Abstract
Contrary to Total Therapy 2 (TT2), FGFR3-translocation bore no adverse effects on outcome in Total Therapy 3 (TT3) with added bortezomib. DelTP53, another poor-risk feature in TT2, was examined for its prognostic consequences in TT3 present in 10% of 441 patients treated. Not affecting rate or duration of complete response, TP53 haplo-insufficiency also did not compromise, in the 83% with genomically defined low-risk myeloma, survival or event-free survival. FGFR+ and FGFR3− molecular subgroups fared worse in the presence of delTP53 when applying TT2 but not TT3. Thus, delTP53’s prognostic implications were protocol- as well as genome-defined risk- and molecular subgroup-dependent.
Keywords: Myeloma, TP53, Bortezomib, Prognosis, Genomics
Introduction
The prognosis of patients with multiple myeloma has been greatly advanced by the introduction of new agents of the immunomodulatory and proteasome-inhibiting varieties. When these agents are used in combination, complete response (CR) rates as high as 40% have been reported (1) approaching levels previously achieved only with high-dose melphalan transplants (2). In Total Therapy 3 (TT3), bortezomib was added for remission induction prior to and with both consolidation and maintenance therapies after melphalan-based tandem transplantation (3). This treatment approach induced CR and near-CR rates beyond 60% and 80%, respectively; at 4 years, approximately 80% are estimated to remain alive and 75% event-free, while 85% of those obtaining CR status have remained in CR 4 years after CR onset. Based on gene expression profiling (GEP) of highly purified plasma cells, the approximately 85% of patients with GEP-defined low-risk MM had a projected 4-year sustained CR rate of 90%, boding well for their cure.
Here we investigated whether TT3, which benefited the FGFR3 molecular subgroup (3), could also overcome the adverse implications of TP53 deletion (delTP53) observed in predecessor Total Therapy 2 (TT2) protocol (4) and in standard chemotherapy regimens (5, 6).
Patients and Methods
The details of TT2 and TT3 protocols and their overall results have been reported previously (2, 3); however, results related to 177 additional patients treated in a TT3 successor protocol to validate bortezomib pharmaco-genomic data have not been reported. The median follow-up times for TT2 and TT3 are 7.2 and 3.9 years, respectively. Both protocols and their revisions had been approved by the Institutional Review Board, and patients had signed a written informed consent in keeping with institutional and federal policies.
The topic of this investigation relates to the prognostic importance of the TP53 suppressor gene deletion, as assessed by GEP analysis, based on an excellent correlation between inter-phase fluorescence in situ hybridization-derived determinations of bi-allelic loss of TP53 and GEP-derived Affymetrix values of less than 727 (4). Our comprehensive myeloma data base was interrogated for standard prognostic variables and the presence of metaphase cytogenetic abnormalities (CA), as well as other GEP-derived parameters such as high-risk (7) and molecular subgroup designations (4). The data base also includes carefully annotated information on initial rate and duration of CR, event-free survival (EFS) and overall survival (OS). All patients had been followed through induction, transplantation and consolidation steps and then at least quarterly for the first year of maintenance and semi-annually thereafter to document disease status.
The Kaplan-Meier method was used to estimate overall and event-free survival with group comparisons made using the log-rank test. Overall survival and event-free survival were measured from the date of registration until death from any cause and disease relapse or death from any cause, respectively; survivors were censored at the time of last contact. Univariate and multivariate analyses of prognostic factors were carried out using Cox regression models.
Results and Discussion
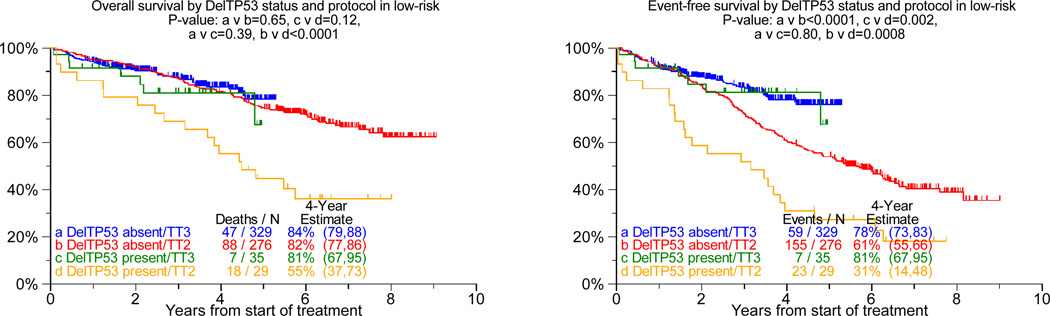
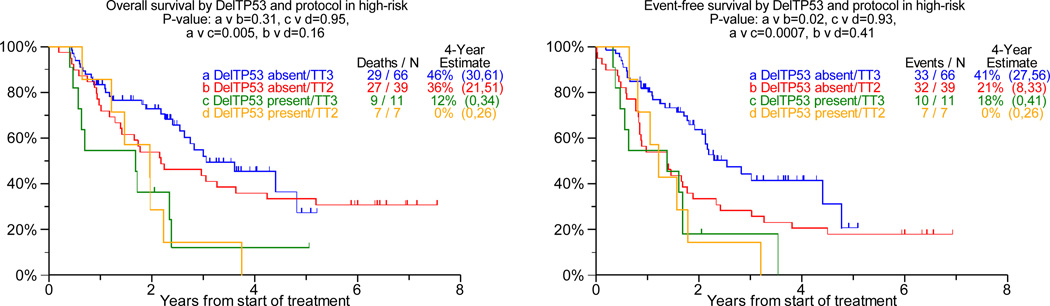
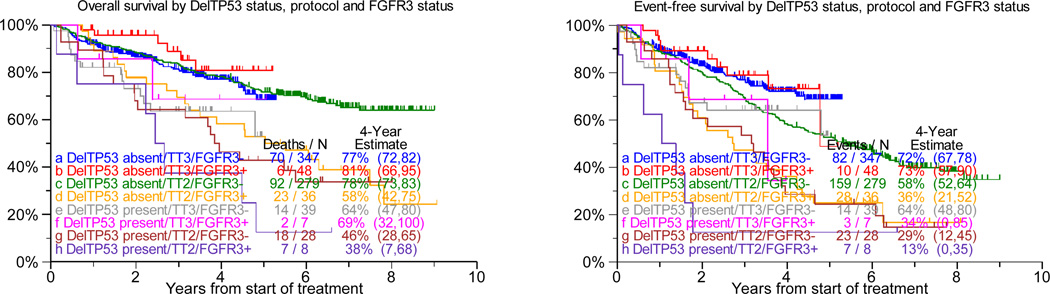
DelTP53, present in 10% each of TT2 and TT3 protocols, was over-represented in patients with elevated levels of lactate dehydrogenase of at least 190IU/L (45% v 28%, p=0.02) and under-represented in the Hyperdiploidy subgroup (7% v 31%, p<0.001). Examining the prognostic consequences of delTP53 in TT2 (regardless of randomization to thalidomide or control arm), we observed that both OS and EFS were markedly inferior in the low-risk setting, with a trend apparent for OS only in high-risk disease (Figure 1a, b). However, with TT3 such detrimental effect of delTP53 was not apparent in low-risk myeloma, while delTP53 further aggravated the poor prognosis in high risk disease. Relevant to FGFR3 status (translocation 4;14), TP53 haplo-insufficiency was an adverse feature regardless of FGFR3 translocation status in TT2, with mainly trends apparent in TT3 (Figure 1c). Examining, on the other hand, the impact of FGFR3 translocation by delTP53 status, clinical outcomes were inferior with TT2 in the absence of delTP53, with trends apparent for furthering the poor prognosis in the presence of delTP53 (see Figure 1c). In case TT3 was applied, FGFR3+ failed to affect outcomes both in the absence and presence of TP53 haplo-insufficiency.
Figure 1. Overall Survival (left panel) and event-free survival (right panels) from start of Total Therapy 2 (TT2, both arms combined) and of Total Therapy 3 (TT3) relative to delTP53.
a: In gene expression profiling-defined low-risk myeloma: In the case of TT2, del-TP53 imparts inferior overall survival and event-free survival, regardless of randomization to control or thalidomide arm (data not shown). By contrast, neither overall nor event-free survival was adversely affected In the case of TT3.
b: In gene expression profiling-defined high-risk myeloma: Del-TP53 imparts inferior overall and event-free survival in TT3, whereas outcomes were equally poor in both TT2 arms.
c: In the context of gene expression profiling-defined FGFR3-type myeloma (present, FGFR3+; absent, FGFR3−): Among patients treated with TT2, delTP53 was associated with shorter overall and event-free survival regardless of FGFR3 status (FGFR3−: compare c and g curves [p=0.0001; p=0.001]; FGFR3+: compare d and h curves [p=0.05; p=0.08]). In case of TT3, overall survival was inferior and a trend was noted for event-free survival in the absence of FGFR3 (FGFR3−: compare a and e curves [p=0.02; p=0.08]); no difference was observed in the presence of FGFR3 (FGFR3+: compare b and f curves [p=0.24; p=0.16]). Viewed differently, FGFR3+ adversely affected both clinical endpoints in TT2 in the absence of delTP53 (compare curves c and d [p=0.0004; p=0.001]) with trends present in delTP53’s presence (compare g and h curves [p=0.25; p=0.10]). In the case of TT3, FGFR3+ failed to affect outcomes both in the absence of delTP53 (compare a and b curves [p=0.22; p=0.72]) and in the presence of delTP53 (compare curves e and f [p=0.65; p=0.79]).
According to multivariate analyses, delTP53 conferred inferior OS and EFS in TT2 but not in TT3 (Table 1). CR rate and duration were not affected by TP53 status in either protocol (data not shown). Additional adverse parameters for OS and EFS in both protocols included the presence of CA and of elevated levels of beta-2-microglobulin, while GEP-defined high-risk designation also imparted shorter CR duration (p<0.001). The beneficial effect of randomization to thalidomide in TT2 applied to both event-free and overall survival.
Table 1.
Multivariate analysis of features linked to overall and event-free survival by protocol
| TT2 | TT3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall Survival | Event-free survival |
Overall Survival | Event-free Survival |
|||||||
| Variable | n/N (%) | HR (95% CI) | P- value |
HR (95% CI) | P- value |
n/N (%) | HR (95% CI) | P- value |
HR (95% CI) | P- value |
| Randomization to Thalidomide | 170/341 (50%) | 0.70 (0.50,0.99) | 0.041 | 0.61 (0.46,0.80) | <.001 | NA | NA | NA | NA | NA |
| B2M > 5.5 mg/L | 70/341 (21%) | 1.70 (1.16,2.48) | 0.006 | 1.62 (1.18,2.22) | 0.003 | 101/419 (24%) | 2.04 (1.30,3.19) | 0.002 | 1.96 (1.29,2.98) | 0.002 |
| LDH >= 190 U/L | 115/341 (34%) | 1.45 (1.01,2.07) | 0.045 | NS | NS | 101/419 (24%) | NS | NS | 1.54 (1.02,2.32) | 0.042 |
| Cytogenetic Abnormalities | 110/341 (32%) | 2.03 (1.43,2.89) | <.001 | 1.60 (1.20,2.13) | 0.002 | 156/419 (37%) | 2.28 (1.44,3.63) | <.001 | 1.62 (1.06,2.47) | 0.025 |
| DelTP53 present | 35/341 (10%) | 2.81 (1.80,4.38) | <.001 | 2.34 (1.58,3.46) | <.001 | 42/419 (10%) | 1.71 (0.98,2.99) | 0.058 | 1.45 (0.85,2.48) | 0.174 |
| GEP high-risk | 45/341 (13%) | 2.62 (1.71,4.01) | <.001 | 2.49 (1.71,3.62) | <.001 | 70/419 (17%) | 2.43 (1.50,3.93) | <.001 | 2.49 (1.58,3.92) | <.001 |
Variables considered for stepwise selection: age, albumin, B2M, creatinine, hemoglobin, LDH, CRP, cytogenetic abnormalities, GEP high-risk and delTP53
We conclude that, with TT3 and in contrast to TT2, delTP53 was not an independent deleterious feature. When examined in the context of GEP-defined risk, the absence TP53 haplo-insufficiency significantly improved the poor prognosis still observed with TT3 in high-risk myeloma beyond the fate observed after treatment with TT2. FGFR3 status did not confer poor outcomes in TT3 as it did with TT2. As the major difference between the two protocols was the incorporation of bortezomib in TT3 for both induction prior to and consolidation therapy after tandem transplantation as well as its use in the maintenance phase, it is tempting to speculate that the use of this proteasome-inhibiting agent negated the adverse consequences of delTP53 at least in the low-risk setting. The underlying mechanism may involve bortezomib’s synergistic interaction with thalidomide in VTD (8) or with melphalan in VMP (9) or other agents (10). Such synergy may result from sensitization of tumor cells to DNA-damaging agents (11) via accumulation of cytosolic TP53 or suppression of cellular response to genotoxic stress. Adding tanespimycine, an inhibitor of HSP90, recently shown to augment bortezomib’s efficacy, may overcome the dire prognosis still observed in case delTP53 occurs in the high-risk setting (12). The currently accruing US Intergroup trial, S0777, performed under the auspices of the Southwest Oncology Group, randomizes newly diagnosed patients between lenalidomide plus dexamethasone and the 3-drug combination of lenalidomide plus dexamethasone plus bortezomib. As state-of-the-art molecular genetic studies are performed as part of this trial, a firm conclusion will be forthcoming whether, in a non-transplant setting, bortezomib indeed overcomes the adverse consequences of TP53 haplo-insufficiency.
Acknowledgment
Support: Program Grant CA55819 from the National Cancer Institute, Bethesda, MD
Footnotes
Author Contributions
Conceptualized work: JDS, FvR, EA, JE, BB
Performed clinical research: FvR, EA, BN, SW, YA, BB
Analyzed data: JDS, YZ, JH, JC
References
- 1.Terpos E, Kastritis E, Roussou M, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22:2247–2256. doi: 10.1038/leu.2008.235. [DOI] [PubMed] [Google Scholar]
- 2.Barlogie B, Tricot G, Anaissie E, et al. Effect of adding thalidomide to the treatment of multiple myeloma with tandem autotransplants. New Engl J Med. 2006;10:1021–1030. [Google Scholar]
- 3.Pineda-Roman M, Zangari M, Haessler J, et al. Sustained complete remission in multiple myeloma linked to bortezomib in total therapy 3: comparison to total therapy 2. Brit J of Haematol. 2008;140:625–634. doi: 10.1111/j.1365-2141.2007.06921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020–2028. doi: 10.1182/blood-2005-11-013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avet-Loiseau H, Andree-Ashley LE, Moore D, II, et al. Molecular cytogenetic abnormalities in multiple myeloma and plasma cell leukemia measured using comparative genomic hybridization. Genes, Chromosomes & Cancer. 2007;19:124–133. doi: 10.1002/(sici)1098-2264(199706)19:2<124::aid-gcc8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Research. 2004;64:1546–1548. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 7.Shaughnessy J, Zhan F, Burington B, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 8.Barlogie B, Shaughnessy J, Tricot G, et al. Treatment of multiple myeloma. Blood. 2004;103:20–32. doi: 10.1182/blood-2003-04-1045. [DOI] [PubMed] [Google Scholar]
- 9.San Miguel J, Schlag R, Khuagena N, et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 10.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic implications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 11.Jacquemont C, Taniguchi T. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res. 2007;67:7395–7405. doi: 10.1158/0008-5472.CAN-07-1015. [DOI] [PubMed] [Google Scholar]
- 12.Richardson P, Chanan-Khan A, Lonial S, et al. Tanespimycin (T) + bortezomib (BZ) in multiple myeloma (MM): confirmation of the recommended dose using a novel formulation. Blood. 2007;110:353a. [Google Scholar]