Abstract
Purpose
To investigate nuclear localized and tyrosine phosphorylated Stat5 (Nuc-pYStat5) as a marker of prognosis in node-negative breast cancer and as a predictor of response to antiestrogen therapy.
Patients and Methods
Levels of Nuc-pYStat5 were analyzed in five archival cohorts of breast cancer by traditional diaminobenzidine-chromogen immunostaining and pathologist scoring of whole tissue sections or by immunofluorescence and automated quantitative analysis (AQUA) of tissue microarrays.
Results
Nuc-pYStat5 was an independent prognostic marker as measured by cancer-specific survival (CSS) in patients with node-negative breast cancer who did not receive systemic adjuvant therapy, when adjusted for common pathology parameters in multivariate analyses both by standard chromogen detection with pathologist scoring of whole tissue sections (cohort I; n = 233) and quantitative immunofluorescence of a tissue microarray (cohort II; n = 291). Two distinct monoclonal antibodies gave concordant results. A progression array (cohort III; n = 180) revealed frequent loss of Nuc-pYStat5 in invasive carcinoma compared to normal breast epithelia or ductal carcinoma in situ, and general loss of Nuc-pYStat5 in lymph node metastases. In cohort IV (n = 221), loss of Nuc-pYStat5 was associated with increased risk of antiestrogen therapy failure as measured by univariate CSS and time to recurrence (TTR). More sensitive AQUA quantification of Nuc-pYStat5 in antiestrogen-treated patients (cohort V; n = 97) identified by multivariate analysis patients with low Nuc-pYStat5 at elevated risk for therapy failure (CSS hazard ratio [HR], 21.55; 95% CI, 5.61 to 82.77; P < .001; TTR HR, 7.30; 95% CI, 2.34 to 22.78; P = .001).
Conclusion
Nuc-pYStat5 is an independent prognostic marker in node-negative breast cancer. If confirmed in prospective studies, Nuc-pYStat5 may become a useful predictive marker of response to adjuvant hormone therapy.
INTRODUCTION
Signal transducer and activator of transcription (Stat5) is a latent cytoplasmic transcription factor and a primary mediator of prolactin signaling in breast epithelia.1,2 After prolactin-induced phosphorylation of Stat5 on a conserved tyrosine residue by Jak2, Stat5 dimers translocate to the cell nucleus and bind to DNA of target genes,1 promoting growth and differentiation of mammary epithelia.2–5 Stat5 is highly activated in terminally differentiated breast epithelial cells during lactation2–5 and is phosphorylated at a basal level in nonpregnant mouse and human epithelia.6 Stat5 has been implicated as a mammary tumor promoter in mice, supported by observations that tumor development was delayed in Stat5-deficient mice and was induced in mice expressing a hyper-active Stat5 transgene.7–9 However, in vitro laboratory studies have indicated that phosphorylated Stat5 promotes cellular differentiation and inhibits invasive characteristics of human breast cancer cell lines.10–12 Consistent with the notion of a prodifferentiation effect of Stat5 in established human breast cancer, several immunohistochemical studies have reported that reduced levels of Stat5 protein or tyrosine phosphorylated and nuclear localized Stat5 (Nuc-pYStat5) were associated with poorly differentiated morphology, higher histologic grade, and more advanced breast cancer.13–16 Importantly, initial tissue microarray analysis suggested that loss of Nuc-pYStat5 was a marker of poor prognosis in human breast cancer, particularly in node-negative breast cancer,13 however, this study did not evaluate a purely prognostic cohort as at least 40% of patients received potentially confounding systemic adjuvant therapy.13 Here, we report the novel prognostic and hormone response–predictive value of Nuc-pYStat5 based on five distinct archival cohorts of breast cancer using both traditional diaminobenzidine (DAB) chromogen immunohistochemistry (IHC) with pathologist scoring and immunofluorescence-based quantification on the Automated Quantitative Analysis (AQUA) platform.17,18
PATIENTS AND METHODS
Breast Tumor Specimens
Archival and deidentified formalin-fixed, paraffin-embedded breast cancer specimens representing five independent clinical cohorts were analyzed, including whole tissue sections and tissue microarrays. The use of tissues was approved by the ethics committee of the respective institutions. Demographic and clinical characteristics of patients in cohorts I, II, IV, and V (not available for progression cohort III) are presented in Table 1.
Table 1.
Characteristics of Cohort I, II, IV, and V
| Variable | Cohort I (n = 233) |
Cohort II (n = 291) |
Cohort IV(n = 221) |
Cohort V (n = 97) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Discrete | ||||||||
| Center | ||||||||
| Fox Chase Cancer Center | 64 | 27 | — | — | — | — | — | — |
| Kaiser Permanente | 79 | 34 | — | — | — | — | — | — |
| University of Miami | 30 | 13 | — | — | — | — | — | — |
| Washington University | 60 | 26 | — | — | — | — | — | — |
| Race | ||||||||
| Asian | 1 | 0.4 | — | — | — | — | — | — |
| Black | 16 | 7 | 3 | 1 | — | — | — | — |
| White | 216 | 93 | 287 | 99 | — | — | — | — |
| Other | — | — | 1 | 0.3 | — | — | — | — |
| Unknown | — | — | — | — | 221 | 100 | 97 | 100 |
| Age, years | ||||||||
| < 50 | 42 | 18 | 86 | 30 | 27 | 12 | 6 | 6 |
| ≥ 50 | 191 | 82 | 205 | 70 | 194 | 88 | 91 | 94 |
| Tumor size, cm | ||||||||
| < 2 | 117 | 50 | 114 | 39 | 53 | 24 | 39 | 40 |
| ≥ 2-< 5 | 107 | 46 | 133 | 46 | 142 | 64 | 43 | 44 |
| ≥ 5 | 9 | 4 | 33 | 11 | 23 | 10 | 11 | 11 |
| Missing | — | — | 11 | 4 | 3 | 1 | 4 | 4 |
| Grade | ||||||||
| 1 | 61 | 26 | 67 | 23 | 56 | 25 | 14 | 14 |
| 2 | 104 | 45 | 137 | 47 | 99 | 45 | 47 | 48 |
| 3 | 68 | 29 | 48 | 16 | 66 | 30 | 32 | 33 |
| Missing | — | — | 39 | 13 | — | — | 4 | 4 |
| ER status | ||||||||
| Negative | 44 | 19 | 97 | 33 | 44 | 20 | 11 | 11 |
| Positive | 186 | 80 | 163 | 56 | 130 | 59 | 80 | 82 |
| Missing | 3 | 1 | 31 | 11 | 47 | 21 | 6 | 6 |
| PR status | ||||||||
| Negative | 65 | 28 | 103 | 35 | 87 | 39 | 25 | 26 |
| Positive | 122 | 52 | 151 | 52 | 134 | 61 | 67 | 69 |
| Missing | 46 | 20 | 37 | 13 | — | — | 5 | 5 |
| HER2 status | ||||||||
| Normal | — | — | 217 | 75 | 173 | 78 | 77 | 79 |
| Overexpressed | — | — | 33 | 11 | 29 | 13 | 13 | 13 |
| Missing | — | — | 41 | 14 | 19 | 9 | 7 | 7 |
| Node status | ||||||||
| Negative | 233 | 100 | 291 | 100 | 70 | 32 | 42 | 43 |
| Positive | 0 | 0 | 0 | 0 | 138 | 62 | 42 | 43 |
| Missing | — | — | — | — | 13 | 6 | 13 | 13 |
| Chemotherapy | ||||||||
| Untreated | 233 | 100 | — | — | 171 | 77 | 95 | 98 |
| Treated | 0 | 0 | — | — | 45 | 20 | 1* | 1 |
| Missing | — | — | — | — | 5 | 2 | 1 | 1 |
| Hormone therapy | ||||||||
| Untreated | 233 | 100 | — | — | 0 | 0 | 5 | 5 |
| Treated | 0 | 0 | — | — | 221 | 100 | 92 | 95 |
| Radiation therapy | ||||||||
| Untreated | 187 | 80 | — | — | 221 | 100 | 36 | 37 |
| Treated | 46 | 20 | — | — | 0 | 0 | 59 | 61 |
| Missing | — | — | — | — | — | — | 2 | 2 |
| CSS events | 52 | 22 | 107 | 37 | 65 | 29 | 56 | 58 |
| Nuc-pYStat5 status | ||||||||
| Low | 129 | 55 | 84 | 29 | 93 | 42 | 10 | 10 |
| High | 94 | 40 | 114 | 39 | 73 | 33 | 55 | 57 |
| Missing | 10 | 4 | 93 | 32 | 55 | 25 | 32 | 33 |
| Mean | Median | Range | SD | |
|---|---|---|---|---|
| Continuous | ||||
| Cohort I | ||||
| Age at diagnosis | 62.3 | 62 | 31-88 | 12.9 |
| Tumor size, cm | 2.08 | 1.8 | 0.6-7.5 | 1.07 |
| Follow-up, months | 129 | 126 | 3-326 | 71 |
| Nuc-pYStat5 score | 5.0 | 0 | 0-40 | 8.6 |
| Year of diagnosis | — | — | 1974-1990 | — |
| Cohort II | ||||
| Age at diagnosis | 57.3 | 57 | 24-86 | 12.3 |
| Tumor size, cm | 2.53 | 2.0 | 0.4-11 | 1.67 |
| Follow-up, months | 165 | 160 | 1-425 | 106 |
| Nuc-pYStat5 score | 852 | 771 | 265-2,236 | 416 |
| Year of diagnosis | — | — | 1953-1980 | — |
| Cohort IV | ||||
| Age at diagnosis | 63.3 | 63 | 35-97 | 12.1 |
| Tumor size, cm | 3.0 | 2.5 | 0.5-13.0 | 1.8 |
| Follow-up, months | 59.0 | 60 | 2-137 | 28.0 |
| Nuc-pYStat5 score | 1 | 0 | 0-4 | 1.3 |
| Positive nodes | 3.8 | 1 | 0-38 | 6.2 |
| Year of diagnosis | — | — | 1985-1996 | — |
| Cohort V | ||||
| Age at diagnosis | 69.8 | 72 | 38-89 | 11.0 |
| Tumor size, cm | 2.7 | 2.1 | 0.4-11 | 1.7 |
| Follow-up, months | 57.5 | 40.7 | 3.6-143 | 40.9 |
| Nuc-pYStat5 score | 1,262 | 1,027 | 533-4,243 | 762 |
| Positive nodes | 2.3 | 0 | 0-22 | 3.7 |
| Year of diagnosis | — | — | 1990-2000 | — |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; CSS, cancer-specific survival; SD, standard deviation; Nuc-pYStat5, nuclear localized and tyrosine phosphorylated Stat5.
Patient received chemotherapy after disease relapse.
Cohort I was obtained from the National Cancer Institute's Cooperative Breast Cancer Tissue Resource as whole tissue sections from patients with node-negative invasive ductal carcinomas (IDC) who did not receive adjuvant systemic therapy (n = 233). Clinical outcome end points included breast cancer–specific survival (CSS) and time to recurrence (TTR) of either local or distant disease. Nuc-pYStat5 DAB scores were obtained for 223 tumor specimens. Cohort II was a breast cancer tissue microarray (0.6 mm cores) from Yale University pathology archives, representing 291 node-negative patients with clinical and CSS data who did not receive adjuvant systemic therapy.19–21 Nuc-pYStat5 AQUA scores were obtained from 198 patients. A significant number of cases were not interpretable by AQUA due to loss of histospots or insufficient staining quality or tumor sampling (< 5% tumor area) required for automated analysis.22 Cohort III was a breast cancer progression array constructed using cutting edge matrix assembly23 representing 180 unmatched patient specimens, including 40 normal breast tissues, 20 ductal carcinoma in situ (DCIS), 100 IDC, and 20 lymph node breast cancer metastases from Thomas Jefferson University Hospital archives. Hormone receptor status of the IDCs was determined by standard IHC and identified as 20% HER2 positive, 59% estrogen receptor (ER) positive, and 42% progesterone receptor (PR) positive. Nuc-pYStat5 AQUA scores were obtained for 130 cases. Cohort IV from the Institute of Pathology at Kantonsspital Basel (Basel, Switzerland)13 comprised 221 patients with CSS and TTR data who received antiestrogen therapy (approximately 20% of cases also received adjuvant chemotherapy). Interpretable Nuc-pYStat5 DAB staining was obtained for 166 patients. Cohort V represented a random series of patients with breast cancer identified through the Alberta Cancer Registry (Calgary, Alberta, Canada) who received adjuvant hormone monotherapy for up to 60 months (average 30 months). The tissue microarray (“Tamoxifen 50/50 array”) was constructed with triplicate 0.6 mm tumor tissue cores from 50 patients who died of breast cancer, classified as resistant to antiestrogen therapy, and 50 patients with longer than 5-years follow-up without breast cancer recurrence, classified as good responders. AQUA analysis yielded informative data on Nuc-pYStat5 for 65 cases.
Detection of Nuc-pYStat5 by DAB Chromogen IHC
The specificity of mouse monoclonal antiphosphoStat5 antibody AX1 (Advantex BioReagents, El Paso, TX) recognizing the conserved phosphotyrosyl residue Y694/Y699 of Stat5a/b, has been extensively validated.6,13 Detection of Nuc-pYStat5 with AX1 was performed as previously described13 except AX1 incubation time was shortened to 45 minutes at a final concentration of 1.2 μg/mL to accommodate the Dako Autostainer (Dako, Carpinteria, CA). Antigen-antibody complexes were detected using biotinylated goat antimouse secondary antibody (Biogenex, San Ramon, CA) followed by streptavidin-horseradish-peroxidase complex, using 3,3-(prime) DAB as chromogen and Mayer hematoxylin as counterstain. Individual breast tumor samples were scored by a pathologist, blinded to clinical outcome, for transcriptionally active Stat5 as defined by nuclear localization and tyrosine phosphorylation of Stat5 by estimating the percent malignant cells with detectable staining and overall staining intensity on a scale ranging from 0 to 3. Positive Nuc-pYStat5 status was defined as staining intensity greater than 0 in 10% or more of the carcinoma cells, corresponding to the original report.13
Quantification of Nuc-pYStat5 by AQUA
Rabbit monoclonal antiphosphoStat5 antibody, E208 (Epitomics, Burlingame, CA), was used for AQUA. E208 recognizes the same phospho-antigen (Y694/Y699 Stat5a/b) as the AX1 antibody and showed similar specificity and dynamic range in side-by-side DAB chromogen IHC and AQUA testing (Figs 1C to 1E). Antigen retrieval was performed using the DAKO PT-module with citric acid buffer (pH 6.0). Immunofluorescent staining was performed on a Dako Autostainer. E208 was diluted 1:1,600 from the supplier-provided stock and coincubated for 30 minutes with mouse monoclonal antipancytokeratin (clone AE1/AE3, DAKO, 1:100 dilution) to define the tumor mask.17 Horseradish peroxidase–conjugated antirabbit immunoglobulin G and Alexa-488-conjugated antimouse immunoglobulin G secondary antibodies were added for 30 minutes, followed by 10 minutes incubation with Cy5-tyramide (Perkin Elmer, Waltham, MA). Slides were coverslipped using 4′,6-diamidino-2-phenylindole (DAPI) -containing mounting media. The AQUA/PM2000 platform (HistoRx, New Haven, CT)17 was used to quantify fluorescence-based immunostaining for Nuc-pYStat5. Tissue array slides were automatically scanned and fluorescent images were captured in three channels, fluorescein isothiocyanate/Alexa-488 (cytokeratin), Cy5 (Nuc-pYStat5), or DAPI (nuclei). AQUA scores, blinded to clinical data, were objectively derived by calculating the mean signal intensities within the cell nuclei of the epithelial compartment, defined by cytokeratin-positive and DAPI-positive mapping.
Fig 1.
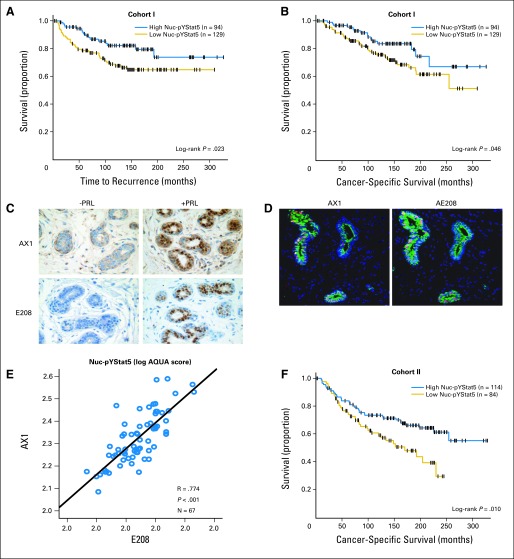
Low levels of nuclear localized and tyrosine phosphorylated Stat5 (Nuc-pYStat5) predict unfavorable breast cancer prognosis. (A-B) Kaplan-Meier analysis of Nuc-pYStat5, detected by mouse monoclonal antipYStat5 AX1 antibody and standard diaminobenzidine (DAB) immunohistochemistry (IHC) with pathologist review of whole tissue sections, in cohort I revealed that low expression of Nuc-pYStat5 was prognostic of (A) reduced time to recurrence of breast cancer and (B) poor breast cancer-specific survival (CSS). (C-E) Comparative validation of mouse monoclonal AX1 with rabbit monoclonal antipYStat5 antibody E208. (C) Detection of nuclear localized and tyrosine phosphorylated Stat5 by DAB chromogen IHC in healthy human breast tissue surgical explants incubated ex vivo with (positive control) or without (negative control) human prolactin (100 nmol/L; 60 minutes) using antipYStat5 antibodies AX1 (16 hours' incubation with manual staining protocol; upper panels) or E208 (20 minutes' incubation with autostainer protocol; lower panels). (D) Representative immunofluorescent images from two serial sections of a breast tissue stained with AX1 or E208 antibodies indicating comparable detection of Nuc-pYStat5. (E) Correlation of Nuc-pYStat5 expression detected by AX1 or E208 and quantified by automated quantitative analysis (AQUA) in serial sections of an assorted breast tissue microarray. (F) Kaplan-Meier analysis of breast CSS in cohort II indicated that risk of death from breast cancer was significantly elevated in patients whose tumors expressed low Nuc-pYStat5 as quantified by immunofluorescence and AQUA analysis. Censored cases (+) and number of patients per group are indicated.
X-Tile Cut Point Analysis and Statistical Methods
Optimal cut points for AQUA-quantified Nuc-pYStat5 as a function of survival in prognostic cohort II and predictive cohort V were derived using X-tile software, which employs crossvalidation to produce corrected P values for multiple cut points.24 End points for survival analysis were TTR (cohorts I, IV and V) and breast CSS (cohorts I, II, IV, and V) according to consensus definitions.25 Survival analyses were performed by constructing Kaplan-Meier curves and using the log-rank test and adjusted Cox or Weibull regression models (SAS version 9.2, SAS Institute, Cary, NC). Cox regression was used when proportional hazard assumption passed (completed globally using a Wald χ2 test for each cohort and outcome multivariate model), otherwise Weibull regression was applied (assessment made graphically). When available, variables included in the adjusted models were tumor grade, tumor size, and status of nodal involvement, ER, PR, HER2, and Nuc-pYStat5. One-way analysis of variation with Dunnett's T3 pairwise posthoc test assuming unequal variances (SPSS version 15.0; SPSS Inc, Chicago, IL) was used to test for differences in Nuc-pYStat5 levels between breast histology groups in progression cohort III.
RESULTS
Nuc-pYStat5 Is an Independent Marker of Prognosis in Node-Negative Breast Cancer
To determine whether levels of Nuc-pYStat5 would predict outcome in patients with lymph node-negative breast cancer who did not receive adjuvant therapy, we first examined the clinically relevant setting of whole tumor tissue sections from cohort I using DAB chromogen IHC and the same mouse monoclonal antipYStat5 antibody (AX1) used in our previous tissue microarray study.13 Evaluation of TTR revealed that low levels of Nuc-pYStat5 were associated with increased risk of breast cancer recurrence (TTR; log-rank P = .023, n = 223, Fig 1A; univariate Cox regression hazard ratio [HR], 2.35; 95% CI, 1.18 to 4.67; P = .015, n = 183, Appendix Table A1, online only). Importantly, low expression of Nuc-pYStat5 also was associated with poor breast CSS (log-rank P = .046, n = 223, Fig 1B; univariate Weibull regression HR, 2.32; 95% CI, 1.10 to 4.89; P = .027, n = 183, Table 2).
Table 2.
Univariate and Multivariate Survival Analyses of Breast CSS in Cohorts I and II
| Variable | No. | Multivariate Adjusted (Weibull) |
Unadjusted (Weibull) |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Cohort I (CSS)* | |||||||
| Tumor grade | |||||||
| 1 | 47 | 1 | — | 1 | — | ||
| 2 | 76 | 1.53 | 0.57 to 4.10 | .399 | 1.87 | 0.72 to 4.87 | .198 |
| 3 | 60 | 1.61 | 0.56 to 4.66 | .381 | 2.74 | 1.05 to 7.11 | .039 |
| Tumor size, cm | |||||||
| < 2 | 88 | 1 | — | 1 | — | ||
| ≥ 2-< 5 | 87 | 2.30 | 1.06 to 4.99 | .036 | 2.47 | 1.20 to 5.08 | .014 |
| ≥ 5 | 8 | 2.50 | 0.62 to 10.04 | .197 | 2.97 | 0.80 to 10.95 | .103 |
| ER status | |||||||
| Negative | 32 | 1 | — | 1 | — | ||
| Positive | 151 | 1.82 | 0.70 to 4.71 | .216 | 0.72 | 0.34 to 1.51 | .380 |
| PR status | |||||||
| Negative | 64 | 1 | — | 1 | — | ||
| Positive | 119 | 0.55 | 0.25 to 1.20 | .133 | 0.55 | 0.29 to 1.04 | .065 |
| Nuc-pYStat5 | |||||||
| Low (0) | 105 | 2.38 | 1.13 to 5.04 | .023 | 2.32 | 1.10 to 4.89 | .027 |
| High (> 0) | 78 | 1 | — | 1 | — | ||
| Cohort II (CSS)† | |||||||
| Tumor grade | |||||||
| 1 | 34 | 1 | — | 1 | — | ||
| 2 | 90 | 1.25 | 0.66 to 2.37 | .500 | 1.16 | 0.62 to 2.19 | .640 |
| 3 | 34 | 0.87 | 0.35 to 2.19 | .772 | 0.81 | 0.36 to 1.82 | .616 |
| Tumor size, cm | |||||||
| < 2 | 60 | 1 | — | 1 | — | ||
| ≥ 2-< 5 | 80 | 2.49 | 1.33 to 4.67 | .004 | 2.11 | 1.15 to 3.88 | .016 |
| ≥ 5 | 18 | 3.89 | 1.63 to 9.26 | .002 | 2.68 | 1.19 to 6.05 | .018 |
| ER status | |||||||
| Negative | 50 | 1 | — | 1 | — | ||
| Positive | 108 | 0.88 | 0.45 to 1.70 | .698 | 1.01 | 0.59 to 1.73 | .958 |
| PR status | |||||||
| Negative | 57 | 1 | — | 1 | — | ||
| Positive | 101 | 1.26 | 0.69 to 2.29 | .459 | 0.92 | 0.55 to 1.55 | .757 |
| HER2 status | |||||||
| Normal | 138 | 1 | — | 1 | — | ||
| Overexpressed | 20 | 1.37 | 0.61 to 3.07 | .440 | 1.04 | 0.49 to 2.19 | .918 |
| Nuc-pYStat5 | |||||||
| Low (< 684) | 67 | 2.39 | 1.37 to 4.17 | .002 | 2.10 | 1.26 to 3.51 | .004 |
| High (≥ 684) | 91 | 1 | — | 1 | — | ||
NOTE. Weibull regression survival analysis was used to evaluate prognostic factors. Global test for Cox regression proportional hazards assumption failed, necessitating Weibull regression. Cohort I: n = 233; 183 (79%) evaluable; 41 (22%) of 183 events. Cohort II: n = 291; 158 (54%) evaluable; 61 (39%) of 158 events.
Abbreviations: CSS, cancer specific survival; HR, hazard ratio; ER, estrogen receptor; PR, progesterone receptor; Nuc-pYStat5, nuclear localized and tyrosine phosphorylated Stat5.
Global test: χ2(5) = 12.93, P = .024.
Global test: χ2(6) = 13.22, P = .040.
Detection of Nuc-pYStat5 by AX1 gives best results after overnight incubation at 4°C and optimal sensitivity is not achieved with the short incubation times (ie, 20 to 45 minutes) required for autostainers. Therefore, we extended the analyses to include a rabbit monoclonal antipYStat5 (E208), which could be optimized for autostainer protocols. The optimized overnight AX1 and autostainer E208 protocols resulted in comparable sensitivity in detecting prolactin-induced Stat5 phosphorylation and nuclear translocation by DAB chromogen IHC (Fig 1C). Levels of Nuc-pYStat5 were also highly correlated and reflected similar dynamic ranges when quantified by AQUA using the AX1 or E208 protocols in two serial sections of an array of assorted normal and malignant breast tissues (Pearson r = 0.774, P < .001, n = 67, Figs 1D to 1E). The reproducibility of the antibodies to detect the same epitope on near-identical serial tissue sections despite differences in antigen retrieval and incubation times was further evidenced by low coefficients of variation (median, 4.7%; range, 0.1% to 9.6%). Therefore, analysis of Nuc-pYStat5 using E208 for automated, objective, and quantitative immunofluorescence was completed in an independent cohort (cohort II), a tissue microarray also limited to primary breast carcinomas from patients who were lymph node negative and did not receive systemic adjuvant therapy. Nuc-pYStat5 levels, quantified using E208 and the AQUA platform, predicted breast cancer survival also in cohort II (CSS; log-rank P = .010, n = 198, Fig 1F; univariate Weibull regression HR, 2.10; 95% CI, 1.26 to 3.51; P = .004, n = 158, Table 2). TTR data was not available for cohort II.
In multivariate analyses, Nuc-pYStat5 remained an independent marker of disease prognosis in both cohorts I and II, when adjusting for standard clinical and pathologic markers. In cohort I, patients with low Nuc-pYStat5 expression had an adjusted 2.5-fold increased risk of disease recurrence (TTR; multivariate Cox regression HR, 2.49; 95% CI, 1.23 to 5.05; P = .012; n = 183, Appendix Table A1) and a 2.4-fold greater risk of dying from breast cancer (CSS; multivariate Weibull regression HR, 2.38; 95% CI, 1.13 to 5.04; P = .023, n = 183, Table 2). Likewise, when quantified by AQUA in cohort II, Nuc-pYStat5 remained an independent marker of breast CSS as reflected in a similar 2.4-fold increased risk of death (CSS; multivariate Weibull regression HR, 2.39; 95% CI, 1.37 to 4.17; P = .002, N = 158, Table 2). Based on two independent cohorts and analytic approaches, we conclude that Nuc-pYStat5 is an independent marker of outcome in patients with lymph node-negative breast cancer.
Levels of Nuc-pYStat5 Are Diminished During Breast Cancer Progression
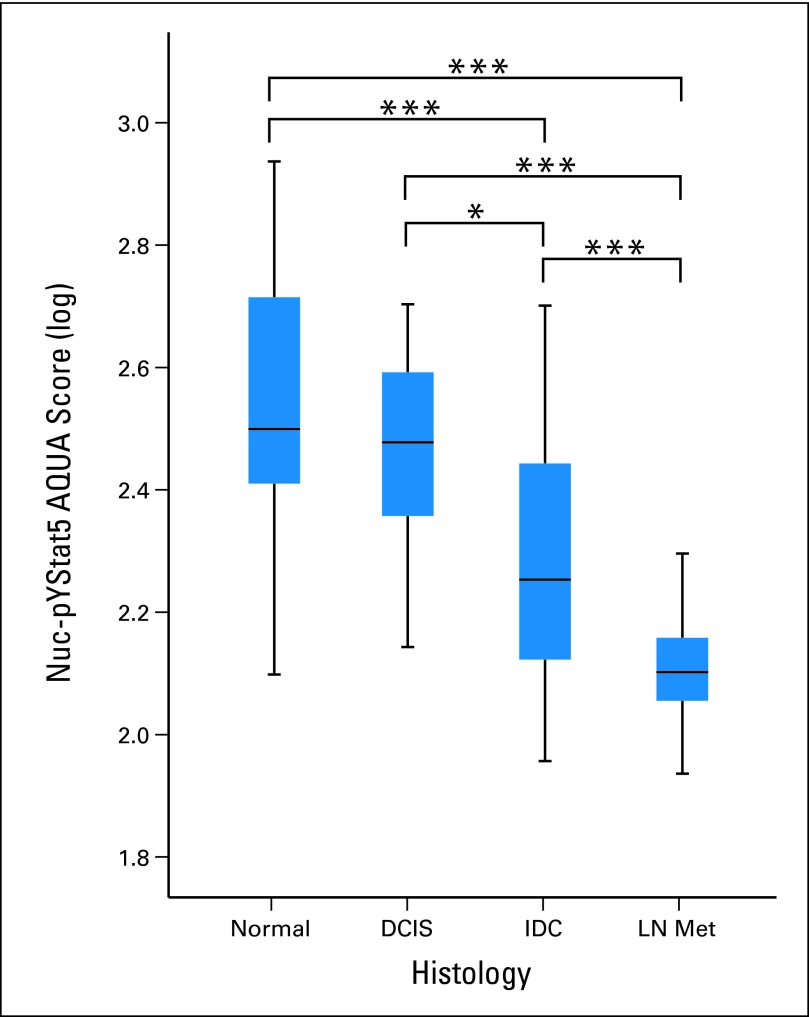
E208 and AQUA were then used to quantify Nuc-pYStat5 levels in a breast tissue progression array (cohort III), which included unmatched normal breast tissue, DCIS, IDC, and breast cancer lymph node metastases. Levels of Nuc-pYStat5 detected by quantitative immunofluorescence were markedly reduced during breast cancer progression (Fig 2). Specifically, while levels of Nuc-pYStat5 remained high and unchanged between normal epithelia and DCIS, Nuc-pYStat5 was significantly reduced in IDC (P < .001) with even greater loss in lymph node metastases (P < .001). These quantitative data provided novel information supporting the observations of frequent loss of Nuc-pYStat5 during breast cancer progression.
Fig 2.
Loss of nuclear localized and tyrosine phosphorylated Stat5 (Nuc-pYStat5) during breast cancer progression. Levels of Nuc-pYStat5 as detected by immunofluorescence and quantified by automated quantitative analysis (AQUA) were significantly reduced in invasive ductal carcinoma (IDC; n = 72) and lymph node metastases (LN Met; n = 17) when compared with normal breast tissue (n = 27) and ductal carcinoma in situ (DCIS; n = 14) in progression array cohort III. (*) P = .012; (***) P < .001.
Nuc-pYStat5 Predicts Responsiveness to Antiestrogen Therapy
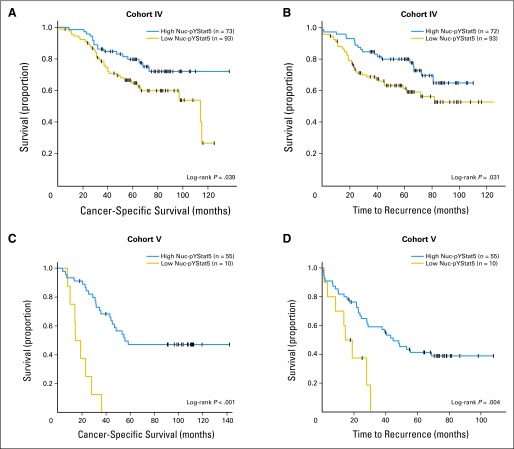
Cohort IV comprised node-negative and node-positive breast cancer tissues from patients who received adjuvant antiestrogen therapy. Levels of Nuc-pYStat5 were assessed using AX1 and DAB chromogen IHC. The absence of detectable Nuc-pYStat5 in tumors from these patients was associated with an increased risk of breast cancer-specific death (CSS; log-rank P = .039, n = 166, Fig 3A; univariate Cox regression, HR, 1.83; 95% CI, 1.02 to 3.29; P = .043, n = 166). Breast cancer recurrence also was evaluated and revealed an increased risk of recurrence in patients with undetectable Nuc-pYStat5 (TTR; log-rank P = .031; n = 165; Fig 3B; HR, 1.83; 95% CI, 1.05 to 3.20; P = .033, n = 166). Although Nuc-pYStat5 did not remain an independent marker of response to antiestrogen therapy in multivariate analyses when detected by AX1 and DAB chromogen IHC in this limited cohort (n = 144, data not shown), univariate analyses were promising and prompted us to employ more sensitive methodology using E208 and AQUA on an independent cohort V.
Fig 3.
Low levels of nuclear localized and tyrosine phosphorylated Stat5 (Nuc-pYStat5) predict increased risk of failure of antiestrogen therapy. (A-B) Low levels of Nuc-pYStat5 detected by diaminobenzidine chromogen immunohistochemistry (IHC) and pathologist scoring in patients treated with antiestrogen therapy (cohort IV) predicted (A) poor breast cancer-specific survival (CSS) and (B) reduced time to recurrence (TTR) of breast cancer. (C-D) Immunofluorescence and quantitative automated quantitative analysis of Nuc-pYStat5 expression in breast cancer patients treated with antiestrogen monotherapy (cohort V) revealed that low expression of Nuc-pYStat5 was predictive of (C) poor CSS and (D) reduced TTR of breast cancer. Kaplan-Meier plots with censored cases (+) and number of patients per group indicated.
Cohort V included tumors from node-negative and node-positive patients treated exclusively with antiestrogen monotherapy. The X-Tile software determined an optimal cut point that identified a subset of patients whose tumors had low levels of Nuc-pYStat5 and were at markedly increased risk of failing antiestrogen treatment and with poor breast CSS (log-rank P < .001; n = 65, Fig 3C). Univariate analysis indicated that approximately 15% of the patients with the lowest levels of Nuc-pYStat5 were at 7.4-fold increased risk of dying from breast cancer (CSS; univariate Cox regression HR, 7.36; 95% CI, 2.94 to 18.42; P < .001, n = 53, Table 3). In multivariate analysis adjusting for tumor size, tumor grade, node status, ER/PR status, and HER2 overexpression, Nuc-pYStat5 remained an independent marker of survival in cohort V (CSS; multivariate Cox regression HR, 21.55; 95% CI, 5.61 to 82.77; P < .001, n = 53, Table 3). Positive node status (CSS; multivariate Cox regression HR, 8.10; 95% CI, 3.03 to 21.64; P < .001, n = 53) and ER/PR status (CSS; multivariate Cox regression HR, 0.10; 95% CI, 0.03 to 0.39; P = .001, n = 53) were also independent predictors of survival (Table 3). Low levels of Nuc-pYStat5 also predicted breast cancer recurrence in these patients in both univariate (TTR; log-rank P = .004, n = 65, Fig 3D; Cox regression HR, 4.71; 95% CI, 1.99 to 11.16; P < .001, n = 53, Table 3) and multivariate analysis (TTR; Cox regression HR, 7.30; 95% CI, 2.34 to 22.78; P = .001, n = 53, Table 3). Collectively, these observations provide novel evidence to suggest that low levels of Nuc-pYStat5 predict failure of antiestrogen treatment and justify further analysis of Nuc-pYStat5 expression in antiestrogen-treated patients.
Table 3.
Univariate and Multivariate Breast CSS and TTR Analysis of Nuc-pYStat5 in Patients With Breast Cancer Treated With Antiestrogen Monotherapy in Cohort V
| Variable | No. | Multivariate Adjusted (Cox) |
Unadjusted (Cox) |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Cohort V (CSS)* | |||||||
| Tumor size, cm | |||||||
| < 2 | 25 | 1 | — | 1 | — | ||
| 2-< 5 | 23 | 1.24 | 0.42 to 3.66 | .700 | 2.92 | 1.33 to 6.41 | .008 |
| ≥ 5 | 5 | 0.97 | 0.21 to 4.41 | .971 | 4.83 | 1.50 to 15.57 | .008 |
| Tumor grade | |||||||
| 1 | 8 | 1 | — | 1 | — | ||
| 2 | 27 | 0.60 | 0.19 to 1.88 | .376 | 0.80 | 0.29 to 2.26 | .677 |
| 3 | 18 | 0.73 | 0.21 to 2.55 | .621 | 1.64 | 0.58 to 4.61 | .347 |
| ER/PR status | |||||||
| Negative | 8 | 1 | — | 1 | — | ||
| Positive | 45 | 0.10 | 0.03 to 0.39 | .001 | 0.37 | 0.16 to 0.88 | .024 |
| HER2 status | |||||||
| Normal | 45 | 1 | — | 1 | — | ||
| Overexpressed | 8 | 1.99 | 0.71 to 5.64 | .193 | 2.53 | 1.08 to 5.93 | .032 |
| Lymph node status | |||||||
| Negative | 28 | 1 | — | 1 | — | ||
| Positive | 25 | 8.10 | 3.03 to 21.64 | < .001 | 4.72 | 2.19 to 10.20 | < .001 |
| Nuc-pYStat5 | |||||||
| Low (< 724) | 8 | 21.55 | 5.61 to 82.77 | < .001 | 7.36 | 2.94 to 18.42 | < .001 |
| High (≥ 724) | 45 | 1 | — | 1 | — | ||
| Cohort V (TTR)† | |||||||
| Tumor size, cm | |||||||
| < 2 | 25 | 1 | — | 1 | — | ||
| 2-< 5 | 23 | 1.56 | 0.56 to 4.35 | .393 | 2.68 | 1.27 to 5.68 | .010 |
| ≥ 5 | 5 | 1.81 | 0.46 to 7.21 | .397 | 5.28 | 1.82 to 15.33 | .002 |
| Tumor grade | |||||||
| 1 | 8 | 1 | — | 1 | — | ||
| 2 | 27 | 0.46 | 0.16 to 1.35 | .156 | 0.67 | 0.26 to 1.75 | .417 |
| 3 | 18 | 0.70 | 0.22 to 2.26 | .553 | 1.53 | 0.59 to 4.00 | .384 |
| ER/PR status | |||||||
| Negative | 8 | 1 | — | 1 | — | ||
| Positive | 45 | 0.16 | 0.05 to 0.49 | .001 | 0.31 | 0.14 to 0.71 | .005 |
| HER2 status | |||||||
| Normal | 45 | 1 | — | 1 | — | ||
| Overexpressed | 8 | 2.02 | 0.74 to 5.50 | .171 | 2.33 | 1.01 to 5.38 | .048 |
| Lymph node status | |||||||
| Negative | 28 | 1 | — | 1 | — | ||
| Positive | 25 | 5.28 | 2.28 to 12.18 | < .001 | 3.65 | 1.80 to 7.42 | < .001 |
| Nuc-pYStat5 | |||||||
| Low (< 724) | 8 | 7.30 | 2.34 to 22.78 | .001 | 4.71 | 1.99 to 11.16 | < .001 |
| High (≥ 724) | 45 | 1 | — | 1 | — | ||
NOTE. Cox regression survival analysis was used to evaluate prognostic factors. Global test for Cox regression proportional hazards passed. Cohort V CSS: n = 97; 53 (55%) evaluable; 31 (58%) of 53 events; Cohort V TTR: n = 97; 53 (55%) evaluable; 34 (64%) of 53 events.
Abbreviations: CSS, cancer specific survival; TTR, time to recurrence; Nuc-pYStat5, nuclear localized and tyrosine phosphorylated Stat5; HR, hazard ratio; ER, estrogen receptor; PR, progesterone receptor.
Global test: χ2(6) = 6.38, P = .38.
Global test: χ2(6) = 5.45, P = .49.
DISCUSSION
This study documents that low levels of Nuc-pYStat5 represent an independent marker of poor prognosis in patients with node-negative breast cancer who did not receive systemic adjuvant therapy. This was based on analyses of cancer-specific survival in two independent clinical cohorts (cohorts I and II) using two distinct antibodies and traditional pathologist scoring of whole tissue sections or quantitative AQUA analysis of a tissue microarray. These findings provide novel support for the previously proposed prognostic value of Nuc-pYStat5 in breast cancer, which was based on tissue array specimens and included patients treated with potentially confounding systemic adjuvant therapy.13 Furthermore, loss of Nuc-pYStat5, as quantified by AQUA, was frequent across a breast cancer progression array (cohort III) with nearly undetectable levels in lymph node metastases. Importantly, analysis of two cohorts of patients with breast cancer who received antiestrogen therapy (cohorts IV and V) suggested that levels of Nuc-pYStat5 constitute a new predictive marker of response to adjuvant hormone therapy.
Antiestrogen therapy is currently guided by positive tumor expression of ERα by IHC. However, approximately 30% of patients with ER-positive breast cancer fail to respond to antiestrogen therapy due to inherent or acquired resistance.26–29 A meta-analysis of 12 studies implicated HER2 as a modest predictor of resistance to antiestrogen therapy,30 but American Society of Clinical Oncology guidelines do not recommend using HER2 as a predictor of response to endocrine therapy due to insufficient evidence.31 In our multivariate analyses, HER2 status reached only borderline significance in predicting outcome in antiestrogen treated patients. In other immunohistochemical studies, markers such as epidermal growth factor receptor,32–34 PR,35–38 and p2739–42 were suggested to predict failure of antiestrogen therapy and it will be of interest to expand future analyses of Nuc-pYStat5 to include epidermal growth factor receptor and p27.
Ongoing studies are exploring the individual prognostic and predictive values of the highly homologous Stat5a and Stat5b proteins, which share greater than 90% amino acid identity.43 AntipYStat5 antibodies, including AX1 and E208 used here, do not distinguish between phosphorylated Stat5a and Stat5b due to their identical phosphotyrosyl-motifs. Increased risk of antiestrogen therapy failure was reportedly associated with loss of Stat5b protein expression in patients with breast cancer relapse.15 However, response to classical adjuvant antiestrogen therapy was not addressed, and nuclear Stat5b was not distinguished from cytoplasmic Stat5b.15 Therefore, it is unclear if the detected protein was transcriptionally active.
In this study, the quantitative analyses of cohort V revealed a strong predictive value of Nuc-pYStat5 for response to antiestrogen therapy. In cohort IV, the predictive value of Nuc-pYStat5 as measured by DAB chromogen IHC and pathologist scoring was statistically significant both for CSS and TTR in univariate but not in multivariate analyses. The greater predictive value of Nuc-pYStat5 in cohort V is likely attributable, at least in part, to the improved dynamic range of quantitative immunofluorescence detection over that provided by DAB chromogen and pathologist scoring17,18,44,45 in cohort IV. In fact, as many as 56% of cases in cohort IV were scored negative for Nuc-pYStat5, while X-Tile applied to the continuous fluorescence-based AQUA data determined a cut point in cohort V that identified 15% of patients whose tumors displayed the lowest levels of Nuc-pYStat5 with distinctly elevated risk of failing adjuvant hormone therapy. Furthermore, a limitation of cohort IV is that approximately 20% of the patients received adjuvant chemotherapy in addition to hormone therapy, whereas all patients in cohort V exclusively received adjuvant hormone therapy. Additional limitations of this study include the limited cohort sizes, loss of evaluable tumor tissue that is characteristic of tissue microarrays, and missing analytic or clinical data. Close examination suggested that some data were not missing at random in the various cohorts although consistent patterns did not emerge across the various cohorts (data not shown). Furthermore, the cohorts differed with regard to period of diagnosis and this may contribute to variability. In general, predictions based on retrospective populations may not adequately reflect current therapeutic strategies.
Collectively, this work supports the notion that Stat5 signaling is frequently lost during breast cancer progression and loss of Nuc-pYStat5 is associated with poor prognosis in node-negative breast cancer. Furthermore, novel evidence is provided suggesting that loss of Nuc-pYStat5 is associated with elevated risk of failure of antiestrogen therapy. However, conclusive validation of the response-predictive value of Nuc-pYStat5 will require quantitative analyses in tumors from patients randomized for antiestrogen therapy, and prospective analyses in a Clinical Laboratory Improvement Amendments–certified laboratory to overcome additional limitations of retrospective studies.
Acknowledgment
Whole tissue sections and clinical data for cohort I were generously provided by the National Cancer Institute's Cooperative Breast Cancer Tissue Resource. We thank Eva Andersson for expert technical help with immunostaining of sections of cohort I; Melanie Girondo for editorial assistance; and Joachim Torhorst, Markus Zuber, Ossi R. Köchli, Frank Mross, and Holger Dieterich for support with pathology and clinical data for cohort IV.
Appendix
Table A1.
Univariate and Multivariate Cox Regression Survival Analyses of TTR of Breast Cancer in Cohort I
| Cohort I* (TTR) Variable | No. | Multivariate Adjusted (Cox)† |
Unadjusted (Cox) |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Tumor grade | |||||||
| 1 | 47 | 1 | — | 1 | — | ||
| 2 | 76 | 2.01 | 0.77 to 5.25 | .156 | 2.24 | 0.89 to 5.67 | .087 |
| 3 | 60 | 2.05 | 0.73 to 5.77 | .174 | 3.41 | 1.32 to 8.84 | .012 |
| Tumor size, cm | |||||||
| < 2 | 88 | 1 | — | 1 | — | ||
| ≥ 2-< 5 | 87 | 2.13 | 1.04 to 4.35 | .038 | 2.41 | 1.25 to 4.65 | .009 |
| ≥ 5 | 8 | 2.54 | 0.67 to 9.57 | .169 | 3.20 | 0.90 to 11.34 | .072 |
| ER status* | |||||||
| Negative | 32 | 1 | — | ||||
| Positive | 151 | 0.83 | 0.40 to 1.73 | .622 | |||
| PR status | |||||||
| Negative | 64 | 1 | — | 1 | — | ||
| Positive | 119 | 0.36 | 0.18 to 0.72 | .004 | 0.35 | 0.18 to 0.69 | .002 |
| Nuc-pYStat5 | |||||||
| Low (0) | 105 | 2.49 | 1.23 to 5.05 | .012 | 2.35 | 1.18 to 4.67 | .015 |
| High (> 0) | 78 | 1 | — | 1 | — | ||
NOTE. Cohort I: n = 233; 183 (79%) evaluable; 45 (25%) of 183 events.
Abbreviations: TTR, time to recurrence; HR, hazard ratio; ER, estrogen receptor; PR, progesterone receptor; Nuc-pYStat5, nuclear localized and tyrosine phosphorylated Stat5.
Stratified by ER status, except for univariate (unadjusted) ER status which was done as Weibull.
Global test for Cox regression proportional hazards passed; Global test:χ2(4) = 3.84, P = .43.
Footnotes
See accompanying editorial on page 2443
Supported by Grants No. R01-CA101841 and R01-CA118740 from the National Institutes of Health (H.R.), Promise Grant No. KG091116 from Komen for the Cure (H.R., A.K.W., C.L., J.A.H., A.J.K., C.D.S., T.H.), Department of Defense CDMRP Predoctoral Fellowship No. BC051251 (A.R.P.), support Grant No. 1P30CA56036 from the National Cancer Institute to the Kimmel Cancer Center; and, in part, under a Commonwealth University Research Enhancement Program grant with the Pennsylvania Department of Health (H.R.). The Department specifically disclaims responsibility for any analyses, interpretations or conclusions. The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense, or US Government.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Marja T. Nevalainen, Advantex BioReagents (U); Hallgeir Rui, Advantex BioReagents (U) Consultant or Advisory Role: Marja T. Nevalainen, Advantex BioReagents (U); David L. Rimm, HistoRX (C); Hallgeir Rui, Advantex BioReagents (U) Stock Ownership: Marja T. Nevalainen, Advantex BioReagents; David L. Rimm, HistoRX; Hallgeir Rui, Advantex BioReagents Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Amy R. Peck, Agnieszka K. Witkiewicz, Marja T. Nevalainen, Terry Hyslop, Guido Sauter, Anthony M. Magliocco, Hallgeir Rui
Financial support: Craig D. Shriver, Terry Hyslop, Guido Sauter, David L. Rimm, Anthony M. Magliocco, Hallgeir Rui
Administrative support: Anne L. Rosenberg, Craig D. Shriver, Terry Hyslop, Guido Sauter, Anthony M. Magliocco, Hallgeir Rui
Provision of study materials or patients: Anne L. Rosenberg, Guido Sauter, David L. Rimm, Anthony M. Magliocco, Hallgeir Rui
Collection and assembly of data: Amy R. Peck, Agnieszka K. Witkiewicz, Chengbao Liu, Ginger A. Stringer, Alexander C. Klimowicz, Edward Pequignot, Boris Freydin, Thai H. Tran, Ning Yang, Anne L. Rosenberg, Jeffrey A. Hooke, Albert J. Kovatich, Marja T. Nevalainen, Craig D. Shriver, Terry Hyslop, Guido Sauter, David L. Rimm, Anthony M. Magliocco, Hallgeir Rui
Data analysis and interpretation: Amy R. Peck, Agnieszka K. Witkiewicz, Chengbao Liu, Ginger A. Stringer, Alexander C. Klimowicz, Edward Pequignot, Boris Freydin, Thai H. Tran, Ning Yang, Jeffrey A. Hooke, Albert J. Kovatich, Marja T. Nevalainen, Craig D. Shriver, Terry Hyslop, Guido Sauter, David L. Rimm, Anthony M. Magliocco,Hallgeir Rui
Manuscript writing: Amy R. Peck, Agnieszka K. Witkiewicz, Chengbao Liu, Ginger A. Stringer, Alexander C. Klimowicz, Edward Pequignot, Boris Freydin, Thai H. Tran, Ning Yang, Anne L. Rosenberg, Jeffrey A. Hooke, Albert J. Kovatich, Marja T. Nevalainen, Craig D. Shriver, Terry Hyslop, Guido Sauter, David L. Rimm, Anthony M. Magliocco,Hallgeir Rui
Final approval of manuscript: Amy R. Peck, Agnieszka K. Witkiewicz, Chengbao Liu, Ginger A. Stringer, Alexander C. Klimowicz, Edward Pequignot, Boris Freydin, Thai H. Tran, Ning Yang, Anne L. Rosenberg, Jeffrey A. Hooke, Albert J. Kovatich, Marja T. Nevalainen, Craig D. Shriver, Terry Hyslop, Guido Sauter, David L. Rimm, Anthony M. Magliocco, Hallgeir Rui
REFERENCES
- 1.Gouilleux F, Wakao H, Mundt M, et al. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. Embo J . 1994;13:4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Robinson GW, Wagner KU, et al. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev . 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Teglund S, McKay C, Schuetz E, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell . 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, LeBaron MJ, Nevalainen MT, et al. Role of tyrosine kinase Jak2 in prolactin-induced differentiation and growth of mammary epithelial cells. J Biol Chem . 2002;277:14020–14030. doi: 10.1074/jbc.M112399200. [DOI] [PubMed] [Google Scholar]
- 5.Wagner KU, Krempler A, Triplett AA, et al. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol Cell Biol . 2004;24:5510–5520. doi: 10.1128/MCB.24.12.5510-5520.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nevalainen MT, Xie J, Bubendorf L, et al. Basal activation of transcription factor signal transducer and activator of transcription (Stat5) in nonpregnant mouse and human breast epithelium. Mol Endocrinol . 2002;16:1108–1124. doi: 10.1210/mend.16.5.0839. [DOI] [PubMed] [Google Scholar]
- 7.Iavnilovitch E, Groner B, Barash I. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol Cancer Res . 2002;1:32–47. [PubMed] [Google Scholar]
- 8.Humphreys RC, Hennighausen L. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ . 1999;10:685–694. [PubMed] [Google Scholar]
- 9.Ren S, Cai HR, Li M, et al. Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene . 2002;21:4335–4339. doi: 10.1038/sj.onc.1205484. [DOI] [PubMed] [Google Scholar]
- 10.Sultan AS, Xie J, Lebaron MJ, et al. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene . 2005;24:746–760. doi: 10.1038/sj.onc.1208203. [DOI] [PubMed] [Google Scholar]
- 11.Nouhi Z, Chughtai N, Hartley S, et al. Defining the role of prolactin as an invasion suppressor hormone in breast cancer cells. Cancer Res. 2006;66:1824–1832. doi: 10.1158/0008-5472.CAN-05-2292. [DOI] [PubMed] [Google Scholar]
- 12.Sultan AS, Brim H, Sherif ZA. Co-overexpression of Janus kinase 2 and signal transducer and activator of transcription 5a promotes differentiation of mammary cancer cells through reversal of epithelial-mesenchymal transition. Cancer Sci . 2008;99:272–279. doi: 10.1111/j.1349-7006.2007.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevalainen MT, Xie J, Torhorst J, et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 14.Bratthauer GL, Strauss BL, Tavassoli FA. STAT 5a expression in various lesions of the breast. Virchows Arch . 2006;448:165–171. doi: 10.1007/s00428-005-0056-6. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita H, Nishio M, Ando Y, et al. Stat5 expression predicts response to endocrine therapy and improves survival in estrogen receptor-positive breast cancer. Endocr Relat Cancer . 2006;13:885–893. doi: 10.1677/erc.1.01095. [DOI] [PubMed] [Google Scholar]
- 16.Cotarla I, Ren S, Zhang Y, et al. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int J Cancer . 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- 17.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med . 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 18.McCabe A, Dolled-Filhart M, Camp RL, et al. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97:1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 19.Kang JY, Dolled-Filhart M, Ocal IT, et al. Tissue microarray analysis of hepatocyte growth factor/Met pathway components reveals a role for Met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res . 2003;63:1101–1105. [PubMed] [Google Scholar]
- 20.Giltnane JM, Moeder CB, Camp RL, et al. Quantitative multiplexed analysis of ErbB family coexpression for primary breast cancer prognosis in a large retrospective cohort. Cancer . 2009;115:2400–2409. doi: 10.1002/cncr.24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolled-Filhart M, Ryden L, Cregger M, et al. Classification of breast cancer using genetic algorithms and tissue microarrays. Clin Cancer Res . 2006;12:6459–6468. doi: 10.1158/1078-0432.CCR-06-1383. [DOI] [PubMed] [Google Scholar]
- 22.Gustavson MD, Bourke-Martin B, Reilly DM, et al. Development of an unsupervised pixel-based clustering algorithm for compartmentalization of immunohistochemical expression using automated quantitative analysis. Appl Immunohistochem Mol Morphol . 2009;17:329–337. doi: 10.1097/PAI.0b013e318195ecaa. [DOI] [PubMed] [Google Scholar]
- 23.LeBaron MJ, Crismon HR, Utama FE, et al. Ultrahigh density microarrays of solid samples. Nat Methods . 2005;2:511–513. doi: 10.1038/nmeth772. [DOI] [PubMed] [Google Scholar]
- 24.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res . 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 25.Punt CJ, Buyse M, Kohne CH, et al. Endpoints in adjuvant treatment trials: A systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst . 2007;99:998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 26.Prat A, Baselga J. The role of hormonal therapy in the management of hormonal-receptor-positive breast cancer with co-expression of HER2. Nat Clin Pract Oncol . 2008;5:531–542. doi: 10.1038/ncponc1179. [DOI] [PubMed] [Google Scholar]
- 27.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med . 1998;339:1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 28.Clarke R, Leonessa F, Welch JN, et al. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev . 2001;53:25–71. [PubMed] [Google Scholar]
- 29.Schiff R, Massarweh SA, Shou J, et al. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res . 2004;10:331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 30.De Laurentiis M, Arpino G, Massarelli E, et al. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res . 2005;11:4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 31.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol . 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 32.Nicholson S, Halcrow P, Sainsbury JR, et al. Epidermal growth factor receptor (EGFr) status associated with failure of primary endocrine therapy in elderly postmenopausal patients with breast cancer. Br J Cancer . 1988;58:810–814. doi: 10.1038/bjc.1988.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arpino G, Green SJ, Allred DC, et al. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A southwest oncology group study. Clin Cancer Res . 2004;10:5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 34.Giltnane JM, Ryden L, Cregger M, et al. Quantitative measurement of epidermal growth factor receptor is a negative predictive factor for tamoxifen response in hormone receptor positive premenopausal breast cancer. J Clin Oncol . 2007;25:3007–3014. doi: 10.1200/JCO.2006.08.9938. [DOI] [PubMed] [Google Scholar]
- 35.Osborne C, Schiff R, Arpino G. Endocrine responsiveness: Understanding how progesterone receptor can be used to select endocrine therapy. Breast . 2005;14:458–465. doi: 10.1016/j.breast.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Stendahl M, Ryden L, Nordenskjold B, et al. High progesterone receptor expression correlates to the effect of adjuvant tamoxifen in premenopausal breast cancer patients. Clin Cancer Res . 2006;12:4614–4618. doi: 10.1158/1078-0432.CCR-06-0248. [DOI] [PubMed] [Google Scholar]
- 37.Ravdin PM, Green S, Dorr TM, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: Results of a prospective Southwest Oncology Group study. J Clin Oncol . 1992;10:1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- 38.Bardou VJ, Arpino G, Elledge RM, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 39.Filipits M, Rudas M, Heinzl H, et al. Low p27 expression predicts early relapse and death in postmenopausal hormone receptor-positive breast cancer patients receiving adjuvant tamoxifen therapy. Clin Cancer Res . 2009;15:5888–5894. doi: 10.1158/1078-0432.CCR-09-0728. [DOI] [PubMed] [Google Scholar]
- 40.Porter PL, Barlow WE, Yeh IT, et al. P27(Kip1) and cyclin E expression and breast cancer survival after treatment with adjuvant chemotherapy. J Natl Cancer Inst . 2006;98:1723–1731. doi: 10.1093/jnci/djj467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pohl G, Rudas M, Dietze O, et al. High p27Kip1 expression predicts superior relapse-free and overall survival for premenopausal women with early-stage breast cancer receiving adjuvant treatment with tamoxifen plus goserelin. J Clin Oncol . 2003;21:3594–3600. doi: 10.1200/JCO.2003.02.021. [DOI] [PubMed] [Google Scholar]
- 42.McCallum M, Baker C, Gillespie K, et al. A prognostic index for operable, node-negative breast cancer. Br J Cancer. 2004;90:1933–1941. doi: 10.1038/sj.bjc.6601826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu X, Robinson GW, Gouilleux F, et al. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A . 1995;92:8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dolled-Filhart M, Gustavson M, Camp RL, et al. Automated analysis of tissue microarrays. Methods Mol Biol . 2010;664:151–162. doi: 10.1007/978-1-60761-806-5_15. [DOI] [PubMed] [Google Scholar]
- 45.Camp RL, Dolled-Filhart M, King BL, et al. Quantitative analysis of breast cancer tissue microarrays shows that both high and normal levels of HER2 expression are associated with poor outcome. Cancer Res. 2003;63:1445–1448. [PubMed] [Google Scholar]