Abstract
Although much progress has been made regarding our knowledge of stem cells and their potential applications for therapeutic angiogenesis, however, there has been less success with the clinical application of this knowledge to patients with critical limb ischemia (CLI). Interestingly, patients with CLI often have chronic wounds, and newer cell-based therapies for chronic wounds show interesting parallels to stem cell therapy for CLI. Several human-derived wound care products and therapies, including human neonatal fibroblast-derived dermis (Dermagraft®), bilayered bioengineered skin substitute (Apligraf®), recombinant human platelet-derived growth factor, and autologous platelet-rich plasma may provide insight into the mechanisms through which differentiated cells can be used as therapy for chronic wounds, and, analogously, by which stem cells might function therapeutically in CLI.
Keywords: therapeutic angiogenesis, wound healing
Introduction
In 1997, Asahara et al published their landmark paper showing that endothelial progenitor cells (EPCs) were capable of being isolated from human peripheral blood and that they also retain the capacity to differentiate into mature and functional endothelial cells [1]. These findings have significant clinical implications and have generated much optimism for new treatment options for critical limb ischemia (CLI). Although the gold-standard treatment of CLI is surgical or endovascular revascularization of the ischemic limb, considerable numbers of patients do not meet criteria for such treatments and have no options other than pain control or amputation [30]. It has now been over a decade since Asahara’s findings were originally published and, although much progress had been made with regard to our knowledge of stem cells and their role in CLI, reliable alternative treatment modalities have been slow to come to clinical use.
Research investigating stem cell based treatment for CLI has, for the most part, focused on the use of bone marrow (BM) derived cells, and has been supported by clinical evidence. The outcomes of the Therapeutic Angiogenesis using Cell Transplantation (TACT) Study, published in 2002, found significant improvement in rest pain, ankle brachial index (ABI), and healing of ischemic ulcers in legs injected with BM-derived cells as compared to those injected with peripherally-derived cells [36]. Further studies have gone on to show that arteriogenesis, a process mediated by BM-derived progenitor cells, holds the capacity to adequately restore arterial flow in an ischemic limb through the remodeling of arterioles into collateral arteries [3]. A question, however, that remains to be fully answered, is how this cell therapy works, and whether angiogenesis occurs secondary to the paracrine effects of BM-derived cells or because of their physical incorporation.
While more recent studies have tended to favor the paracrine theory, a discrepancy has emerged between outcomes in patients treated with cell therapy and those treated with growth factor and cytokine monotherapy [13]. Studies that have solely focused on progenitor cell recruitment through paracrine approaches and the use of granulocyte-colony stimulating factor (G-CSF) have failed to recreate outcomes obtained from stem cell-based clinical trials [5]. Regardless, the only consistency between these debating sides has been the lack of clinical success for growth factor therapy studies to date [9,34]. These outcomes emphasize the complex role of stem cells and suggest that, in addition to cytokine secretion, there is more to be learned regarding the functions of BM-derived cells in angiogenesis.
Interestingly, several human-derived wound care products and therapies currently used may provide insight into the mechanisms through which BM-derived cells function in CLI. Chronic non-healing wounds pose a difficult dilemma in terms of treatment options. Clinical providers must determine whether the etiology of these wounds is secondary to CLI, diabetes, venous disease, etc., and adjust treatment methods accordingly. The use of BM for limb salvage in patients with CLI-induced non-healing wounds and necrotic tissue has been studied in the TACT trial which documented significant improvements in healing [36]. Although BM-based therapies for chronic wounds have yet to become standardized and available, other human-derived cell-based therapies have emerged as treatment options for chronic wounds. These wound care applications are not stem cell therapies, as they use differentiated cells, yet a closer look at the cellular components and outcomes yields definite parallels.
At present, a wide variety of bioengineered tissues are available for use in wound care and the majority are either derived from human adult or animal cells. The properties and functions of neonatally-derived tissues, however, generate interesting considerations and yield a substantial analogy to a more basic stem cell therapy. Representative examples of such tissues can be found in human neonatal fibroblast-derived dermis (Dermagraft®) and bilayered bioengineered skin substitute (Apligraf®).
Neonatal Fibroblast-Derived Dermis
After gaining FDA approval in 2001, Dermagraft® (Advanced Biohealing, La Jolla, CA), a human neonatal fibroblast-derived dermis (HFDD), found clinical success in application to non-healing chronic wounds. Derived from cryopreserved neonatal foreskin fibroblasts, HFDD is then generated through the in vivo culture of these cells onto a polymer scaffold [22]. Studies investigating the clinical value of this dermal substitute have found it to be effective in achieving closure of chronic wounds not previously responsive to conservative standard treatments such as saline-soaked gauze, pressure offloading, and debridement (Figure 1) [22]. Mechanistically, the properties of dermal substitutes have been well studied, and it has been established that they contribute to dermal and epidermal regeneration through their physical properties as well as their paracrine effects. The success of these dermal substitutes lies in the fact that the cultured fibroblasts largely remain viable and metabolically active once thawed and applied to the wound bed [20,29].
Figure 1.
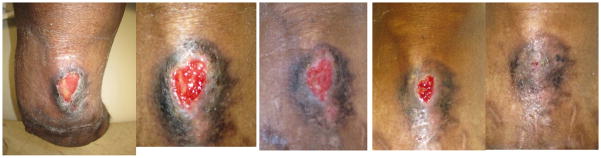
Sequential photographs of a wound unresponsive to conventional dressings treated with human neonatal fibroblast-derived dermis (Dermagraft®); A) At time of presentation, B) 2 weeks, C) 4 weeks, D) 6 weeks, E) 8 weeks
Through topical application, and perhaps analogous to informal stem cell therapy, the HFDD then facilitates deposition of active fibroblasts into the wound base. These fibroblasts generate matrix proteins and a physical scaffold onto which in situ epithelial cells can migrate [17]. Prior studies have found that the composition of this fibroblast-generated matrix is similar in composition to in vivo dermis and consists of types I and III collagen, fibronectin, and glycosaminoglycans (GAGs). These matrix proteins and GAGs facilitate cell migration as well as the binding of critical growth factors that are produced by the HFDD fibroblasts [26].
Proponents of paracrine-mediated BM-therapy have drawn evidence from studies that have documented upregulation of arteriogenesis-related cytokines in hypoxia-induced BM-derived cells and associated proliferation of endothelial and smooth muscles cells [15]. Although fibroblasts were initially thought to largely play a role in remodeling and matrix deposition, we now know that they have an active role in cytokine secretion (Table). Studies of dermal substitutes have shown complimentary data and have found increased mRNA levels of vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in activated neonatal fibroblast cultures [20,29]. Accordingly, it is felt that these proliferative cytokines likewise facilitate the closure of chronic wounds known to be deficient in blood flow.
Table.
Product Comparison of Cytokine Secretion.
| Bone Marrow-Derived Cell Therapy | Human Fibroblast-Derived Dermis | Bilayered Bioengineered Skin Substitute | rhPDGF | Platelet-Rich Plasma | |
|---|---|---|---|---|---|
| Interleukins | |||||
| IL-1 | +++15 | − | +++23,26 | − | +++16 |
| IL-6 | +++12,15 | +++20,28 | ++23 | − | − |
| IL-8 | − | +++20,28 | +++23,26 | − | − |
| Growth Factors | |||||
| HGF | +15 | +++21 | +++26 | − | +++30 |
| TGF-β | +++34 | +++20 | +++23 | − | +++16,30 |
| G-CSF | +++12 | +++12,15 | +++26 | − | − |
| PDGF | ++14 | +++21 | +++26 | +++31 | +++16,30 |
| MCP-1 | ++7 | ++19 | ++26 | − | − |
| FGF | ++14,34 | +21 | ++23,26 | − | +++30 |
| VEGF | +++14,34 | +21 | +++23 | − | ++30 |
Abbreviations: hepatocyte growth factor (HGF), transforming growth factor-beta (TGF-β), granulocyte colony stimulating factor (G-CSF), platelet derived growth factor (PDGF), monocyte chemotactic protein-1(MCP-1), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF)
Human Bilayered Cell Therapy
Similar findings have been likewise been observed in Apligraf® (Organogenesis, MA), a bilayered bioengineered skin substitute (BBSS) that received initial FDA approval in 1998. Much like HFDD, BBSS also derives its cells from culture of neonatal foreskin and is primarily used in treatment of chronic wounds. A distinction regarding BBSS is its recreation of a bilayer through culture and isolation of both fibroblasts and keratinocytes. Sustained viability of these cultures cell lines then allows for the engineering of an allogenic reconstructed fibroblast dermis which is overlaid by a keratinocyte epidermis [28]. This bilayer is then attached to a collagen scaffold which is applied to wound beds. With appreciated similarity to HFDD, review of clinical studies likewise shows BBSS to be effective in the closure of wounds that have failed to respond to conservative treatment options [6].
As has been observed with other skin substitutes and HFDD, the efficacy of engineered tissues relies on their ability to maintain cellular viability once applied. Proposed mechanisms of action for BBSS have included its potential ability to incorporate and adapt as well as its ability to produce cytokines. Studies investigating the characteristics of BBSS have found it to be capable of integrating into the wound site, allowing for vascular ingrowth, and maintaining structural integrity post application [11]. Similar to HFDD, BBSS is believed to facilitate migration and proliferation of surrounding dermal and epidermal cells, and it is felt that both cell types contribute to this function.
Keratinocytes have been shown to be critical in the maintenance phase of wound healing, and a recently published study, attempting to generate a less costly alternative to engineered tissues, documents its use of autologous noncultured epidermal cell suspension to stimulate healing in chronic wounds [33]. Results from this study, although preliminary, illustrate the potency of keratinocytes and their prospective applications for cell-based therapies. Similar to mechanisms that have been documented in BM-therapy for CLI, keratinocytes likewise are capable of recruiting monocytes and accordingly are able to facilitate neovascularization of the wound bed [24]. It has likewise been found that they accelerate extracellular matrix deposition through recruitment of native fibroblasts. Cytokine production that has been found in keratinocytes includes secretion of Fibroblast growth factor (FGF), granulocyte colony-stimulating factor (G-CSF), and platelet-derived growth (PDGF) [24,27]. As noted in the Table, strong similarities exist between cytokine production when comparing engineered tissues and BM-therapy.
Human Platelet-Derived Growth Factor
Alternatively, chronic wound paracrine-mediated therapies, such as recombinant human platelet-derived growth factor (rhPDGF), have chosen to focus on the engineering of topical cytokines. The best recognized example of this has been in the clinical use of Regranex® (Ortho-Mcneil Pharmaceuticals, NJ), a rhPDGF topical gel application that became commercially available in 1997. The importance of PDGF in wound healing and neo-vessel maturation is well recognized, and investigations in the mechanism of topical PDGF have also yielded similarities to BM-therapy and engineered tissues [18,38]. It is felt that that application of rhPDGF acts as an impetus for the stimulation and influx of various cellular mediators including pericytes, macrophages, and fibroblasts [18,32]. Recruitment of pericytes and smooth muscle cells into these ischemic tissues is believed to result in the augmentation of newly formed capillaries that have developed secondary to ischemia-induced angiogenesis [18]. Small clinical trials investing this paracrine monotherapy have gone on to show modest improvements in wound healing [32,38].
The development of this monotherapy however has not yielded tremendous clinical success as many would have expected. Criticisms of topical growth factor monotherapy have drawn on its practical limitations as well as potential concerns regarding its safety. In comparison with engineered tissues, rhPDGF is limited by a short application half-life a well as by its singular modality of action. The benefits ascribed to human-derived engineered tissues lie in their ability to maintain active cellular viability, and the physical presence of metabolically active cells, such as fibroblasts and macrophages, has been shown to be essential in the initiation and sustainability of wound healing. Time limitations on rhPDGF necessitate more frequent applications and affect treatment costs and practicality. These recognized limitations also serve to highlight the importance of cellular presence and viability in successful wound healing [16].
Autologous Platelet-Rich Plasma
Somewhat predictably, the clinical success of platelet-rich plasma (PRP) has fared better. PRP is a human-derived and cell based therapy version of rhPDGF and has been described in maxillofacial and plastic surgical literature since the 1990s [37]. The preparation of PRP varies throughout studies and case reports, however the common basis to all applications lies in the isolation of autologous peripherally-derived platelet concentrates which are suspended in plasma and then activated by thrombin or calcium prior to application. Similar to BM-therapy, the wound healing properties of PRP in tissues has been casually discussed for years; however formal investigations have only recently begun to be initiated. Results from these smaller studies, both human and animal, have provided mixed results with some authors reporting improvements in tissue repair, however, to date there remains a need for larger randomized trials with long-term follow-up [8,25].
Similar to BM-therapy, PRP is delivered locally into affected tissues and is believed to promote healing in ischemic tissues through both cellular and paracrine mechanisms. In comparison to the previously described human-derived tissues, platelet-rich plasma is not an engineered product, but rather, autologously derived through the extraction and fractionation of whole blood; the autologous source of PRP allows it to be a potentially cost-effective alternative in chronic wound care. PRP is believed to exert its beneficial effect through degranulation of alpha granules that contain growth factors that become activated and released once the process of clotting is initiated [23]. Factors specific to wound healing that have been identified in PRP include PDGF, transforming growth factor beta (TGF-β), and VEGF [4]. The activated platelet plug which is delivered in wound beds then acts to maintain growth factor release over the course of days allowing for inflammatory cell recruitment and wound bed remodeling.
PRP appears to functions as a temporary depot for growth factors within areas of tissue injury and initiates cytokine secretion necessary for the recruitment and differentiation of inflammatory cells and fibroblasts much like bioengineered tissues and BM-therapy [31]. Although many questions remain to be answered with regard to mechanism, the common variable present to all of these therapeutic modalities is the introduction of viable cells that remain metabolically active. Results from rhPDGF therapy have also reemphasized the importance of the physical presence of these cellular components, and although advancements remain to be made with regard to stem cell therapy and the treatment of CLI, the modest outcomes we observe today through cell therapy in wound care brings anticipation that future therapies are within reach.
Future Directions for Stem Cell Therapy
Chronic wounds are often relatively deficient in blood flow, lack mechanisms to activate angiogenesis, and contain senescent fibroblasts and epithelial cells; it is the combination of these circumstances that often makes clinical management challenging [19]. All of these aforementioned components are critical for successful wound healing and are not easily replaceable. The success of HFDD, and BBSS in healing chronic wounds lies in their ability to address and correct deficiencies of factors, known and unknown, in wounds and it is likely that that they do so through both paracrine and direct local mechanisms. Prior studies have shown that stem cells are capable of a multitude of complex actions, and that their success in CLI will likely come from a multifaceted approach. The clinical importance of the paracrine and physical mechanisms of BM-derived stem cells remains to be fully determined and, it is our suspicion that, similar to HFDD and BBSS, successful BM-stem cell therapy in CLI will require exploitation of both.
Acknowledgments
Grant support: This review study was supported in part by the National Institute of Health grant R01-HL095498, the American Vascular Association William J. von Liebig Award, as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, Conn.
References
- 1.Asahar T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Curran MP, Plosker GL. Bilayer Bioengineered Skin Substitute (Apgraf) Biodrugs. 2002;16(6):439–455. doi: 10.2165/00063030-200216060-00005. [DOI] [PubMed] [Google Scholar]
- 3.Diehm C, Trampisch H, Haberl R, Darius H, Mahn M, Pittrow D, et al. Prognosis of patients with asymptomatic versus symptomatic peripheral arterial disease: 3-year results of the get ABI study. Vasc Med. 2007;12:141–148. [Google Scholar]
- 4.Eppley BL, Woodell JE, Higgins J. Platelet Quantification and Growth Factor Analysis from Platelet-Rich Plasma: Implications for Wound Healing. Plast Reconstr Surg. 2004;114(6):1502–8. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 5.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Falnga V, Margolis D, Alvarez O, Auletta M, Maggiacomo F, Altman M, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogenic cultured human skin equivalent. Arch Dermatol. 1998;134:293–300. doi: 10.1001/archderm.134.3.293. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs S, Baffour R, Zhou Y. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 8.Gaweda K, Tarczynska M, Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med. 2010;31:577–583. doi: 10.1055/s-0030-1255028. [DOI] [PubMed] [Google Scholar]
- 9.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 10.Hanft JR, Surprenant MS. Healing of Chronic Foot Ulcers in Diabetic Patients Treated with a Human Fibroblast-Derived Dermis. J Foot Ankle Surg. 2002;41(5):291–299. doi: 10.1016/s1067-2516(02)80047-3. [DOI] [PubMed] [Google Scholar]
- 11.Hansbrough JF, Morgan J, Greenleaf G, Parikh M, Nolte C, Wilkins L. Evaluation of Graftskin composite grafts on full-thickness wounds on athymic mice. J Burn Care Rehabil. 1994;15(4):346–353. doi: 10.1097/00004630-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and Il-1alpha. J Cell Physiol. 1996;166(3):585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Heil M, Ziegelhoeffer T, Mees B, Schaper W. A Different Outlook on the Role of Bone Marrow Stem Cells in Vascular Growth: Bone Marrow Delivers Software not Hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 14.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 15.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-Derived Stromal Cells Express Genes Encoding a Broad Spectrum of Arteriogenic Cytokines and Promote In Vitro and In Vivo Arteriogenesis Through Paracrine Mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 16.Lacci KM, Dardik A. Platelet-Rich Plasma: Support for its Use in Wound Healing. Yale J Biol Med. 2010 Mar;83(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Lamme EN, van Leeuwen RTJ, Jonker A, van Marle J, Middelkoop E. Living Skin Substitutes: Survival and Function of Fibroblasts Seeded in a Dermal Substitute in Experimental Wounds. J Invest Dermatol. 1998;111:989–995. doi: 10.1046/j.1523-1747.1998.00459.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 19.Mansbridge J. Commercial considerations in tissue engineering. J Anat. 2006;209:527–532. doi: 10.1111/j.1469-7580.2006.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansbridge J, Liu K, Pinney E, Patch R, Ratcliffe A, Naughton G. Growth factors secreted by fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes Metab. 1999;1:265–279. doi: 10.1046/j.1463-1326.1999.00032.x. [DOI] [PubMed] [Google Scholar]
- 21.Marston WA. Dermagraft®, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Review of Medical Devices. 2004;1(1):21–31. doi: 10.1586/17434440.1.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Marston WA, Hanft J, Norwood P, Pollak R. The Efficacy and Safety of Dermagraft in Improving the Healing of Chronic Diabetic Foot Ulcers. Diabetes Care. 2003;26:1701–1705. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 23.Marx RE. Platelet-Rich Plasma: Evidence to Support its Use. J Oral Maxillofac Surg. 2004;62:498–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.McKay IA, Leigh IM. Epidermal cytokines and their role in cutaneous wound healing. Brit J Derm. 1992;124:513–518. doi: 10.1111/j.1365-2133.1991.tb04942.x. [DOI] [PubMed] [Google Scholar]
- 25.Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 26.Naughton G, Mansbridge J, Gentzkow G. A metabolically active human dermal replacement for the treatment of diabetic foot ulcers. Artificial Organs. 1997;21:1203–1210. doi: 10.1111/j.1525-1594.1997.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 27.Navsaria HA, Myers SR, Leigh IM, McKay IA. Culturing skin in vitro for wound therapy. Trends Biotech. 1995;13:91–100. doi: 10.1016/S0167-7799(00)88913-1. [DOI] [PubMed] [Google Scholar]
- 28.Parenteau NL, Nolte CM, Bilbo P, Rosenberg M, Wilkins LM, Johnson EW, et al. Epidermis Generated In Vitro: Practical Considerations and Applications. J Cell Biochem. 1991;45:245–251. doi: 10.1002/jcb.240450304. [DOI] [PubMed] [Google Scholar]
- 29.Pinnney E, Liu K, Sheeman B, Mansbridge J. Human three-dimensional fibroblasts cultures express angiogenic activity. J Cell Physiol. 2000;183:74–82. doi: 10.1002/(SICI)1097-4652(200004)183:1<74::AID-JCP9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan S, Olin J, Deitcher S, MD, Pieczek A, Laird J, Grossman PM, et al. Use of a Constitutively Active Hypoxia-Inducible Factor-1Transgene as a Therapeutic Strategy in No-Option Critical Limb Ischemia Patients Phase I Dose-Escalation Experience. Circulation. 2007;10:1234–1243. doi: 10.1161/CIRCULATIONAHA.106.607994. [DOI] [PubMed] [Google Scholar]
- 31.Redler LH, Thompson SA, Hsu SH, Ahmad CS, Levine WN. Platelet-Rich Plasma Therapy: A Systematic Literature Review and Evidence For Clinical Use. Phys Sportsmed. 2011;39(1):42–51. doi: 10.3810/psm.2011.02.1861. [DOI] [PubMed] [Google Scholar]
- 32.Robson MC, Thomason A, Pierce GF, Phillips MC, Thomason A, Pierce GF, Phillips LG, Robson LE. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet. 1992;339:23–25. doi: 10.1016/0140-6736(92)90143-q. [DOI] [PubMed] [Google Scholar]
- 33.Shukla VK, Tiwary SK, Barnwal S, Gulati AK, Pandey SS. Effect of autologous epidermal cell suspension transplantation in chronic nonhealing wounds: a pilot study. Can J Surg. 2010;53:6–10. [PMC free article] [PubMed] [Google Scholar]
- 34.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double –blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemia injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 36.Tateishi-Yuyama E, Hiroaki M, Murohara T, Ikeda U, Shintani S, Masaki H, et al. Therapeutic Angiogenesis for Patients with Limb Ischemia by Autologous Transplantation of Bone-Marrow Cells: A Pilot Study and a Randomised Controlled Trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DW, Petrera M, MD, Hendry Mike, BScH, John S, Theodoropoulos MD. A Systematic Review of the Use of Platelet-Rich Plasma in Sports Medicine as a New Treatment for Tendon and Ligament Injuries. J Sport Med. 2011;0:1–9. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 38.Wieman TJ, Smiell JM, Su Y. Efficacy and Safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (Becaplermin) in patients with Neuropathic Diabetic Ulcers. Diabetes Care. 1998;21(5):822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]


