Abstract
Purpose
The optimal management of stage I follicular lymphoma, according to consensus guidelines, is based on uncontrolled experiences of select institutions. Diverse treatment approaches are used despite guidelines that recommend radiation therapy (XRT).
Patients and Methods
We analyzed outcomes of patients with stage I follicular lymphoma enrolled onto the National LymphoCare database.
Results
Of 471 patients with stage I follicular lymphoma, 206 patients underwent rigorous staging as defined by both a bone marrow aspirate and biopsy and an imaging study (a computed tomography [CT] scan of the whole body, a positron emission tomography [PET]/CT scan, or both). Rigorously staged patients had superior progression-free survival (PFS) compared with nonrigorously staged patients (hazard ratio [HR], 0.63). Treatments given to rigorously staged patients were rituximab/chemotherapy (R-chemo; 28%), XRT (27%), observation (17%), systemic therapy + XRT (13%), rituximab monotherapy (12%), and other (3%). With a median follow-up of 57 months for PFS, there were 44 progression events (in 21% of patients) for rigorously staged patients. For these patients, PFS was significantly improved with either R-chemo or systemic therapy + XRT compared with patients receiving XRT alone after adjustment for histology, LDH, and the presence of B symptoms. There were no differences in overall survival.
Conclusion
In this largest, prospectively enrolled group of patients with stage I follicular lymphoma, variable treatment approaches resulted in similar excellent outcomes, which challenges the paradigm that XRT should be standard for this presentation.
INTRODUCTION
Follicular lymphoma is the second most common histology of non-Hodgkin's lymphoma in the United States and has significantly increased in incidence over the past three decades.1 In a recent analysis of the SEER database from the United States, 26% of patients with follicular lymphoma presented with stage I disease, which was defined as a single lymph node site and no bone marrow involvement.2 Practice guidelines, including those from the National Comprehensive Cancer Network, recommend radiation therapy (XRT) as the preferred treatment approach in this setting3 on the basis of several retrospective series from single institutions that demonstrated that a proportion of patients achieve long-term disease control with this modality.4 However, to our knowledge, no prospective randomized trials and few retrospective reports have compared XRT with other modalities. A recent SEER database analysis demonstrated that only 34% of patients in the United States with early-stage follicular lymphoma were treated with XRT, suggesting a need to better understand alternative management strategies.5
The National LymphoCare Study is a disease-specific, prospective registry that enrolled more than 2,700 patients with newly diagnosed follicular lymphoma from 2004 to 2007 from more than 200 practice sites in the United States. We previously analyzed patterns of care in the National LymphoCare Study and demonstrated marked variability in the approach to patients with de novo stage I follicular lymphoma.6 Less than one third of patients were treated with XRT, and other modalities used for treatment included observation, single-agent rituximab, and rituximab/chemotherapy (R-chemo) combinations.
With more than 5 years of follow-up, we analyzed the outcomes of patients with stage I follicular lymphoma treated with these various modalities by using this unique resource.
PATIENTS AND METHODS
Details of the conception and operation of the National LymphoCare Study (supported by Genentech, South San Francisco, CA, and Biogen Idec, Cambridge, MA) have been previously published.6 All authors of this article serve on the advisory board for this study and had full access to data listings and analysis of this cohort of patients. This article was written by the author members of the advisory board. Patients signed informed consent before participation, and the protocol was approved by an institutional review board. Between March 2004 and March 2007, consecutive patients with newly diagnosed (within 6 months) follicular lymphoma at participating sites were recruited. There was no central pathology review; the local pathology report defined follicular lymphoma diagnosis following investigator training on WHO definitions of follicular lymphoma.
All treatment strategies that patients received for follicular lymphoma were recorded, including a watch-and-wait strategy, which is referred to as observation. Patients who did not receive therapy within 90 days of diagnosis date were considered to be in the observation cohort. Patients treated on a clinical trial were coded as clinical trial, even if agents were available commercially. Patients who received XRT within 2 months of systemic therapy without evidence of disease progression were defined as patients treated with a systemic therapy + XRT approach.
Treatment and outcomes (including response, time to progression, and survival) are collected quarterly. The progression-free survival (PFS) for patients initially observed was defined as diagnosis to first progression. Follow-up data are actively solicited from providers at the time of clinical follow-up. Enrolled patients are followed up to 10 years from enrollment or until death, withdrawal of consent, or loss to follow-up.
Patient stage was determined by the treating physician. Staging procedures, including a bone marrow biopsy, computed tomography imaging, [18F]fluorodeoxyglucose, and positron emission tomography (PET) scanning are recorded in the database. For this analysis, rigorously staged patients were defined as patients who were staged with a bone marrow biopsy and either computed tomography scans, [18F]fluorodeoxyglucose-PET scans, or both. Nonrigorously staged patients were missing bone marrow biopsy or complete imaging studies.
Demographics, baseline disease characteristics, and initial treatment strategy, were summarized by using descriptive statistics. Univariate associations between demographic, baseline disease characteristics, and staging method or initial treatment were evaluated by using Pearson's χ2 test or Fisher's exact test if required by sample size. Median PFS, which was defined as documented disease progression or death by any cause, and associated 95% CIs were estimated by using the Kaplan-Meier method. Hazard ratios (HRs) and associated 95% CIs were estimated by using Cox regression (Cox proportional hazards model). Variables that showed different distribution across groups to be compared were included in the Cox models. Cox regression models comparing staging procedures were adjusted for age (≤ 60 v > 60 years), treatment (observation, rituximab, R-chemo, XRT, systemic therapy + XRT, or other, each as an indicator variable in the model), and practice setting (academic v community). Cox regression models comparing first-line treatment were adjusted for histology (grades 1 or 2 v 3), serum LDH (normal v > upper limit of normal), and the presence of B symptoms. Both adjusted and unadjusted HRs are presented.
RESULTS
Patients and Staging Procedures
A total of 471 patients with stage I follicular lymphoma were identified in the National LymphoCare Study. Details of these patients are listed in Table 1. Similar to the entire database, more than 80% of patients were enrolled from community-based practices. Of these 471 patients, 206 patients underwent rigorous staging, as defined in Patients and Methods. Disease characteristics were similar for rigorously and nonrigorously staged patients, with no statistically significant differences in rates of hemoglobin less than 12 g/dL, serum LDH greater than the upper limit of normal, intermediate- or poor-risk features by using the Follicular Lymphoma Prognostic Index (FLIPI), grade 3 histology, or the presence of B symptoms (Table 1). A greater percentage of nonrigorously staged patients were older than age 60 years compared with rigorously staged patients (64% v 52%, respectively; P = .008). There was a statistically significant difference in treatment selection for rigorously versus nonrigorously staged patients (P < .001), with more rigorously staged patients receiving R-chemo (28% v 17% of nonrigorously staged patients) and systemic therapy + XRT (13% v 6% of nonrigorously staged patients) and fewer rigorously staged patients receiving watchful waiting (17% v 39% of nonrigorously staged patients). A greater percentage of patients from academic centers were rigorously staged compared with patients from community centers (52% v 42%, respectively), although the difference was not statistically significant.
Table 1.
Patient Demographics and Clinical Characteristics: All Patients With Stage I Disease
| Demographic or Characteristic | Nonrigorous |
Rigorous |
All Stage I |
P * | |||
|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Age, years | .01 | ||||||
| ≤ 60 | 95 | 36 | 99 | 48 | 194 | 41 | |
| > 60 | 170 | 64 | 107 | 52 | 277 | 59 | |
| Hemoglobin, g/dL | .54 | ||||||
| < 12 | 39 | 16 | 27 | 14 | 66 | 15 | |
| ≥ 12 | 202 | 84 | 165 | 86 | 367 | 85 | |
| Missing | 24 | 14 | 38 | ||||
| Serum LDH | .51 | ||||||
| Normal | 169 | 87 | 135 | 89 | 304 | 88 | |
| > ULN | 25 | 13 | 16 | 11 | 41 | 12 | |
| FLIPI | .55 | ||||||
| Good | 163 | 84 | 140 | 86 | 303 | 85 | |
| Intermediate/poor | 32 | 16 | 23 | 14 | 55 | 15 | |
| Histologic diagnosis | .86 | ||||||
| Grade 1 or 2 | 195 | 80 | 144 | 80 | 339 | 80 | |
| Grade 3 | 48 | 20 | 37 | 20 | 85 | 20 | |
| B symptoms | .58 | ||||||
| No | 231 | 87 | 183 | 89 | 414 | 88 | |
| Yes | 34 | 13 | 23 | 11 | 57 | 12 | |
| Practice setting | .10 | ||||||
| Academic | 41 | 15 | 44 | 21 | 85 | 18 | |
| Community | 224 | 85 | 162 | 79 | 386 | 82 | |
| Treatment | < .001 | ||||||
| Observation | 104 | 39 | 35 | 17 | 139 | 30 | |
| R-mono | 34 | 13 | 25 | 12 | 59 | 13 | |
| R-chemo | 45 | 17 | 57 | 28 | 102 | 22 | |
| XRT | 54 | 20 | 56 | 27 | 110 | 23 | |
| Systemic therapy + XRT | 15 | 6 | 26 | 13 | 41 | 9 | |
| Other | 13 | 5 | 7 | 3 | 20 | 4 | |
Abbreviations: FLIPI, Follicular Lymphoma Prognostic Index; LDH, lactate dehydrogenase; R-chemo, rituximab/chemotherapy; R-mono, rituximab monotherapy; ULN, upper limit of normal; XRT, radiation therapy.
Calculated by using the χ2 test.
In the rigorously staged group of patients, 128 patients had staging that included a PET scan. Patient characteristics were similar for PET and non-PET rigorously staged patients (Table 2). Community centers were more likely to rigorously stage by using PET than were academic centers (48% v 66%; P = .026).
Table 2.
Patient Demographics and Clinical Characteristics: All Rigorously Staged Patients With Stage I Disease
| Demographic or Characteristic | Staged Without PET Scan |
Staged With PET Scan |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .32* | ||||
| ≤ 60 | 34 | 44 | 65 | 51 | |
| > 60 | 44 | 56 | 63 | 49 | |
| Hemoglobin, g/dL | .95* | ||||
| < 12 | 10 | 14 | 17 | 14 | |
| ≥ 12 | 60 | 86 | 105 | 86 | |
| Serum LDH | .73* | ||||
| Normal | 48 | 91 | 87 | 89 | |
| > ULN | 5 | 9 | 11 | 11 | |
| FLIPI | .98* | ||||
| Good | 49 | 86 | 91 | 86 | |
| Intermediate/poor | 8 | 14 | 15 | 14 | |
| Histologic diagnosis | .10* | ||||
| Grade 1 or 2 | 56 | 86 | 88 | 76 | |
| Grade 3 | 9 | 14 | 28 | 24 | |
| B symptoms | .44* | ||||
| No | 71 | 91 | 112 | 88 | |
| Yes | 7 | 9 | 16 | 12 | |
| Practice setting | .03* | ||||
| Academic | 23 | 29 | 21 | 16 | |
| Community | 55 | 71 | 107 | 84 | |
| Treatment | .47† | ||||
| Watchful waiting | 16 | 21 | 19 | 15 | |
| R-mono | 10 | 13 | 15 | 12 | |
| R-chemo | 25 | 32 | 32 | 25 | |
| XRT | 18 | 23 | 38 | 30 | |
| CM:XRT | 8 | 10 | 18 | 14 | |
| Other | 1 | 1 | 6 | 5 | |
Abbreviations: CM:XRT, combined modality with radiation therapy; FLIPI, Follicular Lymphoma Prognostic Index; LDH, lactate dehydrogenase; PET, positron emission tomography; R-chemo, rituximab/chemotherapy; R-mono, rituximab monotherapy; ULN, upper limit of normal; XRT, radiation therapy.
Calculated by using the χ2 test.
Calculated by using Fisher's exact test.
Treatment Selection
As previously reported, diverse treatment selections were made for these patients, as detailed in Table 3. Most patients treated with systemic therapy + XRT received abbreviated chemoimmunotherapy before radiation. Among rigorously staged patients there were statistically significant differences in treatment selection between patients with increased versus normal serum LDH (P = .017), grade 3 versus grade 1 or 2 histology (P < .001), and with B symptoms versus without B symptoms (P = .031; Table 3). There were no patients who were observed and had increased LDH, compared with 20% of rituximab patients and 22% of R-chemo patients. Patients receiving systemic therapy + XRT were most likely to have received R-CHOP or R-CVP as systemic therapy and have grade 3 histology (57%) compared with 27% of chemotherapy/rituximab patients, 12% of XRT patients, 9% of watchful waiting patients, and 8% of rituximab monotherapy patients. Patients receiving systemic therapy + XRT were also most likely to have B symptoms (23%) versus 20% of those receiving rituximab, 15% of XRT patients, 12% of R-chemo patients, and 3% of observation patients.
Table 3.
Patient Demographics and Clinical Characteristics: All Rigorously Staged Patients With Stage I Disease Receiving Watchful Waiting, R-Mono, R-Chemo, XRT, or CM:XRT
| Demographic or Characteristic | Watchful Waiting |
R-Mono |
R-Chemo |
XRT |
CM:XRT |
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Age, years | .30* | ||||||||||
| ≤ 60 | 12 | 34 | 11 | 44 | 32 | 56 | 25 | 45 | 14 | 54 | |
| > 60 | 23 | 66 | 14 | 56 | 25 | 44 | 31 | 55 | 12 | 46 | |
| Hemoglobin, g/dL | .74† | ||||||||||
| < 12 | 4 | 14 | 4 | 16 | 10 | 19 | 5 | 9 | 3 | 13 | |
| ≥ 12 | 25 | 86 | 21 | 84 | 44 | 81 | 48 | 91 | 21 | 88 | |
| Serum LDH | .02† | ||||||||||
| Normal | 22 | 100 | 16 | 80 | 32 | 78 | 38 | 95 | 21 | 95 | |
| > ULN | 0 | 0 | 4 | 20 | 9 | 22 | 2 | 5 | 1 | 5 | |
| FLIPI | .19† | ||||||||||
| Good | 20 | 87 | 16 | 73 | 37 | 80 | 40 | 93 | 20 | 91 | |
| Intermediate/poor | 3 | 13 | 6 | 27 | 9 | 20 | 3 | 7 | 2 | 9 | |
| Histologic diagnosis | < .001† | ||||||||||
| Grade 1 or 2 | 29 | 91 | 22 | 92 | 36 | 73 | 42 | 88 | 9 | 43 | |
| Grade 3 | 3 | 9 | 2 | 8 | 13 | 27 | 6 | 12 | 12 | 57 | |
| B symptoms | .03† | ||||||||||
| No | 34 | 97 | 20 | 80 | 50 | 88 | 53 | 95 | 20 | 77 | |
| Yes | 1 | 3 | 5 | 20 | 7 | 12 | 3 | 15 | 6 | 23 | |
| Practice setting | .28† | ||||||||||
| Academic | 6 | 17 | 2 | 8 | 13 | 23 | 16 | 29 | 7 | 27 | |
| Community | 29 | 83 | 23 | 92 | 44 | 77 | 40 | 71 | 19 | 73 | |
Abbreviations: CM:XRT, combined modality with radiation therapy; FLIPI, Follicular Lymphoma Prognostic Index; LDH, lactate dehydrogenase; R-chemo, rituximab/chemotherapy; R-mono, rituximab monotherapy; ULN, upper limit of normal; XRT, radiation therapy.
Calculated by using the χ2 test.
Calculated by using Fisher's exact test.
Outcome by Staging Procedure
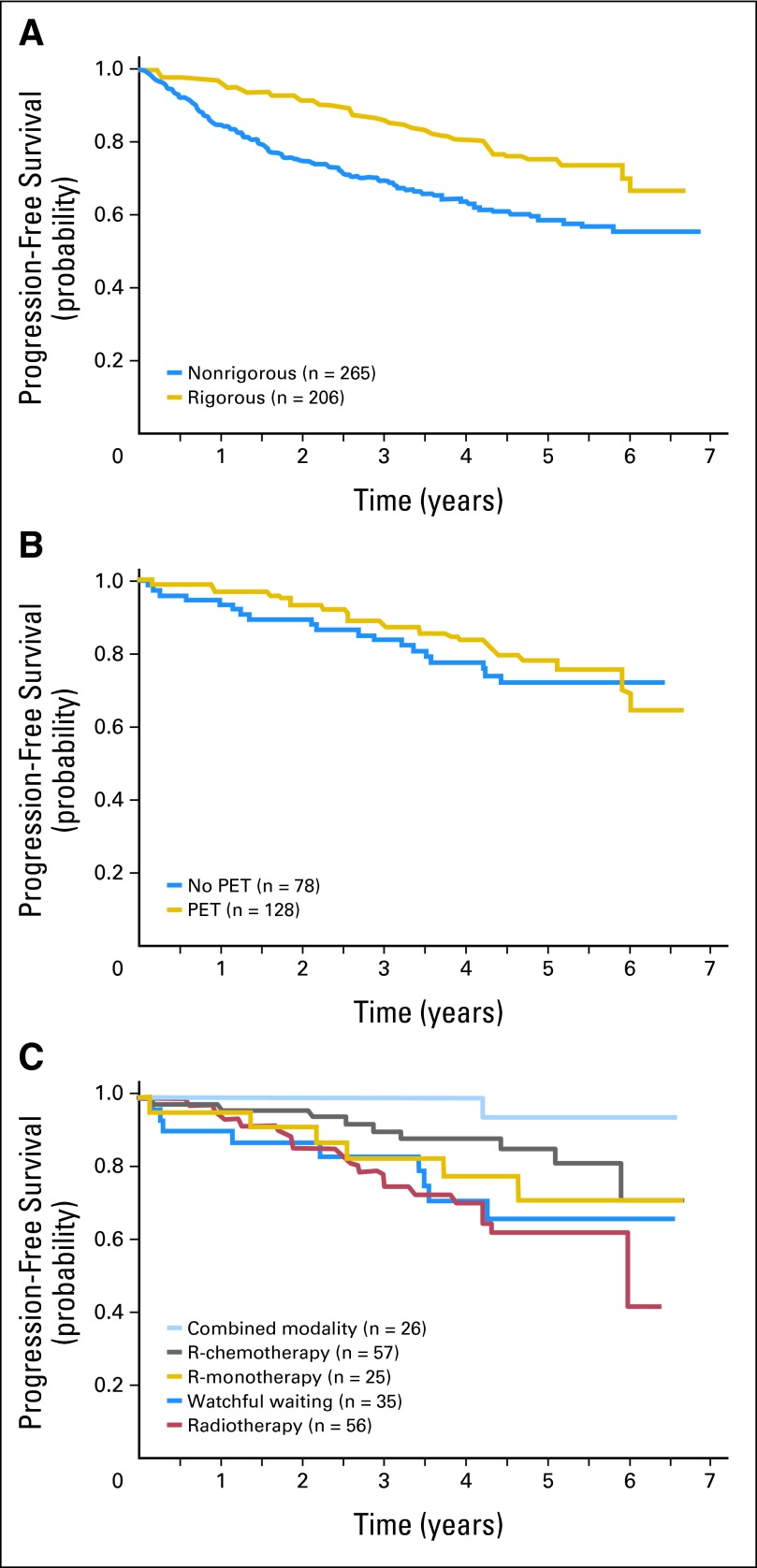
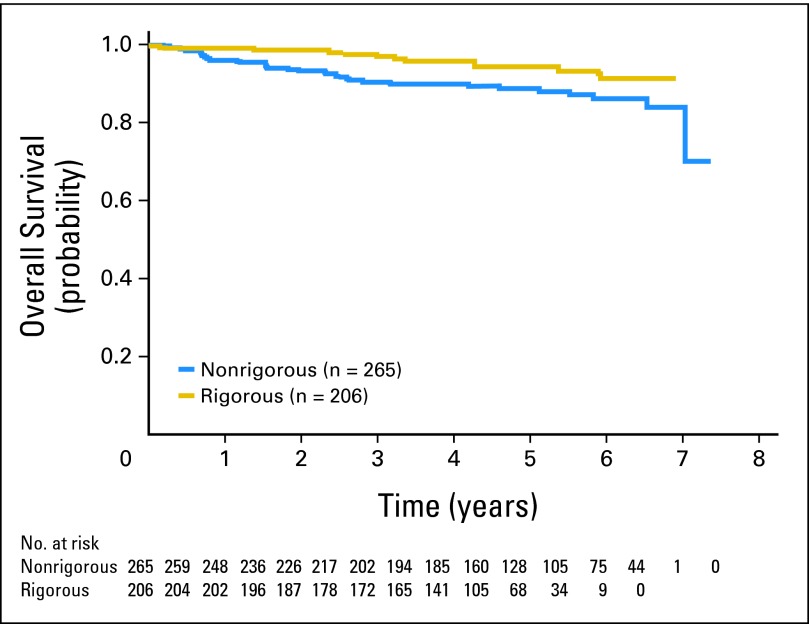
After adjustment for age, treatment, and practice setting, rigorously staged patients had superior PFS compared with nonrigorously staged patients (HR, 0.63; 95% CI, 0.44 to 0.92); there was no difference in overall survival. Even when observed patients were removed, there was a trend toward improved PFS for rigorously staged patients. However, for rigorously staged patients, there was no significant difference in PFS relative to whether or not staging included a PET scan (HR, 0.87; 95% CI, 0.47 to 1.62). PFS and overall survival curves are shown in Figures 1 and 2, respectively.
Fig 1.
(A) Progression-free survival (PFS) of patients with stage I follicular lymphoma in the National LymphoCare Study comparing rigorously staged patients (defined as computed tomography with or without positron emission tomography [PET] and a bone marrow assessment) and nonrigorously staged patients. Nonrigorously staged patients had inferior PFS. (B) PFS of rigorously staged patients with stage I follicular lymphoma in the National LymphoCare study comparing outcome of patients staged with or without PET imaging. There was no difference in outcomes whether or not PET imaging was included in staging. (C) PFS of rigorously staged patients with stage I follicular lymphoma by treatment modality. Compared with patients treated with radiation therapy, patients treated with rituximab-containing chemotherapy (R-chemotherapy) or systemic therapy and radiation therapy had significantly better PFS. R-monotherapy, rituximab monotherapy.
Fig 2.
Overall survival of patients with stage I follicular lymphoma in the National LymphoCare Study. There was no difference between rigorously staged and nonrigorously staged patients.
Outcome by Treatment Regimen
The analysis of outcome by regimen was limited to the rigorously staged group of 206 patients. With a median follow-up of 57 months for PFS, there have been 44 PFS events (21% of patients). For rigorously staged patients, patients receiving either R-chemo or systemic therapy + XRT had significantly improved PFS compared with patients receiving XRT alone after adjustment for histology, LDH, and the presence of B symptoms (HRs of 0.36 [95% CI, 0.16 to 0.82] and 0.11 [95% CI, 0.01 to 0.83], respectively; Table 4). There was no significant difference when R-chemo–treated patients were compared with patients treated with rituximab monotherapy (HR, 0.65; 95% CI, 0.23 to 1.84). There were no differences in overall survival between treatment groups. Survival curves are shown in Figure 1.
Table 4.
PFS by Treatment: All Rigorously Staged Patients With Stage I Disease Receiving Watchful Waiting, R-Mono, R-Chemo, XRT, or CM:XRT
| Outcome | XRT (n = 56) |
Watchful Waiting(n = 35) |
R-Mono (n = 25) |
R-Chemo (n = 57) |
CM:XRT (n = 26) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| PFS events | ||||||||||
| No. | 18 | 9 | 6 | 9 | 1 | |||||
| % | 32 | 26 | 24 | 16 | 4 | |||||
| PFS, months | ||||||||||
| Median | 72 | NR | NR | NR | NR | |||||
| 95% CI | 50.5 to NR | |||||||||
| Relative to XRT | ||||||||||
| Unadjusted | 0.93 | 0.42 to 2.08 | 0.65 | 0.26 to 1.63 | 0.39 | 0.18 to 0.87 | 0.09 | 0.01 to 0.67 | ||
| Adjusted* | 1.02 | 0.45 to 2.34 | 0.56 | 0.21 to 1.48 | 0.36 | 0.16 to 0.82 | 0.11 | 0.01 to 0.83 | ||
| Relative to watchful waiting | ||||||||||
| Unadjusted | 0.69 | 0.25 to 1.95 | 0.42 | 0.17 to 1.06 | 0.10 | 0.01 to 0.76 | ||||
| Adjusted* | 0.55 | 0.18 to 1.62 | 0.35 | 0.13 to 0.94 | 0.10 | 0.01 to 0.88 | ||||
| Relative to R-mono | ||||||||||
| Unadjusted | 0.60 | 0.21 to 1.70 | 0.14 | 0.02 to 1.15 | ||||||
| Adjusted* | 0.65 | 0.23 to 1.84 | 0.19 | 0.02 to 1.67 | ||||||
NOTE. The median PFS follow-up for rigorously staged patients with stage I disease was 57 months.
Abbreviations: CM:XRT, combined modality with radiation therapy; HR, hazard ratio; NR, median PFS was not reached; PFS, progression-free survival; R-chemo, rituximab/chemotherapy; R-mono, rituximab monotherapy; XRT, radiation therapy.
Adjusted for grade, LDH, and B symptoms.
Because grade 3 histology is thought by some individuals to have a unique natural history, a sensitivity analysis was performed limited to patients with grade 1 or 2 histology. Compared with patients treated with XRT alone, rigorously staged patients with grade 1 or 2 histology treated with R-chemo continued to have improved PFS (HR, 0.43; 95% CI, 0.19 to 0.96). Systemic therapy + XRT could not be evaluated as a result of low numbers of events.
DISCUSSION
To our knowledge, this is the largest published series of prospectively enrolled patients with stage I follicular lymphoma in the modern therapy era. We have confirmed the high survival rate of these patients with more than 5 years of follow-up. These excellent outcomes were obtained in a group of rigorously staged patients with diverse treatment approaches. Practice guidelines endorsing XRT were not followed for the majority of patients. A recent analysis of guideline adherence in patients from the Netherlands with lymphoma suggested that both patient- and tumor-related factors impede adherence to guidelines, and the need to improve care guidelines in lymphoma is higher than for several other tumor types.7 A comprehensive approach that targets the physician, team members, hospitals, and care environment is required to improve guideline adherence.8 However, our data question whether XRT, which is the historical standard, is the best choice and whether it has any impact on outcomes in this group of patients compared with other treatment modalities, including observation.
The literature describing outcomes of early-stage follicular lymphoma treated with XRT alone largely consists of retrospective accounts of selected patients from single institutions treated in an era before modern chemotherapy and rituximab and before modern staging procedures.9–16 These series generally included patients with both stage I and stage II follicular lymphoma and used various doses of radiation (generally between 30 and 40 Gy) with heterogeneous field sizes. In these series, the 10-year overall survival rates ranged from 40% to 80%, with relatively few reported events after 8 years. For example, in the series from Stanford University with a median follow-up of 7 years, only five of 47 patients who reached 10 years without a relapse subsequently developed a recurrence.16
More recent efforts have attempted to standardize the radiation dose and field size in indolent lymphoma. Recently, preliminary results from a definitive radiation-dose study from the British National Lymphoma Investigation have been presented. This study randomly assigned patients with indolent lymphoma to either a radiation dose of 24 Gy or a dose of 40 to 45 Gy. The 5-year freedom from local progression was approximately 75% in both arms, and there was no difference in overall survival.17 In a study from Germany that explored extended- versus involved-field XRT, the 5-year PFS was 59%; 10 secondary malignancies were reported, including three cases of myelodysplastic syndrome/acute myelocytic leukemia.18 Some recent studies have shown responses of a long duration with low-dose radiation (4 Gy) in selected patients from single institutions19,20; thus, the optimal dose and field size remain unknown for this patient population.
On the basis of these selected retrospective series, many physicians have determined that XRT is the standard treatment approach for many patients with early-stage follicular lymphoma. However, few studies have evaluated alternative approaches in a comparative way. In a retrospective analysis from Stanford University, 43 patients with early-stage follicular lymphoma were identified who were observed rather than treated with immediate XRT.21 With a median follow-up of over 7 years, 63% of these patients had not required therapy, and the estimated survival at 10 years was 85%. This survival compares favorably with the aforementioned series of patients from the same institution,16 and the authors from Stanford University concluded that having no initial therapy was an acceptable approach in selected patients with early-stage follicular lymphoma.
A relatively small number of patients in our series were treated with systemic therapy and XRT, which included rituximab and abbreviated chemotherapy followed by involved-field XRT, with excellent observed outcomes. Previous experience of combined modality treatment of early-stage follicular lymphoma is limited to small studies from single institutions that did not use rituximab. The largest series is from MD Anderson Cancer Center, which enrolled 102 patients with low-grade lymphoma (defined by Working Formulation, including 85 patients with follicular lymphoma) to 10 cycles of risk-adapted chemotherapy plus involved field XRT.22 With a median follow-up of 10 years, the overall survival was 80% for patients with follicular lymphoma, and 72% of patients were disease free at 10 years. These results compare favorably to those of studies of XRT alone, but therapy-related myelodysplasia and secondary malignancies occurred after this intensive treatment approach. An older study from the British National Lymphoma Investigation randomly assigned patients to XRT alone versus XRT with continuous oral chlorambucil.23 With prolonged follow-up, there was no significant difference in overall survival or disease-free survival between treatment groups. In a retrospective series from the Joint Center for Radiation Therapy, the addition of chemotherapy to XRT in a minority of patients did not affect outcome; however, these patients may have been selected for high-risk features.15
A recent analysis of SEER data compared patients with stage I and II follicular lymphoma who received XRT with patients who did not receive XRT and suggested that immediate XRT was associated with improved disease-specific and overall survival.5 Similar to our study, in this analysis, radiation was not controlled for dose or field. In contrast to the SEER analysis, our patients had similar risk factors as defined by FLIPI, whether treated with XRT or observed.24 The SEER-database analysis did not adjust for FLIPI factors, which is a possible explanation of why our results differed from this SEER analysis.
The definition of stage I disease has changed over time. In our series, one half of patients determined to have stage I disease by their physicians had incomplete staging, with no bone marrow biopsy completed. These incompletely staged patients had inferior outcomes compared with patients who were rigorously staged with both a bone marrow biopsy and modern imaging. This finding emphasizes the importance of complete, rigorous staging to most accurately predict the outcome in follicular lymphoma. Our analysis also supports a consensus statement emphasizing that PET has a limited diagnostic role in the staging of indolent lymphoma.25,26
Thirty-seven patients within our rigorously staged group had grade 3 histology. The histologic grade affected the treatment choice, with more patients with grade 3 histology treated with combined-modality therapy than patients with grade 1 or 2 histology. There was no central review of pathology in our study. Whether grade 3 follicular lymphoma has a unique natural history and should be approached therapeutically, such as with diffuse large B-cell lymphoma, is controversial.27,28–30 Nevertheless, in our study, grade 3 follicular lymphoma had a favorable outcome. We were unable to ascertain whether the favorable outcome was reflective of more aggressive therapy or unique disease biology. However, a sensitivity analysis in which the grade 3 patients in our series were excluded continued to show a superior outcome with R-chemo compared with XRT alone.
Limitations of our study, which were similar to those of previously reported studies, included its observational design and our relatively short follow-up duration. A central pathology review was not included, although it may not be necessary for an accurate diagnosis of follicular lymphoma.31–33 Although patients were enrolled prospectively, and we attempted to analyze known prognostic features, there may have been other unknown confounding variables that affected outcomes and introduced bias in the comparison between treatment groups. For example, increased baseline β2-microglobulin has been shown to be prognostic in some series34 and was not collected in the National LymphoCare Study. Clearly, a randomized study would be preferred, but as a result of the rarity of stage I follicular lymphoma, the long natural history, and the overall excellent outcome without any therapy, it is unlikely there will ever be a definitive study. It should be emphasized that the bulk of the literature that has endorsed XRT has been similarly uncontrolled, from single institutions, and frequently retrospective and not prospective in nature. Moreover, our study may be particularly relevant to current practice because it was conducted in the rituxmab era, during which time significant improvements in overall survival were demonstrated in advanced stage follicular lymphoma35,36 and when modern staging techniques were used.
Our median follow-up for survival exceeded 5 years, which is the time during which the majority of events have generally occurred in other series of early-stage follicular lymphoma. Our results observed with XRT also compare favorably to previously published series. A long-term follow-up of other radiation series suggested few relapses after 10 years, with a possible plateau on the PFS curve. Additional follow-up of our cohort of patients will be required to determine whether long-term disease control is different between treatment modalities. However, the 10-year follow-up of a trial that enrolled patients with low-bulk follicular lymphoma treated systemically with single-agent rituximab (without XRT) suggests that almost one half of newly diagnosed patients who obtained an initial response have maintained that response 8 years later.37 Therefore, it is likely that patients treated with a variety of approaches may enjoy prolonged disease-free intervals.
In conclusion, in this large, prospectively enrolled group of patients with stage I follicular lymphoma in the modern era, diverse treatment approaches resulted in similar excellent outcomes, challenging the paradigm that XRT should be standard for this presentation. The registry nature of this report limited the ability to provide definitive therapy recommendations. Ideally, a randomized trial would be conducted to compare these various strategies but is unlikely to occur as a result of the large sample size required and the rarity of events in this patient population. As our understanding of the biology of follicular lymphoma improves,38 including the ability to define high-risk disease by using genetic and molecular techniques,39 future studies should focus efforts in these molecularly defined patient populations by using rationally targeted therapeutic approaches.
Footnotes
See accompanying editorial on page 3328
Supported by Genentech and in part by the Rochester-Arizona Specialized Program of Research Excellence in Lymphoma (CA-130805; J.W.F and T.P.M). Also supported in part by the Leukemia and Lymphoma Society Scholar in Clinical Research program (J.W.F.).
Presented in part at the 11th International Congress on Malignant Lymphoma, June 15-18, 2011, Lugano, Switzerland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Michelle Byrtek, Genentech (C); Michael Taylor, Genentech (C); Jamie Hirata, Genentech (C) Consultant or Advisory Role: Jonathan W. Friedberg, Genentech (C); Brian K. Link, Genentech (C); Christopher Flowers, Allos Therapeutics (U), Celgene (U), Genentech/Roche (C), Millennium/Takeda Pharmaceuticals (C), OptumRx (C), Seattle Genetics (C), Spectrum Pharmaceuticals (C); John Hainsworth, Genentech (C); James R. Cerhan, Genentech (C); Andrew D. Zelenetz, Cancer Genetics (C), Genentech/Roche (C), Gilead Sciences (U), GlaxoSmithKline (C); Thomas P. Miller, Genentech (U) Stock Ownership: Michelle Byrtek, Roche; Michael Taylor, Roche; Jamie Hirata, Roche Honoraria: None Research Funding: Christopher Flowers, Gilead Sciences, Millennium/Takeda Pharmaceuticals, Novartis, Spectrum Pharmaceuticals; Andrew D. Zelenetz, Genentech/Roche Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan W. Friedberg, Brian K. Link, Michael Taylor, John Hainsworth, James R. Cerhan, Andrew D. Zelenetz, Thomas P. Miller
Provision of study materials or patients: Jonathan W. Friedberg, Brian K. Link, John Hainsworth, James R. Cerhan, Thomas P. Miller
Collection and assembly of data: Jonathan W. Friedberg, Brian K. Link, Christopher Flowers, Michael Taylor, Jamie Hirata, Thomas P. Miller
Data analysis and interpretation: Jonathan W. Friedberg, Michelle Byrtek, Brian K. Link, Christopher Flowers, Michael Taylor, James R. Cerhan, Andrew D. Zelenetz, Jamie Hirata, Thomas P. Miller
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene. 2004;23:6524–6534. doi: 10.1038/sj.onc.1207843. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—Released April 2011 on the basis of the November 2010 submission. Bethesda, MD: National Cancer Institute, Surveillance Research Program, Cancer Statistics Branch; 2011. http://www.seer.cancer.gov. [Google Scholar]
- 3.Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2011;9:484–560. doi: 10.6004/jnccn.2011.0046. [DOI] [PubMed] [Google Scholar]
- 4.Mauch P. Follicular non-Hodgkin's lymphoma: The role of radiation therapy. Ann Hematol. 2001;80(suppl 3):B63–B65. doi: 10.1007/pl00022793. [DOI] [PubMed] [Google Scholar]
- 5.Pugh TJ, Ballonoff A, Newman F, et al. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation: A Surveillance, Epidemiology, and End Results database analysis. Cancer. 2010;116:3843–3851. doi: 10.1002/cncr.25149. [DOI] [PubMed] [Google Scholar]
- 6.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: First report of the National LymphoCare Study. J Clin Oncol. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennekes L, Ottevanger PB, Raemaekers JM, et al. Development and measurement of guideline-based indicators for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2011;29:1436–1444. doi: 10.1200/JCO.2010.30.1622. [DOI] [PubMed] [Google Scholar]
- 8.Grol R, Grimshaw J. From best evidence to best practice: Effective implementation of change in patients' care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence TS, Urba WJ, Steinberg SM, et al. Retrospective analysis of stage I and II indolent lymphomas at the National Cancer Institute. Int J Radiat Oncol Biol Phys. 1988;14:417–424. doi: 10.1016/0360-3016(88)90254-4. [DOI] [PubMed] [Google Scholar]
- 10.Pendlebury S, el Awadi M, Ashley S, et al. Radiotherapy results in early stage low grade nodal non-Hodgkin's lymphoma. Radiother Oncol. 1995;36:167–171. doi: 10.1016/0167-8140(95)01600-l. [DOI] [PubMed] [Google Scholar]
- 11.Soubeyran P, Eghbali H, Bonichon F, et al. Localized follicular lymphomas: Prognosis and survival of stages I and II in a retrospective series of 103 patients. Radiother Oncol. 1988;13:91–98. doi: 10.1016/0167-8140(88)90030-8. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RE, Allan SG, McIntyre MA, et al. Low grade stage I and II non-Hodgkin's lymphoma: Results of treatment and relapse pattern following therapy. Clin Radiol. 1988;39:287–290. doi: 10.1016/s0009-9260(88)80537-3. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan Hudson B, Vaughan Hudson G, MacLennan KA, et al. Clinical stage 1 non-Hodgkin's lymphoma: Long-term follow-up of patients treated by the British National Lymphoma Investigation with radiotherapy alone as initial therapy. Br J Cancer. 1994;69:1088–1093. doi: 10.1038/bjc.1994.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilder RB, Jones D, Tucker SL, et al. Long-term results with radiotherapy for stage I-II follicular lymphomas. Int J Radiat Oncol Biol Phys. 2001;51:1219–1227. doi: 10.1016/s0360-3016(01)01747-3. [DOI] [PubMed] [Google Scholar]
- 15.Guadagnolo BA, Li S, Neuberg D, et al. Long-term outcome and mortality trends in early-stage, grade 1-2 follicular lymphoma treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:928–934. doi: 10.1016/j.ijrobp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Mac Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J Clin Oncol. 1996;14:1282–1290. doi: 10.1200/JCO.1996.14.4.1282. [DOI] [PubMed] [Google Scholar]
- 17.Hoskin P, Lowry L, Smith P, et al. Radiation dose for local control in non-Hodgkin lymphoma: British National Lymphoma Investigation randomized trial. Ann Oncol. 2011;22:iv90. [Google Scholar]
- 18.Engelhard M, Unterhalt M, Hansmann M, et al. Follicular lymphoma: Curability by radiotherapy in limited stage nodal disease? Updated results of a randomized trial. Ann Oncol. 2011;22:iv90. [Google Scholar]
- 19.Haas RL, Poortmans P, de Jong D, et al. High response rates and lasting remissions after low-dose involved field radiotherapy in indolent lymphomas. J Clin Oncol. 2003;21:2474–2480. doi: 10.1200/JCO.2003.09.542. [DOI] [PubMed] [Google Scholar]
- 20.Knoops L, Haas R, de Kemp S, et al. In vivo p53 response and immune reaction underlie highly effective low-dose radiotherapy in follicular lymphoma. Blood. 2007;110:1116–1122. doi: 10.1182/blood-2007-01-067579. [DOI] [PubMed] [Google Scholar]
- 21.Advani R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkin's lymphoma: Long-term follow-up of no initial therapy. J Clin Oncol. 2004;22:1454–1459. doi: 10.1200/JCO.2004.10.086. [DOI] [PubMed] [Google Scholar]
- 22.Seymour JF, Pro B, Fuller LM, et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003;21:2115–2122. doi: 10.1200/JCO.2003.07.111. [DOI] [PubMed] [Google Scholar]
- 23.Kelsey SM, Newland AC, Hudson GV, et al. A British National Lymphoma Investigation randomised trial of single agent chlorambucil plus radiotherapy versus radiotherapy alone in low grade, localised non-Hodgkins lymphoma. Med Oncol. 1994;11:19–25. doi: 10.1007/BF02990087. [DOI] [PubMed] [Google Scholar]
- 24.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 25.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 26.Friedberg JW, Chengazi V. PET scans in the staging of lymphoma: Current status. Oncologist. 2003;8:438–447. doi: 10.1634/theoncologist.8-5-438. [DOI] [PubMed] [Google Scholar]
- 27.Chau I, Jones R, Cunningham D, et al. Outcome of follicular lymphoma grade 3: Is anthracycline necessary as front-line therapy? Br J Cancer. 2003;89:36–42. doi: 10.1038/sj.bjc.6601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dana BW, Dahlberg S, Nathwani BN, et al. Long-term follow-up of patients with low-grade malignant lymphomas treated with doxorubicin-based chemotherapy or chemoimmunotherapy. J Clin Oncol. 1993;11:644–651. doi: 10.1200/JCO.1993.11.4.644. [DOI] [PubMed] [Google Scholar]
- 29.Miller TP, LeBlanc M, Grogan TM, et al. Follicular lymphomas: Do histologic subtypes predict outcome? Hematol Oncol Clin North Am. 1997;11:893–900. doi: 10.1016/s0889-8588(05)70468-8. [DOI] [PubMed] [Google Scholar]
- 30.Shustik J, Quinn M, Connors JM, et al. Follicular non-Hodgkin lymphoma grades 3A and 3B have a similar outcome and appear incurable with anthracycline-based therapy. Ann Oncol. 2011;22:1164–1169. doi: 10.1093/annonc/mdq574. [DOI] [PubMed] [Google Scholar]
- 31.LaCasce AS, Kho ME, Friedberg JW, et al. Comparison of referring and final pathology for patients with non-Hodgkin's lymphoma in the National Comprehensive Cancer Network. J Clin Oncol. 2008;26:5107–5112. doi: 10.1200/JCO.2008.16.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clarke CA, Glaser SL, Dorfman RF, et al. Expert review of non-Hodgkin's lymphomas in a population-based cancer registry: Reliability of diagnosis and subtype classifications. Cancer Epidemiol Biomarkers Prev. 2004;13:138–143. doi: 10.1158/1055-9965.epi-03-0250. [DOI] [PubMed] [Google Scholar]
- 33.Dick F, VanLier S, Banks P, et al. Use of the working formulation for non-Hodgkin's lymphoma in epidemiologic studies: Agreement between reported diagnoses and a panel of experienced pathologists. J Natl Cancer Inst. 1987;78:1137–1144. [PubMed] [Google Scholar]
- 34.Federico M, Guglielmi C, Luminari S, et al. Prognostic relevance of serum β2 microglobulin in patients with follicular lymphoma treated with anthracycline-containing regimens. A GISL study. Haematologica. 2007;92:1482–1488. doi: 10.3324/haematol.11502. [DOI] [PubMed] [Google Scholar]
- 35.Swenson WT, Wooldridge JE, Lynch CF, et al. Improved survival of follicular lymphoma patients in the United States. J Clin Oncol. 2005;23:5019–5026. doi: 10.1200/JCO.2005.04.503. [DOI] [PubMed] [Google Scholar]
- 36.Fisher RI, LeBlanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 37.Martinelli G, Schmitz SF, Utiger U, et al. Long-term follow-up of patients with follicular lymphoma receiving single-agent rituximab at two different schedules in trial SAKK 35/98. J Clin Oncol. 2010;28:4480–4484. doi: 10.1200/JCO.2010.28.4786. [DOI] [PubMed] [Google Scholar]
- 38.Staudt LM. A closer look at follicular lymphoma. N Engl J Med. 2007;356:741–742. doi: 10.1056/NEJMcibr067155. [DOI] [PubMed] [Google Scholar]
- 39.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]