Abstract
Purpose
A key challenge in the treatment of thymoma and thymic carcinoma (TC) is in improving our understanding of the molecular biology of these relatively rare tumors. In recent years, significant efforts have been made to dissect the molecular pathways involved in their carcinogenesis. Here we discuss the results of large-scale genomic analyses conducted to date and review the most active chemotherapies and targeted treatments.
Methods
We reviewed the literature for chemotherapeutic trials in the last 20 years and trials involving targeted therapies between 1999 and 2010. The search was supplemented by a review of abstracts presented at the annual meetings of the American Society of Clinical Oncology (from 1999 to 2010), at the first International Conference on Thymic Malignancies in 2009, and at a follow-up meeting of the newly formed International Thymic Malignancies Interest Group in 2010.
Results
Surgery remains the treatment of choice for operable tumors, whereas chemotherapy is standard in locally advanced and metastatic disease. Thus far, targeted therapies have been developed empirically. Histone deacetylase inhibitors have shown some activity in thymoma whereas sunitinib may be active in TC. There are no data to support the use of HER2- or EGFR-targeted therapies in thymic malignancies.
Conclusion
Drug development for the treatment of thymic malignancies is difficult because of the rarity of these tumors. Ethnic differences are becoming apparent, with aggressive subtypes being observed in Asians and African Americans. Incremental improvements in our understanding of tumor biology suggest that molecular profiling–directed therapies may be the preferred route of investigation in the future.
INTRODUCTION
Thymic epithelial tumors (TETs) are divided into two broad categories: thymomas and thymic carcinomas (TCs). As the site of maturation for T cells, the thymus plays a central role in adaptive immunity. A wide spectrum of autoimmune disorders, including myasthenia gravis (30%), are seen in patients with thymomas, whereas patients with TC rarely if ever have autoantibody-induced phenomena.1 The risk of second malignancies, in particular, non-Hodgkin's lymphoma, and a possible protective effect in the presence of myasthenia gravis suggests inherent immune differences among patients with regard to immune surveillance and autoimmunity.2–4 Surgical resection forms the cornerstone of therapy for early-stage tumors, and approximately 90% of patients with encapsulated thymomas are cured with complete surgical extirpation of disease.5 In advanced or recurrent TETs, a multimodality approach incorporating surgery, radiation, and chemotherapy is required. The rarity of this tumor has precluded it from large phase II and III clinical trial investigations, and new drugs have been slow in development. In the last decade, several targeted agents have been investigated with varying success rates. The cause of thymoma remains unknown; however, our understanding of the aberrant pathways involved is improving. Herein, we summarize the molecular biology of thymic malignancies that defines molecular subsets with potential clinical and therapeutic relevance. We highlight the most important new trends in the treatment of these tumors with respect to current standard-of-care chemotherapeutic regimens and discuss ongoing research involving targeted therapies.
METHODS
Information for this review was derived from searching the PubMed database for all significant chemotherapeutic clinical trials that have occurred in the last 20 years and trials in the last decade that involve targeted agents. Our search limits included thymic malignancies and clinical trials. The search was supplemented by a review of abstracts presented at the American Society of Clinical Oncology (ASCO) annual meetings from 1999 to 2010. In addition, all abstracts presented at the first International Conference on Thymic Malignancies (held in 2009 at the National Institutes of Health) and a follow-up meeting of the newly formed International Thymic Malignancies Interest Group (ITMIG) in 2010 in New York were reviewed.
Incidence and Epidemiology
Although thymic malignancies are relatively rare (0.2% to 1.5% of all malignancies or 0.13 per 100,000 person-years in the United States6), they are among the most common mediastinal primary tumors with up to 50% of anterior mediastinal masses proving to be of thymic descent.7 Males have a slightly higher risk of developing thymomas than females, and the risk rises with age, reaching a peak in the seventh decade of life, which is in direct contrast to the progressive involution of the thymus with age.2 Data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program collected for Hispanic and Asian/Pacific Islander subgroups have been available only since 1992 and 1998, respectively. Thymoma incidence in the United States is higher in African Americans and especially Asian/Pacific Islanders than among whites or Hispanics. Furthermore, thymoma arises at a younger age among African Americans than among whites (median age at diagnosis, 48 v 58 years; SEER data). Similarly, myasthenia gravis may be more common in African Americans than in whites.8 Compared with controls, patients with thymoma are more likely to have an autoimmune disease at some point during their lives (32.7% v 2.4%; P < .001), most frequently myasthenia gravis (24.5%), systemic lupus erythematosus (2.4%), or red cell aplasia (1.2%).9 Ethnic variations in terms of higher incidence rates and younger age at diagnosis suggest a role for genetic factors. The distribution of alleles at the HLA locus on chromosome 6 varies markedly across racial groups.2 Both class I and class II HLA proteins are highly expressed on thymic epithelial cells.10 Further research is needed to understand whether particular genetic variants (at HLA or other loci) predispose to thymoma.
Staging and Histology
The Masaoka staging (Table 1) focuses on the integrity of the thymic capsule, the presence of micro or macroscopic invasion into adjacent structures, and metastatic spread.11 The WHO histologic classification13 focuses on thymic epithelial cell morphology (spindle or epithelioid), the degree of the lymphocytic component, and the presence or absence of epithelial atypia (Table 1). Epithelial morphology corresponds to medullary or cortical differentiation. The classification reports a continuum of tumors from type A, AB, B1, B2, B3, and thymic carcinomas. Thymic epithelial neoplasms have been the subject of much controversy over the years because of difficulties in predicting prognosis and behavior. As a result of the complexity of these tumors, several attempts at classification have been presented in the literature. Moran and Suster14 examined more than 600 thymoma cases to determine morphologic heterogeneity. They concluded that proper evaluation of histology and aggressive potential in thymoma should be based on ample sampling (cutoff number of five sections is required) and assessment of capsular integrity.
Table 1.
Masaoka Staging of Thymic Malignancies11 With Modifications by Koga et al12 and WHO Classification of Thymic Malignancies13
| Stage/Type | Definition |
|---|---|
| Stage | |
| I | Grossly and microscopically completely encapsulated |
| II (a) | Microscopic transcapsular invasion |
| II (b) | Macroscopic invasion into thymic or surrounding fatty tissue or grossly adherent to but not breaking through mediastinal pleura or pericardium |
| III | Macroscopic invasion of neighboring organs (ie, pericardium, great vessel, or lung) |
| IV (a) | Pleural or pericardial dissemination |
| IV (b) | Lymphatic or hematogenous metastasis |
| Histologic type | Description |
| A | Neoplastic oval or spindle cells; no atypia; no lymphocytes |
| AB | Type A with foci of lymphocytes |
| B1 | Plump epithelioid cells resembling normal thymic medulla |
| B2 | Scattered foci of atypical epithelial cells with lymphocytes |
| B3 | Round or polygonal epithelial cells with mild atypia with minor component of lymphocytes |
| Thymic carcinoma (C) | Histologic subtyping required |
Although tumor stage is the single most important prognostic factor in thymoma, a combination of stage and histologic subtype should be considered in predicting survival. Types A, AB, and B1 have an excellent overall survival rate of more than 90% to 95% at 10 years.15 Five-year survival for types B2, B3, and C are 75%, 70%, and 48%, respectively.16 TCs account for less than 1% of thymic malignancies and have a different molecular and clinical profile when compared with thymoma17 (Table 2). A variety of histopathologic subtypes of TC—some being more aggressive than others—have been reported, including squamous cell, lymphoepithelioma-like, sarcomatoid, clear-cell, basaloid, mucoepidermoid, papillary, and undifferentiated carcinoma. Thymomas rarely metastasize, whereas TCs display a more aggressive phenotype, with distant metastases in liver, lymph nodes, and bones.
Table 2.
Molecular Abnormalities in Thymic Malignancies
| Oncogene/Molecular Change | Thymoma (%) | Thymic Carcinoma (%) | Reference |
|---|---|---|---|
| EGFR | |||
| Gene amplification (FISH) | 20 | 25 | Lonescu et al18 |
| Overexpression (IHC) | 23 | 67-100 | Suzuki et al,19 Henley et al,20 Hayashi et al21 |
| HER2 | |||
| Overexpression (IHC) | 6 | 53 | Pan et al22 |
| c-KIT (CD117) | |||
| Overexpression (IHC) | < 5 | 73-86 | Nakagawa et al,23 Pan et al24 |
| BCL2 | |||
| Overexpression (IHC) | 14 | 100 | Pan et al22 |
| Tumor suppressor genes | |||
| TP53 | |||
| LOH | 0 | 38 | Penzel et al25 |
| Mutation | 0 | 11-30 | Tateyama et al,26 Chen et al,27 Pich et al,28 Weirich et al29 |
| Overexpression (IHC) | 12-100 | 80-100 | Tateyama et al,26 Weirich et al,29 Hirabyashi et al30 |
| P16INK4A | |||
| LOH | 0 | 25 | Penzel et al25 |
| Loss of expression | 40-50 | 70 | Hirabyashi et al30 |
Abbreviations: FISH, fluorescent in situ hybridization; IHC, immunohistochemistry; LOH, loss of heterozygosity.
Molecular Biology
The molecular biology of thymic malignancies is poorly understood because of the availability of only a few cell lines, the rarity of the disease, and the lack of specific preclinical animal models. A multistep accumulation of genetic and epigenetic aberrations in thymic epithelial cells is thought to drive neoplastic transformation. Cytogenetic studies16 have revealed chromosomal abnormalities in all histologic subtypes, including t(15;19) translocations and 6p22-p25 deletions, which are the only recurrent abnormalities found in TETs. The t(15;19)(q13:p13.1) translocation generates the fusion gene BRD4-NUT, which has been described in a rare subtype of undifferentiated thymic carcinomas.13 A relatively large body of data exists from comparative genomic hybridization (CGH)31,32 and microsatellite/loss of heterozygosity studies.33,34 The most frequent genetic alterations identified across the spectrum of thymomas occur on chromosome 6p21.3 (major histocompatibility complex locus) and 6q25.2 to 25.3.31,33,35 Interestingly, the identification of 6q25 as a common genetic alteration across all histologic subtypes suggests the presence of an as yet undefined tumor suppressor gene which may be absent in the more aggressive phenotypes. In TC, CGH has demonstrated frequent copy number gains of 1q, 17q, and 18 and loss of 3p, 6, 16q, and 17p.31,32 Although TCs are a distinct entity in the WHO classification scheme, they do share some similarities with type B3 thymoma, most notably gain of 1q and loss of chromosome 6.31 The frequency, extent, and number of genomic aberrations increases from type A thymoma to TC.32
Transgenic mice expressing SV40 large T-antigen and small t-antigen under L-pyruvate kinase promoter develop TETs,36 suggesting the importance of RB and p53 during TET development. Variable expression of p53 has been reported in TETs21,22,26–30,37–44 with p53 being more frequently expressed in invasive thymomas and TCs. p53 expression is a poor prognostic marker in TETs,28,29,39,40 and inactivating mutations have been described with variable frequency.26,29,30,32,38,41 Both RB and P16INK4 have been implicated in TET; P16INK4, through inhibition of CDK4 and CDK6, prevents RB phosphorylation leading to G1→S block. Methylation of the P16INK4 promoter has been observed in 3% to 13% of TETs, which correlates with p16 downregulation albeit in a minority of cases.30,45 No P16INK4 mutations have been described in TETs to date, but deletion of the gene locus is related to invasive phenotypes in rat animal models46 (Table 2).
Autoimmune Disease and the Autoimmune Regulator Gene
Failure to induce self-tolerance may be a key factor leading to the export of autoreactive T-cell clones that subsequently circulate systemically and lead to autoantibody development in the spleen, lymph nodes, and bone marrow.47 Approximately 40% of patients with thymoma develop an autoimmune condition, half of which will be myasthenia gravis.48 Autoimmune regulator gene (AIRE) is important for ectopic expression of peripheral self-antigen and, as such, it is critical for central thymic T-cell education and deletion of autoreactive clones.47,49 This regulatory role is achieved through the activation of nuclear transcription of ectopic tissue–specific autologous antigens in medullary epithelial cells and in their presentation to medullary thymocytes; peptide recognition by self-reactive thymocytes causes their negative selection.47,50 The molecular basis underlying the defective expression of AIRE in most thymomas is enigmatic; however, it has been identified as the gene responsible for autoimmune polyendocrinopathy syndrome type 1, candidiasis, and ectodermal dystrophy (APECED), a rare autosomal recessive disorder characterized by organ-specific autoimmunity affecting the parathyroid and adrenal glands.51,52 The AIRE gene is located on chromosome 21q22.3 and encodes a 58-kDa protein involved in nuclear transcription.53 Defective expression of AIRE in thymoma may play a crucial role in the pathogenesis of autoimmune diseases by favoring the development of self-reactive T-cell clones within the thymus leading to the export of a CD4+ progeny without tolerogenic myoid cells that are actively autoimmunized against autoantigens (eg, acetylcholine receptors). High rates of autoantibodies to cytokines have been described in patients with thymic malignancies. A luciferase immunoprecipitation system panel for autoantibodies against 39 different cytokines54 showed a statistically increased but highly heterogeneous immunoreactivity against 16 of the 39 cytokines in patients with thymoma. The suggestion that AIRE expression may be altered in thymic tumors provides a fascinating link between autoimmunity and malignancy, as demonstrated by patients with thymomas.
Chemotherapy
Chemotherapy is offered to patients with advanced thymomas (stages III to IV) on the basis of evidence from small phase II studies in either the neoadjuvant or refractory/recurrent disease setting. No validated biomarkers exist to predict response to chemotherapy.55,56 Here we discuss treatment options for patients with inoperable refractory or recurrent disease.
Platinum-based combinations.
Cisplatin-based chemotherapy remains the standard of care (Table 3). In the early 1990s, the ADOC regimen (doxorubicin, cisplatin, vincristine and cyclophosphamide) was tested in first-line treatment with an overall response rate (ORR) of 92% and a median survival (MS) of 15 months.58 An intergroup trial57 investigated a three-drug combination—cisplatin, doxorubicin and cyclophosphamide (PAC)—with an ORR of 50% and an MS of 38 months. Another three-drug regimen consists of cisplatin, doxorubicin, and methylprednisone (CAMP). This regimen was administered in the neoadjuvant setting and demonstrated an ORR of 93% with a 5-year overall survival (OS) of 81% in a small phase II trial of 17 patients.66 The European Organisation for Research and Treatment of Cancer (EORTC) investigated cisplatin and etoposide in 16 patients who demonstrated an ORR of 56% and an MS of 4.3 years.59 The addition of ifosfamide to this doublet (VIP) demonstrated a partial response rate of 32% and an MS of 32 months.61 Carboplatin and paclitaxel demonstrated a modest clinical benefit in patients with thymic malignancies with an ORR of 33% and progression-free survival of 19.8 months and 6.2 months for thymomas and TCs, respectively.63 In 2011, the PAC regimen is considered standard first-line chemotherapy in most centers.
Table 3.
Major Prospective Chemotherapy Trials in Extensive-Stage Inoperable Thymoma
| Reference | Regimen | Stage | No. of Patients | CR + PR (%) | Median Survival (years) |
|---|---|---|---|---|---|
| Anthracycline-containing regimens | |||||
| Loehrer et al57 | Cisplatin, doxorubicin, cyclophosphamide | IV | 30 | 50 | 3.2 |
| Fornasiero et al58 | Doxorubicin, cisplatin, vincristine and cyclophosphamide | III/IV | 32 | 90 | 1.25 |
| Non–anthracycline- containing regimens | |||||
| Giaccone et al59 | Cisplatin and etoposide | IV | 16 | 56 | 4.3 |
| Highley et al60 | Ifosfamide | III/IV | 13 | 46 | N/R |
| Loehrer et al61 | Etoposide, ifosfamide, cisplatin | IV | 28 | 32 | 2.5 |
| Grassin et al62 | Etoposide, ifosfamide, cisplatin | IV | 16 | 25 | N/R |
| Lemma at al63 | Carboplatin, paclitaxel | IV | 44 | 35 | N/R |
| Loehrer et al64,65 | Pemetrexed | IV | 23 | 17 | 2.4 |
Abbreviations: CR, complete response; N/R, not recorded; PR, partial response.
Pemetrexed.
A phase II study64 evaluated pemetrexed 500 mg/m2 every 3 weeks for a maximum of six cycles in 27 patients with previously treated unresectable stage IVA (n = 16) or stage IVB (n = 11) recurrent thymic malignancies. In 23 fully evaluable patients, two complete responses (CRs) and two partial responses (PRs) were noted. All four responding patients had stage IVA thymoma. The median time to progression (TTP) for all patients was 45 weeks (thymomas, 45.4 weeks v TC, 5.1 weeks), and the MS was 29 months for all patients.65 Overall, single-agent pemetrexed is an active agent in a heavily pretreated population of patients with recurrent thymomas, but it has limited activity in TC.
Targeted Therapy
Epidermal growth factor receptor signaling pathway.
Somatic activating epidermal growth factor receptor (EGFR) mutations are extremely rare in thymic malignancies except for a few isolated case reports in Asian patients.67,68 EGFR gene amplification by fluorescent in situ hybridization occurs in approximately 20% of thymomas, in particular type B3 thymomas, and in TCs and is associated with more advanced stage and capsule invasion.18 EGFR protein overexpression by immunohistochemistry (IHC) is present in 46% to 85% of thymomas19,20; however, no clear association with clinicopathologic features has been determined. Twenty-six previously treated patients with metastatic thymoma (n = 19) or TC (n = 7) received gefitinib 250 mg orally daily (Table 4). 69 There were no CRs, one PR lasting 5 months, and 14 patients with stable disease (SD). Median TTP was 4 months. None of the patients analyzed (including one PR) had evidence of EGFR or KRAS mutations. A phase II trial studied the combination of erlotinib and bevacizumab in 18 pretreated patients (thymomas, 11; TCs, 7) with progressive TETs. Erlotinib 150 mg orally once daily and bevacizumab 15 mg/kg intravenously was repeated every 21 days.70 Eleven patients achieved SD, and seven had progressive disease. The MS has not been reported, but it appears that this combination has limited activity. Cetuximab has been reported to have activity in two case reports: one patient overexpressed EGFR by IHC and the other did not.74,75 Ras mutations have been detected in three of 63 thymic tumors analyzed: one TC (KRAS mutation, G12V), one type B2 thymoma (KRAS mutation, G12A), and one type A thymoma (HRAS, G13V), respectively.32 No occurrences of HER2 gene amplification by fluorescent in situ hybridization have been detected.22 Overall results from EGFR tyrosine kinase inhibitors have been disappointing in patients with thymic malignancies and, at present, these therapies cannot be recommended.
Table 4.
Targeted Therapies in Thymic Malignancies
| Reference | Drug | No. of Patients | Thymoma | Thymic Carcinoma | RR | SD | PD |
|---|---|---|---|---|---|---|---|
| Kurup et al63 | Gefitinib | 26 | 19 | 7 | 1 | 14 | — |
| Bedano et al70 | Erlotinib + bevacizumab | 18 | 11 | 7 | 0 | 11 | 7 |
| Giaccone et al71 | Imatinib | 7 | 2 | 5 | 0 | 2 | 5 |
| Salter et al72 | Imatinib | 11 | 0 | 11 | 0 | 3 | 4 |
| Giaccone et al73 | Belinostat | 41 | 25 | 16 | 2 | 25 | 13 |
Abbreviations: PD, progressive disease; RR, response rate; SD, stable disease.
Angiogenesis inhibition.
VEGF-A, VEGFR-1, and VEGFR-2 are overexpressed in thymoma and TC,76,77 and microvessel density and VEGF expression levels have been shown to correlate with both tumor invasion and clinical stage.78 However, there are limited data regarding the efficacy of angiogenesis inhibitors in thymic malignancies. A phase I trial79 reports a PR to the combination of aflibercept and docetaxel in a patient with thymoma. Sorafenib and sunitinib have not been assessed in formal clinical trial settings, and case reports have described evidence of the activity of these agents in TC. A PR has been reported in a patient receiving sorafenib who had a missense mutation in exon 17 (D820E) of the c-KIT gene,80 and a second patient has demonstrated prolonged SD (> 9 months) in a nonmutated but high IHC expressing tumor for KIT, p53, and VEGF.81 Of four patients with TC who were treated with sunitinib, three had PRs (2 to 18+ months) and one had prolonged SD (22 months), with OS ranging from 4 to 40+ months.82 In a phase I trial of SU14813,83 a multitargeted tyrosine kinase inhibitor (VEGFRs, PDGFRs, KIT, and FLT-3), four patients with thymoma were treated and two had PRs with progression-free survival of 15.3 and 9.0 months, respectively. From the limited data to date, it appears that multikinase inhibitors may be of interest in targeting angiogenesis with some benefit, especially in TC.
c-KIT signaling pathway.
Thymic malignancies have shown c-KIT positivity by IHC in 73% to 86% of TCs, but there is limited to no overexpression in thymoma, with approximately 2% of patients demonstrating positivity.20,24 Despite a high frequency of KIT expression in TC, the rate of KIT mutations remains low at 9%. From the observed mutations, the two V560 deletions32,84 and the L576P substitution67 are sensitive to imatinib,85 the D820E to nilotinib, and H697Y to sorafenib.86 A small phase II trial evaluated imatinib in seven patients with either type B3 thymomas (two patients) or TC (five patients). c-KIT expression was found in one of four samples by IHC; no mutations in c-KIT or PDGFRA genes were demonstrated in three tumors examined, and no responses were seen.71 A second trial72 investigated imatinib in 11 patients with previously treated, advanced, unresectable TC. IHC expression was confirmed for c-KIT in nine patients and PDGFR in two patients, and there were no objective responses in this study. Future studies should consider selecting patients on the basis of the presence of c-KIT mutations, although they are rare. Second-generation KIT inhibitors currently undergoing clinical trial evaluation would be preferable because KIT mutations in TC are not uniformly sensitive to imatinib.84
Histone deacetylase inhibitors.
Belinostat is a pan–histone deacetylase inhibitor. In a phase I study87 of this agent, a patient with thymoma had some tumor reduction that lasted for 17 months on treatment. We performed a phase II trial of belinostat, given by intravenous infusion at 1 g/m2 on days 1 through 5 of a 21-day cycle until progression or intolerance.73 In total, 41 patients were enrolled, 25 with thymoma and 16 with TC. Treatment was well tolerated, with nausea, vomiting, and fatigue as the major adverse effects. There were two PRs in patients with thymoma, 25 patients with SD, and 13 with progressive disease. There were no responses in TC. TTP and OS were 174 days and 575 days, respectively. This study is one of the largest phase II trials of a targeted agent performed in thymic malignancies to date. An ongoing phase II trial is investigating belinostat in combination with PAC as first-line therapy in patients with advanced thymic malignancies (NCT 01100944).
Octreotide.
Somatostatin (SST) receptors belong to a superfamily of G-protein–coupled receptors with several transmembrane-spanning domains.88 SST receptors are found in TET tissue, and SST receptor scintigraphy that uses radiolabeled octreotide can be used to identify locally advanced and metastatic disease.89 Indium-111 diethylenetriamine pentaacetic acid (111In-labeled DTPA)-octreotide scintigraphy has been evaluated in 18 patients with thymic masses.90 Uptake was increased in patients with thymoma compared with patients who had thymic hyperplasia or other benign disorders. Small metastatic pleural or pericardial metastases can be missed, but lesions larger than 1.5 cm may be detected. Octreotide, an octapeptide SST analog, has high affinity for a selective SST subtype (SST2) receptor and has been demonstrated to have an in vitro inhibitory effect in thymic epithelial cells, perhaps through blockage or inhibition of the insulin-like growth factor 1 (IGF-1) and endothelial growth factor.91 It has also been suggested that an interaction between thymic tissue and SST has an impact on T-lymphocyte development through paracrine mechanisms.92 The Eastern Cooperative Oncology Group (ECOG) conducted a phase II trial5 of octreotide with or without prednisone in patients with advanced, unresectable, octreotide scan–positive thymic malignancies. Thirty-eight patients (32 thymomas, five TCs, and one thymic carcinoid) received octreotide 0.5 mg subcutaneously three times daily for up to 1 year. Two CRs (5.3%) and 10 PRs (25%) were observed (four PRs with octreotide alone). None of the six patients without pure thymoma responded. The 1- and 2-year survival rates were 86.6% and 75.7%, respectively, indicating that octreotide alone has modest activity in patients with octreotide scan–positive thymomas but can be considered for treatment of recurrent disease in selected patients. Prednisone improves the overall response rate but is associated with increased toxicity. An ongoing clinical trial is currently studying the efficacy of octreotide in patients with primary inoperable thymomas.
IGF-1 receptor signaling pathway.
Increased IGF-1 receptor (IGF-1R) expression in TET has a poor prognostic value for OS93 and in primary tumors for TTP.94 We evaluated IGF-1R expression by IHC in 132 operable thymic tumors and demonstrated that 22 (20%) of 111 samples were positive.94 In this study, IGF-1R was less common in type A, AB, and B1 thymomas compared with B2, B3, and C subtypes (3.4% v 37.2%; P < .001). A second study93 evaluated 63 thymic tumors and confirmed a higher degree of IGF-1R expression by IHC in TC versus thymoma (83% v 43%, respectively; P = .039). Figitumumab, an anti–IGF-1R antibody demonstrated clinical activity in a patient with refractory thymoma95 lasting more than 1 year. We are currently performing a phase II trial (NCT00965250) investigating cixutumumab (IMC-A12; ImClone Systems, Bridgewater, NJ), an IGF-1R monoclonal antibody, in advanced and refractory thymoma and TC following at least one platinum-containing chemotherapy treatment. Cixutumumab 20 mg/kg is administered on day 1 of a 21-day cycle until progression and treatment is well tolerated.96 At present, no major signs of activity have been observed in TC trials in which accrual has been halted, whereas major responses have been seen in patients with thymoma in trials in which accrual is still ongoing.
Tropomyosin receptor kinase A and cyclin-dependent kinase A inhibitor.
The tropomyosin receptor kinase (Trk) family comprises three isoforms (TrkA, TrkB, and TrkC), which have an intrinsic tyrosine kinase activity. TrkA is the specific receptor for nerve growth factor; TrkB for brain-derived neurotrophic factor, NT-4, and NT-5; and TrkC for NT-3.97 Decreased expression of cell-cycle proteins p21 and p27, both of which are natural inhibitors of cyclin-dependent kinases (CDKs), predict for a poor response to neoadjuvant chemotherapy in invasive thymoma.98 TrkA activation promotes tumor growth, probably through MAPK pathway activation. The expression of Trk receptors was evaluated in 99 patients with TET by using IHC staining.99 All tumors except one TC demonstrated cytoplasmic TrkA immunostaining, and no tumors showed TrkB or TrkC immunoreactivity. Our laboratory has analyzed 60 paraffin-embedded samples from patients diagnosed with TET. We found copy number gain of TrkA in 45% of all thymoma samples analyzed and 72% of TC samples (unpublished data). Another group has shown that CDK proteins that control the cell cycle G1→S phase transition may be altered through p16INK4 loss in thymoma.30 On the basis of these results, we believe TrkA could potentially be an important therapeutic target in thymic tumors. The pyrazoloquinazoline PHA-848125-AC is an oral compound that is a potent inhibitor of the CDK2/cyclin A complex and TrkA. Preliminary results from a phase I trial demonstrated PRs in two of three patients with thymic malignancies (type B3 and type C). Two phase II studies (NCT01011439 and NCT01301391) in advanced TC are currently investigating PHA-848125-AC. The first is an international study in patients with TC for whom one line of prior treatment has failed, and the second is a single-center study by the National Cancer Institute in patients with type B3 and TC with progressive disease after more than one line of therapy.
Src inhibitors.
The Src family of tyrosine kinases and its ligands are considered important components in thymocyte development.100 A phase II trial investigated saracatinib (AZD0530), a small-molecule inhibitor of Src in patients with previously treated advanced thymic malignancies. A total of 21 patients (14 thymomas and seven TCs) received 175 mg of saracatinib daily. The trial was terminated because of lack of clinical activity.100
DISCUSSION
Although surgery remains the mainstay treatment in thymic malignancies, tumors are often large and require preoperative chemotherapy. Despite radical resection, recurrence is not unusual, especially in more aggressive histologic tumor types. Preliminary data suggest that the expression of some biologic markers may be of prognostic or predictive value.101,102
A better understanding of the biology of these tumors through the integration of results obtained with advanced technologies, such as array CGH, expression array analysis, and next-generation sequencing, will likely help identify potential markers for prognosis and treatment of advanced cases (Figs 1and 2). Gene expression profiling and genomic clustering studies performed to date indicate that thymic tumors as defined histologically by the current 2004 WHO classification do have different molecular features.32 Type B2 thymoma, which has a distinctly more lymphocytic component can be separated from the other subgroups in which epithelial cells predominate. Genomic profiling also distinguishes type B3 thymoma and TC from type A and type B2 thymomas. The presence of KIT mutations in TC further strengthens the notion of subtypes existing among the spectrum of thymic malignancies.
Fig 1.

Summary of array comparative genomic hybridization results for autosomic chromosomes of two patients with thymic epithelial tumors. Regions highlighted in red represent copy number (CN) losses and regions highlighted in green represent CN gains. Blue dots represent the array probes, and their respective positions aligned to the chromosomes are shown on the left. The divergences from the median axis of the blue dots indicate regions of CN aberrations. Tumor DNA was cohybridized with commercially available normal reference DNA on Agilent 180K Sure Print array (Agilent, Palo Alto, CA). Data were collected from the Agilent microarray scanner (Agilent) and analyzed by using Nexus 5.1 software (Biodiscovery, La Jolla, CA). (A) Type A thymoma exhibits a much less aberrant karyotype than (B) thymic carcinoma.
Fig 2.
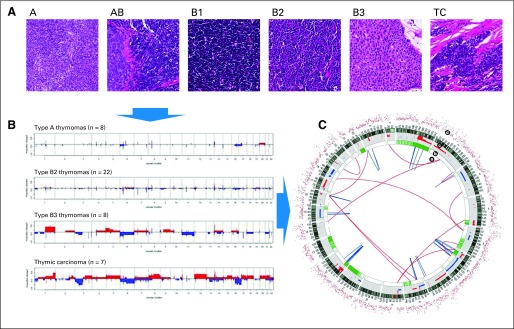
(A) Thymic epithelial tumors are classified according to their histologic appearance into type A, AB, B1, B2, B3, and TC (thymic carcinoma) groups. (B) Correlation of thymic epithelia histotypes with specific genomic abnormalities. Comparative genomic hybridization diagram is derived from Girard et al,32 reprinted by permission from Clinical Cancer Research. Red represents the frequency of copy number (CN) gain, and blue represents the frequency of CN loss for eight type A thymomas, 22 type B2 thymomas, eight type B3 thymomas, and seven thymic carcinomas. (C) Modified chromosome ideograms (Reprinted by permission from Macmillan Publishers Ltd: Lee et al: Nature 465:473-477, 2010) depict complete genome sequencing from a lung adenocarcinoma. Next-generation sequencing technology allows genome-wide identification of single nucleotide mutations (d, red dots), CN aberrations (c, red, regions of CN gain; blue, regions of CN loss), loss of heterozygosity and allelic imbalance (b, green), and somatic structural variations (a, red lines, interchromosomal variations; blue lines, intrachromosomal variations).
The rarity of these tumors is a major limitation in researching thymic malignancies and, for many years, it has prevented us from performing large randomized clinical trials. Rare diseases may, however, lend themselves perfectly to investigation via molecular profiling–directed therapies. Individual case reports that highlight advanced thymic tumors responding to targeted agents suggest that thymic malignancies may comprise clinically relevant subsets that can be defined at the molecular level. We are currently conducting a molecular profiling study (NCT01306045) in which patients with thymic malignancies have a repeat biopsy to enable genome-wide sequencing. Therapy is assigned depending on the molecular profile of the tumor. It is hoped that this protocol may herald a new paradigm of therapies for thymic malignancies and will facilitate a rapid translation of preclinical findings to the clinic.
Footnotes
Supported by the National Cancer Institute (NCI), National Institutes of Health (NIH) and by the NIH Intramural Research Program, Center for Cancer Research, NCI.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organization imply endorsement by the US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Ronan J. Kelly, Iacopo Petrini, Yisong Wang, Giuseppe Giaccone
Data analysis and interpretation: Ronan J. Kelly, Iacopo Petrini, Yisong Wang, Giuseppe Giaccone
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Margaritora S, Cesario A, Cusumano G, et al. Thirty-five-year follow-up analysis of clinical and pathologic outcomes of thymoma surgery. Ann Thorac Surg. 2010;89:245–252. doi: 10.1016/j.athoracsur.2009.08.074. discussion 252. [DOI] [PubMed] [Google Scholar]
- 2.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: Demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–551. doi: 10.1002/ijc.11099. [DOI] [PubMed] [Google Scholar]
- 3.Evoli A, Punzi C, Marsili F, et al. Extrathymic malignancies in patients with thymoma. Ann Oncol. 2004;15:692–693. doi: 10.1093/annonc/mdh155. [DOI] [PubMed] [Google Scholar]
- 4.Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer. 2001;92:2406–2411. doi: 10.1002/1097-0142(20011101)92:9<2406::aid-cncr1589>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Loehrer PJ, Sr, Wang W, Johnson DH, et al. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: An Eastern Cooperative Oncology Group Phase II Trial. J Clin Oncol. 2004;22:293–299. doi: 10.1200/JCO.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5(suppl 4):S260–S265. doi: 10.1097/JTO.0b013e3181f1f62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors: Part 1. Tumors of the anterior mediastinum. Chest. 1997;112:511–522. doi: 10.1378/chest.112.2.511. [DOI] [PubMed] [Google Scholar]
- 8.Phillips LH, 2nd, Torner JC, Anderson MS, et al. The epidemiology of myasthenia gravis in central and western Virginia. Neurology. 1992;42:1888–1893. doi: 10.1212/wnl.42.10.1888. [DOI] [PubMed] [Google Scholar]
- 9.Gadalla SM, Rajan A, Pfeiffer R, et al. A population-based assessment of mortality and morbidity patterns among patients with thymoma. Int J Cancer. 2011;128:2688–2694. doi: 10.1002/ijc.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vukmanović S, Bevan MJ, Hogquist KA. The specificity of positive selection: MHC and peptides. Immunol Rev. 1993;135:51–66. doi: 10.1111/j.1600-065x.1993.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 11.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Koga K, Matsuno Y, Noguchi M, et al. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol Int. 1994;44:359–367. doi: 10.1111/j.1440-1827.1994.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuo TT. Tumours of the thymus. In: Travis WD, Brambilla E, Muller-Hermelink HK, et al., editors. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004. pp. 146–248. [Google Scholar]
- 14.Moran CA, Suster S. On the histologic heterogeneity of thymic epithelial neoplasms: Impact of sampling in subtyping and classification of thymomas. Am J Clin Pathol. 2000;114:760–766. doi: 10.1309/CYJH-9RXM-P2PK-120J. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Marx A, Chen WH, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: A clinicopathologic study of 200 thymoma cases from China. Cancer. 2002;95:420–429. doi: 10.1002/cncr.10665. [DOI] [PubMed] [Google Scholar]
- 16.Herens C, Radermecker M, Servais A, et al. Deletion (6)(p22p25) is a recurrent anomaly of thymoma: Report of a second case and review of the literature. Cancer Genet Cytogenet. 2003;146:66–69. doi: 10.1016/s0165-4608(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn E, Wistuba II. Molecular pathology of thymic epithelial neoplasms. Hematol Oncol Clin North Am. 2008;22:443–455. doi: 10.1016/j.hoc.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Ionescu DN, Sasatomi E, Cieply K, et al. Protein expression and gene amplification of epidermal growth factor receptor in thymomas. Cancer. 2005;103:630–636. doi: 10.1002/cncr.20811. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki E, Sasaki H, Kawano O, et al. Expression and mutation statuses of epidermal growth factor receptor in thymic epithelial tumors. Jpn J Clin Oncol. 2006;36:351–356. doi: 10.1093/jjco/hyl028. [DOI] [PubMed] [Google Scholar]
- 20.Henley JD, Cummings OW, Loehrer PJ., Sr Tyrosine kinase receptor expression in thymomas. J Cancer Res Clin Oncol. 2004;130:222–224. doi: 10.1007/s00432-004-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi Y, Ishii N, Obayashi C, et al. Thymoma: Tumour type related to expression of epidermal growth factor (EGF), EGF-receptor, p53, v-erb B and ras p21. Virchows Arch. 1995;426:43–50. doi: 10.1007/BF00194697. [DOI] [PubMed] [Google Scholar]
- 22.Pan CC, Chen PC, Wang LS, et al. Expression of apoptosis-related markers and HER-2/neu in thymic epithelial tumours. Histopathology. 2003;43:165–172. doi: 10.1046/j.1365-2559.2003.01663.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa K, Matsuno Y, Kunitoh H, et al. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest. 2005;128:140–144. doi: 10.1378/chest.128.1.140. [DOI] [PubMed] [Google Scholar]
- 24.Pan CC, Chen PC, Chiang H. KIT (CD117) is frequently overexpressed in thymic carcinomas but is absent in thymomas. J Pathol. 2004;202:375–381. doi: 10.1002/path.1514. [DOI] [PubMed] [Google Scholar]
- 25.Penzel R, Hoegel J, Schmitz W, et al. Clusters of chromosomal imbalances in thymic epithelial tumours are associated with the WHO classification and the staging system according to Masaoka. Int J Cancer. 2003;105:494–498. doi: 10.1002/ijc.11101. [DOI] [PubMed] [Google Scholar]
- 26.Tateyama H, Eimoto T, Tada T, et al. p53 protein expression and p53 gene mutation in thymic epithelial tumors: An immunohistochemical and DNA sequencing study. Am J Clin Pathol. 1995;104:375–381. doi: 10.1093/ajcp/104.4.375. [DOI] [PubMed] [Google Scholar]
- 27.Chen FF, Yan JJ, Jin YT, et al. Detection of bcl-2 and p53 in thymoma: Expression of bcl-2 as a reliable marker of tumor aggressiveness. Hum Pathol. 1996;27:1089–1092. doi: 10.1016/s0046-8177(96)90289-0. [DOI] [PubMed] [Google Scholar]
- 28.Pich A, Chiarle R, Chiusa L, et al. p53 expression and proliferative activity predict survival in non-invasive thymomas. Int J Cancer. 1996;69:180–183. doi: 10.1002/(SICI)1097-0215(19960621)69:3<180::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 29.Weirich G, Schneider P, Fellbaum C, et al. p53 alterations in thymic epithelial tumours. Virchows Arch. 1997;431:17–23. doi: 10.1007/s004280050064. [DOI] [PubMed] [Google Scholar]
- 30.Hirabayashi H, Fujii Y, Sakaguchi M, et al. P16INK4, pRB, p53 and cyclin D1 expression and hypermethylation of CDKN2 gene in thymoma and thymic carcinoma. Int J Cancer. 1997;73:639–644. doi: 10.1002/(sici)1097-0215(19971127)73:5<639::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 31.Zettl A, Ströbel P, Wagner K, et al. Recurrent genetic aberrations in thymoma and thymic carcinoma. Am J Pathol. 2000;157:257–266. doi: 10.1016/S0002-9440(10)64536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girard N, Shen R, Guo T, et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res. 2009;15:6790–6799. doi: 10.1158/1078-0432.CCR-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue M, Marx A, Zettl A, et al. Chromosome 6 suffers frequent and multiple aberrations in thymoma. Am J Pathol. 2002;161:1507–1513. doi: 10.1016/S0002-9440(10)64426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou R, Zettl A, Ströbel P, et al. Thymic epithelial tumors can develop along two different pathogenetic pathways. Am J Pathol. 2001;159:1853–1860. doi: 10.1016/s0002-9440(10)63031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue M, Starostik P, Zettl A, et al. Correlating genetic aberrations with World Health Organization-defined histology and stage across the spectrum of thymomas. Cancer Res. 2003;63:3708–3715. [PubMed] [Google Scholar]
- 36.Nabarra B, Pontoux C, Godard C, et al. Neoplastic transformation and angiogenesis in the thymus of transgenic mice expressing SV40 T and t antigen under an L-pyruvate kinase promoter (SV12 mice) Int J Exp Pathol. 2005;86:397–413. doi: 10.1111/j.0959-9673.2005.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stefanaki K, Rontogianni D, Kouvidou CH, et al. Expression of p53, mdm2, p21/waf1 and bcl-2 proteins in thymomas. Histopathology. 1997;30:549–555. doi: 10.1046/j.1365-2559.1997.5730805.x. [DOI] [PubMed] [Google Scholar]
- 38.Oyama T, Osaki T, Mitsudomi T, et al. p53 alteration, proliferating cell nuclear antigen, and nucleolar organizer regions in thymic epithelial tumors. Int J Mol Med. 1998;1:823–826. doi: 10.3892/ijmm.1.5.823. [DOI] [PubMed] [Google Scholar]
- 39.Mineo TC, Ambrogi V, Mineo D, et al. Long-term disease-free survival of patients with radically resected thymomas: Relevance of cell-cycle protein expression. Cancer. 2005;104:2063–2071. doi: 10.1002/cncr.21433. [DOI] [PubMed] [Google Scholar]
- 40.Khoury T, Arshad A, Bogner P, et al. Apoptosis-related (survivin, Bcl-2), tumor suppressor gene (p53), proliferation (Ki-67), and non-receptor tyrosine kinase (Src) markers expression and correlation with clinicopathologic variables in 60 thymic neoplasms. Chest. 2009;136:220–228. doi: 10.1378/chest.08-2482. [DOI] [PubMed] [Google Scholar]
- 41.Hino N, Kondo K, Miyoshi T, et al. High frequency of p53 protein expression in thymic carcinoma but not in thymoma. Br J Cancer. 1997;76:1361–1366. doi: 10.1038/bjc.1997.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilhus NE, Jones M, Turley H, et al. Oncogene proteins and proliferation antigens in thymomas: Increased expression of epidermal growth factor receptor and Ki67 antigen. J Clin Pathol. 1995;48:447–455. doi: 10.1136/jcp.48.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engel P, Francis D, Graem N. Expression of bcl-2 in fetal thymus, thymomas and thymic carcinomas: Association with p53 expression and review of the literature. APMIS. 1998;106:449–455. doi: 10.1111/j.1699-0463.1998.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 44.Comin CE, Messerini L, Novelli L, et al. KI-67 antigen expression predicts survival and correlates with histologic subtype in the WHO classification of thymic epithelial tumors. Int J Surg Pathol. 2004;12:395–400. doi: 10.1177/106689690401200412. [DOI] [PubMed] [Google Scholar]
- 45.Hirose Y, Kondo K, Takizawa H, et al. Aberrant methylation of tumour-related genes in thymic epithelial tumours. Lung Cancer. 2009;64:155–159. doi: 10.1016/j.lungcan.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Tsuji T, Ikeda H, Tsuchikawa T, et al. Malignant transformation of thymoma in recipient rats by heterotopic thymus transplantation from HTLV-I transgenic rats. Lab Invest. 2005;85:851–861. doi: 10.1038/labinvest.3700292. [DOI] [PubMed] [Google Scholar]
- 47.Scarpino S, Di Napoli A, Stoppacciaro A, et al. Expression of autoimmune regulator gene (AIRE) and T regulatory cells in human thymomas. Clin Exp Immunol. 2007;149:504–512. doi: 10.1111/j.1365-2249.2007.03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okumura M, Fujii Y, Shiono H, et al. Immunological function of thymoma and pathogenesis of paraneoplastic myasthenia gravis. Gen Thorac Cardiovasc Surg. 2008;56:143–150. doi: 10.1007/s11748-007-0185-8. [DOI] [PubMed] [Google Scholar]
- 49.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 50.Anderson MS, Venanzi ES, Chen Z, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 51.Finnish-German APECED Consortium. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 52.Kekäläinen E, Tuovinen H, Joensuu J, et al. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol. 2007;178:1208–1215. doi: 10.4049/jimmunol.178.2.1208. [DOI] [PubMed] [Google Scholar]
- 53.Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 54.Burbelo PD, Browne SK, Sampaio EP, et al. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 2010;116:4848–4858. doi: 10.1182/blood-2010-05-286161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu JM, Wang LS, Huang MH, et al. Topoisomerase 2alpha plays a pivotal role in the tumor biology of stage IV thymic neoplasia. Cancer. 2007;109:502–509. doi: 10.1002/cncr.22404. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki H, Fukai I, Kiriyama M, et al. Thymidylate synthase and dihydropyrimidine dehydrogenase mRNA levels in thymoma. Surg Today. 2003;33:83–88. doi: 10.1007/s005950300018. [DOI] [PubMed] [Google Scholar]
- 57.Loehrer PJ, Sr, Kim K, Aisner SC, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: Final results of an intergroup trial—The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group. J Clin Oncol. 1994;12:1164–1168. doi: 10.1200/JCO.1994.12.6.1164. [DOI] [PubMed] [Google Scholar]
- 58.Fornasiero A, Daniele O, Ghiotto C, et al. Chemotherapy for invasive thymoma: A 13-year experience. Cancer. 1991;68:30–33. doi: 10.1002/1097-0142(19910701)68:1<30::aid-cncr2820680106>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 59.Giaccone G, Ardizzoni A, Kirkpatrick A, et al. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma: A phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 1996;14:814–820. doi: 10.1200/JCO.1996.14.3.814. [DOI] [PubMed] [Google Scholar]
- 60.Highley MS, Underhill CR, Parnis FX, et al. Treatment of invasive thymoma with single-agent ifosfamide. J Clin Oncol. 1999;17:2737–2744. doi: 10.1200/JCO.1999.17.9.2737. [DOI] [PubMed] [Google Scholar]
- 61.Loehrer PJ, Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: An intergroup trial. Cancer. 2001;91:2010–2015. [PubMed] [Google Scholar]
- 62.Grassin F, Paleiron N, Andre M, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma. A French experience. J Thorac Oncol. 2010;5:893–897. doi: 10.1097/jto.0b013e3181db3dee. [DOI] [PubMed] [Google Scholar]
- 63.Lemma GL, Loehrer PJ, Lee JW, et al. A phase II study of carboplatin plus paclitaxel in advanced thymoma or thymic carcinoma: E1C99. J Clin Oncol. 2008;26(suppl; abstr 8018):428s. doi: 10.1200/JCO.2010.32.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loehrer PJ, Yiannoutsos CT, Dropcho S, et al. A phase II trial of pemetrexed in patients with recurrent thymoma or thymic carcinoma. J Clin Oncol. 2006;24(suppl; abstr 7079):383s. [Google Scholar]
- 65.Schmitt J, Loehrer PJ., Sr The role of chemotherapy in advanced thymoma. J Thorac Oncol. 2010;5(suppl 4):S357–S360. doi: 10.1097/JTO.0b013e3181f21129. [DOI] [PubMed] [Google Scholar]
- 66.Yokoi K, Matsuguma H, Nakahara R, et al. Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. J Thorac Oncol. 2007;2:73–78. doi: 10.1097/JTO.0b013e31802bafc8. [DOI] [PubMed] [Google Scholar]
- 67.Yoh K, Nishiwaki Y, Ishii G, et al. Mutational status of EGFR and KIT in thymoma and thymic carcinoma. Lung Cancer. 2008;62:316–320. doi: 10.1016/j.lungcan.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 68.Yamaguchi H, Soda H, Kitazaki T, et al. Thymic carcinoma with epidermal growth factor receptor gene mutations. Lung Cancer. 2006;52:261–262. doi: 10.1016/j.lungcan.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Kurup A, Burns M, Dropcho S, et al. Phase II study of gefitinib treatment in advanced thymic malignancies. J Clin Oncol. 2005;23(suppl; abstr 7068):381s. [Google Scholar]
- 70.Bedano PM, Perkins S, Burns M, et al. A phase II trial of erlotinib plus bevacizumab in patients with recurrent thymoma or thymic carcinoma. J Clin Oncol. 2008;26(suppl; abstr 19087):713s. [Google Scholar]
- 71.Giaccone G, Rajan A, Ruijter R, et al. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol. 2009;4:1270–1273. doi: 10.1097/JTO.0b013e3181b6be57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salter JT, Lewis D, Yiannoutsos PJ, et al. Imatinib for the treatment of thymic carcinoma. J Clin Oncol. 2008;26(suppl; abstr 8116):452s. [Google Scholar]
- 73.Giaccone G, Rajan A, Berman A, et al. Phase II study of belinostat in patients with recurrent or refractory advanced thymic epithelial tumors. J Clin Oncol. 2011;29:2052–2059. doi: 10.1200/JCO.2010.32.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farina G, Garassino MC, Gambacorta M, et al. Response of thymoma to cetuximab. Lancet Oncol. 2007;8:449–450. doi: 10.1016/S1470-2045(07)70141-9. [DOI] [PubMed] [Google Scholar]
- 75.Palmieri G, Marino M, Salvatore M, et al. Cetuximab is an active treatment of metastatic and chemorefractory thymoma. Front Biosci. 2007;12:757–761. doi: 10.2741/2098. [DOI] [PubMed] [Google Scholar]
- 76.Cimpean AM, Raica M, Encica S, et al. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat. 2008;190:238–245. doi: 10.1016/j.aanat.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Sasaki H, Yukiue H, Kobayashi Y, et al. Elevated serum vascular endothelial growth factor and basic fibroblast growth factor levels in patients with thymic epithelial neoplasms. Surg Today. 2001;31:1038–1040. doi: 10.1007/s005950170021. [DOI] [PubMed] [Google Scholar]
- 78.Tomita M, Matsuzaki Y, Edagawa M, et al. Correlation between tumor angiogenesis and invasiveness in thymic epithelial tumors. J Thorac Cardiovasc Surg. 2002;124:493–498. doi: 10.1067/mtc.2002.124389. [DOI] [PubMed] [Google Scholar]
- 70.Isambert N, Freyer G, Zanetta S, et al. A phase I dose escalation and pharmacokinetic (PK) study of intravenous aflibercept (VEGF trap) plus docetaxel (D) in patients (pts) with advanced solid tumors: Preliminary results. J Clin Oncol. 2008;26(suppl; abstr 3599):177s. [Google Scholar]
- 80.Bisagni G, Rossi G, Cavazza A, et al. Long lasting response to the multikinase inhibitor bay 43-9006 (Sorafenib) in a heavily pretreated metastatic thymic carcinoma. J Thorac Oncol. 2009;4:773–775. doi: 10.1097/JTO.0b013e3181a52e25. [DOI] [PubMed] [Google Scholar]
- 81.Li XF, Chen Q, Huang WX, et al. Response to sorafenib in cisplatin-resistant thymic carcinoma: A case report. Med Oncol. 2009;26:157–160. doi: 10.1007/s12032-008-9100-0. [DOI] [PubMed] [Google Scholar]
- 82.Ströbel P, Bargou R, Wolff A, et al. Sunitinib in metastatic thymic carcinomas: Laboratory findings and initial clinical experience. Br J Cancer. 2010;103:196–200. doi: 10.1038/sj.bjc.6605740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiedler W, Giaccone G, Lasch P, et al. Phase I trial of SU14813 in patients with advanced solid malignancies. Ann Oncol. 2011;22:195–201. doi: 10.1093/annonc/mdq313. [DOI] [PubMed] [Google Scholar]
- 84.Ströbel P, Hartmann M, Jakob A, et al. Thymic carcinoma with overexpression of mutated KIT and the response to imatinib. N Engl J Med. 2004;350:2625–2626. doi: 10.1056/NEJM200406173502523. [DOI] [PubMed] [Google Scholar]
- 85.Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Girard N. Thymic tumors: Relevant molecular data in the clinic. J Thorac Oncol. 2010;5(suppl 4):S291–S295. doi: 10.1097/JTO.0b013e3181f209b9. [DOI] [PubMed] [Google Scholar]
- 87.Steele NL, Plumb JA, Vidal L, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 88.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 89.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: The Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 90.Lastoria S, Vergara E, Palmieri G, et al. In vivo detection of malignant thymic masses by indium-111-DTPA-D-Phe1-octreotide scintigraphy. J Nucl Med. 1998;39:634–639. [PubMed] [Google Scholar]
- 91.Ferone D, van Hagen PM, van Koetsveld PM, et al. In vitro characterization of somatostatin receptors in the human thymus and effects of somatostatin and octreotide on cultured thymic epithelial cells. Endocrinology. 1999;140:373–380. doi: 10.1210/endo.140.1.6398. [DOI] [PubMed] [Google Scholar]
- 92.Fuller PJ, Verity K. Somatostatin gene expression in the thymus gland. J Immunol. 1989;143:1015–1017. [PubMed] [Google Scholar]
- 93.Girard N, Teruya-Feldstein J, Payabyab EC, et al. Insulin-like growth factor-1 receptor expression in thymic malignancies. J Thorac Oncol. 2010;5:1439–1446. doi: 10.1097/JTO.0b013e3181e392a8. [DOI] [PubMed] [Google Scholar]
- 94.Zucali PA, Petrini I, Lorenzi E, et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer. 2010;116:4686–4695. doi: 10.1002/cncr.25367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 96.Rajan A, Berman AW, Kelly RJ, et al. Phase II study of the insulin-like growth factor-1 receptor (IGF-1R) antibody cixutumumab (C) in patients (pts) with thymoma (T) and thymic carcinoma (TC) J Clin Oncol. 2010;28(suppl):e17525. [Google Scholar]
- 97.Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 98.Mineo TC, Mineo D, Onorati I, et al. New predictors of response to neoadjuvant chemotherapy and survival for invasive thymoma: A retrospective analysis. Ann Surg Oncol. 2010;17:3022–3029. doi: 10.1245/s10434-010-1134-9. [DOI] [PubMed] [Google Scholar]
- 99.Kim DJ, Yang WI, Kim SH, et al. Expression of neurotrophin receptors in surgically resected thymic epithelial tumors. Eur J Cardiothorac Surg. 2005;28:611–616. doi: 10.1016/j.ejcts.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 100.Wakelee HA, Gubens MA, Burns M, et al. A phase II study of saracatinib (AZD0530), a SRC inhibitor, administered orally daily to patients with advanced thymic malignancies. Presented at International Thymic Malignancy Group Conference; May 5-6, 2010; New York, NY. [DOI] [PubMed] [Google Scholar]
- 101.Mineo TC, Ambrogi V, Baldi A, et al. Recurrent intrathoracic thymomas: Potential prognostic importance of cell-cycle protein expression. J Thorac Cardiovasc Surg. 2009;138:40–45. doi: 10.1016/j.jtcvs.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 102.Aisner SC, Dahlberg S, Hameed MR, et al. Epidermal growth factor receptor, C-kit, and Her2/neu immunostaining in advanced or recurrent thymic epithelial neoplasms staged according to the 2004 World Health Organization in patients treated with octreotide and prednisone: An Eastern Cooperative Oncology Group study. J Thorac Oncol. 2010;5:885–892. doi: 10.1097/JTO.0b013e3181d86a30. [DOI] [PMC free article] [PubMed] [Google Scholar]


