Abstract
Purpose
To determine the maximum-tolerated dose (MTD), safety, pharmacokinetic and pharmacodynamic profiles, and clinical activity of an oral formulation of azacitidine in patients with myelodysplastic syndromes (MDSs), chronic myelomonocytic leukemia (CMML), or acute myeloid leukemia (AML).
Patients and Methods
Patients received 1 cycle of subcutaneous (SC) azacitidine (75 mg/m2) on the first 7 days of cycle 1, followed by oral azacitidine daily (120 to 600 mg) on the first 7 days of each additional 28-day cycle. Pharmacokinetic and pharmacodynamic profiles were evaluated during cycles 1 and 2. Adverse events and hematologic responses were recorded. Cross-over to SC azacitidine was permitted for nonresponders who received ≥ 6 cycles of oral azacitidine.
Results
Overall, 41 patients received SC and oral azacitidine (MDSs, n = 29; CMML, n = 4; AML, n = 8). Dose-limiting toxicity (grade 3/4 diarrhea) occurred at the 600-mg dose and MTD was 480 mg. Most common grade 3/4 adverse events were diarrhea (12.2%), nausea (7.3%), vomiting (7.3%), febrile neutropenia (19.5%), and fatigue (9.8%). Azacitidine exposure increased with escalating oral doses. Mean relative oral bioavailability ranged from 6.3% to 20%. Oral and SC azacitidine decreased DNA methylation in blood, with maximum effect at day 15 of each cycle. Hematologic responses occurred in patients with MDSs and CMML. Overall response rate (ie, complete remission, hematologic improvement, or RBC or platelet transfusion independence) was 35% in previously treated patients and 73% in previously untreated patients.
Conclusion
Oral azacitidine was bioavailable and demonstrated biologic and clinical activity in patients with MDSs and CMML.
INTRODUCTION
Azacitidine is a cytidine nucleoside analog with a mechanism of action that involves incorporation into DNA and RNA.1,2 Data suggest that patients must be exposed to azacitidine over several treatment cycles for optimal therapeutic effect.3 The requirement for chronic exposure can be explained by drug pharmacokinetics, as azacitidine has a short plasma half-life, and by mechanism of action, as induction of DNA hypomethylation through incorporation into DNA is cell-cycle dependent (S-phase restricted) and DNA remethylation is observed by the end of each treatment cycle.4
A treatment regimen facilitating chronic administration may help achieve optimal efficacy outcomes. An oral azacitidine formulation would improve convenience of administration and expand the possibilities of exploring novel maintenance schedules, targeting different malignancies, and testing multiple combinations. A phase 0 trial demonstrated that a single oral azacitidine dose resulted in detectable levels in the blood.5
This phase I study sought to identify the maximum-tolerated dose (MTD), dose-limiting toxicities (DLTs), safety, pharmacokinetic and pharmacodynamic profiles, and clinical activity of oral azacitidine in patients with myelodysplastic syndromes (MDSs), chronic myelomonocytic leukemia (CMML), or acute myeloid leukemia (AML).
PATIENTS AND METHODS
The trial was approved by the relevant institutional review boards and ethics committees. All patients gave written informed consent.
Patients
Eligible patients were ≥ 18 years, had an Eastern Cooperative Oncology Group performance status score of 0 to 2, and a diagnosis of MDSs, CMML, or AML according to WHO classification.6,7 For patients with AML, eligibility was limited to those for whom standard curative measures did not exist or were no longer effective. Exclusion criteria included a diagnosis of acute promyelocytic leukemia, previous treatment with hypomethylating agents within 4 weeks before cycle 1, and anticancer therapy within 21 days before the first dose of study drug, or less than full recovery from any significant toxic effects of prior treatments.
Study Design and Therapy
This open-label, phase I, dose-escalation trial was performed in four participating institutions and evaluated multiple cycles of oral azacitidine administered daily for the first 7 days of a 28-day cycle. The objectives were to determine the MTD, DLTs, and the safety profile of oral azacitidine. Pharmacokinetic and pharmacodynamic profiles of oral and subcutaneous (SC) azacitidine, administered on the same 7-day schedule, were also compared. A secondary objective was to assess the clinical activity of oral azacitidine.
During cycle 1, patients received azacitidine 75 mg/m2 daily SC for 7 days of a 28-day cycle. During cycle 2 and beyond, patients received oral azacitidine under fasting conditions (ie, no food for 2 hours before and after dosing). The dose of oral azacitidine was escalated following a standard phase I 3 + 3 design. The starting dose was 120 mg and doses were escalated in 60 mg increments up to a dose of 360 mg, followed by 120 mg increments until the MTD was reached. Intrapatient dose escalation was permitted if the dose level to which the patient was escalated was associated with a DLT rate of ≤ 33%. Treatment continued until disease progression, lack of activity, unacceptable toxicity, or patient preference.
The MTD was defined as the highest dose at which no more than 33% of patients experienced a DLT. DLT was defined as: grade ≥ 3 nausea, diarrhea, or vomiting despite adequate/maximal medical intervention; grade ≥ 3 clinically significant nonhematologic toxicity unrelated to underlying disease or intercurrent illness; failure to recover to an absolute neutrophil count (ANC) of higher than 500/μL and/or platelet count of higher than 25,000/μL with hypocellular bone marrow (< 5%) 42 days after starting oral azacitidine (patients with a baseline ANC of ≤ 500/μL and/or platelet count of ≤ 25,000/μL were not evaluable for neutrophil or platelet toxicity); any treatment-related effect resulting in missing ≥ 3 oral azacitidine doses in the 7-day treatment period; or any treatment-related nonhematologic toxicity delaying initiation of the second oral azacitidine cycle by longer than 14 days. Only DLTs that occurred during the first oral azacitidine cycle were considered in determining the MTD. Adverse events were graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0.
Pharmacokinetic Analysis
Plasma and urine pharmacokinetic evaluation of azacitidine was performed on days 1 and 7 in cycles 1 and 2. Samples were collected up to 8 hours after administration and analyzed using a validated high-performance liquid chromatography/tandem mass spectrometric method. Parameters calculated using noncompartmental method, included maximum observed plasma concentration (Cmax), time of maximum observed plasma concentration (Tmax), area under the plasma concentration–time curve from zero to infinity (AUCinf), apparent total clearance (CL/F), relative oral bioavailability (F), and apparent volume of distribution (Vd/F).
Pharmacodynamic Analysis
DNA methylation levels were measured to determine DNA hypomethylating activity of azacitidine when administered SC or orally. Whole blood was collected at baseline and before drug administration on days 3, 8, 15, and 22 of cycle 1, and days 1, 3, 8, 15, 22, and 28 of cycle 2. Genomic DNA was purified from each whole blood sample using the PAXgene Blood DNA System (Qiagen; Valencia, CA). DNA methylation was analyzed using the Infinium Human Methylation27 BeadArray (Illumina; San Diego, CA). In cycle 1, DNA methylation data were generated from blood samples of 15 patients. For 10 of these patients, data were also generated in cycle 2. A methylation ratio, or beta value, for each locus per sample was calculated as methylated signal/(methylated + unmethylated signal). Those with detection P ≤ .05 were considered high-quality measures. Samples with more than 25,200 high-quality beta values and 26,304 autosomal loci with high-quality beta values in at least half of the samples were used for analyses. The low-quality beta values were reimputed using the pamr.knnimpute function from the R package pamr.8 Wilcoxon signed-rank tests were performed to identify loci with significant methylation differences at each post-treatment time point versus baseline; P < .01 was considered statistically significant. All statistical analyses were carried out in R (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org).
Clinical Activity
Data for clinical activity were evaluated using International Working Group (IWG) 2006 criteria, with modifications as described below, for patients with MDSs or CMML9 and IWG 2003 criteria for patients with AML.10 Complete remission (CR), hematologic improvement (HI), and RBC and platelet transfusion independence (TI) were evaluated for patients with MDSs or CMML. Bone marrow CR (mCR) was also evaluated but not included in the overall response rate. RBC transfusion dependence at baseline was defined as ≥ 4 RBC units in the 56 days before cycle 1. Platelet transfusion dependence at baseline was defined as ≥ 2 platelet transfusions in the 56 days before cycle 1 (modification to IWG 2006 criteria). RBC and platelet TI were defined as no transfusions in any 56 consecutive-day period on treatment. Patients who achieved ≥ 50% reduction in platelet transfusion requirement, but not platelet TI, in any 56 consecutive-day period on treatment were counted as having achieved HI platelet (HI-P; modification to IWG 2006 criteria). Patients RBC transfusion dependent at baseline achieving a ≥ 50% reduction in RBC transfusion requirement in any 56-consecutive day period and patients not RBC transfusion dependent at baseline, but who achieved a 1.5 g/dL increase in hemoglobin in any 56-consecutive day period on treatment were considered to have achieved HI erythroid (HI-E; modification to IWG 2006 criteria). All patients who received ≥ 1 cycle of oral azacitidine were included in the response analysis. The cutoff date for data in this article was August 19, 2010.
RESULTS
Patient Characteristics
Forty-five patients were treated on a 7-day once-daily schedule. Four patients received the first cycle of SC azacitidine only; three discontinued due to progressive disease (including one death), and one withdrew consent. Baseline characteristics for the remaining 41 patients who received oral azacitidine are presented in Table 1 .11 Cytogenetic data were available at baseline for 35 of 41 patients treated with oral azacitidine; nearly half of the patients had normal karyotype, approximately 25% had a single abnormality, and nearly 20% had a complex karyotype (≥ 3 chromosomal abnormalities). Overall, 16 (39%) of 41 patients had received prior hypomethylating therapy.
Table 1.
Baseline Patient Characteristics (N = 41)
| Parameter | No. of Patients | % |
|---|---|---|
| Median age, years | 70 | |
| Range | 31-91 | |
| Sex | ||
| Male | 32 | 78 |
| Female | 9 | 22 |
| MDSs (WHO classification) | 29 | 71 |
| RA/RARS/RCMD | 11 | 27 |
| RAEB-1 | 12 | 29 |
| RAEB-2 | 5 | 12 |
| MDSs-U | 1 | 2 |
| CMML | 4 | 10 |
| AML | 8 | 20 |
| De novo | 4 | 10 |
| Transformed from MDSs | 4 | 10 |
| IPSS (MDSs patients)* | ||
| Low risk | 2 | 7 |
| Intermediate 1 risk | 12 | 41 |
| Intermediate 2 risk | 13 | 45 |
| High risk | 1 | 3 |
| Not available† | 1 | 3 |
| Hematology | ||
| Median hemoglobin, g/dL | 9.3 | |
| Range | 6.9-15.1 | |
| Median white blood cell count ×109/L | 2.4 | |
| Range | 0.4-30.2 | |
| Median absolute neutrophil count ×109/L | 0.8 | |
| Range | 0.0-21.7 | |
| Median platelet count ×109/L | 54.0 | |
| Range | 3.0-262.0 | |
| Cytogenetics‡ | ||
| Normal chromosomal karyotype | 17 | 49 |
| 1 chromosomal abnormality | 9 | 26 |
| 2 chromosomal abnormalities | 3 | 9 |
| ≥ 3 chromosomal abnormalities | 6 | 17 |
| Prior treatment with hypomethylating agent | 16 | 39 |
| MDSs | 13 | 32 |
| CMML | 0 | 0 |
| AML | 3 | 7 |
Abbreviations: AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; MDSs, myelodysplastic syndromes; MDSs-U, MDSs unclassified; RA, refractory anemia; RAEB, RA with excess blasts; RARS, RA with ringed sideroblasts; RCMD, refractory cytopenias with multilineage dysplasia.
IPSS score11 was available for 28 patients with MDSs.
Patient had a bone marrow transplantation and therefore IPSS risk was not considered applicable.
Cytogenetic data were available for 35 patients.
Dose Escalation of Oral Azacitidine
No DLTs were observed at dose levels up to 480 mg. DLT was observed at the 600 mg dose, with two (66.7%) of three patients experiencing severe diarrhea, despite adequate medical intervention (grade 3 in one patient and grade 4 in the other). Per protocol, the MTD was exceeded and the previous dose level of 480 mg was determined to be the MTD.
Safety Profile
Table 2 shows the incidence of AEs (any grade) that occurred in ≥ 20% of patients treated with oral azacitidine. The most frequently observed AEs were gastrointestinal disorders, headache, fatigue, and peripheral edema. Other commonly occurring AEs included fever, cough, contusion, dizziness, and febrile neutropenia. Grade 3/4 nausea and grade 3/4 vomiting were each observed in 7% of patients. Grade 3 fatigue was observed in 10% of patients. Diarrhea occurred at grade 3 severity in 10% of patients and grade 4 severity in 2%. Grade 3 febrile neutropenia was observed in eight patients (20%), with four of those having an ANC of ≤ 500/μL at baseline.
Table 2.
Incidence of Adverse Events According to Severity in ≥ 20% of Patients Treated With Oral Azacitidine (n = 41)
| System Organ Class Preferred Term(MeDRA 10.1) | CTCAE Grade |
Total |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Diarrhea | 10 | 24.4 | 12 | 29.3 | 4 | 9.8 | 1 | 2.4 | 27 | 65.9 |
| Nausea | 8 | 19.5 | 10 | 24.4 | 3 | 7.3 | 0 | 21 | 51.2 | |
| Constipation | 9 | 22.0 | 7 | 17.1 | 0 | 0 | 16 | 39.0 | ||
| Vomiting | 4 | 9.8 | 6 | 14.6 | 3 | 7.3 | 0 | 13 | 31.7 | |
| Abdominal pain | 6 | 14.6 | 4 | 9.8 | 0 | 0 | 10 | 24.4 | ||
| Headache | 7 | 17.1 | 5 | 12.2 | 1 | 2.4 | 0 | 13 | 31.7 | |
| Fatigue | 6 | 14.6 | 2 | 4.9 | 4 | 9.8 | 0 | 12 | 29.3 | |
| Peripheral edema | 11 | 26.8 | 1 | 2.4 | 0 | 0 | 12 | 29.3 | ||
| Fever | 6 | 14.6 | 2 | 4.9 | 2 | 4.9 | 0 | 10 | 24.4 | |
| Cough | 7 | 17.1 | 1 | 2.4 | 2 | 4.9 | 0 | 10 | 24.4 | |
| Contusion | 9 | 22.0 | 0 | 0 | 0 | 9 | 22.0 | |||
| Dizziness | 5 | 12.2 | 3 | 7.3 | 0 | 0 | 8 | 19.5 | ||
| Febrile neutropenia | 0 | 0 | 8 | 19.5 | 0 | 8 | 19.5 | |||
NOTE. This Table includes all adverse events which started during any dosing cycle at which oral azacitidine was administered. Percentages are based on the number of patients who received at least one dose of oral azacitidine. Multiple reports of the same preferred term from a patient are counted only once, using the maximum CTCAE grade.
Abbreviations: CTCAE, National Cancer Institute Common Toxicity Criteria for Adverse Events; MedDRA, Medical Dictionary for Regulatory Activities.
Of the 41 patients who received oral azacitidine, 33 terminated from the study as of the date of data analysis, with 17 discontinuing before completing 6 cycles of oral therapy. Reasons for discontinuation included disease progression/treatment failure (n = 10), investigator decision primarily due to absence of observed benefit/response (n = 15), withdrawal of consent (n = 4), AEs (n = 3), and decision to pursue hematopoietic stem-cell transplantation (n = 1). There were three deaths within 28 days of last dose of study drug due to multiple organ failure (n = 1), gastrointestinal hemorrhage (n = 1), and pneumonia plus urinary tract infection (n = 1). No deaths were attributed to study drug. Eight patients remained on the study at the time of data analysis, having each received between 14 and 32 treatment cycles.
Pharmacokinetic Characteristics of Azacitidine
High interpatient variability was noted for all pharmacokinetic parameters. Azacitidine was rapidly absorbed after SC (n = 42) and oral (n = 36) administration, reaching Cmax within 0.5 hours (range, 0.2 to 1.1 hours) and 1.0 hours (range, 0.3 to 3.6 hours) postdose, respectively. Concentration versus time profiles decreased in a pseudobiphasic manner (Fig 1A). The mean elimination half-life was 1.6 ± 0.7 hours for SC and 0.62 ± 0.25 hours for oral azacitidine. Exposure after single oral administration generally increased with dose (Table 3). For the seven oral dose levels, the mean relative azacitidine oral bioavailability (F) ranged from 6.3% to 20%. The MTD had a mean relative bioavailability of 13% ± 9%. CL/F exceeded hepatic blood flow, indicating extrahepatic metabolism, and Vd/F was greater than total body water, suggesting extensive tissue distribution. The amount of azacitidine recovered in urine relative to dose was small (< 2%) for SC and oral administration, suggesting that nonrenal elimination is the predominant pathway for clearance. Results after multiple doses were similar to those obtained after a single dose for both administration routes (data not shown). There was no evidence of azacitidine accumulation.
Fig 1.
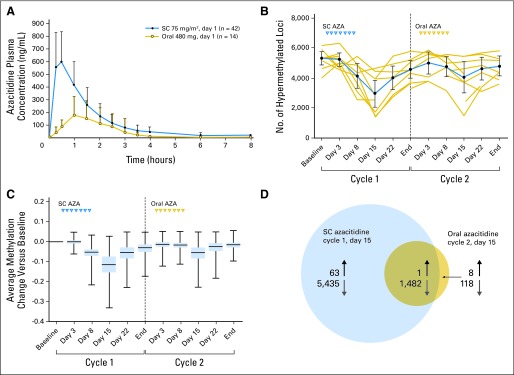
(A) Mean azacitidine (AZA) plasma concentration versus time profiles following single subcutaneous (SC) or oral administration (linear scale). (B) Pharmacodynamics as measured by plotting the numbers of highly methylated loci (beta ≥ 0.7; ± 95% CI) for 10 patients with DNA methylation data in cycles 1 and 2 (gold lines represent individual patients, blue line represents the average). (C) Change in methylation level during treatment with SC or oral AZA for 5,232 loci highly methylated at baseline (blue box represents the 25th to 75th percentile, horizontal band represents the median, vertical line with bars represents minimum and maximum values). (D) Number of significantly differentially methylated loci on day 15 of cycle 1 (SC azacitidine) and on day 15 of cycle 2 (oral azacitidine). Upward arrows denote hypermethylated loci and downward arrows denote hypomethylated loci.
Table 3.
Day 1 Plasma Pharmacokinetics Parameters After Single Subcutaneous or Oral Azacitidine Administration
| Dose | No. of Patients | AUCinf(ng × h/mL) |
CL/F (L/h) |
Cmax (ng/mL) |
Tmax (h) |
Vd/F (L) |
F (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | %CV | Mean | SD | %CV | Mean | SD | %CV | Median | Range | Mean | SD | %CV | Mean | SD | Relative Oral Bioavailability | ||
| Subcutaneous, 75 mg/m2 | 42 | 1,020 | 440 | 43* | 175 | 128 | 73* | 650 | 250 | 39 | 0.50 | 0.2-1.1 | 410 | 410 | 101* | NA | ||
| Oral, mg | ||||||||||||||||||
| 120 | 4 | 62 | 43 | 70 | 4,100 | 4,860 | 118 | 38 | 24 | 64 | 1.48 | 1.0-2.0 | 2,930 | 3,810 | 130 | 8.1 | 5.6 | 69 |
| 180 | 3 | 112 | 64 | 58 | 2,330 | 1,890 | 81 | 72 | 36 | 50 | 1.50 | 1.0-1.5 | 1,700 | 1,580 | 93 | 6.3 | 2.3 | 37 |
| 240 | 3 | 463 | 221 | 48 | 598 | 258 | 43 | 215 | 102 | 47 | 1.00 | 1.0-1.5 | 814 | 421 | 52 | 20.0 | 9.6 | 48 |
| 300 | 5 | 282 | 88 | 31 | 1,180 | 487 | 41 | 144 | 13 | 9.2 | 1.48 | 1.0-2.0 | 1,090 | 626 | 57 | 11.5 | 2.6 | 23 |
| 360 | 5 | 311 | 141 | 45 | 1,360 | 573 | 42 | 195 | 79 | 40 | 1.00 | 0.5-3.6 | 947 | 251 | 27 | 12.8 | 2.4 | 19 |
| 480 | 14 | 362 | 253 | 70 | 2,140 | 1,620 | 76 | 211 | 140 | 66 | 1.00 | 0.3-2.5 | 2,010 | 1,910 | 95 | 12.8 | 9.4 | 74† |
| 600 | 2 | 502 | 100 | 20 | 1,220 | 244 | 20 | 253 | 29 | 12 | 1.50 | 1.0-2.0 | 1,580 | 1,410 | 89 | 14.9 | 0.8 | 5 |
Abbreviations: AUCinf, area under the plasma concentration–time curve from time zero to infinity; CL/F, apparent total clearance; Cmax, maximum observed plasma concentration; F, relative oral bioavailability; NA, not applicable; Tmax, time of maximum observed plasma concentration; Vd/F, apparent volume of distribution.
n = 40.
n = 13.
Pharmacodynamics of Azacitidine: Effect on DNA Methylation
DNA methylation was evaluated during cycles 1 and 2 in 10 patients treated with oral azacitidine. The numbers of highly methylated loci were calculated at each time point by averaging across patients the number of loci with methylation ratios ≥ 0.7 (Fig 1B). These numbers decreased after SC and oral administration, with maximal effects at day 15 of each cycle. The reduction in levels of highly methylated loci was not maintained throughout the entire cycle and returned to near-baseline levels by the end of each cycle. SC azacitidine decreased a greater number of loci in comparison to oral azacitidine. The changes in methylation level from baseline across patients for the 5,232 highly methylated loci (average methylation ratio at baseline ≥ 0.7) are represented as box plots (Fig 1C). As with the analysis of total numbers of highly methylated loci, the median DNA methylation of these loci was reduced by 0.115 on day 15 of cycle 1 (SC azacitidine) and 0.055 on day 15 of cycle 2 (oral azacitidine), and returned to baseline levels at the end of each cycle.
Differentially methylated loci at each post-treatment time point compared with baseline were identified in cycles 1 and 2, with the maximum number observed on day 15 of each cycle; 6,981 loci were differentially methylated (6,917 hypomethylated) on day 15 of cycle 1 (SC azacitidine) and 1,609 loci were differentially methylated (1,600 hypomethylated) on day 15 of cycle 2 (oral azacitidine; P < .01). In total, 1,482 loci were significantly hypomethylated by both SC and oral azacitidine (Fig 1D), representing 92.6% of all loci significantly hypomethylated by oral azacitidine treatment. These data demonstrate comparable biologic activity with SC and oral azacitidine, albeit to a lesser extent with oral azacitidine.
Clinical Activity of Oral Azacitidine
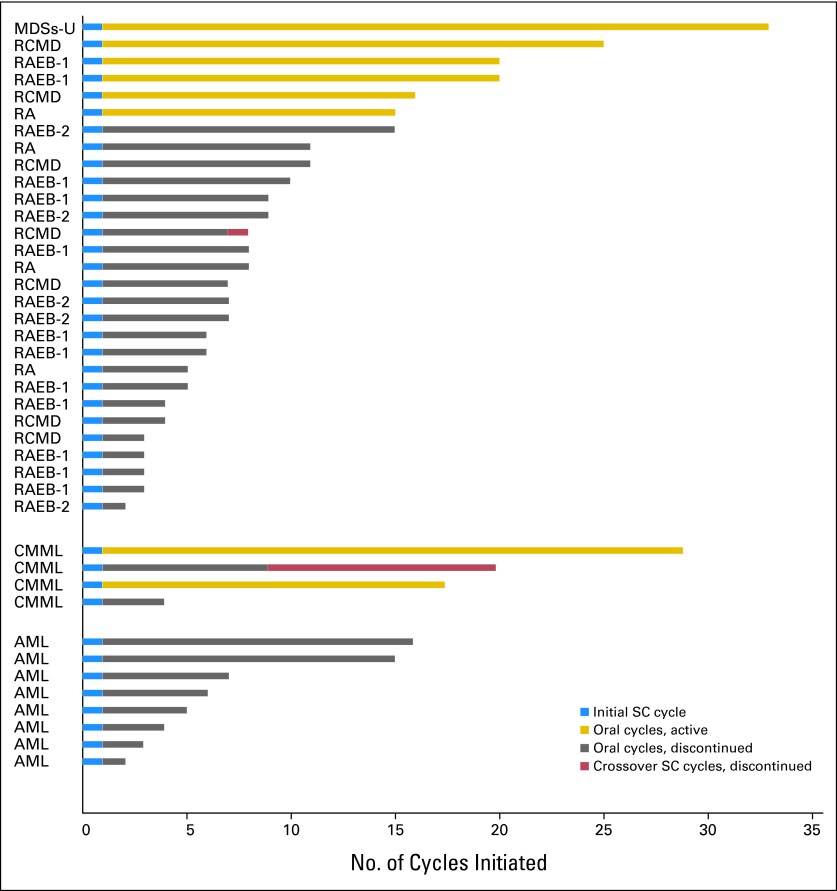
The median number of oral azacitidine cycles administered to patients with MDSs, CMML, and AML was 6 (range, 1 to 32+), 12.5+ (range, 3 to 28+), and 4.5 (range, 1 to 15), respectively. Treatment duration is summarized in Figure 2. The number of patients from the MDSs, CMML, and AML groups who remained on the study at the time of the analysis was 6, 2, and 0, respectively. Response and duration of response data are summarized in Table 4. In the 17 previously treated patients with MDSs and CMML, the overall response rate was 35%, without including patients who only achieved mCR; if those patients were included the response rate would be 65%. In the 15 patients with MDSs and CMML receiving first-line treatment, the overall response rate was 73% and in this group no patients achieved mCR only. Longest duration of response to date was 483 days overall. In one patient who achieved a CR, the response began before oral dosing and ended in cycle 2, thus was likely attributable to the single cycle of SC azacitidine.
Fig 2.
Treatment duration for the 41 patients treated with oral azacitidine (AZA). AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; MDSs-U, myelodysplastic syndromes-unclassified; RA, refractory anemia; RCMD, refractory cytopenias with multilineage dysplasia; RAEB, RA with excess blasts; SC, subcutaneous.
Table 4.
Response in Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia Patients
| Response | Previously Treated Patientsa |
First-Line Treatment |
Duration of Response:Range (days) | ||||
|---|---|---|---|---|---|---|---|
| Responders | Evaluable Patients | % | Responders | Evaluable Patients | % | ||
| Overall responseb | 6 | 17 | 35 | 11 | 15 | 73 | 30-483c |
| CRd | 0 | 17 | 0 | 6 | 15 | 40 | 30-152 |
| Any HIe | 6 | 16 | 38 | 5 | 9 | 56 | 56-483c |
| HI-E | 3 | 10 | 30 | 2 | 4 | 50 | 56-483c |
| HI-N | 0 | 10 | 0 | 2 | 7 | 29 | 82-321c |
| HI-P | 5 | 14 | 36 | 2 | 6 | 33 | 58-351c |
| TI | 0 | 5 | 0 | 1 | 3 | 33 | 76 |
| Red blood cell | 0 | 3 | 0 | 1 | 3 | 33 | 76 |
| Platelet | 0 | 4 | 0 | 0 | NA | ||
| mCRe,f | 6 | 9 | 67 | 2 | 6 | 33 | 63-422g |
NOTE. At any cycle of azacitidine, International Working Group 2006 criteria were used with modifications as described in the Patients and Methods section.
Abbreviations: CR, complete remission; E, erythroid; HI, hematologic improvement; mCR, bone marrow complete remission; N, neutrophil; NA, not applicable; P, platelet; TI, transfusion independence.
Includes erythropoiesis-stimulating agents, chemotherapy, hypomethylating agents, and investigational and/or other agents.
Overall response rate does not include patients achieving mCR only.
One or more responses, including that at upper limit of range, are ongoing. Data were censored as of last visit entered into the clinical database.
Patients achieving CR were not included in any other categories.
One patient with mCR in the previously treated group also achieved HI (both HI-E and HI-P). Two patients with mCR in the first-line treatment group also achieved HI (one patient with HI-P and one patient with both HI-E and HI-N). These patients have been included in both the mCR and HI categories.
In the eight patients who achieved mCR, the response began in cycle 1 of subcutaneous (SC) dosing (n = 4) or very early in cycle 2 of oral dosing (n = 4). Therefore, the contribution of a single SC azacitidine cycle to the induction of these responses is likely relevant.
Bone marrow aspirates were not required after 6 cycles of oral azacitidine treatment, therefore follow-up data were not available to confirm upper limit of duration. Data were censored as of last visit entered into the clinical database.
No responses were observed in patients with AML. Two patients with AML (25%) had stable disease for 14 and 15 cycles, respectively, and five patients with AML (63%) received ≥ 4 oral azacitidine cycles.
DISCUSSION
An oral azacitidine formulation may bring advantages for patients (ease of administration), society (health care cost implications), and disease treatment (extended administration), provided that clinical activity and safety are similar to SC/intravenous azacitidine. This phase I trial demonstrated that oral azacitidine is associated with minimal adverse effects at doses lower than 600 mg. The MTD was 480 mg on a 7-day of 28 days treatment schedule. The 600 mg dose was associated with early onset of severe diarrhea in two of three patients. Diarrhea in patients taking oral azacitidine doses lower than 600 mg was self-limiting and manageable by treatment and/or prophylaxis with antidiarrheal agents and/or dose reduction. Azacitidine, along with one or more ingredients used in its formulation, may contribute to the diarrhea observed because it was a common adverse event at most dose levels tested. Gastrointestinal disturbances may have been exacerbated by the requirement to ingest oral azacitidine in a fasting state. Whether oral azacitidine administration with food can reduce gastrointestinal toxicity will be evaluated in ongoing studies. Grade 3 and 4 AEs consisted primarily of febrile neutropenia, gastrointestinal disturbances, and fatigue. Of the eight patients who experienced grade 3 febrile neutropenia, four entered the study with a baseline ANC of ≤ 500/μL.
After oral administration, maximum azacitidine plasma concentrations were achieved rapidly (within 1 hour), suggesting that absorption occurs from the proximal gastrointestinal tract. Azacitidine exposure increased with increasing oral doses, and the mean relative oral bioavailability ranged from 6.3% to 20%. After multiple doses, there was no evidence of azacitidine accumulation, and no apparent decline in absorption was seen between days 1 and 7. Azacitidine clearance was hepatic and extrahepatic, with little evidence of renal clearance.
Kinetics of the change in DNA methylation levels after SC and oral azacitidine were similar, with maximum hypomethylation achieved on day 15, and methylation levels returned to near-baseline values by the end of each cycle. This pattern has been observed in other azacitidine studies.3,12 At the dosing schedule employed in this study, oral azacitidine affected fewer loci than SC azacitidine; however, 1,482 loci were identified as commonly hypomethylated by both azacitidine formulations.
Significant responses were observed in patients with MDSs and CMML, indicating that oral azacitidine has clinical activity in these settings. Although all patients received an initial cycle of SC azacitidine, which may have contributed to the clinical activity observed, it has been reported that only half of the total hematologic responses to SC azacitidine manifest within 2 cycles.13 Continued treatment with oral azacitidine following the single cycle of SC azacitidine is therefore likely to be associated with the development and/or maintenance of clinical responses observed in this study.
Results from a study investigating alternative SC azacitidine dosing schedules in lower-risk patients with MDSs suggested that for all dosing regimens tested, continued azacitidine treatment may be beneficial.14 The short plasma half-life of azacitidine, S-phase restricted incorporation into DNA, and rapid remethylation of DNA, are contributing factors to the importance of chronic exposure to the drug. It is therefore likely that extended schedules of oral administration will positively affect clinical activity of azacitidine. A follow-up trial has been initiated to investigate the efficacy of such extended schedules.
In conclusion, the MTD for oral azacitidine administered daily for 7 days of a 28-day cycle was determined to be 480 mg, and oral azacitidine is bioavailable and biologically active. Clinical responses were reported in 35% of previously treated patients with MDSs and CMML, and in 73% of patients who received oral azacitidine as first-line therapy. Lower drug exposure and DNA hypomethylation seen with oral azacitidine relative to SC azacitidine provide the rationale for further study of more frequent dosing and extended schedules of oral azacitidine in MDSs, CMML, and AML. While these results show promise for an oral formulation of azacitidine, they are preliminary data and need further research so that these positive early findings can be confirmed in larger numbers of patients.
Footnotes
Supported by Celgene; editorial support in the preparation of this manuscript from Excerpta Medica, sponsored by Celgene.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00528983.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Tao Shi, Celgene (C); Kyle J. MacBeth, Celgene (C); Eric Laille, Celgene (C); Heidi Giordano, Celgene (C); Barry Skikne, Celgene (C) Consultant or Advisory Role: Guillermo Garcia-Manero, Celgene (C); Steven D. Gore, Celgene (C) Stock Ownership: Steven D. Gore, Celgene; Renee Ward, Celgene; Tao Shi, Celgene; Kyle J. MacBeth, Celgene; Eric Laille, Celgene; Heidi Giordano, Celgene; Barry Skikne, Celgene Honoraria: Christopher Cogle, Celgene Research Funding: Guillermo Garcia-Manero, Celgene; Steven D. Gore, Celgene; Christopher Cogle, Celgene; Kyle J. MacBeth, Celgene; Hagop Kantarjian, Celgene; Barry Skikne, Celgene Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Guillermo Garcia-Manero, Steven D. Gore, Christopher Cogle, Renee Ward, Kyle J. MacBeth, Eric Laille, Barry Skikne
Financial support: Barry Skikne
Administrative support: Barry Skikne
Provision of study materials or patients: Guillermo Garcia-Manero, Steven D. Gore, Elias Jabbour, Hagop Kantarjian
Collection and assembly of data: Guillermo Garcia-Manero, Steven D. Gore, Renee Ward, Kyle J. MacBeth, Heidi Giordano, Sarah Sakoian, Barry Skikne
Data analysis and interpretation: Guillermo Garcia-Manero, Steven D. Gore, Christopher Cogle, Renee Ward, Tao Shi, Kyle J. MacBeth, Eric Laille, Heidi Giordano, Elias Jabbour, Hagop Kantarjian, Barry Skikne
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hollenbach PW, Nguyen AN, Brady H, et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS One 5. 2010:e9001. doi: 10.1371/journal.pone.0009001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Alò F, Voso MT, Leone G. Profile of azacitidine. Therapy. 2005;2:717–731. [Google Scholar]
- 3.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 4.Streseman C, Bokelmann I, Mahlknecht U, et al. Azacytidine causes complex DNA methylation responses in myeloid leukemia. Mol Cancer Ther. 2008;7:2998–3005. doi: 10.1158/1535-7163.MCT-08-0411. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Manero G, Stoltz ML, Ward MR, et al. A pilot pharmacokinetic study of oral azacitidine. Leukemia. 2008;22:1680–1684. doi: 10.1038/leu.2008.145. [DOI] [PubMed] [Google Scholar]
- 6.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 8.Troyanskaya O, Cantor M, Sherlock G, et al. Missing value estimation methods for DNA microarrays. Bioinformatics. 2001;17:520–525. doi: 10.1093/bioinformatics/17.6.520. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 12.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110:2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 13.Silverman LR, Fenaux P, Mufti GJ, et al. Continued azacitidine therapy beyond time of first response improves quality of response in patients with higher-risk myelodysplastic syndromes. Cancer. doi: 10.1002/cncr.25774. Epub ahead of print on January 10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyons RM, Cosgriff TM, Modi SS, et al. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol. 2009;27:1850–1856. doi: 10.1200/JCO.2008.17.1058. [DOI] [PubMed] [Google Scholar]