Abstract
Salix caprea is well suited for phytoextraction strategies. In a previous survey we showed that genetically distinct S. caprea plants isolated from metal-polluted and unpolluted sites differed in their zinc (Zn) and cadmium (Cd) tolerance and accumulation abilities. To determine the molecular basis of this difference we examined putative homologues of genes involved in heavy metal responses and identified over 200 new candidates with a suppression subtractive hybridization (SSH) screen. Quantitative expression analyses of 20 genes in leaves revealed that some metallothioneins and cell wall modifying genes were induced irrespective of the genotype's origin and metal uptake capacity while a cysteine biosynthesis gene was expressed constitutively higher in the metallicolous genotype. The third and largest group of genes was only induced in the metallicolous genotype. These data demonstrate that naturally adapted woody non-model species can help to discover potential novel molecular mechanisms for metal accumulation and tolerance.
Keywords: Salicaceae, Phytoremediation, Adaptation, Heavy metals
Highlights
► The transcriptional Zn/Cd response of the willow Salix caprea was quantified. ► Two genotypes from a highly contaminated and a control environment were compared. ► In addition to candidate genes an SSH library revealed over 200 metal induced genes. ► Constitutive upregulation and isolate-specific induction of genes were revealed. ► Willows adapt to environmental Zn/Cd contamination on the transcriptional level.
Expression analyzes reveal different responses to Cd and Zn exposure of S. caprea genotypes with different heavy metal accumulation abilities.
1. Introduction
Metal contaminated soils pose a severe threat to environment, agriculture and consequently via the food chain to human and animal health (Schwitzguébel et al., 2011). A promising approach to substitute costly remediation technologies is phytoextraction, the use of plants to clean up polluted soils (Salt et al., 1998). Efficient phytoextraction species should translocate high quantities of metals into their aboveground biomass without toxicity symptoms, and at the same time produce large amounts of biomass (Pulford and Watson, 2003; Vangronsveld et al., 2009). Metal hyperaccumulators take up and tolerate more than 100 mg kg−1 cadmium (Cd) or 10,000 mg kg−1 zinc (Zn) in their shoots, up to 1000-fold more than excluder species (Rascio and Navari-Izzo, 2011; Verbruggen et al., 2009b). Hyperaccumulation of Zn and Cd has been shown for numerous members of the Brassicaceae family. Especially Noccaea (formerly Thlaspi) caerulescens (J. Presl and C. Presl) F.K. Mey (Escarré et al., 2000; Xing et al., 2008), Thlaspi goesingense Halacsy (Lombi et al., 2000), Thlaspi praecox Wulfen and Arabidopsis halleri (L.) O'Kane and Al-Shehbaz (Bert et al., 2000; Wenzel and Jockwer, 1999) have been serving as model organisms to uncover the molecular basis of hyperaccumulation of essential and non-essential trace metals. However, those species typically do not produce high biomass, are limited in rooting depth and may not be competitive against weed outside their native environments. Therefore, the use of fast-growing, woody metal-accumulating species with larger root systems such as poplar (Populus spp.) or willow (Salix spp.) would enhance the efficiency of the phytoextraction process, making it economically feasible (Marmiroli et al., 2011; Pulford and Watson, 2003; Thewys et al., 2010).
Salix and Populus sp. accumulate considerable amounts of Zn and Cd in their aboveground organs (Dos Santos Utmazian et al., 2007; Landberg and Greger, 1996; Robinson et al., 2000; Unterbrunner et al., 2007). The goat willow Salix caprea (S. caprea) accumulated up to 4680 mg Zn kg−1 and 116 mg Cd kg−1 dry weight in leaves in a metal-polluted habitat (Unterbrunner et al., 2007). There is a remarkable natural genetic variability among poplar and willow species and ecotypes adapted to soils of varying metal contaminations (Laureysens et al., 2004; Puschenreiter et al., 2010). We have shown that genetically distinct S. caprea plants isolated from metallicolous sites accumulate significantly larger amounts of Cd than those from non-metallicolous sites (Puschenreiter et al., 2010). Further analysis of a metallicolous S. caprea genotype, KH21 (Kutná Hora, Czech Republic), and a non-metallicolous genotype, F20 (Forchtenstein, Austria), revealed different root anatomical responses and element distribution pattern upon metal exposure (Vaculík et al., 2012). Moreover, analysis of heavy metal contents in leaves demonstrated that the metallicolous genotype KH21 accumulated significantly more Cd and by trend more Zn than the non-metallicolous genotype F20 (Vaculík et al., 2012).
Whereas the basis of metal hyperaccumulation in woody species is largely unknown, the molecular mechanisms underlying the adaptation to metals in small model plants such as A. halleri or Thlaspi/Noccaea spp. are well studied (Becher et al., 2004; Dräger et al., 2004; Hanikenne et al., 2008; Plessl et al., 2010; van de Mortel et al., 2006). A network of transporters tightly controls uptake into roots, xylem loading and vacuolar sequestration (Broadley et al., 2007; Verbruggen et al., 2009b). Although these transporters are thought to balance the concentration of essential metals such as Zn, they also unselectively transport toxic elements like Cd (Mendoza-Cozatl et al., 2011; Verbruggen et al., 2009b). Inside the cells, metals are chelated with small molecules such as the low molecular weight, cysteine-rich metallothioneins or non-translationally synthesized, glutathione-derived phytochelatins (Cobbett and Goldsbrough, 2002). Remarkable similarity in copy number expansion and transcriptional regulation was found for the xylem loading transporter HEAVY METAL ATPASE 4 (HMA4) in A. halleri and N. caerulescens, indicating parallel evolutionary pathways in these two Brassicaceae species (Hanikenne et al., 2008; Ó Lochlainn et al., 2011). Moreover, HMA4 was recently found to be involved in maintenance of Zn homeostasis also in poplar (Adams et al., 2011). This example of cross-species functionality suggests that well-studied pathways might also act in S. caprea metal tolerance.
However, trees could have evolved also distinct mechanisms to cope with elevated metal concentrations. Their elucidation requires broad scale transcriptional, proteomic and metabolic approaches. Proteomic studies on poplar (Populus tremula L.) revealed numerous proteins influenced by long- and short-term Cd exposure, especially belonging to primary carbon and glutathione metabolism (Kieffer et al., 2009). On the transcriptional level, a recent microarray-based analysis of Populus × euramericana Guinier hybrids identified Zn-responsive genes (Di Baccio et al., 2011). A cDNA-AFLP approach identified chromium-inducible genes in four Salix species (Quaggiotti et al., 2007). However, the transcriptional responses of Salix spp. to Cd and Zn contamination remain unknown.
Genome sequence and microarrays are not yet available for willows. Suppression subtractive hybridization (SSH) analysis allows the simultaneous identification of a multitude of genes in unsequenced species (Diatchenko et al., 1996). Gene networks involved in lead response in the legume Sesbania drummondii (Rydb.) Cory (Srivastava et al., 2007), in chromium metabolism of the oilseed crop Crambe abyssinica Hochst. ex R.E.Fr. (Zulfiqar et al., 2011), and in copper response in birch (Betula pendula Roth) (Keinänen et al., 2007) have been successfully identified using the SSH technique.
The aim of this study was to unravel candidate genes that might be responsible for the divergent Cd accumulation of two natural S. caprea genotypes. In a biased approach the expression of putative S. caprea orthologs of known metal responsive genes was assessed. With an unbiased approach an SSH library of the superior Cd accumulating genotype was created. The gene expression analyses allowed for inferring molecular pathways involved in metal tolerance and accumulation in metallicolous and non-metallicolous S. caprea genotypes.
2. Materials and methods
2.1. Plant material
Two Salix caprea L. genotypes from a metal-polluted and an unpolluted control site in Central Europe were used. Genotype KH21 was collected near Kutná Hora (Czech Republic), a site historically metal contaminated due to ore mining and smelting activities. Genotype F20 originates from Forchtenstein (Austria), an unpolluted control site. KH21 had initially been characterized by higher Zn and Cd accumulation but lower biomass production than F20 in perlite-based cultures (Puschenreiter et al., 2010). The original plants were collected as cuttings at the two sites in 2003 and were kept in unpolluted soil in a tree nursery until preparation of green cuttings for the experiments.
2.2. Perlite-based cultivation and heavy metal treatments
The experimental setup was performed as described in (Vaculík et al., 2012). Briefly, green cuttings with four internodes were rooted in quartz sand under tap water irrigation in a greenhouse for 60 days at 24/18 °C day/night, 60% relative humidity, a 12 h photoperiod and 200 μM m−2 s−1 PAR. Rooted cuttings were transferred into 1 L pots filled with perlite and irrigated with a nutrient solution containing (in μM) 1000 Ca(NO3)2, 500 Mg(SO4)2, 50 KH2PO4, 100 KCl, 5 H3BO3, 0.2 H24Mo7N6O24, 10 MnSO4, 2.5 CuSO4, 0.25 NiSO4, 2.5 ZnSO4 and 50 Fe(III)-ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDHA), adjusted to pH 6.0 with 1 mM 2-(N-morpholino)ethanesulfonic acid (MES) as potassium salt (Shen et al., 1997) for eight weeks. Thereafter, control (normal nutrient solution), and Zn + Cd (nutrient solution with 0.5 mg L−1 (4.5 μM) Cd2+ as CdNO3·4H2O plus 5 mg L−1 (76 μM) Zn2+ as ZnSO4·7 H2O) treatments were continuously applied to four independent replicate plants for three months (14 weeks). Leaves were harvested for gene expression and metal analysis after two, eight and 14 weeks. After eight weeks, material was collected for the construction of the SSH library. Leaves were sampled at the same time on each harvesting day to avoid any possible diurnal variation in gene expression (Zheng et al., 2011). A second independent experiment was performed like the first one with six independent replicates. The Zn and Cd concentrations from four replicate plants are reported in (Vaculík et al., 2012).
2.3. RNA isolation and real-time quantitative PCR (qPCR)
For RNA isolation, the third or fourth young leaf from the apex was used (approx. 150–200 mg). 3 mL preheated extraction buffer (2% [w/v] hexadecyltrimethyl-ammonium bromide, 2% [w/v] polyvinylpyrrolidone, 100 mM Tris/HCl pH 8.0, 25 mM ethylenediaminetetraacetic acid (EDTA), 2 M NaCl, 0.5 g/L spermidine, 2.7% [v/v] 2-mercaptoethanol) were mixed with the frozen leaf powder and incubated at 65 °C for 7 min. After two extraction steps with 3 mL ice-cold chloroform:isoamylalcohol (24:1, v:v), 0.25 volumes of ice-cold 10 M LiCl were added to precipitate RNA at 4 °C for 18 h. After centrifugation at 16 000 × g, 4 °C, 60 min, the pellets were incubated with 4 mL ice-cold 75% ethanol at −80 °C for 60 min, followed by centrifugation at 16 000 × g, 4 °C, 20 min. Dried pellets were dissolved in RNase free water. RNA concentrations were determined using a Qubit Fluorometer (Invitrogen, USA). cDNA synthesis was essentially done as described by Karsai et al. (2002).
Degenerate primers for metal responsive candidate genes were designed in conserved regions of the Populus trichocarpa Torr. & A. Grey, A. thaliana and Betula ssp. genes (Supplementary Table S1). Their specificity was determined by sequencing and subsequent phylogenetic analysis (data not shown). qPCR was performed in the Rotorgene-3000 cycler (Qiagen, Germany) using the SYBR® Green Jumpstart™ Taq Readymix™ kit (Sigma–Aldrich, USA) and the qPCR Core Kit for SYBR® Green (Eurogentec, Belgium). Each cDNA sample was measured in triplicate. Amplification occurred after an initial denaturation (10 min/94 °C) in 40 cycles (94 °C/5 s–55 °C/5 s–72 °C/25 s–81 °C/15 s–85 °C/15 s). At the end of each run, a melting curve was recorded between 65 °C and 99 °C. Data were analysed using standard curves of known PCR fragment copy numbers for each gene. ScACTIN2 served as reference gene. Significance levels between the triplicate qPCR measurements of all biological replicates were calculated with Student's t-tests.
2.4. Construction and screening of the SSH library
To identify further heavy metal induced genes, total leaf RNA of KH21 plants treated with normal nutrient solution or with 5 mg L−1 Zn plus 0.5 mg L−1 Cd for eight weeks in perlite-based hydroponic culture was isolated as described above. Equal amounts of RNA from four replicate plants were pooled to obtain 20 μg. cDNA synthesis, subtraction of the control from the metal-treated cDNA pool and cloning of the differential, i.e. metal induced, cDNAs into the pAL16 vector were performed by Evrogen (Moscow, Russia) according to the protocol of Diatchenko et al. (1996).
2.5. Reverse Northern blot analyses
The pAL16 library of Zn/Cd induced cDNAs was spread on LB plates (1:70 000) containing 75 mg L−1 ampicillin, 12 mg L−1 IPTG and 30 mg L−1 X-Gal. White colonies were grown in 100 μL LB (+ampicillin, 75 mg L−1) in 96-well plates (IBL, Austria) at 37 °C, 180 rpm, for 6 h. 1 μL culture was used for colony PCR using M13 primers to check for presence and length of inserts. To display differentially expressed colonies, 2 μL PCR products were spotted on two positively charged nylon membranes (Roche, Germany) and UV crosslinked at 2400 mJ. After prehybridization with Easy Hyb solution (Roche, Germany), membranes were hybridized with DIG-11-dUTP (Roche, Germany) labeled subtracted cDNA pools from treated or untreated plants over night at 42 °C. Washing at 83 °C and detection were done according the manufactures protocol (Roche, Germany). Differential spots representing highly metal induced cDNAs were selected for sequencing and bioinformatic analysis.
Annotation was performed by BLASTN and TBLASTX in the P. trichocarpa genome database (http://www.phytozome.net/poplar.php; version 5.0) and NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Gene ontology annotations were performed in http://www.geneontology.org.
3. Results
3.1. Combined Zn and Cd treatment induces potential S. caprea orthologs of known metal responsive genes in a genotype-specific manner
To uncover the molecular basis involved in the differential metal storage and tolerance capabilities of S. caprea genotypes, the expression of orthologous genes known to respond to elevated metal concentrations in other plants was quantified in leaves with qPCR. Leaves were selected for the analysis because metal storage and tolerance in harvestable organs is of particular interest for phytoextraction crops that at the same time provide biomass for energy production (Ruttens et al., 2011; Šyc et al., 2012). Based on phylogenetic analyses with poplar, birch and Arabidopsis thaliana (L.) Heynh sequences, degenerated primers were constructed in conserved regions of the genes encoding the metal influx transporter ZRT-IRT-LIKE PROTEIN ZIP6, the metal sequestering protein HEAVY METAL TRANSLOCATING P-TYPE ATPASE HMA1, two metallothioneins (MT2B and MT3), the first three enzymes involved in cysteine and glutathione biosynthesis (SERINE-O-ACETYL TRANSFERASE SAT1, O-ACETYLSERIN (THIOL) LYASE OASA1, γ-GLUTAMYLCYSTEINE SYNTHETASE CAD2), a METAL TOLERANCE PROTEIN MTP1, and two PHYTOCHELATIN SYNTHASES (PCS1 and PCS2). To determine if the primers were specific for the potential orthologous genes, all PCR fragments amplified from S. caprea cDNAs were sequenced and integrated into the phylogenetic analyses (data not shown). These ESTs have been deposited in the NCBI Genbank with the accession numbers JS807771–JS807786 (Supplementary Table S1).
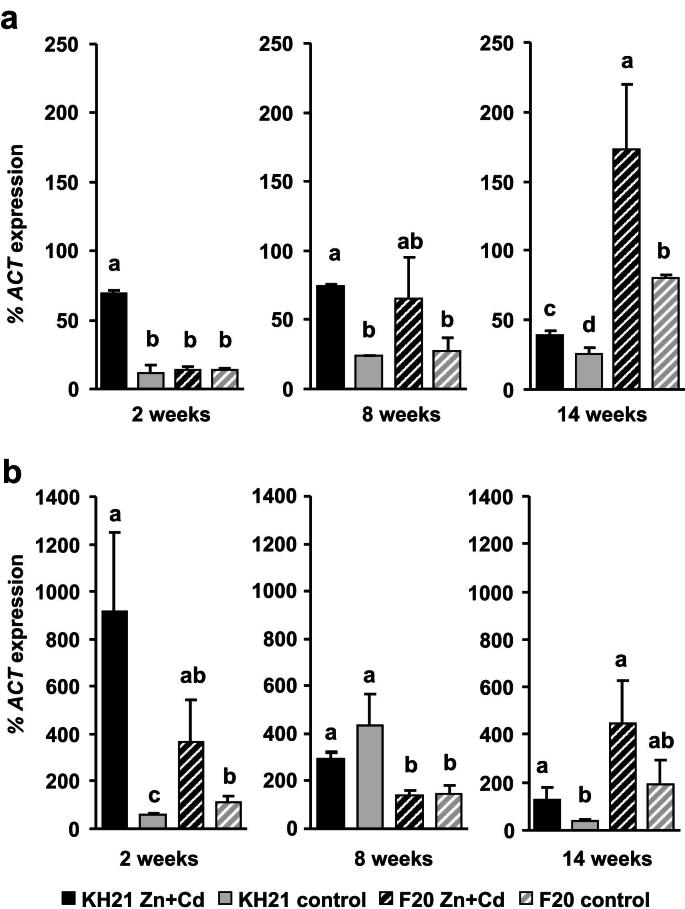
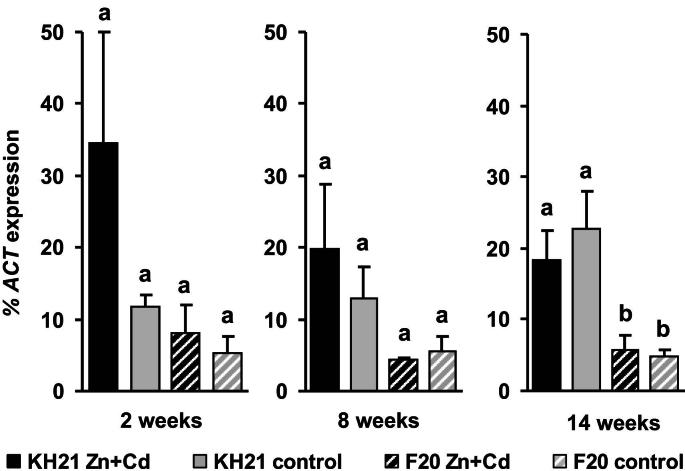
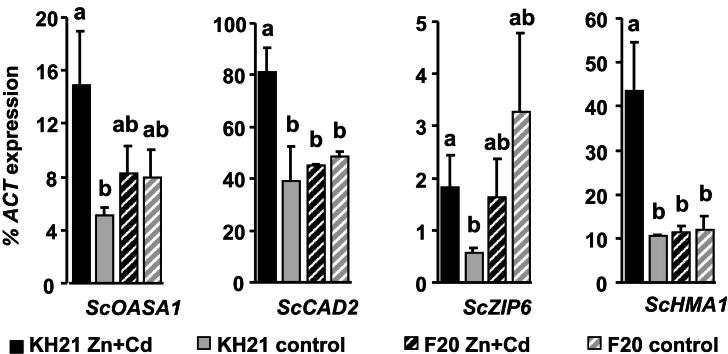
The expression of ScMTP1, ScPCS1 and ScPCS2 were below the detection limit (data not shown). All other S. caprea orthologs of known metal responsive genes were expressed in a genotype-specific manner. According to their expression patterns during a combined Zn and Cd treatment of three months they could be grouped into three categories. The first group included the metallothionein genes, ScMT2B and ScMT3, which were induced under metal stress independent of the genotype's origin and metal uptake capacity (Fig. 1). ScMT2B and ScMT3 were highly induced already after two weeks in the metallicolous genotype KH21, whereas the ScMT2B induction was delayed in F20. The second group contained ScSAT1 which was constitutively higher expressed in the metallicolous genotype throughout the whole experiment and additionally strongly induced at the two week time point of metal treatment (Fig. 2). The third group comprised genes that were inducible only in the metallicolous genotype KH21 and not induced or even downregulated in the non-metallicolous genotype F20 (Fig. 3).
Fig. 1.
Two metallothionein genes are predominantly induced in leaves upon Zn + Cd treatment and thus display category (1) expression pattern. qPCR analysis of genotypes KH21 (full bars) and F20 (striped bars) after 2, 8 and 14 weeks in perlite cultures without (control, gray) and with 5 mg Zn L−1 and 0.5 mg Cd L−1 (Zn + Cd, black). Expression levels of ScMT2B (a) and ScMT3 (b) were normalized to ScACTIN2. Measurements were carried out in triplicates and values represent means ± SE of four biological replicates. Different letters represent significant differences (Student's t-test, p < 0.05).
Fig. 2.
The cysteine biosynthesis gene serine-o-actetyl transferase (ScSAT) is constitutively higher expressed in leaves of the metallicolous isolated KH21 and thus displays a category (2) expression pattern. qPCR analysis of genotypes KH21 (full bars) and F20 (striped bars) after 2, 8 and 14 weeks in perlite cultures without (control, gray) and with 5 mg Zn L−1 and 0.5 mg Cd L−1 (Zn + Cd, black). Expression levels of ScSAT were normalized to ScACTIN2. Measurements were carried out in triplicates and values represent means ± SE of four biological replicates. Different letters represent significant differences (Student‘s t-test, p < 0.05).
Fig. 3.
Glutathione biosynthesis and transporter genes are only induced after Zn + Cd treatment in leaves of the metallicolous genotype and thus display category (3) expression pattern. qPCR analysis of genotypes KH21 (full bars) and F20 (striped bars) after 2 weeks in perlite cultures without (control, gray) and with 5 mg Zn L−1 and 0.5 mg Cd L−1 (Zn + Cd, black). Expression levels of the glutathione biosynthesis genes, ScOASA1 and ScCAD2, and the transporter genes, ScZIP6 and ScHMA1, were normalized to ScACTIN2. Measurements were carried out in triplicates and values are means ± SE of four biological replicates. Note that ScZIP6 is constitutively higher expressed in F20 in control condition and downregulated to the level of KH21 upon Zn + Cd exposure. Different letters represent significant differences (Student's t-test, p < 0.05).
3.2. Novel Zn and Cd-inducible genes were identified through SSH analysis
To identify additional candidate genes a suppression subtractive hybridization (SSH) library containing Zn/Cd induced ESTs was created from KH21 plants (Diatchenko et al., 1996). More than 3000 colonies were screened for differential expression by colony PCR and reverse Northern blot analysis. Clones differing most strikingly in their intensity when hybridized with either the labeled untreated or the metal-treated cDNA library were selected for sequencing and bioinformatic analyses. In total, 405 cDNAs were annotated. The more colonies were screened, the more duplicates were found, leading to the identification of 213 unique expressed sequence tag (EST) clones. Using gene ontology (GO) vocabularies, the ESTs were classified into 29 “biological process” terms (Table 1). A full list of all identified S. caprea cDNAs containing their poplar orthologs, chromosomal positions in the poplar genome and NCBI TBLASTX results is presented in Supplementary Table S2. All the ESTs have been deposited to the NCBI Genbank with the Accessions numbers JK747484–JK747796.
Table 1.
Zn- and Cd-inducible S. caprea genes identified in the SSH library screening, grouped according to GO terms.
| GO term name | GO term number | Unique ESTs in SSH library (total ESTs) |
|---|---|---|
| Amino acid metabolism | GO:0006520 | 4 (9) |
| Carbohydrate Metabolism | GO:0005975 | 4 (6) |
| Carbon fixation | GO:0015977 | 5 (13) |
| Cellular nitrogen compound metabolism | GO:0034641 | 4 (5) |
| Cellular response to unfolded protein | GO:0034620 | 2 (2) |
| Cell wall modification | GO:0042545 | 7 (9) |
| Cytoskeleton organization | GO:0007010 | 4 (4) |
| Developmenta | – | 6 (6) |
| Disease resistance | GO:0009614 | 5 (6) |
| Energy homeostasis | GO:0097009 | 7 (7) |
| Glycolysis | GO:0006096 | 2 (3) |
| Kinase activity (molecular function)b | GO:0016301 | 9 (11) |
| Lipid metabolic process | GO:0006629 | 6 (7) |
| Metal ion transport | GO:0030001 | 5 (8) |
| Nucleic acid metabolic process | GO:0090304 | 5 (5) |
| Oxidation–reduction process | GO:0055114 | 9 (11) |
| Photosynthesis | GO:0015979 | 29 (53) |
| Predicted protein of unknown function | – | 20 (32) |
| Protein binding (molecular function)b | GO:0005515 | 5 (5) |
| Protein transport | GO:0015031 | 4 (4) |
| Regulation of protein metabolism | GO:0051246 | 9 (11) |
| Regulation of transcription | GO:0045449 | 15 (17) |
| Phenylpropanoid biosynthetic process | GO:0009699 | 3 (5) |
| Rapid alkalinization factor-like (RALFL) | – | 2 (120) |
| Signaling | GO:0023052 | 9 (11) |
| Translation | GO:0006412 | 9 (11) |
| Transport | GO:0006810 | 2 (2) |
| No hit | – | 9 (9) |
| Others (miscellaneous)c | – | 13 (13) |
| Total | 213 (405) |
“Development” summarizes several developmental processes of different “biological process” GO terms.
Annotation according to GO terms “molecular function” due to the high diversity of biological processes in which the gene products are involved.
For details, see Supplementary Table S2.
The most abundant EST in the library shows partial homology to a gene coding for an unknown P. trichocarpa protein, which itself exhibits weak similarity to a member of the rapid alkalinization factor (RALF) gene family encoding small secreted peptide hormones (Pearce et al., 2001). This EST clone was identified 120 times, suggesting a considerable transcriptional activation and thus a potentially important role in S. caprea metal tolerance. The second largest group with 53 members comprises genes encoding proteins of the photosynthetic apparatus, accompanied by numerous genes encoding constituents of basic cellular processes. In accordance with the initial hypothesis, eight ESTs of metal transporters and chelators were identified, one of them encoding ScMT3 which had already been selected in the candidate gene approach (Fig. 1). Thirty-two and nine ESTs showed homology to proteins of unknown function or were lacking similarities to already annotated genes, respectively.
3.3. Novel genes are candidates for genotype-specific metal responses
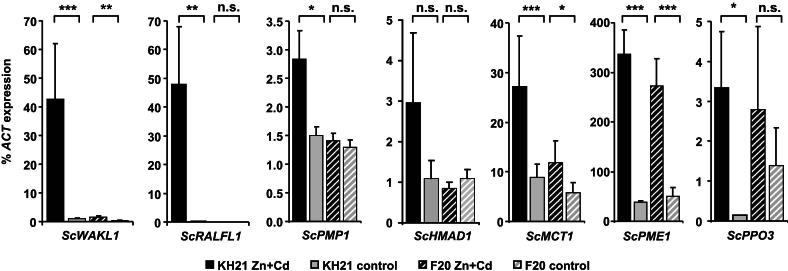
In order to determine the reliability of the SSH screening and their potential genotype-specific transcription, the expression of selected genes was compared in KH21 and F20 using qPCR (Supplementary Table S3, Fig. 4). Of special interest were genes identified several times during the screening, such as ScRALFL1 and a thylakoid membrane phosphoprotein (ScTMP14) which occurred 120 and 10 times during the SSH library screen, respectively. Polyphenol oxidase (ScPPO3) ESTs were found three times. The pectin methylesterase (ScPME1) and a disease resistance protein encoding EST (ScTOL21) were identified twice. The other selected genes are implicated in metal transport (ScHMAD1, ScMCT1, ScMT2A), or might be involved in metal perception or signaling due to their predicted plasma membrane localization (ScPMP1, ScWAKL1).
Fig. 4.
qPCR expression analysis in leaves of selected genes identified in the SSH screen. Expression values were normalized to ScACTIN2 and represent means ± SE of six biological replicates after either 8 or 14 weeks of Zn + Cd treatment. Abbreviations of genes correspond to Supplementary Table S3. Significance levels were calculated with Student's t-test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
All these selected genes showed Zn- and Cd-triggered expression but at varying degrees. Three genes (ScTMP14, ScTOL21, ScMT2A) were only transiently activated in the metallicolous genotype KH21 at the time point of leaf sampling for the construction of the SSH library, i.e. after eight weeks of combined treatment, but not at any other time point (data not shown). The other genes showed comparable expression patterns at multiple time points (Fig. 4). They could be classified into similar categories as the initial candidate genes. ScPME1 and to a lesser extent ScPPO3 were induced in both genotypes. Thus they are grouped to the first category of metal induced genes independent of the genotypes' origins and metal accumulation capacities. The other tested genes grouped to the third category with genes only induced in the metallicolous genotype KH21. ScMCT1, ScHMAD1 and ScPMP1 were induced only in KH21, whereas the basal expression level in F20 did not change. ScWAKL1 and ScRALFL1 could hardly be detected in both genotypes in the control treatments, but were highly expressed in KH21 upon metal exposure (Fig. 4). Because of the highly genotype-specific expression pattern of ScWAKL1 and ScRALFL1, these two genes are promising candidates for future functional analyses.
4. Discussion
Although gene expression does not necessarily imply functional relevance (Roosens et al., 2008), expression profiling was a successful starting point for the identification of genes involved in metal hyperaccumulation (Becher et al., 2004). This approach is particularly useful for non-model plants with no sequence information such as S. caprea. This study presents the first and largest EST collection of S. caprea currently available. Additionally, in-depth expression analyses of 14 genes revealed transcriptional responses to Zn and Cd in S. caprea, which may be a source to explain the different Cd accumulation abilities of the two studied naturally occurring genotypes (Vaculík et al., 2012).
Genes encoding two metallothioneins (ScMT2B and ScMT3), a pectin methylesterase ScPME1, and to a lesser extent a polyphenol oxidase (ScPPO3) were induced irrespective of the genotypes' origin and metal uptake capacity. Metallothioneins are involved in metal sequestration in numerous species (Cobbett and Goldsbrough, 2002). Hybrid aspen (P. trichocarpa × deltoides) PtdMT2b conferred Cd tolerance to a Cd sensitive yeast strain (Kohler et al., 2004). MT2b expression levels of P. tremula × tremuloides correlated with foliar Zn and Cd concentrations on metal contaminated soil (Hassinen et al., 2009). Induced metallothionein expression might constitute a general metal response in S. caprea leaves allowing higher Zn and Cd uptake. Transcriptional upregulation of ScPME1 suggests that modification of cell wall properties might be a general response to metal stress in S. caprea. PMEs modify cell walls by changing their degree of pectin methylesterification and creating free carboxylic groups that retain divalent cations, thereby enhancing the apoplastic metal binding capacity (Pelloux et al., 2007). Numerous metals induce PME expression and activity, such as aluminum in rice (Yang et al., 2008) and Zn in N. caerulescens (Hassinen et al., 2007).
Both S. caprea genotypes accumulated high foliar Zn and Cd concentrations, however, the metallicolous genotype took up significantly more Cd than the non-metallicolous genotype (Vaculík et al., 2012). This differential metal uptake correlated with constitutively higher expression of the cysteine biosynthesis gene ScSAT1 in the metallicolous genotype. Similar constitutively high SAT expression was also observed in the nickel hyperaccumulator T. goesingense and its overexpression in Arabidopsis leads to enhanced tolerance toward cobalt, Zn and Cd (Freeman et al., 2004; Freeman and Salt, 2007).
While ScSAT1 was the only constitutively higher expressed gene, most others, such as the glutathione biosynthesis genes ScOASA1 and ScCAD2, were induced only in the metallicolous genotype. Glutathione is important for metal detoxification in several plant species (Seth et al., 2012). Overexpression of an Arabidopsis ortholog of ScOASA1 increased the Cd tolerance (Dominguez-Solis et al., 2004) while the cad2-1 mutant is Cd sensitive (Howden et al., 1995a,b). Thus cysteine and glutathione quantifications might help to select Zn and Cd tolerant S. caprea genotypes.
Two genes encoding putative orthologs of important metal transporters, ScZIP6 and ScHMA1, were transiently induced only in the metallicolous genotype. Poplar ZIP6.1 and ZIP6.2 belong to a Zn deficiency inducible family (Migeon et al., 2010) and AtHMA1 has been shown to contribute to the detoxification of excess Zn in Arabidopsis (Kim et al., 2009). The metal homeostasis function is corroborated by their induction upon low and high Zn in A. halleri (Becher et al., 2004).
A cell wall-associated kinase-like (ScWAKL1) and an EST with weak similarities to rapid alkalinization factors (ScRALFL1) displayed the most striking expression pattern. For the Arabidopsis AtWAKL4 gene, an involvement in tolerance to multiple metal pollution was reported (Hou et al., 2005). However, metal-related functions are still elusive for ScRALFL1 (Haruta and Constabel, 2003; Haruta et al., 2008; Pearce et al., 2001) and the genotype-specific genes, the putative membrane protein (ScPMP1), the metal-associated domain-containing protein, ScHMAD1, and the mitochondrial copper transporter, ScMCT1.
Furthermore our SSH analysis implicated a wide range of cellular processes in response to long-term Zn and Cd exposure. The homology annotation of the upregulated ESTs predominantly revealed genes involved in photosynthesis, carbon fixation and carbohydrate metabolism which is consistent with a report on metal disturbed mitochondrial electron transfer in poplar (Kieffer et al., 2009). It appears that S. caprea compensates for the metal induced mitochondrial and photosynthesis defects by raising the corresponding mRNA levels.
Metals interfering with photosynthesis generate reactive oxygen species (Verbruggen et al., 2009a). Eleven ESTs were related to redox processes and reactive oxygen species scavenging. Among them a polyphenol oxidase (ScPPO3) was strongly metal inducible in the metallicolous and by trend elevated in the non-metallicolous genotype. High PPO levels created polymerized phenolic crystals serving as Cd “traps” in cell walls of Water Lily (Nymphaeae L.) (Lavid et al., 2001). Whether such crystals are also formed in S. caprea cell walls remains to be determined. Alternatively, PPO was implicated in herbivore defense in hybrid poplar (P. trichocarpa × deltoides) (Constabel et al., 2000). As such, it is not the only EST indicating parallel responses to biotic and abiotic challenges. Six disease resistance ESTs, among them two encoding a thaumatin/osmotin-like protein (ScTOL21) were isolated. The abundance of a thaumatin-like protein is also increased in Cd-challenged poplar leaves (Kieffer et al., 2009).
The present study demonstrates the complexity of the transcriptional network activated by Zn and Cd in S. caprea genotypes with different storage capabilities. It provides new candidate genes that might be responsible for naturally adapted S. caprea genotypes to cope with elevated Zn and Cd levels. This expression survey shows that naturally adapted, woody non-model plants such as S. caprea genotypes provide useful sources to identify novel molecular mechanisms of metal tolerance.
Acknowledgments
We thank A. Lux, W. Adlassnig and I. Lichtscheidl for valuable comments on the manuscript. We acknowledge G. Klanert, M. Kańduła, A. Farid, and S. Neubert for technical assistance. We thank A. Aryan, A. Golestani, P. Brugner, A. Rodler and C. Steidl for help in greenhouse and laboratory work. This work was funded by the WWTF LS149 (Genometalix) project and the FWF project L433_B17.
Footnotes
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.envpol.2013.02.033.
Appendix A. Supplementary material
References
- Adams J.P., Adeli A., Hsu C.-Y., Harkess R.L., Page G.P., dePamphilis C.W., Schultz E.B., Yuceer C. Poplar maintains zinc homeostasis with heavy metal genes HMA4 and PCS1. Journal of Experimental Botany. 2011;62:3737–3752. doi: 10.1093/jxb/err025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher M., Talke I.N., Krall L., Krämer U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant Journal. 2004;37:251–268. doi: 10.1046/j.1365-313x.2003.01959.x. [DOI] [PubMed] [Google Scholar]
- Bert V., Macnair M.R., De Laguerie P., Saumitou-Laprade P., Petit D. Zinc tolerance and accumulation in metallicolous and nonmetallicolous populations of Arabidopsis halleri (Brassicaceae) New Phytologist. 2000;146:225–233. doi: 10.1046/j.1469-8137.2000.00634.x. [DOI] [PubMed] [Google Scholar]
- Broadley M.R., White P.J., Hammond J.P., Zelko I., Lux A. Zinc in plants. New Phytologist. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Cobbett C., Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Reviews in Plant Biology. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- Constabel C.P., Yip L., Patton J.J., Christopher M.E. Polyphenol oxidase from hybrid poplar. Cloning and expression in response to wounding and herbivory. Plant Physiology. 2000;124:285–295. doi: 10.1104/pp.124.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Baccio D., Galla G., Bracci T., Andreucci A., Barcaccia G., Tognetti R., Sebastiani L. Transcriptome analyses of Populus × euramericana clone I-214 leaves exposed to excess zinc. Tree Physiology. 2011;31:1293–1308. doi: 10.1093/treephys/tpr106. [DOI] [PubMed] [Google Scholar]
- Diatchenko L., Lau Y.F., Campbell A.P., Chenchik A., Moqadam F., Huang B., Lukyanov S., Lukyanov K., Gurskaya N., Sverdlov E.D., Siebert P.D. Suppression subtractive hybridization: a method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6025–6030. doi: 10.1073/pnas.93.12.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Solis J.R., Lopez-Martin M.C., Ager F.J., Ynsa M.D., Romero L.C., Gotor C. Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnology Journal. 2004;2:469–476. doi: 10.1111/j.1467-7652.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- Dos Santos Utmazian M.N., Wieshammer G., Vega R., Wenzel W.W. Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environmental Pollution. 2007;148:155–165. doi: 10.1016/j.envpol.2006.10.045. [DOI] [PubMed] [Google Scholar]
- Dräger D.B., Desbrosses-Fonrouge A.G., Krach C., Chardonnens A.N., Meyer R.C., Saumitou-Laprade P., Krämer U. Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant Journal. 2004;39:425–439. doi: 10.1111/j.1365-313X.2004.02143.x. [DOI] [PubMed] [Google Scholar]
- Escarré J., Lefèbvre C., Gruber W., Leblanc M., Lepart J., Rivière Y., Delay B. Zinc and cadmium hyperaccumulation by Thlaspi caerulescens from metalliferous and nonmetalliferous sites in the Mediterranean area: implications for phytoremediation. New Phytologist. 2000;145:429–437. doi: 10.1046/j.1469-8137.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Freeman J.L., Salt D.E. The metal tolerance profile of Thlaspi goesingense is mimicked in Arabidopsis thaliana heterologously expressing serine acetyl-transferase. BMC Plant Biology. 2007;7:63. doi: 10.1186/1471-2229-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J.L., Persans M.W., Nieman K., Albrecht C., Peer W., Pickering I.J., Salt D.E. Increased glutathione biosynthesis plays a role in nickel tolerance in thlaspi nickel hyperaccumulators. Plant Cell. 2004;16:2176–2191. doi: 10.1105/tpc.104.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanikenne M., Talke I.N., Haydon M.J., Lanz C., Nolte A., Motte P., Kroymann J., Weigel D., Krämer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453:391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- Haruta M., Constabel C.P. Rapid alkalinization factors in poplar cell cultures. Peptide isolation, cDNA cloning, and differential expression in leaves and methyl jasmonate-treated cells. Plant Physiology. 2003;131:814–823. doi: 10.1104/pp.014597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M., Monshausen G., Gilroy S., Sussman M.R. A cytoplasmic Ca2+ functional assay for identifying and purifying endogenous cell signaling peptides in Arabidopsis seedlings: identification of AtRALF1 peptide. Biochemistry. 2008;47:6311–6321. doi: 10.1021/bi8001488. [DOI] [PubMed] [Google Scholar]
- Hassinen V.H., Tervahauta A.I., Halimaa P., Plessl M., Peraniemi S., Schat H., Aarts M.G.M., Servomaa K., Karenlampi S.O. Isolation of Zn-responsive genes from two accessions of the hyperaccumulator plant Thlaspi caerulescens. Planta. 2007;225:977–989. doi: 10.1007/s00425-006-0403-0. [DOI] [PubMed] [Google Scholar]
- Hassinen V., Vallinkoski V.M., Issakainen S., Tervahauta A., Kärenlampi S., Servomaa K. Correlation of foliar MT2b expression with Cd and Zn concentrations in hybrid aspen (Populus tremulaxtremuloides) grown in contaminated soil. Environmental Pollution. 2009;157:922–930. doi: 10.1016/j.envpol.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Hou X., Tong H., Selby J., Dewitt J., Peng X., He Z.H. Involvement of a cell wall-associated kinase, WAKL4, in Arabidopsis mineral responses. Plant Physiology. 2005;139:1704–1716. doi: 10.1104/pp.105.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R., Andersen C.R., Goldsbrough P.B., Cobbett C.S. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiology. 1995;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R., Goldsbrough P.B., Andersen C.R., Cobbett C.S. Cadmium-sensitive, cad1 mutants of Arabidopsis thaliana are phytochelatin deficient. Plant Physiology. 1995;107:1059–1066. doi: 10.1104/pp.107.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsai A., Müller S., Platz S., Hauser M.T. Evaluation of a homemade SYBR green I reaction mixture for real-time PCR quantification of gene expression. Biotechniques. 2002;32(790–792):794–796. doi: 10.2144/02324st05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinänen S.I., Hassinen V.H., Kärenlampi S.O., Tervahauta A.I. Isolation of genes up-regulated by copper in a copper-tolerant birch (Betula pendula) clone. Tree Physiology. 2007;27:1243–1252. doi: 10.1093/treephys/27.9.1243. [DOI] [PubMed] [Google Scholar]
- Kieffer P., Schroder P., Dommes J., Hoffmann L., Renaut J., Hausman J. Proteomic and enzymatic response of poplar to cadmium stress. Journal of Proteomics. 2009;72:379–396. doi: 10.1016/j.jprot.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Kim Y.Y., Choi H., Segami S., Cho H.T., Martinoia E., Maeshima M., Lee Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant Journal. 2009;58:737–753. doi: 10.1111/j.1365-313X.2009.03818.x. [DOI] [PubMed] [Google Scholar]
- Kohler A., Blaudez D., Chalot M., Martin F. Cloning and expression of multiple metallothioneins from hybrid poplar. New Phytologist. 2004;164:83–93. doi: 10.1111/j.1469-8137.2004.01168.x. [DOI] [PubMed] [Google Scholar]
- Landberg T., Greger M. Differences in uptake and tolerance to heavy metals in Salix from unpolluted and polluted areas. Applied Geochemistry. 1996;11:175–180. [Google Scholar]
- Laureysens I., Blust R., De Temmerman L., Lemmens C., Ceulemans R. Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture: I. Seasonal variation in leaf, wood and bark concentrations. Environmental Pollution. 2004;131:485–494. doi: 10.1016/j.envpol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Lavid N., Schwartz A., Lewinsohn E., Tel-Or E. Phenols and phenol oxidases are involved in cadmium accumulation in the water plants Nymphoides peltata (Menyanthaceae) and Nymphaeae (Nymphaeaceae) Planta. 2001;214:189–195. doi: 10.1007/s004250100610. [DOI] [PubMed] [Google Scholar]
- Lombi E., Zhao F.J., Dunham S.J., McGrath S.P. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytologist. 2000;145:11–20. [Google Scholar]
- Marmiroli M., Pietrini F., Maestri E., Zacchini M., Marmiroli N., Massacci A. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy metals and organics. Tree Physiology. 2011;31:1319–1334. doi: 10.1093/treephys/tpr090. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cozatl D., Jobe T., Hauser F., Schroeder J. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Current Opinion in Plant Biology. 2011;14:554–562. doi: 10.1016/j.pbi.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon A., Blaudez D., Wilkins O., Montanini B., Campbell M.M., Richaud P., Thomine S., Chalot M. Genome-wide analysis of plant metal transporters, with an emphasis on poplar. Cellular and Molecular Life Sciences. 2010;67:3763–3784. doi: 10.1007/s00018-010-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ó Lochlainn S., Bowen H.C., Fray R.G., Hammond J.P., King G.J., White P.J., Graham N.S., Broadley M.R. Tandem quadruplication of HMA4 in the zinc (Zn) and cadmium (Cd) hyperaccumulator Noccaea caerulescens. PLoS One. 2011;6:e17814. doi: 10.1371/journal.pone.0017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Moura D.S., Stratmann J., Ryan C.A. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12843–12847. doi: 10.1073/pnas.201416998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux J., Rustérucci C., Mellerowicz E.J. New insights into pectin methylesterase structure and function. Trends in Plant Science. 2007;12:267–277. doi: 10.1016/j.tplants.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Plessl M., Rigola D., Hassinen V.H., Tervahauta A., Kärenlampi S., Schat H., Aarts M.G., Ernst D. Comparison of two ecotypes of the metal hyperaccumulator Thlaspi caerulescens (J. & C. PRESL) at the transcriptional level. Protoplasma. 2010;239:81–93. doi: 10.1007/s00709-009-0085-0. [DOI] [PubMed] [Google Scholar]
- Pulford I.D., Watson C. Phytoremediation of heavy metal-contaminated land by trees – a review. Environment International. 2003;29:529–540. doi: 10.1016/S0160-4120(02)00152-6. [DOI] [PubMed] [Google Scholar]
- Puschenreiter M., Türktaş M., Sommer P., Wieshammer G., Laaha G., Wenzel W.W., Hauser M.T. Differentiation of metallicolous and non-metallicolous Salix caprea populations based on phenotypic characteristics and nuclear microsatellite (SSR) markers. Plant, Cell & Environment. 2010;33:1641–1655. doi: 10.1111/j.1365-3040.2010.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggiotti S., Barcaccia G., Schiavon M., Nicole S., Galla G., Rossignolo V., Soattin M., Malagolia M. Phytoremediation of chromium using Salix species: cloning ESTs and candidate genes involved in the Cr response. Gene. 2007;402:68–80. doi: 10.1016/j.gene.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Rascio N., Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Science. 2011;180:169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Robinson B.H., Mills T.M., Petit D., Fung L.E., Green S.R., Clothier B.E. Natural and induced cadmium-accumulation in poplar and willow: implications for phytoremediation. Plant and Soil. 2000;227:301–306. [Google Scholar]
- Roosens N.H., Willems G., Saumitou-Laprade P. Using Arabidopsis to explore zinc tolerance and hyperaccumulation. Trends in Plant Science. 2008;13:208–215. doi: 10.1016/j.tplants.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Ruttens A., Boulet J., Weyens N., Smeets K., Adriaensen K., Meers E., Van Slycken S., Tack F., Meiresonne L., Thewys T., Witters N., Carleer R., Dupae J., Vangronsveld J. Short rotation coppice culture of willows and poplars as energy crops on metal contaminated agricultural soils. International Journal of Phytoremediation. 2011;13(Suppl. 1):194–207. doi: 10.1080/15226514.2011.568543. [DOI] [PubMed] [Google Scholar]
- Salt D.E., Smith R.D., Raskin I. Phytoremediation. Annual Reviews in Plant Physiology and Plant Molecular Biology. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- Schwitzguébel J.P., Comino E., Plata N., Khalvati M. Is phytoremediation a sustainable and reliable approach to clean-up contaminated water and soil in Alpine areas? Environmental Science and Pollution Research International. 2011;18:842–856. doi: 10.1007/s11356-011-0498-0. [DOI] [PubMed] [Google Scholar]
- Seth C.S., Remans T., Keunen E., Jozefczak M., Gielen H., Opdenakker K., Weyens N., Vangronsveld J., Cuypers A. Phytoextraction of toxic metals: a central role for glutathione. Plant Cell and Environment. 2012;35:334–346. doi: 10.1111/j.1365-3040.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- Shen Z.G., Zhao F.J., McGrath S.P. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the non-hyperaccumulator Thlaspi ochroleucum. Plant, Cell & Environment. 1997;20:898–906. [Google Scholar]
- Srivastava A.K., Venkatachalam P., Raghothama K.G., Sahi S.V. Identification of lead-regulated genes by suppression subtractive hybridization in the heavy metal accumulator Sesbania drummondii. Planta. 2007;225:1353–1365. doi: 10.1007/s00425-006-0445-3. [DOI] [PubMed] [Google Scholar]
- Šyc M., Pohořelý M., Kameníková P., Habart J., Svoboda K., Punčochář M. Willow trees from heavy metals phytoextraction as energy crops. Biomass and Bioenergy. 2012;37:106–113. [Google Scholar]
- Thewys T., Witters N., Van Slycken S., Ruttens A., Meers E., Tack F.M., Vangronsveld J. Economic viability of phytoremediation of a cadmium contaminated agricultural area using energy maize. Part I: effect on the farmer's income. International Journal of Phytoremediation. 2010;12:650–662. doi: 10.1080/15226514.2010.493187. [DOI] [PubMed] [Google Scholar]
- Unterbrunner R., Puschenreiter M., Sommer P., Wieshammer G., Tlustos P., Zupan M., Wenzel W.W. Heavy metal accumulation in trees growing on contaminated sites in Central Europe. Environmental Pollution. 2007;148:107–114. doi: 10.1016/j.envpol.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Vaculík M., Konlechner C., Langer I., Adlassnig W., Puschenreiter M., Lux A., Hauser M.-T. Root anatomy and element distribution vary between two Salix caprea isolates with different Cd accumulation capacities. Environmental Pollution. 2012;163:117–126. doi: 10.1016/j.envpol.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Mortel J.E., Almar Villanueva L., Schat H., Kwekkeboom J., Coughlan S., Moerland P.D., Ver Loren van Themaat E., Koornneef M., Aarts M.G.M. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiology. 2006;142:1127–1147. [Google Scholar]
- Vangronsveld J., Herzig R., Weyens N., Boulet J., Adriaensen K., Ruttens A., Thewys T., Vassilev A., Meers E., Nehnevajova E., van der Lelie D., Mench M. Phytoremediation of contaminated soils and groundwater: lessons from the field. Environmental Science and Pollution Research International. 2009;16:765–794. doi: 10.1007/s11356-009-0213-6. [DOI] [PubMed] [Google Scholar]
- Verbruggen N., Hermans C., Schat H. Mechanisms to cope with arsenic or cadmium excess in plants. Current Opinion in Plant Biology. 2009;12:364–372. doi: 10.1016/j.pbi.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Verbruggen N., Hermans C., Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- Wenzel W., Jockwer F. Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environmental Pollution. 1999;104:145–155. [Google Scholar]
- Xing J.P., Jiang R.F., Ueno D., Ma J.F., Schat H., McGrath S.P., Zhao F.J. Variation in root-to-shoot translocation of cadmium and zinc among different accessions of the hyperaccumulators Thlaspi caerulescens and Thlaspi praecox. New Phytologist. 2008;178:315–325. doi: 10.1111/j.1469-8137.2008.02376.x. [DOI] [PubMed] [Google Scholar]
- Yang J.L., Li Y.Y., Zhang Y.J., Zhang S.S., Wu Y.R., Wu P., Zheng S.J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiology. 2008;146:602–611. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Fujii M., Yamaji N., Sasaki A., Yamane M., Sakurai I., Sato K., Ma J.F. Isolation and characterization of a barley yellow stripe-like gene, HvYSL5. Plant and Cell Physiology. 2011;52:765–774. doi: 10.1093/pcp/pcr009. [DOI] [PubMed] [Google Scholar]
- Zulfiqar A., Paulose B., Chhikara S., Dhankher O. Identifying genes and gene networks involved in chromium metabolism and detoxification in Crambe abyssinica. Environmental Pollution. 2011;159:3123–3128. doi: 10.1016/j.envpol.2011.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.