Abstract
Skeletal morbidity is a prominent burden to men with advanced prostate cancer throughout the natural history of the disease. Bone metastases can cause pain and greatly elevate the risk for fractures and other structural complications. Distinct from the problem of metastases, treatment-related osteoporosis and associated fragility fractures are potential complications of androgen-deprivation therapy. Bone-targeted therapies for prostate cancer have therefore been the focus of considerable research and drug development efforts. The osteoclast is a validated therapeutic target in the management of prostate cancer. Osteoclast inhibition with zoledronic acid (a bisphosphonate) or with denosumab (a monoclonal antibody to RANK ligand) reduces risk for skeletal events in men with castration-resistant prostate cancer metastatic to bone. Osteoclast inhibition with any of several bisphosphonates improves bone mineral density, a surrogate for osteoporotic fracture risk. Denosumab and toremifene (a selective estrogen receptor modulator) have each been shown to reduce osteoporotic fracture risk among men receiving androgen-deprivation therapy. Beta-emitting radiopharmaceuticals reduce pain due to metastatic disease. Investigations involving alpha-emitting radium-223, endothelin-A receptor antagonists atrasentan and zibotentan, proto-oncogene tyrosine-protein kinase (SRC) inhibitor dasatinib, and tyrosine kinase inhibitor cabozantinib (XL184) are ongoing in clinical trials and are also discussed.
INTRODUCTION
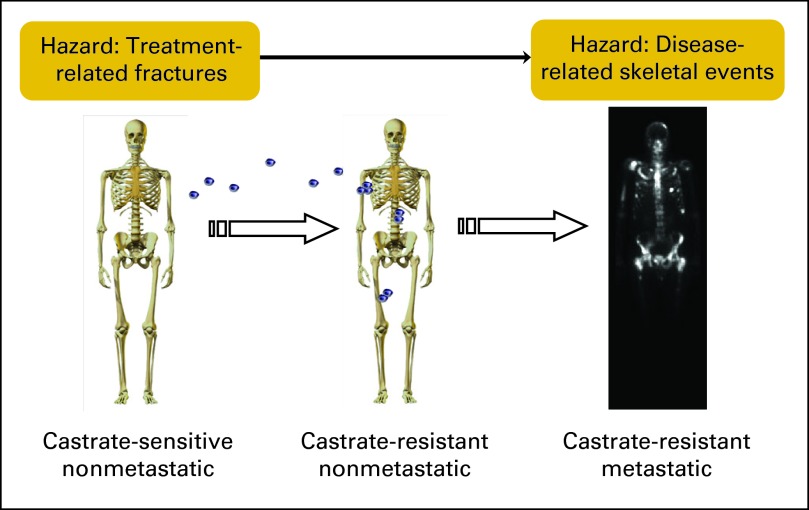
Approximately 32,000 men in the United States will die as a result of prostate cancer in 2010, making it the second most common cause of cancer death in men.1 Risks for skeletal morbidity are present throughout the natural history of the disease (Fig 1). The two major clinical problems are bone metastases and treatment-related osteoporosis.
Fig 1.
Spectrum of bone disease in prostate cancer. Men with prostate cancer are vulnerable to skeletal morbidity throughout the natural history of the disease and its treatment. Treatment-related osteoporosis and fractures are an early danger. The development and progression of micrometastases is later followed by risk for skeletal-related events.
Bone Complications of Prostate Cancer
Advanced prostate cancer has a strong propensity to metastasize to bone. Among men with metastatic castration-resistant prostate cancer (CRPC), close to 90% have radiographically detectable bone metastases.2,3 The most common sites of bone metastases are throughout the axial skeleton (vertebral bodies, pelvis, ribs, skull) although long bones can be involved as well.
Clinically, there are several potential manifestations of prostate cancer bone metastases. Pain is the most common symptom. Hypocalcemia also occurs frequently but is usually asymptomatic. Skeletal events such as pathologic fractures and spinal cord compression are less common but can abruptly cause devastating problems.
Older men are distinctly vulnerable to morbidity and mortality due to fragility fractures. Although osteoporotic fractures are more common in women, men suffer from one fourth of all hip fractures,4 with a lifetime incidence of approximately 20%.5 In the general population, the most prevalent risk factors for osteoporosis are hypogonadism, excessive alcohol intake, and chronic glucocorticoid therapy.6 Androgen-deprivation therapy (ADT) for prostate cancer causes severe hypogonadism.
ADT accelerates loss of bone mineral density (BMD) and is associated with an increased incidence of fragility fractures. Prospective studies of men receiving ADT reproducibly demonstrate BMD declines of approximately 3% at the lumbar spine (range, 1.4% to 3.3%) and 2% at the hip (range, 0.7% to 3.3%) in the first year of therapy.7–10 Population-based studies have shown that gonadotropin-releasing hormone (GnRH) agonist treatment, a form of ADT, is associated with an increase in the incidence of fractures.11,12 Because age13 and ADT each elevate fracture risk, men receiving treatment for prostate cancer are a distinctly vulnerable population.
Normal Bone Physiology
The health and structural integrity of normal bone is the result of an active and continuous process of bone resorption by osteoclasts and new bone formation by osteoblasts. Osteoclasts differentiate from monocyte or macrophage precursors14 and attach to bone matrix to form a resorption vacuole which they acidify and into which they secrete resorptive enzymes. Resultant bone resorption liberates several osteoblast-activating growth factors, including transforming growth factor beta, basic fibroblast growth factor, platelet-derived growth factor, and insulin-like growth factor 1 and 2. Osteoblasts differentiate from stromal stem cells and produce an organic matrix that is mineralized over the course of several weeks.
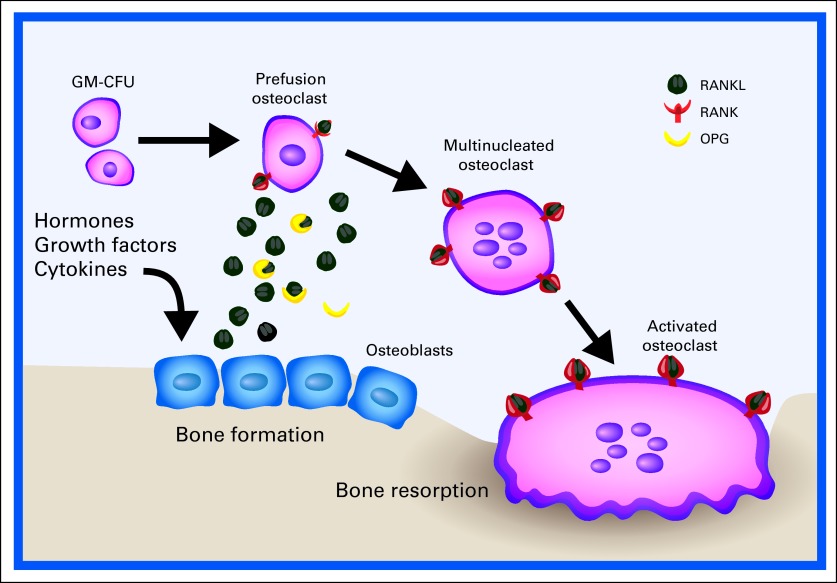
Receptor activator of nuclear factor kappa B (RANK) is a receptor that is present on the surface of osteoclasts. RANK signaling is a central regulator at several points in the osteoclast life cycle (Fig 2). RANK ligand (RANKL) is expressed by osteoblasts and stromal cells within the marrow. RANKL binding to RANK leads to differentiation of osteoclast precursors as well as to activation and survival of mature osteoclasts. Osteoprotegerin is a decoy receptor for RANKL and can therefore competitively inhibit this signaling.15
Fig 2.
The role of the receptor activator of nuclear factor kappa B (RANK) and RANK ligand (RANKL) in normal bone physiology. RANK signaling is a central regulator of osteoclast differentiation, activity, and survival. Osteoblasts promote this by secreting RANKL. Osteoprotegerin (OPG) is a soluble decoy receptor for RANKL and serves as a negative regulator. GM-CFU, granulocyte-macrophage colony-forming unit. Adapted.14
Pathophysiology of Treatment-Related Osteoporosis
The hormonal environment is an important determinant of the balance between bone resorption and bone mineralization. Testosterone and estrogen are each correlated with BMD16–18 and fracture risk19–21 among older men in the general population. ADT for prostate cancer drastically reduces serum testosterone, generally to below 20 ng/mL.22,23 In men, most estrogen is produced by the peripheral aromatization of testosterone. Estrogen levels are therefore also low during ADT. For example, in a prospective study of ADT-naive men initiating GnRH agonist therapy, mean estradiol was 26 pg/mL at baseline and 7 pg/mL after 48 weeks of treatment.10
The drastic hypogonadism produced by medical castration (GnRH agonist or antagonist therapy) or surgical castration (bilateral orchiectomies) increases biochemical markers of osteoclast and osteoblast activity. This accelerated bone turnover results in a loss of bone mass.10 ADT is associated with increased fracture risk, particularly with longer duration of therapy.11,12 This increased fracture risk is an important concern because many men receive GnRH agonist treatment in conjunction with potentially curative radiation or as first-line therapy for advanced or recurrent disease. Such men may live for years to decades at increased risk for fractures.
Pathophysiology of Bone Metastases
Bone is the most common site of metastasis in advanced prostate cancer. The bone microenvironment features a high concentration of growth factors within the marrow and even more growth factors immobilized within the matrix.24 Laboratory evaluation of men with bone metastases reveals evidence of increased bone turnover with activation of both osteoblasts (as reflected by bone-specific alkaline phosphatase) and osteoclasts (as reflected by urinary N-telopeptide or NTx).25,26
Woven bone laid down by osteoblasts under these circumstances produces the characteristically dense osteoblastic appearance of prostate cancer bone metastases as seen on plain radiographs. New bone deposition by osteoblasts also commonly causes hypocalcemia. Parathyroid hormone increase due to hypocalcemia can worsen the cycle by inducing RANKL expression. Despite their dense appearance, bone metastases due to prostate cancer feature high osteoclast activity and cause compromised structural integrity.27 In men with such bone metastases, increased NTx levels are associated with higher incidence of skeletal events, disease progression, and death.28 Osteoclast inhibition is therefore a rational therapeutic approach in this disease state.
Available Bone-Targeted Therapies
Bisphosphonates.
The first and most widely prescribed class of osteoclast-targeted agent is the bisphosphonates. Their activity is derived from structural similarity to pyrophosphate, a normal component of bone. When administered orally or intravenously, bisphosphonates are incorporated into bone matrix by binding to exposed hydroxyapatite crystals. This binding provides a barrier to osteoclast-mediated bone resorption and has direct inhibitory effects on osteoblasts.29 Although bisphosphonates remain present within bone long after dosing, their serum half-lives are short. For example, the serum concentration of zoledronic acid falls to less than 1% of its maximum by 24 hours after infusion. The organic side chain of a given bisphosphonate determines its relative potency. Bisphosphonates that have an amino group side chain (eg, pamidronate, zoledronic acid) are more potent than those that do not (eg, clodronate). Zoledronic acid is the most potent available bisphosphonate, 1,000 times more potent in vitro than clodronate.
Denosumab.
RANKL-induced signaling plays an important role in osteoclast regulation, making it a logical target for therapeutic intervention.30 Denosumab is a subcutaneously administered monoclonal antibody with a high binding affinity for RANKL (dissociation constant, 3 × 10−12 M). It has a half-life of more than 30 days at its highest doses and can produce sustained inhibition of bone turnover markers (eg, NTx) for more than 6 months in certain clinical settings.31 Denosumab has been tested for several potential applications in both benign and malignant clinical settings.
Radiopharmaceuticals.
Given the burden of skeletal metastases in advanced prostate cancer, systemic bone-seeking radiopharmaceuticals are also a management strategy. These agents differentially concentrate within osteoblastic bone metastases in which they locally radiate. Two available examples of beta-emitting agents are strontium-89 (89Sr) chloride and samarium-153 (153Sm) lexidronam. Each has been shown to palliate pain due to bone metastases.32 The most prominent toxicity of these agents is myelosuppression produced by collateral radiation of bone marrow.
BONE-TARGETED THERAPIES: CLINICAL EVIDENCE AND USE
Several classes of bone-targeted drugs have been shown to benefit men with prostate cancer. Choice of drug, dose, and schedule is directed by the clinical application. Notable contemporary trials of bone-targeted therapies are summarized in Table 1.
Table 1.
Notable Contemporary Trials of Bone-Targeted Therapies for Prostate Cancer
| Trial Name | Identifier | Reference | No. of Patients | Population | Treatment Arm | Primary Analysis | Outcome/Comments |
|---|---|---|---|---|---|---|---|
| Prevention of fractures among men receiving androgen deprivation therapy | |||||||
| Denosumab HALT 138 | Smith et al39 | 1,468 | Maintained on ADT and at least one risk factor: age ≥ 70 years; history of fracture; low BMD | Denosumab 60 mg SC every 6 months v placebo | Change in lumbar spine BMD at 24 months | Incident fractures was a secondary end point. Denosumab improved BMD at all measured sites. Three-year incidence of new vertebral fractures decreased by 62% (1.5% v 3.9%; P = .006) with denosumab treatment. | |
| Toremifene G300203 | Smith et al42 | 1,389 | Maintained on ADT and at least one risk factor: age ≥ 70 years; low BMD, T < −1.5 | Toremifene 80 mg orally daily v placebo | Incidence of new vertebral fractures | Toremifene reduced the incidence of new vertebral fractures (2.5% v 4.9%; relative risk, 0.50; P < .05). Toremifene also improved BMD, LDL, triglycerides, HDL, breast pain, and hot flashes. | |
| Metastasis prevention (nonmetastatic castration-resistant prostate cancer) | |||||||
| Denosumab147 | 1,432 | Nonmetastatic CRPC with at least one risk factor: PSA ≥ 8; PSA dt ≤ 10 months | Denosumab 120 mg SC every 4 weeks v placebo | Metastasis-free survival | Press release in December 2010 stated that denosumab improved median bone metastasis-free survival by 4.2 months (HR, 0.85; 95% CI, 0.73 to 0.98; P = .03). | ||
| ZEUS | 1,433 (target) | Nonmetastatic CRPC with at least one risk factor: PSA ≥ 20 ng/mL; node metastasis; Gleason score ≥ 8 | Zoledronic acid 4 mg IV every 3 months v placebo | Proportion of men with at least one bone metastasis after 48 months of therapy | The trial is ongoing. | ||
| Metastatic castration-sensitive prostate cancer | |||||||
| CALGB/CTSU 90202 | NCT00079001 | 680 (target) | Bone metastases and within 6 months of initiation of ADT | Zoledronic acid 4 mg IV every 4 weeks v placebo | Time to first SRE or death due to prostate cancer | Men receiving placebo cross over to zoledronic acid treatment if they develop CRPC or if they experience an SRE. The trial is currently recruiting participants. | |
| Zoledronic acid | NCT00242567 | 550 (target) | At least one bone metastasis, responding to ongoing ADT | Zoledronic acid every 4 weeks + ADT v ADT | Skeletal event–free survival at 18 months | The study is ongoing. Estimated primary completion date is January 2012. | |
| Metastatic castration-resistant prostate cancer | |||||||
| Zoledronic acid Zometa 039 | Saad et al54 | 643 | CRPC with bone metastases | Zoledronic acid (4 mg or 8 mg) every 3 weeks v placebo | Proportion of men who experienced at least one SRE during the first 15 months of therapy | There was a significant decrease in proportion of men who experienced SREs and a nonsignificant trend toward improved survival. This trial led to FDA approval of zoledronic acid for this indication. | |
| Denosumab 103 | Fizazi et al 33 | 1,901 | CRPC with bone metastases | Denosumab 120 mg SC every 4 weeks v zoledronic acid 4 mg IV every 4 weeks | Time to first SRE | Time to first SRE was significantly better in the denosumab arm (20.7 v 17.1 months; P < .001 for noninferiority; P = .008 for superiority). Overall survival and disease progression did not differ | |
| Notable bone-targeted agents in clinical development | |||||||
| Radium-223: ALSYMPCA | NCT00699751 | 900 (target) | CRPC with at least two skeletal metastases on bone scan and no visceral metastases | Radium-223; 50 kBq/kg every 4 weeks (six cycles) v placebo | Overall survival | Participants were randomly assigned 2:1 to treatment with the radiopharmaceutical or to placebo. In June 2011, the study was stopped early on the basis of a recommendation of an independent data monitoring committee after a preplanned interim efficacy analysis.33a Compared with placebo, radium-223 chloride was associated with improved overall survival (median, 14.0 v 11.2 months; HR, 0.699; P = .0022). | |
| Atrasentan | Nelson et al34 | 941 | Nonmetastatic CRPC | Atrasentan 10 mg orally daily v placebo | Time to disease progression | This ETA receptor inhibitor trial was negative because it found only a nonsignificant (P = .288) 93-day delay in time to progression. | |
| Atrasentan | Carducci et al35 | 809 | Metastatic CRPC | Atrasentan 10 mg orally daily v placebo | Time to disease progression | Atrasentan did not reduce risk of disease progression relative to placebo. BAP and PSA rose more slowly with atrasentan than with placebo. | |
| Atrasentan: SWOG 0421 | NCT00134056 | 930 (target) | Metastatic CRPC, taxane naive | Atrasentan + docetaxel-prednisone v placebo + docetaxel-prednisone | Overall survival and progression-free survival | The trial is ongoing. Estimated primary completion date is March 2014. | |
| Zibotentan: ENTHUSE M1 Study 14 | NCT00554229 | 848 (target) | CRPC metastatic to bone, mild pain or no pain | Zibotentan 10 mg orally daily v placebo | Overall survival | Press release in September 2010 stated that the ETA receptor inhibitor did not produce significant improvement in the primary end point, overall survival. Full results have not yet been published. | |
| Zibotentan: ENTHUSE M0 Study 15 | NCT00626548 | 1,500 (target) | CRPC that is not metastatic to bone or viscera | Zibotentan 10 mg orally daily v placebo | Progression-free survival and overall survival | The study was halted in February 2011 prior to completion because it was judged to be unlikely to meet its primary efficacy end points. | |
| Zibotentan: ENTHUSE M1C Study 33 | NCT00617669 | 1,445 (target) | CRPC metastatic to bone, chemotherapy naive | Zibotentan 10 mg orally daily + docetaxel v docetaxel | Overall survival | The study is ongoing. Estimated study completion date is May 2012. | |
| Dasatinib | NCT00744497 | 1,500 (target) | Metastatic CRPC | Dasatinib + docetaxel-prednisone v placebo + docetaxel-prednisone | Overall survival | This SRC inhibitor trial is currently recruiting participants. Estimated study completion date is December 2012. | |
| AMG 102 | NCT00770848 | 162 (target) | Metastatic CRPC, prior taxane-based treatment | AMG 102 every 3 weeks + mitoxantrone-prednisone v mitoxantrone-prednisone | Overall survival (phase II portion) | AMG 102 is a fully human monoclonal antibody that blocks the action of hepatocyte growth factor/scatter factor (c-MET receptor signaling). Estimated study completion date is March 2013. |
Abbreviations: ADT, androgen-deprivation therapy; ALSYMPCA, Alpharadin in Symptomatic Prostate Cancer; BAP, bone alkaline phosphatase; BMD, bone mineral density; CALGB/CTSU, Cancer and Leukemia Group B/Cancer Trials Support Unit; CRPC, castration-resistant prostate cancer; dt, doubling time; ETA, endothelin A; FDA, US Food and Drug Administration; HDL, high-density lipoprotein; HR, hazard ratio; IV, intravenous; LDL, low-density lipoprotein; PSA, prostate-specific antigen; SC, subcutaneous; SRC, proto-oncogene tyrosine-protein kinase; SRE, skeletal-related event; SWOG, Southwest Oncology Group; ZEUS, Zometa European Study.
End points in clinical trials of bone-targeted agents are an important consideration. Trials examining treatment for therapy-induced osteoporosis typically feature a primary end point of either BMD (sample size generally < 100) or fracture (sample size generally > 1,000). The primary end point for metastasis prevention trials is the first appearance of a scan-detectable bone metastasis. Trials examining treatment of prostate cancer metastatic to bone typically feature a composite clinical end point called skeletal-related events (SREs). That end point includes pathologic fracture, spinal cord compression, need for surgery to bone, and need for radiation to bone.
Treatment-Related Osteoporosis
Bisphosphonates, denosumab, and selective estrogen receptor modulators (SERMs) represent three classes of agents that have been shown to benefit men with treatment-induced osteoporosis. When examining the evidence in support of these agents, it is important to make note of the doses and schedules used for this indication. Treatment of therapy-induced osteoporosis requires considerably lower dose and/or frequency than does the prevention of SREs in patients with metastatic cancer.
Several bisphosphonates improve BMD in men receiving ADT, including alendronate,7 pamidronate,10,36 zoledronic acid,8,37 and neridronate.38 These trials were not powered to detect differences in fracture rate (range of sample sizes, 21 to 112). The results of two phase III fracture prevention trials have recently been published (sample sizes, 1,284 for toremifene and 1,468 for denosumab). Together, these drugs provide options for men receiving ADT and at increased risk for osteoporotic fracture.
Denosumab significantly reduces the incidence of vertebral fractures in high-risk men receiving ADT.39 Denosumab HALT 138 (A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Denosumab in the Treatment of Bone Loss in Subjects Undergoing Androgen Deprivation Therapy (ADT) for Non-Metastatic Prostate Cancer) enrolled 1,468 men who were maintained on ADT and were at increased fracture risk due to age ≥ 70 years, history of osteoporotic fracture, or low BMD. Participants were randomly assigned to denosumab (60 mg subcutaneously [SC] every 6 months) or to placebo. The trial demonstrated significant improvements in BMD at the lumbar spine (6.7%), total hip (4.8%), and distal one third of the radius (5.5%) at 24 months. In addition, it demonstrated significant improvement relative to placebo in the 3-year incidence of new vertebral fractures (1.5% v 3.9%, decreased by 62%; P = .006). Adverse events did not significantly differ between the denosumab and placebo arms. On the basis of these data, the European Commission granted marketing authorization for denosumab in this population.
Given the important role of estrogen in maintenance of bone health, SERMs have been examined as therapy for treatment-induced bone loss. Raloxifene and toremifene are both orally dosed SERMs that exert estrogenic effects on bone and have been shown to improve BMD in men receiving ADT.40,41 In a phase III study with 1,389 patients, toremifene was also shown to significantly reduce the incidence of vertebral fractures in that same population (2.5% v 4.9%; relative risk 0.50; P < .05 by modified intent-to-treat analysis).42 One notable adverse effect of SERM therapy is an increased risk for venous thromboembolism. In the phase III study of toremifene, venous thromboembolic events were more common in the treatment arm (17 patients; 2.6% incidence) than in the placebo arm (seven patients; 1.1% incidence). No SERM has yet been approved by the US Food and Drug Administration (FDA) for the reduction of osteoporotic fracture risk in men receiving ADT for prostate cancer.
Therapy-induced osteoporosis is a morbid problem for which available treatments are effective. Rational selection of treatment candidates is an important clinical challenge. Historically, fracture risk assessment has been strongly driven by BMD measurement. BMD measurement at the femoral neck is the most reliable value, because spine BMD can be confounded by degenerative joint disease, osteophyte formation, and calcification of the posterior ligaments. Although low BMD is associated with increased fracture risk, it is not a sensitive surrogate. Most men who suffer from fractures have BMD measurements that are not in the osteoporotic range.43
Other factors must be taken into account. Several clinical characteristics such as age, body mass index, and smoking status are predictive of fracture independent of their effects on BMD. WHO has developed an algorithm that uses these and other clinical factors with or without measured BMD to generate a quantitative assessment of fracture risk. Risk assessment based on clinical factors (ie, without BMD) is an important option for men with bone metastases that would render BMD testing inaccurate. National Comprehensive Cancer Network (NCCN) guidelines for the management of prostate cancer recommend this online algorithm (http://www.shef.ac.uk/FRAX/tool.jsp) for fracture risk assessment.44 In this population, the algorithm identifies a substantial proportion of patients as candidates for drug therapy to reduce fracture risk.45,46
In summary, therapy-induced osteoporosis is an important clinical problem among men receiving ADT for prostate cancer. Accepted guidelines recommend the use of the WHO/FRAX algorithm to assess fracture risk and guide treatment decisions. Among men who merit treatment, several options exist. Denosumab and toremifene are supported by the strongest evidence in this population (fracture prevention) but neither is approved by the FDA for men with prostate cancer. Several bisphosphonates are available and have been shown to improve BMD, a surrogate for fracture risk.
Metastasis Prevention
Until recently, no osteoclast-targeted agent had been shown to delay or prevent the development of bone metastases due to prostate cancer. Clodronate was studied in a completed but negative trial. Zoledronic acid was studied in a trial that closed early because the event rate was lower than projected. Denosumab and zoledronic acid are each currently the subject of phase III metastasis prevention trials. The denosumab trial has preliminarily been reported to demonstrate a benefit.
Clodronate.
Clodronate did not produce a significant benefit in the Medical Research Council PR04 (MRC PR04) trial. The trial enrolled 508 men with localized T2-4 prostate cancer and without evidence of bone metastases. Men were randomly assigned to 5 years of treatment with clodronate (2,080 mg orally daily) or placebo. The primary end point was time to symptomatic bone metastases or death due to prostate cancer. The trial found no significant difference between clodronate and placebo with short- and long-term follow-up.47,48
Zoledronic acid.
Zoledronic acid was the subject of a metastasis prevention trial that was closed early because of an event rate that was lower than expected. Zometa 704 enrolled men with nonmetastatic CRPC and randomly assigned them to zoledronic acid (4 mg intravenously [IV] every 4 weeks) or placebo. The primary end point was time to first bone metastasis. The Data Safety Monitoring Board for this trial halted the study after accrual of 398 of 991 planned subjects because the low event rate was projected to yield inadequate statistical power. Time to first bone metastasis did not differ significantly between the treatment and placebo arms.
Although the primary analysis of the trial was not informative, data from the placebo arm have better defined the natural history of CRPC and have informed the design of subsequent trials. The median time to development of skeletal metastasis was 30 months. Two characteristics that predicted shorter time to first metastasis were baseline PSA more than 10 ng/mL (relative risk, 3.18; 95% CI, 1.74 to 5.80; P < .01) and PSA velocity (relative risk, 4.34 for each 0.01 increase in PSA velocity; 95% CI, 2.30 to 8.21; P < .01).49
Zoledronic acid is also the subject of an ongoing prostate cancer metastasis prevention trial, the Zometa European Study (ZEUS). The study enrolls men without bone metastases who have at least one of the following factors that put them at high risk: PSA ≥ 20 ng/mL, lymph node metastasis, or Gleason score ≥ 8 primary tumor. It will randomly assign a total of 1,433 men to zoledronic acid (4 mg IV every 3 months for 48 months) or to standard care without zoledronic acid. The primary end point is the proportion of men with at least one bone metastasis after 48 months of therapy.50
Denosumab.
Denosumab is the subject of a metastasis prevention trial (Denosumab Trial 147) that enrolled 1,432 men with CRPC but no bone metastases. All patients had at least one of the following factors that put them at high risk for metastases: PSA ≥ 8 ng/mL or PSA doubling time of ≤ 10 months. Participants were randomly assigned to denosumab (120 mg SC every 4 weeks) or to placebo. The primary end point is metastasis-free survival. Secondary end points include time to first metastasis and overall survival (OS). The trial has preliminarily been reported to show a significant 4.2-month improvement in metastasis-free survival (Amgen, data on file).
In summary, management of nonmetastatic CRPC is an open question. At present, bone-targeted therapy should not be used for the prevention of metastases. Denosumab has been preliminarily reported to be the first agent to significantly improve metastasis-free survival. The results of this trial and the ongoing zoledronic acid trial have not yet been formally reported.
Metastatic Castration-Sensitive Prostate Cancer
In other malignancies such as multiple myeloma and breast cancer, clinical trials have demonstrated benefit with immediate use of osteoclast-targeted therapies for patients with bone involvement by cancer. In contrast, clinical trials of osteoclast-targeted therapies for men with prostate cancer have demonstrated significant benefit only for patients with bone metastases and cancer progression despite first-line ADT. Given that osteoclast inhibition prevents SREs in men with CRPC metastatic to bone, it is logical to investigate its potential earlier in the natural history of the disease. Although no agent has demonstrated a benefit in this population, study of zoledronic acid is ongoing.
Clodronate.
The MRC PR05 trial examined the use of clodronate for the treatment of men with bone metastases who were initiating or responding to first-line ADT. Clodronate failed to reach its primary objective but later showed a benefit in OS. A total of 311 men were randomly assigned to clodronate (2,080 mg orally daily) or to placebo. At 59 months follow-up, clodronate had produced nonsignificant improvements in bone progression-free survival (PFS; hazard ratio [HR], 0.79; 95% CI, 0.61 to 1.02; P = .066) and OS (HR, 0.80; 95% CI, 0.62 to 1.03; P = .082).51 Interestingly, long-term follow-up revealed a significant improvement in survival (8-year OS, 22% v 14%; HR, 0.77; 95% CI, 0.60 to 0.98; P = .032).48 This benefit with clodronate, a comparatively weak bisphosphonate, demonstrates the potential of an osteoclast-targeted treatment strategy.
Zoledronic acid.
Zoledronic acid is compared with placebo for the treatment of bone metastatic castration-sensitive prostate cancer in the ongoing Cancer and Leukemia Group B/Cancer Trials Support Unit 90202 (CALGB/CTSU 90202) trial. A total of 680 men who are within 6 months of initiation of ADT will be randomly assigned to either zoledronic acid (4 mg IV every 4 weeks) or placebo. The primary end point is time to first SRE or death due to prostate cancer. Men receiving placebo cross over to receive zoledronic acid if they experience an SRE or if they progress to CRPC.
In summary, management of metastatic castration-sensitive prostate cancer is an open question. Currently available evidence does not support the use of osteoclast-targeted therapy in this setting. Denosumab has not been studied and should not be used for this indication. Early versus late addition of zoledronic acid for men with prostate cancer metastatic to bone will be examined by the ongoing CALGB/CTSU 90202 trial.
Metastatic Castration-Resistant Prostate Cancer
Men with bone metastatic prostate cancer that progresses despite ADT belong to a poor prognosis population that is at high risk for skeletal morbidity. Although relatively weaker bisphosphonates clodronate and pamidronate failed in this setting, zoledronic acid was later demonstrated to reduce the incidence of skeletal events and became the standard-of-care treatment. Denosumab has more recently been shown to be superior to zoledronic acid and was approved by the FDA for this indication in 2010.
Pamidronate.
Pamidronate failed to produce a significant benefit in this clinical setting. Two similar trials, CGP 032 and INT 05, examined the use of pamidronate (90 mg IV every 3 weeks) to treat men with CRPC and symptomatic bone metastases. Each featured 27 weeks of treatment and 1:1 randomization between pamidronate and placebo. The trials together enrolled 350 men and were analyzed as one. The primary end points were self-reported pain score, analgesic use, and proportion of patients who experienced an SRE. When compared with placebo, treatment did not produce significant improvements in any of the primary end points.52
Clodronate.
Clodronate also failed to produce a significant benefit in this clinical setting. The National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) PR.6 study compared clodronate (1,500 mg IV every 3 weeks) with placebo for the treatment of men with CRPC and symptomatic bone metastases. All 209 participants also received mitoxantrone and prednisone. The primary end point was palliative response (improvement in self-reported pain index or a ≥ 50% drop in analgesic use). Palliative response was similar in the two arms (46% with clodronate and 39% with placebo; P = .54),53 as were PFS, OS, and quality of life.
Zoledronic acid.
The Zometa 039 trial established zoledronic acid as the first osteoclast-targeted agent to benefit men with CRPC and bone metastases. In the trial, 643 men were randomly assigned to receive treatment every 3 weeks with zoledronic acid (4 mg or 8 mg) or with placebo. The primary end point was the proportion of men who experienced at least one SRE during the first 15 months of therapy.54
Nephrotoxicity of zoledronic acid during this trial led to two notable changes in the protocol. First, zoledronic acid infusion time was lengthened from 5 minutes to 15 minutes. Second, the 8-mg dose arm was eliminated in favor of 4 mg dosing. After these changes, adverse events related to kidney function were not significantly more frequent in the treatment cohort.
Zoledronic acid was associated with fewer SREs at 15 months when compared with placebo (33.2% v 44.2%; P = .021). Time to first SRE was also improved with zoledronic acid (488 v 321 days; P = .009).55 There was a statistically nonsignificant trend toward improved survival with zoledronic acid (546 v 464 days; P = .091). The positive result with zoledronic acid compared with negative trials that used other bisphosphonates may be attributable to differences in patient selection, differences in study end points, or the increased potency of zoledronic acid. The results of this trial led to FDA approval of zoledronic acid for the prevention of SREs in men with CRPC and bone metastases.
Denosumab.
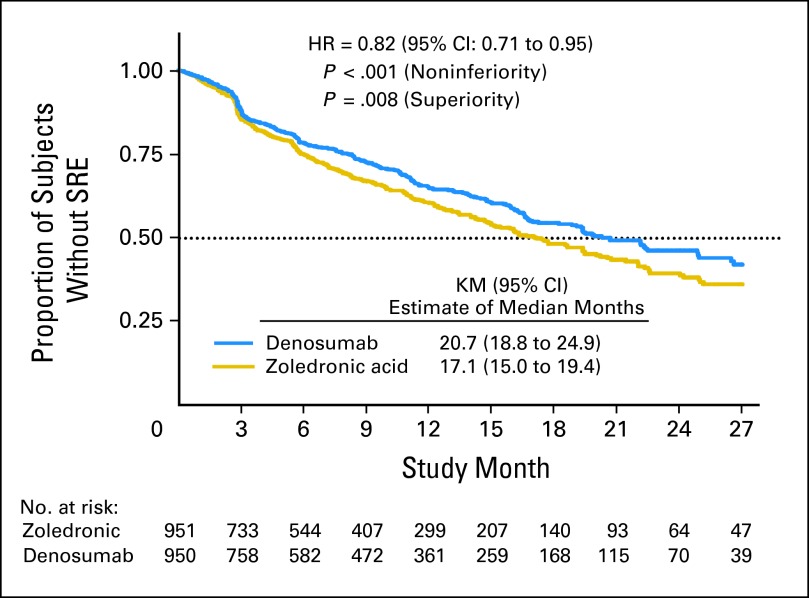
Given that zoledronic acid had been established as standard of care for men with CRPC and bone metastases, Denosumab Trial 103 compared denosumab (120 mg SC every 4 weeks) with zoledronic acid (4 mg IV every 4 weeks). The primary end point was time to first SRE. The trial was powered to demonstrate noninferiority and enrolled 1,901 men. The trial was positive because median time to first SRE was significantly better in the denosumab arm (20.7 v 17.1 months; P < .001 for noninferiority; P = .008 for superiority; Fig 3). OS (HR, 1.03; 95% CI, 0.91 to 1.17) and overall disease progression (HR, 1.06; 95% CI, 0.95 to 1.18) were equivalent. Men treated with denosumab experienced higher incidence of hypocalcemia (12.8% v 5.8%) and a nonsignificant trend toward higher osteonecrosis of the jaw (ONJ; 2.3% v 1.3%; P = .09), possibly a reflection of more potent osteoclast inhibition.
Fig 3.
Primary analysis of the Denosumab 103 trial. Denosumab (120 mg subcutaneously every 4 weeks) was compared with zoledronic acid (4 mg intravenously every 4 weeks) in men with castration-resistant prostate cancer metastatic to bone. Median time to first skeletal-related event (SRE) was significantly longer in the denosumab arm (20.7 v 17.1 months). Analyses for noninferiority and for superiority were both significant.33 HR, hazard ratio; KM, Kaplan-Meier.
Two additional similarly designed studies compared denosumab with zoledronic acid in women with breast cancer metastatic to bone (n = 2,046)56 and in patients with solid tumors or multiple myeloma metastatic to bone (n = 1,776).57 Those studies each showed that denosumab significantly delayed time to first on-study SRE. On the basis of the results of these three phase III trials, the FDA approved denosumab in November 2010 for the treatment of solid tumors metastatic to bone.
Toxicity is an important concern with either zoledronic acid or denosumab. Because both drugs can cause hypocalcemia, it is important to appropriately replete vitamin D before initiation of therapy and to monitor calcium while on therapy. Zoledronic acid requires dose modification for stable renal insufficiency (glomerular filtration rate 30 to 60 mL/min/1.7m2) and is not recommended if glomerular filtration rate is less than 30 mL/min/1.7m2. Although the phase III trial comparing the two drugs excluded men with creatinine clearance less than 30 mL/min, denosumab is thought to be safe regardless of renal function. Denosumab has not been reported to cause nephrotoxicity, and its clearance is not dependent on kidney function. ONJ seems to be a potential complication of any form of potent osteoclast inhibition. Zoledronic acid and denosumab produce similar rates of ONJ when compared directly.33 Retrospective data have shown that intensity of dosing, duration of therapy, and dental extractions during therapy convey the highest risk of ONJ.58 Some have recommended risk reduction through oral examination before initiation of therapy, extraction of nonrestorable teeth before therapy, and a 2- to 3-week wait between extractions before initiation of therapy.59
In summary, monthly denosumab or zoledronic acid reduces the incidence of SREs among men with CRPC metastatic to bone. Either drug is a reasonable choice in this setting. Zoledronic acid is more familiar to many clinicians because it has been available for years. Denosumab is slightly superior in delaying time to first SRE and was recently approved by the FDA for this indication. Toxicity is an important factor favoring denosumab among men with significant renal insufficiency.
NOTABLE BONE-TARGETED THERAPIES IN CLINICAL DEVELOPMENT
Radiopharmaceuticals
Beta-emitting pharmaceuticals strontium-89 chloride (89Sr) and samarium-153 (153Sm) are approved for the palliation of pain due to bone metastases.32 Radium-223 (223Ra) is an alpha-emitting pharmaceutical that is under current investigation for the treatment of CRPC with symptomatic skeletal metastases. One notable theoretical advantage of alpha emission over beta emission is a more limited range that may reduce toxicity due to marrow irradiation. A phase II study of 223Ra suggested that this is the case.60 In the 900-patient phase III study of 223Ra, patients were randomly assigned 2:1 to 223Ra (six IV administrations separated by 4-week intervals) or to placebo. The primary end point was OS. In June 2011, the study was stopped early on the basis of the recommendation of an independent data monitoring committee after a preplanned interim efficacy analysis.33a Compared with placebo, 223Ra chloride was associated with improved OS (median, 14.0 v 11.2 months; HR, 0.699; P = .0022).
Endothelin A Receptor–Targeted Therapies
Endothelin signaling modulates vasomotor tone, nociception, hormone production, and cellular proliferation.61 Of the three peptide factors (ET-1, ET-2, and ET-3) composing the endothelin family, ET-1 is known to stimulate osteoblast activity62 and is thought to play a role in promoting prostate cancer growth and metastasis. The effects of ET-1 are mediated by the endothelin A (ETA) receptor,63 making the ETA receptor a rational target for drug development.64 Atrasentan and zibotentan (ZD4054) are oral ETA receptor antagonists.
Atrasentan has been the subject of three phase III trials. Two have been negative and one is ongoing. One placebo-controlled trial enrolled 941 men with nonmetastatic CRPC and found no significant delay in time to progression, the primary end point.34 Atrasentan did significantly slow the progression of bone-specific alkaline phosphatase and lengthen PSA doubling time. Another placebo-controlled trial enrolled 809 men with metastatic CRPC and found that atrasentan did not delay time to progression.35 A third ongoing trial is designed to enroll 930 taxane-naive men with metastatic CRPC and randomly assign them to docetaxel-prednisone with either atrasentan or placebo. OS and PFS are end points.
Zibotentan is an ETA inhibitor that, unlike atrasentan, has no detectable activity at ETB. A randomized phase II study of zibotentan showed no effect on time to progression (the primary end point) but demonstrated a promising OS benefit.65,66 Zibotentan is in current clinical development that involves three phase III studies as summarized in Table 1. These trials feature longer-term follow-up than the atrasentan studies and do not allow crossover from placebo to treatment. One of those studies enrolled 848 men with CRPC metastatic to bone and randomly assigned them to zibotentan or placebo. That study was reported in a September 2010 press release to have failed to show an improvement in OS, its primary end point. A second study was halted early because it was found to be unlikely to demonstrate an improvement in PFS or OS with zibotentan monotherapy for nonmetastatic CPRC. The third study is ongoing.
SRC-Targeted Therapies
The proto-oncogene SRC is a nonreceptor tyrosine kinase that mediates downstream signals from several cell surface receptors (epidermal growth factor receptor [EGFR], human epidermal growth factor receptor 2 [HER2], vascular endothelial growth factor receptor [VEGFR], and insulin-like growth factor receptors).67 It is thought to be involved in bone remodeling, cancer metastasis, and tumor growth. In particular, preclinical studies have suggested that SRC signaling promotes prostate cancer growth and metastatic potential in response to androgens.68,69 Dasatinib is one of several inhibitors of SRC that are in clinical development for the treatment of prostate and breast cancer.67 It has been shown to suppress markers of bone turnover70 and is currently under study in a phase III trial for men with CRPC that compares docetaxel monotherapy and docetaxel with dasatinib.
MET-Targeted Therapies
MET is a receptor tyrosine kinase that has roles in oncogenic signaling, angiogenesis, and metastasis. Androgen deprivation activates MET signaling in prostate cancer cells. Activated MET is particularly highly expressed in bone. Preclinical studies have suggested that MET signaling may promote survival of prostate cancer cells.71 Two notable MET-targeted therapies are in clinical development. AMG 102 is a fully human monoclonal antibody that blocks the action of hepatocyte growth factor/scatter factor and therefore MET receptor-mediated signaling. It is the subject of a randomized phase II trial in which it is combined with mitoxantrone and prednisone. Cabozantinib (XL184) is a small-molecule inhibitor of multiple kinase signaling pathways, including MET, RET, VEGFR2/KDR, and KIT. In an ongoing phase II study, cabozantinib has shown promising activity in men with bone metastases with improvement in bone scans in most patients.72
SUMMARY
In conclusion, skeletal complications are an important consideration in the lives of men with prostate cancer. From osteoporotic fractures caused by ADT to skeletal events caused by metastases, bone-related hazards are present at diverse time points within the natural history of treated prostate cancer. Currently available evidence supports the use of bone-targeted therapy for the treatment of men with increased risk for osteoporotic fracture. Current evidence also supports the monthly use of either zoledronic acid or denosumab for the reduction of SREs in men with bone-metastatic castration-resistant disease. Preliminary report of a study demonstrating improved metastasis-free survival with denosumab will require scrutiny of officially reported data but offers promise for near-term clinical application. Additional applications are under investigation.
Footnotes
Supported by Young Investigator Awards from the Prostate Cancer Foundation and American Society of Clinical Oncology (ASCO) Cancer Foundation (P.J.S.), by a Physician Research Training Award (Prostate Cancer Research Program, Department of Defense) and a Career Development Award from the ASCO Cancer Foundation (R.J.L.), and by National Institutes of Health K24 Midcareer Investigator Award No. 5K24CA121990-02 and awards from the Prostate Cancer Foundation (M.R.S.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Richard J. Lee, Amgen (C); Matthew R. Smith, Amgen (C), GTx (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 6.Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab. 1999;84:3431–3434. doi: 10.1210/jcem.84.10.6060. [DOI] [PubMed] [Google Scholar]
- 7.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: A randomized trial. Ann Intern Med. 2007;146:416–424. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 8.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–1042. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittan D, Lee S, Miller E, et al. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab. 2002;87:3656–3661. doi: 10.1210/jcem.87.8.8782. [DOI] [PubMed] [Google Scholar]
- 10.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 11.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 12.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 13.Kanis JA, Borgstrom F, De Laet, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 14.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 15.Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(suppl 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greendale GA, Edelstein S, Barrett-Connor E. Endogenous sex steroids and bone mineral density in older women and men: The Rancho Bernardo Study. J Bone Miner Res. 1997;12:1833–1843. doi: 10.1359/jbmr.1997.12.11.1833. [DOI] [PubMed] [Google Scholar]
- 17.Khosla S, Melton LJ, 3rd, Atkinson EJ, et al. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 18.Slemenda CW, Longcope C, Zhou L, et al. Sex steroids and bone mass in older men: Positive associations with serum estrogens and negative associations with androgens. J Clin Invest. 1997;100:1755–1759. doi: 10.1172/JCI119701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett-Connor E, Mueller JE, von Mühlen DG, et al. Low levels of estradiol are associated with vertebral fractures in older men, but not women: The Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:219–223. doi: 10.1210/jcem.85.1.6327. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc ES, Nielson CM, Marshall LM, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94:3337–3346. doi: 10.1210/jc.2009-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellström D, Johnell O, Ljunggren O, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21:529–535. doi: 10.1359/jbmr.060110. [DOI] [PubMed] [Google Scholar]
- 22.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: Implications for clinical decision making. Urology. 2000;56:1021–1024. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13:241–245. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 25.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88(suppl 12):2919–2926. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Cook RJ, Coleman R, Brown J, et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res. 2006;12:3361–3367. doi: 10.1158/1078-0432.CCR-06-0269. [DOI] [PubMed] [Google Scholar]
- 27.Clarke NW, McClure J, George NJ. Osteoblast function and osteomalacia in metastatic prostate cancer. Eur Urol. 1993;24:286–290. doi: 10.1159/000474311. [DOI] [PubMed] [Google Scholar]
- 28.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 29.Rogers MJ, Watts DJ, Russell RG. Overview of bisphosphonates. Cancer. 1997;80(suppl 8):1652–1660. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Bekker PJ, Holloway DL, Rasmussen AS, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- 32.Paes FM, Serafini AN. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med. 2010;40:89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Bayer HealthCare. Bayer's investigational compound radium-223 chloride met its primary endpoint of significantly improving overall survival in a phase III trial in patients with castration-resistant prostate cancer that has spread to the bone. http://pharma.bayer.com/html/pdf/news_room115.pdf.
- 34.Nelson JB, Love W, Chin JL, et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–2487. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carducci MA, Saad F, Abrahamsson PA, et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 36.Diamond TH, Winters J, Smith A, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: A double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–1450. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 37.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–2012. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 38.Morabito N, Gaudio A, Lasco A, et al. Neridronate prevents bone loss in patients receiving androgen deprivation therapy for prostate cancer. J Bone Miner Res. 2004;19:1766–1770. doi: 10.1359/JBMR.040813. [DOI] [PubMed] [Google Scholar]
- 39.Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MR, Fallon MA, Lee H. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: A randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–3846. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 41.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: Interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–155. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith MR, Morton RA, Barnette KG, et al. Toremifene to reduce fracture risk in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2010;184:1316–1321. doi: 10.1016/j.juro.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeman E, Bianchi G, Khosla S, et al. Bone fragility in men: Where are we? Osteoporos Int. 2006;17:1577–1583. doi: 10.1007/s00198-006-0160-8. [DOI] [PubMed] [Google Scholar]
- 44.National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Prostate Cancer. 2010. http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf.
- 45.Adler RA, Hastings FW, Petkov VI. Treatment thresholds for osteoporosis in men on androgen deprivation therapy: T-score versus FRAX. Osteoporos Int. 2010;21:647–653. doi: 10.1007/s00198-009-0984-0. [DOI] [PubMed] [Google Scholar]
- 46.Saylor PJ, Kaufman DS, Michaelson MD, et al. Application of a fracture risk algorithm to men treated with androgen deprivation therapy for prostate cancer. J Urol. 2010;183:2200–2205. doi: 10.1016/j.juro.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mason MD, Sydes MR, Glaholm J, et al. Oral sodium clodronate for nonmetastatic prostate cancer: Results of a randomized double-blind placebo-controlled trial—Medical Research Council PR04 (ISRCTN61384873) J Natl Cancer Inst. 2007;99:765–776. doi: 10.1093/jnci/djk178. [DOI] [PubMed] [Google Scholar]
- 48.Dearnaley DP, Mason MD, Parmar MK, et al. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: Long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10:872–876. doi: 10.1016/S1470-2045(09)70201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 50.Wirth M, Tammela T, Debruyne F, et al. Effectiveness of Zoledronic acid for the prevention of bone metastases in high-risk prostate cancer patients: A randomised, open label, multicenter study of the European Association of Urology (EAU) in Cooperation with the Scandinavian Prostate Cancer Group (SPCG) and the Arbeitsgemeinschaft Urologische Onkologie (AUO)—A report of the ZEUS study. Genitourinary Cancers Symposium. 2008 abstr 184. [Google Scholar]
- 51.Dearnaley DP, Sydes MR, Mason MD, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial) J Natl Cancer Inst. 2003;95:1300–1311. doi: 10.1093/jnci/djg038. [DOI] [PubMed] [Google Scholar]
- 52.Small EJ, Smith MR, Seaman JJ, et al. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21:4277–4284. doi: 10.1200/JCO.2003.05.147. [DOI] [PubMed] [Google Scholar]
- 53.Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21:3335–3342. doi: 10.1200/JCO.2003.03.042. [DOI] [PubMed] [Google Scholar]
- 54.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 55.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 56.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 57.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2010;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 58.Hoff AO, Toth BB, Altundag K, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Advisory Task Force on Bisphosphonate-Related Osteonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson S, Franzén L, Parker C, et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587–594. doi: 10.1016/S1470-2045(07)70147-X. [DOI] [PubMed] [Google Scholar]
- 61.Battistini B, Chailler P, D'Orléans-Juste P, et al. Growth regulatory properties of endothelins. Peptides. 1993;14:385–399. doi: 10.1016/0196-9781(93)90057-n. [DOI] [PubMed] [Google Scholar]
- 62.Nelson JB, Nguyen SH, Wu-Wong JR, et al. New bone formation in an osteoblastic tumor model is increased by endothelin-1 overexpression and decreased by endothelin A receptor blockade. Urology. 1999;53:1063–1069. doi: 10.1016/s0090-4295(98)00658-x. [DOI] [PubMed] [Google Scholar]
- 63.Nelson JB, Chan-Tack K, Hedican SP, et al. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- 64.Nelson JB, Carducci MA. The role of endothelin-1 and endothelin receptor antagonists in prostate cancer. BJU Int. 2000;85(suppl 2):45–48. doi: 10.1046/j.1464-410x.2000.00063.x. [DOI] [PubMed] [Google Scholar]
- 65.James ND, Caty A, Borre M, et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: A double-blind, placebo-controlled, randomised, phase 2 trial. Eur Urol. 2009;55:1112–1123. doi: 10.1016/j.eururo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 66.James ND, Caty A, Payne H, et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: A double-blind, placebo-controlled, randomized Phase II trial. BJU Int. 2010;106:966–973. doi: 10.1111/j.1464-410X.2010.09638.x. [DOI] [PubMed] [Google Scholar]
- 67.Saad F, Lipton A. SRC kinase inhibition: Targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev. 2010;36:177–184. doi: 10.1016/j.ctrv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Asim M, Siddiqui IA, Hafeez BB, et al. Src kinase potentiates androgen receptor transactivation function and invasion of androgen-independent prostate cancer C4-2 cells. Oncogene. 2008;27:3596–3604. doi: 10.1038/sj.onc.1211016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–2356. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- 70.Yu EY, Wilding G, Posadas E, et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2009;15:7421–7428. doi: 10.1158/1078-0432.CCR-09-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang S, Zhau HE, Osunkoya AO, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer. 2010;9:9. doi: 10.1186/1476-4598-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith DC, Spira A, De Grève J, et al. Phase 2 study of XL184 in a cohort of patients with castration resistant prostate cancer (CRPC) and measurable soft tissue disease. 22nd European Organisation for Research and Treatment of Cancer-National Cancer Institute-American Association for Cancer Research (EORTC-NCI-AACR) Symposium on Molecular Targets and Cancer Therapeutics; November 16-19, 2010; Berlin, Germany. [Google Scholar]