. Chichester: Wiley-Blackwell; 2011. [Google Scholar]
- Asche F, Guttormsen AG, Tveterås R. Environmental problems, productivity and innovations in Norwegian aalmon aquaculture. Aquaculture Economics & Management. 1999;3:19–29. [Google Scholar]
- Bailey RJE, Birkett MA, Ingvarsdottir A, Mordue AJ, Mordue W, O'shea B, Pickett JA, Wadhams LJ. The role of semiochemicals in host location and non-host avoidance by salmon louse (Lepeophtheirus salmonis) copepodids. Canadian Journal of Fisheries and Aquatic Sciences. 2006;63:448–456. [Google Scholar]
- Bakke TA, Harris PD. Diseases and parasites in wild Atlantic salmon (Salmo salar) populations. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:247–266. [Google Scholar]
- Beamish RJ, Neville CM, Sweeting RM, Ambers N. Sea lice on adult Pacific salmon in the coastal waters of Central British Columbia, Canada. Fisheries Research. 2005;76:198–208. [Google Scholar]
- Berg AGT, Horsberg TE. Plasma concentrations of emamectin benzoate after Slice (TM) treatments of Atlantic salmon (Salmo salar): differences between fish, cages, sites and seasons. Aquaculture. 2009;288:22–26. [Google Scholar]
- Berland B. Salmon lice on wild salmon (Salmo salar L.) in western Norway. In: Boxshall GA, Defaye D, editors. Pathogens of Wild and Farmed Fish: Sea Lice. West Sussex, UK: Ellis Horwood Ltd; 1993. pp. 179–187. [Google Scholar]
- Berland B, Margolis L. The early history of ‘Lakselus’ and some nomenclatural questions relating to copepod parasites of salmon. Sarsia. 1983;68:281–288. [Google Scholar]
- Bjelland R, Løkke J, Simensen L, Kvenseth PG. Successful survival of wrasse through winter in submersible netcages in a fjord in western Norway. In: Sayer MDJ, Treasurer JW, Costello MJ, editors. Wrasse: Biology and Use in Aquaculture. Oxford, UK: Fishing News Books; 1996. pp. 265–271. [Google Scholar]
- Bjordal Å. Cleaning Symbiosis between Wrasses (Labridae) and Lice Infested Salmon (Salmo salar) in Mariculture. Copenhagan: International Council for the Exploration of the Sea, Committee on Mariculture; 1988a. [Google Scholar]
- Bjordal Å. Rense fisk mot lakselus (Cleanerfish against sea lice) Norsk Fiskeoppdrett. 1988b:37. [Google Scholar]
- Bjordal Å. Sea lice infestation on farmed salmon: possible use of cleaner-fish as an alternative method for de-lousing. Canadian Technical Reports of Fisheries and Aquatic Sciences. 1990;1761:85–89. [Google Scholar]
- Bjørn PA, Finstad B, Asplin L, Skilbrei OT, Nilsen R, Llinares RMS, Boxaspen KK. Rapport fra Havforskningsinstituttet. Bergen, Norway: Havforskningsinstituttet; 2011. Metodeutvikling for overvåking og telling av lakselus på viltlevende laksefisk (Development of Methods for Surveillance of Salmon lice Infestation on Wild Salmonids) p. 92. [Google Scholar]
- Blom G. Fiskeristatistikk for leppefisk – 1998–2010 (Fishery Statistics for Wrasses – (1998–2010) Bergen, Norway: Fiskeridirektoratet; 2010. [Google Scholar]
- Brandal PO, Egidius E. Preliminary report on oral treatment against salmon lice, Lepeophtheirus salmonis, with Neguvon. Aquaculture. 1977;10:177–178. [Google Scholar]
- Brandal PO, Egidius E. Treatment of salmon lice (Lepeophtheirus salmonis Krøyer, 1838) with Neguvon® — description of method and equipment. Aquaculture. 1979;18:183–188. [Google Scholar]
- Brandal PO, Egidius E, Romslo I. Host blood: a major food component for the parasitic copepod Lepeophtheirus salmonis, Kroyeri, 1838 (Crustacea: Caligidae) Norwegian Journal of Zoology. 1976;24:341–343. [Google Scholar]
- Branson EJ, Ronsberg SS, Ritchie G. Efficacy of teflubenzuron (Calicide((R))) for the treatment of sea lice, Lepeophtheirus salmonis (Kroyer 1838), infestations of farmed Atlantic salmon (Salmo salar L.) Aquaculture Research. 2000;31:861–867. [Google Scholar]
- Bravo S, Sevatdal S, Horsberg TE. Sensitivity assessment of Caligus rogercresseyi to emamectin benzoate in Chile. Aquaculture. 2008;282:7–12. [Google Scholar]
- Bricknell IR, Dalesman SJ, O'shea B, Pert CC, Luntz AJM. Effect of environmental salinity on sea lice Lepeophtheirus salmonis settlement success. Diseases of Aquatic Organisms. 2006;71:201–212. doi: 10.3354/dao071201. [DOI] [PubMed] [Google Scholar]
- Bron JE, Sommerville C, Rae GH. Aspects of the behaviour of copepodid larvae of the salmon louse Lepeophtheirus salmonis (Krøyer, 1837) In: Boxshall GA, Defaye D, editors. Pathogens of Wild and Farmed Fish: Sea Lice. New York: Ellis Horwood; 1993. pp. 125–142. [Google Scholar]
- Brooks KM, Jones SRM. Perspectives on pink salmon and sea lice: scientific evidence fails to support the extinction hypothesis. Reviews in Fisheries Science. 2008;16:403–412. [Google Scholar]
- Butler JRA. Wild salmonids and sea louse infestations on the west coast of Scotland: sources of infection and implications for the management of marine salmon farms. Pest Management Science. 2002;58:595–608. doi: 10.1002/ps.490. [DOI] [PubMed] [Google Scholar]
- Carpio Y, Basabe L, Acosta J, Rodriguez A, Mendoza A, Lisperger A, Zamorano E, Gonzalez M, Rivas M, Contreras S, Haussmann D, Figueroa J, Osorio VN, Asencio G, Mancilla J, Ritchie G, Borroto C, Estrada MP. Novel gene isolated from Caligus rogercresseyi: a promising target for vaccine development against sea lice. Vaccine. 2011;29:2810–2820. doi: 10.1016/j.vaccine.2011.01.109. [DOI] [PubMed] [Google Scholar]
- Committee IA. Report of the ICES Advisory Committee, 2011. Copenhagen: International Council for the Exploration of the Sea; 2011. [Google Scholar]
- Copley L, Tierney TD, Kane F, Naughton O, Kennedy S, O'Donohoe P, Jackson D, McGrath D. Sea lice, Lepeophtheirus salmonis and Caligus elongatus, levels on salmon returning to the west coast of Ireland, 2003. Journal of the Marine Biological Association of the United Kingdom. 2005;85:87–92. [Google Scholar]
- Costello MJ. The global economic cost of sea lice to the salmonid farming industry. Journal of Fish Diseases. 2009;32:115–118. doi: 10.1111/j.1365-2761.2008.01011.x. [DOI] [PubMed] [Google Scholar]
- Costello MJ, Burridge L, Chang B, Robichaud L. Sea Lice 2003 – Proceedings of the sixth international conference on sea lice biology and control. Aquaculture Research. 2004;35:711–712. [Google Scholar]
- Costelloe M, Costelloe J, Coghlan N, O'Donohoe G, O'connor B. Distribution of the larval stages of Lepeophtheirus salmonis in three bays on the west coast of Ireland. ICES Journal of Marine Science. 1998;55:181–187. [Google Scholar]
- Dalvin S, Frost P, Biering E, Hamre LA, Eichner C, Krossoy B, Nilsen F. Functional characterisation of the maternal yolk-associated protein (LsYAP) utilising systemic RNA interference in the salmon louse (Lepeophtheirus salmonis) (Crustacea: Copepoda) International Journal for Parasitology. 2009;39:1407–1415. doi: 10.1016/j.ijpara.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Denholm I, Devine GJ, Horsberg TE, Sevatdal S, Fallang A, Nolan DV, Powell R. Analysis and management of resistance to chemotherapeutants in salmon lice, Lepeophtheirus salmonis (Copepoda: Caligidae) Pest Management Science. 2002;58:528–536. doi: 10.1002/ps.482. [DOI] [PubMed] [Google Scholar]
- Eichner C, Frost P, Dysvik B, Jonassen I, Kristiansen B, Nilsen F. Salmon louse (Lepeophtheirus salmonis) transcriptomes during post molting maturation and egg production, revealed using EST-sequencing and microarray analysis. BMC Genomics. 2008;9:126. doi: 10.1186/1471-2164-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland SH, Nedreaas K, Mortensen S, Skiftesvik AB, Agnalt A-L, Durif C, Harkestad LS, Karlsbakk E, Knutsen H, Thangstad T, Jørstad K, Bjordal Å, Gjøsæter J. Kunnskapsstatus leppefisk: Utfordringer i et økende fiskeri (Knowledge status wrasses: challenges in an increased fishery) Fisken og havet. 2010:35. [Google Scholar]
- Fallang A, Ramsay JM, Sevatdal S, Burka JF, Jewess P, Hammell KL, Horsberg TE. Evidence for occurrence of an organophosphate-resistant type of acetylcholinesterase in strains of sea lice (Lepeophtheirus salmonis Kroyer) Pest Management Science. 2004;60:1163–1170. doi: 10.1002/ps.932. [DOI] [PubMed] [Google Scholar]
- Fallang A, Denholm I, Horsberg TE, Williamson MS. Novel point mutation in the sodium channel gene of pyrethroid-resistant sea lice Lepeophtheirus salmonis (Crustacea: Copepoda) Diseases of Aquatic Organisms. 2005;65:129–136. doi: 10.3354/dao065129. [DOI] [PubMed] [Google Scholar]
- Fast MD, Burka JF, Johnson SC, Ross NW. Enzymes released from Lepeophtheirus salmonis in response to mucus from different salmonids. Journal of Parasitology. 2003;89:7–13. doi: 10.1645/0022-3395(2003)089[0007:ERFLSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fast MD, Ross NW, Craft CA, Locke SJ, Mackinnon SL, Johnson SC. Lepeophtheirus salmonis: characterization of prostaglandin E-2 in secretory products of the salmon louse by RP-HPLC and mass spectrometry. Experimental Parasitology. 2004;107:5–13. doi: 10.1016/j.exppara.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Fast MD, Ross NW, Johnson SC. Prostaglandin E-2 modulation of gene expression in an Atlantic salmon (Salmo salar) macrophage-like cell line (SHK-1) Developmental and Comparative Immunology. 2005;29:951–963. doi: 10.1016/j.dci.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Fast MD, Ross NW, Muise DM, Johnson SC. Differential gene expression in Atlantic salmon infected with Lepeophtheirus salmonis. Journal of Aquatic Animal Health. 2006;18:116–127. [Google Scholar]
- Fast MD, Johnson SC, Eddy TD, Pinto D, Ross NW. Lepeophtheirus salmonis secretory/excretory products and their effects on Atlantic salmon immune gene regulation. Parasite Immunology. 2007;29:179–189. doi: 10.1111/j.1365-3024.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- Finstad B, Bjorn PA, Grimnes A, Hvidsten NA. Laboratory and field investigations of salmon lice Lepeophtheirus salmonis (Kroyer) infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture Research. 2000;31:795–803. [Google Scholar]
- Fiskeridirektoratet . Foreløpig statistikk for akvakultur 2011. Bergen, Norway: Fiskeridirektoratet (Directorate of Fisheries); 2012. p. 53. [Google Scholar]
- FOR-2009-08-18-1095 . In: Forskrift om bekjempelse av lus i akvakulturanlegg (luseforskriften) (Directive on Prevention of Sea Lice in Aquaculture) Fiskeri- og Kystdepartementet (Ministry of Fisheries and Coastal Affairs), editor. Oslo, Norway: 2009. http://www.lovdata.no/cgi-wift/wiftldles?doc=/app/gratis/www/docroot/for/sf/fi/fi-20090818-1095.html. [Google Scholar]
- Gargan PG, Forde G, Hazon N, Russell DJF, Todd CD. Evidence for sea lice-induced marine mortality of Atlantic salmon (Salmo salar) in western Ireland from experimental releases of ranched smolts treated with emamectin benzoate. Canadian Journal of Fisheries and Aquatic Sciences. 2012;69:343–353. [Google Scholar]
- Gharbi K, Glover KA, Stone LC, Macdonald ES, Matthews L, Grimholt U, Stear MJ. Genetic dissection of MHC-associated susceptibility to Lepeophtheirus salmonis in Atlantic salmon. BMC Genetics. 2009;10:20. doi: 10.1186/1471-2156-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerde B, Odegard J, Thorland I. Estimates of genetic variation in the susceptibility of Atlantic salmon (Salmo salar) to the salmon louse Lepeophtheirus salmonis. Aquaculture. 2011;314:66–72. [Google Scholar]
- Glover KA, Hamre LA, Skaala O, Nilsen F. A comparison of sea louse (Lepeophtheirus salmonis) infection levels in farmed and wild Atlantic salmon (Salmo salar L.) stocks. Aquaculture. 2004;232:41–52. [Google Scholar]
- Glover KA, Aasmundstad T, Nilsen F, Storset A, Skaala O. Variation of Atlantic salmon families (Salmo salar L.) in susceptibility to the sea lice Lepeophtheirus salmonis and Caligus elongatus. Aquaculture. 2005;245:19–30. [Google Scholar]
- Glover KA, Grimholt U, Bakke HG, Nilsen F, Storset A, Skaala O. Major histocompatibility complex (MHC) variation and susceptibilty to the sea louse Lepeophtheirus salmonis in Atlantic salmon Salmo salar. Diseases of Aquatic Organisms. 2007;76:57–65. doi: 10.3354/dao076057. [DOI] [PubMed] [Google Scholar]
- Glover KA, Samuelsen OB, Skilbrei OT, Boxaspen K, Lunestad BT. Pharmacokinetics of emamectin benzoate administered to Atlantic salmon, Salmo salar L., by intra-peritoneal injection. Journal of Fish Diseases. 2010;33:183–186. doi: 10.1111/j.1365-2761.2009.01099.x. [DOI] [PubMed] [Google Scholar]
- Glover KA, Stolen AB, Messmer A, Koop BF, Torrissen O, Nilsen F. Population genetic structure of the parasitic copepod Lepeophtheirus salmonis throughout the Atlantic. Marine Ecology-Progress Series. 2011;427:161–172. [Google Scholar]
- Grave K, Engelstad M, Søli N. Utilization of dichlorvos and trichlorfon in salmonid farming in Norway during 1981–1988. Acta Veterinaria Scandinavica. 1991;31:1–7. doi: 10.1186/BF03546991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grave K, Horsberg TE, Lunestad BT, Litleskare I. Consumption of drugs for sea lice infestations in Norwegian fish farms: methods for assessment of treatment patterns and treatment rate. Diseases of Aquatic Organisms. 2004;12:3–131. doi: 10.3354/dao060123. [DOI] [PubMed] [Google Scholar]
- Grayson TH, Jenkins PG, Wrathmell AB, Harris JE. Serum responses to the salmon louse, Lepeophtheirus salmonis (Kroyer, 1838), in naturally infected salmonids and immunised rainbow trout, Oncorhynchus mykiss (Walbaum), and rabbits. Fish & Shellfish Immunology. 1991;1:141–155. [Google Scholar]
- Grayson TH, John RJ, Wadsworth S, Greaves K, Cox D, Roper J, Wrathmell AB, Gilpin ML, Harris JE. Immunization of Atlantic salmon against the salmon louse: identification of antigens and effects on louse fecundity. Journal of Fish Biology. 1995;47:85–94. [Google Scholar]
- Guttormsen AG. Input factor sustainability in salmon aquaculture. Marine Resource Economics. 2002;17:91–102. [Google Scholar]
- Hamre LA, Nilsen F. Individual fish tank arrays in studies of Lepeophtheirus salmonis and lice loss variability. Diseases of Aquatic Organisms. 2011;97:47–56. doi: 10.3354/dao02397. [DOI] [PubMed] [Google Scholar]
- Hamre LA, Glover KA, Nilsen F. Establishment and characterisation of salmon louse (Lepeophtheirus salmonis (Kroyer 1837)) laboratory strains. Parasitology International. 2009;58:451–460. doi: 10.1016/j.parint.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Hart JL, Thacker JRM, Braidwood JC, Fraser NR, Matthews JE. Novel cypermethrin formulation for the control of sea lice on salmon (Salmo salar. Veterinary Record. 1997;140:179–181. doi: 10.1136/vr.140.7.179. [DOI] [PubMed] [Google Scholar]
- Hayward CJ, Andrews M, Nowak BF. Introduction: Lepeoptheirus salmonis – a remarkable success story. In: Jones S, Beamish R, editors. Salmon Lice: An Integrated Approach to Understanding Parasite Abundance and Distribution. Chichester, UK: John Wiley & Sons Ltd; 2011. pp. 1–28. [Google Scholar]
- Helgesen KO, Horsberg TE. Single dose field bioassay for sensitivity testing in sea lice (Lepeophtheirus salmonis): development of a rapid diagnostic tool. Journal of Fish Diseases. 2012 doi: 10.1111/jfd.12053. (in press) [DOI] [PubMed] [Google Scholar]
- Hesthagen T, Hansen LP. Estimates of the annual loss of Atlantic salmon, Salmo salar L., in Norway due to acidification. Aquaculture and Fisheries Management. 1991;22:85–91. [Google Scholar]
- Heuch PA. Experimental evidence for aggregation of salmon louse copepodids (Lepeophtheirus salmonis) in step salinity gradients. Journal of the Marine Biological Association of the United Kingdom. 1995;75:927–939. [Google Scholar]
- Heuch PA, Karlsen HE. Detection of infrasonic water oscillations by copepodids of Lepeophtheirus salmonis (Copepoda: Caligida) Journal of Plankton Research. 1997;19:735–747. [Google Scholar]
- Heuch PA, Parsons A, Boxaspen K. Diel vertical migration – a possible host-finding mechanism in salmon louse (Lepeophtheirus salmonis) copepodids. Canadian Journal of Fisheries and Aquatic Sciences. 1995;52:681–689. [Google Scholar]
- Heuch PA, Bjorn PA, Finstad B, Holst JC, Asplin L, Nilsen F. A review of the Norwegian ‘national action plan against salmon lice on salmonids’: the effect on wild salmonids (vol 246, pg 79, 2005) Aquaculture. 2005;250:535. [Google Scholar]
- Heuch PA, Olsen RS, Malkenes R, Revie CW, Gettinby G, Baillie M, Lees F, Finstad B. Temporal and spatial variations in lice numbers on salmon farms in the Hardanger fjord 2004–06. Journal of Fish Diseases. 2009;32:89–100. doi: 10.1111/j.1365-2761.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- Heumann J, Carmichael S, Bron JE, Tildesley A, Sturm A. Molecular cloning and characterisation of a novel P-glycoprotein in the salmon louse Lepeophtheirus salmonis. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 2012;155:198–205. doi: 10.1016/j.cbpc.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Holm M, Holst JC, Hansen LP. Spatial and temporal distribution of post-smolts of Atlantic salmon (Salmo salar L.) in the Norwegian Sea and adjacent areas. ICES Journal of Marine Science. 2000;57:955–964. [Google Scholar]
- Holst JC, Jakobsen P, Nilsen F, Holm M, Asplin L, Aure J. Mortality of seaward-migrating post-smolt of Atlantic salmon due to lice infection in Norwegian salmon stock. In: Mills D, editor. Salmon at the Edge. Oxford: Blackwell Publishing; 2003. pp. 136–137. [Google Scholar]
- Horsberg TE. Avermectin use in aquaculture. Current Pharmaceutical Biotechnology. 2012;13:1095–1102. doi: 10.2174/138920112800399158. [DOI] [PubMed] [Google Scholar]
- Horsberg TE, Hoy T. Tissue distribution of 14C-diflubenzuron in Atlantic salmon (Salmo salar. Acta Veterinaria Scandinavica. 1991;32:527–533. doi: 10.1186/BF03546954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidsten NA, Finstad B, Kroglund F, Johnsen BO, Strand R, Arnekleiv JV, Bjorn PA. Does increased abundance of sea lice influence survival of wild Atlantic salmon post-smolt? Journal of Fish Biology. 2007;71:1639–1648. [Google Scholar]
- ICES. Report of the ICES Advisory Committee, 2011. Copenhagen: International Council for the Exploration of the Sea; 2011. [Google Scholar]
- Jackson D. Ireland: the development of sea lice management methods. In: Jones S, Beamish R, editors. Salmon Lice: An Integrated Approach to Understanding Parasite Abundance and Distribution. Oxford, UK: Wiley-Blackwell; 2011. pp. 177–203. [Google Scholar]
- Jackson D, Minchin D. Aspects of the reproductive output of two caligid copepod species parasitic on cultivated salmon. Invertebrate Reproduction & Development. 1992;22:87–90. [Google Scholar]
- Jackson D, Deady S, Leahy Y, Hassett D. Variations in parasitic caligid infestations on farmed salmonids and implications for their management. ICES Journal of Marine Science. 1997;54:1104–1112. [Google Scholar]
- Jackson D, Hassett D, Deady S, Leahy Y. Lepeophtheirus salmonis (Copepoda: Caligidae) on farmed salmon in Ireland. Contributions to Zoology. 2000;69:71–77. [Google Scholar]
- Jackson D, Hassett D, Copley L. Integrated lice management on Irish salmon farms. Fish Veterinary Journal. 2002;6:28–38. [Google Scholar]
- Jackson D, Cotter D, Omaoileidigh N, O'Donohoe P, White J, Kane F, Kelly S, McDermott T, McEvoy S, Drumm A, Cullen A. Impact of early infestation with the salmon louse Lepeophtheirus salmonis on the subsequent survival of outwardly migrating Atlantic salmon smolts from a number of rivers on Ireland's south and west coasts. Aquaculture. 2011a;319:37–40. [Google Scholar]
- Jackson D, Cotter D, Omaoileidigh N, O'Donohoe P, White J, Kane F, Kelly S, McDermott T, McEvoy S, Drumm A, Cullen A, Rogan G. An evaluation of the impact of early infestation with the salmon louse Lepeophtheirus salmonis on the subsequent survival of outwardly migrating Atlantic salmon, Salmo salar L., smolts. Aquaculture. 2011b;320:159–163. [Google Scholar]
- Jackson D, Cotter D, Newel J, McEvoy S, O'Donohoe P, Kane F, McDermott T, Kelly S, Drumm A. Impact of Lepeophtheirus salmonis infestations on migrating Atlantic salmon smolts at eight locations in Ireland with an analysis of lice-induced marine mortality. Journal of Fish Diseases. 2012 doi: 10.1111/jfd.12054. doi: 10.1111/jfd.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JA, Gaard E. Open-ocean infestation by salmon lice (Lepeophtheirus salmonis): comparison of wild and escaped farmed Atlantic salmon (Salmo salar L.) ICES Journal of Marine Science. 1997;54:1113–1119. [Google Scholar]
- Jakobsen PJ, Holm JC. Lovende forsøk med nytt middel mot lakselus [Promising tests with a new agent against salmon lice] Norsk Fiskeoppdrett. 1990:16–18. [Google Scholar]
- Jansen PA, Kristoffersen AB, Viljugrein H, Jimenez D, Aldrin M, Stien A. Sea lice as a density-dependent constraint to salmonid farming. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2012;279:2314–2322. doi: 10.1098/rspb.2012.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen A. Lakselus [Salmon lice] Fisken og Havet Ser B. 1974;2:21–31. [Google Scholar]
- Johannessen A. Early stages of Lepeophtheirus salmonis (Copepoda, Caligidae) Sarsia. 1978;63:169–176. [Google Scholar]
- Johansen LH, Jensen I, Mikkelsen H, Bjørn PA, Jansen PA, Bergh O. Disease interaction and pathogens exchange between wild and farmed fish populations with special reference to Norway. Aquaculture. 2011;315:167–186. [Google Scholar]
- Johnson SC, Albright LJ. Development, growth, and survival of Lepeophtheirus salmonis (Kroyer, 1837) (Copepoda: Caligidae) under laboratory conditions. Journal of the Marine Biological Association of the United Kingdom. 1991;71:425–436. [Google Scholar]
- Johnson SC, Albright LJ. Comparative susceptibility and histopathology of the response of naive Atlantic. Chinook and coho salmon to experimental infection with Lepeophtheirus salmonis (Copepoda: Caligidae) Diseases of Aquatic Organisms. 1992;14:179–193. [Google Scholar]
- Johnson SC, Blaylock RB, Elphick J, Hyatt KD. Disease induced by the sea louse (Lepeophtheirus salmonis) (Copepoda: Caligidae) in wild sockeye salmon (Oncorhynchus nerka) stocks of Alberni Inlet, British Columbia. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:2888–2897. [Google Scholar]
- Johnson SC, Treasurer JW, Bravo S, Nagasawa K, Kabata Z. A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies. 2004;43:229–243. [Google Scholar]
- Jones SRM, Beamish RJ. Lepeophtheirus salmonis on salmonids in the northeast Pacific Ocean. In: Jones S, Beamish R, editors. Salmon Lice: An Integrated Approach to Understanding Parasite Abundance and Distribution. Chichester, UK: John Wiley & Sons, Ltd; 2011. pp. 307–329. [Google Scholar]
- Jones SRM, Hargreaves NB. Infection threshold to estimate Lepeophtheirus salmonis-associated mortality among juvenile pink salmon. Diseases of Aquatic Organisms. 2009;84:131–137. doi: 10.3354/dao02043. [DOI] [PubMed] [Google Scholar]
- Jones MW, Sommerville C, Wootten R. Reduced sensitivity of the salmon louse, Lepeophtheirus salmonis, to the organophosphate dichlorvos. Journal of Fish Diseases. 1992;15:197–202. [Google Scholar]
- Jones SRM, Fast MD, Johnson SC, Groman DB. Differential rejection of salmon lice by pink and chum salmon: disease consequences and expression of proinflammatory genes. Diseases of Aquatic Organisms. 2007;75:229–238. doi: 10.3354/dao075229. [DOI] [PubMed] [Google Scholar]
- Jones S, Kim E, Bennett W. Early development of resistance to the salmon louse, Lepeophtheirus salmonis (Kroyer), in juvenile pink salmon, Oncorhynchus gorbuscha (Walbaum) Journal of Fish Diseases. 2008a;31:591–600. doi: 10.1111/j.1365-2761.2008.00933.x. [DOI] [PubMed] [Google Scholar]
- Jones SRM, Fast MD, Johnson SC. Influence of reduced feed ration on Lepeophtheirus salmonis infestation and inflammatory gene expression in juvenile pink salmon. Journal of Aquatic Animal Health. 2008b;20:103–109. doi: 10.1577/H07-014.1. [DOI] [PubMed] [Google Scholar]
- Kabata Z. Parasitic Copepoda of British Fishes. London, UK: The Ray Society, British Museum; 1979. [Google Scholar]
- Kolstad K, Heuch PA, Gjerde B, Gjedrem T, Salte R. Genetic variation in resistance of Atlantic salmon (Salmo salar) to the salmon louse Lepeophtheirus salmonis. Aquaculture. 2005;247:145–151. [Google Scholar]
- Krkosek M, Lewis MA, Volpe JP. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proceedings of the Royal Society of London. Series B, Biological Sciences. 2005;272:689–696. doi: 10.1098/rspb.2004.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkosek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA. Declining wild salmon populations in relation to parasites from farm salmon. Science. 2007;318:1772–1775. doi: 10.1126/science.1148744. [DOI] [PubMed] [Google Scholar]
- Krkosek M, Connors BM, Morton A, Lewis MA, Dill LM, Hilborn R. Effects of parasites from salmon farms on productivity of wild salmon. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14700–14704. doi: 10.1073/pnas.1101845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees F, Baillie M, Gettinby G, Revie CW. The efficacy of emamectin benzoate against infestations of Lepeophtheirus salmonis on farmed Atlantic Salmon (Salmo salar L) in Scotland, 2002–2006. PLoS ONE. 2008a;3:e1549. doi: 10.1371/journal.pone.0001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees F, Gettinby G, Revie CW. Changes in epidemiological patterns of sea lice infestation on farmed Atlantic salmon, Salmo salar L., in Scotland between 1996 and 2006. Journal of Fish Diseases. 2008b;31:259–268. doi: 10.1111/j.1365-2761.2007.00897.x. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Olaussen JO, Skonhoft A. Wild and farmed salmon in Norway-a review. Marine Policy. 2011;35:413–418. [Google Scholar]
- Maritz-Olivier C, Van Zyl W, Stutzer C. A systematic, functional genomics, and reverse vaccinology approach to the identification of vaccine candidates in the cattle tick, Rhipicephalus microplus. Ticks and Tick-borne Diseases. 2012;3:179–187. doi: 10.1016/j.ttbdis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Marshall S. The incidence of sea lice infestations on wild sea trout compared to farmed salmon. Bulletin of the European Association of Fish Pathologists. 2003;23:72–79. [Google Scholar]
- Marty GD, Saksida SM, Quinn TJ. Relationship of farm salmon, sea lice, and wild salmon populations. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22599–22604. doi: 10.1073/pnas.1009573108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay SJ, Devlin RH, Smith MJ. Phylogeny of Pacific salmon and trout based on growth hormone type-2 and mitochondrial NADH dehydrogenase subunit 3 DNA sequences. Canadian Journal of Fisheries and Aquatic Sciences. 1996;53:1165–1176. [Google Scholar]
- McVicar AH. Management actions in relation to the controversy about salmon lice infections in fish farms as a hazard to wild salmonid populations. Aquaculture Research. 2004;35:751–758. [Google Scholar]
- Mennerat A, Nilsen F, Ebert D, Skorping A. Intensive farming: evolutionary implications for parasites and pathogens. Evolutionary Biology. 2010;37:59–67. doi: 10.1007/s11692-010-9089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerat A, Hamre L, Ebert D, Nilsen F, Davidova M, Skorping A. Life history and virulence are linked in the ectoparasitic salmon louse Lepeophtheirus salmonis. Journal of Evolutionary Biology. 2012;25:856–861. doi: 10.1111/j.1420-9101.2012.02474.x. [DOI] [PubMed] [Google Scholar]
- Messmer AM, Rondeau EB, Jantzen SG, Lubieniecki KP, Davidson WS, Koop BF. Assessment of population structure in Pacific Lepeophtheirus salmonis (Kroyer) using single nucleotide polymorphism and microsatellite genetic markers. Aquaculture. 2011;320:183–192. [Google Scholar]
- Morton A, Routledge R, Peet C, Ladwig A. Sea lice (Lepeophtheirus salmonis) infection rates on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon in the nearshore marine environment of British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:147–157. [Google Scholar]
- Muncaster S. Reproductive Physiology of Ballan Wrasse (Labrus bergylta) Bergen, Norway: University of Bergen; 2008. [Google Scholar]
- Nagasawa K. Prevalence and abundance of Lepeophtheirus salmonis (Copepoda, Caligidae) on high-seas salmon and trout in the north Pacific-Ocean. Nippon Suisan Gakkaishi. 1987;53:2151–2156. [Google Scholar]
- Nagasawa K. Annual changes in the population size of the salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae) on high-seas Pacific Salmon (Oncorhynchus spp.), and relationship to host abundance. Hydrobiologia. 2001;453:411–416. [Google Scholar]
- NASCO. Report of the Twenty-Eighth Annual Meeting of the Council. Ilulissat, Greenland: North Atlantic Salmon Conservation Organization; 2011. pp. 1–349. [Google Scholar]
- Nendick L, Sackville M, Tang S, Brauner CJ, Farrell AP. Sea lice infection of juvenile pink salmon (Oncorhynchus gorbuscha): effects on swimming performance and postexercise ion balance. Canadian Journal of Fisheries and Aquatic Sciences. 2011;68:241–249. [Google Scholar]
- Noakes DJ, Beamish RJ. Shifting the balance: towards sustainable salmon populations and fisheries of the future. In: Taylor WW, Lynch AJ, Schechter MGMG, editors. Sustainable Fisheries: Multi-Level Approaches to a Global Problem. Bethesda, MD, USA: American Fisheries Society; 2011. pp. 23–50. [Google Scholar]
- O'Donohoe P, Kane F, Kelly S, McDermott T, Drumm A, Jackson D. National survey of Sea lice (Lepeophtheirus salmonis Krøyer and Caligus elongatus Nordmann) on Fish Farms in ireland – 2010. Irish Fisheries Bulletin. 2011;34:1–37. [Google Scholar]
- Olavsen T, Sandberg M, Henriksen K, Bull-Berg H, Johansen U, Stokka A. SINTEF Report. Trondheim, Norway: SINTEF; 2011. Betydningen av fiskeri- og havbruksnæringen for Norge i 2009 – En nasjonal og regional ringvirkningsanalyse (In Norwegian: The impact of the fishing and aquaculture industry in Norway in 2009 – A national and regional analysis of multiplicator effect) [Google Scholar]
- Olaussen JO, Liu YJ. On the willingness-to-pay for recreational fishing: escaped farmed versus wild Atlantic salmon. Aquaculture Economics & Management. 2011;15:245–261. [Google Scholar]
- Otero J, Jensen AJ, L'abee-Lund JH, Stenseth NC, Storvik GO, Vollestad LA. Quantifying the ocean, freshwater and human effects on year-to-year variability of one-sea-winter Atlantic salmon angled in multiple Norwegian rivers. PLoS ONE. 2011;6:e24005. doi: 10.1371/journal.pone.0024005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R, Rodger H, Drinan E, Dwyer C, Smith PR. Preliminary trials on the efficacy of ivermectin against parasitic copepods of Atlantic salmon. Bulletin of the European Association of Fish Pathologists. 1987;7:47–54. [Google Scholar]
- Penston MJ, Davies IM. An assessment of salmon farms and wild salmonids as sources of Lepeophtheirus salmonis (Kroyer) copepodids in the water column in Loch Torridon, Scotland. Journal of Fish Diseases. 2009;32:75–88. doi: 10.1111/j.1365-2761.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- Penston MJ, Millar CP, Zuur A, Davies IM. Spatial and temporal distribution of Lepeophtheirus salmonis (Kroyer) larvae in a sea loch containing Atlantic salmon, Salmo salar L., farms on the north-west coast of Scotland. Journal of Fish Diseases. 2008;31:361–371. doi: 10.1111/j.1365-2761.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- Quignard JP, Pras A. Labridae. In: Whitehead PJP, Bauchot ML, Hureau JC, Nielsen J, Tortonese E, editors. Fishes of the North-Eastern Atlantic and the Mediterranean. Paris, France: UNESCO; 1986. pp. 919–942. [Google Scholar]
- Rae GH. On the trail of the sea lice. Fish Farmer. 1979;2:22–25. [Google Scholar]
- Raynard RS, Bricknell IR, Billingsley PF, Nisbet AJ, Vigneau A, Sommerville C. Development of vaccines against sea lice. Pest Management Science. 2002;58:569–575. doi: 10.1002/ps.474. [DOI] [PubMed] [Google Scholar]
- Reilly P, Mulcahy MF. Humoral antibody response in Atlantic salmon (Salmo salar L.) immunised with extracts derived from the ectoparasitic caligid copepods, Caligus elongatus (Nordmann, 1832) and Lepeophtheirus salmonis (Kroyer, 1838) Fish & Shellfish Immunology. 1993;3:59–70. [Google Scholar]
- Rikardsen AH. Seasonal occurrence of sea lice Lepeophtheirus salmonis on sea trout in two north Norwegian fjords. Journal of Fish Biology. 2004;65:711–722. [Google Scholar]
- Roberts TR, Hutson DH. Metabolic Pathways of Agrochemicals. Part 2: Insecticides and Fungicides. Camebridge, UK: The Royal Society of Chemistry; 1999. [Google Scholar]
- Roper J, Grayson TH, Jenkins PG, Hone JV, Wrathmell AB, Russell PM, Harris JE. The immunocytochemical localisation of potential candidate vaccine antigens from the salmon louse Lepeophtheirus salmonis (Kroyer 1837) Aquaculture. 1995;132:221–232. [Google Scholar]
- Roth M. The availability and use of chemotherapeutic sea lice control products. Contributions to Zoology. 2000;69:109–118. [Google Scholar]
- Roth M, Richards RH, Dobson DP, Rae GH. Field trials on the efficacy of the organophosphorus compound azamethiphos for the control of sea lice (Copepoda: Caligidae) infestations of farmed Atlantic salmon (Salmo salar. Aquaculture. 1996;140:217–239. [Google Scholar]
- Sackville M, Tang S, Nendick L, Farrell AP, Brauner CJ. Pink salmon (Oncorhynchus gorbuscha) osmoregulatory development plays a key role in sea louse (Lepeophtheirus salmonis) tolerance. Canadian Journal of Fisheries and Aquatic Sciences. 2011;68:1087–1096. [Google Scholar]
- Saksida S, Constantine J, Karreman GA, Donald A. Evaluation of sea lice abundance levels on farmed Atlantic salmon (Salmo salar L.) located in the Broughton Archipelago of British Columbia from 2003 to 2005. Aquaculture Research. 2007a;38:219–231. [Google Scholar]
- Saksida S, Karreman GA, Constantine J, Donald A. Differences in Lepeophtheirus salmonis abundance levels on Atlantic salmon farms in the Broughton Archipelago, British Columbia, Canada. Journal of Fish Diseases. 2007b;30:357–366. doi: 10.1111/j.1365-2761.2007.00814.x. [DOI] [PubMed] [Google Scholar]
- Saksida SM, Morrison D, Revie CW. The efficacy of emamectin benzoate against infestations of sea lice, Lepeophtheirus salmonis, on farmed Atlantic salmon, Salmo salar L., in British Columbia. Journal of Fish Diseases. 2010;33:913–917. doi: 10.1111/j.1365-2761.2010.01192.x. [DOI] [PubMed] [Google Scholar]
- Saksida SM, Greba L, Morrison D, Revie CW. Sea lice on wild juvenile Pacific salmon and farmed Atlantic salmon in the northernmost salmon farming region of British Columbia. Aquaculture. 2011;320:193–198. [Google Scholar]
- Sevatdal S, Horsberg TE. Determination of reduced sensitivity in sea lice (Lepeophtheirus salmonis Krøyer) against the pyrethroid deltamethrin using bioassays and probit modelling. Aquaculture. 2003;218:21–31. [Google Scholar]
- Sevatdal S, Copley L, Wallace C, Jackson D, Horsberg TE. Monitoring of the sensitivity of sea lice (Lepeophtheirus salmonis) to pyrethroids in Norway, Ireland and Scotland using bioassays and probit modelling. Aquaculture. 2005a;244:19–27. [Google Scholar]
- Sevatdal S, Fallang A, Ingebrigtsen K, Horsberg TE. Monooxygenase mediated pyrethroid detoxification in sea lice (Lepeophtheirus salmonis. Pest Management Science. 2005b;61:772–778. doi: 10.1002/ps.1057. [DOI] [PubMed] [Google Scholar]
- Skern-Mauritzen R, Malde K, Furmanec T, Reinhardt R, Koop BF, Nilsen F. Sea Lice 2012. Bergen, Norway: 2012. The Salmon Louse Genome Project. [Google Scholar]
- Skilbrei OT, Wennevik V. Survival and growth of sea-ranched Atlantic salmon, Salmo salar L., treated against sea lice before release. ICES Journal of Marine Science. 2006;63:1317–1325. [Google Scholar]
- Skilbrei OT, Glover KA, Samuelsen OB, Lunestad BT. A laboratory study to evaluate the use of emamectin benzoate in the control of sea lice in sea-ranched Atlantic salmon (Salmo salar L.) Aquaculture. 2008;285:2–7. [Google Scholar]
- Skilbrei OT, Holst JC, Asplin L, Holm M. Vertical movements of “escaped” farmed Atlantic salmon (Salmo salar L.)-025EFa simulation study in a western Norwegian fjord. ICES Journal of Marine Science. 2009;66:278–288. [Google Scholar]
- Skilbrei OT, Finstad B, Urdal K, Bakke G, Kroglund F, Strand R. Impact of early salmon louse (Lepeophtheirus salmonis) infestation, and differences in survival and marine growth of sea ranched Atlantic salmon (Salmo salar) smolts 1997–2009. Journal of Fish Diseases. 2012;36:249–260. doi: 10.1111/jfd.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skugor S, Glover KA, Nilsen F, Krasnov A. Local and systemic gene expression responses of Atlantic salmon (Salmo salar L.) to infection with the salmon louse (Lepeophtheirus salmonis. BMC Genomics. 2008;9:498. doi: 10.1186/1471-2164-9-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Roheim CA, Crowder LB, Halpern BS, Turnipseed M, Anderson JL, Asche F, Bourillon L, Guttormsen AG, Khan A, Liguori LA, McNevin A, O'connor MI, Squires D, Tyedmers P, Brownstein C, Carden K, Klinger DH, Sagarin R, Selkoe KA. Sustainability and global seafood. Science. 2010;327:784–786. doi: 10.1126/science.1185345. [DOI] [PubMed] [Google Scholar]
- Stien A, Bjorn PA, Heuch PA, Elston DA. Population dynamics of salmon lice Lepeophtheirus salmonis on Atlantic salmon and sea trout. Marine Ecology-Progress Series. 2005;290:263–275. [Google Scholar]
- Stone J, Sutherland IH, Sommerville C, Richards RH, Endris RG. The duration of efficacy following oral treatment with emamectin benzoate against infestations of sea lice, Lepeophtheirus salmonis (Kroyer), in Atlantic salmon Salmo salar L. Journal of Fish Diseases. 2000a;23:185–192. [Google Scholar]
- Stone J, Sutherland IH, Sommerville C, Richards RH, Varma KJ. Field trials to evaluate the efficacy of emamectin benzoate in the control of sea lice, Lepeophtheirus salmonis (Kroyer) and Caligus elongatus Nordmann, infestations in Atlantic salmon Salmo salar L. Aquaculture. 2000b;186:205–219. [Google Scholar]
- Stucchi DJ, Guo M, Foreman MGG, Czajko P, Galbraith M, Mackas DL, Gillibrand PA. Modeling sea lice production and concentrations in the Broughton Archipelago, British Columbia. In: Jones S, Beamish R, editors. Salmon Lice: An Integrated Approach to Understanding Parasite Abundance and Distribution. Chichester, UK: John Wiley & Sons, Ltd; 2011. pp. 117–150. [Google Scholar]
- Sutherland BJG, Jantzen SG, Sanderson DS, Koop BF, Jones SRM. Differentiating size-dependent responses of juvenile pink salmon (Oncorhynchus gorbuscha) to sea lice (Lepeophtheirus salmonis) infections. Comparative Biochemistry and Physiology D: Genomics & Proteomics. 2011;6:213–223. doi: 10.1016/j.cbd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sutherland BJG, Jantzen SG, Yasuike M, Sanderson DS, Koop BF, Jones SRM. Transcriptomics of coping strategies in free-swimming Lepeophtheirus salmonis (Copepoda) larvae responding to abiotic stress. Molecular Ecology. 2012 doi: 10.1111/mec.12072. doi: 10.1111/mec.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadiso TM, Krasnov A, Skugor S, Afanasyev S, Hordvik I, Nilsen F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genomics. 2011;12:141. doi: 10.1186/1471-2164-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranger GL, Svåsand T, Bjørn PA, Jansen PA, Heuch PA, Grøntvedt RN, Asplin L, Skilbrei OT, Glover K, Skaala Ø, Wennevik V, Boxaspen KK. Forslag til førstegenerasjons målemetode for miljøeffekt (effektindikatorer) med hensyn til genetisk påvirkning fra oppdrettsfisk til villaks, og påvirkning av lakselus fra oppdrett på viltlevende laksebestander. (Suggestions for a first generation indicators for environmental impacts of salmon farming with respect to genetical influence and effect of sea lice on wild salmon populations) 2012. pp. 1–40. Rapport fra Havforskningsinstituttet.
- The Norwegian Institute of Public Health. Forbruk av legemidler i norsk fiskeoppdrett i 2001–2008 (Consumption of Pharmaceuticals in Norwegian Aquaculture 2001–2008) The Norwegian Institute of Public Health; 2012. [Google Scholar]
- Thomassen JM. Hydrogen peroxide as a delousing agent for Atlantic salmon. In: Boxshall GA, Defaye D, editors. Pathogens of Wild and Farmed Fish: Sea Lice. New York, USA: Ellis Horwood; 1993. pp. 290–295. [Google Scholar]
- Tjensvoll K, Glover KA, Nylund A. Sequence variation in four mitochondrial genes of the salmon louse Lepeophtheirus salmonis. Diseases of Aquatic Organisms. 2006;68:251–259. doi: 10.3354/dao068251. [DOI] [PubMed] [Google Scholar]
- Todd CD, Walker AM, Hoyle JE, Northcott SJ, Walker AF, Ritchie MG. Infestations of wild adult Atlantic salmon (Salmo salar L.) by the ectoparasitic copepod sea louse Lepeophtheirus salmonis Kroyer: prevalence, intensity and the spatial distribution of males and females on the host fish. Hydrobiologia. 2000;429:181–196. [Google Scholar]
- Todd CD, Walker AM, Ritchie MG, Graves JA, Walker AF. Population genetic differentiation of sea lice (Lepeophtheirus salmonis) parasitic on Atlantic and Pacific salmonids: analyses of microsatellite DNA variation among wild and farmed hosts. Canadian Journal of Fisheries and Aquatic Sciences. 2004;61:1176–1190. [Google Scholar]
- Torrissen O, Olsen RE, Toresen R, Hemre GI, Tacon AGJ, Asche F, Hardy RW, Lall S. Atlantic salmon (Salmo salar): the “super-chicken” of the sea? Reviews in Fisheries Science. 2011;19:257–278. [Google Scholar]
- Tribble ND, Burka JF, Kibenge FSB. Evidence for changes in the transcription levels of two putative P-glycoprotein genes in sea lice (Lepeophtheirus salmonis) in response to emamectin benzoate exposure. Molecular and Biochemical Parasitology. 2007;153:59–65. doi: 10.1016/j.molbiopara.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Trudel M, Jones SRM, Thiess ME, Morris JFT, Welch DW, Sweeting RM, Moss JH, Wing BL, Farley EV, Murphy JM. Infestations of motile salmon lice on Pacific salmon along the west coast of North America. In: Grimes CB, Brodeur RD, Haldorsen LJ, McKinnell SM, editors. The Ecology of juvenile salmon in the Northeast Pacific ocean: regional comparisons. Bethesda, MD: Published by the American Fisheries Society; 2007. pp. 157–182. Symposium 57. [Google Scholar]
- Tucker CS, Sommerville C, Wootten R. An investigation into the larval energetics and settlement of the sea louse, Lepeophtheirus salmonis, an ectoparasitic copepod of Atlantic salmon, Salmo salar. Fish Pathology. 2000;35:137–143. [Google Scholar]
- Tucker CS, Sommerville C, Wootten R. Does size really matter? Effects of fish surface area on the settlement and initial survival of Lepeophtheirus salmonis, an ectoparasite of Atlantic salmon Salmo salar. Diseases of Aquatic Organisms. 2002;49:145–152. doi: 10.3354/dao049145. [DOI] [PubMed] [Google Scholar]
- Tully O, Whelan KF. Production of nauplii of Lepeophtheirus salmonis (Krøyer) (Copepoda: Caligidae) from farmed and wild salmon and its relation to the infestation of wild sea trout (Salmo trutta L.) off the west coast of Ireland in 1991. Fisheries Research. 1993;17:187–200. [Google Scholar]
- Tveterås R. Industrial agglomeration and production costs in Norwegian salmon aquaculture. Marine Resource Economics. 2002;17:1–22. [Google Scholar]
- Wagner GN, Fast MD, Johnson SC. Physiology and immunology of Lepeophtheirus salmonis infections of salmonids. Trends in Parasitology. 2008;24:176–183. doi: 10.1016/j.pt.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Westcott JD, Stryhn H, Burka JF, Hammell KL. Optimization and field use of a bioassay to monitor sea lice Lepeophtheirus salmonis sensitivity to emamectin benzoate. Diseases of Aquatic Organisms. 2008;79:119–131. doi: 10.3354/dao01887. [DOI] [PubMed] [Google Scholar]
- White HC. Sea lice (Lepeophtheirus) and death of salmon. Journal of the Fisheries Research Board of Canada. 1940;5:172–175. [Google Scholar]
- Yazawa R, Yasuike M, Leong J, Von Schalburg KR, Cooper GA, Beetz-Sargent M, Robb A, Davidson WS, Jones SRM, Koop BF. EST and mitochondrial DNA sequences support a distinct Pacific form of salmon louse, Lepeophtheirus salmonis. Marine Biotechnology. 2008;10:741–749. doi: 10.1007/s10126-008-9112-y. [DOI] [PubMed] [Google Scholar]


