Abstract
Cognitive changes associated with cancer and cancer treatments have become an increasing concern. Using breast cancer as the prototype, we reviewed the research from neuropsychological, imaging, genetic, and animal studies that have examined pre- and post-treatment cognitive change. An impressive body of research supports the contention that a subgroup of patients is vulnerable to post-treatment cognitive problems. We also propose that models of aging may be a useful conceptual framework for guiding research in this area and suggest that a useful perspective may be viewing cognitive change in patients with cancer within the context of factors that influence the trajectory of normal aging.
INTRODUCTION
Cognitive changes associated with treatments for CNS and pediatric cancers have long been recognized.1,2 However, over the last 15 to 20 years, increasing evidence has suggested that treatments for non-CNS tumors can have both acute and long-term effects on cognitive functioning, which can affect educational and occupational goals and quality of life. Understanding these cognitive changes and the impact on survivors' functioning is critical, because hundreds of thousands of patients are treated worldwide each year, and the number of long-term survivors who may have to cope with these cognitive changes is growing dramatically. This review focuses on cognitive changes associated with adjuvant treatment for breast cancer as an example of the emerging findings in this field. Furthermore, we will explore the value of viewing this literature within the larger context of models of aging.
NEUROPSYCHOLOGICAL STUDIES
Although references to cognitive changes associated with chemotherapy can be found dating back to the 1980s,3 serious scientific attention was not paid to the topic until the mid 1990s.1 Post-treatment cognitive changes frequently include problems in attention, concentration, working memory, and executive function. Cross-sectional studies of breast cancer survivors have found that 17% to 75% of women experienced cognitive deficits in these domains from 6 months to 20 years after exposure to chemotherapy.4–6 The lack of prechemotherapy assessment of cognitive performance limited the conclusions that could be drawn from these studies; consequently, investigators began longitudinal studies that included pretreatment neuropsychological assessments. To date, 21 longitudinal studies7–27 including pre- and post-treatment assessments have been reported, and a majority of studies16 have found evidence for post-treatment cognitive change (Table 1). Consistent with the cross-sectional studies, the longitudinal studies suggest that a subgroup of patients experience post-treatment cognitive problems. Estimates of the frequency of post-treatment cognitive change vary among studies, likely because of differences in patient populations, assessment instruments used, criteria for defining change, and other aspects of study methods. Many investigators cite the incidence of post-treatment cognitive problems as ranging from 15% to 25%,28 although percentages as high as 61% have been reported.7 However, results of the longitudinal studies have challenged some basic assumptions made in the field and have shown a less consistent pattern of post-treatment cognitive decline (five studies had negative findings12,16,18,22,23).
Table 1.
Longitudinal Studies of Cognitive Effects of Adjuvant Therapy in Women With Breast Cancer
| Study | Participants | Assessment Schedule | Cognitive Domains | Outcomes |
|---|---|---|---|---|
| Wefel et al7 | Chemotherapy (n = 18; mean age, 45.4 years) | Baseline, 3 weeks postchemotherapy, and 1 year postchemotherapy | Attention, processing speed, learning, memory, executive function, visuospatial function, and motor skills | Decline in attention, learning, and processing speed |
| Mar Fan et al8 | Adjuvant chemotherapy (n = 104); healthy controls (n = 102) | Baseline and 1 and 2 years after adjuvant chemotherapy | Memory, language, attention/concentration, visual motor, spatial, psychomotor speed, and executive functions | Moderate to severe cognitive dysfunction decreased from 16% to 4% over 2-year follow-up; no difference in cognition between ER-positive patients who started hormonal therapy (mainly tamoxifen) after chemotherapy and ER-negative patients who did not |
| Schilling et al9 | Patients to receive chemotherapy and patients receiving radiotherapy and/or endocrine therapy (n = 50; mean age, 51.1 years); healthy controls (n = 43; mean age, 52.3 years) | Baseline, 4 weeks after completion of chemotherapy (6 months for controls), and 18 months | Intelligence, verbal memory, visual memory, working memory, executive function, and processing speed and vigilance | Decline in cognitive performance compared with controls |
| Bender et al10 | Chemotherapy only, chemotherapy and tamoxifen, and no chemotherapy or tamoxifen (n = 46; mean age, 42.57 years) | T1, postsurgery, before starting adjuvant therapy; T2, within 2 weeks of completing chemotherapy; T3, 1 year after T2 | Attention, learning, memory, psychomotor speed, mental flexibility, executive function, visuoconstructional ability, and general intelligence | Chemotherapy plus tamoxifen group declined in visual memory and verbal working memory; chemotherapy alone group showed decline in verbal working memory only |
| Hurria et al11 | Chemotherapy only (age > 65 years; n = 31; mean age, 71 years) | Before and after chemotherapy | Attention, verbal memory, visual memory, and verbal, spatial, psychomotor, and executive functions | Women who received CMF (91%) declined in visual memory, spatial function, attention, and psychomotor function |
| Jenkins et al12 | Chemotherapy (n = 85; mean age 51.49 years); endocrine therapy and/or radiotherapy (n = 43; mean age, 58.93 years); healthy controls (n = 49; mean age, 51.90 years) | Baseline, postchemotherapy or 6 months postbaseline, and 18 months postbaseline | Intelligence, verbal memory, visual memory, working memory, executive function, and processing speed and vigilance | Treatment regimens were not found to affect cognitive performance at group or individual level |
| Schagen et al13 | High-dose chemotherapy (n = 28; mean age, 45.5 years); standard-dose chemotherapy (n = 39; mean age, 45.2 years); stage I disease, not receiving chemotherapy (n = 57; mean age, 50.5 years); healthy controls (n = 60; mean age, 48.8 years) | High- and standard-dose groups, before and 6 months after chemotherapy (12-month interval); stage I disease with no chemotherapy, baseline and 12-month interval; healthy controls, baseline and 6-month interval | Attention, working, verbal, and visual memory, processing speed, executive function, and verbal and motor function | Patients who received CTC chemotherapy declined in cognitive performance |
| Hermelink et al14 | Chemotherapy (n = 101; mean age, 48.6 years); standard dose (n = 48); dose intense (n = 53) | Before neoadjuvant chemotherapy and toward end of neoadjuvant chemotherapy | Verbal memory, attention, working memory, information processing speed, and executive function | Before chemotherapy, subgroup showed cognitive decline; during chemotherapy, most remained stable; subgroup declined (27%) and another improved (28%); no effects were associated with treatment arm |
| Stewart et al15 | Adjuvant chemotherapy (n = 61; mean age, 57.5 years); adjuvant hormonal therapy (n = 51; mean age, 57.9 years) | Baseline (before any adjuvant treatment) and follow-up (after last chemotherapy cycle or equivalent time point in hormonal group) | Executive function, language function, motor, processing speed, verbal learning and memory, visual learning and memory, visuospatial function, and working memory | Chemotherapy patients were 3.3× more likely to show reliable cognitive decline; working memory was most vulnerable to chemotherapy |
| Collins et al16 | Postmenopausal patients: chemotherapy (n = 53; mean age, 57.9 years); hormone therapy only (n = 40; mean age, 57.6 years) | After surgery but before adjuvant chemotherapy, within 1 month of completing chemotherapy or 5-6 months after baseline (T2), and 1 year after T2 (T3) | Executive function, language function, motor, processing speed, verbal learning and memory, visual learning and memory, visuospatial function, and working memory | Chemotherapy plus hormone therapy group performed more poorly on measures of processing speed and verbal memory at T3 |
| Hermelink et al17 | Premenopausal (n = 11); induced menopause at T3 (n = 31); postmenopausal (n = 49; mean age, 48.4 years); received one of two neoadjuvant chemotherapy regimens; received tamoxifen or AIs (n = 62) | Before start of cancer therapy, toward end of neoadjuvant chemotherapy, and 1 year after baseline | Verbal memory, attention, working memory, information processing speed, psychomotor function, and executive function | No effects of treatment-induced hormonal changes on cognitive functioning |
| Mehlsen et al18 | Patients with breast cancer (n = 34; mean age, 48.6 years); cardiac patients hospitalized with MI (n = 12; mean age, 50.4 years); healthy controls (n = 12; mean age, 39.3 years) | Baseline; patients with cancer, 0-7 days before chemotherapy; cardiac patients, 4 days after hospitalization; follow-up (25 weeks later for patients with cancer; 3 months later for cardiac patients); healthy controls, 12-16 weeks between assessments | Processing speed, working memory, visuospatial ability, visual memory, verbal memory, verbal fluency, and response inhibition | No differences in cognitive performance between three groups |
| Quesnel et al19 | Chemotherapy and radiotherapy (n = 41; mean age, 50.3 years); radiotherapy only (n = 40; mean age, 57.7 years); healthy controls matched to those receiving chemotherapy and radiotherapy (n = 23; mean age, 47.9 years); healthy controls matched to those receiving only radiotherapy (n = 22; mean age, 55.0 years) | Before and after adjuvant chemotherapy and/or radiotherapy and 3 months post-treatment; healthy controls at baseline only | Verbal and visual memory, attention, concentration, executive functions, speed of information, and verbal fluency | At baseline, patients showed lower performance on attention measures compared with healthy controls; both patient groups declined in verbal memory; chemotherapy group also declined in verbal fluency |
| Vearncombe et al20 | Standard-dose adjuvant chemotherapy with or without endocrine treatment and radiotherapy (n = 138; mean age, 49.4 years); no adjuvant chemotherapy (n = 21; mean age, 53.9 years) | After surgery but before chemotherapy (T1) and 4 weeks after last cycle of chemotherapy (T2); no-chemotherapy group assessed at matched intervals | Verbal learning and memory, visual memory, working memory, processing speed, attention, executive function, and motor coordination | Declines in verbal learning and memory, abstract reasoning, and motor functioning were seen in 16.9% after chemotherapy; decline in hemoglobin and increased anxiety over course of chemotherapy predicted impairment in ≥ two cognitive domains |
| Ahles et al21 | Chemotherapy (n = 60; mean age, 51.7 years); no chemotherapy (n = 72; mean age, 56.6 years); healthy controls (n = 45; mean age, 52.9 years) | Before chemotherapy and 1, 6, and 18 months postchemotherapy; no-chemotherapy group and healthy controls assessed at matched intervals | Verbal ability, verbal memory, visual memory, working memory, processing speed, and executive function | Chemotherapy patients who were older and had lower baseline cognitive reserve had slower processing speed; in no-chemotherapy group, negative effect of tamoxifen on processing speed and verbal memory; healthy controls and no-chemotherapy group improved in verbal ability over time; chemotherapy group improved at 6 and 18 months |
| Debess et al22 | Patients with stage I-III disease identified after surgery (n = 124): chemotherapy (n = 75; mean age, 47.2 years); chemotherapy and tamoxifen (n = 26; mean age, 56.2 years); not receiving chemotherapy or tamoxifen (n = 19; mean age, 49.7 years); healthy controls (n = 208; mean age, 48.1 years) | Baseline and after 6 months of chemotherapy | Concentration, episodic memory (intermediate and long-term memory), simple and complex attention, cognitive speed and flexibility, visual scanning, and executive functioning | No differences in cognitive functioning |
| Tager et al23 | Postmenopausal women: adjuvant chemotherapy (n = 30; mean age, 60.3 years); no adjuvant chemotherapy (n = 31; mean age, 61.1 years) | Before adjuvant chemotherapy, 6 months after adjuvant therapy, and 6 months after second evaluation | Motor, language, attention/concentration/working memory, visuospatial, verbal memory, and visual memory | Time by treatment interaction with slower motor functioning among women treated with chemotherapy (but possibly result of peripheral neuropathy) |
| Wefel et al24 | Patients with stage I-III disease (n = 42; mean age, 48.8 years) | Before adjuvant chemotherapy, during and shortly after adjuvant therapy, and 1 year after chemotherapy | Attention, processing speed, learning and memory, and executive function | Decline most common in learning and memory, executive function, and processing speed; before chemotherapy, 21% had cognitive decline; 71% exhibited continuous decline; 29% had new-onset decline |
| Hedayati et al25 | Chemotherapy (n = 18; mean age, 52 years); hormone therapy (n = 45; mean age, 61 years); no adjuvant therapy (n = 14; mean age, 61 years); healthy controls (n = 69; mean age, 51 years) | Before diagnosis (T1), after surgery and before adjuvant treatment (T2), 6 months after start of adjuvant treatment (T3), and after another 3 months of follow-up (T4) | Response speed, processing speed, memory, and attention | Memory was lower for patients than controls; memory and response speed were lower after chemotherapy and remained low at T4; processing speed and attention improved consistent with practice effect |
| Jansen et al26 | Patients divided by regimen (n = 71; mean age, 50 years): AC alone (n = 22); AC followed by a taxane (n = 49) | Before chemotherapy, 1 week after AC chemotherapy, 1 week after completing all chemotherapy, and 6 months after completing all chemotherapy | Attention, immediate memory, delayed memory, visuospatial, language, motor, and executive function | 23% had impairment before chemotherapy; decreases after chemotherapy but improvement in visuospatial ability, attention, and delayed memory 6 months after completing chemotherapy; deficits in motor function almost exclusively in patients receiving taxane (likely result of peripheral neuropathy) |
| Biglia et al27 | Patients (n = 40; mean age, 51 years) | Before and after 6 months of chemotherapy | Attention, verbal fluency, verbal memory, processing speed, and global intelligence | Decline in selective attention and global cognitive functioning postchemotherapy; processing speed improved (attributed to practice effect) |
Abbreviations: AC, cyclophosphamide and doxorubicin; AI, aromatase inhibitor; CMF, cyclophosphamide, methotrexate, and fluorouracil; CTC, cyclophosphamide, thiotepa, and carboplatin; ER, estrogen receptor; MI, myocardial infarction.
Two basic assumptions were: one, patients with breast cancer have normal cognitive functioning before treatment; and two, chemotherapy is the major cause of post-treatment cognitive problems, hence the colloquial term chemobrain. Several studies have found that 20% to 30% of patients with breast cancer have lower than expected cognitive performance based on age and education at the pretreatment assessment.29,30 Interestingly, lower than expected level of performance does not seem to be related to psychological factors (eg, depression or anxiety), fatigue, or surgical factors (eg, type or length of general anesthesia).29 No explanation for this phenomenon currently exists; however, two nonmutually exclusive hypotheses have been proposed: one, the biology of cancer (eg, inflammatory response triggering neurotoxic cytokines) may contribute to lower than expected cognitive performance; and/or two, common risk factors for the development of both breast cancer and mild cognitive changes over years may exist (eg, poor DNA repair mechanisms have been linked to risk of cancer and neurodegenerative disorders).31
The assumption that cognitive changes result from chemotherapy exposure has also been questioned as evidence has emerged suggesting that the combination of chemotherapy and endocrine therapy or endocrine therapy alone may cause cognitive change.32 Initial examination of this issue has produced mixed results; however, most studies were not powered to adequately examine the independent effects of endocrine therapy. A longitudinal study examining patients not treated with chemotherapy who were randomly assigned to treatment with tamoxifen or exemestane demonstrated that those treated with tamoxifen, but not exemestane, experienced cognitive problems compared with healthy controls.33 Early investigators assumed they were studying the effects of chemotherapy; however, most patients with breast cancer receive multimodality treatment (eg, surgery with exposure to general anesthesia, radiation therapy, and endocrine therapy in addition to chemotherapy). This in combination with the evidence for pretreatment cognitive problems led Hurria et al34 to propose that the phenomenon is more accurately described as cancer- and cancer treatment–associated cognitive change.
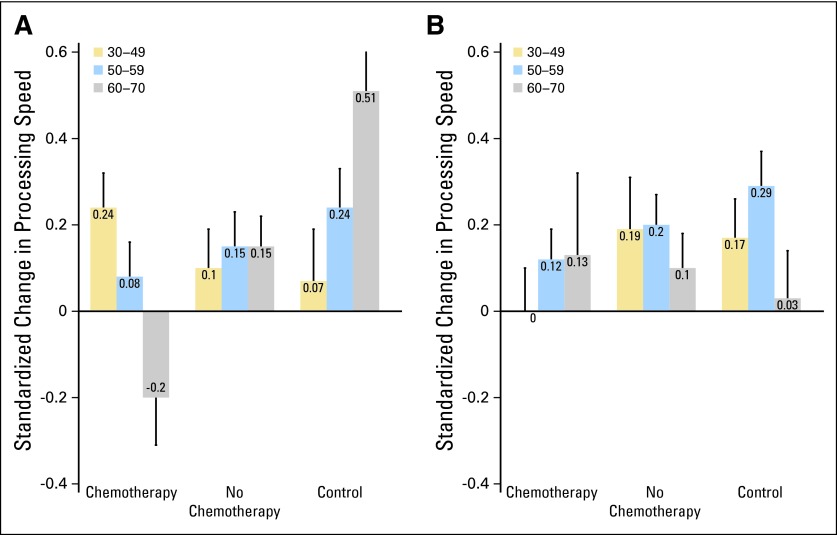
Furthermore, if only a subgroup of patients experience persistent post-treatment cognitive decline, a critical step is to examine risk factors for cognitive change. Age is a well-established risk factor for cognitive decline, and researchers have speculated that older adults may be more vulnerable to cognitive adverse effects of cancer treatments. Cognitive reserve, which represents innate and developed cognitive capacity (influenced by education, occupational attainment, and lifestyle), has also been associated with resiliency (high) or vulnerability (low) to cognitive decline after various brain insults. Support for an interaction of age, cognitive reserve, and exposure to chemotherapy as a risk factor for cognitive decline has been reported21; older patients with lower levels of pretreatment cognitive reserve exposed to chemotherapy demonstrated significantly reduced performance on post-treatment processing speed (Figs 1A and 1B). Exploratory analyses conducted by Schilder et al33 also revealed that in older patients with breast cancer (age > 65 years), tamoxifen had an effect on more cognitive domains, suggesting an age dependency of the impact of tamoxifen on cognitive functioning.
Fig 1.
Pre- to post-treatment change in processing speed by treatment, age group, and level of cognitive reserve, assessed by the Wide Range Achievement Test (WRAT) –Reading. (A) WRAT below median; (B) WRAT above median.
Genetic factors such as apolipoprotein E (APOE) and catechol-O-methyltransferase (COMT) have been associated with age-related cognitive decline.35 APOE is a complex glycolipoprotein that facilitates the uptake, transport, and distribution of lipids and plays a role in neuronal repair and plasticity after injury. The E4 allele has been associated with cognitive decline related to Alzheimer's disease, brain trauma, and aging. Ahles et al36 demonstrated that long-term cancer survivors who had been treated with chemotherapy and had at least one E4 allele scored significantly lower on a variety of domains of cognitive function, as compared with survivors who did not carry an E4 allele.
Small et al37 studied COMT, which influences the metabolic breakdown of catecholamines through the methylation of dopamine. Individuals homozygous for the Val allele have lower levels of dopamine in the frontal cortex, because they metabolize dopamine more rapidly than those with the Met allele. These researchers found that patients with breast cancer who had the COMT–Val allele combination and were treated with chemotherapy performed more poorly on tests of attention, verbal fluency, and motor speed, as compared with COMT-Met homozygotes.
Several studies did not find evidence for cognitive changes associated with chemotherapy or other treatments. This inconsistent pattern of results may be related to variability in study design and choice of comparison groups. Two of the studies compared patients treated with chemotherapy with patients treated with endocrine therapy but did not include a healthy control group.16,23 However, both chemotherapy- and endocrine-treated patients may experience cognitive change, which could explain the lack of group differences. Furthermore, the pattern of post-treatment cognitive deficits may be influenced by sample characteristics like age and cognitive reserve. Therefore, if a study population consists of young, highly educated (one proxy for cognitive reserve) patients, one might expect less evidence of post-treatment cognitive deficits, as compared with a study that includes older, less educated individuals. In two of the studies with negative findings, the mean ages of the patients with cancer were in the 40s.18,22 Furthermore, in modest-sized studies, the mix of patients with vulnerable alleles of genes like APOE and COMT can vary significantly.
IMAGING STUDIES
Several cross-sectional, post-treatment studies38–42 (Table 2) using magnetic resonance imaging (MRI) have documented reductions in gray matter, primarily in frontal structures and the hippocampus, and white matter integrity in cancer survivors treated with chemotherapy, although negative results have also been reported.43 Longitudinal studies have reported similar results: first, decreased gray matter density in bilateral frontal, temporal (including hippocampus), and cerebellar regions and right thalamus at 1 month postchemotherapy, with only partial recovery at 1 year postchemotherapy in several structures, compared with no significant changes in gray matter over time in the no-chemotherapy cancer group or the healthy controls44; and second, decreased frontal, parietal, and occipital white matter integrity in chemotherapy-exposed patients, with no changes in the no-chemotherapy group or healthy controls post-treatment.45
Table 2.
Structural Imaging Studies
| Study | Design/ Modality | Assessment Schedule | Participants |
Endocrine Therapy | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Group | No. | Age (years) | SD | |||||
| Yoshikawa et al43 | Cross-sectional MRI | T1, 12 months post-treatment | CTX+ | 44 | 48.3 | 5.69 | 31 (tamoxifen) | No difference in hippocampal volume or memory performance between CTX+ and CTX− at 12 months post-treatment |
| CTX− | 31 | 48.2 | 5.7 | 0 (tamoxifen) | ||||
| Inagaki et al38 | Cross-sectional MRI | T1, > 12 months post-treatment | CTX+ | 51 | 47.3 | 5.2 | 20 | Smaller gray and white matter in prefrontal, parahippocampal, cingulate, and precuneus in CTX+ compared with CTX− at 12 months post-treatment |
| CTX− | 54 | 46.3 | 6.1 | 11 | ||||
| HC | 55 | 46.2 | 6.7 | — | ||||
| Inagaki et al38 | Cross-sectional MRI | T1, > 36 months post-treatment | CTX+ | 73 | 48.2 | 5.6 | 21 | No difference between CTX+ and CTX− at 36 months post-treatment |
| CTX− | 59 | 48.4 | 4.8 | 5 | ||||
| HC | 37 | 48 | 6.4 | — | ||||
| Abraham et al39 | Cross-sectional DTI | T1, 22 months post-treatment | CTX+ | 10 | 49.8 | 8 | 4 (tamoxifen); 6 (anastrazole) | Lower FA in genu and slower processing speed in CTX+ compared with HCs at 22 months post-treatment |
| HC | 9 | 46.8 | 6.8 | — | ||||
| McDonald et al44 | Longitudinal MRI | T1, pretreatment; T2, 1 month post-treatment; T3, 12 months post-treatment | CTX+ | 17 | 52.4 | 8.5 | Baseline: 0; month 1: 3 (tamoxifen), 1 (anastrazole); year 1: 9 (tamoxifen), 1 (anastrazole), 3 (letrozole) | Decreased gray matter density in both CTX+ and CTX− compared with HCs at 1 month post-treatment; decreased frontal, temporal, thalamic, and cerebellar gray matter density in CTX+ at 1 month post-treatment compared with pretreatment; gray matter density recovered in CTX+ group, with areas of reduced density remaining at 1 year post-treatment |
| CTX− | 12 | 52.7 | 7.2 | Baseline: 1 (tamoxifen); month 1: 6 (tamoxifen), 1 (tamoxifen/goserelin), 2 (anastrazole); year 1: 6 (tamoxifen), 1 (tamoxifen/goserelin), 2 (anastrazole) | ||||
| HC | 18 | 50.6 | 6.5 | — | ||||
| Koppelmans et al40 | Cross-sectional MRI | T1, 21 years post-treatment | CTX+ | 184 | 64 | 6.5 | Smaller total brain volume and gray matter volume in CTX+ compared with HCs at 21 years post-treatment | |
| HC | 368 | 64 | 6.5 | |||||
| Deprez et al41 | Cross-sectional MRI; DTI | T1, 80-160 days post-treatment | CTX+ | 18 | 45.4 | 4.2 | 12 (novaldex) | Decreased frontal and temporal FA and increased frontal MD in CTX+ compared with CTX− and HCs 80-160 days post-treatment |
| CTX− | 10 | 45.2 | 3.9 | 9 (novaldex) | ||||
| HC | 18 | 45.2 | 3.9 | — | ||||
| Deprez et al45 | Longitudinal MRI; DTI | T1, pretreatment; T2, 3-4 months post-treatment | CTX+ | 34 | 43.7 | 6.1 | 18 (tamoxifen) | Decreased frontal, parietal, and occipital FA in CTX+, with no changes in either CTX− or HCs at 3-4 months post-treatment |
| CTX− | 16 | 43.1 | 5.7 | 14 (tamoxifen) | ||||
| HC | 19 | 43.8 | 4.9 | — | ||||
| de Ruiter et al42 | Cross-sectional MRI; DTI; MRS | T1, > 9 years post-treatment | CTX+ | 17 | 56.5 | 5.1 | 17 (tamoxifen; 3.8 ± 1.7 years) | Reduced white matter integrity in CTX+ compared with CTX− > 9 years post-treatment; reduced N-acetylaspartate/creatine in left centrum semiovale in CTX+ compared with CTX− > 9 years post-treatment; smaller posterior parietal volume in CTX+ compared with CTX− > 9 years post-treatment |
| CTX− | 15 | 58.2 | 5.8 | 1 (tamoxifen; > 5 years) | ||||
Abbreviations: CTX+, chemotherapy; CTX−, no chemotherapy; DTI, diffusion tensor imaging; FA, fractional anisotropy; HC, healthy control; MD, mean diffusivity; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; SD, standard deviation.
Cross-sectional studies of cancer survivors using functional imaging techniques, including functional MRI (fMRI)46–49 and functional positron emission tomography (fPET),50 have demonstrated areas of decreased activation during performance of a cognitive task in survivors exposed to chemotherapy, as compared with controls, in areas similar to the structural differences described (Table 3). McDonald et al51 conducted a longitudinal study using fMRI and found frontal lobe hyperactivation to support a working memory task before treatment, decreased activation 1 month postchemotherapy, and a return to pretreatment hyperactivation at 1 year post-treatment. A similar pattern was seen in patients treated with endocrine therapy. Interestingly, two other studies reported overactivation during a memory task before treatment in patients with cancer compared with healthy controls, consistent with the reports of neuropsychological deficits at pretreatment.52,53 One interpretation is that pretreatment overactivation represents an attempt to compensate for preexisting deficits; however, over years, patients lose the ability for compensatory activation as a result of exposure to cancer treatments and/or age-associated changes in the brain.
Table 3.
Functional Imaging Studies
| Study | Design/ Modality | Assessment Schedule | Participants |
Endocrine Therapy | In-Scanner Task | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|
| Group | No. | Age (years) | SD | ||||||
| Pretreatment | |||||||||
| Cimprich et al52 | Cross-sectional fMRI | T1, pretreatment only | BC | 10 | 45 | 8 | Verbal working memory | Greater bilateral activation during verbal working memory task in BC group compared with HCs pretreatment | |
| HC | 9 | 52 | 10 | ||||||
| Scherling et al53 | Cross-sectional fMRI | T1, pre-treatment only | BC | 23 | 51.5 | 8.47 | Visual n-back | Greater inferior frontal gyrus, insula, thalamus, and midbrain activations during working memory task in BC group compared with HCs pretreatment | |
| HC | 23 | 50.4 | 8.82 | ||||||
| Post-Treatment | |||||||||
| Ferguson et al46 | Cross-sectional MRI; fMRI | T1, 22 months post-treatment | CTX+ | 1 | 60 | Auditory n-back | Greater WM hyperintensities and greater spatial extent of frontal activation during working memory in CTX+ case compared with twin HC case | ||
| HC | 1 | 60 | |||||||
| Silverman et al50 | Cross-sectional PET | T1, 5-10 year post-treatment | CTX+ | 5 | 47.6 | 6 | Paired word memory task; 10-minute delay; 1-day delay | Lower inferior frontal gyrus metabolism in CTX+ compared with CTX− and HCs 5-10 years post-treatment; lower basal ganglia metabolism in CTX + tamoxifen group compared with CTX+, CTX−, and HCs 5-10 years post-treatment | |
| CTX + tamoxifen | 7 | 51.7 | 4.7 | ||||||
| CTX− | 5 | 53.2 | 4.1 | ||||||
| HC | 3 | 57.9 | 7.1 | ||||||
| Kesler et al48 | Cross-sectional fMRI | T1, 3 years post-treatment | CTX+ | 14 | 55.1 | 8.0 | 11 (tamoxifen); 6 (after chemotherapy/ irradiation); 3 (concurrent with tamoxifen); 2 (before tamoxifen); 0 (currently receiving tamoxifen) | Verbal declarative encoding and verbal declarative recognition | Lower prefrontal cortex activation during encoding in CTX+ compared with HCs 3 years post-treatment; greater regional activations during recall in CTX+ compared with HCs 3 years post-treatment |
| HC | 14 | 54.2 | 8.0 | — | |||||
| Kesler et al47 | Cross-sectional fMRI | T1, 5 years post-treatment | CTX+ | 25 | 56.2 | 7.8 | 14 (tamoxifen) | Card-sorting task | Lower left middle dorsolateral prefrontal cortex activation and premotor cortex activation in BC group compared with HCs; lower left caudal lateral prefrontal cortex activation in CTX+ compared with CTX− and HCs 5 years post-treatment |
| CTX− | 19 | 58.1 | 6.5 | 10 (tamoxifen) | |||||
| de Ruiter et al49 | Cross-sectional fMRI | T1, 10 years post-treatment | CTX+ | 19 | 56.3 | 5.5 | 19 (tamoxifen) | Tower of London, Paired Associates | Lower dorsolateral prefrontal cortex activity during Tower of London task; lower parahippocampal gyrus activity during paired associates task in CTX+ compared with CTX− 10 years post-treatment |
| CTX− | 15 | 58.2 | 5.8 | 1 (tamoxifen) | |||||
| McDonald et al51 | Longitudinal fMRI | T1, pretreatment; T2, 1 month post-treatment; T3, 1 year post-treatment | CTX+ | 16 | 52.9 | 8.6 | 0 (baseline); 4 (month 1); 12 (year 1) | N-back test | Greater frontal activation and lower parietal activation at baseline in BC group compared with HCs; lower frontal activation in BC group compared with HCs immediately after treatment; greater frontal activation in BC group compared with HCs 1 year after treatment |
| CTX− | 12 | 52.7 | 7.2 | 1 (baseline); 9 (month 1); 9 (year 1) | |||||
| HC | 15 | 50.5 | 6 | — | |||||
Abbreviations: BC, breast cancer; CTX+, chemotherapy; CTX−, no chemotherapy; fMRI, functional magnetic resonance imaging; HC, healthy control; PET, positron emission tomography; SD, standard deviation; WM, white matter.
ANIMAL STUDIES
Seigers et al54 recently reviewed the animal studies of chemotherapy-induced cognitive impairment. Studies using common chemotherapeutic agents demonstrated changes in memory and learning that parallel the deficits seen in cancer survivors. Furthermore, animal studies have demonstrated evidence for a variety of potential mechanisms for the effect of chemotherapy on the brain, including: one, inhibition of hippocampal neurogenesis; two, oxidative damage; three, white matter damage, including progressive change associated with fluorouracil (FU); four, decreased hypothalamic-pituitary-adrenal axis activity; and five, reduced brain vascularization and blood flow. Also, concentrations of chemotherapy agents that are ineffective in killing tumor cells increased cell death and decreased cell division in brain regions including the hippocampus, suggesting that small amounts of chemotherapy crossing the blood-brain barrier can have toxic effects.55
Emerging evidence supports the efficacy of antioxidants in blocking behavioral and physiologic effects when coadministered with chemotherapy.54 Although this is an interesting proof of principal, antioxidants may not be a treatment option because of concerns that they may decrease the efficacy of chemotherapy. Fluoxetine has been shown to prevent deficits in behavior and hippocampal function when administered before and during administration of FU and may represent a more promising preventative approach.56,57
Data from imaging and animal studies support the hypothesis that chemotherapy affects brain structure and function and begin to provide evidence for candidate mechanisms of chemotherapy-induced cognitive change. Similar studies examining other aspects of cancer treatments such as endocrine therapy for breast cancer and hormone ablation therapy for prostate cancer are clearly needed.
CANCER, COGNITION, AND AGING
One gap in the field is the lack of a model to guide research. A potentially useful perspective is viewing cognitive change within the context of factors that influence the trajectory of normal aging. Cancer and aging are linked, although the molecular mechanisms responsible for the increasing risk of cancer with increasing age are not completely understood. Aging is associated with a variety of biologic changes, including increased cell senescence, DNA damage, oxidative stress, inflammation, and decreased telomere length (telomerase activity).58,59 Chemotherapy has been associated with increased DNA damage, oxidative stress, inflammation, and shortened telomeres.31,60 Furthermore, research has suggested that the targets for certain cancer treatments negatively affect biologic markers of aging (eg, increases in tumor suppressor mechanisms through the p53 pathway are associated with increased cell senescence systemically).61 Tamoxifen has also been shown to be genotoxic, and other endocrine therapies may be associated with increased DNA damage because of decreased antioxidant capacity.62 Finally, all of these processes have been implicated in cognitive decline and the development of neurodegenerative diseases.31,60 This research suggests that biologic processes underlying cancer, the impact of cancer treatments, aging, neurodegeneration, and cognitive decline are linked, leading to the hypothesis that cancer treatments may accelerate the aging process.60
In addition to examining specific pathways associated with aging, theoreticians have elucidated systems theories of aging, which provide interesting insights and hypotheses regarding cognition and cancer treatment. The reliability theory of aging is an example of a model of aging that is not specific to a particular biologic process but is consistent with a systems biology perspective.63 Reliability theory proposes that complex biologic systems have developed a high level of redundancy to support survival. In a highly redundant system, failure of one or more components may not be problematic if other components are available to support a specific pathway. Therefore, aging is determined by the failure rate of systems (loss of redundancy), which is influenced by the initial extent of system redundancy, the systems repair potential, and factors that increase failure rate such as poor health care, lifestyle risk factors, and/or exposure to environmental toxins. Someone with a low failure rate and/or high repair potential will show fewer signs of biologic aging as they age chronologically, whereas someone with a high failure rate and/or low repair potential will age more rapidly, as evidenced by the development of a disease associated with a specific set of system failures or frailty with a patchwork of failures across multiple systems.
One implication of reliability theory is that vulnerability to post-treatment cognitive change does not necessarily depend on a given treatment affecting a specific biologic pathway. Rather, different patterns of failure rate (redundancy loss) across various biologic systems may confer more or less vulnerability to specific treatments for each individual. Therefore, one patient may be vulnerable to the DNA damaging effects of a chemotherapy regimen, whereas another patient may be vulnerable to the impact on the hormonal milieu of endocrine treatments. This vulnerability may be strongly influenced by the pattern of systems failure before cancer diagnosis.
Furthermore, investigators have assumed that long-term cognitive problems result from the lack of recovery from the acute effects of treatment but remain stable after initial recovery.28 However, viewed within the context of models of aging, two additional hypotheses emerge: first, the initial effect of cancer treatment may produce a cascade of biologic events, which causes continued cognitive decline with aging; and second, a given treatment may not be sufficient to cause enough redundancy loss to immediately effect cognitive function but may produce a delayed effect as aging continues. Support for each of these patterns was reported by Wefel et al,24 who studied patients treated with regimens that included FU: first, stable cognitive functioning over time after an acute post-treatment decline; second, continued cognitive decline over 1 year; and third, no acute cognitive decline with new evidence of cognitive decline at 1 year post-treatment.
These considerations suggest the need for studying the short- and long-term effects of cancer treatments in older patients with cancer. Despite the fact that a majority of patients with breast cancer are diagnosed at age 65 years or older and that the number of older breast cancer survivors is growing dramatically, nearly all of the published research has focused on younger patients with breast cancer (mean age, < 60 years). Longitudinal studies11 suggest that older patients with breast cancer experience objective cognitive declines shortly after treatment; however, larger-scale prospective studies are needed. Additionally, a cross-sectional study of older (age > 65 years) long-term breast cancer survivors found lower performance on measures of executive function, working memory, and divided attention, as compared with healthy controls.64
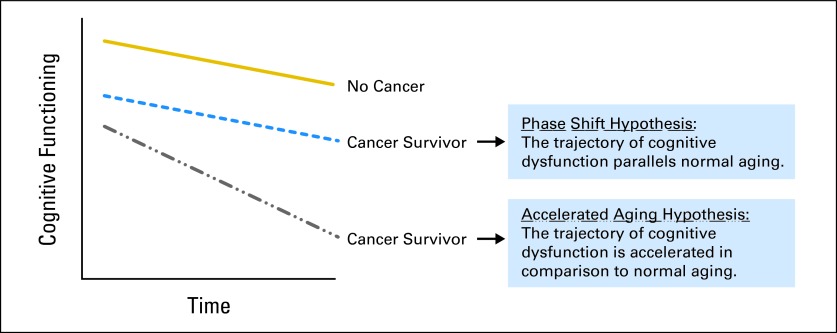
Although the recent focus of research has been on longitudinal studies with pretreatment assessments, data suggesting the possibility of continued or delayed cognitive decline demonstrate the critical need for studies examining the impact of cancer and cancer treatments on the trajectory of age-associated cognitive change, particularly in older long-term survivors. Cross-sectional studies suggest that older long-term cancer survivors will have lower performance in various areas of neurocognitive functioning, as compared with matched older adults without a cancer history.64–66 However, longitudinal assessments are important to define whether age-associated declines parallel those of older adults with no cancer history (phase shift hypothesis) or follow a steeper slope of decline (accelerated aging hypothesis; Fig 2). These are not mutually exclusive hypotheses, in that one group of survivors may demonstrate the phase shift pattern, whereas another vulnerable population may demonstrate the accelerated aging pattern. Furthermore, it is critical to define whether the impact on the trajectory of cognitive aging is the same for someone treated as a younger versus older adult.
Fig 2.
Trajectories of cognitive change.
To the extent that cancer treatments may accelerate the effects of aging, some overlap in brain structures affected by cancer treatments and aging would be expected. Imaging studies have demonstrated that total gray matter volume reliably decreases with advancing age (beginning in the mid 40s), with regional changes exhibited mainly in the frontal cortex and in regions around the central sulcus.67 Global white matter decreases with advancing age, and a trend for anterior white matter integrity decreasing earlier than posterior sites has been found.67,68 Therefore, change in brain structure and function may be an interaction between the effects of cancer treatments and changes associated with aging.
INTERVENTIONS
Few studies designed to evaluate interventions to treat cognitive changes have been reported. In terms of medication management of cognitive deficits, two studies have found support for the efficacy of modafinil, a psychostimulant, in improving memory and attention and reducing fatigue.69,70 Cognitive rehabilitation approaches are also being developed, with initial reports of positive results.71 A recent review of factors associated with prevention of cognitive decline with aging reported evidence for cognitive training, physical exercise, and possibly diet as efficacious interventions.72 These data suggest the value of testing exercise and dietary interventions to preserve cognitive function in cancer survivors.
GENERALIZABILITY OF RESULTS
A legitimate question is the extent to which the breast cancer studies are generalizable to other types of cancers and treatment regimens. Research examining treatment-related cognitive change in other cancers is difficult to evaluate, because there are generally fewer studies. However, evidence for treatment-related cognitive changes has been found for patients with various tumors, including lymphoma,65 leukemia,73 ovarian,74 and prostate (hormone ablation75) cancers, although negative studies have been reported. On the other hand, studies of patients with testicular cancer suggest that cognitive deficits can be identified on self-report measures of cognitive functioning, but not on objective neuropsychological testing.76,77 Interestingly, the chemotherapy agents included in treatment regimens for testicular cancer (cisplatin, etoposide, bleomycin) have been implicated in cognitive change in other cancers. Therefore, questions remain as to whether there are aspects of the treatment regimen (eg, dose, timing) or the biology of the disease that are responsible for the lack of results on neurocognitive testing. Alternatively, patients with testicular cancer tend to be younger than most other cohorts studied. Consistent with the discussion of models of aging, it may be that younger patients have more physical and cognitive reserve, which allows them to maintain performance on neuropsychological testing. However, children treated for non-CNS cancers and adult survivors of these childhood cancers can experience persistent cognitive changes78; therefore, there may be a curvilinear relationship with age, in that younger and older patients with cancer are more vulnerable to cognitive change, whereas younger to middle-aged adults may be more resilient. Clearly, additional research is necessary to test this hypothesis.
DISCUSSION
A convincing body of evidence from neuropsychological, imaging, and animal studies demonstrates cognitive changes associated with cancer and cancer treatments in a subgroup of individuals. Future research will require larger sample sizes to identify predictors of vulnerability to pre- and post-treatment cognitive change and define the impact of cancer and cancer treatments on the trajectory of cognitive change in long-term, particularly older, cancer survivors. Models of aging may provide a conceptual framework to guide future research. Finally, this area represents an excellent example of how translational and team science can result in significant scientific progress.
Footnotes
Supported by Grants No. R01 CA87845, R01 CA101318, R01 CA129769, and U54 CA132378 from the National Cancer Institute, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Ahles TA, Correa DD. Neuropsychological impact of cancer and cancer treatments. In: Holland JC, editor. Psycho-Oncology. ed 2. New York, NY: Oxford University Press; 2010. pp. 251–257. [Google Scholar]
- 2.Anderson FS, Kunin-Batson AS. Neurocognitive late effects of chemotherapy in children: The past 10 years of research on brain structure and function. Pediatr Blood Cancer. 2009;52:159–164. doi: 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- 3.Oxman TE, Silberfarb PM. Serial cognitive testing in cancer patients receiving chemotherapy. Am J Psychiatry. 1980;137:1263–1265. doi: 10.1176/ajp.137.10.1263. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, Ahles TA, Ganz PA, et al. Cognitive impairment associated with chemotherapy for cancer: Report of a workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 5.Vardy J, Wefel JS, Ahles TA, et al. Cancer and cancer-therapy related cognitive dysfunction: An international perspective from the Venice Cognitive Workshop. Ann Oncol. 2008;19:623–629. doi: 10.1093/annonc/mdm500. [DOI] [PubMed] [Google Scholar]
- 6.Wefel JS, Vardy J, Ahles TA, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in cancer patients. Lancet Oncol. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 7.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal study. J Clin Oncol. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 8.Mar Fan HG, Houédé-Tchen, Yi QL, et al. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2- year follow-up of a prospective study. J Clin Oncol. 2005;23:8025–8032. doi: 10.1200/JCO.2005.01.6550. [DOI] [PubMed] [Google Scholar]
- 9.Schilling V, Jenkins V, Morris R, et al. The effects of adjuvant chemotherapy on cognition in women with breast cancer-preliminary results of an observational study. Breast. 2005;14:142–150. doi: 10.1016/j.breast.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15:422–430. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 11.Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: A pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54:925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schagen SB, Muller MJ, Boogerd W, et al. Change in cognitive function after chemotherapy: A prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- 14.Hermelink K, Untch M, Lux MP, et al. Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- 15.Stewart A, Collins B, Mackenzie J, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: A prospective study. Psychooncology. 2008;17:122–130. doi: 10.1002/pon.1210. [DOI] [PubMed] [Google Scholar]
- 16.Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18:134–143. doi: 10.1002/pon.1379. [DOI] [PubMed] [Google Scholar]
- 17.Hermelink K, Henschel V, Untch M, et al. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: Results of a multicenter, prospective, longitudinal study. Cancer. 2008;113:2431–2439. doi: 10.1002/cncr.23853. [DOI] [PubMed] [Google Scholar]
- 18.Mehlsen M, Pedersen AD, Jensen AB, et al. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psychooncology. 2009;18:248–257. doi: 10.1002/pon.1398. [DOI] [PubMed] [Google Scholar]
- 19.Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res Treat. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- 20.Vearncombe KJ, Rolfe M, Wright M, et al. Predictors of cognitive decline after chemotherapy in breast cancer patients. J Int Neuropsychol Soc. 2009;15:951–962. doi: 10.1017/S1355617709990567. [DOI] [PubMed] [Google Scholar]
- 21.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: The impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debess J, Riis JØ, Engebjerg MC, et al. Cognitive function after adjuvant treatment for early breast cancer: A population-based longitudinal study. Breast Cancer Res Treat. 2010;121:91–100. doi: 10.1007/s10549-010-0756-8. [DOI] [PubMed] [Google Scholar]
- 23.Tager FA, McKinley PS, Schnabel FR, et al. The cognitive effets of chemotherapy in post-menopausal breast cancer patients: A controlled longitudinal study. Breast Cancer Res Treat. 2010;123:25–34. doi: 10.1007/s10549-009-0606-8. [DOI] [PubMed] [Google Scholar]
- 24.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 25.Hedayati E, Alinaghizadeh H, Schedin A, et al. Effects of adjuvant treatment on cognitive function in women with early breast cancer. Eur J Oncol Nurs. 2012;16:315–322. doi: 10.1016/j.ejon.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Jansen CE, Cooper BA, Dodd MJ, et al. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19:1647–1656. doi: 10.1007/s00520-010-0997-4. [DOI] [PubMed] [Google Scholar]
- 27.Biglia N, Bounous VE, Malabaila A, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: A prospective study. Eur J Cancer Care (Engl) 2012;21:485–492. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 28.Ahles TA, Schagen S, Vardy J. Neurocognitive effects of anti-cancer treatments. In: Grassi L, Riba M, editors. Clinical Psycho-Onoclogy: An International Perspective. Hoboken, NJ: Wiley-Blackwell; 2010. in press. [Google Scholar]
- 29.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110:143–152. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wefel JS, Lenzi R, Theriault R, et al. Chemobrain in breast carcinoma? A prologue. Cancer. 2004;101:466–475. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 31.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellon SA, Ganz PA, Bower JE, et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 33.Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive function of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. J Clin Oncol. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 34.Hurria A, Somlo G, Ahles T. Renaming “chemobrain.”. Cancer Invest. 2007;25:373–377. doi: 10.1080/07357900701506672. [DOI] [PubMed] [Google Scholar]
- 35.Harris SE, Deary IJ. The genetics of cognitive ability and cognitive ageing in healthy older people. Trends Cogn Sci. 2011;15:388–394. doi: 10.1016/j.tics.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12:612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 37.Small BJ, Rawson KS, Walsh E, et al. Catechol-o-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117:1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 38.Inagaki M, Yoshikawa E, Matsuoka Y, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 39.Abraham J, Haut MW, Moran MT, et al. Adjuvant chemotherapy for breast cancer: Effects on cerebral white matter seen in diffusion tensor imaging. Clin Breast Cancer. 2008;8:88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- 40.Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132:1099–1106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 41.Deprez S, Amant F, Yigit R, et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired functioning in breast cancer patients. Hum Brain Mapp. 2011;32:480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Ruiter MB, Reneman L, Boogerd W, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum Brain Mapp. doi: 10.1002/hbm.21422. epub ahead of print on September 23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshikawa E, Matsuoka Y, Inagaki M, et al. No adverse effects of adjuvant chemotherapy on hippocampal volume in Japanese breast cancer survivors. Breast Cancer Res Treat. 2005;92:81–84. doi: 10.1007/s10549-005-1412-6. [DOI] [PubMed] [Google Scholar]
- 44.McDonald BC, Conroy SK, Ahles TA, et al. Gray matter reduction associated with systemic chemotherapy for breast cancer: A prospective MRI study. Breast Cancer Res Treat. 2010;123:819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deprez S, Amant F, Smeets A, et al. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J Clin Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson RJ, McDonald BC, Saykin AJ, et al. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25:3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kesler SR, Kent JS, O'Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68:1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kesler SR, Bennett FC, Mahaffey ML, et al. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15:6665–6673. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Ruiter MD, Reneman L, Boogerd W, et al. Cerebral hyperresponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;38:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silverman DH, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–331. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 51.McDonald BC, Conroy SK, Ahles TA, et al. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30:2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cimprich B, Reuter-Lorenz P, Nelson J, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324–331. doi: 10.1080/13803390903032537. [DOI] [PubMed] [Google Scholar]
- 53.Scherling C, Collins B, MacKenzie J, et al. Pre-chemotherapy differences in visuospatial working memory in breast cancer patients compared to controls: An fMRI study. Front Hum Neurosci. 2011;5:1–21. doi: 10.3389/fnhum.2011.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seigers R, Fardell JE. Neurobiological basis of chemotherapy-induced cognitive impairment: A review of rodent research. Neurosci Biobehav Rev. 2011;35:729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Dietrich J, Han R, Yang Y, et al. CNS progenitor cells and oligodendrocytes ae targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyons L, ElBeltagy M, Bennett G, et al. Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS One. 2012;1:e30010. doi: 10.1371/journal.pone.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ElBeltagy M, Mustafa S, Umka J, et al. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav Brain Res. 2010;208:112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Irminger-Finger I. Science of cancer and aging. J Clin Oncol. 2007;25:1844–1851. doi: 10.1200/JCO.2007.10.8928. [DOI] [PubMed] [Google Scholar]
- 59.Campisi J, Yaswen P. Aging and cancer cell biology, 2009. Aging Cell. 2009;8:221–225. doi: 10.1111/j.1474-9726.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 60.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: A cause of early onset frailty? Med Hypotheses. 2006;67:212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 61.Campisi J, d'Adda di Fagagna F. Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 62.Brown K. Is tamoxifen a genotoxic caricinogen in women? Mutagenesis. 2009;24:391–404. doi: 10.1093/mutage/gep022. [DOI] [PubMed] [Google Scholar]
- 63.Gavrilov LA, Gavrilova NS. Reliability theory of aging and longevity. In: Masoro EJ, Sustad ST, editors. Handbook of the Biology of Aging. ed 6. Burlington, MA: Academic Press; 2006. pp. 3–42. [Google Scholar]
- 64.Yamada TH, Denburg NL, Beglinger LJ, et al. Neuropsychological outcomes of older breast cancer survivors: Cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychological impact of standard-dose chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 66.Koppelmans V, Breteler MM, Boogerd W, et al. Neuropsychological performance in breast cancer survivors more than 20 years after adjuvant chemotherapy. J Clin Oncol. 2012;30:1080–1086. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- 67.Peelle JE, Cusack R, Henson RN. Adjusting for global effects in voxel-based morphometry: Gray matter decline in normal aging. Neuroimage. 2012;60:1503–1516. doi: 10.1016/j.neuroimage.2011.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunning-Dixon FM, Brickman AM, Cheng JC, et al. Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kohli SS, Fisher SG, Tra Y, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–2616. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lundorff LE, Jønsson BH, Sjøgren P. Modafinil for attentional and psychomotor dysfunction in advanced cancer: A double-blind, randomised, cross-over trial. Palliat Med. 2009;23:731–738. doi: 10.1177/0269216309106872. [DOI] [PubMed] [Google Scholar]
- 71.Ferguson JR, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: Results of a waitlist control trial. Psychooncology. 2012;21:176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Plassman BL, Williams JW, Burke JR, et al. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 73.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 74.Correa DD, Hess LM. Cognitive function and quality of life in ovarian cancer. Gynecol Oncol. 2012;124:404–409. doi: 10.1016/j.ygyno.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Nelson CJ, Lee JS, Gamboa MC, et al. Cognitive effects of hormone therapy in men with prostate cancer: A review. Cancer. 2008;113:1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skaali T, Fosså SD, Andersson S, et al. Self-reported problems in testicular cancer patients: Relation to neuropsychological performance, fatigue, and psychological distress. J Psychosom Res. 2011;70:403–410. doi: 10.1016/j.jpsychores.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Schagen SB, Boogerd W, Muller MJ, et al. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol. 2008;47:63–70. doi: 10.1080/02841860701518058. [DOI] [PubMed] [Google Scholar]
- 78.Edelstein K, D'Agostino N, Berstein LJ, et al. Long-term neurocognitive outcomes in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2011;33:450–458. doi: 10.1097/MPH.0b013e31820d86f2. [DOI] [PubMed] [Google Scholar]