Abstract
Purpose
Estrogens and androgens are elevated in obesity and associated with increased postmenopausal breast cancer risk, but the effect of weight loss on these biomarkers is unknown. We evaluated the individual and combined effects of a reduced-calorie weight loss diet and exercise on serum sex hormones in overweight and obese postmenopausal women.
Patients and Methods
We conducted a single-blind, 12-month, randomized controlled trial from 2005 to 2009. Participants (age 50 to 75 years; body mass index > 25.0 kg/m2, exercising < 100 minutes/wk) were randomly assigned using a computer-generated sequence to (1) reduced-calorie weight loss diet (“diet”; n = 118), (2) moderate- to vigorous-intensity aerobic exercise (“exercise”; n = 117), (3) combined reduced-calorie weight loss diet and moderate- to vigorous-intensity aerobic exercise (“diet + exercise”; n = 117), or (4) control (n = 87). Outcomes were estrone concentration (primary) and estradiol, free estradiol, total testosterone, free testosterone, androstenedione, and sex hormone–binding globulin (SHBG) concentrations (secondary).
Results
Mean age and body mass index were 58 years and 30.9 kg/m2, respectively. Compared with controls, estrone decreased 9.6% (P = .001) with diet, 5.5% (P = .01) with exercise, and 11.1% (P < .001) with diet + exercise. Estradiol decreased 16.2% (P < .001) with diet, 4.9% (P = .10) with exercise, and 20.3% (P < .001) with diet + exercise. SHBG increased 22.4% (P < .001) with diet and 25.8% (P < .001) with diet + exercise. Free estradiol decreased 21.4% (P < .001) with diet and 26.0% (P < .001) with diet + exercise. Free testosterone decreased 10.0% (P < .001) with diet and 15.6% (P < .001) with diet + exercise. Greater weight loss produced stronger effects on estrogens and SHBG.
Conclusion
Weight loss significantly lowered serum estrogens and free testosterone, supporting weight loss for risk reduction through lowering exposure to breast cancer biomarkers.
INTRODUCTION
Overweight, obesity, and a sedentary lifestyle are associated with an increased risk of postmenopausal breast cancer,1–3 possibly through adiposity-induced excess levels of sex hormones.1,4 Serum concentrations of estrogens and androgens have been positively associated with risk for breast cancer in several cohort studies.5–9 Adipose tissue is the main storage site in postmenopausal women for aromatase and 17β-hydroxysteroid dehydrogenases, enzymes that catalyze the formation of estrone, estradiol, and testosterone.10
In observational studies, lower body weight/body fat and higher levels of physical activity are associated with lower circulating postmenopausal blood estrogens and androgens and higher sex hormone–binding globulin (SHBG), which reduces their bioactivity.11–17 Previous randomized controlled exercise trials reported modest or no reductions in estradiol and free estradiol, with little change in androgens,18–21 although one found significant reductions in exercisers who lost body fat.18,19 Low-fat dietary interventions with minimal or no weight loss have also modestly reduced estrogens and increased SHBG.22–29 To our knowledge, no previous randomized controlled trials have tested the effects of a weight loss intervention on sex hormones in postmenopausal women, a group at elevated risk for breast cancer.
The purpose of this investigation was to assess the independent and combined effects of reduced-calorie weight loss and moderate-to-vigorous aerobic exercise interventions with achievable goals on serum sex hormones. We hypothesized that the combined reduced-calorie weight loss diet and moderate-to-vigorous aerobic exercise intervention would produce a greater reduction in estrogens and androgens, and a greater increase in SHBG, compared with either condition alone or with controls. Because the association between obesity and breast cancer risk could be multifactorial, we also report weight loss and exercise effects on other breast cancer biomarkers: fasting insulin, C-reactive protein, adiponectin, and leptin.30–34
PATIENTS AND METHODS
The Nutrition and Exercise for Women randomized controlled trial, conducted in Seattle, WA, from 2005 to 2009, tested the effects of three 12-month-long weight loss and exercise interventions on estrone, estradiol, free estradiol, total testosterone, free testosterone, androstenedione, and SHBG. Study procedures were approved by the Fred Hutchinson Cancer Research Center institutional review board in Seattle, WA. All participants provided signed informed consent.
Participant Recruitment and Inclusion and Exclusion Criteria
Participants were postmenopausal (no menstrual cycles for ≥ 1 year or follicle-stimulating hormone level of > 23.0 IU/L for women 50 to 59 years of age without a uterus), age 50 to 75 years, body mass index (BMI) ≥ 25.0 kg/m2 (≥ 23.0 kg/m2 if Asian-American), and participating in less than 100 minutes/wk in moderate-intensity physical activity. Exclusion criteria included use of estrogen, progesterone, or testosterone hormones (past 3 months); history of breast cancer or other serious medical conditions; diabetes, fasting glucose ≥ 126 mg/dL, or use of diabetes medications; more than two alcohol drinks/d; currently smoking; or current use of weight loss medications or programs.
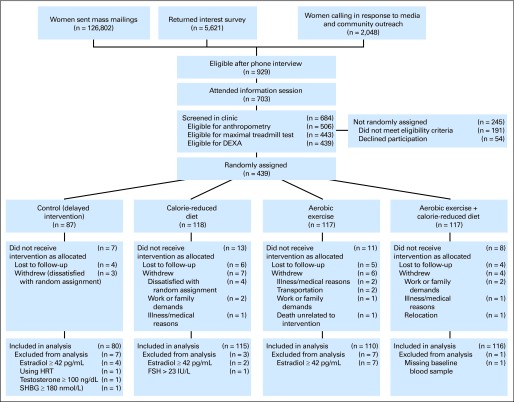
The trial design and recruitment are depicted in Figure 1.35 Eligible women were randomly assigned, stratified according to BMI (< 30 kg/m2 or ≥ 30 kg/m2) and race/ethnicity (non-Hispanic white, black, other), into one of four groups: (1) reduced-calorie weight loss diet (“diet”; n = 118), (2) moderate- to vigorous-intensity aerobic exercise (“exercise”; n = 117), (3) combined reduced-calorie weight loss diet and moderate- to vigorous-intensity aerobic exercise (“diet + exercise”; n = 117), or (4) no diet or exercise change (“control”; n = 87). A permuted blocks, computer-generated randomization (ratio 0.75:1:1:1) was used to assign a proportionally smaller number of women to the control group.
Fig 1.
CONSORT diagram: Flow of participants through the Nutrition and Exercise for Women Trial. DEXA, dual x-ray absorptiometry; FSH, follicle-stimulating hormone; HRT, hormone-replacement therapy; SHBG, sex hormone–binding globulin.
Interventions and Control Group
The weight loss diet intervention35 was a modification of the dietary component of the Diabetes Prevention Program36 and the Look AHEAD (Action for Health in Diabetes)37 lifestyle intervention programs, with a goal of daily energy intake of 1200 to 2000 kcal/d based on baseline weight, less than 30% daily energy intake from fat, and a 10% reduction in body weight by 6 months with maintenance to 12 months. In months 1 to 6, participants met individually with a study dietitian at least twice, attended weekly group dietitian-led meetings, and kept daily food logs. In each of months 7 to 12, participants had one face-to-face individual or group contact and one phone or e-mail contact with a dietician.
The exercise intervention goal was ≥ 45 minutes of moderate- to vigorous-intensity aerobic exercise, 5 days per week (225 minutes/wk).18,19 Each week, participants attended three monitored exercise sessions at our study facility and two at home. The program progressed to the maintenance target of 70% to 85% maximal heart rate for 45 minutes by week 7. Activities with four or more metabolic equivalents,38 such as brisk walking, were counted toward the prescribed exercise target.
Women randomly assigned to the diet + exercise intervention received both interventions, but with separate diet sessions and instructions not to discuss the diet intervention at the exercise facility.
Women randomly assigned to the control group were requested not to change their diet or exercise habits and were offered four weight loss classes and 8 weeks of facility exercise training at study end.
Outcome Measures
The primary outcome of the trial was estrone, and secondary outcomes included the sex hormones estradiol, free estradiol, total testosterone, free testosterone, androstenedione, and SHBG. Twelve-hour fasting blood was collected at baseline and 12 months, processed within 1 hour, and stored at −70°C. Laboratory assays were performed at the Reproductive Endocrine Research Laboratory (University of Southern California, Los Angeles, CA). Estrone, estradiol, total testosterone, and androstenedione were quantified by radioimmunoassays after organic solvent extraction and Celite column partition chromatography.39,40 SHBG was quantified via chemiluminescent immunometric assay using the Immulite Analyzer (Siemens Medical Solutions Diagnostics, Malvern, PA). Free estradiol and free testosterone were calculated using the measured values for estradiol, total testosterone, SHBG, and an assumed constant for albumin.41–43 The interassay coefficients of variation (CVs) ranged from 8% to 13% for the steroid hormone assays and 5% to 7% for the SHBG assay. Insulin (quantified by a 48-hour, polyethylene glycol-accelerated, double antibody radioimmunoassay) and high-sensitivity C-reactive protein (assay kits from Siemens Healthcare Diagnostics Products, Tarrytown, NY) were analyzed at the University of Washington Clinical Nutrition Research Unit Laboratory (Seattle, WA), and intra-assay CVs were 4.5% and 4.1%, respectively.44 Leptin and adiponectin (quantified by radioimmunoassays from Millipore [Billerica, MA] and Linco Research [St Charles, MO], respectively) were analyzed at the Northwest Lipid Metabolism and Diabetes Research Laboratories (Seattle, WA), and intra-assay CVs were 9.1% and 8.4%, respectively.
Covariate Measures
Study measures were obtained by trained study personnel blinded to randomization status. Participants completed questionnaires on demographic information, medical history, and food frequency dietary patterns45 and a physical activity interview.46 Height and weight were measured. Body composition was estimated using a dual x-ray absorptiometry scanner (GE Lunar, Madison, WI). All participants wore pedometers (Accusplit, Silicon Valley, CA) for 7 consecutive days to estimate average daily pedometer steps. Cardiorespiratory fitness (VO2max reported in liters per minute47) was measured by a maximal graded treadmill test and a metabolic cart (MedGraphics, St Paul, MN).48,49 Intervention women completed daily diet logs (for the first 6 months) and/or facility and home activity logs (for all 12 months), depending on assigned group.
Adverse Outcome Measures
Self-reported injuries and hot flash symptoms50 were ascertained via standard questionnaires, and total bone mineral density (in grams per square centimeter) was measured with dual x-ray absorptiometry.51 Participants were asked to report any musculoskeletal injuries to staff.
Statistical Analysis
Descriptive data are presented as means ± standard deviation (SD). Blood measures were log-transformed and presented as geometric means with 95% CIs. Intervention effects were examined based on the assigned treatment (ie, intent to treat). Mean 12-month changes in diet, exercise, and diet + exercise groups were compared with controls and with the other intervention groups using the generalized estimating equations (GEE) approach to random effects regression to account for the correlation within individuals over time.35 The GEE analysis based on available data was conducted to deal with missing outcome values at 12 months. We used the Bonferroni correction (two-sided α = .05/6; critical P value of P = .008) to adjust for the six comparisons of the four intervention arms.
We also assessed changes in sex hormones for each intervention arm using GEE models by preplanned subgroups reflecting intervention adherence, including (1) weight loss, (2) body fat loss, (3) change in pedometer steps per day, (4) change in VO2max, (5) diet session attendance, and (6) exercise adherence (in minutes per day). For these subgroup analyses, a P value of .05 was considered significant. Incidence and severity of injuries and hot flashes were compared across groups using χ2 tests. All statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS
At 12 months, 399 participants (91%) completed physical examinations and provided blood (Fig 1). Women whose respective estradiol (n = 2) or estrone (n = 1) levels were below the limit of detection, less than 3 pg/mL and less than 5 pg/mL, respectively, were assigned a value halfway between 0 and the limit of detection (1.5 and 2.5 pg/mL) and included in the analyses. One participant was missing baseline blood values, and 17 patients were excluded from analyses for sex hormone concentrations outside acceptable postmenopausal ranges: follicle-stimulating hormone less than 23.0 IU/L (n = 1), estrogen use (n = 1), serum estradiol ≥ 42 pg/mL (n = 13), total testosterone ≥ 100 ng/dL (n = 1), or SHBG ≥ 180 nmol/L (n = 1). Therefore, 421 women were included in the analyses.
Baseline Data
Groups were balanced on main demographic factors at baseline (Table 1). The mean age of participants was 58 years. The majority were non-Hispanic white (85%) and well-educated (65.4% college graduate or above). Participants had a mean BMI of 30.9 kg/m2, 47.2% body fat, and VO2max of 1.90 L/min (indicating mean poor aerobic fitness).
Table 1.
Baseline Characteristics of Randomly Assigned Participants
| Characteristic | All (n = 439) |
Control(n = 87) |
Diet (n = 118) |
Exercise(n = 117) |
Diet + Exercise(n = 117) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||
| Mean | 58.0 | 57.4 | 58.1 | 58.1 | 58.0 | |||||
| SD | 5.0 | 4.4 | 6.0 | 5.0 | 4.5 | |||||
| Range | 50-76 | 51-70 | 50-76 | 51-72 | 51-73 | |||||
| Weight, kg | ||||||||||
| Mean | 83.6 | 84.2 | 84.0 | 83.7 | 82.5 | |||||
| SD | 11.8 | 12.5 | 11.8 | 12.3 | 10.8 | |||||
| BMI, kg/m2 | ||||||||||
| Mean | 30.9 | 30.7 | 31.1 | 30.7 | 31.0 | |||||
| SD | 4.0 | 3.9 | 3.9 | 3.7 | 4.3 | |||||
| Body fat, % | ||||||||||
| Mean | 47.2 | 47.3 | 47.0 | 47.3 | 47.4 | |||||
| SD | 4.3 | 4.4 | 4.3 | 4.1 | 4.5 | |||||
| Body fat mass, kg | ||||||||||
| Mean | 39.8 | 40.1 | 39.8 | 39.9 | 39.4 | |||||
| SD | 8.1 | 8.5 | 8.1 | 8.2 | 7.9 | |||||
| Lean mass, % | ||||||||||
| Mean | 48.5 | 48.5 | 48.7 | 48.3 | 48.3 | |||||
| SD | 4.2 | 4.3 | 4.3 | 3.9 | 4.4 | |||||
| Lean mass, kg | ||||||||||
| Mean | 40.2 | 40.6 | 40.7 | 40.2 | 39.6 | |||||
| SD | 5.0 | 5.3 | 5.1 | 5.3 | 4.3 | |||||
| VO2max, L/min | ||||||||||
| Mean | 1.90 | 1.93 | 1.89 | 1.85 | 1.93 | |||||
| SD | 0.33 | 0.37 | 0.31 | 0.31 | 0.34 | |||||
| Usual physical activity, MET min/wk | ||||||||||
| Mean | 151 | 109 | 160 | 173 | 151 | |||||
| SD | 208 | 196 | 230 | 202 | 197 | |||||
| Total calories, kcal/d* | ||||||||||
| Mean | 1,934 | 1,988 | 1,884 | 1,986 | 1,890 | |||||
| SD | 638 | 669 | 661 | 589 | 638 | |||||
| Alcohol, g/d* | ||||||||||
| Mean | 7.3 | 8.2 | 8.8 | 7.1 | 5.4 | |||||
| SD | 10.8 | 11.5 | 13.9 | 9.7 | 69 | |||||
| Ethnicity | ||||||||||
| Non-Hispanic white | 373 | 85.0 | 74 | 85.1 | 101 | 85.6 | 98 | 83.8 | 100 | 85.4 |
| African American | 35 | 8.0 | 6 | 6.9 | 9 | 7.6 | 15 | 12.8 | 5 | 4.3 |
| Asian/Pacific Islander | 8 | 1.8 | 2 | 2.3 | 2 | 1.7 | 2 | 1.7 | 2 | 1.7 |
| Hispanic/Latino | 12 | 2.7 | 3 | 3.4 | 2 | 1.7 | 2 | 1.7 | 5 | 4.3 |
| Other | 11 | 2.5 | 2 | 2.3 | 4 | 3.4 | 0 | 0.0 | 5 | 4.3 |
| Education | ||||||||||
| Some high school or high school graduate | 18 | 4.1 | 5 | 5.7 | 3 | 2.5 | 6 | 5.1 | 4 | 3.4 |
| Some college | 134 | 30.5 | 23 | 26.5 | 39 | 33.1 | 41 | 35.1 | 31 | 26.5 |
| College graduate and above | 287 | 65.4 | 59 | 67.8 | 76 | 64.4 | 70 | 59.8 | 82 | 70.1 |
| Family history of breast cancer | ||||||||||
| None | 286 | 62.1 | 54 | 67.8 | 80 | 65.0 | 76 | 65.0 | 76 | 65.0 |
| First degree | 81 | 16.1 | 14 | 18.6 | 22 | 22.2 | 26 | 22.2 | 19 | 16.2 |
| Second degree | 72 | 21.8 | 19 | 13.6 | 16 | 12.8 | 15 | 12.8 | 22 | 18.8 |
| Parity† | ||||||||||
| Nulliparous | 118 | 26.9 | 22 | 25.3 | 32 | 27.4 | 29 | 24.8 | 35 | 29.9 |
| 1 | 72 | 16.4 | 15 | 17.2 | 23 | 19.7 | 16 | 13.7 | 18 | 15.4 |
| 2 | 145 | 33.1 | 27 | 31.0 | 32 | 27.4 | 44 | 37.6 | 42 | 35.9 |
| ≥3 | 103 | 23.5 | 23 | 26.4 | 30 | 25.6 | 28 | 23.9 | 22 | 18.8 |
| History of breastfeeding, months‡ | ||||||||||
| 0 | 174 | 40.8 | 33 | 39.8 | 44 | 37.9 | 47 | 41.2 | 50 | 43.9 |
| 1-6 | 54 | 12.7 | 11 | 13.3 | 19 | 16.4 | 11 | 9.7 | 13 | 11.4 |
| ≥ 6 | 199 | 46.6 | 39 | 47.0 | 53 | 45.7 | 56 | 49.1 | 51 | 44.7 |
| History of breast biopsy | ||||||||||
| Yes | 58 | 13.2 | 14 | 16.1 | 17 | 14.4 | 10 | 8.6 | 17 | 14.5 |
| No | 381 | 86.8 | 73 | 83.9 | 101 | 85.6 | 107 | 91.5 | 100 | 85.5 |
| Age at randomization, years | ||||||||||
| < 60 | 304 | 69.3 | 62 | 74.7 | 83 | 70.3 | 80 | 68.4 | 79 | 67.5 |
| ≥ 60 | 135 | 30.8 | 25 | 28.7 | 35 | 29.7 | 37 | 31.6 | 38 | 32.5 |
| Age at menarche, years§ | ||||||||||
| < 13 | 209 | 48.0 | 44 | 50.6 | 54 | 46.2 | 55 | 47.0 | 56 | 48.7 |
| ≥ 13 | 227 | 52.0 | 43 | 49.4 | 63 | 53.9 | 62 | 53.0 | 59 | 51.3 |
| Age at menopause, years‖ | ||||||||||
| < 50 | 178 | 42.4 | 28 | 33.3 | 52 | 46.0 | 42 | 38.2 | 56 | 49.6 |
| ≥ 50 | 242 | 57.6 | 56 | 66.7 | 61 | 54.0 | 68 | 61.8 | 57 | 50.4 |
| Ever used hormone therapy | ||||||||||
| No | 178 | 40.5 | 37 | 42.5 | 53 | 44.9 | 49 | 41.9 | 39 | 33.3 |
| Yes | 261 | 59.5 | 50 | 57.5 | 65 | 55.1 | 68 | 58.1 | 78 | 66.7 |
| History of hysterectomy | ||||||||||
| No | 345 | 78.6 | 67 | 77.0 | 93 | 78.8 | 92 | 78.6 | 93 | 79.5 |
| Yes | 94 | 21.4 | 20 | 23.0 | 25 | 21.2 | 25 | 21.4 | 24 | 20.5 |
| History of bilateral oophorectomy | ||||||||||
| No | 416 | 94.8 | 83 | 95.4 | 112 | 94.9 | 109 | 93.2 | 112 | 95.7 |
| Yes | 23 | 5.2 | 4 | 4.6 | 6 | 5.1 | 8 | 6.8 | 5 | 4.3 |
| Sex hormones¶ | ||||||||||
| Estrone, pg/mL | ||||||||||
| Mean | 37.2 | 35.8 | 38.1 | 37.4 | 36.9 | |||||
| SD | 15.5 | 17.1 | 15.5 | 14.8 | 15.3 | |||||
| Estradiol, pg/mL | ||||||||||
| Mean | 12.7 | 12.4 | 12.7 | 12.6 | 12.8 | |||||
| SD | 5.8 | 6.1 | 5.4 | 5.4 | 6.3 | |||||
| Testosterone, ng/dL | ||||||||||
| Mean | 26.5 | 26.3 | 26.6 | 26.8 | 26.2 | |||||
| SD | 12.9 | 15.2 | 13.5 | 11.3 | 12.1 | |||||
| Androstenedione, ng/dL | ||||||||||
| Mean | 55.5 | 53.9 | 56.4 | 54.6 | 56.4 | |||||
| SD | 24.9 | 23.9 | 28.9 | 24.6 | 21.4 | |||||
| SHBG, nmol/L | ||||||||||
| Mean | 39.3 | 38.1 | 39.3 | 43.4 | 36.2 | |||||
| SD | 17.6 | 16.5 | 16.9 | 22.2 | 12.8 | |||||
| Free estradiol,# pg/mL | ||||||||||
| Mean | 0.35 | 0.34 | 0.35 | 0.34 | 0.36 | |||||
| SD | 0.17 | 0.16 | 0.16 | 0.16 | 0.18 | |||||
| Free testosterone, pg/mL | ||||||||||
| Mean | 5.7 | 5.6 | 5.8 | 5.5 | 5.8 | |||||
| SD | 2.8 | 2.9 | 2.9 | 2.5 | 2.7 | |||||
NOTE. SI conversion factors: To convert estrone to pmol/L, multiply by 3.699; estradiol to pmol/L, multiply by 3.67; testosterone to nmol/L, multiply by 0.0347; androstenedione to nmol/L, multiply by 0.0349.
Abbreviations: BMI, body mass index; SD, standard deviation; SHBG, sex hormone–binding globulin.
N = 427 for total; n = 85 for control; n = 114 for diet, exercise, and diet + exercise groups.
N = 438 for total; n = 87 for control; n = 117 for diet, exercise, and diet + exercise groups.
N = 427 for total; n = 83 for control; n = 116 for diet; n = 114 for exercise and diet + exercise groups.
N = 436 for total; n = 87 for control; n = 117 for diet and exercise groups; n = 115 for diet + exercise.
N = 420 for total; n = 84 for control; n = 113 for diet; n = 110 for exercise; n = 113 for diet + exercise.
N = 421 for total; n = 80 for control; n = 115 for diet; n = 110 for exercise; n = 116 for diet + exercise groups.
N = 420 for total; n = 79 for control; n = 115 for diet; n = 110 for exercise; n = 116 for diet + exercise group.
Weight and Body Composition Changes
Weight and body composition results were previously reported.35 Mean weight changes at 12 months were as follows: diet, −10.8% (P < .001); exercise, −3.3% (P = .02); diet + exercise, −11.9% (P < .001); and controls, −0.6% (Table 2).
Table 2.
Adherence to the Interventions by Study Group
| Outcome | Baseline |
6-Month |
12-Month |
Δ0-6-mo | %Δ0-6-mo | Δ0-12 mo | %Δ0-12 mo | P * | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Weight, kg | |||||||||||
| Control | 84.2 | 12.5 | 83.8 | 13.0 | 83.7 | 12.3 | −0.5 | −0.6 | −0.5 | −0.6 | |
| Diet | 84.0 | 11.8 | 77.0 | 12.8 | 74.9 | 12.3 | −7.1 | −8.4 | −9.1 | −10.8 | PC< .001† PE< .001‡ PD+E = .17‡ |
| Exercise | 83.7 | 12.3 | 82.1 | 12.3 | 80.9 | 12.2 | −1.6 | −1.9 | −2.8 | –3.3 | PC = .02† PD< .001‡ PD+E< .001‡ |
| Diet + exercise | 82.5 | 10.8 | 74.9 | 10.8 | 72.7 | 10.9 | −7.7 | −9.3 | −9.8 | −11.9 | PC< .001† PD = .10‡ PE< .001‡ |
| Body fat, % | NA | NA | |||||||||
| Control | 47.3 | 4.4 | 47.2 | 5.3 | −0.3 | −0.5 | |||||
| Diet | 47.0 | 4.3 | 42.1 | 6.4 | −5.0 | −10.6 | PC< .001† PE = .02‡ PD+E< .001‡ | ||||
| Exercise | 47.3 | 4.1 | 45.5 | 5.0 | −1.8 | −3.8 | PC< .001† PD< .001‡ PD+E < .001‡ | ||||
| Diet + exercise | 47.4 | 4.5 | 41.1 | 7.0 | −6.4 | −13.4 | PC< .0001† PD = .02‡ PE< .001‡ | ||||
| Pedometer, steps/wk§ | NA | NA | |||||||||
| Control | 5,605 | 2,334 | 6,136 | 2,909 | 530.9 | 9.5 | |||||
| Diet | 5,539 | 2,257 | 6,365 | 2,841 | 825.7 | 14.9 | PC = .67† PE < .001‡ PD+E< .001‡ | ||||
| Exercise | 5,777 | 2,129 | 9,139 | 3,120 | 3362.6 | 58.2 | PC< .001† PD< .001‡ PD+E = .06‡ | ||||
| Diet + exercise | 5,980 | 2,393 | 10,069 | 2,944 | 4088.5 | 68.4 | PC< .001† PD< .001‡ PE = .06‡ | ||||
| VO2max, L/min | NA | NA | |||||||||
| Control | 1.93 | 0.37 | 1.91 | 0.33 | −0.02 | −0.9 | |||||
| Diet | 1.89 | 0.31 | 1.84 | 0.32 | −0.04 | −2.3 | PC = .83† PD< .001‡ PD+E< .001‡ | ||||
| Exercise | 1.85 | 0.31 | 2.04 | 0.35 | 0.19 | 10.1 | PC< .001† PD< .001‡ PD+E = .30‡ | ||||
| Diet + exercise | 1.93 | 0.34 | 2.07 | 0.41 | 0.15 | 7.6 | PC< .001† PD< .001‡ PE = .30‡ | ||||
| Total calories, kcal/d‖ | NA | NA | |||||||||
| Control | 1,988 | 669 | 1,733 | 616 | −255 | −13 | |||||
| Diet | 1,885 | 661 | 1,564 | 539 | −320 | −17 | PC = .54† PE = .30‡ PD+E = .75‡ | ||||
| Exercise | 1,987 | 589 | 1,768 | 501 | −219 | −11 | PC = .73† PD = .30‡ PD+E = .17‡ | ||||
| Diet + exercise | 1,891 | 638 | 1,549 | 525 | −342 | −18 | PC = .36† PD = .75‡ PE = .10‡ | ||||
| Fat intake, % of kcal/d‖ | NA | NA | |||||||||
| Control | 35.6 | 6.9 | 33.3 | 6.9 | −2.3 | −6.3 | |||||
| Diet | 33.1 | 6.3 | 26.2 | 6.9 | −6.9 | −21.0 | PC< .001† PE< .001‡ PD+E = .17‡ | ||||
| Exercise | 33.6 | 6.9 | 31.8 | 7.0 | −1.8 | −5.4 | PC = .30† PD< .001‡ PD+E< .001‡ | ||||
| Diet + exercise | 35.3 | 7.3 | 27.0 | 6.6 | −8.4 | −23.7 | PC< .001† PD = .17‡ PE< .001‡ | ||||
Abbreviations: SD, standard deviation; Δ, change.
Change from baseline to 12 months.
PC, P values for comparing the changes between control group and three intervention groups.
PD or PE, P values for comparing the changes between exercise group and diet group; PD+E, P values for comparing the changes between diet + exercise group and two other intervention groups (exercise group or diet group).
N = 428 for total; n = 82 for control; n = 117 for diet; n = 114 for exercise; and n = 115 for diet + exercise groups.
N = 427 for total; n = 85 for control; n = 114 for diet; n = 114 for exercise; n = 114 for diet + exercise groups.
Main Hormone Effects
Estrone significantly decreased with diet (−9.6%, P = .001) and diet + exercise (−11.1%, P < .001), and to a smaller extent with exercise (−5.5%, P = .01), versus controls (+8.1%; Table 3). Estradiol decreased significantly with diet (−16.2%, P < .001) and diet + exercise (−20.3%, P < .001) and minimally with exercise (−4.9%, P = .10), versus controls (+4.9%). Total testosterone decreased nonsignificantly with diet + exercise, (−5.9%, P = .02). No changes in androstenedione were noted in any of the groups. SHBG increased with diet (+22.4%) and diet + exercise (+25.8%; both P < .001 v controls), with no change with exercise (−0.7%, P = .41) versus controls (−2.7%). Free estradiol decreased with diet (−21.4%) and diet + exercise (−26.0%) versus controls (+6.3%, both P < .001); little change was observed with exercise (−4.7%, P = .08). Free testosterone decreased with diet (−10.0%, P < .001) and diet + exercise (−15.6%, P < .001) versus controls.
Table 3.
Change in Sex Hormones by Study Group, Geometric Mean, and 95% CI
| Biomarker | Baseline |
12 Months |
Δ* | %Δ | P† | ||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| Estrone, pg/mL | |||||||
| Control | 32.0 | 28.6 to 35.8 | 34.6 | 31.4 to 38.1 | 2.6 | 8.1 | |
| Diet | 35.2 | 32.7 to 37.9 | 31.8 | 29.4 to 34.4 | −3.4 | −9.6 | PC = .001‡ PE = .30§ PD+E = .17§ |
| Exercise | 34.8 | 32.4 to 37.4 | 32.9 | 30.5 to 35.5 | −1.9 | −5.5 | PC = .01‡ PD = .30§ PD+E = .01§ |
| Diet + exercise | 33.9 | 31.5 to 36.6 | 30.2 | 28.0 to 32.5 | −3.8 | −11.1 | PC< .001‡ PD = .17§ PE = .01§ |
| Estradiol, pg/mL | |||||||
| Control | 10.9 | 9.6 to 12.3 | 11.4 | 10.2 to 12.8 | 0.5 | 4.9 | |
| Diet | 11.6 | 10.7 to 12.5 | 9.7 | 8.9 to 10.6 | −1.9 | −16.2 | PC< .001‡ PE = .002§ PD+E = .07§ |
| Exercise | 11.5 | 10.6 to 12.5 | 11.0 | 10.1 to 11.9 | −0.6 | −4.9 | PC = .10‡ PD = .002§ PD+E< .001§ |
| Diet + exercise | 11.5 | 10.6 to 12.5 | 9.2 | 8.4 to 10.0 | −2.3 | −20.3 | PC< .001‡ PD = .07§ PE< .001§ |
| Total testosterone, ng/dL | |||||||
| Control | 22.8 | 20.2 to 25.7 | 23.2 | 20.9 to 25.7 | 0.4 | 1.8 | |
| Diet | 23.9 | 21.9 to 26.0 | 23.6 | 21.6 to 25.8 | −0.2 | −0.9 | PC = .40‡ PE = .67§ PD+E = .07§ |
| Exercise | 24.8 | 23.0 to 26.7 | 23.6 | 21.6 to 25.7 | −1.2 | −4.9 | PC = .24† PD = .67§ PD+E = .24§ |
| Diet + exercise | 23.9 | 22.1 to 25.8 | 22.5 | 20.8 to 24.3 | −1.4 | −5.9 | PC = .02‡ PD = .07§ PE = .24§ |
| Androstenedione, ng/dL | |||||||
| Control | 48.7 | 43.9 to 54.0 | 49.4 | 45.4 to 53.7 | 0.7 | 1.5 | |
| Diet | 51.1 | 47.1 to 55.3 | 51.8 | 47.7 to 56.2 | 0.7 | 1.4 | PC = .83‡ PE = .93§ PD+E = .26§ |
| Exercise | 50.2 | 46.6 to 54.1 | 49.6 | 45.6 to 54.0 | −0.6 | −1.2 | PC = .75‡ PD = .93§ PD+E = .25§ |
| Diet + exercise | 52.6 | 49.1 to 56.4 | 50.8 | 47.1 to 54.7 | −1.9 | −3.5 | PC = .22‡ PD = .26§ PE = .25§ |
| SHBG, nmol/L | |||||||
| Control | 34.7 | 31.5 to 38.2 | 33.7 | 30.3 to 37.5 | −1.0 | −2.7 | |
| Diet | 35.8 | 33.0 to 38.8 | 43.8 | 40.4 to 47.5 | 8.0 | 22.4 | PC< .001‡ PE< .001§ PD+E = .48§ |
| Exercise | 39.1 | 35.9 to 42.6 | 38.8 | 35.6 to 42.4 | −0.3 | −0.7 | PC = .41‡ PD< .001§ PD+E< .001§ |
| Diet + exercise | 34.1 | 31.9 to 36.4 | 42.9 | 40.2 to 45.6 | 8.8 | 25.8 | PC< .001‡ PD = .48§ PE< .001§ |
| Free estradiol, pg/mL‖ | |||||||
| Control | 0.30 | 0.27 to 0.34 | 0.33 | 0.30 to 0.36 | 0.02 | 6.3 | |
| Diet | 0.31 | 0.28 to 0.34 | 0.24 | 0.22 to 0.27 | −0.07 | −21.4 | PC< .001‡ PE< .001§ PD+E = .06§ |
| Exercise | 0.30 | 0.27 to 0.33 | 0.29 | 0.26 to 0.32 | −0.01 | −4.7 | PC = .08‡ PD< .001§ PD+E< .001§ |
| Diet + exercise | 0.32 | 0.29 to 0.35 | 0.23 | 0.21 to 0.26 | −0.08 | −26.0 | PC< .001‡ PD = .06§ PE< .001§ |
| Free testosterone, pg/mL | |||||||
| Control | 4.9 | 4.4 to 5.6 | 5.1 | 4.6 to 5.7 | 0.13 | 2.6 | |
| Diet | 5.1 | 4.7 to 5.6 | 4.6 | 4.2 to 5.1 | −0.51 | −10.0 | PC< .001‡ PE = .02§ PD+E = .02§ |
| Exercise | 5.1 | 4.7 to 5.5 | 4.9 | 4.5 to 5.3 | −0.23 | −4.5 | PC = .20‡ PD = .02§ PD+E< .001§ |
| Diet + exercise | 5.3 | 4.9 to 5.7 | 4.5 | 4.1 to 4.8 | −0.82 | −15.6 | PC< .001‡ PD = .02§ PE< .001§ |
Abbreviations: SHBG, sex hormone–binding globulin; Δ, change.
Change at 12 months from baseline.
P values for comparing the change from baseline to 12 months between groups. There are six between-group comparisons (P < .05/6; .008 is the critical value).
PC, P values for comparing the changes between control group and three intervention groups.
PD or PE, P values for comparing the changes between exercise group and diet group; PD+E, P values for comparing the changes between diet + exercise group and two other intervention groups (exercise group or diet group).
Free estradiol, not calculated for individuals with estradiol below the level of detection (N = 420; n = 79 for control; n = 115 for diet; n = 110 for exercise; n = 116 for diet + exercise groups).
No comparisons of diet with diet + exercise groups reached Bonferroni-corrected statistical significance (Table 3). Compared with exercise, diet + exercise produced significantly greater reductions in estradiol, free estradiol, and free testosterone and a larger increase in SHBG (all P < .001).
There were greater reductions in fasting insulin44 and C-reactive protein with diet and diet + exercise (both P < .001), compared with control, with no changes in the exercise group (Table 4). A significant reduction in leptin was noted in all intervention groups compared with controls, whereas an increase in adiponectin was only noted in the diet and diet + exercise groups (both P < .001).
Table 4.
Change in Insulin, CRP, Leptin, and Adiponectin by Study Group, Geometric Mean, and 95% CI
| Biomarker | Baseline |
12 Months |
Δ* | %Δ | P† | ||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||||
| Insulin,‡ μU/mL | |||||||
| Control | 11.99 | 10.83 to 13.28 | 11.55 | 10.38 to 12.85 | −0.44 | −3.7 | |
| Diet | 11.00 | 9.89 to 12.23 | 8.12 | 7.31 to 9.02 | −2.87 | −26.1 | PC< .001§ PE< .01‖ PD+E = .87‖ |
| Exercise | 10.94 | 9.97 to 12.0 | 10.05 | 9.14 to 11.04 | −0.89 | −8.2 | PC = .26§ PD< .01‖ PD+E< .001‖ |
| Diet + exercise | 10.67 | 9.65 to 11.81 | 7.85 | 7.07 to 8.71 | −2.83 | −26.5 | PC< .001§ PD = .87‖ PE< .001‖ |
| High-sensitivity CRP,‡ mg/L | |||||||
| Control | 1.89 | 1.50 to 2.39 | 1.89 | 1.48 to 2.42 | 0.00 | −0.2 | |
| Diet | 2.60 | 2.18 to 3.11 | 1.53 | 1.28 to 1.82 | −1.07 | −41.2 | PC< .001§ PE< .001‖ PD+E = .47‖ |
| Exercise | 2.50 | 2.10 to 2.99 | 2.23 | 1.79 to 2.78 | −0.27 | −10.9 | PC = .37§ PD< .001‖ PD+E< .001‖ |
| Diet + exercise | 2.12 | 1.79 to 2.52 | 1.23 | 0.99 to 1.53 | −0.89 | −42.1 | PC< .001§ PD = .47‖ PE< .001‖ |
| Leptin,¶ ng/mL | |||||||
| Control | 24.85 | 22.94 to 26.92 | 24.64 | 22.23 to 27.30 | −0.21 | −0.9 | |
| Diet | 23.07 | 21.41 to 24.86 | 15.71 | 14.18 to 17.40 | −7.37 | −31.9 | PC< .001§ PE< .001‖ PD+E = .001‖ |
| Exercise | 23.50 | 21.73 to 25.41 | 20.34 | 18.52 to 22.33 | −3.16 | −13.4 | PC< .01§ PD< .001‖ PD+E< .001‖ |
| Diet + exercise | 23.75 | 22.02 to 25.63 | 13.77 | 12.40 to 15.30 | −9.98 | −42.0 | PC< .001§ PD = .001‖ PE< .001‖ |
| Adiponectin,¶ μg/mL | |||||||
| Control | 12.78 | 11.70 to 13.96 | 12.44 | 11.36 to 13.64 | −0.34 | −2.6 | |
| Diet | 12.36 | 11.37 to 13.44 | 13.46 | 12.41 to 14.60 | 1.10 | 8.9 | PC < .001§ PE < .001‖ PD+E = .34‖ |
| Exercise | 12.46 | 11.47 to 13.54 | 12.09 | 11.03 to 13.25 | −0.38 | −3.0 | PC = .86§ PD < .001‖ PD+E< .01‖ |
| Diet + exercise | 12.76 | 11.72 to 13.88 | 14.00 | 12.89 to 15.20 | 1.24 | 9.7 | PC < .001§ PD = .34‖ PE< .01‖ |
Abbreviations: CRP, C-reactive protein; Δ, change.
Change at 12 months from baseline.
P values for comparing the change from baseline to 12 months between groups.
For insulin and CRP, N = 406 (control, n = 79; diet, n = 105; exercise, n = 106; diet + exercise, n = 108).
PC, P values for comparing the changes between control group and three intervention groups.
PD or PE, P values for comparing the changes between exercise group and diet group; PD+E, P values for comparing the changes between diet + exercise group and two other intervention groups (exercise group or diet group).
For adiponectin and leptin, N = 399 (control, n = 79; diet, n = 105; exercise, n = 106; diet + exercise, n = 109).
Subgroup Analyses
Reductions in estrone, estradiol, free estradiol, and free testosterone and increases in SHBG were larger with greater degrees of weight loss (Fig 2 and Data Supplement). Weight loss more than 10% in the diet, but not diet + exercise, group produced markedly greater reductions in estrone (Fig 2A), estradiol (Fig 2B), free estradiol (Fig 2E), and free testosterone (Fig 2F) and greater increase in SHBG (Fig 2D) compared with lower amounts of weight loss. We observed similar results when hormone changes were assessed by fat loss (Data Supplement).
Fig 2.
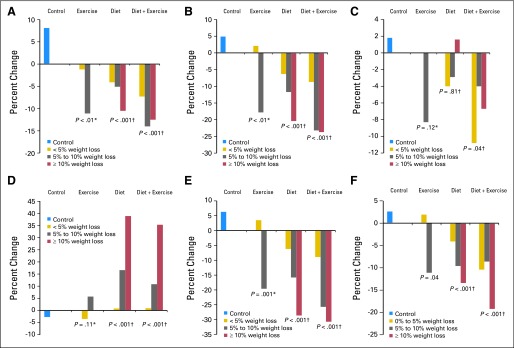
Change in hormone levels by weight loss category. (A) Estrone; (B) estradiol; (C) total testosterone; (D) sex hormone–binding globulin; (E) free estradiol; (F) free testosterone. (*) Testing for a trend in change from baseline to 12 months in hormones from controls through weight loss < 5% and ≥ 5% (for exercise only). For exercise: n = 80 for control, n = 72 for weight loss < 5%, n = 27 for weight loss ≥ 5%. (†) Testing for a trend in change from baseline to 12 months in hormones from controls through weight loss ≥ 10%. For diet: n = 80 for control, n = 28 for weight loss < 5%, n = 27 for weight loss 5% to 10%, n = 46 for weight loss ≥ 10%. For diet + exercise: n = 80 for control, n = 18 for weight loss < 5%; n = 21 for weight loss 5% to 10%; n = 69 for weight loss ≥ 10%. Statistical significance P value < .05.
Intervention Adherence Effects
Higher attendance at diet sessions was variably associated with greater reductions in some of the hormones (Data Supplement). Measures of exercise adherence, conversely, were unrelated to hormone changes (Data Supplement).
Adverse Outcomes
Compared with controls (24%), musculoskeletal injuries were reported in 34%, 18%, and 19% of patients , respectively, in the exercise, diet + exercise, and diet groups (all P > .10). Total bone mineral density was reduced in all intervention groups: diet, −1.2% (P < .001); exercise, −0.8% (P = .03); and diet + exercise, −1.7% (P < .001) versus no change in controls. Hot flash number or severity did not change differently in the four arms.
DISCUSSION
A 12-month reduced-calorie weight loss, with or without exercise, produced large and statistically significant reductions in postmenopausal serum estrone, estradiol, free estradiol, and free testosterone and increases in SHBG. The weight loss interventions also significantly reduced insulin, C-reactive protein, and leptin and increased adiponectin. Exercise had little effect on sex hormones or the other potential breast cancer biomarkers.
Elevated blood estrogen and testosterone concentrations have consistently been associated with increased breast cancer risk, with doubling or greater effect on risk in women in the highest versus lowest quartiles or quintiles in prospective cohort studies.5,7,52,53 In one of these, Woolcott et al7 measured sex hormones in the same laboratory as for the present study and found that a doubling of free estradiol raised breast cancer risk by a factor of 2.26. Therefore, the 30% drop in free estradiol we observed with ≥ 5% of weight loss (achieved by 78% and 65% of the diet + exercise and diet groups, respectively) could be associated with a 22% decrease in breast cancer risk. Our participants' mean baseline estradiol concentration is in the top estradiol quartile range for the Woolcott study, and the diet and diet + exercise groups' mean 12-month values fall into the third highest quartile range. Because the odds ratio for breast cancer decreased from 6.4 to 2 in the fourth and third quartiles in that study, respectively, our weight loss intervention's decrease in mean estradiol could represent a ≥ 50% reduction in breast cancer risk. However, the results of these prospective cohort studies are based on a one-time sample, rather than serial samples. Therefore, our study suggests that a modest degree of weight loss could have a powerful effect on breast cancer risk; however, the impact of a reduction in sex hormones on breast cancer risk reduction is still unknown.
Low-fat dietary interventions without weight loss have reported either no or small change in estrogens.22–24,26,27,29,54 Two previous randomized controlled trials in postmenopausal women found modest reductions of 2% to 14% in estrogens after 1-year aerobic exercise interventions.19,20 In one of these, those exercisers who reduced percent body fat by more than 2% (mean absolute value) experienced a 15% decline in estradiol.19 A third 1-year randomized controlled trial found a significant lowering of testosterone in postmenopausal women randomly assigned to exercise who lost more than 2% body fat.21 In the present study, weight loss ≥ 5% was associated with significantly greater reductions in estradiol, free estradiol, and free testosterone and significantly increased SHBG. Taken together, these findings suggest that weight loss is the key factor linking alterations in diet or exercise to sex hormone changes. The effect of weight loss on estrogens may occur through a reduction in adipose tissue aromatase levels.10,55 In addition to being the first study to examine the effect of weight loss on sex hormones in postmenopausal women, the Nutrition and Exercise for Women Trial achieved greater adherence to a higher exercise goal and greater weight loss than the original Diabetes Prevention Program lifestyle intervention.36
The lack of effect of exercise alone does not agree with epidemiologic studies in which physical activity is associated with decreased risk of breast cancer.3,56 Therefore, exercise could play a role in reducing risk of postmenopausal breast cancer though different biologic mechanisms than were examined in this study. Exercise may also play a role in reducing breast cancer risk by augmenting dietary weight loss57 and maintenance,58 which will be critical for long-term risk reduction.1
Strengths of our study include a large sample size and long duration with excellent adherence and low attrition. The weight loss intervention was a group-based modification of the Diabetes Prevention Program intervention,36 which has publically available materials that have been tested in a variety of populations.59–63 This suggests that successful replication without the high costs of individually delivered weight loss programs is feasible. The exercise intervention, which consisted primarily of brisk walking, should be easily adoptable by most women in a clinical or community setting. However, the effects of these dietary weight loss and exercise interventions on breast cancer incidence are unknown.
Our study had some limitations. We tested only one dietary weight loss and one exercise intervention and cannot speculate on effects of other weight loss or exercise modalities. The study population was primarily non-Hispanic whites, and intervention effects in women from other race or ethnic groups cannot be inferred. Furthermore, the trial did not test whether weight loss or exercise reduced incidence of breast cancer, which would require a trial with a much larger scope.64
Total bone mineral density declined in all intervention groups, although the clinical significance of the change in total bone density is not defined.65 Future weight loss studies should consider the inclusion of resistance exercise to avoid loss of bone mass.65–68 The exercise group reported more musculoskeletal injuries than the diet and diet + exercise groups, which suggests that weight loss protects women from exercise-induced injury.69,70
The results of this study could be relevant even to women who choose to use breast cancer chemoprevention. Tamoxifen may have a lower breast cancer risk reduction effect in obese than normal weight women.71 Aromatase inhibitors, which have been found to reduce risk of new72 or recurrent breast cancer, had lower effectiveness in obese versus normal-weight patients in some,73 but not other,72,74 trials. Conditions that are increased with some of these agents, such as deep vein thrombosis and endometrial cancer, may be increased to a greater extent in obese than normal-weight women.71,75 Therefore, even for women who chose treatment with these agents, weight reduction if overweight or obese may be beneficial. Furthermore, although recent reports indicate that tamoxifen and raloxifene treatment confers long-term (10+ years) protection against breast cancer risk,76–80 the long-term efficacy of aromatase inhibitors on breast cancer risk reduction has not yet been established.72 Weight loss in overweight or obese women therefore represents an additional option for long-term breast cancer risk reduction.
In summary, a moderate degree of weight loss achieved through a reduced-calorie weight loss diet intervention reduced serum concentrations of estrogens, free testosterone, and other potential breast cancer biomarkers in overweight or obese postmenopausal women. These results have implications for the significant majority of postmenopausal women who are overweight or obese81,82 and therefore at elevated risk for breast cancer incidence2 and mortality.83
Supplementary Material
Acknowledgment
We thank the study staff and the participants for their dedication to the study.
Footnotes
Supported by National Cancer Institute at the National Institutes of Health (Grants No. R01 CA102504; 5KL2RR025015-03 to K.F.S.; R25 CA94880 and 2R25CA057699 to A.K.); and Canadian Institutes of Health Research (Fellowships to K.L.C. and C.M.). None of the funding agencies were involved in the trial design or conduct.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00470119.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Anne McTiernan, Merck (C), Metagenics (C), Novartis (C), Proctor & Gamble (C), ZymoGenetics (C) Stock Ownership: Anne McTiernan, Merck Honoraria: Anne McTiernan, Novartis, Wyeth Research Funding: Anne McTiernan, Wyeth Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Catherine M. Alfano, Ching-Yun Wang, Anne McTiernan
Administrative support: Catherine R. Duggan, Ikuyo Imayama, Angela Kong, Liren Xiao, Carolyn E. Bain
Provision of study materials or patients: George L. Blackburn
Collection and assembly of data: Kristin L. Campbell, Karen E. Foster-Schubert, Catherine M. Alfano, Catherine R. Duggan, Angela Kong, Carolyn E. Bain
Data analysis and interpretation: Kristin L. Campbell, Karen E. Foster-Schubert, Chia-Chi Wang, Ching-Yun Wang, Catherine R. Duggan, Caitlin Mason, Ikuyo Imayama, Liren Xiao, George L. Blackburn, Frank Z. Stanczyk
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Vainio H, Bianchini F International Agency for Research on Cancer WHO, editor. IARC Handbooks of Cancer Prevention. Lyon, France: IARC Press; 2002. Weight control and physical activity. [Google Scholar]
- 2.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 4.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8:205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 5.Key T, Appleby P, Barnes I, et al. Endogenous Hormones and Breast Cancer Collaborative Group: Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studie. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 6.Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: Reanalysis of 13 studies. Br J Cancer. 2011;105:709–722. doi: 10.1038/bjc.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolcott CG, Shvetsov YB, Stanczyk FZ, et al. Plasma sex hormone concentrations and breast cancer risk in an ethnically diverse population of postmenopausal women: The Multiethnic Cohort Study. Endocr Relat Cancer. 2010;17:125–134. doi: 10.1677/ERC-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamimi RM, Byrne C, Colditz GA, et al. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99:1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 9.Sieri S, Krogh V, Bolelli G, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: The ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:169–176. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 10.Gruber CJ, Tschugguel W, Schneeberger C, et al. Production and action of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 11.Chan MF, Dowsett M, Folkerd E, et al. Usual physical activity and endogenous sex hormones in postmenopausal women: The European prospective investigation into cancer-norfolk population study. Cancer Epidemiol Biomarkers Prev. 2007;16:900–905. doi: 10.1158/1055-9965.EPI-06-0745. [DOI] [PubMed] [Google Scholar]
- 12.Madigan MP, Troisi R, Potischman N, et al. Serum hormone levels in relation to reproductive and lifestyle factors in postmenopausal women (United States) Cancer Causes Control. 1998;9:199–207. doi: 10.1023/a:1008838412423. [DOI] [PubMed] [Google Scholar]
- 13.Verkasalo PK, Thomas HV, Appleby PN, et al. Circulating levels of sex hormones and their relation to risk factors for breast cancer: A cross-sectional study in 1092 pre- and postmenopausal women. Cancer Causes Control. 2001;12:47–59. doi: 10.1023/a:1008929714862. [DOI] [PubMed] [Google Scholar]
- 14.van Gils CH, Peeters PH, Schoenmakers MC, et al. Physical activity and endogenous sex hormone levels in postmenopausal women: A cross-sectional study in the Prospect-EPIC Cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:377–383. doi: 10.1158/1055-9965.EPI-08-0823. [DOI] [PubMed] [Google Scholar]
- 15.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 16.McTiernan A, Wu L, Chen C, et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 2006;14:1662–1677. doi: 10.1038/oby.2006.191. [DOI] [PubMed] [Google Scholar]
- 17.Baglietto L, English DR, Hopper JL, et al. Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 2009;115:171–179. doi: 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- 18.McTiernan A, Tworoger SS, Rajan KB, et al. Effect of exercise on serum androgens in postmenopausal women: A 12-month randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2004;13:1099–1105. [PubMed] [Google Scholar]
- 19.McTiernan A, Tworoger SS, Ulrich CM, et al. Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res. 2004;64:2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 20.Friedenreich CM, Woolcott CG, McTiernan A, et al. The Alberta Physical Activity and Breast Cancer Prevention Trial: Sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monninkhof EM, Velthuis MJ, Peeters PH, et al. Effect of exercise on postmenopausal sex hormone levels and role of body fat: A randomized controlled trial. J Clin Oncol. 2009;27:4492–4499. doi: 10.1200/JCO.2008.19.7459. [DOI] [PubMed] [Google Scholar]
- 22.Berrino F, Bellati C, Secreto G, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: The diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10:25–33. [PubMed] [Google Scholar]
- 23.Boyar AP, Rose DP, Loughridge JR, et al. Response to a diet low in total fat in women with postmenopausal breast cancer: A pilot study. Nutr Cancer. 1988;11:93–99. doi: 10.1080/01635588809513975. [DOI] [PubMed] [Google Scholar]
- 24.Crighton IL, Dowsett M, Hunter M, et al. The effect of a low-fat diet on hormone levels in healthy pre- and postmenopausal women: Relevance for breast cancer. Eur J Cancer. 1992;28A:2024–2027. doi: 10.1016/0959-8049(92)90252-w. [DOI] [PubMed] [Google Scholar]
- 25.DeWaard F, Schwarz F. Weight reduction and postmenopausal estrogenic effect. Acta Cytol. 1964;8:449–453. [PubMed] [Google Scholar]
- 26.Heber D, Ashley JM, Leaf DA, et al. Reduction of serum estradiol in postmenopausal women given free access to low-fat high-carbohydrate diet. Nutrition. 1991;7:137–139. discussion 139-140. [PubMed] [Google Scholar]
- 27.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 28.Tymchuk CN, Tessler SB, Barnard RJ. Changes in sex hormone-binding globulin, insulin, and serum lipids in postmenopausal women on a low-fat, high-fiber diet combined with exercise. Nutr Cancer. 2000;38:158–162. doi: 10.1207/S15327914NC382_3. [DOI] [PubMed] [Google Scholar]
- 29.Wu AH, Pike MC, Stram DO. Meta-analysis: Dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst. 1999;91:529–534. doi: 10.1093/jnci/91.6.529. [DOI] [PubMed] [Google Scholar]
- 30.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heikkilä K, Harris R, Lowe G, et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: Findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 2009;20:15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- 32.Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 33.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: A systematic review. Br J Cancer. 2006;94:1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MH, Chou YC, Chou WY, et al. Circulating levels of leptin, adiposity and breast cancer risk. Br J Cancer. 2009;100:578–582. doi: 10.1038/sj.bjc.6604913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) doi: 10.1038/oby.2011.76. epub ahead of print on April 14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 38.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 39.Goebelsmann U, Bernstein GS, Gale JA. Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. In: Lepow IH, Crozier R, editors. Vasectomy: Immunologic and Pathophysiologic Effects in Animals and Man. New York, New York: Academic Press; 1979. p. 165. [Google Scholar]
- 40.Probst-Hensch NM, Ingles SA, Diep AT, et al. Aromatase and breast cancer susceptibility. Endocr Relat Cancer. 1999;6:165–173. doi: 10.1677/erc.0.0060165. [DOI] [PubMed] [Google Scholar]
- 41.Södergård R, Bäckström T, Shanbhag V, et al. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 43.Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11:1065–1071. [PubMed] [Google Scholar]
- 44.Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41:366–375. doi: 10.1016/j.amepre.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson RE, Kristal AR, Tinker LF, et al. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 46.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 47.McArdle WD, Katch FI, Katch VL. Exercise Physiology. ed 7. Baltimore, MD: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 48.Schauer JE, Hanson P. Usefulness of a branching treadmill protocol for evaluation of cardiac functional capacity. Am J Cardiol. 1987;60:1373–1377. doi: 10.1016/0002-9149(87)90622-9. [DOI] [PubMed] [Google Scholar]
- 49.Pate R, Blair S, Durstine J. Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Lea & Febinger; 1991. pp. 70–72. [Google Scholar]
- 50.Hays J, Ockene JK, Brunner RL, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 51.Mazess RB, Barden HS, Bisek JP, et al. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 52.Missmer SA, Eliassen AH, Barbieri RL, et al. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 53.Farhat GN, Cummings SR, Chlebowski RT, et al. Sex hormone levels and risks of estrogen receptor-negative and estrogen receptor-positive breast cancers. J Natl Cancer Inst. 2011;103:562–570. doi: 10.1093/jnci/djr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turcato E, Zamboni M, De Pergola G, et al. Interrelationships between weight loss, body fat distribution and sex hormones in pre- and postmenopausal obese women. J Intern Med. 1997;241:363–372. doi: 10.1046/j.1365-2796.1997.120129000.x. [DOI] [PubMed] [Google Scholar]
- 55.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 56.Friedenreich CM. Physical activity and breast cancer: Review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 57.Shaw K, Gennat H, O'Rourke P, et al. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 59.Ackermann RT, Finch EA, Brizendine E, et al. Translating the Diabetes Prevention Program into the community: The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: A comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Vadheim LM, Brewer KA, Kassner DR, et al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health. 2010;26:266–272. doi: 10.1111/j.1748-0361.2010.00288.x. [DOI] [PubMed] [Google Scholar]
- 62.Vadheim LM, McPherson C, Kassner DR, et al. Adapted diabetes prevention program lifestyle intervention can be effectively delivered through telehealth. Diabetes Educ. 2010;36:651–656. doi: 10.1177/0145721710372811. [DOI] [PubMed] [Google Scholar]
- 63.Boltri JM, Davis-Smith M, Okosun IS, et al. Translation of the National Institutes of Health Diabetes Prevention Program in African American churches. J Natl Med Assoc. 2011;103:194–202. doi: 10.1016/s0027-9684(15)30301-1. [DOI] [PubMed] [Google Scholar]
- 64.Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: Clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villareal DT, Apovian CM, Kushner RF, et al. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 66.Daly RM, Dunstan DW, Owen N, et al. Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int. 2005;16:1703–1712. doi: 10.1007/s00198-005-1906-4. [DOI] [PubMed] [Google Scholar]
- 67.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 68.Baim S, Binkley N, Bilezikian JP, et al. Official Positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2008;11:75–91. doi: 10.1016/j.jocd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Anandacoomarasamy A, Caterson I, Sambrook P, et al. The impact of obesity on the musculoskeletal system. Int J Obes (Lond) 2008;32:211–222. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- 70.Hootman JM, Macera CA, Ainsworth BE, et al. Predictors of lower extremity injury among recreationally active adults. Clin J Sport Med. 2002;12:99–106. doi: 10.1097/00042752-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Dignam JJ, Wieand K, Johnson KA, et al. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95:1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 73.Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 74.Goodwin PJ, Pritchard KI. Obesity and hormone therapy in breast cancer: An unfinished puzzle. J Clin Oncol. 2010;28:3405–3407. doi: 10.1200/JCO.2010.29.5113. [DOI] [PubMed] [Google Scholar]
- 75.Connolly GC, Khorana AA. Emerging risk stratification approaches to cancer-associated thrombosis: Risk factors, biomarkers and a risk score. Thromb Res. 2010;125(suppl 2):S1–S7. doi: 10.1016/S0049-3848(10)00227-6. [DOI] [PubMed] [Google Scholar]
- 76.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cuzick J, Forbes JF, Sestak I, et al. Long-term results of tamoxifen prophylaxis for breast cancer: 96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99:272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 78.Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 79.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 80.Veronesi U, Maisonneuve P, Rotmensz N, et al. Tamoxifen for the prevention of breast cancer: Late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99:727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- 81.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 82.World Health Organization, editor. Obesity and Overweight Fact Sheet No. 311. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 83.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.