Abstract
Purpose
The platinum chemotherapy agents cisplatin and carboplatin are widely used in the treatment of adult and pediatric cancers. Cisplatin causes hearing loss in at least 60% of pediatric patients. Reducing cisplatin and high-dose carboplatin ototoxicity without reducing efficacy is important.
Patients and Methods
This review summarizes recommendations made at the 42nd Congress of the International Society of Pediatric Oncology (SIOP) in Boston, October 21-24, 2010, reflecting input from international basic scientists, pediatric oncologists, otolaryngologists, oncology nurses, audiologists, and neurosurgeons to develop and advance research and clinical trials for otoprotection.
Results
Platinum initially impairs hearing in the high frequencies and progresses to lower frequencies with increasing cumulative dose. Genes involved in drug transport, metabolism, and DNA repair regulate platinum toxicities. Otoprotection can be achieved by acting on several these pathways and generally involves antioxidant thiol agents. Otoprotection is a strategy being explored to decrease hearing loss while maintaining dose intensity or allowing dose escalation, but it has the potential to interfere with tumoricidal effects. Route of administration and optimal timing relative to platinum therapy are critical issues. In addition, international standards for grading and comparing ototoxicity are essential to the success of prospective pediatric trials aimed at reducing platinum-induced hearing loss.
Conclusion
Collaborative prospective basic and clinical trial research is needed to reduce the incidence of irreversible platinum-induced hearing loss, and optimize cancer control. Wide use of the new internationally agreed-on SIOP Boston ototoxicity scale in current and future otoprotection trials should help facilitate this goal.
INTRODUCTION
Platinum drugs are effective chemotherapeutic agents commonly used in the treatment of a variety of adult and pediatric cancers.1 Sixty percent of children treated with cisplatin develop permanent bilateral hearing loss.2,3 Although cisplatin is more ototoxic than other platinum-based drugs, carboplatin is also ototoxic, especially when delivered at myeloablative doses for autologous bone marrow transplantation or when administered in conjunction with osmotic opening of the blood-brain barrier.4,5 Once clinically significant toxicity is observed on audiologic monitoring, current practice suggests dose reductions or omissions, potentially reducing cure,6 but the ototoxic damage is already done and the hearing loss is permanent.7 In a young child, this will have a detrimental effect on speech, language, and social development.2,3 Further research is needed to clarify the mechanisms of platinum ototoxicity, improve methods of reducing irreversible hearing loss,2,3,8,9 and permit maintenance or escalation of platinum dose intensity to improve cancer control. The development of cotherapies aimed at preventing platinum ototoxicity requires collaboration between experts in auditory systems, cancer therapeutics, drug interactions, and clinical oncology to ensure that proposed otoprotectants do not reduce the platinum agents' potent tumoricidal activity.10–12
This article summarizes the work of four groups of experts (Appendix Table A1, online only) in the fields of basic science, genetics, ototoxicity monitoring, and clinical trials in otoprotection. Each of the groups included European and American experts who met through telephone conferences and prepared a working document that was presented at a symposium on chemotherapy-induced ototoxicity at the 42nd Congress of the International Society of Pediatric Oncology (SIOP) in Boston in October 2010. Attendees at the international symposium were invited to join breakout sessions following the symposium to share their expertise and contribute to a draft report. The essence of those four working group summary reports and recommendations are presented here.
MECHANISMS OF PLATINUM-INDUCED OTOTOXICITY
In preclinical studies, cisplatin has been the platinum agent most frequently investigated in guinea pigs, mice, rats, and other rodents. Induction of consistent ototoxicity with cisplatin requires a high dose with either intraperitoneal or intravenous administration; however, a single low dose is ototoxic if infused retrograde into the common carotid artery,13,14 likely because of first-pass high-dose perfusion of the vertebral arteries feeding the cochlea.
Platinum agents induce dose-dependent death of cochlear hair cells, with outer hair cells more susceptible to cisplatin and inner hair cells more susceptible to carboplatin15,16 in some animal models. However, in the rat, carboplatin primarily targets outer hair cells.17 Cochlear hair cell death is first evident at the cochlear base and progresses apically with continued exposure to the drug.15,18 Platinum agents target the DNA of proliferating cells to exert tumoricidal effects.19,20 Inside the cell, cisplatin is activated by the replacement of one of its two chloride groups by a water molecule, and carboplatin is activated by replacement of the cyclobutane moiety. The activated monoaqua-platin binds to DNA, forming intra- and interstrand complexes that lead to inhibition of DNA synthesis, suppression of RNA transcription, cell cycle arrest, and apoptosis. In contrast to tumor cells, cochlear and proximal tubule cells proliferate slowly, and mammalian cochlear hair cells not at all. In these cells, cisplatin alkylation in mitochondria leads to release of proapoptotic factors and generation of toxic levels of reactive oxygen species (ROS), both of which can initiate cell death mechanisms through caspase activation.21–23 Hair cell death is significantly inhibited (or at least delayed) by broad-spectrum inhibition of caspases, which are highly involved in apoptosis, thought to be a mechanism of hair cell death.24–27 Cytosolic ROS formation has also been implicated as a major mediator of cisplatin-induced hair cell death.28–30 Increased pools of ROS not only damage proteins and lipids but also deplete the cell's intrinsic antioxidant molecules potentiating further damage.31,32
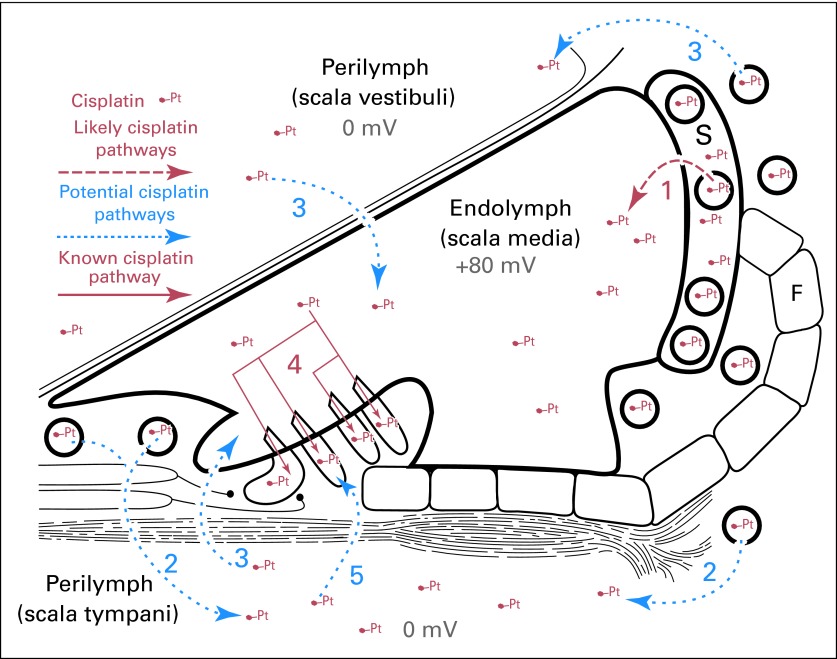
Cisplatin also induces degeneration of the stria vascularis, decreasing the number of marginal and intermediate cells as well as spiral ganglion cells.33,34 Inner ear sensory cells reside within a blood-labyrinth barrier (BLB; Fig 1), similar to the blood-brain barrier. Any breakdown in the cellular integrity or increase in paracellular permeability (decoupling of tight junctions) between adjacent endothelial cells in the BLB rapidly induces loss of the endolymphatic potential with consequent loss of hearing sensitivity. Although platinum is largely excluded by the blood-brain barrier,36,37 it can be detected in cochlear tissues, indicating that it does cross the intact BLB (Fig 1),38,39 but the trafficking mechanism remains poorly understood.40,41 A clear understanding of BLB function is critical to studies aimed at inhibiting the entry of platinum (and other ototoxic agents) into cochlear tissues or delivering potential otoprotective molecules to the cochlea to reduce ototoxicity.
Fig 1.
Model of the cochlea and cisplatin (Pt) trafficking routes. Potential pathways for systemic Pt to cross the blood-labyrinth barrier and enter hair cells include (1) a trans-strial trafficking route from strial capillaries to marginal cells, followed by clearance into endolymph; (2,3) traversing the blood-labyrinth barrier into perilymph and subsequently into endolymph via transcytosis across the epithelial perilymph/endolymph barrier. (4) Once in endolymph, Pt enters hair cells across their apical membranes. (5) Pt in the scala tympani could also pass through the basilar membrane into extracellular fluids within the organ of Corti and enter hair cells across their basolateral membranes. S, stria vascularis; F, fibrocytes in spiral ligament (data adapted35).
GENETICS OF OTOTOXICITY
Platinum toxicity shows significant interindividual variability since 20% or more of children are seemingly not affected, and there is some evidence to support ethnic/racial variability.42 These observations have led to the hypothesis that genetic factors may render certain individuals more susceptible to the adverse effects of cisplatin.43–45 The field of pharmacogenomics seeks to explore this interindividual variability in drug response and identify genetic predictors of cisplatin-induced hearing loss. A literature search of candidate genes involved in platinum-induced ototoxicity is summarized in Table 1.
Table 1.
Results of Published Studies in Cisplatin Pharmacogenomics Using Candidate Gene Approach
| Gene/Protein | Summary of Results |
|---|---|
| Megalin | Selected for candidate gene approach because it is highly expressed in renal proximal tubular cells and marginal cells of the inner ear. Also associated with the uptake of ototoxic aminoglycosides.46 |
| GSTs | Animal studies suggest GSTs are found in the cochlea and have a role in protection from ototoxicity. The GSTM1, GSTT1, and GSTP1 genes are polymorphic in humans, and nonfunctional variants are commonly found in whites.47 |
| TPMT, COMT | Two cohorts (identified through the Canadian Pharmacogenomics Network for Drug Safety) were evaluated for cisplatin toxicity.42 They used a gene chip composed of variants in 220 drug metabolism genes and found that genetic variants of TPMT (odds ratio, 17) and COMT (odds ratio, 5.5) were significantly associated with cisplatin-induced hearing loss. The combination of TPMT and COMT genotypes could be used as a clinical test to identify those who will have cisplatin-induced deafness with a positive predictive value of 92.9% and a negative predictive value of 48.6%.42 Mechanisms of toxicity include increased efficiency of cisplatin cross-linking, as well as a possible role of the methionine pathway through a common substrate, S-adenosylmethionine.42 |
| ERCC1, ERCC2 | ERCC1 encodes an excision repair enzyme involved in platinum DNA adduct repair.48 Two common single nucleotide polymorphisms in ERCC1 are correlated with an increased risk of both toxicity and survival in adults with non–small-cell lung tumors.49,50 |
| Mitochondrial gene mutations | No studies have been performed that have evaluated for associations between mitochondrial gene mutations and cisplatin-induced hearing loss. Aminoglycoside-induced deafness is thought to be associated with mutations in the mitochondrial 12S ribosomal RNA gene.51–53 |
Abbreviations: COMT, catechol-O-methyltransferase; ERCC1, excision repair cross-complementation group 1; ERCC2, excision repair cross-complementation group 2; GST, glutathione-S-transferase; TPMT, thiopurine S-methyltransferase.
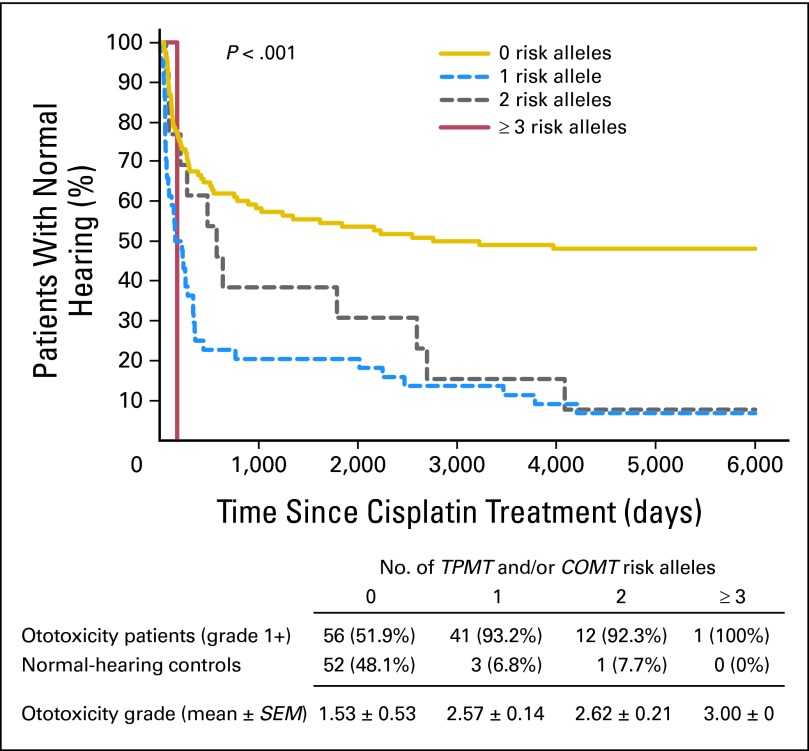
In a recent study,42 genetic variations in two specific genes, thiopurine S-methyltransferase (TPMT) and catechol-O-methyltransferase (COMT), were identified as having a strong association with cisplatin-induced ototoxicity in children (Fig 2). TPMT and COMT variants were found to be associated with severe cisplatin-induced hearing loss (combined odds ratio, 42.2; P < .001). Furthermore, the number of risk alleles carried by an individual was inversely related to time to deafness; those who carried at least three of four risk alleles had a rapid decline in their hearing, often with their first dose of cisplatin. The combination of TPMT and COMT genotypes could be used as a clinical test to identify individuals more likely to develop cisplatin-induced deafness with a positive predictive value of 92.9% and a negative predictive value of 48.6%.42 Whether treatment can be adapted for an individual patient following on from these results will depend on the potential alternative treatments available and balance of risks for each child and each tumor type. Similarly, genes involved in cisplatin-DNA adduct repair (ERCC1, ERCC2) can increase the risk of cisplatin-associated toxicity but may also carry a tumor cell survival advantage. This is because there are molecular factors that not only play a role in platinum's mode of action but also interfere with the ability of the drug to induce apoptosis (Table 1).19,47,49,50,54 Target tissues within the cochlea may show a variable genetic susceptibility to platinum, and genetic variation in the detoxification of platinum within the cochlea may contribute to the severity of ototoxicity.47 Investigators have also been interested in genetic variation in the glutathione S-transferase genes with somewhat conflicting results in adults: one group identifies an association with GSTM3,47 and another group identifies an association with GSTP1.55 Replication of genotype-phenotype findings is needed in both candidate gene and genome-wide approaches to evaluate validity and applicability in the clinic.56,57 All of the studies done to date are limited by the fact that they are retrospective in nature; thus, prospective evaluation of these genetic variations through future studies is urgently needed.
Fig 2.
Kaplan-Meier graph of cisplatin ototoxicity and number of thiopurine S-methyltransferase (TMPT) and catechol-O-methyltransferase (COMT) risk alleles. An increasing number of TPMT rs12201199 and COMT rs9332377 risk alleles is associated with earlier onset of cisplatin-induced hearing loss (P < .001) and with more severe cisplatin-induced hearing loss (P < .001; adapted by permission from Macmillan Publishers: Nature Genetic, 200942).
The challenges to these studies are that false-positive findings may occur that are not reproducible because of small sample size, inadequate phenotyping, poor case-control definition and/or use of patients from different ancestries. Moreover, monogenic approaches may underestimate susceptibility to platinum ototoxicity because it is likely that multiple genetic pathways are involved in the metabolism, transport, and detoxification of platinum. Future research will require a polygenic approach and novel methodologies.58 Cost reduction and new techniques in whole-genome sequencing should permit large-scale projects if adequate sample sizes of well-characterized phenotypes become available. Inclusion of genetic studies in pediatric treatment studies with standardized audiologic assessment is essential so that the phenotyping will be adequate to identify ototoxicity susceptibility alleles.
OTOTOXICITY GRADING
Platinum ototoxicity is sensorineural and typically bilateral, initially impairing hearing in the high frequencies and progressing to lower frequencies with increasing cumulative dose.6,7 The risk is greatest in young children, and there are significant long-term implications, particularly if the children are prelingual or in the early stages of language development2 or have other functional impairments such as visual deficits or cognitive dysfunction. Since high-frequency speech sounds are critical to speech intelligibility, even mild hearing loss in the high frequencies may affect academic and social-emotional development in young children.59–63 Acquired hearing loss can be addressed with hearing assistive technology, speech-language therapy, and/or the use of communication strategies. It is essential to appreciate that although these interventions may reduce the negative consequences of the hearing loss, they do not restore normal hearing. If we are to succeed in conducting prospective pediatric clinical trials to reduce platinum ototoxicity and compare patients, disease groups, candidate genes, and otoprotective agents, it is critically important to adopt an international standard for grading and comparing ototoxicity at the end of therapy.64
Impact of Ototoxicity in Children
Compared with adults and adolescents, young children require greater audibility for speech recognition and comprehension. Young children do not have the language base or neurologic maturity to fill in the gaps when acoustic access is compromised.65 Hearing loss decreases the audibility of speech and also reduces the clarity of speech.61,62 Platinum ototoxicity initially affects high-frequency hearing. When low-frequency hearing is preserved, children continue to hear vowel sounds, intonation, nasality, and consonants that have primary energy in the lower frequencies. High-frequency hearing loss causes difficulty in distinguishing high-frequency consonants (s, sh, f, t, z, th, h, k, p) that are critical for speech intelligibility, and it significantly impairs recognition of speech in the presence of background noise.66–68 For children developing language and vocabulary who are learning spoken language through listening, high-frequency hearing loss is communicatively and educationally significant.65 Gurney et al63 studied educational achievement and quality of life in 137 neuroblastoma survivors. Children with hearing loss were reported as having twice the rate of educational difficulties and need for support services or special education.
High-frequency hearing loss in older children and adolescents has an impact on ease of listening and may negatively affect educational achievement and social-emotional development.60 Learning in a classroom environment is highly dependent on hearing and listening. Poor classroom acoustics (noise and reverberation) compound the perceptual deficits caused by hearing loss.
Ototoxicity Grades and Classification
Numerous ototoxicity criteria or grading systems have been developed and used to classify hearing loss in children, but in the clinical trial setting, uniformity is essential. There are currently two main types of ototoxicity assessment criteria: (1) those that rely on change of hearing from baseline, including WHO Common Toxicity Criteria,69 National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE),70 protocol criteria from Children's Cancer Group A9961 (CCG-A9961; phase III intergroup average-risk medulloblastoma protocol71), and the Children's Hospital Boston (CHB) scale72), and (2) those specifically written for children that measure absolute hearing levels, including Brock et al7 and Chang and Chinosornvatana73 (hereafter Brock and Chang), and the new SIOP Boston scale proposed in this article. The new scale detailed in Table 2, which all participants agreed on, combines the best elements from all the assessment criteria. This new scale will make it possible to compare clinical trial outcomes world-wide.
Table 2.
SIOP Boston Ototoxicity Scale
| Grade | Parameters |
|---|---|
| 0 | ≤ 20 dB HL at all frequencies |
| 1 | > 20 dB HL (ie, 25 dB HL or greater) SNHL above 4,000 Hz (ie, 6 or 8 kHz) |
| 2 | > 20 dB HL SNHL at 4,000 Hz and above |
| 3 | > 20 dB HL SNHL at 2,000 Hz or 3,000 Hz and above |
| 4 | > 40 dB HL (ie, 45 dB HL or more) SNHL at 2,000 Hz and above |
NOTE. Scale is based on sensorineural hearing thresholds in dB hearing level (HL; bone conduction or air conduction with a normal tympanogram). Bone conduction thresholds are used to determine the grade in the case of abnormal tympanometry and/or suspected conductive or mixed hearing loss. Even when the tympanogram is normal, bone conduction is strongly recommended at the single frequency that is determining the ototoxicity grade to fully confirm that the hearing loss at that frequency is sensorineural. Temporary, fluctuating conductive hearing loss due to middle ear dysfunction or cerumen impaction is common in the pediatric population, and decreases in hearing thresholds that include conductive hearing losses do not reflect ototoxicity to the cochlea.
Abbreviations: SIOP, International Society of Pediatric Oncology; SNHL, sensorineural hearing loss.
Classification of ototoxicity in children should be objective, sensitive, reliable, valid, functionally relevant, applicable to results obtained at any age, and simple to understand and describe. The primary intent of any scale will depend on whether its purpose is to guide treatment decisions, identify ototoxicity at the soonest possible opportunity during treatment, or report the incidence and severity of acquired hearing loss in children at the completion of treatment for comparison of clinical trials. The SIOP scale is intended to be used for patients at the end of treatment on a clinical trial (Table 2). It is sensitive to high-frequency hearing losses that result in reduced audibility of the average speech spectrum, and it uses the criteria that correspond to functional outcomes, including the need for audiologic interventions such as hearing aids and other assistive technologies. The scale was based on a modification of the CHB functional scale,72 which classifies hearing loss as grade 1, 2, or 3 on the basis of change in hearing threshold of 20 dB or more compared with baseline measures. The CHB scale was validated by using the Brock scale which, in a multivariate analysis, showed that cisplatin dose was a significant predictor of hearing loss. The CHB scale was favored for its simplicity and objectivity, but two main modifications were recommended. The first was to use absolute hearing levels similar to those of Brock and Chang. The second was to add a grade 4 that was equivalent to Brock and Chang grade 3.
The reason for opting for absolute hearing levels is that, although baseline evaluation is the gold standard for ototoxicity monitoring and obtaining a baseline is recommended for all children who are treated with cisplatin, it has been recognized for many years that a complete and reliable baseline evaluation is not always possible in young children with cancer. Children are often quite sick, they may be fearful in the clinical setting, and attention or cooperation may be limited. When grading is based on change from baseline, audiograms from children without a baseline are not gradable. Furthermore, absolute hearing threshold levels after cessation of treatment, rather than change from baseline, determine whether an individual child has sufficient acoustic access to all of the speech sounds for everyday listening situations, including distance hearing and the ability to understand speech in a noisy environment.
Grade 4 was added, equivalent to Brock and Chang grade 3, to distinguish children who acquire moderate or greater ototoxic hearing loss from those with milder impairment, since there are important functional and clinical differences as the degree of hearing loss increases. A minor modification was to expand grade 3 to include hearing loss greater than 20 dB at 2,000 or 3,000 Hz, since audibility at both 2,000 and 3,000 Hz is critical for speech intelligibility, and loss at either of these frequencies is commonly used as the indication for hearing aids in children.
The SIOP Boston ototoxicity scale is being validated on existing data that include international multicenter audiologic results in very young children treated with cisplatin. Results will be directly compared with existing scales (CTCAE versions 3 and 4; Brock and Chang) to determine whether the SIOP scale better correlates with functional outcomes and offers improved simplicity and inter-rater reliability. Results will be submitted for future publication and the SIOP scale will be recommended if the study outcomes are positive.
OTOPROTECTION
Several promising otoprotective agents are in preclinical and clinical development. The challenge is how to select the best products for further investigation, how to evaluate their efficacy and safety, and how to introduce them into clinical practice.
Preclinical Studies
Activated platinum agents react preferentially with antioxidant molecules, particularly glutathione and metallothioneins.74 In cancer cells with high levels of glutathione, platinum can be effectively bound by the glutathione, inhibiting DNA binding and reducing the chemotherapeutic efficacy in these tumors.75 Cisplatin-induced ototoxicity is reduced in animal models by a variety of antioxidants, including N-acetyl-cysteine (NAC),13,14,76–78 alpha-tocopherol,79,80 lipoic acid,81–83 sodium thiosulfate (STS),14,17,84–87 salicylate,88 ebselen,82,89 d-methionine,90 and amifostine.86,91
Preclinical studies have demonstrated that choice of species, dosing protocol, route of administration, and optimal timing relative to platinum therapy are critical issues.13,14,72,87,92–94 Intravenous or intra-arterial administration of NAC is required to achieve the high concentration necessary for otoprotection, since oral administration does not provide effective concentrations.13,14,78,87 Strategies for localized delivery of protective molecules include transtympanic or round window delivery,77,92,95 although this method has had variable success in animal models to date, depending on the agent.96,97 [scap]d-methionine has shown complete otoprotection with round window application or oral administration in animal studies.96,98,99 As is the case with any chemoprotectant, systemic administration of otoprotectants must address whether the protective agent interferes with the tumoricidal effect of platinum. Delayed administration of a protective agent such as STS or NAC may provide hearing protection4,5,13,14 without compromising anticancer therapy87,100,101 (Fig 3).
Fig 3.
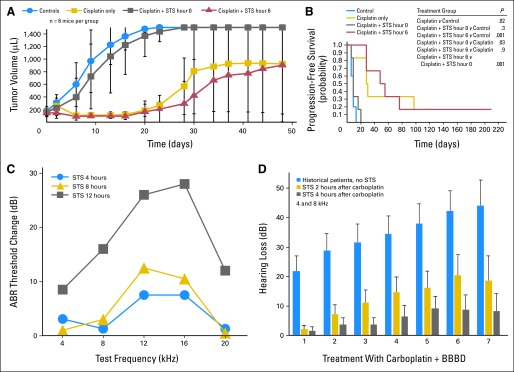
Chemoprotection studies. (A) Effect of sodium thiosulfate (STS) and cisplatin on subcutaneous human neuroblastoma xenograft growth. Nude mice were inoculated subcutaneously with 3.2 × 107 SMS-SAN neuroblastoma cells and were treated with no treatment (blue circles; n = 5), cisplatin 4 mg/kg intraperitoneally (IP) × 4 days (gold squares; n = 6), cisplatin 4 mg/kg per day × 4 days plus STS 3.5 g/kg per day IP × 4 days immediately (0 hours) after cisplatin (gray squares; n = 6), or cisplatin 4 mg/kg per day IP × 4 days plus STS 3.5 g/kg per day IP × 4 days at 6 hours after cisplatin (red triangles; n = 6). Tumor volumes were measured twice per week (data adapted100). (B) Time to tumor progression (tumor volume of > 600 μL or last measurement taken) was determined. The probability of progression-free survival for the four treatment groups was determined by using the permutated log-rank test. STS given 6 hours after cisplatin daily for 4 days did not significantly (P = .9) affect cisplatin antitumor activity in SMS-SAN xenografts in athymic nu/nu mice compared with cisplatin alone. However, STS given simultaneously with cisplatin daily for 4 days did significantly (P = .03) protect tumors from cisplatin (data adapted100). (C) Cisplatin induced changes in auditory brainstem response (ABR) threshold when STS was given at 4, 8, or 12 hours after cisplatin in a rat model. As illustrated, delay of 4 or 8 hours was highly otoprotective, whereas delay of 12 hours was not otoprotective (data adapted14). (D) Comparison of threshold shift against carboplatin treatment number (at 4,000 Hz) in historical comparison patients who received carboplatin without STS and in patients treated with STS delayed 2 hours or 4 hours after carboplatin. There was a significant difference between the STS treatment groups and the historical comparison control group (P = .0075; Adapted and reprinted by permission from the American Association for Cancer Research4). BBBD, blood-brain barrier disruption.
Clinical Studies
Antioxidants that have been tested as otoprotectants in clinical trials in humans receiving platinum-based chemotherapy are amifostine69–71,102 and STS.4,5 To the best of our knowledge, the only otoprotection study completed in the cooperative oncology group setting to date is CCG-P9645, a randomized controlled trial of amifostine for prevention of cisplatin-induced hearing loss in children with newly diagnosed hepatoblastoma, conducted by the Children's Oncology Group (COG) from 1999 to 2006.102 Amifostine did not provide otoprotection when the suggested dose and schedule were used, and it was accompanied by hypocalcemia. However, a more dose-intensive schedule of amifostine tested in a comparative cohort study71 was reported to reduce ototoxicity in children treated with cisplatin for medulloblastoma. Both the COG, which fully accrued as of March 2012, and the International Pediatric Oncology Epithelial Liver Tumor Strategy Group (SIOPEL) are currently conducting randomized controlled trials of STS to prevent cisplatin-induced hearing loss. Further information can be found at the National Cancer Institute Physician Data Query (NCI-PDQ) Clinical Trials Web site.103 Both of these phase III STS studies are based on phase II studies of carboplatin followed by delayed STS (Fig 3D).4 In addition, these studies have also incorporated DNA collection from patients, which allows pharmacogenomic studies to be performed.
Emerging Agents
Several properties or characteristics constitute the ideal pediatric otoprotectant: it must be effective (reliable otoprotection), be safe (no tumor protection), have minimal adverse effects, use simple administration techniques, be suitable for use with various platinum compounds and schedules of administration, and be of sufficient interest to the pharmaceutical industry for investment in research and development.
Currently no pharmacologic agents have US Food and Drug Administration (FDA) approval to prevent or reverse platinum-induced hearing loss, although STS has orphan-drug designation as an otoprotectant. The development of amifostine, STS, NAC, d-methionine, and ebselen are summarized in Table 3. NAC is well established as being safe in humans, shows good promise as an otoprotectant, has the added benefit of providing nephroprotection from cisplatin,11,12 and when combined with STS, it may protect the bone marrow from carboplatin toxicity.101 We recommend completing or initiating pediatric clinical trials with STS, NAC, d-methionine, and possibly ebselen, depending on further findings as these drugs are developed.
Table 3.
Representative Emerging Otoprotectants for Use With Platinum-Based Chemotherapy
| Agent | Route | Mechanism | Comment |
|---|---|---|---|
| STS | IV | Thiol-reducing agent | In rats, STS protects against ototoxicity14 without reducing antitumor efficacy.101 Currently in phase III trials. Possible approaches include delayed administration, 14,87,100 two-compartment models, 4,5,104 and cochlear application.85,96 |
| Amifostine | IV | Metabolized to WR-1065, a thiol-reducing agent | Most trials show no otoprotection; dose intensity may be critical; routine use of amifostine to prevent platinum-associated neurotoxicity or ototoxicity is not currently supported by the American Society of Clinical Oncology 2008 Clinical Practice Guideline.105 |
| NAC | IV | Thiol-reducing agent | High dose (1,000 mg/kg) IV or intra-arterial NAC protects against cisplatin ototoxicity in the rat when given either 30 minutes prior to or 4 hours after chemotherapy and also blocks kidney toxicity and weight loss.14,78 Delayed IV NAC does not block chemotherapy antitumor efficacy.101 |
| d-methionine | PO, IV, or delivery to the round window | Glutathione modulator, free-radical scavenger | Animal studies have confirmed d-methionine protection from carboplatin- and cisplatin-induced ototoxicity.99 Effective delivered PO,99 systemically, or to the round window.96 Animal studies have not shown significant antitumor interference.106 One small-scale clinical trial showed complete otoprotection.107 Larger-scale clinical trials will be needed. |
| Ebselen | PO | Glutathione peroxidase promoter | In animal studies, ebselen, a selenium-containing compound, has reduced cisplatin-induced outer hair cell loss with and without allopurinol co-administration89 and does not appear to comprise cisplatin's antitumor efficacy.108 To date, ebselen has not been tested in clinical trials, but trials are in the planning stages. |
| Ringer's solution or dexamethasone | Intratympanic injection | Agent dependent (anti-inflammatory) | Compartmental therapy via tympanostomy tubes.92,95 |
Abbreviations: IV, intravenous; NAC, N-acetylcysteine; PO, orally; STS, sodium thiosulfate.
Another approach to otoprotection is that of anatomic or compartmental therapy, that is, delivery of d-methionine to the round window before systemic treatment with platinum-based chemotherapy.96
CONSIDERATIONS IN CLINICAL STUDY DESIGN FOR OTOPROTECTION
Potential characteristics of clinical trials of otoprotectants will vary according to their phase of drug development. After phase I studies to assess pharmacokinetics, pharmacodynamics, and dose-limiting toxicity, each otoprotectant must be tested in patients receiving ototoxic chemotherapy. Phase II studies will estimate dose-timing response curves and efficacy range with hearing threshold change or proportional incidence of hearing loss as the outcome measure. Randomized phase III study designs will be necessary to confirm protection against ototoxicity (n = 100 to 250 patients). Exact sample sizes will depend on the characteristics of the study agent and the statistical parameters used (eg, effect size and desired significance level). Pharmacogenomic testing that leads to only patients at high risk for ototoxicity being included in clinical trials may enable smaller sample sizes to be used for these protectant trials.
Pursuing the two major goals of efficacy (reducing ototoxicity) and safety (not compromising chemotherapeutic antitumor activity) within the same clinical trial presents serious challenges in statistical design. The sample size required for proving superiority in otoprotection is usually substantially smaller than that required for demonstrating noninferiority in tumor control.109 Study designers must choose which end point should control power calculations. Given the justifiable concern for ensuring patient safety (ie, lack of tumor protection), there may be a temptation to insist on completion of classical noninferiority trials involving sample sizes of several hundred patients. However, a classical noninferiority trial of this type is not feasible in pediatric oncology, because accrual of adequate numbers of children with cisplatin-sensitive cancers would take many years and would lock up limited clinical trials resources in the interim.109 A trial in adults to justify the pediatric indication may not provide a definitive solution because hearing loss is not the dose-limiting cisplatin toxicity in adults that it is in children,110 and common adult tumors treated with cisplatin are insufficiently chemotherapy-sensitive to serve as a marker for tumor protection.
A more novel approach than the traditional noninferiority study is critically needed to optimize safety in a practical way that permits effective otoprotectants to be developed and made commercially available. Lacking such innovation, the field of otoprotection and the children who stand to benefit from protective agents are condemned to the status quo—life with significant hearing loss, the associated educational and social costs, and the risk of reduced cancer control with platinum reduction or omission. One approach for these unique pediatric situations may be for regulatory agencies such as the FDA to accept a combination of preclinical studies that are unequivocal on the tumor protection question plus smaller clinical trials in children that are compelling for hearing protection and at least reassuring against tumor protection. Development of such a strategy will require a partnership of committed individuals in academic medicine, the pharmaceutical industry, and FDA.
Strategies for improving the safety and efficiency of trial designs include combining trials (eg, phase IIIA and IIIB could be designed in one trial with an interim analysis of otoprotection and a final analysis of antitumor efficacy). Safety can be enhanced by incorporating an interim futility analysis on the otoprotection question, which limits risk to future patients by identifying an ineffective agent before study completion. Another strategy is to devise a method for monitoring early tumor responses in an initial study cohort. Although this approach will likely lack statistical significance because of the small number of patients, it may serve as an early warning system to detect major, unanticipated treatment failures. Once one or more safe and effective otoprotectants have been identified, future trials of a new agent may need to incorporate an established agent, rather than observation, as the control arm.
Clinical trials of otoprotection may be conducted in the setting of single institutions, multiple collaborating institutions (a consortium), or larger cooperative oncology groups. In planning and designing future studies, it is imperative that anticipated concerns of treating pediatric oncologists about tumor protection be addressed as thoroughly as possible in the concept proposal stage by using available preclinical and clinical data, and for experienced pediatric audiologists to be involved in determining the study end points and methods.111 Central review of the audiologic data are recommended to ensure that maximal evaluable data will be available during the analytic phase of the study.
RECOMMENDATIONS
Mechanisms to foster translation from basic science to clinical practice are needed, as is more research regarding mechanisms of platinum ototoxicity, trafficking of platinum to cochlear sensory cells, and development of clinically relevant animal models for studying ototoxicity and otoprotectants. Collaboration between the pharmacogenomic community and basic scientists to investigate potential new pathways and biologic understanding could result in novel strategies.
New technologies and cost reduction now make relevant pharmacogenomic research possible. Identification of genotypes that are at high risk for ototoxicity and novel clinical trial design could increase the power of clinical studies and decrease the sample size needed to demonstrate effect. Cooperative groups that focus on hearing loss should collect DNA samples for research. Audiologic results are a key end point in the study of otoprotective agents, and they provide the phenotypes for pharmacogenomic ototoxicity research. It is critical that high-quality, reliable audiologic data be obtained. International standardization and wide use of the SIOP Boston ototoxicity scale (Table 2) will allow for comparison between studies and replication of results.
It is feasible to conduct otoprotection trials in the pediatric cooperative oncology group setting as with STS, but cooperative groups need to include otoprotection as a high scientific priority. Additional innovative study designs that measure otoprotection need to be generated to modify standard tumor-related phase III trials, possibly through a task force that involves key scientific disciplines and stakeholders, including pediatric oncologists, audiologists, basic and translational researchers, biostatisticians, clinical pharmacologists, pharmaceutical companies, and patient advocates (particularly parents and childhood cancer survivors). This testing paradigm could be readily applied to new otoprotectants as they become available.
Acknowledgment
We thank Leslie Muldoon and Aliana Culp for their editorial support and Emily Hochhalter for her administrative assistance.
Appendix
Table A1.
Co-Chairs and Members of the Four Working Groups
| Basic Science Research in Ototoxicity |
| Co-Chairs: |
| Peter S. Steyger, Oregon Health & Science University, Portland, OR |
| Brian W. Blakley, University of Manitoba, Winnipeg, Manitoba, Canada |
| Contributors: |
| Kathleen C.M. Campbell, Southern Illinois University School of Medicine, Springfield, IL |
| Lisa L. Cunningham, National Institute on Deafness and Other Communication Disorders, Bethesda, MD |
| Federico Kalinec, House Ear Institute, Los Angeles, CA |
| Leslie L. Muldoon, Oregon Health & Science University, Portland, OR |
| Genetics of Ototoxicity |
| Co-Chairs: |
| Sharad R. Rassekh, University of British Columbia, Vancouver, British Columbia, Canada |
| Michael Sullivan, University of Otago, Christchurch, New Zealand |
| Contributors: |
| Colin J. Ross, University of British Columbia, Vancouver, British Columbia, Canada |
| Jamie L. Renbarger, Indiana University, Indianapolis, IN |
| John M. Maris, The Children's Hospital of Philadelphia, Philadelphia, PA |
| Sharon Diskin, The Children's Hospital of Philadelphia, Philadelphia, PA |
| Nathan Fischel-Ghodsian, University of California at Los Angeles, Los Angeles, CA |
| Ototoxicity Grading System |
| Co-Chairs: |
| Kay W. Chang, Stanford University, Palo Alto, CA |
| Kristin R. Knight, Oregon Health & Science University, Portland, OR |
| Contributors: |
| Carmen Brewer, National Institute on Deafness and Other Communication Disorders, Bethesda, MD |
| Penelope R. Brock, Great Ormond Street Hospital National Health Service Trust, London, United Kingdom |
| Beth Brooks, BC Children's Hospital, Vancouver, British Columbia, Canada |
| Brian J. Fligor, Children's Hospital Boston, Boston, MA |
| Alison Grimes, University of California at Los Angeles Medical Center, Los Angeles, CA |
| Wendy Landier, City of Hope, Duarte, CA |
| Arnold Paulino, The Methodist Hospital System, Houston, TX |
| Kaukab Rajput, Great Ormond Street Hospital National Health Service Trust, London, United Kingdom |
| Lillian Sung, The Hospital for Sick Children, Toronto, Ontario, Canada |
| Joined at breakout meeting in Boston: Barry Pizer, Alder Hey Children's National Health Service Foundation Trust, Liverpool, United Kingdom; Sue Picton, The Leeds Teaching Hospitals National Health Service Trust, Leeds, United Kingdom; and Leanne Super, The Royal Children's Hospital, Melbourne, Australia |
| Clinical Trials of Otoprotectants |
| Co-Chairs: |
| Penelope R. Brock, Great Ormond Street Hospital for Children National Health Service Trust, London, United Kingdom |
| David R. Freyer, Children's Hospital Los Angeles, Los Angeles, CA |
| Contributors: |
| Kathleen C.M. Campbell, Southern Illinois University School of Medicine, Springfield, IL |
| Lillian Sung, The Hospital for Sick Children, Toronto, Ontario, Canada |
| Kristin R. Knight, Oregon State University, Portland, OR |
| Dale Kraemer, Oregon State University, Portland, OR |
| Rudolf Maibach, International Pediatric Oncology Epithelial Liver Tumor Strategy Group (SIOPEL), Bern, Switzerland |
| Leonard Rybak, Southern Illinois University School of Medicine, Springfield, IL |
| Clinton Stewart, St Jude Children's Research Hospital, Memphis, TN |
| Nancy Doolittle, Oregon Health & Science University, Portland, OR |
| Brian W. Blakley, University of Manitoba, Winnipeg, Manitoba, Canada |
Footnotes
See accompanying editorial on page 2303
Supported by Grants No. NS044687 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH), No. CA137488 from the National Cancer Institute, NIH, and by funding from the Walter S. and Lucienne Driskill Foundation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: Oregon Health & Science University (OHSU), Portland Veterans Affairs Medical Center (PVAMC), and the Department of Veterans Affairs have a significant financial interest in Adherex, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest was reviewed and managed by the OHSU Integrity Program Oversight Council and the PVAMC Conflict of Interest in Research Committee. E.A.N. has divested his financial interests in Adherex.
AUTHOR CONTRIBUTIONS
Conception and design: Penelope R. Brock, Kristin R. Knight, David R. Freyer, Kathleen C.M. Campbell, Brian W. Blakley, Kay W. Chang, Edward A. Neuwelt
Collection and assembly of data: Kristin R. Knight, David R. Freyer, Kathleen C.M. Campbell, Brian W. Blakley, Shahrad R. Rassekh, Kay W. Chang, Kaukab Rajput, Michael Sullivan, Edward A. Neuwelt
Data analysis and interpretation: Kristin R. Knight, David R. Freyer, Kathleen C.M. Campbell, Peter S. Steyger, Brian W. Blakley, Shahrad Rod Rassekh, Kay W. Chang, Brian J. Fligor, Edward A. Neuwelt
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.National Cancer Institute. Cancer Drug Information: Cisplatin, May 10, 2011 update. http://www.cancer.gov/cancertopics/druginfo/cisplatin.
- 2.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 3.Knight KR, Kraemer DF, Winter C, et al. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 4.Doolittle ND, Muldoon LL, Brummett RE, et al. Delayed sodium thiosulfate as an otoprotectant against carboplatin-induced hearing loss in patients with malignant brain tumors. Clin Cancer Res. 2001;7:493–500. [PubMed] [Google Scholar]
- 5.Neuwelt EA, Brummett RE, Doolittle ND, et al. First evidence of otoprotection against carboplatin-induced hearing loss with a two-compartment system in patients with central nervous system malignancy using sodium thiosulfate. J Pharmacol Exp Ther. 1998;286:77–84. [PubMed] [Google Scholar]
- 6.Brock P, Pritchard J, Bellman S, et al. Ototoxicity of high-dose cis-platinum in children. Med Pediatr Oncol. 1988;16:368–369. doi: 10.1002/mpo.2950160517. [DOI] [PubMed] [Google Scholar]
- 7.Brock PR, Bellman SC, Yeomans EC, et al. Cisplatin ototoxicity in children: A practical grading system. Med Pediatr Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 8.Brock PR, Yeomans EC, Bellman SC, et al. Cisplatin therapy in infants: Short and long-term morbidity. Br J Cancer Suppl. 1992;18:S36–S40. [PMC free article] [PubMed] [Google Scholar]
- 9.Walker DA, Pillow J, Waters KD, et al. Enhanced cis-platinum ototoxicity in children with brain tumours who have received simultaneous or prior cranial irradiation. Med Pediatr Oncol. 1989;17:48–52. doi: 10.1002/mpo.2950170110. [DOI] [PubMed] [Google Scholar]
- 10.Bhutani M, Pathak AK. Re: Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:1334. doi: 10.1093/jnci/djn279. author reply 1334-1335. [DOI] [PubMed] [Google Scholar]
- 11.Lawenda BD, Kelly KM, Ladas EJ, et al. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100:773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 12.Block K, Koch A, Mead M, et al. Re: Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2009;101:124–125. doi: 10.1093/jnci/djn446. author reply 125-126. [DOI] [PubMed] [Google Scholar]
- 13.Thomas Dickey D, Muldoon LL, Kraemer DF, et al. Protection against cisplatin-induced ototoxicity by N-acetylcysteine in a rat model. Hear Res. 2004;193:25–30. doi: 10.1016/j.heares.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Dickey DT, Wu YJ, Muldoon LL, et al. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther. 2005;314:1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 15.Laurell G, Bagger-Sjöbäck D. Dose-dependent inner ear changes after i.v. administration of cisplatin. J Otolaryngol. 1991;20:158–167. [PubMed] [Google Scholar]
- 16.Hofstetter P, Ding D, Powers N, et al. Quantitative relationship of carboplatin dose to magnitude of inner and outer hair cell loss and the reduction in distortion product otoacoustic emission amplitude in chinchillas. Hear Res. 1997;112:199–215. doi: 10.1016/s0378-5955(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 17.Neuwelt EA, Brummett RE, Remsen LG, et al. In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res. 1996;56:706–709. [PubMed] [Google Scholar]
- 18.Schaefer SD, Post JD, Close LG, et al. Ototoxicity of low- and moderate-dose cisplatin. Cancer. 1985;56:1934–1939. doi: 10.1002/1097-0142(19851015)56:8<1934::aid-cncr2820560807>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 20.Neuwelt AJ, Wu YJ, Knap N, et al. Using acetaminophen's toxicity mechanism to enhance cisplatin efficacy in hepatocarcinoma and hepatoblastoma cell lines. Neoplasia. 2009;11:1003–1011. doi: 10.1593/neo.09688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park MS, De Leon M, Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J Am Soc Nephrol. 2002;13:858–865. doi: 10.1681/ASN.V134858. [DOI] [PubMed] [Google Scholar]
- 22.Bragado P, Armesilla A, Silva A, et al. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007;12:1733–1742. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 23.García-Berrocal JR, Nevado J, Ramírez-Camach R, et al. The anticancer drug cisplatin induces an intrinsic apoptotic pathway inside the inner ear. Br J Pharmacol. 2007;152:1012–1020. doi: 10.1038/sj.bjp.0707405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Staecker H, Stupak H, et al. Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. Neuroreport. 1998;9:2609–2614. doi: 10.1097/00001756-199808030-00034. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng AG, Cunningham LL, Rubel EW. Hair cell death in the avian basilar papilla: Characterization of the in vitro model and caspase activation. J Assoc Res Otolaryngol. 2003;4:91–105. doi: 10.1007/s10162-002-3016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopke RD, Liu W, Gabaizadeh R, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18:559–571. [PubMed] [Google Scholar]
- 29.Dehne N, Lautermann J, Petrat F, et al. Cisplatin ototoxicity: Involvement of iron and enhanced formation of superoxide anion radicals. Toxicol Appl Pharmacol. 2001;174:27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- 30.Bánfi B, Malgrange B, Knisz J, et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 31.Rybak LP, Whitworth CA, Mukherjea D, et al. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Rybak LP, Husain K, Morris C, et al. Effect of protective agents against cisplatin ototoxicity. Am J Otol. 2000;21:513–520. [PubMed] [Google Scholar]
- 33.Sergi B, Ferraresi A, Troiani D, et al. Cisplatin ototoxicity in the guinea pig: Vestibular and cochlear damage. Hear Res. 2003;182:56–64. doi: 10.1016/s0378-5955(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 34.Laurell G, Ekborn A, Viberg A, et al. Effects of a single high dose of cisplatin on the melanocytes of the stria vascularis in the guinea pig. Audiol Neurootol. 2007;12:170–178. doi: 10.1159/000099020. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Kachelmeier A, Steyger PS. Competitive antagonism of fluorescent gentamicin uptake in the cochlea. Hearing Res. 2010;268:250–259. doi: 10.1016/j.heares.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gormley PE, Gangji D, Wood JH, et al. Pharmacokinetic study of cerebrospinal fluid penetration of cis-diamminedichloroplatinum (II) Cancer Chemother Pharmacol. 1981;5:257–260. doi: 10.1007/BF00434394. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs SS, Fox E, Dennie C, et al. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin Cancer Res. 2005;11:1669–1674. doi: 10.1158/1078-0432.CCR-04-1807. [DOI] [PubMed] [Google Scholar]
- 38.van Ruijven MW, de Groot JC, Hendriksen F, et al. Immunohistochemical detection of platinated DNA in the cochlea of cisplatin-treated guinea pigs. Hear Res. 2005;203:112–121. doi: 10.1016/j.heares.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Thomas JP, Lautermann J, Liedert B, et al. High accumulation of platinum-DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not carboplatin ototoxicity. Mol Pharmacol. 2006;70:23–29. doi: 10.1124/mol.106.022244. [DOI] [PubMed] [Google Scholar]
- 40.Choi YK, Kim KW. Blood-neural barrier: Its diversity and coordinated cell-to-cell communication. BMB Rep. 2008;41:345–352. doi: 10.5483/bmbrep.2008.41.5.345. [DOI] [PubMed] [Google Scholar]
- 41.Neuwelt E, Abbott NJ, Abrey L, et al. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 42.Ross CJ, Katzov-Eckert H, Dubé MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 43.Shord SS, Thompson DM, Krempl GA, et al. Effect of concurrent medications on cisplatin-induced nephrotoxicity in patients with head and neck cancer. Anticancer Drugs. 2006;17:207–215. doi: 10.1097/00001813-200602000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Ekborn A, Laurell G, Andersson A, et al. Cisplatin-induced hearing loss: Influence of the mode of drug administration in the guinea pig. Hear Res. 2000;140:38–44. doi: 10.1016/s0378-5955(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 45.Brock PR, Koliouskas DE, Barratt TM, et al. Partial reversibility of cisplatin nephrotoxicity in children. J Pediatr. 1991;118:531–534. doi: 10.1016/s0022-3476(05)83372-4. [DOI] [PubMed] [Google Scholar]
- 46.Riedemann L, Lanvers C, Deuster D, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 47.Peters U, Preisler-Adams S, Hebeisen A, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: The role of DNA repair pathways. Clin Cancer Res. 2008;14:1291–1295. doi: 10.1158/1078-0432.CCR-07-2238. [DOI] [PubMed] [Google Scholar]
- 49.Zhou W, Gurubhagavatula S, Liu G, et al. Excision repair cross-complementation group 1 polymorphism predicts overall survival in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Clin Cancer Res. 2004;10:4939–4943. doi: 10.1158/1078-0432.CCR-04-0247. [DOI] [PubMed] [Google Scholar]
- 50.Suk R, Gurubhagavatula S, Park S, et al. Polymorphisms in ERCC1 and grade 3 or 4 toxicity in non-small cell lung cancer patients. Clin Cancer Res. 2005;11:1534–1538. doi: 10.1158/1078-0432.CCR-04-1953. [DOI] [PubMed] [Google Scholar]
- 51.Prezant TR, Agapian JV, Bohlman MC, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289–294. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- 52.Torroni A, Cruciani F, Rengo C, et al. The A1555G mutation in the 12S rRNA gene of human mtDNA: Recurrent origins and founder events in families affected by sensorineural deafness. Am J Hum Genet. 1999;65:1349–1358. doi: 10.1086/302642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casano RA, Johnson DF, Bykhovskaya Y, et al. Inherited susceptibility to aminoglycoside ototoxicity: Genetic heterogeneity and clinical implications. Am J Otolaryngol. 1999;20:151–156. doi: 10.1016/s0196-0709(99)90062-5. [DOI] [PubMed] [Google Scholar]
- 54.Caronia D, Patiño-García A, Milne RL, et al. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009;9:347–353. doi: 10.1038/tpj.2009.19. [DOI] [PubMed] [Google Scholar]
- 55.Oldenburg J, Kraggerud SM, Cvancarova M, et al. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 56.Impicciatore M. Pharmacogenomic can give children safer medicines. Arch Dis Child. 2003;88:366. doi: 10.1136/adc.88.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.NCI-NHGRI Working Group on Replication in Association Studies. Chanock SJ, Manolio T, et al. Replicating genotype-phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- 58.Eichelbaum M, Ingelman-Sundberg M, Evans WE. Pharmacogenomics and individualized drug therapy. Annu Rev Med. 2006;57:119–137. doi: 10.1146/annurev.med.56.082103.104724. [DOI] [PubMed] [Google Scholar]
- 59.Davis JM, Elfenbein J, Schum R, et al. Effects of mild and moderate hearing impairments on language, educational, and psychosocial behavior of children. J Speech Hear Disord. 1986;51:53–62. doi: 10.1044/jshd.5101.53. [DOI] [PubMed] [Google Scholar]
- 60.Bess FH, Dodd-Murphy J, Parker RA. Children with minimal sensorineural hearing loss: Prevalence, educational performance, and functional status. Ear Hear. 1998;19:339–354. doi: 10.1097/00003446-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 61.Horwitz AR, Dubno JR, Ahlstrom JB. Recognition of low-pass-filtered consonants in noise with normal and impaired high-frequency hearing. J Acoust Soc Am. 2002;111:409–416. doi: 10.1121/1.1427357. [DOI] [PubMed] [Google Scholar]
- 62.Stelmachowicz PG, Pittman AL, Hoover BM, et al. The importance of high-frequency audibility in the speech and language development of children with hearing loss. Arch Otolaryngol Head Neck Surg. 2004;130:556–562. doi: 10.1001/archotol.130.5.556. [DOI] [PubMed] [Google Scholar]
- 63.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: A report from the Children's Oncology Group. Pediatrics. 2007;120:e1229–e1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 64.Neuwelt EA, Brock P. Critical need for international consensus on ototoxicity assessment criteria. J Clin Oncol. 2010;28:1630–1632. doi: 10.1200/JCO.2009.26.7872. [DOI] [PubMed] [Google Scholar]
- 65.Boothroyd A. Developmental factors in speech recognition. Int J Audiol. 1970;9:30–38. [Google Scholar]
- 66.Beattie RC, Barr T, Roup C. Normal and hearing-impaired word recognition scores for monosyllabic words in quiet and noise. Br J Audiol. 1997;31:153–164. doi: 10.3109/03005364000000018. [DOI] [PubMed] [Google Scholar]
- 67.Crandell CC. Speech recognition in noise by children with minimal degrees of sensorineural hearing loss. Ear Hear. 1993;14:210–216. doi: 10.1097/00003446-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 68.Finitzo-Hieber T, Tillman TW. Room acoustics effects on monosyllabic word discrimination ability for normal and hearing-impaired children. J Speech Hear Res. 1978;21:440–458. doi: 10.1044/jshr.2103.440. [DOI] [PubMed] [Google Scholar]
- 69.Gallegos-Castorena S, Martínez-Avalos A, Mohar-Betancourt A, et al. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatr Hematol Oncol. 2007;24:403–408. doi: 10.1080/08880010701451244. [DOI] [PubMed] [Google Scholar]
- 70.Fisher MJ, Lange BJ, Needle MN, et al. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr Blood Cancer. 2004;43:780–784. doi: 10.1002/pbc.20132. [DOI] [PubMed] [Google Scholar]
- 71.Fouladi M, Chintagumpala M, Ashley D, et al. Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma. J Clin Oncol. 2008;26:3749–3755. doi: 10.1200/JCO.2007.14.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis MJ, DuBois SG, Fligor B, et al. Ototoxicity in children treated for osteosarcoma. Pediatr Blood Cancer. 2009;52:387–391. doi: 10.1002/pbc.21875. [DOI] [PubMed] [Google Scholar]
- 73.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 74.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 75.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 76.Feghali JG, Liu W, Van De Water TR. L-n-acetyl-cysteine protection against cisplatin-induced auditory neuronal and hair cell toxicity. Laryngoscope. 2001;111:1147–1155. doi: 10.1097/00005537-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Choe WT, Chinosornvatana N, Chang KW. Prevention of cisplatin ototoxicity using transtympanic N-acetylcysteine and lactate. Otol Neurotol. 2004;25:910–915. doi: 10.1097/00129492-200411000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Dickey DT, Muldoon LL, Doolittle ND, et al. Effect of N-acetylcysteine route of administration on chemoprotection against cisplatin-induced toxicity in rat models. Cancer Chemother Pharmacol. 2008;62:235–241. doi: 10.1007/s00280-007-0597-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fetoni AR, Sergi B, Ferraresi A, et al. Protective effects of alpha-tocopherol and tiopronin against cisplatin-induced ototoxicity. Acta Otolaryngol. 2004;124:421–426. doi: 10.1080/00016480410016559. [DOI] [PubMed] [Google Scholar]
- 80.Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114:538–542. doi: 10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- 81.Rybak LP, Husain K, Whitworth C, et al. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: Antioxidant defense system. Toxicol Sci. 1999;47:195–202. doi: 10.1093/toxsci/47.2.195. [DOI] [PubMed] [Google Scholar]
- 82.Rybak LP, Whitworth C, Somani S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope. 1999;109:1740–1744. doi: 10.1097/00005537-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Mukherjea D, Jajoo S, Whitworth C, et al. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci. 2008;28:13056–13065. doi: 10.1523/JNEUROSCI.1307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaltenbach JA, Church MW, Blakley BW, et al. Comparison of five agents in protecting the cochlea against the ototoxic effects of cisplatin in the hamster. Otolaryngol Head Neck Surg. 1997;117:493–500. doi: 10.1016/S0194-59989770020-2. [DOI] [PubMed] [Google Scholar]
- 85.Wang J, Lloyd Faulconbridge RV, Fetoni A, et al. Local application of sodium thiosulfate prevents cisplatin-induced hearing loss in the guinea pig. Neuropharmacology. 2003;45:380–393. doi: 10.1016/s0028-3908(03)00194-1. [DOI] [PubMed] [Google Scholar]
- 86.Church MW, Kaltenbach JA, Blakley BW, et al. The comparative effects of sodium thiosulfate, diethyldithiocarbamate, fosfomycin and WR-2721 on ameliorating cisplatin-induced ototoxicity. Hear Res. 1995;86:195–203. doi: 10.1016/0378-5955(95)00066-d. [DOI] [PubMed] [Google Scholar]
- 87.Muldoon LL, Pagel MA, Kroll RA, et al. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res. 2000;6:309–315. [PubMed] [Google Scholar]
- 88.Li G, Sha SH, Zotova E, et al. Salicylate protects hearing and kidney function from cisplatin toxicity without compromising its oncolytic action. Lab Invest. 2002;82:585–596. doi: 10.1038/labinvest.3780453. [DOI] [PubMed] [Google Scholar]
- 89.Lynch ED, Gu R, Pierce C, et al. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res. 2005;201:81–89. doi: 10.1016/j.heares.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 90.Campbell KC, Rybak LP, Meech RP, et al. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- 91.Hussain AE, Blakley BW, Nicolas M, et al. Assessment of the protective effects of amifostine against cisplatin-induced toxicity. J Otolaryngol. 2003;32:294–297. doi: 10.2310/7070.2003.11264. [DOI] [PubMed] [Google Scholar]
- 92.Hill GW, Morest DK, Parham K. Cisplatin-induced ototoxicity: Effect of intratympanic dexamethasone injections. Otol Neurotol. 2008;29:1005–1011. doi: 10.1097/MAO.0b013e31818599d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poirrier AL, Van den Ackerveken P, Kim TS, et al. Ototoxic drugs: Difference in sensitivity between mice and guinea pigs. Toxicol Lett. 2010;193:41–49. doi: 10.1016/j.toxlet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Blakley BW, Hochman J, Wellman M, et al. Differences in ototoxicity across species. J Otolaryngol Head Neck Surg. 2008;37:700–703. [PubMed] [Google Scholar]
- 95.Paksoy M, Ayduran E, Sanli A, et al. The protective effects of intratympanic dexamethasone and vitamin E on cisplatin-induced ototoxicity are demonstrated in rats. Med Oncol. 2011;28:615–621. doi: 10.1007/s12032-010-9477-4. [DOI] [PubMed] [Google Scholar]
- 96.Wimmer C, Mees K, Stumpf P, et al. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol Neurotol. 2004;25:33–40. doi: 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 97.More SS, Akil O, Ianculescu AG, et al. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Korver KD, Rybak LP, Whitworth C, et al. Round window application of D-methionine provides complete cisplatin otoprotection. Otolaryngol Head Neck Surg. 2002;126:683–689. doi: 10.1067/mhn.2002.125299. [DOI] [PubMed] [Google Scholar]
- 99.Campbell KC, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 100.Harned TM, Kalous O, Neuwelt A, et al. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res. 2008;14:533–540. doi: 10.1158/1078-0432.CCR-06-2289. [DOI] [PubMed] [Google Scholar]
- 101.Neuwelt EA, Pagel MA, Kraemer DF, et al. Bone marrow chemoprotection without compromise of chemotherapy efficacy in a rat brain tumor model. J Pharmacol Exp Ther. 2004;309:594–599. doi: 10.1124/jpet.103.063347. [DOI] [PubMed] [Google Scholar]
- 102.Katzenstein HM, Chang KW, Krailo M, et al. Amifostine does not prevent platinum-induced hearing loss associated with the treatment of children with hepatoblastoma: A report of the Intergroup Hepatoblastoma Study P9645 as a part of the Children's Oncology Group. Cancer. 2009;115:5828–5835. doi: 10.1002/cncr.24667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.National Cancer Institute's List of Cancer Clinical Trials. http://www.cancer.gov/clinicaltrials/search/results?protocolsearchid=7215792.
- 104.Zuur CL, Simis YJ, Lansdaal PE, et al. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol. 2007;25:3759–3765. doi: 10.1200/JCO.2006.08.9540. [DOI] [PubMed] [Google Scholar]
- 105.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 106.Cloven NG, Re A, McHale MT, et al. Evaluation of D-methionine as a cytoprotectant in cisplatin treatment of an animal model for ovarian cancer. Anticancer Res. 2000;20:4205–4209. [PubMed] [Google Scholar]
- 107.Campbell K, Nayar R, Borgonha S, et al. Oral D-methionine significantly protects against cisplatin induced hearing loss in humans. Abstracts of the 32nd Annual Midwinter Research Meeting of the Association for Research in Otolarynogology; 2009. p. 7. abstr 22. [Google Scholar]
- 108.Lynch ED, Gu R, Pierce C, et al. Combined oral delivery of ebselen and allopurinol reduces multiple cisplatin toxicities in rat breast and ovarian cancer models while enhancing anti-tumor activity. Anticancer Drugs. 2005;16:569–579. doi: 10.1097/00001813-200506000-00013. [DOI] [PubMed] [Google Scholar]
- 109.Lesaffre E. Superiority, equivalence, and non-inferiority trials. Bull NYU Hosp Jt Dis. 2008;66:150–154. [PubMed] [Google Scholar]
- 110.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: The influence of age and the cumulative dose. Eur J Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 111.Chang KW. Clinically accurate assessment and grading of ototoxicity. Laryngoscope. 2011;121:2649–2657. doi: 10.1002/lary.22376. [DOI] [PubMed] [Google Scholar]