Abstract
Purpose
To measure longitudinal changes in body mass and composition in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem-cell transplantation (HSCT).
Patients and Methods
Body mass index (BMI) was analyzed in 179 survivors by category (underweight, healthy-weight, overweight, and obese) and by z score. Fat and lean body mass measured by dual-energy x-ray absorptiometry was analyzed as z scores.
Results
Over a median 6.6 years of follow-up, BMI z scores diminished significantly (0.32 pre-HSCT v −0.60 at 10 years post-HSCT; P < .001). Mean z scores for fat mass stayed within population norms, but those for lean mass remained below normal levels and diminished significantly over time (P = .018). Pre-HSCT BMI category and/or z score were strongly predictive of post-HSCT BMI (P < .001) and of fat and lean mass z scores (both P < .001). Survivors with extensive chronic graft-versus-host disease were more likely than others to have low BMI (P = .004) and low lean mass (P < .001) post-HSCT. Older age at HSCT (P = .015) and T-cell–depleted graft (P = .018) were predictive of lower post-HSCT BMI. Female patients had higher body fat (P = .002) and lower lean mass (P = .013) z scores than male patients, and black patients had higher fat mass z scores than white patients (P = .026).
Conclusion
BMI declines significantly after allogeneic HSCT for childhood hematologic malignancies, reflecting primarily a substantial decrease in lean mass but not fat mass. Monitoring and preservation of BMI and lean mass are vital, especially in those with the identified risk factors.
INTRODUCTION
Allogeneic hematopoietic stem-cell transplantation (alloHSCT) offers an effective treatment for a variety of diseases, both malignant and nonmalignant.1 Its success has led to an increasing number of long-term survivors of pediatric diseases.2 Because developing children are particularly susceptible to transplantation-related toxicities, the long-term consequences of alloHSCT are of increasing interest.3–7 Recent studies showed that survivors of childhood hematologic malignancies who have undergone hematopoietic stem-cell transplantation (HSCT) have greater morbidity than not only noncancer populations but also survivors who have undergone conventional chemotherapy only.7,8
In the general population, obesity has reached epidemic proportions in recent decades.9 In the United States, the National Health and Nutrition Examination Survey (NHANES) data for 2007 to 2008 indicate that more than 72 million adults (32.2% of men and 35.5% of women) have a body mass index (BMI) that indicates obesity.10 During the same period, 16.9% of 2- to 19-year-old children had a BMI ≥ 95th percentile for age according to the 2000 Centers for Disease Control and Prevention (CDC) growth chart, derived from previous pediatric data.11
Body mass and composition have been studied in survivors of childhood cancer, but there is little information about these factors in the subset of survivors who have undergone alloHSCT. Past reports from our group and others have been limited by small sample sizes, short duration of follow-up, heterogeneous diagnoses including benign and malignant diseases, and combined evaluation of recipients of autologous and allogeneic HSCT.8,12–15 We therefore studied longitudinally survivors of pediatric hematologic malignancies, which are the most common indication for alloHSCT in children, to determine the effects of alloHSCT on body composition. Comprehensive evaluations included standard parameters such as height, weight, and BMI, as well as fat and lean mass measurement by dual energy x-ray absorptiometry (DXA).
PATIENTS AND METHODS
Patients
Patients included in this study were participants in a longitudinal follow-up cohort study of recipients of HSCT at St Jude Children's Research Hospital. The study was approved by the St Jude institutional review board, and informed consent was obtained from all patients or their legal guardians. Patients included in this study underwent alloHSCT for hematologic malignancy at age ≥ 2 years between 1990 and 2007 and remained alive more than 1 year after alloHSCT. Patients younger than 2 years old at alloHSCT were excluded because of the absence of population norm BMI data. Patients received follow-up for at least 10 years post-HSCT or until age 18 years, whichever was longer.
BMI, Height, and Weight
Patients received follow-up from the time of transplantation through 2009. Patient weight and height before HSCT and at annual follow-up visits were obtained from patient records. Data were censored at the date of relapse. BMI (measured in kg/m2) was calculated for patients older than 2 years. For patients 2 to 20 years old, z scores and percentiles of BMI, height, and weight were calculated from normative data by using SAS code downloaded from the CDC Web site (http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm). These patients were categorized on the basis of the calculated percentiles as underweight (< fifth percentile for age), healthy-weight (fifth to 84th percentile), overweight (85th to 94th percentile), or obese (≥ 95th percentile). For adult patients (age ≥ 20 years), the BMI was used to define weight categories as follows: underweight (BMI, < 18.5), healthy-weight (BMI, 18.5 to 24.9), overweight (BMI, 25.0 to 29.9), or obese (BMI, ≥ 30). BMI, height, and weight z scores of adult patients were calculated using normative data from the NHANES 2007 to 2008.16
Dual Energy X-Ray Absorptiometry
Whole-body DXA data after HSCT were acquired by using a Discovery QDR 4500A fan beam densitometer (Hologic, Inc, Bedford, MA), as recommended by the manufacturer. For patients age ≥ 8 years at the time of examination, z scores for total body fat percentage and lean mass/height2 were calculated from reference curves obtained from the 2008 NHANES DXA whole-body data set.17
Statistical Analysis
To identify risk factors for obesity and overweight after HSCT, patients in the obese and overweight BMI categories were combined and those in the healthy-weight and underweight categories were combined. The generalized estimating equations (GEE) method was used to fit the resulting longitudinal binomial data, assuming an exchangeable dependence structure over time. A similar model was used to identify risk factors for underweight in survivors in the healthy-weight or underweight BMI category before HSCT and in the underweight BMI category after HSCT. Potential risk factors selected by univariate GEE models at a significance level of P < .10 were combined in a multiple GEE model to determine their independent effect.
A linear regression model for longitudinal data was used to investigate the relationship between clinical factors (Table 1) to z scores for BMI, height, weight, and total body fat percentage and lean mass/height2 (Proc Mixed, SAS, Cary, NC). An autoregressive structure with order one was selected by the Bayesian information criterion to model intrapatient dependence. The independent effect of each factor that had a P value < .10 in univariate models was determined by using a multiple regression model. The two-way interaction terms between any two factors were entered in the multiple regression models through backward selection. Normal assumptions were confirmed to hold in all analyses. Both visual inspection and statistical testing verified that all z score values could be modeled as a linear function of time.
Table 1.
Patient and Clinical Characteristics (N = 179)
| Characteristic | No. of Patients | % |
|---|---|---|
| Sex | ||
| Female | 76 | 42.5 |
| Male | 103 | 57.5 |
| Race | ||
| White | 132 | 73.7 |
| Black | 20 | 11.2 |
| Other | 27 | 15.1 |
| Primary malignancy | ||
| AML | 68 | 38.0 |
| Lymphoid | 61 | 34.1 |
| CML | 33 | 18.4 |
| MDS | 17 | 9.5 |
| Body mass index before HSCT | ||
| Healthy weight | 117 | 65.4 |
| Obese | 26 | 14.5 |
| Overweight | 28 | 15.6 |
| Underweight | 8 | 4.5 |
| Donor | ||
| Parent | 33 | 18.4 |
| Sibling | 68 | 38.0 |
| Unrelated | 78 | 43.6 |
| Donor sex | ||
| Female | 95 | 53.1 |
| Male | 84 | 46.9 |
| Donor/recipient CMV status | ||
| D+/R+ | 57 | 31.8 |
| D−/R+ | 24 | 13.4 |
| D+/R− | 32 | 17.9 |
| D−/R− | 66 | 36.9 |
| Graft product | ||
| Bone marrow | 150 | 83.8 |
| Peripheral blood | 29 | 16.2 |
| T-cell–depleted graft | ||
| No | 109 | 60.9 |
| Yes | 70 | 39.1 |
| Radiation | ||
| TBI | 165 | 92.2 |
| Non-TBI | 14 | 7.8 |
| Acute GVHD, grade | ||
| None | 86 | 48.0 |
| 1 | 55 | 30.7 |
| 2 | 24 | 13.4 |
| 3 | 8 | 4.5 |
| 4 | 6 | 3.4 |
| Chronic GVHD | ||
| None | 131 | 73.2 |
| Limited | 29 | 16.2 |
| Extensive | 19 | 10.6 |
Abbreviations: AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CMV, cytomegalovirus; D, donor; GVHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation; MDS, myelodysplastic syndrome; R, recipient; TBI, total body irradiation.
RESULTS
Patient Characteristics
Of 256 participants in the larger follow-up study, 191 had hematologic malignancies and 179 of these 191 were ≥ 2 years old at the time of HSCT (Table 1). Median age at HSCT was 11.3 years (range, 2.1 to 21.3 years) and median follow-up after HSCT was 6.6 years (range, 1.0 to 17.7 years). Twenty-four patients received growth hormone replacement and 55 received sex hormone therapy during follow-up. All patients were regularly screened for thyroid dysfunction; patients with hypothyroidism (n = 55) were adequately treated.
Longitudinal Analysis of BMI Categories
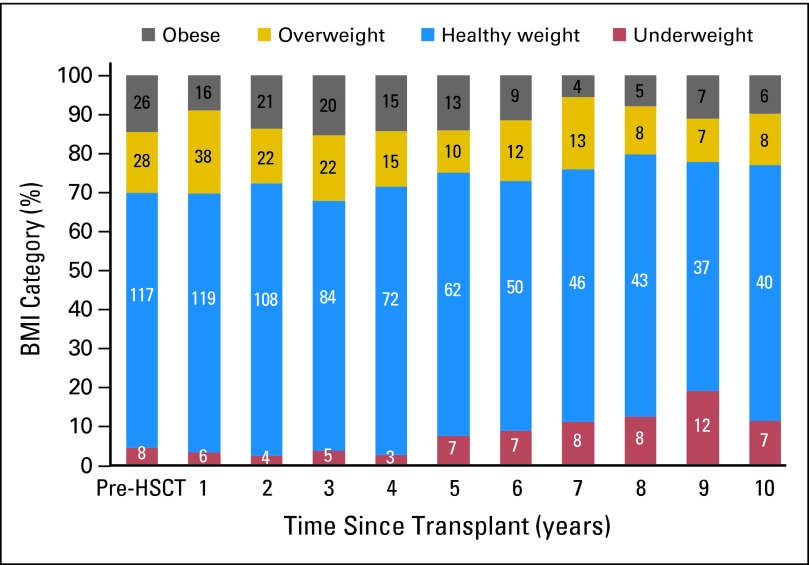
The number and proportion of patients in each BMI category before and during each year after HSCT (1,231 observed instances) are illustrated in Figure 1. The proportion of underweight patients increased from 4.5% pre-HSCT to 11.5% at 10 years post-HSCT, while the proportion of obese or overweight patients decreased from 30.1% to 23.0%. No patient who was obese or overweight pre-HSCT became underweight at any time point after HSCT. Similarly, no patients who were underweight pre-HSCT became obese or overweight during follow-up.
Fig 1.
Longitudinal changes in body mass index (BMI) category. The number and proportion of patients in each BMI category are shown for each year of follow-up after allogeneic hematopoietic stem-cell transplantation (HSCT).
Risk Factors for Post-HSCT Obesity/Overweight and Underweight
A multivariable logistic GEE analysis identified pre-HSCT obesity/overweight as an independent risk factor for post-HSCT obesity/overweight (P < .001; Table 2). The odds ratio (OR) for post-HSCT obesity/overweight was 14.02 (95% CI, 5.44 to 36.09; P < .001) in survivors who were obese before HSCT and 2.89 (95% CI, 1.45 to 5.76; P = .003) in survivors who were overweight pre-HSCT, compared with survivors in the healthy-weight/underweight BMI categories pre-HSCT (Table 2).
Table 2.
Factors Associated With Changes in BMI After HSCT
| Variable | Univariate P | Multivariate |
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Estimate | SE | ||
| Factors associated with overweight/obese BMI category after HSCT | ||||||
| BMI category before HSCT | ||||||
| Obese | < .001 | 14.02 | 5.44 to 36.09 | < .001 | ||
| Overweight | 2.89 | 1.45 to 5.76 | ||||
| Healthy/underweight | ||||||
| Acute GVHD | ||||||
| None or grade 1 | .025 | 0.58 | 0.26 to 1.31 | .22 | ||
| Grades 2-4 | ||||||
| Donor CMV status | ||||||
| Negative | .061 | 1.74 | 0.91 to 3.32 | .094 | ||
| Positive | ||||||
| T-cell depletion | ||||||
| No | .017 | 1.55 | 0.82 to 2.91 | .18 | ||
| Yes | ||||||
| Factors associated with underweight BMI category after HSCT | ||||||
| Chronic GVHD | ||||||
| Extensive | .078 | 4.08 | 1.23 to 13.55 | .070 | ||
| Limited | 3.50 | 1.24 to 9.90 | ||||
| None | ||||||
| Sex | ||||||
| Female | .072 | 2.10 | 0.85 to 5.16 | .11 | ||
| Male | ||||||
| Factors associated with lower BMI z scores after HSCT | ||||||
| BMI z score before HSCT | < .001 | < .001 | 0.51 | 0.05 | ||
| Years after HSCT | < .001 | < .001 | −0.07 | 0.01 | ||
| Age at HSCT | .045 | .015 | −0.03 | 0.01 | ||
| Chronic GVHD | ||||||
| Extensive | .030 | .004 | −0.67 | 0.20 | ||
| Limited | −0.27 | 0.16 | ||||
| None | 0 | |||||
| T-cell depletion | ||||||
| No | .003 | .018 | 0.30 | 0.12 | ||
| Yes | 0 | |||||
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; GVHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation.
Because no patient who was obese or overweight pre-HSCT became underweight during follow-up, we analyzed patients who were healthy-weight or underweight pre-HSCT for risk factors associated with patients who were underweight post-HSCT. A multivariable GEE model analysis of 800 observed instances in 125 patients found that chronic graft-versus-host disease (GVHD; compared with its absence) was significantly predictive of underweight post-HSCT, whether it was extensive (OR, 4.08; 95% CI, 1.23 to 13.55; P = .022) or limited (OR, 3.50; 95% CI, 1.24 to 9.90; P = .018; Table 2).
Longitudinal Analysis of BMI, Height, and Weight Z Scores
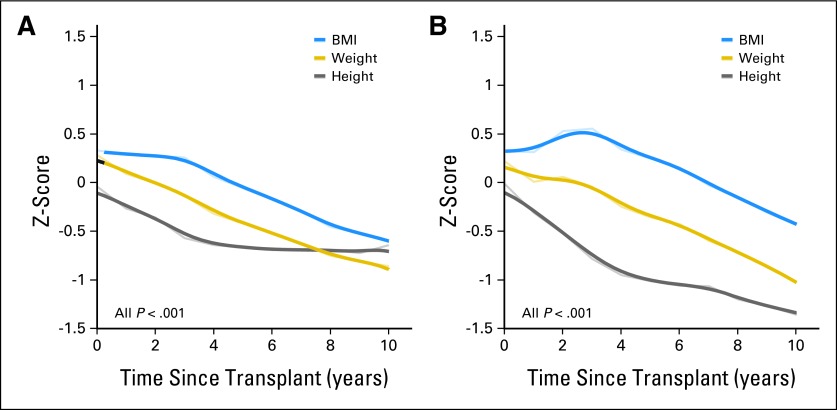
Because the healthy-weight BMI category (spanning the fifth to the 84th percentiles) included the majority of our patients, we sought to identify subtle but clinically important longitudinal changes by using continuous variables (BMI, height, and weight z scores) rather than categoric variables. Before HSCT, patients had slightly higher BMI z scores (mean, 0.33; range, −3.34 to 3.14) and weight z scores (mean, 0.28; range, −2.74 to 3.80) than did age- and sex-matched controls, although their mean height z scores were comparable (mean, −0.04; range, −2.66 to 2.76). Figure 2A shows that the BMI, height, and weight z scores all decreased over time (all P < .001), but with distinct patterns. While the mean BMI and weight z scores declined progressively throughout follow-up, dropping to −0.60 and −0.86, respectively, at 10 years post-HSCT, the mean height z score declined only during the first 4 years and then reached a plateau (−0.64 at 10 years after HSCT). No plateau in the height z score was observed when we repeated the analysis in the 95 patients who were younger than 12 years old at the time of HSCT (592 observed instances; mean z score, −0.02 before HSCT and −1.37 10 years after HSCT; Fig 2B),18 indicating that the observed plateau likely resulted from the older patients' proximity to final height attainment.
Fig 2.
Longitudinal changes in mean body mass index (BMI), weight, and height z scores in (A) all survivors (n = 179) and (B) in those younger than 12 years old at the time of hematopoietic stem-cell transplantation (n = 95). Narrow lines connect the observed mean z scores, whereas corresponding broader lines represent the statistically smoothed curves.
Clinical Factors Associated With Changes in BMI, Height, and Weight Z Scores
Multivariable analysis identified lower BMI z scores before HSCT (P < .001), a longer time period since HSCT (P < .001), older age at HSCT (P = .015), extensive chronic GVHD (P = .004), and T-cell–depleted graft (P = .018) as independent predictors of lower BMI z scores post-HSCT (Table 2). To evaluate the effect of limited chronic GVHD, we excluded patients with extensive chronic GVHD. Patients with limited chronic GVHD had a lower BMI z score than those without chronic GVHD (univariate analysis, P = .020; multivariate analysis, P = .017; estimate, −0.41; SE, 0.17).
We also evaluated clinical factors associated with longitudinal changes in height z scores (in patients < 12 years old at HSCT) and weight z scores (all patients). Lower height z score before HSCT and longer time since HSCT were independently associated with lower post-HSCT height z score (both P < .001; Appendix Table A1, online only). Lower weight z scores before HSCT (P < .001), longer time since HSCT (P < .001), and T-cell–depleted graft (P = .021) were independent predictors of low post-HSCT weight z score (Appendix Table A1).
Longitudinal Analysis of Body Composition by DXA
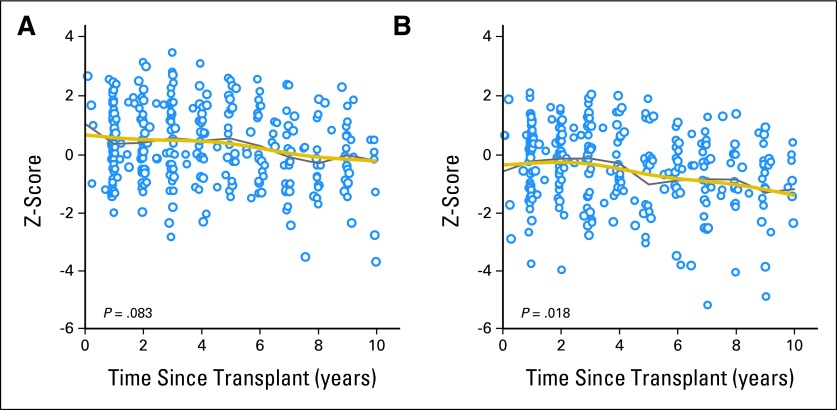
To investigate whether the decrease in BMI was primarily owing to loss of fat mass, lean mass, or both, we analyzed data from 324 DXA scans of 134 patients. Figures 3A and 3B show the scatter plots and mean z scores for total body fat percentage and lean mass/height2, respectively, after HSCT. The mean z score for total body fat percentage was close to the population mean (0.31) at 1 year after HSCT and declined only slightly (−0.27) at 10 years after HSCT (Fig 3A). In contrast, mean z scores for lean mass/height2 remained below 0 (−0.30) 1 year after HSCT and decreased substantially thereafter, falling as low as −1.26 at 10 years post-HSCT (P = .018; Fig 3B and Table 3). Table 3 lists other clinical factors associated with longitudinal changes in body composition in univariate and multivariate regression models. The pre-HSCT BMI z score was predictive of post-HSCT total body fat percentage (P < .001) and lean mass/height2 (P < .001); patients who had high BMI z scores before HSCT had significantly greater total body fat percentage and lean mass/height2 z scores after HSCT. Female patients had significantly greater total body fat percentage (P = .002) and lower lean mass/height2 (P = .013) z scores than did male patients, and black patients had higher total body fat percentage z scores than white patients (P = .026). Patients with extensive chronic GVHD had a lower lean mass/height2 than those with no chronic GVHD (P < .001). When patients with extensive chronic GVHD were excluded, patients with limited chronic GVHD had slightly less lean mass than those without chronic GVHD in univariate analysis (P = .17; estimate, −0.40; SE, 0.29).
Fig 3.
Scatter plot showing longitudinal changes in z scores for (A) total body fat percentage and (B) lean mass/height2 after hematopoietic stem-cell transplantation. Data points represent 324 dual energy x-ray absorptiometry scans in 134 patients. Gray lines connect the observed mean z scores, whereas gold lines represent the statistically smoothed curves.
Table 3.
Factors Associated With Change in Body Composition After HSCT
| Variable | Univariate P | Multivariate |
||
|---|---|---|---|---|
| P | Estimate | SE | ||
| Factors associated with higher z scores for total body fat percentage | ||||
| BMI z score before HSCT | < .001 | < .001 | 0.36 | 0.08 |
| Sex | ||||
| Female | .067 | .002 | 0.56 | 0.18 |
| Male | 0 | |||
| Race | ||||
| Black | .068 | .026 | 0.62 | 0.22 |
| Other | 0.08 | 0.10 | ||
| White | 0 | |||
| Years after HSCT | .003 | .083 | −0.04 | 0.02 |
| Diagnosis | ||||
| Lymphoid | .006 | .053 | 0.38 | 0.20 |
| Myeloid | 0 | |||
| Acute GVHD | ||||
| None or grade 1 | .073 | .28 | −0.24 | 0.22 |
| Grades 2-4 | 0 | |||
| Factors associated with lower z scores for lean body mass/height2 | ||||
| BMI z score before HSCT | < .001 | < .001 | 0.47 | 0.07 |
| Years after HSCT | < .001 | .018 | −0.06 | 0.03 |
| Sex | ||||
| Female | .020 | .013 | −0.47 | 0.19 |
| Male | 0 | |||
| Chronic GVHD | ||||
| Extensive | .021 | < .001 | −1.68 | 0.36 |
| Limited | −0.40 | 0.24 | ||
| None | 0 | |||
| Age at HSCT | .066 | .074 | 0.04 | 0.02 |
| T-cell depletion | ||||
| No | .023 | .25 | 0.22 | 0.19 |
| Yes | 0 | |||
Abbreviations: BMI, body mass index; GVHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation.
DISCUSSION
This longitudinal analysis of 179 pediatric alloHSCT recipients with hematologic malignancies showed that BMI declines significantly over time in this vulnerable population. Importantly, lean body mass consistently remained below normal levels and declined significantly over time. Our observations underscore the unique impact of HSCT in survivors of childhood hematologic malignancies.
Few longitudinal studies of body mass after childhood HSCT have been reported. Cohen et al12 found that BMI values in adults remained unchanged after HSCT, whereas those among 88 pediatric patients increased, paralleling a normal population. Although Cohen et al concluded that nutritional status was maintained in the pediatric patients, they did not adjust BMI values for sex and age. In contrast, Couto-Silva et al13 found persistently reduced BMI without significant longitudinal changes after HSCT in 36 children who received total-body irradiation (TBI). However, Couto-Silva et al included patients with hematologic malignancies and those with neuroblastoma, and the source of stem cells (autologous v allogeneic) and preconditioning regimens were unclear. Our study of a much larger group of patients with only hematologic malignancies was conducted over a longer follow-up period and we found that BMI z scores declined significantly over time. This finding differs from those of several studies of acute lymphoblastic leukemia (ALL) survivors, most of whom did not undergo HSCT and whose BMIs were comparable to or higher than normal control values.19–21 BMI at the time of HSCT was significantly associated with post-HSCT BMI category and BMI z score in our study, consistent with findings in ALL survivors at our institution.20
Another novel finding of our study is that the mean z scores of lean mass/height2 fell below those of healthy controls during follow-up, although the mean values for total body fat percentage remained at the general population level. This finding underscores the importance of assessing lean body mass as well as BMI during long-term follow-up. Propitiously, lean body mass may be measured conveniently during assessment of bone density by routine DXA scan and it is useful in interpreting BMI data. If a decline in lean mass is observed, early intervention is necessary to prevent metabolic syndrome and further muscle wasting.
Low BMI in general is an important predictor of morbidity and mortality and is associated with severe physiologic, psychologic, and immunologic consequences.22 We found that chronic GVHD, especially in its extensive form, was a risk factor for low BMI and lean mass. The association between chronic GVHD and lower BMI has been reported in other pediatric and adult studies.23,24 T-cell–depleted grafts, which were typically from nonsibling alternative donors, were also associated with low BMI and weight, possibly reflecting the effect of alloreactive T cells after engraftment as well as complications associated with delayed immune reconstitution. In chronic GVHD, a state of catabolism is induced by elevated levels of proinflammatory cytokines, such as tumor necrosis factor-α, interleukin-1, and interleukin-6, increasing patients' resting energy expenditure.25,26 In addition, extensive chronic GVHD requires prolonged steroid therapy, which may further contribute to the loss of lean mass. Intensive nutritional support is necessary for patients with chronic GVHD and for those who show a significant decline in BMI or lean body mass during follow-up.
Younger age at the time of HSCT was associated with higher BMI and continuous decline of height z scores after HSCT. Similar findings have been reported in survivors of ALL and brain tumors.19–21 The effects of cranial irradiation on BMI in survivors of ALL remain unclear. As the majority of our patients received TBI as part of their preconditioning regimen, we were unable to meaningfully compare irradiated and nonirradiated groups. However, higher BMIs in some of the survivors may reflect the impact of growth hormone deficiency and relative decrease in height caused by preconditioning with TBI at a young age.27 TBI may also blunt hypothalamic leptin sensitivity, altering the response to leptin and the regulation of body weight and metabolism.28 This effect, together with limited vertical growth, may explain the higher post-HSCT BMI of patients who underwent HSCT at a younger age. In univariate analysis, patients with lymphoid malignancies had higher z scores for total body fat percentage than did those with myeloid diseases; this finding could be associated with pre-HSCT chemotherapy that included steroids or additional cranial irradiation. As non-TBI regimens are now often used for myeloid malignancies and reduced-intensity conditioning, it will be important to evaluate body mass and composition in this set of survivors.
Although BMI z scores did not differ according to sex or race, female patients had higher fat and lower lean mass z scores than male patients, and black patients had higher fat z scores than white patients. Several explanations have been offered for this difference. Female brains may be more vulnerable to HSCT preconditioning, as girls experience more rapid brain growth than do boys during early childhood; therefore, preconditioning may cause greater neurocognitive impairment and inactivity.29,30 A study in childhood ALL survivors showed that girls were less likely than boys to meet Centers for Disease Control and Prevention physical activity recommendations.31 Female survivors of childhood ALL who had a homozygous Gln223Arg polymorphism in the leptin receptor gene were markedly more obese than those with a Gln223 allele, and this difference was not observed in male survivors.32 African Americans, including cancer survivors, are reported to have lower levels of physical activity and greater fat consumption than other ethnic groups.33,34
This study was not without limitations. First, population values were unavailable for BMI in patients younger than 2 years old and for DXA-based body composition in those younger than 8 years old. However, we found no significant difference in clinical factors between patients who did and who did not undergo DXA assessment. Second, as supportive care and nutritional interventions were nonstandardized, varied over time, and were initiated as clinically needed, we were unable to assess their impact on body composition in the study population.
In conclusion, we found a significant decline in BMI z scores after alloHSCT in survivors of childhood hematologic malignancies, primarily owing to a decrease in lean mass. Most, if not all, of the findings in this study may be attributable to TBI and/or the degree of chronic GVHD. Prospective endocrine evaluation, including not only growth hormone secretion, thyroid function, and sex hormone production but also hormonal regulation of glucose and lipid metabolism, will improve our understanding of the observed changes in body composition and their impact. The declines in BMI and lean mass persisted throughout the follow-up period. Therefore, health care providers should be alert to losses in not only BMI but also lean mass in these survivors and ensure early, appropriate intervention by a registered dietitian and physical therapist. We suggest that dietary education and exercise counseling are essential to improve the physical status and overall health of survivors with the risk factors identified in this study.
Acknowledgment
We thank Sharon Naron, ELS, for her editorial review of the manuscript.
Appendix
Table A1.
Factors Associated With Change in Height and Weight After HSCT
| Variable | Univariate P | Multivariate |
||
|---|---|---|---|---|
| P | Estimate | SE | ||
| Factors associated with lower height zscores, patients < 12 years old | ||||
| Height z score before HSCT | < .001 | < .001 | 0.73 | 0.08 |
| Years after HSCT | < .001 | < .001 | −0.11 | 0.01 |
| BMI zscore before HSCT | < .001 | .26 | 0.07 | 0.06 |
| Graft product | ||||
| Peripheral blood | .022 | .65 | 0.09 | 0.20 |
| Bone marrow | 0 | |||
| Factors associated with lower weight z scores, all patients | ||||
| Weight zscore before HSCT | < .001 | < .001 | 0.66 | 0.12 |
| Years after HSCT | < .001 | < .001 | −0.08 | 0.01 |
| BMI z score before HSCT | < .001 | .64 | −0.08 | 0.12 |
| Acute GVHD | ||||
| None or grade 1 | .079 | .35 | 0.16 | 0.17 |
| Grades 2-4 | 0 | |||
| T-cell depletion | ||||
| No | .001 | .021 | 0.31 | 0.13 |
| Yes | 0 | |||
Abbreviations: BMI, body mass index; GVHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation.
Footnotes
Supported by Cancer Center Support Grant No. P30 CA021765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Hiroto Inaba, Sue C. Kaste, Wing Leung
Financial support: Ching-Hon Pui, Wing Leung
Administrative support: Hiroto Inaba, Ching-Hon Pui, Wing Leung
Provision of study materials or patients: Hiroto Inaba, Sue C. Kaste, Christine M. Hartford, Wassim Chemaitilly, Brandon M. Triplett, David R. Shook, Ching-Hon Pui, Wing Leung
Collection and assembly of data: Hiroto Inaba, Jie Yang, Sue C. Kaste, Christine M. Hartford, Megan S. Motosue, David R. Shook, Wing Leung
Data analysis and interpretation: Hiroto Inaba, Jie Yang, Sue C. Kaste, Megan S. Motosue, Wassim Chemaitilly, Brandon M. Triplett, Ching-Hon Pui, Wing Leung
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Leung W, Campana D, Yang J, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118:223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socié G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: Report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86:215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 6.Baker KS, Armenian S, Bhatia S. Long- term consequences of hematopoietic stem cell transplantation: Current state of the science. Biol Blood Marrow Transplant. 2010;16(suppl 1):S90–S96. doi: 10.1016/j.bbmt.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: A report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118:1413–1420. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung W, Hudson MM, Strickland DK, et al. Late effects of treatment in survivors of childhood acute myeloid leukemia. J Clin Oncol. 2000;18:3273–3279. doi: 10.1200/JCO.2000.18.18.3273. [DOI] [PubMed] [Google Scholar]
- 9.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Duell T, Socié G, et al. Nutritional status and growth after bone marrow transplantation (BMT) during childhood: EBMT Late-Effects Working Party retrospective data—European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1999;23:1043–1047. doi: 10.1038/sj.bmt.1701769. [DOI] [PubMed] [Google Scholar]
- 13.Couto-Silva AC, Trivin C, Esperou H, et al. Changes in height, weight and plasma leptin after bone marrow transplantation. Bone Marrow Transplant. 2000;26:1205–1210. doi: 10.1038/sj.bmt.1702718. [DOI] [PubMed] [Google Scholar]
- 14.Nysom K, Holm K, Michaelsen KF, et al. Degree of fatness after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant. 2001;27:817–820. doi: 10.1038/sj.bmt.1703012. [DOI] [PubMed] [Google Scholar]
- 15.Leung W, Hudson M, Zhu Y, et al. Late effects in survivors of infant leukemia. Leukemia. 2000;14:1185–1190. doi: 10.1038/sj.leu.2401818. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES), 2007-2008. http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm.
- 17.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemaitilly W, Boulad F, Heller G, et al. Final height in pediatric patients after hyperfractionated total body irradiation and stem cell transplantation. Bone Marrow Transplant. 2007;40:29–35. doi: 10.1038/sj.bmt.1705694. [DOI] [PubMed] [Google Scholar]
- 19.Dalton VK, Rue M, Silverman LB, et al. Height and weight in children treated for acute lymphoblastic leukemia: Relationship to CNS treatment. J Clin Oncol. 2003;21:2953–2960. doi: 10.1200/JCO.2003.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25:1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 21.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra RK. Nutrition, immunity, and infection: Present knowledge and future directions. Lancet. 1983;1:688–691. doi: 10.1016/s0140-6736(83)91980-3. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsohn DA, Margolis J, Doherty J, et al. Weight loss and malnutrition in patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29:231–236. doi: 10.1038/sj.bmt.1703352. [DOI] [PubMed] [Google Scholar]
- 24.Browning B, Thormann K, Seshadri R, et al. Weight loss and reduced body mass index: A critical issue in children with multiorgan chronic graft-versus-host disease. Bone Marrow Transplant. 2006;37:527–533. doi: 10.1038/sj.bmt.1705268. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zauner C, Rabitsch W, Schneeweiss B, et al. Energy and substrate metabolism in patients with chronic extensive graft-versus-host disease. Transplantation. 2001;71:524–528. doi: 10.1097/00007890-200102270-00007. [DOI] [PubMed] [Google Scholar]
- 27.Cohen A, Rovelli A, Bakker B, et al. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: A study by the Working Party for Late Effects-EBMT. Blood. 1999;93:4109–4115. [PubMed] [Google Scholar]
- 28.Brennan BM, Rahim A, Blum WF, et al. Hyperleptinaemia in young adults following cranial irradiation in childhood: Growth hormone deficiency or leptin insensitivity? Clin Endocrinol (Oxf) 1999;50:163–169. doi: 10.1046/j.1365-2265.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 29.Christie D, Leiper AD, Chessells JM, et al. Intellectual performance after presymptomatic cranial radiotherapy for leukaemia: Effects of age and sex. Arch Dis Child. 1995;73:136–140. doi: 10.1136/adc.73.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong GT, Sklar CA, Hudson MM, et al. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25:4477–4489. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
- 31.Florin TA, Fryer GE, Miyoshi T, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16:1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 32.Ross JA, Oeffinger KC, Davies SM, et al. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2004;22:3558–3562. doi: 10.1200/JCO.2004.11.152. [DOI] [PubMed] [Google Scholar]
- 33.Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118:4024–4031. doi: 10.1002/cncr.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison JA, Glueck CJ, Wang P. Preteen insulin levels interact with caloric intake to predict increases in obesity at ages 18 to 19 years: A 10-year prospective study of black and white girls. Metabolism. 2010;59:718–727. doi: 10.1016/j.metabol.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]