Abstract
Background
Cryptococcal meningitis has been described in immunocompromised patients as well as in those for whom no immune defect has been identified. GM-CSF regulates the function of phagocytes and pulmonary alveolar macrophages, critical elements in cryptococcal control.
Methods
We performed clinical histories, immunological evaluation, and anticytokine autoantibody screening in 4 current patients with cryptococcal meningitis, and identified and tested 103 archived plasma/CSF samples from patients with cryptococcal meningitis. We assessed the ability of anti-GM-CSF autoantibody containing plasmas to inhibit GM-CSF signaling.
Results
We recognized anti-GM-CSF autoantibodies in an otherwise healthy female with cryptococcal meningitis who later developed pulmonary alveolar proteinosis. Her diagnosis prompted screening of patients with cryptococcal meningitis for anticytokine autoantibodies. We identified 7 HIV uninfected patients with cryptococcal meningitis who tested positive for high-titer anti-GM-CSF autoantibodies. Two of the 7 later developed evidence of PAP. Plasma from all patients prevented GM-CSF-induced STAT-5 phosphorylation and MIP-1α production in normal PBMC. This effect was limited to their IgG fraction.
Conclusions
Anti-GM-CSF autoantibodies are associated with some cases of cryptococcal meningitis in otherwise immunocompetent patients. These cases need not have associated pulmonary alveolar proteinosis.
Introduction
Anti-granulocyte macrophage-colony stimulating factor (GM-CSF) autoantibodies have been shown to cause acquired pulmonary alveolar proteinosis (PAP)(1, 2), a chronic lung disease characterized by abnormalities of surfactant metabolism. PAP generally occurs in adulthood and is characterized by accumulation of dense proteinaceous infiltrates and associated respiratory insufficiency. Both respiratory and extrapulmonary infections, often with unusual pathogens, have also been reported(3-11). The role of serum anti-GM-CSF autoantibodies in infection susceptibility outside the lung has not been clearly defined.
Anti-GM-CSF autoantibodies in patients with PAP contribute to a range of defects in alveolar macrophage function including impaired chemotaxis, adhesion, phagocytosis, microbicidal activity, and phagolysosome fusion (12, 13). GM-CSF receptor-deficient mice show that GM-CSF induces PU.1, which is critical to both surfactant homeostasis and TLR signaling (14), potentially explaining not only surfactant accumulation but also the infection susceptibility seen in PAP. GM-CSF-/- mice and PAP patients have defective neutrophil phagocytosis, adhesion, oxidative burst and bacterial killing(15). Furthermore, many of the infecting organisms in PAP, such as Nocardia spp(3, 5, 6, 8-10), Histoplasma (7), and Cryptococcus(4, 9, 16, 17), are ones against which phagocytes are known to be important. However, the majority of these reports originated prior to the identification of anti-GM-CSF autoantibodies as a major cause of acquired PAP.
Anticytokine autoantibodies are now known to be a cause of adult-onset infection susceptibility(18), with examples including anti-IFNγ autoantibodies with disseminated nontuberculous mycobacterial infections(19-22) and anti-IL-17 autoantibodies with chronic mucocutaneous candidiasis(23, 24). Cryptococcal meningitis is a recognized opportunistic infection in those with immunocompromise, such as lymphoma, steroid use, or HIV infection, but has also been described in those without identified immune defects(25-31).
Identification of a patient with cryptococcal meningitis and PAP prompted us to recognize anti-GM-CSF autoantibodies. To evaluate whether anti-GM-CSF autoantibodies were specifically associated with cryptococcal meningitis, we screened banked archived sera or plasma, and in some cases cerebrospinal fluid (CSF), of previously healthy, HIV negative adults with cryptococcal meningitis.
Methods
Subjects
Three patients were seen at the National Institutes of Health and consented to evaluation and treatment of cryptococcal meningitis under IRB-approved protocols 93-I-0119 or 93-I-0106. One patient was seen and consented in Thailand under National Institutes of Health IRB-approved protocol 09-I-N060. Normal peripheral blood mononuclear cells (PBMC) were obtained though the National Institutes of Health Blood Bank under appropriate IRB-approved protocols. Plasma from healthy controls (n=64) and diseased controls (n=43) that had not been previously tested for autoantibodies included healthy adult volunteers and adult patients with an undiagnosed immunodeficiency respectively. All subjects were seen and consented under appropriate National Institutes of Allergy and Infectious Diseases IRB-approved protocols.
One hundred and three patients were identified as having been treated at the NIH for cryptococcal meningitis between 1955 and 1984. Archived plasma samples that had been stored at -80°C were screened on IRB-approved protocol 09-I-N162. CSF collected for diagnosis and management of cryptococcal meningitis was stored at -80°C for some patients and when available was screened. Anonymous CSF collected at NIH for other purposes and designated as medical waste by the clinical laboratory was used as controls.
All current patients underwent a history and physical examination, routine laboratories including HIV testing, complete blood count with differential, serum electrolytes, renal and hepatic function chemistries, quantitative immunoglobulin levels and lymphocyte markers including total T cells (CD3+; BD Pharmigen); CD4+ T cells (Immunotech), CD8+ T cells (Immunotech); CD20+ B cells (BD Pharmigen); CD20+/CD27+ memory B cells (BD Pharmigen); CD16+ or CD56+/CD3- NK cells (BD Pharmigen) and CD56+/CD3+ NKT cells. Medical charts were reviewed on patients for whom archived serum had been collected to document microbiological evidence of cryptococcal meningitis and to identify possible comorbidities that could contribute to cryptococcal susceptibility.
Determination of anti-cytokine autoantibody titers
Plasma from patients and healthy controls was screened for anti-cytokine autoantibodies using a particle-based technology described previously (32). Briefly, 6 sets of differentially fluorescing magnetic beads (Bio-Rad) were conjugated to 2.5 μg recombinant human GM-CSF, IFNα, IFNγ, IL-12p70, IL-17A, or IL-22 (R&D Systems), respectively. Beads were combined and incubated for 1 hour with subject or control plasma at 1:100 dilution, washed, and incubated with biotinylated mouse anti-human total IgG as well as IgG subclasses, and IgA, IgM and IgE (Sigma). Beads were washed again and incubated with Streptavadin-PE (Bio-Rad) before being run in a multiplex assay on the Bio-plex (Bio-Rad) instrument. Fluorescence intensity for each bead type was plotted as a function of antibody titer (Graphpad Prism, version 5.0c). Stored CSF from patients identified as having anti-GM-CSF autoantibodies was examined in the same manner using neat fluid.
Plasma isolation, cell culture and stimulation
Plasma from each subject was isolated from whole blood and stored at -80°C until testing. For current patients, PBMCs were obtained from the remaining cell pellets by density gradient centrifugation as previously described (33), and cultured at 106 cells/mL in complete medium consisting of RPMI 1640 (Gibco BRL), 2mM glutamine, 20mM Hepes, 0.01 mg/mL penicillin/streptomycin with 10% plasma from patients or from healthy blood bank donors.
Detection of phosphoSTAT-5 by flow cytometry
To demonstrate that patient cells had intact GM-CSF signaling when washed free of autologous plasma, PBMC (5 × 105 cells) from patients were cultured in complete RPMI media containing control or patient plasma (10%) and left unstimulated or stimulated with GM-CSF (10 ng/mL) or IL-3 (10 ng/mL) (R&D) for 30 minutes at 37°C. To demonstrate that patient plasma blocked GM-CSF signaling, PBMC (5 × 105 cells) from normals were cultured in complete RPMI media containing control or patient plasma (10%) and left unstimulated or stimulated with GM-CSF (10 ng/mL) or IL-3 (10 ng/mL) (R&D) for 30 minutes at 37°C. Monocytes were identified by CD14 (BD Pharmingen) surface staining before being fixed and permeabilized for intracellular staining with phosphorylated signal transducer and activator of transcription 5 (pSTAT-5) (Y694), antibody (BD Pharmingen) as previously described (22). Data were collected using FACSCalibur (BD Biosciences), analyzed using FlowJo (Treestar), and graphed with Prism5 (Graphpad).
Plasma inhibition of GM-CSF induced MIP-1α
To evaluate cellular immune function and plasma inhibition of GM-CSF activity, normal PBMC in normal plasma or subject plasma (10%) were left unstimulated or stimulated overnight with GM-CSF (10 ng/mL) (R&D). Supernatants were collected and stored at -20°C until measurement of MIP-1α levels using the Bio-Plex cytokine assay (Bio-Rad) per the manufacturer's instructions.
Statistics
To evaluate differences between healthy controls and patients, a two-tailed unpaired t-test was applied using Prism 5 (Graphpad).
Results
Case Reports
Patient 1
An otherwise healthy HIV-uninfected 20-year-old woman from California presented in June, 2008 after experiencing a few days of fever, debilitating headache, neck pain, double vision, vomiting, and confusion (Table 1). Lumbar puncture found an opening pressure of 250 mmH2O, 17 white blood cells per cubic millimeter, lymphocyte predominant, glucose 51 (mg/dL) and protein 33 (mg/dL). The CSF cryptococcal antigen was positive at 1:256, and culture grew Cryptococcus neoformans. Brain MRI showed three hyperintense gadolinium-enhancing lesions in bilateral occipital lobes and right parietal temporal lobe (Figure 1A). A left lower lobe mass was confirmed to be a cryptococcoma by needle aspiration. She received liposomal amphotericin B, flucytosine, and later fluconazole with resolution of her meningitis. Lymphocyte phenotyping was normal except for low NK cell number (Table 2).
Table 1.
Summary of clinical features.
| Clinical Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age at diagnosis | 20 | 31 | 48 | 47 | 26 | 34 | 32 |
| Gender | F | F | M | M | M | M | M |
| Presenting symptoms | Fever, headache, neck pain, diplopia, confusion | Headache | Back pain, cough, fever | Cough, weakness and tremors | Back/neck pain, nausea, vomiting, ptosis | Blurred vision, nausea, vomiting, fever, chills | Headache, fatigue, confusion |
| Infections | C. neoformans (CNS/ lung) | C. gattii (CNS / lung) | C. neoformans (CNS / lung); MTB* (lung) | C. neoformans (CNS / lung / blood / skin) | Cryptococcus (CNS) | Cryptococcus (CNS / lung) | Cryptococcus (CNS / lung) |
| Peripheral total WBC** count (cells/μL) | 8600 | 9900 | 6800 | 8200 | 6300 | 10800 | 5000 |
| Granulocytes (cells/μL) | 7482 | 8400 | 3046 | 5035 | 3150 | 9288 | 3500 |
| Lymphocytes (cells/μL) | 795 | 800 | 1714 | 1870 | 1071 | 972 | 1250 |
| Monocytes (cells/μL) | 353 | 700 | 802 | 713 | 378 | 540 | 250 |
| Serum cryptococcal antigen | 1:128 | 1:256 | 1:1024 | 1:512 | Not available | Not available | Not available |
| Lumbar puncture | |||||||
| Opening pressure (mmH20) | 250 | 400 | 250 | Not done | 200 | 360 | 210 |
| WBC (cells/mm3) | 17 | 219 | 98 | 241 | 158 | 60 | 53 |
| Granulocytes (cells/mm3) | 1 | 6 | 2 | 22 | 48 | 14 | Not available |
| Lymphocytes (cells/mm3) | 16 | 82 | 94 | 219 | 0 | 32 | Not available |
| Monocytes (cells/mm3) | 0 | 12 | 0 | 0 | 110 | 4 | Not available |
| Microbiology | Cryptococcal antigen + (1:256) | Cryptococcal antigen + (1:8) and culture + | Cryptococcal antigen + and culture + | Cryptococcal antigen + (1:16) | Budding yeast + / India ink + | India ink and culture + | Direct stain and culture + |
| Protein (mg/dL) | 33 | 91 | 85 | 75 | 68 | 36 | Not available |
| Glucose (mg/dL) | 51 | 47 | 36 | 36 | 43 | 140 | Not available |
| PAP | Yes, 2 years later | No | No | Asymptomatic ground glass opacities on chest CT | No | No | No |
| Anti-infectives | AmB§ + 5FC¶; then fluconazole | AmB + 5FC; then fluconazole + 5FC | AmB; then fluconazole; anti-tuberculous therapy | AmB + fluconazole | AmB + 5FC | AmB IT and IV+ 5FC | AmB IT and IV + 5FC |
| Outcome and sequelae | Required whole lung lavage for PAP with subsequent improvement | Full recovery | Full recovery | Recovered; remains on maintenance fluconazole | Full recovery | Seizures, homonymous hemianopsia, normal pressure hydrocephalus, chronic brain syndrome | Seizures, neurogenic bladder |
*MTB= Mycobacterium tuberculosis;
WBC = White blood cell
AmB = Amphotericin B
5FC = Flucytosine
Figure 1.
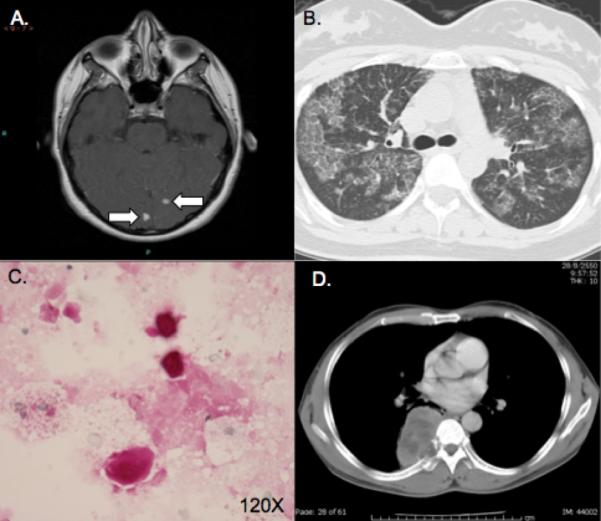
Radiographic and cytopathologic manifestations. A. MRI brain with gadolinium of patient 1 showing two enhancing lesions. B. Later chest CT of patient 1 demonstrating PAP. C. Periodic Acid Schiff (PAS) Diastase stain of BAL fluid showing characteristic findings of PAP with granular proteinaceous material and globules that are PAS positive, diastase resistant. D. Chest CT of patient 3 demonstrating pulmonary cryptococcal lesion with local bony invasion.
Table 2.
Baseline immunophenotyping.
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Mean ± SEM | Normal range |
|---|---|---|---|---|---|---|
| CD4/CD3 (cells/μL) | 1399 | 950 | 817 | 773 | 985 ± 143 | 362-1275 cells/μL |
| CD8/CD3 (cells/μL) | 490 | 305 | 340 | 1076 | 553 ± 179 | 344-911 cells/μL |
| CD20 (cells/μL) | 246 | 163 | 365 | 251 | 256 ± 41 | 49-424 cells/μL |
| CD16+or CD56+/CD3- (cells/μL) | 56 | 105 | 157 | 86 | 101 ± 21 | 87-505 cells/μL |
| CD16+or CD56+/CD3+ (cells/μL) | 86 | 42 | 19 | 145 | 73 ± 28 | 24-516 cells/μL |
| IgG | 843 | 901 | 1490 | 1350 | 1146 ± 161 | 642-1730 mg/dL |
| IgM | 160 | 64 | 120 | 106 | 113 ± 20 | 91-499 mg/dL |
| IgA | 168 | 154 | 175 | 286 | 196 ± 30 | 34-342 mg/dL |
| IgE | 86 | 16 | ND | 49 | 50 ± 20 | 0-90 IU/mL |
Chest CT two years later showed resolution of the cryptococcoma but new scattered, patchy, ground glass opacities in both lungs (Figure 1B). She denied pulmonary symptoms, despite regularly dancing and singing. Bronchoalveolar lavage with transbronchial biopsy was consistent with PAP (Figure 1C). As she was asymptomatic, no intervention was performed. About one year later, exertional dyspnea and inspiratory chest pain prompted repeat chest CT, which showed worsened bilateral ground-glass opacities associated with a decrease in total lung capacity from 4.13 L (85% predicted) to 3.77 L (77%) but no significant change in diffusing capacity 14.4 mL/mmHg/min (46%) or exertional oxyhemoglobin desaturation. Bilateral whole lung lavage led to improvement in symptoms.
Patient 2
An otherwise healthy 31-year-old HIV uninfected woman from southern California presented in November 2010 with a severe headache. Lumbar puncture showed OP of 400 mmH2O, 219 white blood cells/mm3, with lymphocytic predominance, glucose of 47 mg/dL, and protein of 91 mg/dL (Table 1). CSF cryptococccal antigen was positive at 1:8 and culture grew Cryptococcus gattii. Brain MRI was normal. Chest CT scan showed a sharply circumscribed solid mass in the left lung apex that on biopsy contained yeast consistent with Cryptococcus. She received intravenous liposomal amphotericin B and flucytosine for 8 days, then fluconazole plus flucytosine. Diarrhea led to discontinuation of flucytosine. Five months later she remained on fluconazole and felt well; her lung mass improved and she resumed working full-time.
Patient 3
A previously healthy HIV-uninfected 48-year-old Thai man had two months of severe back pain, chronic cough and low grade fever. Chest CT showed a broad based mass in the posterior right thorax, invading the right seventh rib with fibronodular infiltration of the left upper lung (Figure 1D). CT-guided aspiration demonstrated numerous mucoid encapsulated yeasts consistent with Cryptococcus; serum cryptococcal antigen was 1:1024. Lumbar puncture had a leukocyte count of 98 white blood cells/mm3 with 96% lymphocytes, a glucose level of 36 mg/dL, a protein level of 85 mg/dL, and a positive cryptococcal antigen and C. neoformans culture. The patient received 2 weeks of Amphotericin B deoxycholate, which was then changed to fluconazole due to nephrotoxicity.
Eleven months later the patient complained of a low-grade fever, productive cough and 3-kg weight loss. New consolidations were seen in the right upper lung and medial segment of the right middle lung as well as with cavitation in the left lower lung. Bronchoscopy identified M. tuberculosis. Standard anti-tuberculosis treatment was promptly initiated and continued for 9 months. The patient was well off all antimicrobial therapy after one year.
Patient 4
A previously healthy HIV-uninfected 47-year-old Mexican man living in California since age 12 presented in 2008 with persistent cough prompting chest X-ray. A large perihilar mass led to bronchoscopic biopsy that showed C. neoformans. His serum cryptococcal antigen was 1:512 and fluconazole was initiated. Approximately one week later he complained of weakness and tremors. Lumbar puncture showed cryptococcal antigen positive at 1:16 with 241 white blood cells/mm3, lymphocyte predominant, glucose of 36 mg/dL and protein of 75 mg/dL. Amphotericin B was given for 14 days but was changed to fluconazole due to renal toxicity. The patient was maintained on fluconazole and has had no evidence of recurrence of infection, however a chest CT scan performed April, 2012 demonstrated a new small region with ground-glass opacification. A repeat chest CT in July, 2012 demonstrated expansion of this infiltrate with multiple new regions of identified bilaterally and bronchoalveolar lavage revealed PAS positive material on cytology, consistent with a diagnosis of PAP. Since the patient remains asymptomatic with normal pulmonary function testing, clinical monitoring will be continued without therapeutic intervention at this time.
Archived samples
Plasma samples, and CSF when available, were obtained for 103 patients with microbiologically proven cryptococcal meningitis collected between 1958 and 1982. Clinical histories were reviewed and patients were categorized as those without evidence of immunodeficiency (n=67) and those with either a history of iatrogenic immunosuppression or an underlying medical condition, including diabetes mellitus or hematological malignancy, prior to their diagnosis of cryptococcal meningitis (n=36).
Anticytokine autoantibody detection
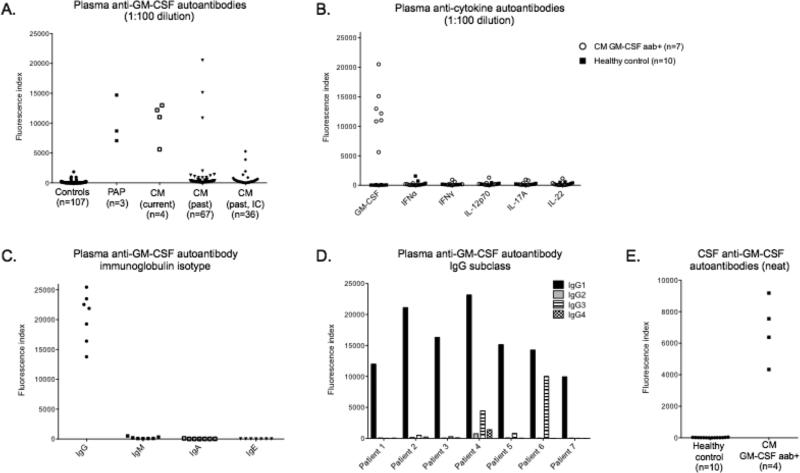
Plasma from controls (n=107), patients with known PAP (n=3), the 4 patients described above with cryptococcal meningitis, and the archived samples from patients with cryptococcal meningitis (n=103) were tested for anti-GM-CSF autoantibodies at 1:100 plasma dilution. Patients with PAP and the 4 current patients with cryptococcal meningitis were positive for anti-GM-CSF autoantibodies (Figure 2A). Three of 67 archived patient samples (Patients 5-7, Table 1) without recognized immunodeficiency were positive for anti-GM-CSF autoantibodies compared with none of the healthy controls (n=64), diseased controls (n=43) or patients with recognized immunocompromise (n=36). Anticytokine autoantibodies against IFNα, IFNγ, IL-12p70, IL-17A, and IL-22 were absent in those patients with GM-CSF autoantibodies (Figure 2B). The anti-GM-CSF autoantibodies were all IgG isotype (Figure 2C), and were predominantly IgG1 subclass (Figure 2D). Anti-GM-CSF autoantibodies were also identified at lower titers in CSF in 4 patients (Patients 1, 4, 6 and 7) for whom CSF was available (Figure 2E).
Figure 2.
Anticytokine autoantibody evaluation. A. Anti-GM-CSF autoantibodies in controls, both diseased (n=60) and healthy (n=43), patients with known acquired pulmonary alveolar proteinosis (PAP), and patients with cryptococcal meningitis (CM) with and without known immunocompromise (IC). B. Multiplex screen for anticytokine autoantibodies against GM-CSF, IFNα, IFNγ, IL-12p70, IL-17A and IL-22 in anti-GM-CSF autoantibody positive (aab+) cryptococcal meningitis patients and 10 normal controls. C. Evaluation of anti-GM-CSF autoantibody positive cryptococcal meningitis patients for other anti-GM-CSF immunoglobulin isotypes and D. anti-GM-CSF IgG subclasses. E. Anti-GM-CSF autoantibodies in CSF.
Anti-GM-CSF IgG inhibits GM-CSF induced pSTAT-5
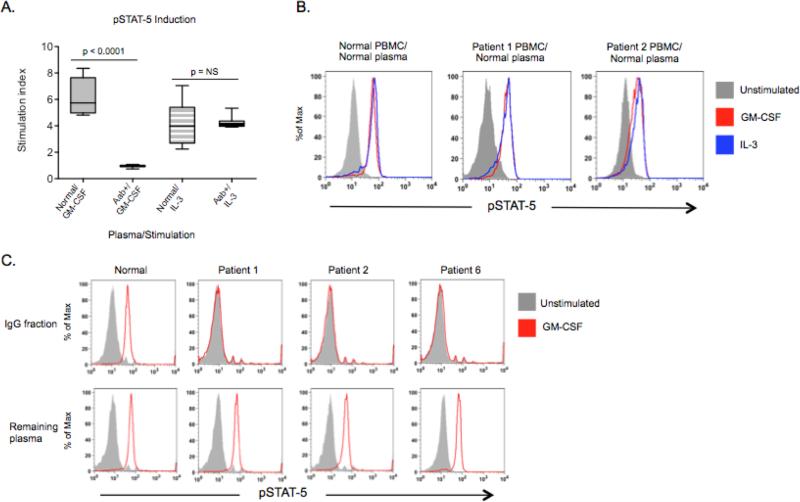
To demonstrate biological activity of plasma containing anti-GM-CSF autoantibodies, normal PBMC were left unstimulated or stimulated with GM-CSF or IL-3 in the presence of patient or normal plasma (10%) to evaluate for pSTAT-5 production. Only plasma containing anti-GM-CSF autoantibodies prevented GM-CSF-induced pSTAT-5, while signaling induced by IL-3, which shares the β unit of the GM-CSF receptor and also causes STAT-5 phosphorylation, was not affected (Figures 3A) (p<0.0001). However, in the absence of autologous plasma, PBMC from patients 1 and 2 were normally responsive to both GM-CSF and IL-3 (Figure 3B), indicating that their defect was humoral and not cell-intrinsic.
Figure 3.
Inhibitory capacity of anti-GM-CSF autoantibody-containing plasma. A. Normal PBMC were incubated with patient or normal plasma and left unstimulated or stimulated with GM-CSF or IL-3. Intracellular staining for pSTAT-5 was measured by flow cytometry and a stimulation index (ratio of stimulated to unstimulated geometric mean channels) was calculated for each plasma sample tested. B. GM-CSF and IL-3 induction of pSTAT-5 in washed PBMC from 2 patients with anti-GM-CSF autoantibodies. C. Normal PBMC were left unstimulated or stimulated in the presence of either purified IgG or the remaining plasma fraction for all 7 patients. Three of the 7 patients (2 current patients and 1 archived sample) and one normal are shown.
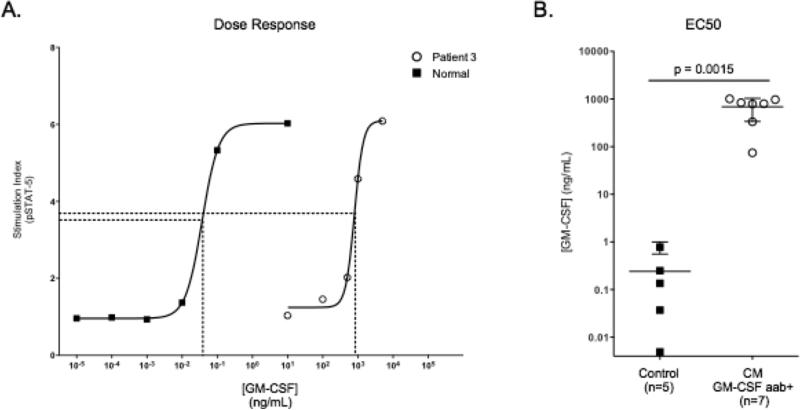
To confirm that the IgG fraction contained the inhibitory factor, normal PBMC were stimulated with GM-CSF in the presence of the purified IgG or the other plasma fractions. Only the IgG fraction in all 7 patients, but not the remaining plasma fractions, prevented GM-CSF-induced STAT-5 phosphorylation, as shown for patients 1, 2 and 6 (Figure 3C). To further demonstrate the specificity of GM-CSF autoantibodies in patient plasma we generated dose response curves by stimulating normal PBMC in patient or normal plasmas (10%) with increasing amounts of GM-CSF and evaluating pSTAT-5 production (Figure 4A). Anti-GM-CSF-autoantibody-containing plasma required a 2 to 3 log higher concentration of GM-CSF to obtain 50% of maximum pSTAT-5 production (EC50) when compared to normal plasma (Figure 4B) (p=0.0015).
Figure 4.
Anti-GM-CSF autoantibody-containing plasma inhibits GM-CSF induced pSTAT-5 in dose-dependent fashion. A. Representative dose-response curves for pSTAT-5 production in normal PBMC incubated with plasma from Patient 3 or normal plasma and stimulated with increasing amounts of GM-CSF. The concentration of GM-CSF required for 50% STAT-5 phosphorylation (EC50) was 798 ng/mL (R2=0.9954) and 0.0371 ng/mL (R2= 0.9999) for Patient 3 and normal plasma, respectively. B. The EC50 was determined from the dose-response curves generated for each of the 7 patient plasmas and 5 normal controls.
Anti-GM-CSF autoantibody containing plasma inhibits GM-CSF-induced MIP-1α
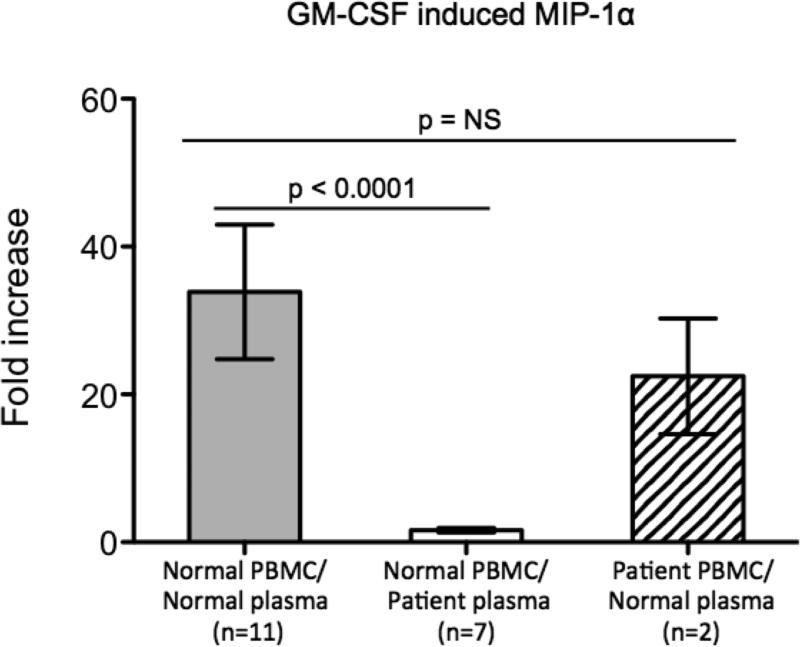
To further evaluate the downstream effects of anti-GM-CSF autoantibodies, plasma was tested for its ability to prevent GM-CSF-induced MIP-1α production from normal PBMC. Normal and patient PBMC washed of autologous plasma demonstrated that GM-CSF induced a 34-fold and 22-fold increase in MIP-1α production respectively (p=NS), whereas normal PBMC in the presence of anti-GM-CSF autoantibody-containing plasma demonstrated no increase in MIP-1α from baseline (p<0.0001) (Figure 5).
Figure 5.
Anti-GM-CSF autoantibody-containing plasma inhibits GM-CSF-induced MIP-1α protein expression. Normal PBMC incubated with normal or patient plasma and patient PBMC washed of autologous plasma were left unstimulated or stimulated with GM-CSF and fold-induction of MIP-1α production was determined. For each patient with anti-GM-CSF autoantibodies, values represent an average of 3 independent experiments.
Discussion
Cryptococcal meningitis is typically seen in immunocompromised hosts, but also occurs in those for whom no underlying immune defect has been found(25-31). Here we describe an association between biologically inhibitory anti-GM-CSF autoantibodies and cryptococcal meningitis in seven otherwise healthy HIV uninfected individuals. None of these patients carried the diagnosis of PAP at presentation of meningitis, and only one patient developed symptomatic PAP in the following years; a second patient had radiographic and cytopathologic changes without symptoms. Review of the medical records related to archived patient samples found no reports of pulmonary disease consistent with PAP. To our knowledge, Cryptococcus has been described in 4 patients with active PAP, including 2 with meningitis(4, 9, 17, 34), but all of these reports antedated the recognition of anti-GM-CSF autoantibodies as a cause of PAP.
Anticytokine autoantibodies, including those against GM-CSF, have been reported in normal hosts(18, 35),(36). Therefore, it is possible that there are asymptomatic people in the general population who have anti-GM-CSF autoantibodies that could lead to cryptococcal infection following sufficient exposure. Alternatively, GM-CSF is induced by cryptococcal infection and anti-GM-CSF autoantibodies might develop as a reactive phenomenon. The absence of high-titer anti-GM-CSF autoantibodies identified in both healthy and diseased controls (n=107, Figure 2A) as well as over 180 previously published controls (healthy volunteers and patients with Tuberculosis)(1, 37, 38), is consistent with a direct relationship between cryptococcal disease and anti-GM-CSF autoantibodies in a subset of patients.
Anti-GM-CSF autoantibodies in the context of PAP have broad immunological effects on monocytes, macrophages, and neutrophils(12-15), which appear to be important for host control of Cryptococcus. Studies in mice and in humans with HIV have shown that GM-CSF therapy may augment the effects of triazole therapy and increase cryptococcal killing by monocytes,(39, 40) suggesting a physiologic role for GM-CSF in host control of Cryptococcus. Additionally, Cryptococcus may diminish GM-CSF production by natural killer cells and T lymphocytes, thereby decreasing phagocyte and macrophage activity(41, 42). The IgG fraction of plasma from all 7 patients with anti-GM-CSF autoantibodies inhibited GM-CSF-induced STAT-5 phosphorylation (Figure 3) and eliminated GM-CSF-induced MIP-1α protein expression (Figure 5), proving that these autoantibodies are biologically active. GM-CSF plays a critical role in alveolar macrophage function and activation and pulmonary host defense, as clearly manifest by PAP and its complications. We hypothesize that GM-CSF-mediated macrophage function against Cryptococcus is important and that autoantibodies that block its function are etiologic and precede the infection causing cryptococcal susceptibility. As is the case with many autoantibody-mediated diseases, such as anti-IFNγ autoantibodies and adult-onset immunodeficiency, the trigger for the culprit autoantibody is unknown.
Complex factors such as titer, epitope, IgG subclass, and antibody avidity may contribute to disease severity and phenotype (i.e., PAP, crytptococcal meningitis, or both). Indeed, these factors have been shown to contribute to disease activity in other autoantibodies mediated syndromes.(43-45) Furthermore, susceptibility to Cryptococcus may only be identified in the case of sufficient exposure to this environmentally restricted organism.
In those for whom CSF was available, our patients with anti-GM-CSF autoantibodies in serum also had detectable levels in CSF, albeit at lower levels (Figure 2E). It is unclear whether these antibodies play a pathogenic role in permitting cryptococcal entry to the central nervous system. GM-CSF does not appear to influence CNS microglial killing of Cryptococcus, implicating other host defense mechanisms(46). For example, the lung may be an important portal of entry where defective alveolar macrophage(47, 48) activity allows for dissemination and subsequent CNS penetration.
Two patients from our archived samples had low positive anti-GM-CSF autoantibody titers (Figure 2A) that were not inhibitory in vitro (not shown). Given that these samples had been stored for more than 40 years, it is possible that their binding was nonspecific, or alternatively that they lost activity over the time they were in storage. Additionally, these patients had alternative explanations for their infection susceptibilities: one had end-stage cirrhosis and the other had diabetes mellitus and systemic lupus erythematosis for which he was receiving chronic steroid therapy. Conversely, Patient 4 had low anti-GM-CSF autoantibody titers but they were fully inhibitory. The plasma sample for this patient was collected four years after his initial meningitis diagnosis and in the setting of inactive disease, raising the possibility that his titers may have declined over time. Decreasing titers in concordance with clinical improvement have been demonstrated in other anticytokine autoantibody syndromes(44, 49).
Consistent with previous reports (50), all 7 patients with anti-GM-CSF autoantibodies and the 3 with PAP had IgG1 subclass antibodies. Conversely, in other autoantibody syndromes, such as anti-IFNγ autoantibodies and pemphigus vulgaris (anti-desmoglein autoantibodies) the predominating subclasses were either IgG3 or IgG4(37, 51). The influence of infection, cytokine, age and gender on pathologic IgG autoantibody subclass and disease pathogenesis remain to be dissected.
All four contemporary patients with cryptococcal meningitis and anti-GM-CSF autoantibodies responded well to standard antifungal therapy. One patient had symptomatic PAP, which was treated with whole lung lavage alone and one patient has radiographic and cytopathologic evidence of PAP without clinical manifestations. Somewhat surprisingly and in contrast to the experience with anti-IFNγ autoantibody-associated disseminated mycobacterial disease32, the cases of cryptococcal meningitis have remained well to date after successful therapy. Subcutaneous and inhaled GMCSF have shown activity in other PAP patients, and anti-CD20 therapy (rituximab) has demonstrated encouraging results for refractory acquired PAP (49, 52) as well as against anti-IFNγ autoantibodies (44).
Further prospective work must be done to definitively establish the relationship between anti-GM-CSF autoantibodies in otherwise immunocompetent patients and the development of cryptococcal meningitis. Finding anti-GM-CSF autoantibodies should prompt consideration of PAP, which may develop after meningitis. Together, these data implicate GM-CSF as a critical actor in host defense against Cryptococcus and support a direct role for anti-GM-CSF autoantibodies in some cases of cryptococcal infection.
Acknowledgments
Funding
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
References
- 1.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka N, Watanabe J, Kitamura T, Yamada Y, Kanegasaki S, Nakata K. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte-macrophage colony stimulating factor. FEBS Lett. 1999;442:246–250. doi: 10.1016/s0014-5793(98)01668-8. [DOI] [PubMed] [Google Scholar]
- 3.Andersen BR, Ecklund RE, Kellow WF. Pulmonary alveolar proteinosis with systemic nocardiosis. A case report. Jama. 1960;174:28–31. doi: 10.1001/jama.1960.03030010030008. [DOI] [PubMed] [Google Scholar]
- 4.Bergman F, Linell F. Cryptococcosis as a cause of pulmonary alveolar proteinosis. Acta pathologica et microbiologica Scandinavica. 1961;53:217–224. doi: 10.1111/j.1699-0463.1961.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 5.Clague HW, Harth M, Hellyer D, Morgan WK. Septic arthritis due to Nocardia asteroides in association with pulmonary alveolar proteinosis. J Rheumatol. 1982;9:469–472. [PubMed] [Google Scholar]
- 6.Fried J, Hinthorn D, Ralstin J, Gerjarusak P, Liu C. Cure of brain abscess caused by Nocardia asteroides resistant to multiple antibiotics. South Med J. 1988;81:412–413. doi: 10.1097/00007611-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 7.Hartung M, Salfelder K. Pulmonary alveolar proteinosis and histoplasmosis: report of three cases. Virchows Arch A Pathol Anat Histol. 1975;368:281–287. doi: 10.1007/BF00432306. [DOI] [PubMed] [Google Scholar]
- 8.Oerlemans WG, Jansen EN, Prevo RL, Eijsvogel MM. Primary cerebellar nocardiosis and alveolar proteinosis. Acta Neurol Scand. 1998;97:138–141. doi: 10.1111/j.1600-0404.1998.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. The New England journal of medicine. 1958;258:1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 10.Walker DA, McMahon SM. Pulmonary alveolar proteinosis complicated by cerebral abscess: report of a case. J Am Osteopath Assoc. 1986;86:447–450. [PubMed] [Google Scholar]
- 11.Witty LA, Tapson VF, Piantadosi CA. Isolation of mycobacteria in patients with pulmonary alveolar proteinosis. Medicine (Baltimore) 1994;73:103–109. doi: 10.1097/00005792-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Golde DW, Territo M, Finley TN, Cline MJ. Defective lung macrophages in pulmonary alveolar proteinosis. Ann Intern Med. 1976;85:304–309. doi: 10.7326/0003-4819-85-3-304. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Rothi RJ, Harris JO. Pulmonary alveolar proteinosis. Further evaluation of abnormal alveolar macrophages. Chest. 1986;90:656–661. doi: 10.1378/chest.90.5.656. [DOI] [PubMed] [Google Scholar]
- 14.Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, Puchalski JT, Hauck DM, Trapnell BC. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. The New England journal of medicine. 2007;356:567–579. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- 16.Lee YC, Chew GT, Robinson BW. Pulmonary and meningeal cryptococcosis in pulmonary alveolar proteinosis. Australian and New Zealand journal of medicine. 1999;29:843–844. doi: 10.1111/j.1445-5994.1999.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 17.Sunderland WA, Campbell RA, Edwards MJ. Pulmonary alveolar proteinosis and pulmonary cryptococcosis in an adolescent boy. J Pediatr. 1972;80:450–456. doi: 10.1016/s0022-3476(72)80503-1. [DOI] [PubMed] [Google Scholar]
- 18.Browne SK, Holland SM. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. The Lancet infectious diseases. 2010;10:875–885. doi: 10.1016/S1473-3099(10)70196-1. [DOI] [PubMed] [Google Scholar]
- 19.Doffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, Espitia-Pinzon C, Barnes N, Bothamley G, Casanova JL, Longhurst HJ, Kumararatne DS. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38:e10–14. doi: 10.1086/380453. [DOI] [PubMed] [Google Scholar]
- 20.Hoflich C, Sabat R, Rosseau S, Temmesfeld B, Slevogt H, Docke WD, Grutz G, Meisel C, Halle E, Gobel UB, Volk HD, Suttorp N. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 2004;103:673–675. doi: 10.1182/blood-2003-04-1065. [DOI] [PubMed] [Google Scholar]
- 21.Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, Nicol M, Scholvinck E, Relman D, Waddell S, Langford P, Sheehan B, Semple L, Wilkinson KA, Wilkinson RJ, Ress S, Hibberd M, Levin M. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115:2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, Martyak LA, Kubak B, Holland SM. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175:4769–4776. doi: 10.4049/jimmunol.175.7.4769. [DOI] [PubMed] [Google Scholar]
- 23.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, Macdougall L, Boekhout T, Kwon-Chung KJ, Meyer W. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17258–17263. doi: 10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, Papanaoum K, Richards MJ, Gottlieb T. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis. 1995;20:611–616. doi: 10.1093/clinids/20.3.611. [DOI] [PubMed] [Google Scholar]
- 27.Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Henderson H, Kauffman CA, Haas DW, Saccente M, Hamill RJ, Holloway MS, Warren RM, Dismukes WE. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 28.Chau TT, Mai NH, Phu NH, Nghia HD, Chuong LV, Sinh DX, Duong VA, Diep PT, Campbell JI, Baker S, Hien TT, Lalloo DG, Farrar JJ, Day JN. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC infectious diseases. 2010;10:199. doi: 10.1186/1471-2334-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lui G, Lee N, Ip M, Choi KW, Tso YK, Lam E, Chau S, Lai R, Cockram CS. Cryptococcosis in apparently immunocompetent patients. QJM : monthly journal of the Association of Physicians. 2006;99:143–151. doi: 10.1093/qjmed/hcl014. [DOI] [PubMed] [Google Scholar]
- 30.Sanchetee P. Cryptococcal meningitis in immunocompetent patients. The Journal of the Association of Physicians of India. 1998;46:617–619. [PubMed] [Google Scholar]
- 31.Bichile LS, Gokhale YA, Sridhar V, Gill NH. Disseminated cryptococcal infection in immune competent patients. The Journal of the Association of Physicians of India. 2001;49:377–378. [PubMed] [Google Scholar]
- 32.Ding L, Mo A, Jutivorakool K, Pancholi M, Holland SM, Browne SK. Determination of Human Anticytokine Autoantibody Profiles Using a Particle-Based Approach. J Clin Immunol. 2011 doi: 10.1007/s10875-011-9621-8. [DOI] [PubMed] [Google Scholar]
- 33.Holland SM, Dorman SE, Kwon A, Pitha-Rowe IF, Frucht DM, Gerstberger SM, Noel GJ, Vesterhus P, Brown MR, Fleisher TA. Abnormal regulation of interferon-gamma, interleukin-12, and tumor necrosis factor-alpha in human interferon-gamma receptor 1 deficiency. The Journal of infectious diseases. 1998;178:1095–1104. doi: 10.1086/515670. [DOI] [PubMed] [Google Scholar]
- 34.Lee MG, Spencer H, Clarke WF, Rao BN, Lowe M, Nelson M. Pulmonary alveolar proteinosis in Jamaica. West Indian Med J. 1982;31:103–110. [PubMed] [Google Scholar]
- 35.Watanabe M, Uchida K, Nakagaki K, Trapnell BC, Nakata K. High avidity cytokine autoantibodies in health and disease: pathogenesis and mechanisms. Cytokine Growth Factor Rev. 2010;21:263–273. doi: 10.1016/j.cytogfr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, Stevens CA, Beck DC, Denson LA, Carey BC, Keicho N, Krischer JP, Yamada Y, Trapnell BC. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113:2547–2556. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, Kirk JL, Jutivorakool K, Zaman R, Ding L, Hsu AP, Patel SY, Olivier KN, Lulitanond V, Mootsikapun P, Anunnatsiri S, Angkasekwinai N, Sathapatayavongs B, Hsueh PR, Shieh CC, Brown MR, Thongnoppakhun W, Claypool R, Sampaio EP, Thepthai C, Waywa D, Dacombe C, Reizes Y, Zelazny AM, Saleeb P, Rosen LB, Mo A, Iadarola M, Holland SM. Adult-onset immunodeficiency in Thailand and Taiwan. The New England journal of medicine. 2012;367:725–734. doi: 10.1056/NEJMoa1111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, Kristosturyan E, Rajan A, Ding L, Ching KH, Berman A, Oliveira JB, Hsu AP, Klimavicz CM, Iadarola MJ, Holland SM. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 2010;116:4848–4858. doi: 10.1182/blood-2010-05-286161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiller T, Farrokhshad K, Brummer E, Stevens DA. Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on polymorphonuclear neutrophils, monocytes or monocyte-derived macrophages combined with voriconazole against Cryptococcus neoformans. Medical mycology : official publication of the International Society for Human and Animal Mycology. 2002;40:21–26. doi: 10.1080/mmy.40.1.21.26. [DOI] [PubMed] [Google Scholar]
- 40.Tascini C, Vecchiarelli A, Preziosi R, Francisci D, Bistoni F, Baldelli F. Granulocyte-macrophage colony-stimulating factor and fluconazole enhance anticryptococcal activity of monocytes from AIDS patients. AIDS. 1999;13:49–55. doi: 10.1097/00002030-199901140-00007. [DOI] [PubMed] [Google Scholar]
- 41.Murphy JW, Zhou A, Wong SC. Direct interactions of human natural killer cells with Cryptococcus neoformans inhibit granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha production. Infection and immunity. 1997;65:4564–4571. doi: 10.1128/iai.65.11.4564-4571.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen GH, Curtis JL, Mody CH, Christensen PJ, Armstrong LR, Toews GB. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol. 1994;152:724–734. [PubMed] [Google Scholar]
- 43.Bhol K, Natarajan K, Nagarwalla N, Mohimen A, Aoki V, Ahmed AR. Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigus vulgaris: a model for autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:5239–5243. doi: 10.1073/pnas.92.11.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, Pancholi MJ, Yang LM, Long Priel D, Uzel G, Freeman AF, Hayes CE, Baxter R, Cohen SH, Holland SM. Anti-CD20 (Rituximab) therapy for anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012 doi: 10.1182/blood-2011-12-395707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson SJ, Ferbas J, Mayeux P, Casadevall N. Evaluation of methods to detect and characterize antibodies against recombinant human erythropoietin. Nephron. Clinical practice. 2004;96:c88–95. doi: 10.1159/000076746. [DOI] [PubMed] [Google Scholar]
- 46.Lipovsky MM, Juliana AE, Gekker G, Hu S, Hoepelman AI, Peterson PK. Effect of cytokines on anticryptococcal activity of human microglial cells. Clin Diagn Lab Immunol. 1998;5:410–411. doi: 10.1128/cdli.5.3.410-411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olszewski MA, Zhang Y, Huffnagle GB. Mechanisms of cryptococcal virulence and persistence. Future microbiology. 2010;5:1269–1288. doi: 10.2217/fmb.10.93. [DOI] [PubMed] [Google Scholar]
- 48.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, Eishi Y, Kitamura T, Yamada Y, Hanaoka K, Keicho N. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 49.Kavuru MS, Malur A, Marshall I, Barna BP, Meziane M, Huizar I, Dalrymple H, Karnekar R, Thomassen MJ. An Open-Label Trial of Rituximab Therapy in Pulmonary Alveolar Proteinosis. Eur Respir J. 2011 doi: 10.1183/09031936.00197710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura T, Uchida K, Tanaka N, Tsuchiya T, Watanabe J, Yamada Y, Hanaoka K, Seymour JF, Schoch OD, Doyle I, Inoue Y, Sakatani M, Kudoh S, Azuma A, Nukiwa T, Tomita T, Katagiri M, Fujita A, Kurashima A, Kanegasaki S, Nakata K. Serological diagnosis of idiopathic pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;162:658–662. doi: 10.1164/ajrccm.162.2.9910032. [DOI] [PubMed] [Google Scholar]
- 51.Bhol K, Mohimen A, Ahmed AR. Correlation of subclasses of IgG with disease activity in pemphigus vulgaris. Dermatology. 1994;189(Suppl 1):85–89. doi: 10.1159/000246938. [DOI] [PubMed] [Google Scholar]
- 52.Borie R, Debray MP, Laine C, Aubier M, Crestani B. Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1503–1506. doi: 10.1183/09031936.00160908. [DOI] [PubMed] [Google Scholar]