Abstract
Objectives
Several studies have indicated that adult patients with spondyloarthritis (SpA) have elevated levels of antibodies associated with celiac disease (CD). This has not been studied in children, and none of the studies corrected for total IgA levels.
Methods
We measured total IgA and IgA against tissue transglutaminase (TTG) levels in 42 children with Juvenile Idiopathic Arthritis (JIA), of whom 11 had SpA, along with 10 non-inflammatory control subjects.
Results
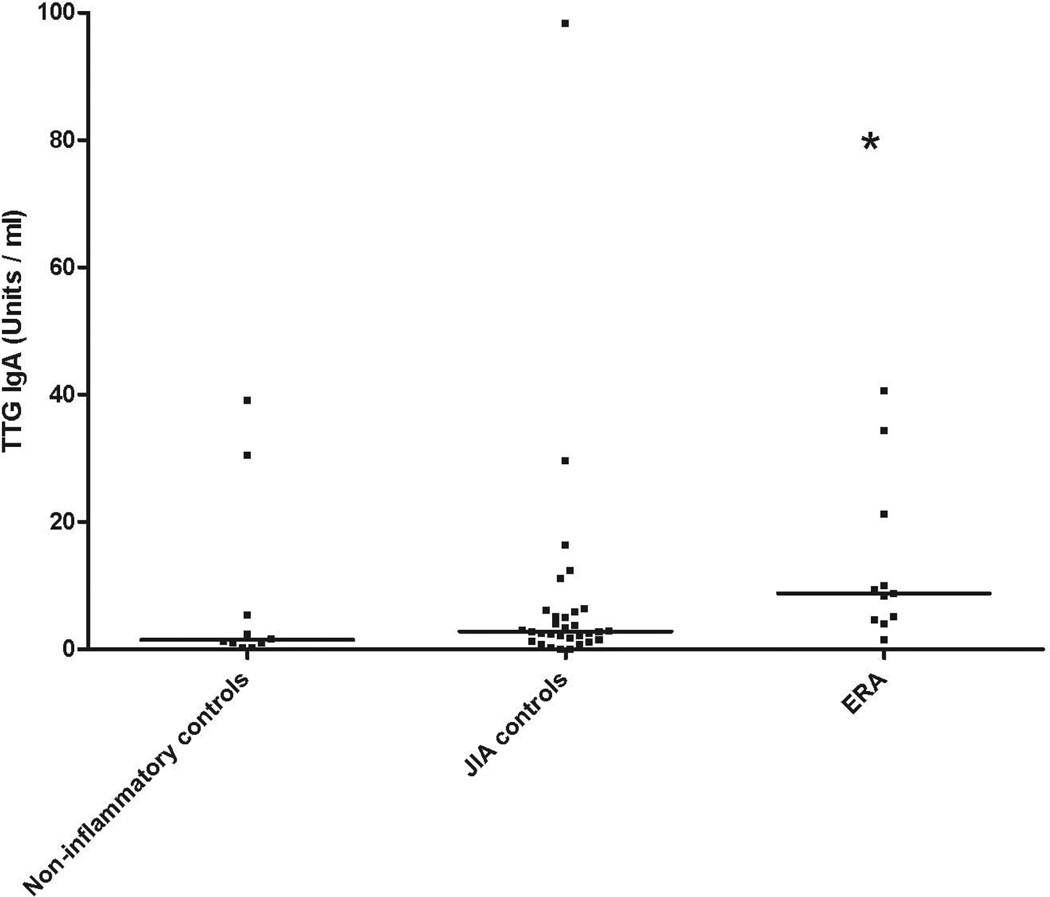
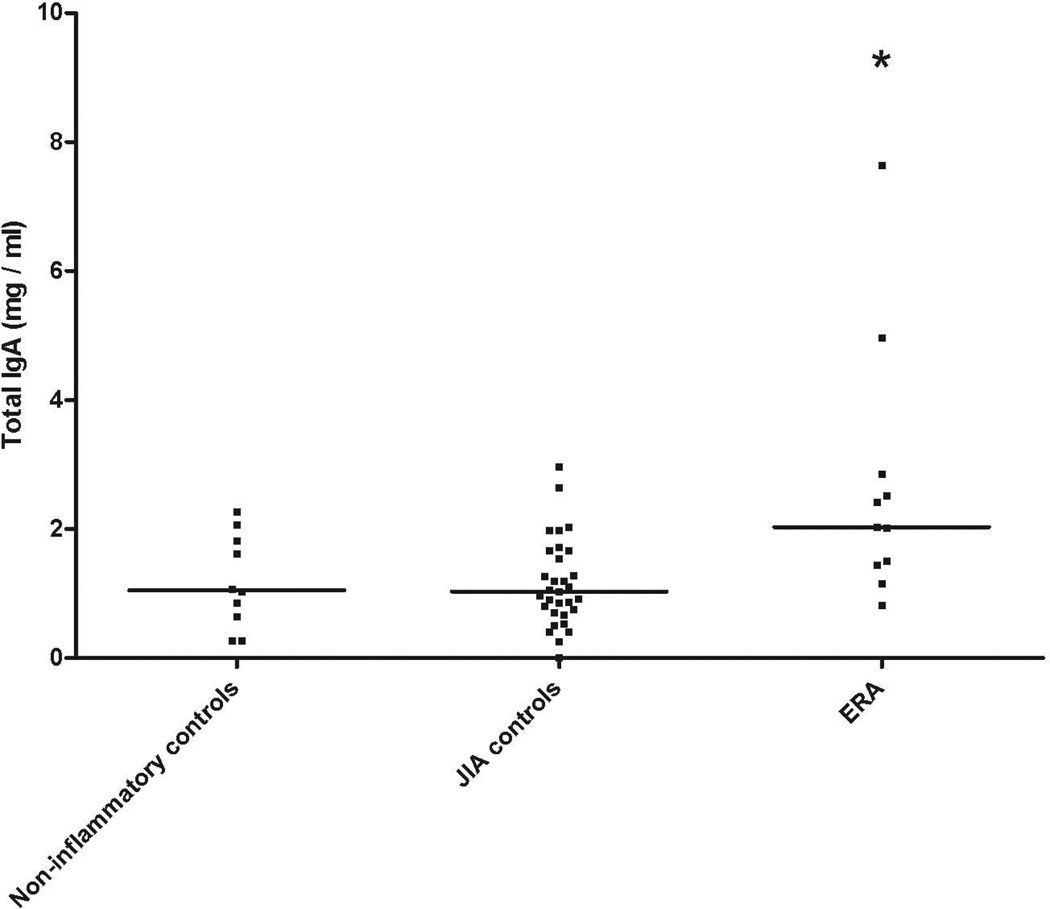
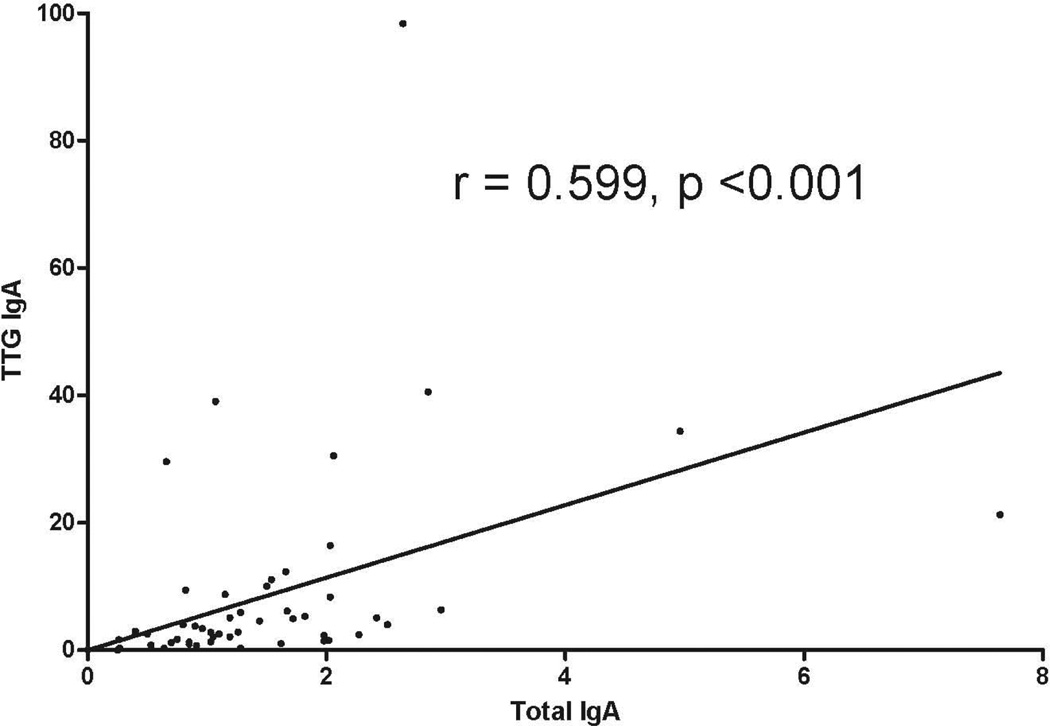
Median (IQR) TTG IgA levels were elevated among children with ERA (8.8, 4.6 – 21) compared to ‘JIA controls’ (JIA subgroups other than ERA) (2.8, 1.5 – 5.9) and a healthy non-inflammatory control group (1.5, 0.82 – 12), p = 0.017, Kruskal-Wallis. There was no correlation between TTG IgA levels and measures of disease activity or with medicine use. None of the children were diagnosed endoscopically with celiac disease. Patients with ERA likewise had elevated total IgA level compared to the other groups (p = 0.001), and total IgA levels correlated highly with TTG IgA (r = 0.599, p<0.001).
Conclusion
These findings suggest that elevations in TTG IgA may reflect increased polyclonal IgA production, rather than a specific intestinal inflammatory process.
Keywords: Spondyloarthritis, celiac disease, transglutaminase, immunoglobulin A
Introduction
It is unclear whether there is an association between spondyloarthritis (SpA) and celiac disease (CD). One study showed that arthritis may be common in patients with CD [1], but this finding has not been confirmed. There have been several case reports of children with Juvenile Idiopathic Arthritis (JIA) who were diagnosed with CD, in whom the underlying arthritis improved with the institution of gluten-free diet [2, 3]. However, there is contradictory data with respect to the presence of antibodies associated with CD in patients with pediatric or adult arthritis. Several studies have identified increased anti-gliadin IgA or anti-tissue transglutaminase (TTG) IgA antibodies in adults with psoriatic arthritis (PsA) or ankylosing spondylitis (AS) [4–7], while others found no differences in antibody titers [8, 9]. Similar data exist in children, and several studies have shown increased frequency of CD-associated antibodies in patients with JIA [10–13], although none of the studies evaluated children with SpA. Most of the above studies did not measure total IgA levels, In order to explore a potential association of CD with juvenile SpA, we evaluated a cohort of children with JIA, comparing TTG IgA levels among children of the various JIA subgroups (enthesitis-related arthritis (ERA), psoriatic JIA (psJIA), oligo and poly-articular JIA) and healthy pediatric control subjects (with non-inflammatory musculoskeletal conditions).
Methods
Patients
Children with JIA
42 children who met the International League of Associations for Rheumatology (ILAR) criteria for JIA evaluated at Texas Scottish Rite Hospital for Children (TSRHC), were recruited into the study [14]. 11 of them were diagnosed with ERA, while the remainder had oligoarticular JIA, polyarticular JIA, or psoriatic arthritis (collectively termed as ‘JIA controls’). Information on patient demographics, clinical phenotype, joint count, medication use, and routine laboratory studies at the time of the study is shown in Table 1. This study was approved by the Institutional Review Board at UT Southwestern Medical Center.
Table 1.
Patient population.
| Non-inflammatory Controls |
JIA controls | ERA | p-value | |
|---|---|---|---|---|
| n | 10 | 31 | 11 | N/A |
| Sex (F:M) | 4 : 6 | 28 : 3 | 2 : 9 | <0.001 |
| Age (years; mean ± SD) | 9.4 ± 3.6 | 6.9 ± 3.7 | 12.9 ± 2.6 | <0.001 |
| Disease duration (years; mean ± SD) | N/A | 2.2 ± 2.7 | 2.1 ± 2.2 | 0.912 |
| Race / ethnicity (%) | ||||
| Latino | 20 | 9.7 | 9.1 | |
| Non-Latino White | 60 | 84 | 73 | 0.541 |
| African-American | 20 | 6.5 | 18 | |
| Labs (mean ± SD) | ||||
| ESR | 8.8 ± 4.5 | 14 ± 9.5 | 28.4 ± 28 | 0.119* |
| WBC | 7.6 ± 3.0 | 8.0 ± 2.4 | 7.8 ± 2.3 | 0.810* |
| Hemoglobin | 12.8 ± 1.1 | 12.6 ± 1.0 | 12.8 ± 1.7 | 0.662* |
| Joint count (mean ± SD) | 0 | 5.3 ± 6.5 | 4.4 ± 5.3 | 0.694* |
| Medicines (%) | ||||
| NSAIDs | 10 | 68 | 36 | 0.086* |
| Methotrexate | 0 | 36 | 18 | 0.453* |
| TNF inhibitors | 0 | 6.5 | 18 | 0.277* |
| Prednisone | 0 | 3.2 | 0 | 1.000* |
Abbreviations: ERA = enthesitis-related arthritis, JIA = juvenile idiopathic arthritis, NSAID = non-steroidal anti-inflammatory drugs, TNF = tumor necrosis factor, TTG = tissue transglutaminase..
Significance testing limited to patients with JIA.
Control subjects
10 healthy children evaluated at TSRHC with a chief complaint of joint pain but found to have a non-inflammatory etiology (e.g. benign hypermobility, amplified pain syndrome) were enrolled as non-inflammatory controls.
Measurement of TTG and total IgA
Serum was obtained and stored in aliquots at −80°C. TTG IgA and total IgA were measured using commercially available kits (Alpco; Salem, NH) as per the instructions from the manufacturer. Briefly, human TTG or IgA was pre-loaded onto plates provided by the manufacturer and incubated for 30 minutes at room temperature (RT) with the patient samples, as well as standards and reference sera provided by the manufacturer. After washing, plates were incubated with anti-human IgA conjugated to horseradish peroxidase for 15 (for TTG) or 30 (for total IgA) minutes at RT, washed again, and incubated for 10 – 15 minutes at RT in the dark with the tetramethylbenzidine substrate. Phosphoric acid as stop solution was added and absorbance at 450 nm was measured. Units of anti-TTG IgA or total IgA were calculated from a standard curve; cutoff values for anti-TTG IgA were obtained from standards provided by the manufacturer.
Statistical analysis
Baseline characteristics among all of the groups were compared with ANOVA for continuous data and the Chisquare test for nominal data. Because TTG levels were not normally distributed (not shown), comparisons were performed with the non-parametric Kruskal-Wallis test. Comparison of serum IgA levels was performed with ANOVA. Correlation analysis was performed with the Spearman test, limited to JIA patients for ESR and the joint count. All analyses were performed with SPSS version 16 (Chicago, IL).
Results
Tissue transglutaminase IgA
Median (IQR) TTG IgA levels were elevated among children with ERA (8.8, 4.6 – 21) compared to the JIA con trols (2.8, 1.5 – 5.9) and the non-inflammatory control subjects (1.5, 0.82 – 12), p = 0.017, Kruskal-Wallis (Figure 1.) The majority of patients in all groups had TTG levels below the cutoff of 15 Units / ml.
Figure 1.
Tissue transglutaminase IgA levels in children with JIA and control subjects
ERA = enthesitis-related arthritis, oJIA = oligoarticular JIA, pJIA = polyarticular JIA, psJIA = psoriatic arthritis, TTG = tissue transglutaminase.
We looked for predictors of elevated TTG IgA levels among children with JIA. On a continuous scale no correlation was observed between TTG IgA levels and age (r = 0.236, p = 0.092), the swollen joint count (r=0.024; p=0.878) or ESR (r = 0.135, p= 0.398). The latter two comparisons were limited to patients with JIA. Additionally, there was no association between use of nonsteroidal anti-inflammatory drugs (NSAIDs) or disease-modifying anti-rheumatic drugs (DMARDs) and TTG levels among JIA patients, with median (intra-quartile range) TTG IgA levels of 3.8 (2.3 – 6.7) among untreated children, 4.9 (2.7 – 10.2) among those receiving NSAIDs alone, and 2.1 (0.7 – 10.0) among those receiving DMARDs. Similar results were obtained if the study group was limited to the ERA subset, although the numbers were too small to permit meaningful statistical analysis. Overall, boys had higher TTG levels compared to girls, 6.8 (2.6 – 23.6) versus 2.5 (1.3 – 5.3), p = 0.021.
Total serum IgA
Serum IgA was measured in all 52 subjects. Of them, one (1.92%) had undetectable levels. Patients with ERA had elevated total IgA levels compared to subjects in each of the other groups (Figure 2; p = 0.001). TTG IgA and total IgA levels were highly correlated (r = 0.599, p < 0.001; Figure 3.) When TTG IgA levels were corrected for total IgA levels, observed differences between the groups were abrogated (data not shown).
Figure 2.
Total IgA levels in children with JIA and control subjects. (Abbreviations as per Figure 1)
Figure 3.
Correlation between total and TTG IgA levels. (Abbreviations as per Figure 1)
Endoscopic diagnosis
Two children included in the study were referred to gastroenterology for evaluation; only one kept the appointment. This child, with a TTG IgA level of 8.8, underwent upper endoscopy and colonoscopy to evaluate for inflammatory bowel disease, with findings negative for CD.
Discussion
Our study confirms prior findings of elevated CD-associated antibodies in patients with SpA [4–7], extending these observations for the first time to pediatric SpA. We also confirm prior observations of elevated total serum IgA levels in SpA patients [15].
Our data however does not, appear to indicate that CD is present in large numbers of our patients. Prior studies showing elevated CD-associated antibodies in adult or pediatric patients with arthritis have shown that upper endoscopy of antibody-positive patients typically yields negative findings [4, 6, 10–13]. Only one study showed a higher than expected incidence of CD in patients with SpA, with histological diagnosis in 5 /114 (4.4%) of psA patients [5]. In our study, most of the patients had TTG levels below the manufacturer’s suggested cutoff of 15 Units / ml, and only one child, who had a TTG level of 9, underwent upper endoscopy, with negative findings. Most importantly, we also found that patients with ERA had elevated TTG IgA levels that correlated highly with total IgA levels, potentially indicative of polyclonal IgA production. Most prior studies involving CD-associated antibodies did not measure total IgA levels [4, 6, 8–13]. Of the two studies that did measure total serum IgA, both found higher levels in SpA patients compared to concurrent or historical controls [5, 7]. Amongst these, the study by Lindqvist et al. (2002) did not involve performing confirmatory biopsies [5] and the one by Togrol et al. (2009) reported findings consistent with CD in only one of 11 biopsied patients [7]. Thus, we suspect that our results and possibly results from prior teams showing elevated CD-associated antibodies in SpA patients may be suggestive of non-specific inflammation in ERA patients, potentially at the mucosal surfaces. Indeed, prior studies have reached similar conclusions that elevated celiac antibodies in children with JIA reflect non-specific immunologic abnormalities rather than true CD [10, 11].
This study has several limitations. Patient numbers are limited, and the results may have been impacted by medical therapy, albeit no obvious association was observed. In addition, only one child with elevated TTG IgA levels underwent endoscopy, raising the possibility that some cases may have gone undetected. Despite these limitations, in light of the elevated total IgA levels in the ERA patients and the strong correlation between TTG and total IgA, we submit that future studies measuring IgA antibodies in SpA patients should take into account the possibility of differences secondary to polyclonal IgA production.
Acknowledgements
Dr. Stoll was supported by Grant Number UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., PI) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Footnotes
Competing interests: The authors declared no competing interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.1Lubrano E, Ciacci C, Ames PR, Mazzacca G, Oriente P, Scarpa R. The arthritis of coeliac disease: prevalence and pattern in 200 adult patients. Br J Rheumatol. 1996;35:1314–1318. doi: 10.1093/rheumatology/35.12.1314. [DOI] [PubMed] [Google Scholar]
- 2.Maki M, Hallstrom O, Verronen P, Reunala T, Lähdeaho ML, Holm K, et al. Reticulin antibody, arthritis, and coeliac disease in children. Lancet. 1988;1:479–480. doi: 10.1016/s0140-6736(88)91280-9. [DOI] [PubMed] [Google Scholar]
- 3.Pinals RS. Arthritis associated with gluten-sensitive enteropathy. J Rheumatol. 1986;13:201–204. [PubMed] [Google Scholar]
- 4.Kallikorm R, Uibo O, Uibo R. Coeliac disease in spondyloarthropathy: usefulness of serological screening. Clin Rheumatol. 2000;19:118–122. doi: 10.1007/s100670050028. [DOI] [PubMed] [Google Scholar]
- 5.Lindqvist U, Rudsander A, Bostrom A, Nilsson B, Michaelsson G. IgA antibodies to gliadin and coeliac disease in psoriatic arthritis. Rheumatology (Oxford) 2002;41:31–37. doi: 10.1093/rheumatology/41.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Teichmann J, Voglau MJ, Lange U. Antibodies to human tissue transglutaminase and alterations of vitamin D metabolism in ankylosing spondylitis and psoriatic arthritis. Rheumatol Int. 2010;30:1559–1563. doi: 10.1007/s00296-009-1186-y. [DOI] [PubMed] [Google Scholar]
- 7.Togrol RE, Nalbant S, Solmazgul E, Ozyurt M, Kaplan M, Kiralp MZ, et al. The significance of coeliac disease antibodies in patients with ankylosing spondylitis: a case-controlled study. J Int Med Res. 2009;37:220–226. doi: 10.1177/147323000903700127. [DOI] [PubMed] [Google Scholar]
- 8.Kia KF, Nair RP, Ike RW, Hiremagalore R, Elder JT, Ellis CN. Prevalence of antigliadin antibodies in patients with psoriasis is not elevated compared with controls. Am J Clin Dermatol. 2007;8:301–305. doi: 10.2165/00128071-200708050-00005. [DOI] [PubMed] [Google Scholar]
- 9.Riente L, Chimenti D, Pratesi F, Delle Sedie A, Tommasi S, Tommasi C, et al. Antibodies to tissue transglutaminase and Saccharomyces cerevisiae in ankylosing spondylitis and psoriatic arthritis. J Rheumatol. 2004;31:920–924. [PubMed] [Google Scholar]
- 10.1Pellegrini G, Scotta MS, Soardo S, Avanzini MA, Ravelli A, Burgio GR, et al. Elevated IgA anti-gliadin antibodies in juvenile chronic arthritis. Clin Exp Rheumatol. 1991;9:653–656. [PubMed] [Google Scholar]
- 11.George EK, Hertzberger-ten Cate R, Van Suijlekom-Smit LW, von Blomberg BM, Stapel SO, van Elburg RM, et al. Juvenile chronic arthritis and coeliac disease in The Netherlands. Clin Exp Rheumatol. 1996;14:571–575. [PubMed] [Google Scholar]
- 12.Al-Mayouf SM, Al-Mehaidib AI, Alkaff MA. The significance of elevated serologic markers of celiac disease in children with juvenile rheumatoid arthritis. Saudi J Gastroenterol. 2003;9:75–78. [PubMed] [Google Scholar]
- 13.Lepore L, Pennesi M, Ventura A, Torre G, Falcini F, Lucchesi A, et al. Anti-alpha-gliadin antibodies are not predictive of celiac disease in juvenile chronic arthritis. Acta Paediatr. 1993;82:569–573. doi: 10.1111/j.1651-2227.1993.tb12756.x. [DOI] [PubMed] [Google Scholar]
- 14.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 15.Cowling P, Ebringer R, Ebringer A. Association of inflammation with raised serum IgA in ankylosing spondylitis. Ann Rheum Dis. 1980;39:545–549. doi: 10.1136/ard.39.6.545. [DOI] [PMC free article] [PubMed] [Google Scholar]