Abstract
Purpose
Febrile neutropenia commonly complicates cancer chemotherapy. Outpatient treatment may reduce costs and improve patient comfort but risk progression of undetected medical problems.
Patients and Methods
By using our validated algorithm, we identified medically stable inpatients admitted for febrile neutropenia (neutrophils < 500/μL) after chemotherapy and randomly assigned them to continued inpatient antibiotic therapy or early discharge to receive identical antibiotic treatment at home. Our primary outcome was the occurrence of any serious medical complication, defined as evidence of medical instability requiring urgent medical attention.
Results
We enrolled 117 patients with 121 febrile neutropenia episodes before study termination for poor accrual. We excluded five episodes as ineligible and three because of inadequate documentation of the study outcome. Treatment groups were clinically similar, but sociodemographic imbalances occurred because of block randomization. The median presenting absolute neutrophil count was 100/μL. Hematopoietic growth factors were used in 38% of episodes. The median neutropenia duration was 4 days (range, 1 to 15 days). Five outpatients were readmitted to the hospital. Major medical complications occurred in five episodes (8%) in the hospital arm and four (9%) in the home arm (95% CI for the difference, −10% to 13%; P = .56). No study patient died. Patient-reported quality of life was similar on both arms.
Conclusion
We found no evidence of adverse medical consequences from home care, despite a protocol designed to detect evidence of clinical deterioration. These results should reassure clinicians who elect to treat rigorously characterized low-risk patients with febrile neutropenia in suitable outpatient settings with appropriate surveillance for unexpected clinical deterioration.
INTRODUCTION
Febrile neutropenia remains the most common iatrogenic cause of emergency hospitalization of patients with cancer.1 Although neutropenia is usually self-limited and often aggressively treated with hematopoietic growth factors, patients who develop fever require prompt, sustained, broad-spectrum antibiotic therapy.2 For nearly three decades, the standard of care for febrile neutropenic patients was hospitalization until both fever and neutropenia resolved.3 More recently, validated decision rules became available to identify patients at low risk of significant new medical problems, justifying less intensive surveillance.4–6 As a result, outpatient therapy has partially replaced inpatient therapy for some low-risk patients in therapeutic trials7–12 or ad hoc treatment plans developed to reduce costs and increase patient comfort.13–17 Trials confirming the equivalence of intravenous monotherapy18 and oral antibiotic combinations19,20 to traditional intravenous combination therapy have simplified antibiotic treatment. However, outpatient management reduces medical observation, potentially delaying detection and treatment of medical problems, although inpatient care may introduce risks that outpatient care may avoid. Therefore, making outpatient management of febrile neutropenia an accepted standard requires high-level evidence that it does not worsen medical outcomes.21,22 Prior trials comparing inpatient and outpatient management11,23–26 had insufficient power to detect harm to patients and also had inconsistent methodology.27 In addition, the quality of life (QOL) and economic consequences of outpatient treatment have been inadequately documented.28 Therefore, we performed a multicenter randomized trial comparing early discharge with continued inpatient care for low-risk patients with cancer who had febrile neutropenia.
PATIENTS AND METHODS
Eligibility
Adult outpatients at participating sites with postchemotherapy fever (≥ 100.5°F at presentation or by patient measurement at home) and neutropenia (absolute neutrophil count [ANC] less than 500/μL) that persisted after at least 24-hour inpatient observation were evaluated by the risk assessment criteria of Talcott et al.4–6 Briefly, patients are at low risk if they are outpatients at presentation; exhibit no indication for hospitalization other than fever and neutropenia, such as systemic hypotension, altered mental status, respiratory failure, or inadequate oral fluid intake during 24-hour observation; and have adequately controlled cancer. For leukemia patients, cancer control is defined as bone marrow–proven complete remission, and for patients without leukemia, no evidence of disease progression after either the initial chemotherapy regimen or at least two cycles of a subsequent regimen. Required diagnostic tests included a chest radiograph without evidence of infectious pneumonitis and blood cultures without pathogenic growth at enrollment. Exclusions included AIDS-associated malignancy, neutropenia arising more than 21 days after chemotherapy, and intensive chemotherapy requiring bone marrow or peripheral stem-cell support. Nonmedical criteria for home care included the ability to use available emergency medical assistance (but not a 24-hour caregiver), residence within 2 hours by surface transportation to a hospital experienced in emergency care of patients with cancer, permission of the patient's treating physician, and documented informed consent. Study sites included Cancer and Leukemia Group B (CALGB) members from 1994 to 1999 and three Boston area hospitals (Massachusetts General Hospital, Brigham and Women's Hospital, and the Lahey Clinic) during the final year of the study. Each site identified a designated commercial home care provider who agreed to provide protocol care for patients without out-of-pocket charges.
Random Assignment
Random assignments were computer-generated by using blocks and stratified by use of colony-stimulating factors (CSFs), participating institution, and whether random assignment occurred on weekends, holidays, or after hours, for which sequenced sealed envelopes were used.
Treatment Program
Patients randomly assigned to home treatment were discharged when home antibiotics became available. All patients were required to continue the antibiotic regimen in use at the time of enrollment. The treating physician could make subsequent antibiotic changes, but changes intended solely to simplify the delivery of antibiotics at home were not permitted. Suggested broad-spectrum antibiotic regimens for patients without penicillin allergy included a semisynthetic penicillin and aminoglycoside combination or ceftazidime alone; for penicillin- and cephalosporin-allergic patients imipenem alone or an aztreonam-containing regimen was suggested. However, other regimens were evaluated at the investigators' request. Additional antibiotic agents indicated by clinical circumstances, including agents to increase Gram-positive coverage,23 were at the discretion of the treating physician.
Use of CSF, a stratification factor, was optional. To reduce nephrotoxicity observed in prior outpatient trials,7,8 oral fluid intake of at least 2 L daily was strongly encouraged. Required laboratory studies included daily CBCs with differential WBC counts; serum creatinine levels twice weekly, every other day for patients receiving an aminoglycoside, and daily for patients receiving both an aminoglycoside and vancomycin; and blood cultures daily for recurrent fever and every other day for continuing fever. Aminoglycoside and vancomycin peak and trough levels documented acceptable levels after dosing changes and at least weekly. All patients received continued broad-spectrum antibiotic therapy until granulocytopenia had resolved (neutrophils and band forms > 500/μL) and patients were afebrile.
Follow-Up Care
Home treatment was supervised by the patient's treating physician, with additional assistance available from the research team. Patients at home were required to measure their temperature and, by using an automated device, measure their blood pressure at least four times daily. They were examined daily by a home care nurse who used a written protocol and who was instructed to contact the primary physician if abnormal findings occurred. In addition, a physician examined each home care patient 2 to 4 days after discharge, at least weekly thereafter, and whenever the patient, the home care nurse, or any physician felt that the patient's condition had significantly worsened. Outpatients were readmitted to the hospital whenever a physician felt the patient's condition warranted it, the patient requested it, or it proved infeasible to administer the prescribed antibiotic regimen at home. Specific medical events also mandated readmission, including pathogenic growth in a blood culture drawn 24 hours or more after the initiation of antibiotics, initiation of amphotericin or empirical antiviral therapy, or a serious medical complication (see Primary End Point). If readmitted, patients remained as inpatients until the episode had resolved. Study observation continued until resolution of neutropenia, discontinuation of antibiotics, and resolution of any new medical problems. In most cases, antibiotics were discontinued when resolution of neutropenia was documented, but the treating physician could order additional treatment or observation.
Primary End Point
Because death, the definitive failure outcome, is rare in low-risk febrile neutropenia, requiring prohibitively large trials, the primary study outcome was the occurrence of any medical complication during the study period, broadly defined as any medical event requiring urgent diagnostic or therapeutic intervention. Predefined complications included systemic hypotension (systolic blood pressure < 90 mmHg), respiratory failure (partial pressure of oxygen [PO2] < 60 torr, adjusted for hyperventilation), altered mental status, congestive heart failure documented by chest radiograph, serious bleeding (three or more unit transfusions within 24 hours), and transfer to an intensive care unit. Two physicians determined whether a major medical complication had occurred via blinded review of a clinical summary form, which documented the most severe medical events, extreme laboratory values, and any radiographic abnormalities during the episode. Before review, the principal investigator (J.A.T.) ensured that the form contained all essential medical information but no reference to the treatment site. If disagreement arose, a third reviewer made the final determination.
QOL was measured by using self-reported questionnaires completed immediately after study enrollment and after discharge from the study. Instruments included the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30)29; the General Well-Being Schedule (GWS),30 an assessment of global QOL validated in a broad range of populations including patients with cancer31–33; the Technical Quality, Communication, Interpersonal Care and Outcomes subscales of the Consumer Satisfaction Survey of the Group Health Association of America,34 a measure of patient satisfaction with medical care; and a study-specific measure developed in a pilot study to assess the effect of treatment site, including relationships with family and physicians and their sense of personal safety. In addition, we assessed direct and indirect medical costs, including out-of-pocket costs to patients and informal caregivers, and, for the latter, time lost, reported in the accompanying article by Hendricks et al.35 Data management was a collaborative project of the Center for Outcomes Research at the Massachusetts General Hospital Cancer Center, the CALGB Statistical Center, and the Quality Assurance Office for Clinical Trials at the Dana-Farber Cancer Institute.
Statistical Analysis
The primary study outcome was the frequency of medical complications. The study was initially designed to detect a clinically significant increase from 4% of inpatient episodes in which at least one medical complication occurred to an 8% outpatient complication rate. However, because comfort with outpatient management of febrile neutropenia increased over time, we revised our goal to a 6% increase, producing a goal of 224 episodes per arm assuming 80% power, one-sided α of .05, and a 5% ineligibility rate. We constructed an unconditional exact 95% CI for the difference in complication rates between arms inverting two one-sided tests that were based on the standardized score statistic, each at the 0.0025 significance level (StatXact 6.0, Cytel Software, Cambridge, MA). We assumed that home antibiotic therapy would improve QOL and decrease costs, making home care the preferred option unless it increased medical risk. Therefore, the study used a one-sided statistical test of whether outpatient care increased complications compared with hospital care. Episodes of febrile neutropenia resolve without a detectable influence on the risk of medical complications in subsequent episodes,8,9,29 particularly for low-risk patients. Therefore, the unit of analysis for this study was an episode of febrile neutropenia; patients could be enrolled for more than one episode. Our assumption of independence was tested in the data analysis. However, because a favorable study experience could encourage a patient to re-enroll, we restricted QOL assessment to the first episode.
RESULTS
Between September 1994 and January 1999, 102 patient episodes were registered, before closure by the CALGB for poor accrual. Between August 15, 1999, and June 30, 2000, an additional 19 episodes were registered when the trial briefly reopened in the Boston area before the study was terminated. The process of enrolling patients was more difficult than anticipated: eligible patients were more reluctant to enroll than anticipated, particularly early in the study, and febrile neutropenia occurred less often and recovery occurred earlier than historical precedent, likely reflecting the increasing use of hematopoietic growth factors. Enrollment was largely confined to five study sites: Massachusetts General Hospital, the Dana-Farber Cancer Institute/Brigham and Women's Hospital, University of North Carolina Hospitals, the Southeast Cancer Care Consortium, and the University of Massachusetts at Worcester, although eight other sites contributed at least one other patient. As a result of the sparse accrual at many sites and block randomization, imbalances between treatment arms occurred in patient numbers and some sociodemographic characteristics.
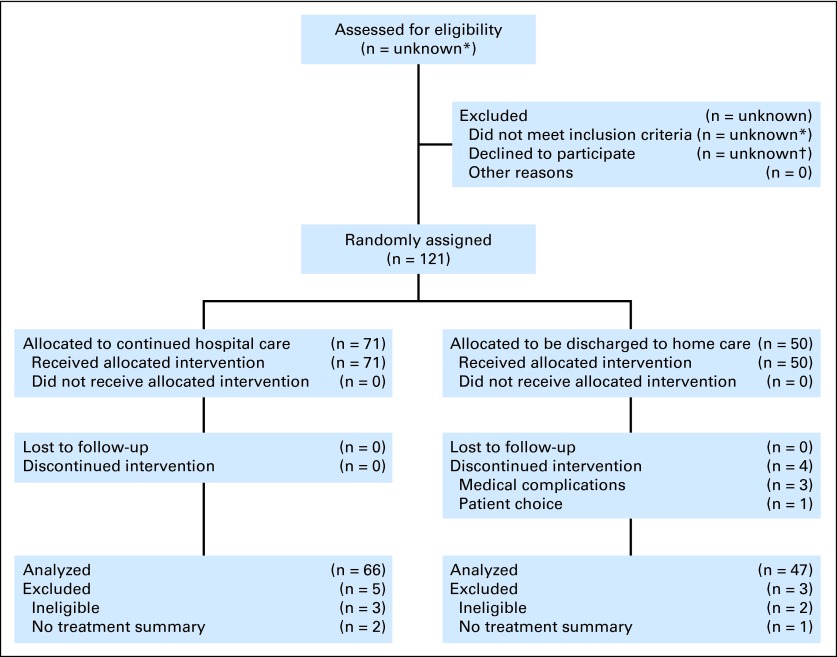
Of the 121 episodes, we excluded five—three because consent was withdrawn before treatment and two in which neutropenia had resolved at enrollment. For three additional episodes, the primary study end point, major medical complications, could not be determined because of incomplete clinical documentation (Fig 1). One patient participated in three different episodes and another in two episodes.
Fig 1.
CONSORT diagram. (*) The study design did not collect these data, in part because the number of patients reviewed varied according to the screening approach. At the Boston sites, we screened approximately 10 patients for each eligible patient. (†) We did not keep the data. In Boston, the average acceptance rate (1-refusal rate) was approximately 30%, increasing from 15% in the first year of the study to 45% in the final year, when the concept of discharge home was more familiar.
We report the remaining 113 patient episodes (Table 1). The median age, 47 years, was the same in both study arms. Home care patients were more often African American and less likely to have high-status jobs and private medical insurance or Medicare. Among hospital arm patients, there was a trend toward more full-time employment and greater educational attainment.
Table 1.
Sociodemographic Characteristics at Random Assignment
| Characteristic | Hospital Care |
Early Discharge |
All Patients |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Episodes | 66 | 47 | 113 | ||||
| Age, years | .55 | ||||||
| Median | 47 | 47 | 47 | ||||
| Range | 20-81 | 25-74 | 20-81 | ||||
| Sex | .34 | ||||||
| Female | 33 | 50 | 28 | 60 | 61 | 54 | |
| Male | 33 | 50 | 19 | 40 | 52 | 46 | |
| Race/ethnicity | .03 | ||||||
| White, non-Hispanic | 60 | 91 | 34 | 72 | 94 | 83 | |
| African American | 4 | 6 | 9 | 19 | 13 | 12 | |
| Hispanic, Asian, American Indian, other | 2 | 3 | 4 | 8 | 6 | 5 | |
| Marital status | .75 | ||||||
| Single | 13 | 21 | 8 | 17 | 21 | 19 | |
| Married | 37 | 60 | 31 | 67 | 68 | 63 | |
| Separated, divorced, widowed | 12 | 19 | 7 | 15 | 19 | 18 | |
| Unknown | 4 | 1 | 5 | ||||
| Education | .06 | ||||||
| High school or less | 17 | 27 | 20 | 48 | 37 | 36 | |
| Attended college | 16 | 26 | 5 | 12 | 21 | 20 | |
| Graduate work | 29 | 47 | 17 | 40 | 46 | 44 | |
| Unknown | 4 | 5 | 9 | ||||
| Living situation | .46 | ||||||
| Lives alone | 8 | 14 | 3 | 7 | 11 | 11 | |
| Lives with family/partner | 44 | 77 | 33 | 79 | 77 | 78 | |
| Lives with others | 5 | 9 | 6 | 14 | 11 | 11 | |
| Unknown | 9 | 5 | 14 | ||||
| Medical insurance | .05 | ||||||
| Insured | 50 | 88 | 29 | 67 | 79 | 79 | |
| Disability/Medicaid | 4 | 7 | 7 | 16 | 11 | 11 | |
| Uninsured/self-pay | 3 | 5 | 7 | 16 | 10 | 10 | |
| Unknown | 9 | 4 | 13 | ||||
| Occupation | .01 | ||||||
| Professional/technical/management/administration | 40 | 61 | 17 | 38 | 57 | 51 | |
| Clerical/sales/skilled labor/service | 16 | 24 | 14 | 31 | 30 | 27 | |
| Machinery operator/laborer/farmer/other | 0 | 0 | 5 | 11 | 5 | 5 | |
| Not reported | 10 | 15 | 9 | 20 | 19 | 17 | |
| Unknown | 0 | 2 | 2 | ||||
| Employment status | .07 | ||||||
| Employed full-time/part-time, homemaker, student | 34 | 52 | 16 | 35 | 50 | 45 | |
| Unemployed, retired | 21 | 32 | 14 | 30 | 35 | 31 | |
| Disabled | 11 | 17 | 16 | 35 | 27 | 24 | |
| Unknown | 0 | 1 | 1 | ||||
Clinical characteristics for the treatments groups were similar (Table 2), including initial peak fever (median, 101.4°F), hematologic values, serum creatinine, chest radiograph findings, and time since chemotherapy. The ANC at presentation was low (median, 100/μL). and CSFs were used in 38% of episodes.
Table 2.
Clinical Characteristics at Presentation
| Characteristic | Hospital Care |
Early Discharge |
All Patients |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Cancer diagnosis | .61 | ||||||
| Acute leukemia | 6 | 9 | 7 | 15 | 13 | 12 | |
| Lymphoma | 13 | 20 | 13 | 28 | 26 | 23 | |
| Breast cancer | 16 | 24 | 13 | 28 | 29 | 26 | |
| Sarcoma | 9 | 14 | 4 | 9 | 13 | 12 | |
| Lung cancer | 6 | 9 | 3 | 6 | 9 | 8 | |
| Other | 16 | 24 | 7 | 15 | 23 | 20 | |
| Fever (maximum inpatient in initial 24 hours), °F | .90 | ||||||
| Median | 101.4 | 101.4 | 101.4 | ||||
| Range | 98.6-106.0 | 98.8-104.3 | 98.8-104.3 | ||||
| WBC per microliter | .34 | ||||||
| Median | 0.7 | 0.8 | 0.7 | ||||
| Range | 0.1-8.1 | 0.1-32.0 | 0.1-32.0 | ||||
| ANC × 103/μL | .13 | ||||||
| Median | 57 | 100 | 100 | ||||
| Range | 0-980 | 0-600 | 0-980 | ||||
| Platelets × 103/μL | .88 | ||||||
| Median | 89 | 97 | 91 | ||||
| Range | 1-657 | 8-365 | 1-657 | ||||
| Hematocrit | .41 | ||||||
| Median | 29.3 | 30.6 | 29.9 | ||||
| Range | 9.4-46.8 | 8.1-42.1 | 8.1-46.8 | ||||
| Creatinine, mg/dL | .35 | ||||||
| Median | 0.9 | 0.8 | 0.8 | ||||
| Range | 0.4-2.0 | 0.5-1.9 | 0.4-2.0 | ||||
| Chest x-ray on admission | .42 | ||||||
| Normal | 48 | 74 | 36 | 78 | 84 | 76 | |
| Stable/improved | 11 | 17 | 4 | 9 | 15 | 14 | |
| Other | 6 | 9 | 6 | 13 | 12 | 11 | |
| Unknown | 1 | 1 | 2 | ||||
| No. of days since previous chemotherapy | .89 | ||||||
| Median | 11 | 11 | 11 | ||||
| Range | 1-18 | 2-21 | 1-21 | ||||
| Use of G-CSF | 1.0 | ||||||
| Yes | 25 | 38 | 17 | 37 | 42 | 38 | |
| No | 41 | 62 | 29 | 63 | 70 | 63 | |
| Unknown | 0 | 1 | 1 | ||||
Abbreviations: ANC, absolute neutrophil count; G-CSF, granulocyte colony-stimulating factor.
For patients in both arms, the median duration of fever was 3 days, duration of neutropenia was 4 days, and duration of the febrile neutropenia episode was 4 days, consistent with their assessment as low-risk patients, although episodes extended to 15 days (Table 3). The most common antibiotic regimen was single-agent anti-Pseudomonas monotherapy, usually ceftazidime (37%), or with an anti-staphylococcal agent (an additional 16%) and was similar between study arms (data not shown). Subsequent antibiotic changes were more frequent for hospitalized patients (24% v 9%; P = .04). Patients receiving CSFs had shorter neutropenia (median, 3 v 4 days; P < .001) but similar duration of fever and frequency of antibiotic changes (data not shown).
Table 3.
Clinical Outcomes
| Outcome | Hospital Care |
Early Discharge |
All Patients |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Duration of fever (admission for T ≤ 99.5°F for 24 hours) | |||||||
| Median | 3 | 3 | 3 | .94 | |||
| Mean | 3.2 | 3.4 | 3.3 | ||||
| Range | 0-13 | 1-14 | 0-14 | ||||
| Duration of neutropenia (initial admission for ANC ≥ 500/μL) | |||||||
| Median | 4 | 4 | 4 | .80 | |||
| Mean | 4.1 | 4.2 | 4.1 | ||||
| Range | 1-10 | 1-15 | 1-15 | ||||
| Duration of fever and neutropenia (admission for T ≤ 99.5°F for 24 hours, ANC ≥ 500/μL, and resolution of all medical problems) | |||||||
| Median | 4 | 4 | 4 | .70 | |||
| Mean | 4.6 | 4.5 | 4.6 | ||||
| Range | 2-13 | 1-15 | 1-15 | ||||
| Antibiotics changed after random assignment | 16 | 24 | 4 | 9 | 20 | 18 | .04 |
| Hospital readmission | — | 4 | 9 | — | |||
| Major medical complications | |||||||
| Hypotension | 5 | 8 | 3 | 6 | 8 | 8 | |
| Other (anal pain) | 1 | 1 | 1 | 2 | 2 | 2 | |
| Any major complication | 5 | 8 | 4 | 9 | 9 | 8 | .56* |
Abbreviations: ANC, absolute neutrophil count; T, temperature.
One-sided test.
Four outpatient episodes resulted in hospital readmission. Major medical complications occurred in five episodes (8%) on the hospital arm and four episodes (9%) in the home arm (exact 95% CI for outpatient v hospital complication rate, −10% to 13%; P = .56). Eight complications involved hypotension (systolic blood pressure < 90 mmHg), which was transient. One episode also included progressive pain from a perianal wound. One patient had a pulmonary embolus the day after discharge from the study. No study patient died.
QOL Results
One hundred ten patients completed the on-study assessment, and 105 patients completed the off-study assessment. The three repeat enrollments were excluded from the QOL portion of the study.
Reported pain decreased for home care patients and slightly increased for hospitalized patients (change, −13.1 v 2.72; P = .01). The Role Function subscale of the EORTC QLQ C-30 increased less for home care patients than for hospitalized patients (change, 0.58 v 0.78; P = .05), but Emotional Function scores increased for home care patients while declining for hospitalized patients (change, 3.27 v −6.94; P = .04). No other QLQ-C30 subscale differences were evident. Similarly, few differences appeared in the instrument comparing the inpatient and outpatient care experience. Home care patients more readily endorsed the statement “It is hard for me to relax … when I am disturbed… by activities of the hospital” after completing treatment, although agreement for hospital patients declined (change, 0.41 v −0.15; P = .08). The pattern was reversed for the statement “When out of the hospital I become worried about being able to reach my doctor when I need him or her” (change, −0.22 v 0.23; P = .07). No differences were noted in response to the Consumer Satisfaction or General Well-Being instruments.
DISCUSSION
Outpatient treatment of febrile neutropenia is attractive because of potential cost savings and improved patient comfort. However, the decision to discharge patients from the hospital requires that clinicians be confident that the reduced opportunity for medical surveillance of outpatients will not increase their medical risk. This question lends itself to empirical evaluation: inpatient treatment is standard, the episode's end is numerically defined (by an ANC ≥ 500/μL), and validated measures exist to identify low-risk patients.4–6 In this multicenter trial, we randomly assigned rigorously identified low-risk patients with febrile neutropenia to either early discharge to home antibiotic therapy or continued hospital care. By using a sensitive indicator of medical instability (ie, the occurrence of even transient medical complications), we found little evidence that home care increased risk.
Prior studies documented few medical problems for patients with low-risk febrile neutropenia when given protocol-specified outpatient antibiotic treatment.7–10,13–16 Despite these broadly reassuring results, rigorous assurance that discharging low-risk patients does not compromise patient safety has been unavailable. The most common end point in outpatient therapy trials has been “response to therapy,” or prompt resolution of infection without antibiotic changes.27 Developed to compare empirical antibiotic regimens for febrile neutropenia, that outcome is less directly relevant to the fundamental question of the safety of home therapy than our main outcome, occurrence of serious medical problems potentially averted by earlier detection and treatment. As a result, “response to therapy” cannot address the more fundamental question addressed here: Are occasionally reported catastrophic outpatient events such as the apparently preventable death from sepsis in the study of Malik et al23 anomalies equally likely in the hospital, or are they insensitive indicators of usually less serious but common adverse events attributable to outpatient treatment? Our data support the former interpretation.
However, these encouraging results may depend on elements of our treatment plan omitted by other outpatient treatment protocols. We used a validated clinical decision rule to identify patients for our study, as recommended.27 Two risk assessment decision rules for febrile neutropenia have been validated: our own4,5 and the Risk Index of the Multinational Association for Supportive Care in Cancer (MASCC).6 That validated risk assessment measures exist does not justify assessing risk by using ad hoc clinical judgment, imprecisely defined criteria such as “brief anticipated duration of neutropenia,” or broad and sometimes misleading indicators of risk, such as the diagnosis of leukemia or lymphoma. For example, Elting et al16 found that all 121 of the 712 patients assigned to an outpatient treatment clinical pathway who were subsequently readmitted to the hospital would have been designated as high-risk by using the MASCC risk index. Patients in this study did well despite putative high-risk characteristics, such as leukemia or lymphoma diagnoses and neutropenia with neutrophils below 100/μL. Safe discharge of patients with febrile neutropenia requires an adequate structure of clinical support, including regular patient evaluation, which our protocol provided, to identify evidence of clinical instability or other evidence that the initial assessment of low risk has changed, meriting closer surveillance.
Despite our hypothesis that home care would improve patient QOL, we found, at most, modest supporting evidence. This result, surprising given that study patients were self-selected by their interest in possible home treatment, may be due to unexpected challenges in receiving home intravenous antibiotics; failure to identify a suitable, sensitive measure of QOL; inadequate study power; or the brief course of low-risk febrile neutropenia. Finally, voluntary participants in a trial offering possible home care may have lower risk than unwilling patients discharged early.
Although limited by reduced power secondary to low accrual, our study documents important constraints on the risk of outpatient treatment, ranging from a 13% increase to a 10% decrease in the risk of developing medical instability (in most cases transient hypotension). It provides the most stringent evidence to date that rigorously characterized low-risk patients are put at minimal medical risk if discharged to home antibiotic therapy under daily clinical evaluation. Given the increasing acceptance of outpatient management of febrile neutropenia, our study may represent the last major effort to rigorously characterize its risk. Our inability to find evidence of adverse medical consequences from home care, despite an aggressive search for indicators of clinical deterioration, should reassure patients and physicians considering appropriately designed outpatient treatment for low-risk febrile neutropenia.
Acknowledgment
We thank Anita E. Rodrigues, Sonya Mitchell, Sahirah Muhammad, Linda Herman, Cathy Collins, and Lisa Wasson who helped with patient accrual and data collection, collaborators within Cancer and Leukemia Group B (CALGB), and enrolled patients. We thank our clinical contributors to this program, including James N. Atkins, MD; Diana M.F. Savarese, MD; Chris Desch, MD; John D. Roberts, MD; Vincent Vinciguerra, MD; Santo DiFino, MD; Neal J. Weiner, MD, John Ellerton, MD; Herbert Mauer, MD, Ronald Hart, MD; Brian Lipman, MD; and John P. Flaherty, MD.
Footnotes
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: James A. Talcott, Jack A. Clark, Paul A. Godley
Financial support: James A. Talcott
Administrative support: James A. Talcott
Provision of study materials or patients: James A. Talcott,Paul A. Godley
Collection and assembly of data: James A. Talcott, Beow Y. Yeap, Robert D. Siegel, Charles Lu, Paul A. Godley
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stokes ME, Muehlenbein CE, Marciniak MD, et al. Neutropenia-related costs in patients treated with first-line chemotherapy for advanced non-small cell lung cancer. J Manag Care Pharm. 2009;15:669–682. doi: 10.18553/jmcp.2009.15.8.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schimpff S, Satterlee W, Young VM, et al. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971;284:1061–1065. doi: 10.1056/NEJM197105132841904. [DOI] [PubMed] [Google Scholar]
- 3.Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328:1323–1332. doi: 10.1056/NEJM199305063281808. [DOI] [PubMed] [Google Scholar]
- 4.Talcott JA, Finberg R, Mayer RJ, et al. The medical course of cancer patients with fever and neutropenia. Clinical identification of a low-risk subgroup at presentation. Arch Intern Med. 1988;148:2561–2568. [PubMed] [Google Scholar]
- 5.Talcott JA, Siegel RD, Finberg R, et al. Risk assessment in cancer patients with fever and neutropenia: A prospective, two-center validation of a prediction rule. J Clin Oncol. 1992;10:316–322. doi: 10.1200/JCO.1992.10.2.316. [DOI] [PubMed] [Google Scholar]
- 6.Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000;18:3038–3051. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 7.Talcott JA, Whalen A, Clark J, et al. Home antibiotic therapy for low-risk cancer patients with fever and neutropenia: A pilot study of 30 patients based on a validated prediction rule. J Clin Oncol. 1994;12:107–114. doi: 10.1200/JCO.1994.12.1.107. [DOI] [PubMed] [Google Scholar]
- 8.Rubenstein EB, Rolston K, Benjamin RS, et al. Outpatient treatment of febrile episodes in low-risk neutropenic patients with cancer. Cancer. 1993;71:3640–3646. doi: 10.1002/1097-0142(19930601)71:11<3640::aid-cncr2820711128>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Escalante CP, Rubenstein EB, Rolston KV. Outpatient antibiotic therapy for febrile episodes in low-risk neutropenic patients with cancer. Cancer Invest. 1997;15:237–242. doi: 10.3109/07357909709039721. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein EB, Rolston K. Outpatient management of febrile episodes in neutropenic cancer patients. Support Care Cancer. 1994;2:369–373. doi: 10.1007/BF00344049. [DOI] [PubMed] [Google Scholar]
- 11.Klastersky J, Paesmans M, Georgala A, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006;24:4129–4134. doi: 10.1200/JCO.2005.03.9909. [DOI] [PubMed] [Google Scholar]
- 12.Innes H, Lim SL, Hall A, et al. Management of febrile neutropenia in solid tumours and lymphomas using the Multinational Association for Supportive Care in Cancer (MASCC) risk index: Feasibility and safety in routine clinical practice. Support Care Cancer. 2008;16:485–491. doi: 10.1007/s00520-007-0334-8. [DOI] [PubMed] [Google Scholar]
- 13.Bash RO, Katz JA, Cash JV, et al. Safety and cost effectiveness of early hospital discharge of lower risk children with cancer admitted for fever and neutropenia. Cancer. 1994;74:189–196. doi: 10.1002/1097-0142(19940701)74:1<189::aid-cncr2820740130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Gardembas-Pain M, Desablens B, Sensebe L, et al. Home treatment of febrile neutropenia: An empirical oral antibiotic regimen. Ann Oncol. 1991;2:485–487. doi: 10.1093/oxfordjournals.annonc.a057996. [DOI] [PubMed] [Google Scholar]
- 15.Mullen CA, Buchanan GR. Early hospital discharge of children with cancer treated for fever and neutropenia: Identification and management of the low-risk patient. J Clin Oncol. 1990;8:1998–2004. doi: 10.1200/JCO.1990.8.12.1998. [DOI] [PubMed] [Google Scholar]
- 16.Elting LS, Lu C, Escalante CP, et al. Outcomes and cost of outpatient or inpatient management of 712 patients with febrile neutropenia. J Clin Oncol. 2008;26:606–611. doi: 10.1200/JCO.2007.13.8222. [DOI] [PubMed] [Google Scholar]
- 17.Freifeld A, Sankaranarayanan J, Ullrich F, et al. Clinical practice patterns of managing low-risk adult febrile neutropenia during cancer chemotherapy in the USA. Support Care Cancer. 2008;16:181–191. doi: 10.1007/s00520-007-0308-x. [DOI] [PubMed] [Google Scholar]
- 18.Pizzo PA, Hathorn JW, Hiemenz J, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986;315:552–558. doi: 10.1056/NEJM198608283150905. [DOI] [PubMed] [Google Scholar]
- 19.Kern WV, Cometta A, De Bock R, et al. Oral versus intravenous empirical antimicrobial therapy for fever in patients with granulocytopenia who are receiving cancer chemotherapy: International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med. 1999;341:312–318. doi: 10.1056/NEJM199907293410502. [DOI] [PubMed] [Google Scholar]
- 20.Freifeld A, Marchigiani D, Walsh T, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999;341:305–311. doi: 10.1056/NEJM199907293410501. [DOI] [PubMed] [Google Scholar]
- 21.Finberg RW, Talcott JA. Fever and neutropenia: How to use a new treatment strategy. N Engl J Med. 1999;341:362–363. doi: 10.1056/NEJM199907293410509. [DOI] [PubMed] [Google Scholar]
- 22.Marti FM, Cullen MH, Roila F, et al. Management of febrile neutropenia: ESMO clinical recommendations. Ann Oncol. 2009;20(suppl 4):166–169. doi: 10.1093/annonc/mdp163. [DOI] [PubMed] [Google Scholar]
- 23.Malik IA, Khan WA, Karim M, et al. Feasibility of outpatient management of fever in cancer patients with low-risk neutropenia: Results of a prospective randomized trial. Am J Med. 1995;98:224–231. doi: 10.1016/s0002-9343(99)80367-2. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo M, Hornedo J, Lumbreras C, et al. Outpatient therapy with oral ofloxacin for patients with low risk neutropenia and fever: A prospective, randomized clinical trial. Cancer. 1999;85:213–219. [PubMed] [Google Scholar]
- 25.Innes HE, Smith DB, O'Reilly SM, et al. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: A prospective randomised controlled single centre study. Br J Cancer. 2003;89:43–49. doi: 10.1038/sj.bjc.6600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta A, Swaroop C, Agarwala S, et al. Randomized controlled trial comparing oral amoxicillin-clavulanate and ofloxacin with intravenous ceftriaxone and amikacin as outpatient therapy in pediatric low-risk febrile neutropenia. J Pediatr Hematol Oncol. 2009;31:635–641. doi: 10.1097/MPH.0b013e3181acd8cd. [DOI] [PubMed] [Google Scholar]
- 27.Feld R, Paesmans M, Freifeld AG, et al. Methodology for clinical trials involving patients with cancer who have febrile neutropenia: Updated guidelines of the Immunocompromised Host Society/Multinational Association for Supportive Care in Cancer, with emphasis on outpatient studies. Clin Infect Dis. 2002;35:1463–1468. doi: 10.1086/344650. [DOI] [PubMed] [Google Scholar]
- 28.Talcott JA. Outpatient management of febrile neutropenia: Should we change the standard of care? Oncologist. 1997;2:365–373. [PubMed] [Google Scholar]
- 29.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 30.Fazio AF. A concurrent validational study of the NCHS General Well-Being Schedule. Vital Health Stat 2. 1977;73:1–53. [PubMed] [Google Scholar]
- 31.Poston WS, 2nd, Olvera NE, Yanez C, et al. Evaluation of the factor structure and psychometric characteristics of the General Well-Being Schedule (GWB) with Mexican American women. Women Health. 1998;27:51–64. doi: 10.1300/J013v27n03_04. [DOI] [PubMed] [Google Scholar]
- 32.Himmelfarb S, Murrell SA. Reliability and validity of five mental health scales in older persons. J Gerontol. 1983;38:333–339. doi: 10.1093/geronj/38.3.333. [DOI] [PubMed] [Google Scholar]
- 33.Costa PT, Jr, McCrae RR. Cross-sectional studies of personality in a national sample: 1. Development and validation of survey measures. Psychol Aging. 1986;1:140–143. doi: 10.1037//0882-7974.1.2.140. [DOI] [PubMed] [Google Scholar]
- 34.Davies AR, Ware JE. GHAA's Consumer Satisfaction Survey and User's Manual. Washington, DC: Group Health Association of America; 1991. [Google Scholar]
- 35.Hendricks AM, Loggers ET, Talcott JA. Costs of home versus inpatient treatment for fever and neutropenia: Analysis of a multicenter randomized trial. J Clin Oncol. 2011;29:3984–3989. doi: 10.1200/JCO.2011.35.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]