Abstract
Background and purpose
To compare the dosimetric impact of organ and target variations relative to the applicator for intracavitary brachytherapy by a multicentre analysis with different application techniques and fractionation schemes.
Material and methods
DVH data from 363 image/contour sets (120 patients, 6 institutions) were included for 1–6 fractions per patient, with imaging intervals ranging from several hours to ∼20 days. Variations between images acquired within one (intra-application) or between consecutive applicator insertions (inter-application) were evaluated. Dose plans based on a reference MR or CT image series were superimposed onto subsequent image sets and for the bladder, rectum and sigmoid and D90 for HR CTV were recorded.
Results
For the whole sample, the systematic dosimetric variations for all organs at risk, i.e. mean variations of , were found to be minor (<5%), while random variations, i.e. standard deviations were found to be high due to large variations in individual cases. The variations (mean ± 1SD) were 0.6 ± 19.5%, 4.1 ± 21.7% and 1.6 ± 26.8%, for the bladder, rectum and sigmoid. For HR CTV, the variations of D90 were found to be −1.1 ± 13.1% for the whole sample.
Grouping of the results by intra- and inter-application variations showed that random uncertainties for bladder and sigmoid were 3–7% larger when re-implanting the applicator for individual fractions. No statistically significant differences between the two groups were detected in dosimetric variations for the HR CTV.
Using 20% uncertainty of physical dose for OAR and 10% for HR CTV, the effects on total treatment dose for a 4 fraction HDR schedule at clinically relevant dose levels were found to be 4–8 Gy EQD2 for OAR and 3 Gy EQD2 for HR CTV.
Conclusions
Substantial variations occur in fractionated cervix cancer BT with higher impact close to clinical threshold levels. The treatment approach has to balance uncertainties for individual cases against the use of repetitive imaging, adaptive planning and dose delivery.
Keywords: Image guided brachytherapy, Cervix cancer brachytherapy, Interfraction variations, Adaptive brachytherapy
3D image guided brachytherapy (BT) of cervical cancer is becoming more and more used by centres with access to MRI or CT imaging facilities for BT planning. This technique allows delivery of high doses to target structures while doses to organs at risk can be reduced with the help of 3D images, which provide detailed information of the anatomical situation and applicator position at the time of BT planning. (e.g. [1]). Dose-effect relationships for cervix cancer BT have been previously reported for the bladder, rectum and sigmoid [2,3–5] and target [6]. When balancing target versus OAR dose, the dose levels are usually in the region of a steep dose-effect curve gradient. Therefore it seems necessary to report the applied dose with high precision, to identify systematic and random uncertainties and design treatment schedules and application techniques to reduce the uncertainties accordingly.
The present study focuses on the dosimetric impact of anatomical variations of target and organ structures in relation to the brachytherapy applicator as a fixed reference coordinate system.
The possibility of target and organ motion, i.e. changes in location relative to the applicator, variations of shape and/or filling status of organs at risk (OAR), occurring between two individual BT fractions or within the time of delivery of one fraction, plays an important role in the assessment of total treatment doses for multi-fractional brachytherapy treatment and correlations with clinical outcome. This is especially the case when one treatment plan is used for multiple fractions, or when organ movement occurs in between imaging and dose delivery.
Dosimetric variations caused by such movement have been reported previously in various treatment planning studies. [7–16]. The majority of these studies focussed on the question whether or not a single treatment plan may be applied for a multi-fractionated BT treatment or whether repetitive imaging and, consequently, dose plan adaptation to modified OAR anatomy is generally required. Although it is commonly assumed that the relative position between the applicator and the target structure remains constant throughout the whole treatment, and little shrinkage of the target (HR CTV) will occur during BT, some studies have also analysed dosimetric changes for HR CTV. For all these studies repetitive 3D image series were obtained for a number of patients.
The aim of the current study is to compare the dosimetric impact of target and OAR variations by a multicentre analysis based on pooled data from different institutions with different application techniques and fractionation schemes, introducing a common method for reporting such variations.
As no general recommendations for the reporting of dosimetric variations caused by relative motions between structures and applicators during cervix cancer BT exist up to this date, we propose a general method to report dosimetric uncertainties in percentage of planned dose of a reference image set. By doing so, it becomes possible to compare variations observed at different centres with different treatment strategies, independent of the absolute dose values obtained. Derived uncertainties could thus be converted to different treatment schedules and expected ranges of true delivered doses could be calculated.
The results of our study could help to uncover systematic correlations of time between image acquisitions and dosimetric variations, and to identify which of the critical organs is affected most by motions occurring during BT treatment.
Materials and methods
Six participants in the GEC ESTRO GYN network performed repeated imaging for the analysis of dosimetric changes caused by anatomical variations during cervix cancer BT. (Medical University of Vienna (MUV), Mount Vernon Cancer Center (MVCC), University Medical Center Utrecht (UMCU), Oslo University Hospital (OUH), Tata Memorial Hospital (TMH), Aarhus University Hospital (AUH)). These centres were invited to submit their raw data and to participate in a direct comparison of their individually reported observations with other institutions.
Table 1 gives an overview of centre-specific treatment details and available image data. Details about the imaging protocols, application techniques, patient selection, contouring and treatment planning were reported in the individual publications listed in Table 1. Four of the participants in the current study (MUV, UMCU, OUH, AUH) have previously published mono-institutional data for variations due to anatomical changes during BT treatment. For centres MVCC and TMH, additional unpublished data were included in this study. Detailed descriptions of their clinical imaging and treatment protocols have been reported by Wills et al. and Mahantshetty et al. [17,18].
Table 1.
Overview of participating centres and the data submitted to this study. Intra-application means that the applicators stayed in place between two image acquisitions, while inter-application means that applicators were removed and reinserted between image acquisitions. References for detailed descriptions of the individual study setups and/or individual centre’s standard practice are given in the last column.
| Centre | No. of patients | Treatment type | Applicator type | Time between image acquisitions | Image type | No. of image sets | Variation type analysed | References |
|---|---|---|---|---|---|---|---|---|
| MUV | 21 | HDR | T/R (ic ± is) | 12–16 h | MRI | 84 | Intra-application | [9,10] |
| MVCC | 21 | HDR | T/R (ic ± is) | 5 h (average) | MRI | 72 | Intra-application | [17] |
| UMCU | 9 | PDR | O (ic + is) | 22 h (average) | MRI | 36 | Intra-aplication | [11] |
| OUH | 11 | HDR | T/R (ic) | 1–20 days | CT | 55 | Inter-application | [12] |
| TMH | 27 | HDR | T/R (ic ± is) | 7–10 days | MRI | 54 | Inter-application | [18] |
| AUH | 31 | PDR | T/R (ic) | 7 days | MRI | 62 | Inter-application | [7,8] |
For the present study each centre contributed a set of DVH data generated with similar workflows as described hereafter. An overview of the data included in this study is given in Table 1. At every institution cervix cancer patients were treated with intracavitary (ic) applicators (tandem/ring (5) or tandem/ovoid (1)) with or without interstitial (is) needles. Two of the centres treated patients with PDR BT, four centres used HDR treatment schedules in addition to EBRT treatment of 45–50 Gy. Data for 120 patients (363 image/contour sets, 308 MRI, 55 CT) were included in this study. For each patient at least two 3D image sets (MRI (5) or CT (1)) were acquired over the course of BT [19]. In four centres 2–6 image series were analysed for each patient. Three centres analysed images obtained during the same applicator insertion (intra-application variations) while the other three analysed DVH parameters based on images acquired for subsequent applicator insertions (inter-application variations). Time intervals between two images for one patient spanned a large range between 3 and 5 h within one applicator insertion and up to three weeks between different insertions. For bladder filling protocols the centres used: constant bladder filling procedures before imaging and before dose delivery, empty bladders, or open catheters.
HR CTV and OAR were contoured for each image series according to GEC ESTRO recommendations [20,21] in five centres. For centre OUH, CT based contours of bladder wall and rectal wall had been included in their original analysis and DVH data included in the present study are based on these contours. For all centres a treatment plan generated on the basis of images taken at the beginning of BT with applicator in place was transferred to consecutive images of the same patient and DVH parameters (D90 for target and for OAR) were reported for HR CTV, bladder, rectum and sigmoid for all image sets available. The original CT based data from centre OUH allowed only to investigate minimum doses to 5% of the bladder and rectum walls, instead of the nowadays used for OAR dose reporting. Given the volumes of bladder wall (61.5 ± 21.0 cm3, mean ± 1SD) and rectum wall 51.4 ± 18.8 cm3) contours these parameters translate to ∼. It was therefore considered that these parameters were comparable to the used for analysis by other centres, especially since the current study is focussed on investigating relative changes between DVH parameters at different time points during BT, rather than comparing absolute DVH values between centres.
For assessment of dosimetric changes due to variations of shape, position or volume of the delineated structures, the relative difference between the doses calculated for the reference image and a subsequent image was calculated as ΔD = (Di−Dref)/Dref [%].
For each individual centre mean, median and standard deviations of the relative differences were calculated in order to distinguish between systematic and random variations. In order to test whether a systematic difference in DVH variations can be observed for intra-application and inter-application scenarios, the mean, median and standard deviation of the relative differences were calculated for the two groups. Finally the mean, median and standard deviations (SD) of the relative differences were calculated for the whole data sample.
The dosimetric variations for delineated structures in this study are related to relative changes between target or OAR anatomy and the BT applicators. In the case of intra-application measurements, the observed variations are related to applicator reconstruction accuracy and change of location or shape of OAR and targets. For inter-application measurements, additional differences in applicator positioning relative to target and OAR influence the dosimetric variations between consecutive implants.
For testing statistical significance of the differences between uncertainties derived for different groups, a two-tailed t-test (for systematic variations – mean values) or two-tailed f-test (for random variations – SD) was performed. Results were considered to be statistically significant if p < 0.05.
In order to evaluate the clinical importance of the observed dosimetric uncertainties, typical clinical scenarios for an HDR BT treatment were simulated to assess the effect of uncertainties on total accumulated dose. Relevant dose levels (EBRT + BT) were analysed for OARs and HR CTV, by calculating biologically equivalent doses in 2 Gy fractions (EQD2) according to the linear quadratic model, using α/β = 10 Gy for target and α/β = 3 Gy for OAR [21]. The dose ranges 60–95 Gy EQD2 and 70−95 Gy EQD2 were simulated in steps of 5 Gy for OARs and targets, respectively, by varying the physical BT dose and keeping the EBRT dose constant (45 Gy in 25 fractions). A BT schedule with four HDR fractions was used.
For each dose level, 40,000 simulations were run. In each simulation, uncertainties were picked randomly for each brachytherapy fraction according to a N(di,σ2) distribution where di was the physical fractional dose and σ the standard deviation typical for intra-application and inter-application variations in target or OAR. Relevant σ levels were chosen according to the results below: 10% and 15% for target, and 20% and 30% for OARs. Each simulated fractional dose was transformed into EQD2 dose, and thereafter all fractions were added and summed up with the EBRT EQD2 dose in order to obtain total EBRT + BT EQD2 dose. Mean and SD of the 40,000 simulations were evaluated and compared with the expected dose.
Results
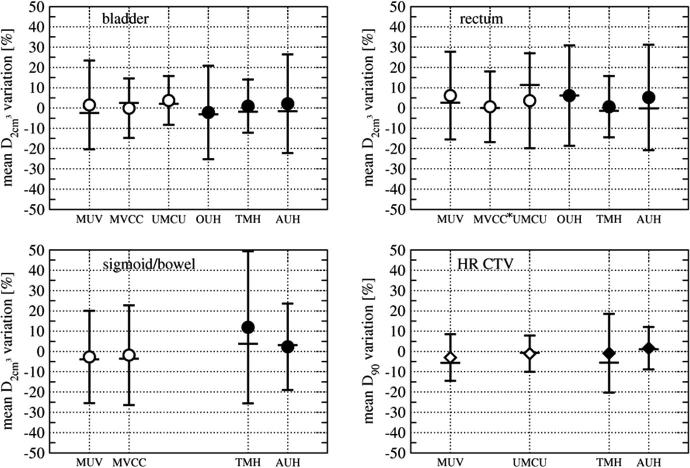
The relative systematic and random changes of D90 HR CTV and for OAR between consecutive image acquisitions are reported as mean and SD uncertainties in percentage of physical dose obtained from the reference images in Table 2. Positive values mean that the dose obtained from consecutive images was higher than in the reference image. Results are shown for individual centres (Fig. 1), as well as for the whole sample and grouped by intra- and inter-application data (Fig. 1, Table 2).
Table 2.
Observed dosimetric variations for the bladder, rectum, sigmoid/bowel and HR CTV. Relative differences between dose parameters ( or D90) from two images/structure sets were calculated as ΔD = (Di−Dref)/Dref (on reference image and subsequent image number i = 1–4). Mean and SD of the variations are given in % of physical dose. A positive value means that the dose obtained for image set i was higher than on the reference image.
| Structure |
between 2 acquisitions [%] (fixed plan, variable anatomy) |
|||||
|---|---|---|---|---|---|---|
| Intra-application only |
Inter-application only |
Total |
||||
| Mean | SD | Mean | SD | Mean | SD | |
| Bladder | 1.3 | ±17.7 | −0.1 | ±21.2 | 0.6 | ±19.5 |
| Rectum⁎ | 3.8 | ±20.5 | 4.3 | ±22.8 | 4.1 | ±21.7 |
| Sigmoid | −2.3 | ±23.5 | 6.8 | ±30.2 | 1.6 | ±26.8 |
| ΔD90 between 2 acquisitions [%] (fixed plan, variable anatomy) |
||||||
| Intra-application only |
Inter-application only |
Total |
||||
| Mean |
SD |
Mean |
SD |
Mean |
SD |
|
| HR CTV | −2.5 | ±10.8 | 0.4 | ±15.1 | −1.1 | ±13.1 |
3 Extreme outliers (Δ = 119–240%) were excluded from the overall analysis. Inclusion would result in 11.2 ± 38.3% (mean ± SD) for rectum for intra-application group and 7.7 ± 31.4% for total sample.
Fig. 1.
Results for 6 individual centres. Mean dosimetric variations between consecutive image sets are shown. Error bars indicate SD, median values are shown as horizontal bars. Open symbols correspond to the intra-application group, filled symbols to the inter-application group. ∗3 extreme outliers were excluded from the analysis shown in this graph. Inclusion would result in 20.5 ± 54.5% (mean ± SD) for rectum for MVCC, as compared to 0.6 ± 17.4% without outliers.
In one centre (MVCC) three extreme outliers were detected in the analysis of the data for the rectum. In patients with very low values of for the first MRI, i.e. in cases of small rectum volumes and large distances between organ and applicator at the time of planning, relative changes between first and second MRI of 119–240% were observed. Comparable changes were not detectable in any other dataset of the remaining 5 institutions. They are likely due to a different bowel preparation in these patients. For this reason, the outliers were excluded from the analysis. Results for the whole sample, including these data are stated in the captions of Fig. 1 and Table 2.
The mean ± SD of the relative variations for the whole sample were 0.6+/−19.5%, 4.1 ± 21.7% and 1.6 ± 26.8 for the bladder, rectum and sigmoid, respectively. The random uncertainties (SD) for the inter-application data were found to be significantly larger than the random uncertainties for the intra-application group for bladder (p = 0.04), sigmoid (p = 0.02) and HR CTV (p < 0.01). Variances of inter- and intra-application data for the rectum were not significantly different from each other.
The differences between random uncertainties (SD) in the inter-application group were 3–7 percentage points higher than in the intra-application data, for OARs and target. Observed SD ranges for random uncertainties of all OARs were 17.7–23.5% and 21.2–30.2% for intra- and inter-application, respectively. Analysing the whole sample the observed SD range was 19.5–26.8%. Overall, the random uncertainties for sigmoid were significantly larger than those for the rectum and bladder (p < 0.01), while variances of the rectum and bladder did not differ from each other with statistical significance. Mean values, i.e. systematic uncertainties, did not differ significantly between the analysed groups.
The mean ± SD of the relative HR CTV D90 variations for the whole sample were −1.1 ± 13.1%. Intra-application data showed lower random (SD) uncertainties (10.8%) than the inter-application data (15.1%). The statistically significant difference (p < 0.01) between random uncertainties in inter- and intra-applications was therefore four percentage points. Systematic uncertainties for HR CTV were smaller than for OAR with the largest value of −3.1% found in an intra-fraction data set (MUV).
The largest random uncertainties for D90 HR CTV were found in a centre with inter-application data acquired one week apart (SD 19.4% (TMH)).
For all investigated DVH parameters no systematic increase or decrease of the random variation with increasing time between two image acquisitions was observed.
Based on information of the implant type for each patient available for two centres in the intra-application group in our analysis, no significant differences between cases with and without interstitial needles were detected.
No systematic differences related to different applicator types or fractionation schedules (HDR or PDR centres) were detected.
Results of the simulation of total treatment dose in EQD2, including EBRT and four fractions of HDR BT, and taking into account dosimetric uncertainties of individual BT fractions are shown in Fig. 2. Comparison of the expected total doses (EQD2) and the corresponding means of the simulated total doses, indicates a small systematic underestimation of total EQD2 if uncertainties are not taken into account, e.g., for a random uncertainty of 30% in OAR dose during BT, the mean simulated dose is 3.1 Gy higher for an expected dose of 95 Gy. For clinically relevant expected total dose levels for OAR, e.g. 70, 75 and 90 Gy EQD2, observed random dosimetric uncertainties of 20% and 30% of physical dose between consecutive image acquisitions lead to random uncertainties (SD) of the total simulated dose of ±4.3, ±5.2, and ±7.9 Gy EQD2 (for physical dose SD 20%) and ±6.5, ±7.9 and ±12.0 Gy EQD2 (for physical dose SD 30%). For HR CTV, at clinically relevant dose values of, e.g., 85 and 90 Gy EQD2, an observed interfractional dose variation of 10% leads to simulated random uncertainties of ±2.9 and ±3.3 Gy while 15% resulted in ±4.4 and ±4.9 Gy, in EQD2.
Fig. 2.
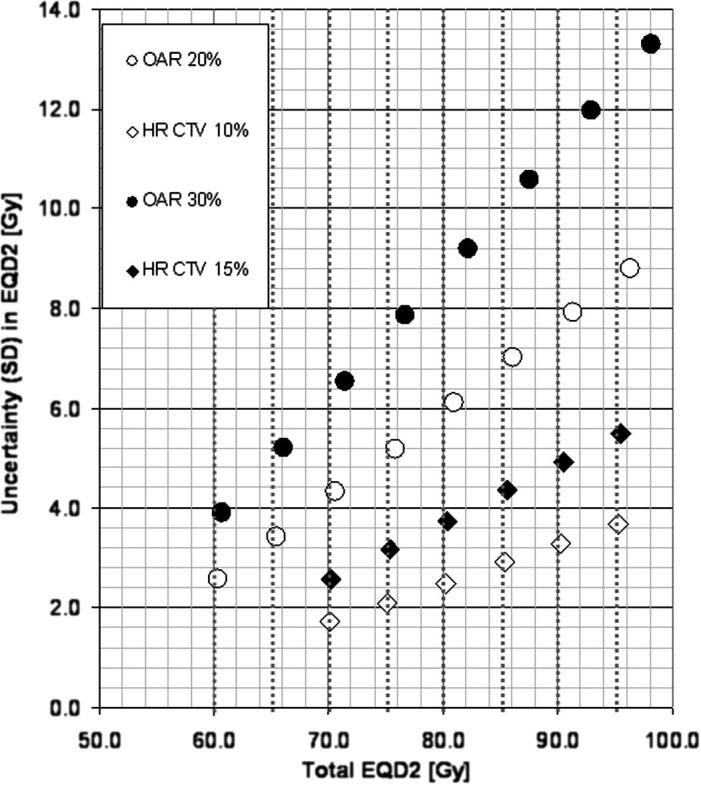
Results of the simulation of total treatment dose for EBRT + 4 fractions of HDR BT. The mean of the total simulated dose in EQD2 is shown for typical expected dose levels for OAR and HR CTV, assuming typical values for random uncertainties as observed in our analysis. Expected total doses (without taking into account dosimetric uncertainties) are indicated by vertical dotted lines. Total EQD2 (with α/β = 3 Gy for OAR and α/β = 10 Gy for HR CTV) are plotted against the simulated random uncertainty (SD) of the whole treatment dose, in Gy EQD.
Discussion
We have presented here the first comprehensive overview of the impact of anatomical variations on dosimetric planning parameters in cervix cancer BT in a multicentre setting based on pooled data. In contrast to BT these effects have been studied extensively in the field of EBRT, for more than 20 years. For 3D BT only limited information on this topic was reported until this day [11,12,14]. Compared to dosimetric uncertainties caused by interfractional variation in EBRT [22], the uncertainties reported in the BT context are different, due to non-uniform dose distributions in the target area [23]. Therefore the present detailed analysis of uncertainties based on a sample of 123 patients has the potential to provide important information for future optimization of adaptive planning strategies.
The dosimetric uncertainties for HR CTV presented in the current study involve re-imaging, re-reconstruction of applicators and re-contouring. Therefore, the uncertainties presented in this paper include inevitably intra- and/or inter-observer uncertainties of reconstruction and contouring. Dosimetric effects of inter-observer reconstruction uncertainties have been found to be of the order <4–5% (SD) [12,23,24] for OARs and target. Inter-observer contouring uncertainties [25] can lead to dosimetric uncertainties of the order 10% (SD) for HR CTV D90 and 5–10% for OARs [26]. Although intra-observer variations may be smaller than inter-observer variations, these data indicate that the intra-application target variations (SD 11%) found in this study are likely to be dominated by contouring uncertainties whereas the inter-application variation (SD 15%) also involves uncertainties due to relation between target and applicator. However, the intra- and inter-application variations evaluated for OARs in this paper (SD 20–27%) are clearly not dominated by reconstruction or contouring uncertainties, and they originate as such from intra- and inter-application movement of organs relative to the applicator.
No correlation of observed uncertainties and time between image acquisitions was detected with the method applied in this study. Therefore the data do not allow drawing any conclusions about how frequently re-imaging and, consequently, plan adaptation is necessary in general. In order to trace temporal evolution of anatomical changes during one course of BT it would be necessary to monitor individual patients using a larger number of images spanning the treatment period, which would be most profitable for assessing total uncertainties of PDR BT.
Kirisits et al. [14] and Mohamed et al. [7] pointed out that re-planning of individual fractions is advisable for consecutive applicator insertions when interstitial needles are used. Based the limited data available in our sample, no significant differences of dosimetric variations for different implant types were detected for the intra-application setting.
The mean values of observed dosimetric differences indicate systematic variations while the standard deviation is a measure for the random uncertainties caused by anatomical variations relative to the applicator during BT treatment. In cases where the BT is started early and target shrinkage is still pronounced systematic increase in OAR dose may appear because the organs may move close to the applicator while the target regresses. Kirisits et al. [14] evaluated inter-application uncertainties by investigating the impact of using one treatment plan for multiple BT fractions based on structures delineated on MRI taken prior to each fraction. They reported a mean increase of D90 HR CTV by 6 Gy EQD2. At the time of that study, BT was delivered during the course of EBRT, when substantial tumour shrinkage was still in progress. Mohamed et al. [7] also reported a correlation of observed dosimetric variations with reduction of contoured HR CTV volumes between consecutive PDR applications. Due to the limited target volume data available for the patients in our present multicentre study, a detailed analysis of target shrinkage was not feasible.
In case there is no target shrinkage, systematic variations are expected to be small and not significant as no preferred direction of the shift between structures and applicators are known. This is true except for changes in bladder shape and volume if no bladder filling protocol is applied and bladder volume systematically increases, (as well as for possible systematic increase of rectal volume due to filling with air, between image acquisitions within one applicator insertion). While systematic dosimetric interfraction uncertainties are therefore expected to be low, large standard deviations of the observed dosimetric differences indicate that for individual patients variations might be large and even become clinically significant if planning DVH parameters are close to the tolerable dose limits.
For the whole data sample from six centres, the derived random uncertainties were lowest for the bladder (20%) and the rectum (22%) and highest for sigmoid (27%). This observation fits well with basic anatomical knowledge of the mobility of the pelvic organs. The posterior inferior bladder wall and the anterior rectal wall are relatively fixed in relation to the applicator, whereas sigmoid loops have more freedom to move independently from the cervix/uterus and the applicator.
Volumetric bladder data were available for three centres. No significant correlation of the change of overall bladder volume with the change of could be detected.
The largest random dosimetric variations were observed for the rectum (26%, AUH) and sigmoid (37%, TMH) in centres who analysed inter-application variations between images acquired at least 1 week apart. Overall, observed random uncertainties (SD) for sigmoid were found to be significantly larger than for the rectum or bladder. The systematic uncertainties investigated, i. e. mean values of dosimetric variations, did not differ significantly between inter- and intra- application groups, while random uncertainties were found to be significantly larger for inter-application variations compared to variations occurring within one applicator insertion.
In one centre (MVCC), extreme outliers were detected in the analysis of dosimetric variations for the rectum (119–240% variation between two MRI acquisitions). These underlying large changes in rectal volume and relative position to the applicator are suspected to be due to a different bowel preparation procedure used in these patients, as compared to the practice of other centres participating in this study. The use of a common prospective bowel preparation protocol might be necessary for a correct interpretation of such observations in future studies.
Plan adaptation by repetitive CT imaging might be an advisable strategy, to take into account movement of OAR relative to the applicator over the course of HDR BT, in centres with limited access to MRI for planning of all BT fractions. A method for combined MRI/CT-based adaptive planning was recently presented by [27].
Our simulations of delivered dose ranges for an example of a treatment with four fractions of BT support the necessity to try to minimize delivered doses to critical structures by repetitive imaging and plan adaptation, especially in patients where planning DVH parameters were close to the limits. The simulated example indicated variations of up to ±8 Gy at e.g. bladder doses less than 90 Gy EQD2(α/β = 3) and a typical 20% uncertainty level. Due to lower total dose, rectum variations are typically lower than bladder variations: up to ±5 Gy at doses lower than 75 Gy EQD2(α/β = 3) and 20% uncertainty level. Sigmoid has higher uncertainty level (e.g. 30%) and therefore variation up to ±8 Gy at total dose lower than 75 Gy EQD2(α/β = 3). Such OAR uncertainties of up to ±5–8 Gy could lead to otherwise unexpected side effects. At clinically relevant dose levels for HR CTV, e.g. 90 Gy EQD2(α/β = 10), random uncertainties were found to be of the order of 3–5 Gy for the total treatment dose including EBRT and HDR BT. These deviations have a direct impact on outcome for target and OAR effects depending on the non-linear dose-effect curves. The combination of dosimetric uncertainty studies with accurate dose-effect relationships has to be studied in further detail.
The total effect of interfractional organ and target changes on the total delivered dose depends on the fractionation schedule of the BT treatment. The observational material available for this study did not allow analysing intrafractional variations with high time resolution. Therefore simulation of the total treatment dose, taking into account the dosimetric effect of anatomical variations occurring during different PDR pulse schemes was not possible based on this material.
One current limitation of our study is the use of only one DVH parameter as a representative parameter for the anatomical variations of OARs and, consequently, their dosimetric impact. Current clinical evidence points to the being the most important parameter for OAR morbidity. Future research may show that intermediate dose levels influenced by EBRT may be relevant too. This should be taken into account for the development of a common protocol for reporting interfraction variations.
By comparison of the relative change of alone, we cannot take into account that the subvolume of the organ wall receiving the highest dose by each treated BT fraction might not always be the same. The method applied to calculate accumulated dose is therefore based on the assumed “worst case assumption”. In order to assess the cumulative dose in volumes receiving these high doses, non-rigid registration of OAR structures of different fractions would allow to sum up the doses received by each individual voxel. Currently there is no solution available for this highly complex problem of non-rigid MRI fusion for cervix cancer BT.
The findings of this study show substantial variations in fractionated cervix cancer brachytherapy, which have to be taken into account. The impact of the dosimetric variations on dose-effect depends on the prescribed dose level for a specific target or OAR. Variations close to clinical threshold level or in the steep part of the dose effect-curve have a higher impact on dose effects. Each centre performing this technique should be aware of the variations of their individual imaging, planning and dose delivery protocol and balance uncertainties for individual cases against the use of repetitive imaging, adaptive treatment planning and dose delivery.
Conflict of interest
The Department of Radiotherapy at the Medical University of Vienna receives financial and/or equipment support for research and educational purposes from Nucletron B.V. and Varian Medical Systems, Inc. CK is a consultant to Nucletron, an Elekta company.
Acknowledgements
This work was supported by the Austrian Science Fund (FWF), project L562-B19.
The GEC ESTRO network was supported by Varian Corporate Systems and Nucletron.
Aarhus University Hospital was supported by research Grants from the European School of Oncology (ESO), Danish Cancer Society, Danish Council for Strategic Research, and CIRRO – the Lundbeck Foundation Centre for Interventional Research in Radiation Oncology.
References
- 1.Pötter R., Georg P., Dimopoulos J.C. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100:116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koom W.S., Sohn D.K., Kim J.Y. Computed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: preliminary demonstration of correlation between dose-volume parameters and rectal mucosal changes observed by flexible sigmoidoscopy. Int J Radiat Oncol Biol Phys. 2007;68:1446–1454. doi: 10.1016/j.ijrobp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Georg P., Kirisits C., Goldner G. Correlation of dose-volume parameters, endoscopic and clinical rectal side effects in cervix cancer patients treated with definitive radiotherapy including MRI-based brachytherapy. Radiother Oncol. 2009;91:173–180. doi: 10.1016/j.radonc.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Georg P., Lang S., Dimopoulos J. Dose-volume histogram parameters and late side effects in magnetic resonance image-guided adaptive cervical cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;79:356–362. doi: 10.1016/j.ijrobp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Georg P., Pötter R., Georg D. Dose effect relationship for late side effects of the rectum and urinary bladder in magnetic resonance image-guided adaptive cervix cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2011;22:22. doi: 10.1016/j.ijrobp.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos J., Lang S., Kirisits C. Dose-effect relationship for local control of cervical cancer by magnetic resonance image guided brachytherapy. Radiother Oncol. 2009;93:311–315. doi: 10.1016/j.radonc.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed S.M.I., Nielsen S.K., Fokdal L.U., Lindegaard J.C., Tanderup K. Feasibility of applying one treatment plan for succeeding fractions in image guided brachytherapy in cervix cancer. Radiother Oncol. 2011;99:S56. doi: 10.1016/j.radonc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed S. Nielsen S K, Fokdal L U et al, Feasibility of applying a single treatment plan for both fractions in PDR image guided brachytherapy in cervix cancer. Radiother Oncol. 2013 doi: 10.1016/j.radonc.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Lang S., Georg P., Kirisits C., Dimopoulos J., Kuzucan A., Georg D. Uncertainty analysis for 3D image based cervix cancer brachytherapy by repeated MRI examinations: DVH-variations between two HDR fractions within one applicator insertion. Radiother Oncol. 2006;81:S79. doi: 10.1016/j.radonc.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Lang S., Nesvacil N., Kirisits C. Uncertainty analysis for 3D image-based cervix cancer brachytherapy by repetitive MR imaging: Assessment of DVH-variations between two HDR fractions within one applicator insertion and their clinical relevance. Radiother Oncol. 2013;107:26–31. doi: 10.1016/j.radonc.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 11.De Leeuw A.C., Moerland M.A., Nomden C. Applicator reconstruction and applicator shifts in 3D MR-based PDR brachytherapy of cervical cancer. Radiother Oncol. 2009;93:341–346. doi: 10.1016/j.radonc.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Hellebust T., Dale E., Skjonsberg A., Olsen D.R. Inter fraction variations in rectum and bladder volumes and dose distributions during high dose rate brachytherapy treatment of the uterine cervix investigated by repetitive CT-examinations. Radiother Oncol. 2001;60:273–280. doi: 10.1016/s0167-8140(01)00386-3. [DOI] [PubMed] [Google Scholar]
- 13.Jamema S.V., Mahantshetty U., Tanderup K. Inter-application variation of dose and spatial location of volumes of OARs during MR image based cervix brachytherapy. Radiother Oncol. 2013;107:58–62. doi: 10.1016/j.radonc.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Kirisits C., Lang S., Dimopoulos J. Uncertainties when using only one MRI-based treatment plan for subsequent high-dose-rate tandem and ring applications in brachytherapy of cervix cancer. Radiother Oncol. 2006;81:269–275. doi: 10.1016/j.radonc.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Beriwal S., Kim H., Coon D. Single magnetic resonance imaging vs magnetic resonance imaging/computed tomography planning in cervical cancer brachytherapy. Clin Oncol (R Coll Radiol) 2009;21:483–487. doi: 10.1016/j.clon.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Anderson C., Lowe G., Wills R. Critical structure movement in cervix brachytherapy. Radiother Oncol. 2013;107:39–45. doi: 10.1016/j.radonc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Wills R., Lowe G., Inchley D. Applicator reconstruction for HDR cervix treatment planning using images from 0 35 T open MR scanner. Radiother Oncol. 2010;94:346–352. doi: 10.1016/j.radonc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Mahantshetty U., Swamidas J., Khanna N. Magnetic resonance image-based dose volume parameters and clinical outcome with high dose rate brachytherapy in cervical cancers–a validation of GYN GEC-ESTRO brachytherapy recommendations. Clin Oncol (R Coll Radiol) 2011;23:376–377. doi: 10.1016/j.clon.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos J.A., Petrow P., Tanderup K., Petric P., Berger D., Kirisits C. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103:113–122. doi: 10.1016/j.radonc.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haie-Meder C., Pötter R., Van Limbergen E. Recommendations from Gynaecological (GYN) GEC ESTRO Working Group (I): concepts and terms in 3D image-based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Pötter R., Haie-Meder C., Van Limbergen E. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Andersen E.S., Muren L.P., Sørensen T.S. Bladder dose accumulation based on a biomechanical deformable image registration algorithm in volumetric modulated arc therapy for prostate cancer. Phys Med Biol. 2012;57:7089–7100. doi: 10.1088/0031-9155/57/21/7089. [DOI] [PubMed] [Google Scholar]
- 23.Tanderup K., Pötter R., Lindegaard J.C., Berger D., Wambersie A., Kirisits C. PTV margins should not be used to compensate for uncertainties in 3D image guided intracavitary brachytherapy. Radiother Oncol. 2010;97:495–500. doi: 10.1016/j.radonc.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Hellebust T.P., Kirisits C., Berger D., Pérez Calatayud J., De Brabandere M., De Leeuw A. Recommendations from gynaecological (GYN) GEC-ESTRO working group: considerations and pitfalls in commissioning and applicator reconstruction in 3D image-based treatment planning of cervix cancer brachytherapy. Radiother Oncol. 2010;96:153–160. doi: 10.1016/j.radonc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Petric P., Dimopoulos J., Kirisits C. Inter- and intraobserver variation in HR-CTV contouring: intercomparison of transverse and paratransverse image orientation in 3D-MRI assisted cervix cancer brachytherapy. Radiother Oncol. 2008;89:164–171. doi: 10.1016/j.radonc.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 26.Hellebust T.P., Petric P., Tanderup K. Spatial dosimetric sensitivity analysis of contouring uncertainties in GYN 3D based brachytherapy. Radiother Oncol. 2012;103:34. doi: 10.1016/j.radonc.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Nesvacil N., Pötter R., Sturdza A. Adaptive image guided brachytherapy for cervical cancer: a combined MRI-/CT-based planning technique with MRI only at first fraction. Radiother Oncol. 2013;107:75–81. doi: 10.1016/j.radonc.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]