Abstract
Purpose
Brachytherapy has disseminated into clinical practice as an alternative to whole-breast irradiation (WBI) for early-stage breast cancer; however, current national treatment patterns and associated complications remain unknown.
Patients and Methods
We constructed a national sample of Medicare beneficiaries ages 66 to 94 years who underwent breast-conserving surgery from 2008 to 2009 and were treated with brachytherapy or WBI. We used hospital referral regions (HRRs) to assess national treatment variation and an instrumental variable analysis to compare complication rates between treatment groups, adjusting for patient and clinical characteristics. We compared overall, wound and skin, and deep-tissue and bone complications between brachytherapy and WBI at 1 year of follow-up.
Results
Of 29,648 women in our sample, 4,671 (15.8%) received brachytherapy. The percent of patients receiving brachytherapy varied substantially across HRRs, ranging from 0% to over 70% (interquartile range, 7.5% to 23.3%). Of women treated with brachytherapy, 34.3% had a complication compared with 27.3% of women undergoing WBI (P < .001). After adjusting for patient and clinical characteristics, 35.2% of women treated with brachytherapy (95% CI, 28.6 to 41.9) had a complication compared with 18.4% treated with WBI (95% CI, 15.5 to 21.3; P value for difference, <.001). Brachytherapy was associated with a 16.9% higher rate of wound and skin complications compared with WBI (95% CI, 10.0 to 23.9; P < .001), but there was no difference in deep-tissue and bone complications.
Conclusion
Brachytherapy is commonly used among Medicare beneficiaries and varies substantially across regions. After 1 year, wound and skin complications were significantly higher among women receiving brachytherapy compared with those receiving WBI.
INTRODUCTION
Radiation therapy after breast-conserving surgery (BCS) decreases the rate of local recurrence for early-stage disease, and whole-breast irradiation (WBI) has been the standard of care for 20 years.1–7 Over the last decade, newer radiation therapy modalities, such as accelerated partial breast irradiation (APBI) with brachytherapy, have disseminated into clinical practice.3,5,8,9
Breast brachytherapy temporarily implants radiation sources within single or multichannel balloon catheters within the lumpectomy cavity or within a parallel array of implanted interstitial catheters. This technique facilitates larger and fewer radiation dose-fractions directly to the breast tissue around the local excision site.7 The resultant shortened radiation treatment course relative to WBI may potentially decrease toxicity to distant breast tissue.3,7,8,10 Data demonstrating improved outcomes is lacking; however, brachytherapy is increasingly being incorporated into clinical practice, particularly in Medicare patients.5,9,11
Currently, there are no large randomized controlled trials or population-based studies confirming brachytherapy as a safe and effective alternative to WBI.5,8,12–14 A recent study among Medicare patients demonstrated an increase in subsequent mastectomy rates and a higher incidence of acute complications among women receiving brachytherapy compared with WBI.15 In theory, brachytherapy decreases the amount of normal tissue exposed to radiation, thus diminishing radiation exposure of the heart, lungs, and skin. However, because brachytherapy involves surgical implantation of catheters and relatively high surface radiation doses to and around the lumpectomy cavity, it may increase the risk of acute skin reactions, infections, and wound complications.9,15 Acute complications described include wound complications from implantation of the catheter, infection, skin toxicity, fat necrosis, seromas, and catheter failure.7,10,13,15–19
Although a large National Surgical Adjuvant Breast and Bowel Project–Radiation Therapy Oncology Group trial is underway comparing WBI with APBI, it may take years to produce meaningful disease-control data.14,20 An industry-sponsored registry trial of the Mammosite device has been published demonstrating outcomes among experienced users with careful patient selection for brachytherapy.21
As observational comparative-effectiveness studies face increased scrutiny, it is critical to use robust analytic techniques to control for treatment selection bias and to account for both measured and unmeasured confounders.5,9,11,15 To address these knowledge gaps, we sought to analyze brachytherapy use in a national sample of Medicare beneficiaries. An instrumental variable (IV) analysis was used to assess the complication rate among patients receiving brachytherapy compared with WBI.
PATIENTS AND METHODS
Data Source and Study Sample
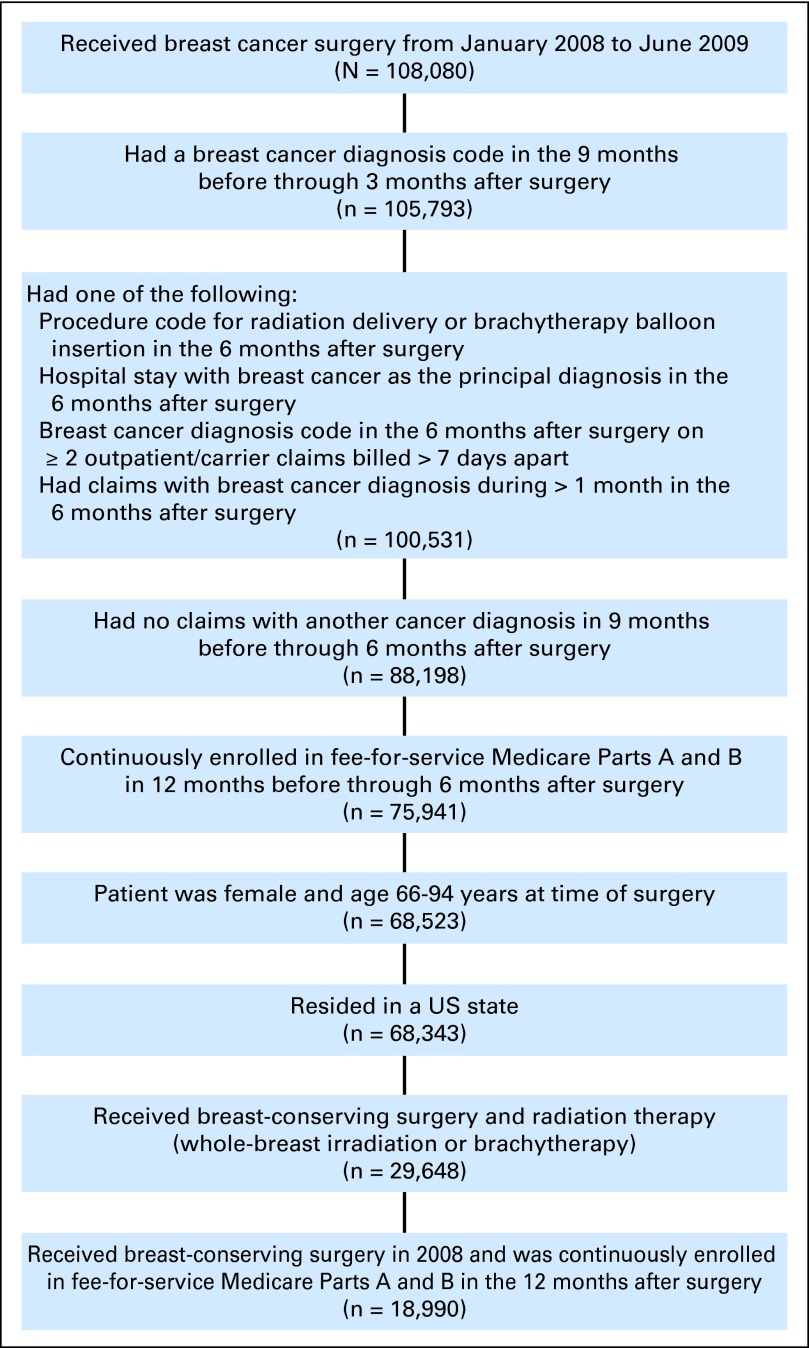
In this retrospective study, we assembled a sample of Medicare beneficiaries who received BCS and adjuvant radiation therapy for invasive breast cancer between 2008 and 2009. The Centers for Medicare and Medicaid Services Chronic Condition Warehouse (CCW) is a national database, which contains 100% of fee-for-service Medicare claims. We used the CCW database to identify all women ages 66 to 94 years who received BCS from January 2008 through June 2009, had an International Classification of Diseases, ninth revision (ICD-9) diagnosis code for invasive breast cancer (174.x), and received brachytherapy or WBI as adjuvant radiation therapy (see Appendix Table A1 [online-only]).22 This time period was selected to ensure that all women had a full 6 months of claims after BCS for assessment of radiation therapy. We excluded women with ICD-9 diagnosis codes for any other cancers in the 9 months before through 6 months after BCS. We excluded women with ductal carcinoma in situ (ICD-9 233), because this study focused on the treatment of invasive breast cancer in older women in which the role of radiation is clearly defined. We also excluded women who did not have continuous Medicare Parts A and B fee-for-service coverage during the study period because their claims are not reported to Medicare (Appendix Fig A1). The sample used for the analysis of complications was further restricted to patients who received BCS in 2008, in order to have a full year of follow-up for assessment of complications. This study was classified as nonhuman subjects research by the Yale Human Investigation Committee.
Radiation Treatment
We searched the Medicare claims for Healthcare Common Procedure Coding System codes indicative of delivery of radiation treatment initiated within 6 months of BCS (Appendix Table A1). The WBI group consisted of traditional external-beam as well as intensity-modulated radiation therapy. Patients who received fewer than four WBI treatments were not included. Any patient who had codes for both WBI and brachytherapy was assigned to the brachytherapy group. Less than 0.5% of the sample received both types of radiation, so reclassifying or excluding these patients would not have changed our results.
Brachytherapy Complications
We used Medicare claims to identify the following complications in the year after BCS: wound complications, fat necrosis, infection, blood vessel injury, pericarditis, lung injury, nerve damage, and rib fracture (Appendix Table A1). This list of complications was based on previous studies and was internally verified by breast cancer surgeons and radiation oncologists.10,12,19,23–25 Our primary outcome was whether a patient experienced any complications. For secondary outcomes, we divided the complications into two groups: those expected to be higher among patients receiving brachytherapy (wound and skin complications, including wound complications, fat necrosis, unspecified complications, radiation complications, and infections) and those expected to be higher among patients receiving WBI (deep-tissue and bone complications, including blood vessel injury, pericarditis, lung injury, nerve damage, and rib fractures).18,19
Construction of Variables
Patient characteristics included age, race, year of surgery, metropolitan status based on Core Based Statistical Areas, and median household income at the ZIP code level. Clinical characteristics included the number of comorbidities, tumor laterality, type of radiation facility (free-standing, hospital-based), receipt of chemotherapy, and axillary node dissection. We incorporated health system and clinical characteristics into our analyses. For instance, we hypothesized that type of facility could affect type of radiation received, and possibly the complication rate. Patients with any radiation claims in the outpatient file were assumed to have received treatment at a hospital-based facility; patients who only had radiation claims in the physician file were assumed to have received treatment at a free-standing facility. Hospital admission, receipt of screening mammogram or a flu shot, or a visit to a primary care physician (all in the year before surgery) were used as markers for access to health care. This is especially important in this analysis because we were limited to identifying complications that were recorded in diagnosis or procedure codes and patients with greater access to care will have more opportunities for a provider to record one of these codes. Prior hospital admission was considered a proxy for health of the patient. We hypothesized that having had a screening mammogram could be a proxy for earlier-stage disease. All clinical characteristics were assessed using Medicare claims.
Patients were assigned to their hospital referral region (HRR) based on ZIP code. HRRs, as defined by Wennberg et al,26 represent regional healthcare markets. The HRR-level brachytherapy rate was calculated as the number of patients who received brachytherapy divided by the total number of patients in the sample residing in that HRR. We summarized brachytherapy rates for each HRR and reported the range across HRRs.
To identify comorbid conditions, we searched inpatient, outpatient, and physician claims billed between 12 months and 1 month before BCS. We used ICD-9 diagnosis codes that appeared on at least one inpatient claim or two or more outpatient/physician claims billed at least 30 days apart. We searched for the comorbidity categories outlined by Elixhauser et al27 that we previously found to be significantly associated with survival in a sample of noncancer patients. The number of conditions a patient had was summed to create a comorbidity score.
Statistical Analysis
χ2 tests assessed the bivariate associations between the independent variables and receipt of brachytherapy. We used an IV analysis to compare complications between patients who received brachytherapy versus those who received WBI. An IV analysis is used to account for unmeasured confounders that are also correlated with the outcome; apparent effects of treatment may be a result of treatment-selection bias. An IV is a factor that is associated with the primary independent variable (brachytherapy), but is only associated with the outcome (complications) through its effect on the independent variable.28 In the absence of randomization, an IV analysis can be used to control for both measured and unmeasured confounders, making it a useful technique for observational health services research.29–33
Our IV was the differential distance that a patient would have to travel to receive brachytherapy beyond what she would have to travel to receive WBI. It was calculated as the distance to the nearest hospital at which five or more patients in our sample went on to receive brachytherapy minus the distance to the nearest hospital at which any patient in our sample received BCS. Differential distance has been used in prior research as an IV for treatment.29,34–38 To determine whether differential distance was an appropriate IV for our study, we qualitatively evaluated the distribution of patient and clinical characteristics across strata of differential distance.37 Because the instrument is in essence pseudorandomization, the different strata should seem similar with respect to baseline sample characteristics. We also assessed the association between differential distance and receipt of brachytherapy and the association between differential distance and the primary outcome of complications; with a valid instrument, the first association should be strong, and the second association should be weak. Thus, we calculated an F-statistic for each using a linear probability model with differential distance categorized into quintiles.
We then estimated a two-stage linear probability model, with complication as the outcome, specifying treatment with brachytherapy as dependent on differential distance and all patient and clinical characteristics in the first stage. We used a linear probability model because of the availability of estimation routines for IVs; such models are an appropriate alternative to logit models when the treatment and complication rates are between 20% and 80%.38,39 We used the Hausmann test of endogeneity to assess whether the IV contributed to the model.
To better interpret the results of the model, we estimated the average effect of brachytherapy on complication rates. First, we calculated the predicted probability of each outcome for each patient, assuming she did and did not receive brachytherapy, using the estimated coefficients from the two-stage linear probability model. We also calculated the SE of each prediction and simulated a random outcome for each patient under the probability from each assumption. Averaging these simulated outcomes over all patients in the sample provided an average expected outcome rate for brachytherapy and WBI.
All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC), Stata version 12.0 (StataCorp, College Station, TX), and ArcMap version 10 (ESRI, Redlands, CA).
RESULTS
We identified 29,648 women with early-stage invasive breast cancer who received BCS between 2008 and 2009; 4,671 (15.8%) received brachytherapy (Table 1). In the bivariate analysis, residence in a metropolitan area, higher income, not receiving chemotherapy, and having greater access to care as measured by prior receipt of screening mammogram and visiting a primary care physician were significantly associated with higher receipt of brachytherapy (all P < .001). Conversely, age was not a significant determinant of brachytherapy, as 15% to 16% of women were treated across all age categories (P = .95). Race, year of surgery, number of comorbidities, and receipt of the flu shot were also not associated with receipt of brachytherapy.
Table 1.
Patient and Clinical Characteristics and Receipt of Brachytherapy
| Characteristic | No. of Patients | % | Patients Who Received Brachytherapy (%) | P* |
|---|---|---|---|---|
| Total No. of patients | 29,648 | 15.8 | ||
| Age at breast-conserving surgery, years | .95 | |||
| 66-69 | 7,879 | 26.6 | 16.0 | |
| 70-74 | 8,835 | 29.8 | 15.7 | |
| 75-79 | 6,871 | 23.2 | 15.6 | |
| 80-84 | 4,435 | 15.0 | 15.8 | |
| 85-94 | 1,628 | 5.5 | 15.5 | |
| Race | .11 | |||
| White | 27,050 | 91.2 | 15.9 | |
| Black | 1,787 | 6.0 | 14.1 | |
| Other | 811 | 2.7 | 14.9 | |
| Year of surgery | .66 | |||
| 2008 | 19,304 | 65.1 | 15.7 | |
| 2009 | 10,344 | 34.9 | 15.9 | |
| Residence in metro county | < .001 | |||
| Yes | 23,913 | 80.7 | 16.4 | |
| No | 5,735 | 19.3 | 13.2 | |
| Median household income | < .001 | |||
| Q1 (≤ $33,208) | 5,724 | 19.3 | 15.4 | |
| Q2 ($33,209-$39,659) | 5,723 | 19.3 | 14.5 | |
| Q3 ($39,661-$47,295) | 5,728 | 19.3 | 16.0 | |
| Q4 ($47,300-$60,118) | 5,727 | 19.3 | 16.1 | |
| Q5 (≥ $60,124) | 5,723 | 19.3 | 16.0 | |
| Unknown | 1,023 | 3.5 | 19.9 | |
| Comorbidity | .41 | |||
| 0 conditions | 16,925 | 57.1 | 15.6 | |
| 1-2 conditions | 10,478 | 35.3 | 16.1 | |
| ≥ 3 conditions | 2,245 | 7.6 | 15.1 | |
| Tumor laterality | < .001 | |||
| Right-sided | 12,527 | 42.3 | 15.8 | |
| Left-sided | 12,828 | 43.3 | 16.6 | |
| Unknown | 4,293 | 14.5 | 13.3 | |
| Type of radiation facility | .01 | |||
| Hospital-based | 18,221 | 61.4 | 15.3 | |
| Free-standing | 11,435 | 38.6 | 16.4 | |
| Axillary node dissection | < .001 | |||
| No | 8,684 | 29.3 | 12.1 | |
| Yes | 20,964 | 70.7 | 17.3 | |
| Chemotherapy | < .001 | |||
| No chemotherapy | 25,956 | 87.6 | 16.4 | |
| Chemotherapy started in month prior through month after surgery | 1,211 | 4.1 | 7.0 | |
| Chemotherapy started in 31-365 days after surgery | 2,481 | 8.4 | 13.3 | |
| Hospital admission† | .005 | |||
| No | 25,671 | 86.6 | 16.0 | |
| Yes | 3,977 | 13.4 | 14.2 | |
| Screening mammogram† | < .001 | |||
| No | 6,840 | 23.1 | 13.5 | |
| Yes | 22,808 | 76.9 | 16.4 | |
| Flu shot† | .13 | |||
| No | 12,440 | 42.0 | 15.4 | |
| Yes | 17,208 | 58.0 | 16.0 | |
| Visit to primary care physician† | .002 | |||
| No | 876 | 3.0 | 12.0 | |
| Yes | 28,772 | 97.1 | 15.9 |
P value is for χ2 test between each covariate and receipt of brachytherapy.
In the year before breast-conserving surgery.
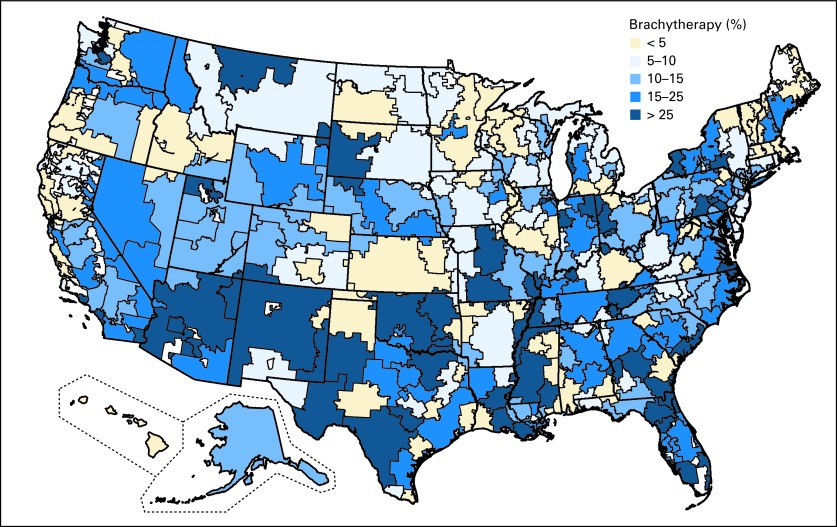
Significant regional variation existed in the receipt of brachytherapy at the HRR level (Fig 1). The median percent of patients receiving brachytherapy across HRRs was 13.8% (interquartile range, 7.5% to 23.3%). Use of brachytherapy tended to be higher in the southwestern portions of the country and along the east coast, with lower levels in the middle, north, and western regions of the country. After restricting to HRRs with at least 20 patients, there were 20 HRRs in which no patients received brachytherapy. The two HRRs with the highest use of brachytherapy were Ogden, UT (65.4%) and Lafayette, IN (71.4%).
Fig 1.
Use of brachytherapy by hospital referral region. White areas indicate hospital referral regions where no patients in the sample resided.
There were 18,990 patients included in the complications analysis. Of the 2,980 women treated with brachytherapy, 34.3% had a complication compared with 27.3% of the 16,010 women who received WBI (P < .001; Table 2). Rates of wound complication, fat necrosis, infection, and rib fracture were significantly higher in women treated with brachytherapy compared with WBI. Among women who received brachytherapy, 20.4% experienced wound complications versus 12.7% among those treated with WBI (P < .001). Similarly, infection occurred in 12.0% of women treated with brachytherapy compared with 10.2% treated with WBI (P = .004). Lung injury was the only complication that was significantly higher in women treated with WBI (2.0%) compared with those treated with brachytherapy (1.5%; P = .04).
Table 2.
One-Year Complication Rates for Patients Receiving Brachytherapy and Whole-Breast Irradiation
| Complication | Brachytherapy (n = 2,980) |
Whole-Breast Irradiation (n = 16,010) |
P* | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Any complication | 1,021 | 34.3 | 4,370 | 27.3 | < .001 |
| Wound and skin complications | 937 | 31.4 | 3,834 | 24.0 | < .001 |
| Wound complication/damage to skeletal muscle, connective tissue, bone | 608 | 20.4 | 2,037 | 12.7 | < .001 |
| Fat necrosis | 203 | 6.8 | 923 | 5.8 | .03 |
| Unspecified complication | 12 | 0.4 | 38 | 0.2 | .11 |
| Radiation complication | 20 | 0.7 | 106 | 0.7 | .96 |
| Infection | 358 | 12.0 | 1,639 | 10.2 | .004 |
| Severe infection | 30 | 1.0 | 224 | 1.4 | .09 |
| Deep-tissue complications | 135 | 4.5 | 792 | 5.0 | .33 |
| Blood vessel injury† | 0 | 0.0 | < 11 | < 0.1 | .29 |
| Pericarditis† | < 11 | < 0.4 | 24 | 0.1 | .51 |
| Lung injury | 44 | 1.5 | 327 | 2.0 | .04 |
| Nerve damage | 64 | 2.1 | 369 | 2.3 | .60 |
| Rib fracture | 27 | 0.9 | 92 | 0.6 | .04 |
NOTE. Data are restricted to the 18,990 patients for whom we had 1 full year of claims after breast-conserving surgery.
P value is for χ2 test between each complication and type of radiation received.
The Centers for Medicare and Medicaid Services prohibit the publication of cell sizes < 11.
The distribution of patient and clinical characteristics was well-balanced across strata of differential distance, thus supporting our use of differential distance as our IV. In addition, the F-statistic for the linear model of receipt of brachytherapy was 343.7, indicating a strong association between differential distance and treatment. The F-statistic for the linear model of differential distance and any complications was 5.7, indicating a weak association. These F-statistics were consistent with a valid IV.
In the IV analysis, which adjusted for patient risk factors, brachytherapy was associated with a 16.8% higher rate of complications compared with WBI (95% CI, 9.6 to 24.1; P < .001; Table 3). In the secondary outcomes analysis, women treated with brachytherapy compared with WBI had a 16.9% higher rate of wound and skin complications (95% CI, 10.0 to 23.9; P < .001). There was no significant association between treatment modality and the incidence of deep-tissue and bone complications (effect of brachytherapy v WBI, 1.9%; 95% CI, −1.5 to 5.4; P = .28). The full results of the two-stage linear probability model are available in Appendix Table A2 (online only).
Table 3.
Estimated Adjusted 1-Year Complication Rates for Patients Receiving Brachytherapy and Whole-Breast Irradiation
| Treatment | Any Complication |
Wound and Skin Complications |
Deep-Tissue and Bone Complications |
|||
|---|---|---|---|---|---|---|
| % of Patients | 95% CI | % of Patients | 95% CI | % of Patients | 95% CI | |
| Brachytherapy | 35.2 | 28.6 to 41.9 | 33.7 | 27.3 to 40.1 | 4.4 | 1.3 to 7.6 |
| Whole-breast irradiation | 18.4 | 15.5 to 21.3 | 16.8 | 14.0 to 19.5 | 2.5 | 1.1 to 3.9 |
| Difference | 16.8 | 9.6 to 24.1 | 16.9 | 10.0 to 23.9 | 1.9 | −1.5 to 5.4 |
NOTE. Rates are adjusted for age at breast-conserving surgery, race, income, comorbidity, type of radiation facility, axillary node dissection, receipt of chemotherapy, prior hospital admission, prior screening mammogram, and prior visit to primary care physician.
The tests of endogeneity for the outcomes of any complication and wound and skin complications were both significant (P < .01), indicating that treatment was endogenous and that therefore the IV analysis did correct for treatment-selection bias. For the outcome of deep-tissue and bone complications, the test of endogeneity was not significant (P = .18), indicating that brachytherapy was not endogenous and that the IV analysis did not adjust for appreciable selection bias.
Based on the final model, the estimated overall complication rate associated with brachytherapy was 35.2% (95% CI, 28.6 to 41.9) compared with 18.4% (95% CI, 15.5 to 21.3) associated with receiving WBI (P < .001; predicted difference in complications, 16.8%; 95% CI, 9.6 to 24.1; Table 3). Women treated with brachytherapy had a 33.7% (95% CI, 27.3 to 40.1) estimated rate of wound and skin complications compared with 16.8% (95% CI, 14.0 to 19.5) in those treated with WBI. Similarly, 4.4% (95% CI, 1.3 to 7.6) of women treated with brachytherapy had deep-tissue and bone complications compared with 2.5% (95% CI, 1.1 to 3.9) in those treated with WBI.
DISCUSSION
In this national study of approximately 30,000 women, 15.8% of Medicare beneficiaries undergoing adjuvant radiotherapy received brachytherapy from 2008 to 2009. This rate is substantially higher than the previously reported rates of approximately 10% in 2006 and less than 1% in 2000.5,9 We also found substantial national variation in the use of brachytherapy.
Brachytherapy had a 16.8% higher complication rate compared with WBI. After adjusting for patient characteristics and treatment-selection bias, the estimated complication rate for brachytherapy was 35.2%, nearly double the rate for WBI (18.4%). Randomized control trials comparing outcomes and complications between brachytherapy and WBI are not available; however, brachytherapy has been promoted in part because of a hypothetical lower risk of complications.40
In our sample, age was not a significant determinant of receipt of brachytherapy, as women younger than 80 years received approximately equal rates of brachytherapy as those older than 80 years. In contrast, prior studies have reported markedly increasing use of brachytherapy among women older than 80 years.5,9 Although the American Brachytherapy Society,41 the American Society of Therapeutic Radiation Oncology,42 and the American Society of Breast Surgeons43 have all issued guidelines stating that brachytherapy is safe for women ages 45 to 60 years, additional evidence is needed to guide treatment decisions among women older than 60 years.
Brachytherapy was associated with several factors consistent with earlier-stage disease, such as not receiving chemotherapy, recent screening mammography, or seeing their primary care physician in the year before BCS. There was a slightly higher rate of brachytherapy treatment among women treated at free-standing versus hospital-based radiation facilities, which may be in part because of potential financial incentives.44
Our study has several limitations. This was an observational study and was therefore not randomized. Because the CCW data do not contain information on tumor characteristics, we were unable to adjust for potential confounders such as tumor stage. Billing codes are also limited in that we cannot know the functional status of patients and the quality of treatment delivery. Because we used administrative claims, we could not assess patient-reported outcomes or the grade of complications. Future work is therefore needed to assess these factors as well as longer-term outcomes and the effect of newer technologies, which minimize radiation to the skin.
Our study had several strengths, including the use of a representative, national Medicare sample, as well as the most current data available, suggesting that our results represent patterns and outcomes similar to current clinical care. The use of a national database resulted in a large sample. In the National Surgical Adjuvant Breast and Bowel Project trial currently underway,20 approximately 28% of patients randomly assigned to APBI thus far are receiving brachytherapy as opposed to three-dimensional conformal external radiotherapy, so it is possible that the study will be underpowered to detect differences in complications among the patients receiving brachytherapy compared with WBI. Though we were unable to account for tumor stage, we were able to account for chemotherapy use and axillary node dissection as well as other patient factors. We also used an IV analysis with an established instrument (differential distance), which allowed us to account for treatment-selection bias.
In conclusion, we found that a substantial proportion of Medicare beneficiaries were treated with brachytherapy for breast cancer from 2008 to 2009. The marked regional variation in utilization suggests that nonclinical factors play an important role in its dissemination. Given the higher costs associated with brachytherapy, the higher risk of complications suggests that clinicians, patients, and policy makers should closely scrutinize the use of this treatment modality. Clinicians and policy makers should aggressively pursue the generation and dissemination of additional evidence about outcomes, complications, and cost to better inform decision-making about specific radiation modalities.
Acknowledgment
We thank General Dynamics Information Technology (Buccaneer Computer Systems and Service, Inc) and Chronic Condition Data Warehouse (under contract with CMS).
Appendix
Table A1.
Procedure and Diagnosis Codes Used in Analysis
| Treatment and Complications | Health Care Common Procedure Coding System | International Classification of Disease, 9th Revision |
|
|---|---|---|---|
| Procedure Codes | Diagnosis Codes | ||
| Breast surgery | 19110, 19120, 19125, 19126, 19160, 19162, 19301, 19302, 19180, 19182, 19200, 19220, 19240, 19303, 19304, 19305, 19306, 19307 | 85.20, 85.21, 85.22, 85.23, 85.25, 85.41, 85.42, 85.43, 85.44, 85.45, 85.46, 85.47, 85.48 | |
| Whole-breast irradiation | 77402, 77403, 77404, 77406, 77407, 77408, 77409, 77411, 77412, 77413, 77414, 77416, 77418, 0073T, G0174 | ||
| Brachytherapy | 77761, 77762, 77763, 77776, 77777, 77778, 77781, 77782, 77783, 77784, 77785, 77786, 77787, 77799, 0182T | ||
| Brachytherapy balloon insertion | 19296, 19297, 19298, C9714, C9715 | ||
| Wound complication/damage to skeletal muscle, connective tissue, bone | 10121, 11000, 11001, 11040-11044, 12020, 12021, 13160, 97597, 97598, 97601, 97602, 97605, 97606, 99183 | 86.22, 86.28, 93.59, 93.95, 96.59, 97.16 | 709.4, 998.6, 998.83, 998.1x, 998.3x |
| Fat necrosis | 611.3 | ||
| Unspecified complication | 998.9 | ||
| Blood vessel injury | 900.x, 903.x, | ||
| Pericarditis | 33010, 33011, 33015, 33025, 33030, 33031 | 37.0, 37.29 | 420.0 420.90, 420.91, 423.2 |
| Lung injury | 508.0, 508.1, 514, 518.4, 518.82, 799.1 | ||
| Radiation complication, unspecified | 990 | ||
| Nerve damage | 353, 353.0, 353.1, 353.3, 353.4, 353.5, 353.8, 353.9, 354.2, 354.3, 354.4, 723.4, 953.x, 955.x | ||
| Infection | 10060, 10061, 10160, 10180 | 041.9, 682.9, 999.31, 998.5x | |
| Severe infection | 785.59, 790.7, 999.3, 038.x, 785.5x | ||
| Fractures | 807.0x, 807.1x | ||
Table A2.
Results of Two-Stage Linear Probability Model, Using Differential Distance As Instrumental Variable
| Variable | Any Complication |
Wound and Skin Complications |
Deep-Tissue and Bone Complications |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Intercept | 0.184 | 0.141 to 0.227 | < .001 | 0.168 | 0.126 to 0.209 | < .001 | 0.025 | 0.004 to 0.046 | .017 |
| Radiation modality | |||||||||
| Whole-breast irradiation | Ref | Ref | Ref | ||||||
| Brachytherapy | 0.168 | 0.096 to 0.240 | < .001 | 0.169 | 0.100 to 0.238 | < .001 | 0.019 | −0.015 to 0.054 | .28 |
| Age at breast-conserving surgery, years | |||||||||
| 66-69 | Ref | Ref | Ref | ||||||
| 70-74 | 0.008 | −0.009 to 0.025 | .36 | 0.012 | −0.005 to 0.028 | .16 | −0.005 | −0.013 to 0.003 | .23 |
| 75-79 | 0.010 | −0.008 to 0.028 | .28 | 0.007 | −0.011 to 0.025 | .44 | 0.001 | −0.008 to 0.009 | .88 |
| 80-84 | 0.014 | −0.007 to 0.035 | .19 | 0.014 | −0.006 to 0.034 | .17 | 0.004 | −0.006 to 0.014 | .41 |
| 85-94 | 0.011 | −0.019 to 0.041 | .48 | 0.001 | −0.028 to 0.030 | .93 | 0.012 | −0.003 to 0.026 | .12 |
| Race | |||||||||
| White | Ref | Ref | Ref | ||||||
| Black | −0.026 | −0.054 to 0.001 | .06 | −0.017 | −0.043 to 0.010 | .22 | −0.011 | −0.024 to 0.002 | .09 |
| Other | −0.030 | −0.069 to 0.009 | .13 | −0.028 | −0.066 to 0.009 | .14 | −0.003 | −0.021 to 0.016 | .78 |
| Income | |||||||||
| Q1 (≤ $33,208) | Ref | Ref | Ref | ||||||
| Q2 ($33,209-$39,659) | 0.002 | −0.019 to 0.023 | .84 | 0.005 | −0.015 to 0.025 | .64 | −0.002 | −0.012 to 0.008 | .70 |
| Q3 ($39,661-$47,295) | 0.012 | −0.009 to 0.032 | .27 | 0.011 | −0.009 to 0.031 | .29 | 0.002 | −0.008 to 0.011 | .77 |
| Q4 ($47,300-$60,118) | 0.010 | −0.011 to 0.031 | .35 | 0.007 | −0.013 to 0.027 | .47 | 0.003 | −0.007 to 0.013 | .53 |
| Q5 (≥ $60,124) | 0.011 | −0.010 to 0.032 | .29 | 0.013 | −0.008 to 0.033 | .22 | 0.001 | −0.009 to 0.011 | .89 |
| Unknown | 0.013 | −0.024 to 0.051 | .49 | 0.003 | −0.033 to 0.039 | .87 | 0.005 | −0.013 to 0.023 | .55 |
| Comorbidity | |||||||||
| 0 conditions | Ref | Ref | Ref | ||||||
| 1-2 conditions | 0.052 | 0.038 to 0.066 | < .001 | 0.041 | 0.028 to 0.055 | < .001 | 0.017 | 0.011 to 0.024 | < .001 |
| ≥ 3 conditions | 0.114 | 0.087 to 0.142 | < .001 | 0.085 | 0.059 to 0.111 | < .001 | 0.055 | 0.042 to 0.068 | < .001 |
| Type of radiation facility | |||||||||
| Hospital-based | Ref | Ref | Ref | ||||||
| Free-standing | −0.009 | −0.022 to 0.004 | .18 | −0.008 | −0.021 to 0.005 | .21 | 0.000 | −0.007 to 0.006 | .95 |
| Axillary node dissection | |||||||||
| No | Ref | Ref | Ref | ||||||
| Yes | −0.006 | −0.020 to 0.009 | .46 | −0.002 | −0.016 to 0.012 | .76 | −0.001 | −0.008 to 0.006 | .88 |
| Chemotherapy | |||||||||
| No chemotherapy | Ref | Ref | Ref | ||||||
| Chemotherapy started in month prior through month after surgery | 0.032 | −0.003 to 0.067 | .07 | 0.025 | −0.009 to 0.058 | .15 | 0.010 | −0.007 to 0.026 | .24 |
| Chemotherapy started 31-365 days after surgery | 0.061 | 0.038 to 0.085 | < .001 | 0.057 | 0.035 to 0.080 | < .001 | 0.019 | 0.007 to 0.030 | .001 |
| Hospital admission* | |||||||||
| No | Ref | Ref | Ref | ||||||
| Yes | 0.022 | 0.001 to 0.042 | .04 | 0.014 | −0.006 to 0.034 | .16 | 0.017 | 0.007 to 0.027 | .001 |
| Screening mammogram* | |||||||||
| No | Ref | Ref | Ref | ||||||
| Yes | −0.017 | −0.033 to −0.002 | .03 | −0.015 | −0.030 to 0.000 | .05 | −0.006 | −0.013 to 0.002 | .14 |
| Visit to primary care physician* | |||||||||
| No | Ref | Ref | Ref | ||||||
| Yes | 0.047 | 0.009 to 0.085 | .02 | 0.033 | −0.003 to 0.070 | .07 | 0.011 | −0.007 to 0.030 | .22 |
Abbreviation: Ref, reference.
In the year before breast-conserving surgery.
Fig A1.
Sample selection.
Footnotes
See accompanying editorial on page 4283; listen to the podcast by Dr Buchholz at www.jco.org/podcasts
Supported by Grant No. 1R01CA149045 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Cary P. Gross, Fair Health (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: Beth-Ann Lesnikoski, Cianna Medical; Cary P. Gross, Medtronic grant for work on sharing of clinical trial data
AUTHOR CONTRIBUTIONS
Conception and design: Carolyn J. Presley, Pamela R. Soulos, Jeph Herrin, Kenneth B. Roberts, James B. Yu, Brigid Killelea, Beth-Ann Lesnikoski, Cary P. Gross
Collection and assembly of data: Carolyn J. Presley, Pamela R. Soulos, Jessica B. Long, Cary P. Gross
Data analysis and interpretation: Carolyn J. Presley, Pamela R. Soulos, Jeph Herrin, Kenneth B. Roberts, James B. Yu, Jessica B. Long, Cary P. Gross
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.NIH consensus conference: Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 2.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): An international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 3.Njeh CF, Saunders MW, Langton CM. Accelerated partial breast irradiation (APBI): A review of available techniques. Radiat Oncol. 2010;5:90. doi: 10.1186/1748-717X-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartelink H, Horiot JC, Poortmans P, et al. Recurrence rates after treatment of breast cancer with standard radiotherapy with or without additional radiation. N Engl J Med. 2001;345:1378–1387. doi: 10.1056/NEJMoa010874. [DOI] [PubMed] [Google Scholar]
- 5.Abbott AM, Habermann EB, Tuttle TM. Trends in the use of implantable accelerated partial breast irradiation therapy for early stage breast cancer in the United States. Cancer. 2011;117:3305–3310. doi: 10.1002/cncr.25927. [DOI] [PubMed] [Google Scholar]
- 6.Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Natl Compr Canc Netw. 2011;9:136–222. doi: 10.6004/jnccn.2011.0016. [DOI] [PubMed] [Google Scholar]
- 7.Aristei C, Palumbo I, Cucciarelli F, et al. Partial breast irradiation with interstitial high-dose-rate brachytherapy in early breast cancer: Results of a phase II prospective study. Eur J Surg Oncol. 2009;35:144–150. doi: 10.1016/j.ejso.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Polgár C, Fodor J, Major T, et al. Breast-conserving treatment with partial or whole breast irradiation for low-risk invasive breast carcinoma: Five-year results of a randomized trial. Int J Radiat Oncol Biol Phys. 2007;69:694–702. doi: 10.1016/j.ijrobp.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Smith GL, Xu Y, Buchholz TA, et al. Brachytherapy for accelerated partial-breast irradiation: A rapidly emerging technology in breast cancer care. J Clin Oncol. 2011;29:157–165. doi: 10.1200/JCO.2009.27.0942. [DOI] [PubMed] [Google Scholar]
- 10.Harper JL, Jenrette JM, Vanek KN, et al. Acute complications of MammoSite brachytherapy: A single institution's initial clinical experience. Int J Radiat Oncol Biol Phys. 2005;61:169–174. doi: 10.1016/j.ijrobp.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Hattangadi JA, Taback N, Neville BA, et al. Accelerated partial breast irradiation using brachytherapy for breast cancer: Patterns in utilization and guideline concordance. J Natl Cancer Inst. 2012;104:29–41. doi: 10.1093/jnci/djr495. [DOI] [PubMed] [Google Scholar]
- 12.Chao KK, Vicini FA, Wallace M, et al. Analysis of treatment efficacy, cosmesis, and toxicity using the MammoSite breast brachytherapy catheter to deliver accelerated partial-breast irradiation: The William Beaumont hospital experience. Int J Radiat Oncol Biol Phys. 2007;69:32–40. doi: 10.1016/j.ijrobp.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Benitez PR, Keisch ME, Vicini F, et al. Five-year results: The initial clinical trial of MammoSite balloon brachytherapy for partial breast irradiation in early-stage breast cancer. Am J Surg. 2007;194:456–462. doi: 10.1016/j.amjsurg.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Radiation Therapy Oncology Group. RTOG 0413 Protocol Information. [Accessed January 26, 2012]. http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0413.
- 15.Smith GL, Xu Y, Buchholz TA, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA. 2012;307:1827–1837. doi: 10.1001/jama.2012.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragun AE, Harper JL, Jenrette JM, et al. Predictors of cosmetic outcome following MammoSite breast brachytherapy: A single-institution experience of 100 patients with two years of follow-up. Int J Radiat Oncol Biol Phys. 2007;68:354–358. doi: 10.1016/j.ijrobp.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Keisch M, Vicini F, Kuske RR, et al. Initial clinical experience with the MammoSite breast brachytherapy applicator in women with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;55:289–293. doi: 10.1016/s0360-3016(02)04277-3. [DOI] [PubMed] [Google Scholar]
- 18.Vicini F, Beitsch P, Quiet C, et al. Five-year analysis of treatment efficacy and cosmesis by the American Society of Breast Surgeons MammoSite Breast Brachytherapy Registry Trial in patients treated with accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;79:808–817. doi: 10.1016/j.ijrobp.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 19.Khan AJ, Vicini FA, Beitsch P, et al. Local control, toxicity, and cosmesis in women > 70 years enrolled in the American Society of Breast Surgeons accelerated partial breast irradiation registry trial. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.12.027. [epub ahead of print February 21, 2012] [DOI] [PubMed] [Google Scholar]
- 20.Julian TB, Costantino JP, Vicini FA, et al. Early toxicity results with 3D conformal external beam therapy (CEBT) from the NSABP B-39/RTOG 0413 accelerated partial-breast irradiation (APBI) trial. Int J Radiat Oncol Biol Phys. 2011;81:S7. [Google Scholar]
- 21.Shah C, Vicini F, Keisch M, et al. Outcome after ipsilateral breast tumor recurrence in patients who receive accelerated partial breast irradiation. Cancer. doi: 10.1002/cncr.27400. 10.1002/cncr.27400 [epub ahead of print on January 17, 2012] [DOI] [PubMed] [Google Scholar]
- 22.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuttino LW, Todor D, Rosu M, et al. Skin and chest wall dose with multi-catheter and MammoSite breast brachytherapy: Implications for late toxicity. Brachytherapy. 2009;8:223–226. doi: 10.1016/j.brachy.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Dickler A, Kirk MC, Choo J, et al. Cosmetic outcome and incidence of infection with the MammoSite breast brachytherapy applicator. Breast J. 2005;11:306–310. doi: 10.1111/j.1075-122X.2005.00014.x. [DOI] [PubMed] [Google Scholar]
- 25.Richards GM, Berson AM, Rescigno J, et al. Acute toxicity of high-dose-rate intracavitary brachytherapy with the MammoSite applicator in patients with early-stage breast cancer. Ann Surg Oncol. 2004;11:739–746. doi: 10.1245/ASO.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Wennberg JE, Cooper MM. Chicago, IL: American Hospital Publishing; 1998. The Dartmouth Atlas of Health Care in the United States. [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Newhouse JP, McClellan M. Econometrics in outcomes research: The use of instrumental variables. Annu Rev Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 29.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? JAMA. 1994;272:859–866. [PubMed] [Google Scholar]
- 30.Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–181. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earle CC, Tsai JS, Gelber RD, et al. Effectiveness of chemotherapy for advanced lung cancer in the elderly: Instrumental variable and propensity analysis. J Clin Oncol. 2001;19:1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 32.Wisnivesky JP, Halm E, Bonomi M, et al. Effectiveness of radiation therapy for elderly patients with unresected stage I and II non-small cell lung cancer. Am J Respir Crit Care Med. 2010;181:264–269. doi: 10.1164/rccm.200907-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: Effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297:278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck CA, Penrod J, Gyorkos TW, et al. Does aggressive care following acute myocardial infarction reduce mortality? Analysis with instrumental variables to compare effectiveness in Canadian and United States patient populations. Health Serv Res. 2003;38:1423–1440. doi: 10.1111/j.1475-6773.2003.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McConnell KJ, Newgard CD, Mullins RJ, et al. Mortality benefit of transfer to level I versus level II trauma centers for head-injured patients. Health Serv Res. 2005;40:435–457. doi: 10.1111/j.1475-6773.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pracht EE, Tepas JJ, III, Celso BG, et al. Survival advantage associated with treatment of injury at designated trauma centers: A bivariate probit model with instrumental variables. Med Care Res Rev. 2007;64:83–97. doi: 10.1177/1077558706296241. [DOI] [PubMed] [Google Scholar]
- 37.Xian Y, Holloway RG, Chan PS, et al. Association between stroke center hospitalization for acute ischemic stroke and mortality. JAMA. 2011;305:373–380. doi: 10.1001/jama.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahn JM, Ten Have TR, Iwashyna TJ. The relationship between hospital volume and mortality in mechanical ventilation: An instrumental variable analysis. Health Serv Res. 2009;44:862–879. doi: 10.1111/j.1475-6773.2009.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reference deleted.
- 40.Shaitelman SF, Vicini FA, Beitsch P, et al. Five-year outcome of patients classified using the American Society for Radiation Oncology consensus statement guidelines for the application of accelerated partial breast irradiation: An analysis of patients treated on the American Society of Breast Surgeons MammoSite Registry Trial. Cancer. 2010;116:4677–4685. doi: 10.1002/cncr.25383. [DOI] [PubMed] [Google Scholar]
- 41.Keisch M, Arthur D, Patel R, et al. Breast brachytherapy guidelines. 2007. [Accessed January 26, 2012]. http://www.americanbrachytherapy.org/guidelines/abs_breast_brachytherapy_taskgroup.pdf.
- 42.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO) Int J Radiat Oncol Biol Phys. 2009;74:987–1001. doi: 10.1016/j.ijrobp.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 43.American Society of Breast Surgeons. Consensus statement for accelerated partial breast irradiation 2008. [Accessed January 26, 2012]. https://www.breastsurgeons.org/statements/APBI_statement_revised_100708.pdf.
- 44.Centers for Medicare & Medicaid Services. Radiology Services and Other Diagnostic Procedures. Medicare Claims Processing Manual. [Accessed January 26, 2012]. https://www.cms.gov/manuals/downloads/clm104c13.pdf.