Abstract
We have recently developed a general liquid chromatography-tandem mass spectrometric (LC-MS/MS) method using a stable isotope-labeled (SIL) monoclonal antibody (mAb) as an internal standard (IS) for single-analyte quantification of mAb (Li et al. Anal Chem 84(3):1267–1273, 2012). The method offers an advantage over ligand binding assay in reducing the time and resources needed for bioanalytical support in preclinical stages of drug development. In this paper, we report another marked increase in assay efficiency for multi-analyte bioanalysis using unique surrogate peptides for each analyte and the strategic choice of the SIL-IS peptide. The method was qualified for the simultaneous determinations of four mAbs in rat plasma and applied to samples from discrete- and cassette-dosed rats. The pharmacokinetic parameters of the four mAbs of cassette dosing were comparable to those of discrete dosing and of enzyme-linked immunosorbent assay results. Although there may be limitations and special considerations for cassette-dosing of biologics, these results demonstrate the robust performance of the multi-analyte LC-MS/MS method allowing cassette-dosing that would ultimately reduce animal use and improve efficiency.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-012-9435-5) contains supplementary material, which is available to authorized users.
Key words: cassette dosing of biologics, immunoaffinity-mass spectrometry, ligand binding assay, monoclonal antibody biotherapeutics, multi-analyte LC-MS/MS
INTRODUCTION
Pharmacokinetic (PK) study samples from subjects dosed with large-molecule biotherapeutics are usually analyzed by ligand binding assays (LBA). Liquid chromatography-tandem mass spectrometric (LC-MS/MS) methods coupled with immunoaffinity sample enrichment have gained interest due to the potential advantages of additional specificity, reduced need for specific reagents, and faster method development time for the analysis of biologics (1–6). We have recently reported the approach of using a common immunoaffinity capture and a general stable isotope-labeled whole-antibody internal standard (SIL-mAb IS) incorporating an essential SIL-amino acid. This approach has been applied to the quantification of eight monoclonal antibodies (mAbs) (7).
During the early phase of drug development, the availability of multiplex assays can be advantageous for the evaluation of multiple candidates such as from cassette-dosing experiments (8,9). However, specific reagents for LBA are not available during the early development phase, and the assays typically could not distinguish between simultaneously administered large-molecule drug entities. Additionally, for novel mAb-fusion constructs, the non-specific LBA could not differentiate the intact molecule from its metabolites (8). On the other hand, LC-MS/MS methods for small molecules are widely applied for multi-analyte quantification for drugs and metabolites as well as for cassette-dosing (9–11) and have high selectivity even with limited method development. Therefore, our objective was to develop a multiplex LC-MS/MS method for multiple mAb candidates from cassette dosing and for metabolites of novel large-molecule drug entities. We have increased the capability of our single-analyte LC-MS/MS method for the simultaneous quantification of four mAbs and tested the feasibility of application to a cassette-dosing study.
The major benefits of cassette-dosing of candidates would be the reduction in animal use and savings in resource, time, and cost (12). However, cassette-dosing during small-molecule drug development has been limited due to multiple concerns (10,12–15). For large-molecule biotherapeutic candidates, the preclinical PK studies often involve nonhuman primates and/or xenograft mice, which demand innovative ways to reduce animal use. However, there are issues of mAb cassette-dosing that are unique and different from those of small-molecule drugs. Therefore, the opportunities and risks as related to cassette-dosing should be weighed carefully with the anticipated application of any multiplex LC-MS/MS method.
There are two objectives of this paper: (1) to present the method development and qualification of a multiplex LC-MS/MS method for four mAbs and (2) to demonstrate the applicability of the multiplex LC-MS/MS method in the analysis of samples from cassette-dosed rats and compare the results to those of discrete-dosed rats. The potential effects of immunogenicity and target-binding on the PK results from cassette-dosing of mAbs were discussed.
EXPERIMENTAL
Materials and Methods
The four mAb analytes, SIL-mAb IS, and biotinylated anti-human Fc (clone 1.35.1) were from Amgen Inc. (Thousand Oaks, CA). The four mAbs were IgG2 against various antigens that were not endogenous in the test animals: two anti-dinitrophenol clones (αDA and αDB), anti-KLH (αK), and mAC. The internal standard (IS) was αDA uniformly labeled with [13C6]-Leu during recombinant synthesis in cell culture. The isotopic purity was ∼95% (7).
Streptavidin-bonded magnetic beads (Dynal MPC-1) were purchased from Invitrogen (Carlsbad, CA). The solid-phase extraction plates (Oasis HLB μElution Plate 30 μm) were from Waters, Corp. (Milford, MA). Trypsin was obtained from Promega Corp. (Madison, WI). Iodoacetamide and enzyme-linked immunosorbent assay (ELISA) reagents other than the mAbs were from Thermo Fisher (Rockford, IL). Acetonitrile (ACN), methanol, and water (HPLC-grade) were obtained from Burdick and Jackson (Muskegon, MI). Formic acid (reagent-grade) was from Aldrich, Inc. (St. Louis, MO). Control rat plasmas were supplied by Bioreclamation Inc. (East Meadow, NY). Bovine serum albumin (BSA), Dulbecco phosphate-buffered saline (DPBS), tris(carboxyethyl)phosphine (TCEP), and Tween 20 were purchased from Sigma (Saint Louis, MO).
Preparation of Calibration Standards and Quality Control (QC) Samples
The working solution of mixed mAbs, each at 100 μg/ml was diluted from the 1 mg/ml stock of each mAb with rat plasma and stored at 2°C to 8°C. Mixed calibration standards at concentrations of 0.10, 0.25, 0.50, 1.00, 2.50, 5.00, 10.0, and 15.0 μg/ml of each mAb were prepared by serial dilution of a freshly prepared standard (15.0 μg/ml of each mAb) in rat plasma. Mixed QC samples at concentrations of 0.30, 1.50, 6.00, and 12.0 μg/ml were prepared by spiking the mixed mAb working solution into control rat plasma with appropriate dilutions. The QC samples were aliquoted into polypropylene tubes and stored at −70°C. The 100 μg/ml SIL-αDA IS stock solution in DPBS containing 1% BSA was stored at 2°C to 8°C. The working IS solution was diluted to 2 μg/ml with 250 mM Tris buffer prior to use.
Workflow of LC-MS/MS
The workflow was similar to Li et al. (7) with modification as shown in Fig. 1: The immunocapture was carried out using magnetic streptavidin beads (10 mg/ml) coated with biotinylated anti-human Fc (b-Ab35, 100 μg/ml). To 25 μl of rat plasma sample in a 96-deep-well plate, 25 μl of SIL-αDA IS (2 μg/ml) and 50 μl of b-Ab35-coated magnetic beads were introduced and incubated for 1 h with mixing at ambient temperature. After incubation, the beads were washed twice with DPBS using a Tomtec Quadra3 instrument (Tomtec, Hamden, CT) with a magnetic nest attachment. Instead of performing trypsin digestion after antibody elution from the magnetic beads as described previously (7), the enzymatic digestion was performed directly on the beads to simplify the analytical workflow and minimize sample loss. In brief, the antibody analytes on the beads were denatured and reduced with 45 μl of 7.5 mM TCEP in denaturing buffer (8M urea, 250 mM Tris, pH 7.5) for 45 min at 55°C after the beads were resuspended with 30 μL DPBS. Cysteine alkylation to protect the reactive thiols was performed with 25 μl of 40 mM iodoacetamide in 250 mM Tris, pH 7.5 for 45 min at 55°C in the dark. The sample was then digested with 300 μl of 2 μg/ml trypsin overnight at ambient temperature. The digestion was stopped with 50 μl 10% acetic acid, and the digests were desalted and concentrated using a 96-well Oasis HLB μElution plate. The LC-MS/MS injection volume was 10 μl.
Fig. 1.
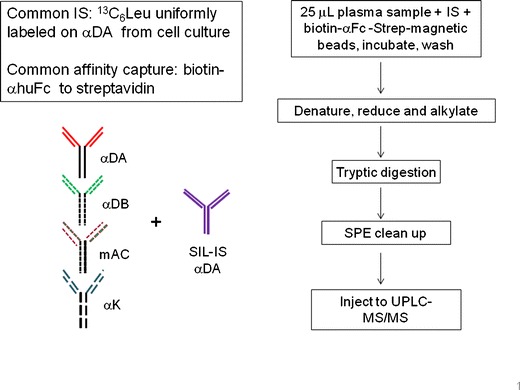
LC-MS/MS method workflow
Instrumentation
The selected peptides were separated and quantified by ultra-performance liquid chromatography (UPLC)-MS/MS, which consisted of an Acquity UPLC (Waters, Milford, MA) coupled to a QTRAP® 5500 mass spectrometer (AB SCIEX, Toronto, Canada) operated in the positive ion multiple reaction monitoring (MRM) mode. The analytical column was a UPLC Acquity BEH C18 2.1 × 100 mm of 1.7 μm particle size (Waters, Milford, MA) and maintained at 70°C. The mobile phases were: (a) 0.1% formic acid in ACN/water (5/95, v/v); (b) 0.1% formic acid in ACN/water (95/5, v/v). The LC gradients in minutes per percent of mobile phase B were 0.0/2, 0.5/2, 4.0/40, 4.1/95, 4.6/95, 4.7/2, and 5.0/2. The flow rate was 0.60 ml/min, and the run time was 5 min. The MS settings were—ESI spray voltage, 5,000 V; source temperature, 500°C; curtain gas, 30; nebulizer gas, 40; and auxiliary gas setting, 50. The ion transitions for the MRM quantification of the selected peptides are listed in Table I.
Table I.
Signature Peptides from CDR and SIL-IS
| Analyte | Signature peptide | Q1 m/z | Q3 m/z |
|---|---|---|---|
| αDA | LLIYAASSLQSGVPSR.3y6 | 554.65 | 602.33 |
| mAC | LLIYDASTR.2y7 | 526.29 | 825.41 |
| αK | LIYAASSLQSGVPSR.2y7 | 774.92 | 730.38 |
| αDB | LIYAASSLQSGVPLR.3y6 | 525.63 | 628.38 |
| IS αDA | aLaLIYAASSaLQSGVPSR.3y6 | 560.65 | 602.33 |
αD anti-dinitrophenol (clone A or B)
αK anti-KLH, IS internal standard
aDesignates [13C6]-Leu
Data Regression
The chromatographic peak areas of selected signature peptides that were unique for each mAb along with an appropriate SIL-IS peptide were used for data regression. Data were collected and processed using AB Sciex Analyst® software (version 1.5.1). Individual standard curves were constructed with the ratios of peak areas of the selected peptide for each analyte over those of the SIL-IS and using a 1/concentration2 weighted linear least-squares regression. The concentrations of the QCs and unknown samples were calculated for each analyte.
ELISA Method for Total mAb Determination
Rat plasma samples were also analyzed using an ELISA method. The immunocapture reagent was the same human anti-Fc clone as that used for the LC-MS/MS method. The detector anti-human Fc antibody was of a different epitope (clone 1.21.1 from Amgen, Thousand Oaks, CA), which was conjugated to horseradish peroxidase. Samples were diluted 1:10 or 1:50 in rat plasma and then further diluted 1:20 with the assay buffer before assay (7).
Detection of Anti-drug Antibody (ADA) Presence by Electrochemiluminescence ELISA
The ADA response as signal/noise over the negative control (pooled normal rat serum) was determined and the response specificity assessed according to Bautista et al. case study #3 (16). Briefly, a microtiter plate was coated with 1 μg/ml of the individual mAb antigen in PBS overnight at 4°C. The plate was washed and blocked. Samples were pre-incubated 15 min at ambient temperature at 200-fold dilution into each of three tubes containing: (a) assay buffer (ADA detection), (b) 50 μg/ml of the mAb antigen (to confirm ADA presence with signal depletion by the antigen), or (c) 50 μg/ml of an irrelevant human IgG2 mAb (to confirm specificity). One hundred microliters of each pre-incubated sample were then added to the plate. After incubating for 3 h at ambient temperature, the plate was washed, and the rabbit anti-rat IgG-ruthenium conjugate (Amgen, Inc.) was added. The plate was incubated for 1.5 h and washed. The signal was developed with 2× Read Buffer T (Meso Scale Discovery, Gaithersburg, MD) and immediately read with a Sector Imager 6000 (Meso Scale Discovery).
PK Studies of mAb in Rat
Plasma samples were collected from Sprague–Dawley rats after dosing subcutaneously with a combined solution of the four mAbs (cassette-dosing) or individually with each mAb (discrete-dosing) at 5 mg/kg according to a protocol approved by the Institutional Animal Care and Use Committee of Amgen Inc. The samples were frozen and stored at −70°C until analysis. The same set of PK study samples were analyzed by LC-MS/MS and ELISA.
RESULTS
Method Development
Choice of Surrogate Peptides in the CDR at Similar Location
A unique surrogate peptide for each mAb was selected at a similar complementarity determining region (CDR) location; a single-signature peptide from the same CDR of the SILαDA IS was used as the IS (SIL-IS). Sequence alignment and in silico analysis of the four mAb sequences as shown in Fig. 2 allowed the identification of the unique tryptic peptides that could be used. MS/MS experiments of these peptides confirmed the peptide identities and specificity without cross-talk, and the MS ion transitions were chosen based on signal intensity. The peptide sequences and the MS/MS transitions for the four mAbs and the common SIL-IS are listed in Table I.
Fig. 2.

Sequence alignment and selection of unique tryptic peptides in the CDR in the light chains of four IgG2
Optimization of Immunoaffinity Extraction and Tryptic Digestion
The previous method (7) was modified to incorporate on-bead digestion to improve yield and increase sensitivity, which allowed the use of a smaller sample volume. The immunocapture was performed using 25 μl rat plasma with 50 μl of b-Ab35-coated magnetic beads. The amount of magnetic beads per sample were confirmed by LC-MS/MS to have sufficient binding capacity for the calibration concentration range of four mixed mAbs with an upper limit of quantification of 15 μg/ml each (60 μg/ml total). After immunocapture, in contrast to the previously described method, the captured analyte mAb were not eluted from the beads with organic solvent at low pH. The tryptic peptides were desalted and enriched using solid-phase extraction after the on-bead digestion as described in the “Experimental” section.
Method Qualification
Sensitivity and Selectivity
Representative chromatograms of the control blank, lower limits of quantification (LLOQ), and IS are shown in Fig. 3. Peptide LLI of αDA was five times more sensitive than LIY of αDB, even though the former only differs by one additional Leu. On the other hand, peptide LIY of αDB and LIY of αK had similar sensitivity while they differed by one amino acid. As a result, analytes αDB and αK were observed to have LLOQs of 0.5 μg/ml (Fig. 3a). The stronger ion signal of the unique surrogate peptides of αDA and mAC resulted in greater sensitivity with LLOQs of 0.1 μg/ml (Fig. 3b).
Fig. 3.
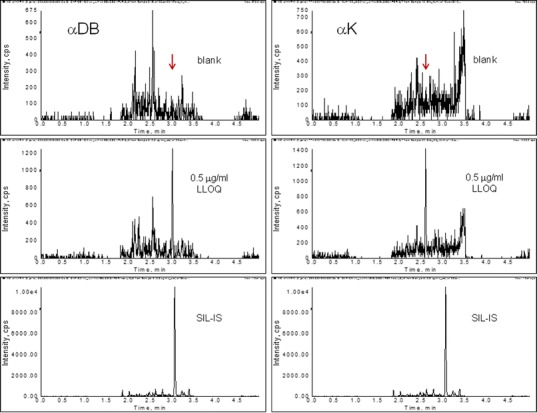
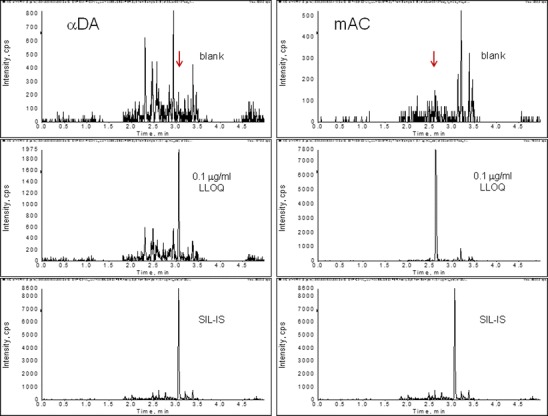
Typical chromatograms demonstrating sensitivity. Top panels: blank control plasma; middle panels: LLOQ of the analyte; bottom panels: SIL-IS peptide. Y-axis: Signal intensity of the corresponding ion transitions monitored for the signature peptide of each analyte in the control and LLOQ panels, or that of the SIL-IS peptide. a Sensitivity of αDB and αK of 0.5 μg/ml
Selectivity was evaluated in six different lots of control rat plasma. Responses at each of the surrogate peptide retention times of these samples were compared with the response of the LLOQ samples at 0.1 or 0.5 μg/ml (n = 6). No response observed in the control matrix was more than 20% of the mean response at the LLOQ.
Linearity, Accuracy, and Precision
A method qualification run was performed. It consisted of mixed standard calibrators at concentrations of 0.10, 0.25, 0.50, 1.0, 2.5, 5.0, 10.0, and 15.0 μg/ml of each mAb to accommodate two standard curve ranges of 0.5–15 and 0.1–15 μg/ml, and four QC levels at 0.3, 1.5, 6.0, and 12.0 μg/ml of each mAb. Two sets of standards were used to bracket the six sets of QCs in the run. Linearity was observed with all the back-calculated concentrations being within the 20% acceptance criteria and correlation coefficients (R2) being greater than 0.99. The slope of αDB and αK was 0.355 and 0.344, respectively, which reflected the low sensitivity of the surrogate peptides for these compounds. The highest slope was observed with mAC at 3.40, which reflected the highest ionization efficiency of the surrogate peptide for mAC. The slope of αDA was 1.47.
QC accuracy and precision data were obtained from six replicates at each QC concentration as shown in Table II. The data for the αDA analyte/SIL-αDA IS peptide pair served as the ideal case for correction by a SIL-mAb IS with identical structure to the analyte. As expected, good assay precision (%CV) and accuracy (%Bias) of αDA were observed to be within 10% as shown in Table II. On the other hand, QC accuracy and precision of mAC, αDB, and αK were all within the 20% acceptance criteria, indicating that the use of the analog peptide SIL-IS situated at the similar CDR location provided acceptable IS correction.
Table II.
Within-Batch Accuracy and Precision of the Mixed QCs Containing Four mAbs
| mAC | αDA | αDB | αK | |||||
|---|---|---|---|---|---|---|---|---|
| %CV | %Bias | %CV | %Bias | %CV | %Bias | %CV | %Bias | |
| QC 0.3 | 8.09 | 6.50 | 4.08 | 4.89 | NA | NA | NA | NA |
| QC 1.5 | 7.93 | 7.44 | 5.10 | 5.78 | 9.55 | 0.889 | 17.7 | 2.78 |
| QC 6 | 9.03 | 0.0833 | 6.59 | 0.0556 | 6.43 | 0.944 | 6.66 | 5.94 |
| QC 12 | 11.2 | −5.94 | 2.40 | −8.19 | 5.23 | −7.78 | 10.4 | 1.53 |
Data from six replicates of QCs at levels of 0.3, 1.5, 6, and 12 μg/ml of each mAb
CV coefficient of variation, αD anti-dinitrophenol (clone A or B), αK anti-KLH, NA not applicable
Method Application
Analysis of Samples from Rats after Discrete Dosing
Aliquots from the same set of samples from rats dosed with each mAb (N = 3 per group) were subjected to ELISA and LC-MS/MS bioanalysis. Table III shows the PK parameters of the LC-MS/MS method in comparison to those of ELISA for each mAb in individual rats. The occurrence of ADA was indicated in each group by the lower area under curve (AUC0-inf) and higher clearance (CL) (17). Samples from the last two time points (840 and 1,008 h) were then tested for ADA against the corresponding mAb. The ADA results in Table III show that in general ADA were present in rats with lower AUC0-inf and higher CL, and the occurrence was variable among the rats dosed with different mAbs. The concentrations of the four mAbs analyzed by LC-MS/MS and ELISA correlated well in a correlation plot (Fig. 4). The slope was near unity (1.06), and the R2 was 0.954 from a data of 121 quantifiable samples.
Table III.
Comparison of LC-MS/MS Versus ELISA Results from Discrete-Dosed Rats
| AUC0-inf, mg h/ml | CL, ml/h/kg | ADA test-positive/negative | ||||
|---|---|---|---|---|---|---|
| LC-MS | ELISA | LC-MS | ELISA | 804 h | 1,008 h | |
| αDB | ||||||
| Rat #1 | 33.6 | 37.1 | 0.149 | 0.135 | − | − |
| Rat #2 | 44.5 | 41.6 | 0.112 | 0.120 | − | − |
| Rat #3 | 26.3 | 29.0 | 0.190 | 0.173 | − | + |
| αDA | ||||||
| Rat #1 | 7.88 | 14.8 | 0.635 | 0.337 | + | + |
| Rat #2 | 28.6 | 33.4 | 0.175 | 0.150 | − | − |
| Rat #3 | 18.1 | 16.3 | 0.276 | 0.306 | − | − |
| αK | ||||||
| Rat #1 | 28.2 | 25.6 | 0.177 | 0.195 | − | − |
| Rat #2 | 8.17 | 7.58 | 0.612 | 0.659 | + | + |
| Rat #3 | 5.62 | 5.97 | 0.889 | 0.837 | + | + |
| mAC | ||||||
| Rat #1 | 10.2 | 9.48 | 0.493 | 0.528 | ND | ND |
| Rat #2 | 33.2 | 39.6 | 0.146 | 0.126 | − | − |
| Rat #3 | 10.0 | 11.1 | 0.498 | 0.450 | ND | ND |
AUC area under curve, CL clearance, ADA anti-drug antibodies, ND not done due to insufficient sample volumes, LC-MS liquid chromatography-mass spectrometry, ELISA enzyme-linked immunosorbent assay, αK anti-KLH, αD anti-dinitrophenol (clone A or B)
Each group of three rats was dosed individually by subcutaneous injection of 5 mg/kg with one of the mAbs
Fig. 4.
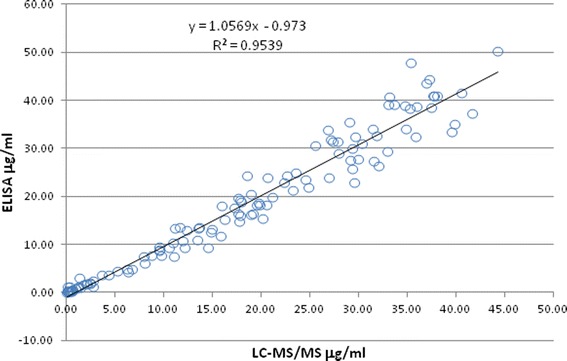
Correlation plot of LC-MS/MS to ELISA results from discrete-dosing
Analysis of Samples from Rats after Cassette Dosing
The cassette-dosed samples were analyzed by ELISA and LC-MS/MS methods. For the LC-MS/MS method a single run produced four sets of data, one for each mAb using its own unique peptide and the SIL-IS peptide LLI. On the other hand, the non-specific ELISA could only quantify the total mAbs.
Figure 5 shows the PK profiles of each of the three rats simultaneously dosed with all four mAbs. The presence of ADA of each rat was determined against each mAb antigen at the last two time points. Samples from both time points were found to be positive for rats #1 and 3 and negative for rat #2 against the CDR of all four mAbs and not the Fc region. The presence of ADA also coincide with a decrease in concentrations at the late time points for rats #1 and 3. This is in agreement with the general observation that the formation of the immune complex may lead to higher CL of mAb drugs (17,18). The formation of immune complex of the mAbs with ADA probably caused the increases in CL of rat #3 over that of #2 as shown in Table IV. This decrease in PK concentration also occurred in rat #1 but to a lesser extent (left panel of Fig. 5). While the absorption phase and Cmax values were similar for the three rats, the higher CL of rat #1 and #3 resulted in lower exposures (AUC0-inf).
Fig. 5.
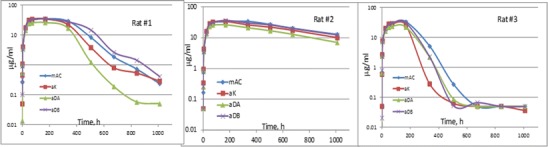
PK profiles of individual rats simultaneously dosed with four mAbs. The concentrations of each mAb were simultaneously determined by the LC-MS/MS method in a single analytical run. For BQL values, the corresponding LLOQ/2 was used
Table IV.
PK Parameters of Four mAbs after Cassette Dosing
| Compound | C max | CL | AUC0-inf |
|---|---|---|---|
| (μg/ml) | (ml/h/kg) | (mg h/ml) | |
| Rat #2 (ADA-negative) | |||
| mAC | 34.7 | 0.153 | 32.7 |
| αDB | 36.6 | 0.164 | 30.5 |
| αK | 34.0 | 0.191 | 26.2 |
| αDA | 26.4 | 0.258 | 19.4 |
| Rat #1 (ADA-positive) | |||
| mAC | 35.0 | 0.358 | 14.0 |
| αDB | 34.2 | 0.334 | 15.0 |
| αK | 33.9 | 0.428 | 11.7 |
| αDA | 25.8 | 0.599 | 8.35 |
| Rat #3 (ADA-positive) | |||
| mAC | 33.7 | 0.676 | 7.40 |
| αDB | 31.4 | 0.769 | 6.51 |
| αK | 29.0 | 0.948 | 5.28 |
| αDA | 23.6 | 0.992 | 5.04 |
AUC area under curve, CL clearance, αD anti-dinitrophenol (clone A or B), αK anti-KLH, ADA anti-drug antibodies
Three rats were each administered by subcutaneous injection at 5 mg/kg of a combined solution of the four mAbs. The samples were analyzed by LC-MS/MS in a single run. The presence of ADA of each rat was determined against each mAb antigen using the last two time points at 840 and 1,008 h. Samples from both time points were found to be positive for rats #1 and 3 and negative for rat #2 against the CDR of all four mAbs and not the Fc region
Table IV shows the PK parameters of the four mAbs in the cassette-dosed rat. The LC-MS/MS data from cassette-dosing of rat #2 that were ADA-negative showed that mAC and αDB have higher exposure and lower CL than the other two mAbs. It is interesting to observe that a similar trend also existed in the two ADA-positive rats.
Comparison of LCMS Results to Those of ELISA
The ELISA method employed the same anti-huFc clone as the capturing agent as the LC-MS/MS method, and another anti-huFc clone was used for detection. For the cassette-dosed samples, the ELISA therefore quantified the total amount of the four mAbs. To compare the LC-MS/MS results with those of the ELISA, the sum of the individual mAb concentrations was calculated for the total mAbs. Figure 6 shows the comparative PK profiles of each cassette-dosed rat from LC-MS/MS and ELISA methods. Only minor differences were observed at the late time points after 840 or 336 h of rats #1 and 3, respectively.
Fig. 6.
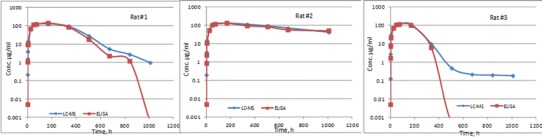
Comparison of PK profiles of total mAbs measured by LC-MS/MS and ELISA. The ELISA concentrations at 1,008 h of rat #1 and last four time points of rat #3 were BQL. The LC-MS/MS concentrations were the sum of the mAb concentrations shown in Fig. 5; the concentrations at the last three time points of rat #3 were BQL
DISCUSSIONS
Method Sensitivity, Specificity, and Robustness
In addition to serum albumin, endogenous IgGs are among the most abundant proteins in plasma. The anti-human Fc antibody (clone 35) does not bind to IgGs from other species and has been proven to be highly efficient in extracting human mAbs from animal biomatrices while removing endogenous plasma proteins to minimize matrix interferences (7,8). The immunoaffinity sample clean-up as well as the in silico choice of the unique peptides contributed to method specificity. The background of peptides from endogenous biomatrix did not interfere with the unique peptides of the analyte as shown in Fig. 3.
The amount of biotinylated anti-human Fc b-Ab35 was optimized to saturate the streptavidin on the bead surface and provided excess binding capacity for the target mAbs. The on-bead digestion approach provided more than an eightfold increase in sensitivity, allowing the use of only ¼ of the sample volume of the previous method. It also circumvented the elution and vacuum dry-down steps used in previous methods (7), which simplified the analytical workflow.
In addition to the specificity against the biological matrix, simultaneous analysis of multiple mAbs requires the selection of surrogate peptides that are specific to each mAb. We strategically selected the surrogate peptides of the analyte antibodies and the IS antibody (SIL-αDA-IS) that are situated at the similar CDR location for similar digestion behavior. MS/MS experiments confirmed the peptide identities and specificities and indicated an absence of cross-talk. The SIL-IS did not interfere with the unlabeled peptide of the αDA analyte or any of the other three mAb analyte peptides as shown in Fig. 3. Therefore, the specificity of the unique peptides enabled a simultaneous multiple-analyte assay, which would not be feasible with ELISA at the early stage of mAb development due to the lack of specific binding reagents.
We showed that the whole mAb IS with uniformly stable isotope labels was important for accurate protein quantitative analysis by LC-MS/MS to track the multiple analytical steps (7). For the single-analyte method, the same pairs of surrogate analyte peptide and SIL-IS peptide were used, and method robustness was demonstrated in the application to eight mAbs (7). For the multiple analyte method, only the αDA quantification was from the same surrogate analyte/SIL-IS peptide pair. For the quantification of the other three mAbs, the SIL-IS peptide represented an analog peptide. The method qualification data demonstrated adequate linearity, accuracy, and precision under the conditions employed. In addition, the analytical run of the cassette dosed samples also demonstrated method robustness by the QC accuracy, as well as the data agreement with those of the ELISA from both discrete and cassette-dosed samples.
Figure 6 shows the PK profiles of the total mAbs of the ELISA and the sum of LC-MS/MS concentrations from the cassette-dosed animals. There was an overall agreement except that the LC-MS/MS results were higher at the late time points after 840 or 336 h of rats #1 and 3, respectively. The overall agreement demonstrated the method applicability and robustness. The minor differences at the late time points might indicate interference of ADA on the ELISA, while the LC-MS method would measure total (bound and unbound to ADA) concentration. The below-quantitative-limit (BQL) concentrations at the late time points (such as the last three time points of rat #3) were plotted as half of the LLOQs of the ELISA or LC-MS/MS method in Fig. 6. It should be noted that the ELISA method was more sensitive than the LC-MS/MS method—the LLOQ of ELISA was 0.01 μg/ml, while those of the LC-MS/MS were 0.5 and 0.1 μg/ml.
Potential Advantage of Cassette Dosing
Cassette-dosing offers the advantage of minimizing biological variability between rats and to reduce animal use. Good agreement was generally observed between both concentrations and PK data generated by either ELISA or LC-MS/MS methods. Actual comparison of PK parameters among the mAb compounds was complicated in the presence of ADA in the study. Since ADA can randomly and frequently occur in preclinical subcutaneous dosing, the use of intravenous instead of subcutaneous dosing and/or non-human primates instead of rodent species may diminish the ADA issue allowing better PK interpretation (17).
Our first cassette-dosing experiment achieved the goal of animal reduction, using only three rats compared with 12 for discrete-dosing. It also demonstrated the efficiency in time- and resource-saving of performing only one analytical run instead of four for sample analysis.
It should be noted that cassette pooling of small-molecule drugs has been used to improve analytical efficiency without the risk of drug–drug interaction (19). However, there is still little published experience of pooling for biologics in preclinical species, and the overall reliability of this approach still needs to be further demonstrated, particularly given the variable formation of ADA that can occur in animal species. The incidence of ADA formation was variable in the discrete-dosed animals and relatively high for the cassette-dosed group (two out of three). It is interesting to note that the ADA-positive animals showed positive responses against all four mAbs.
Specific Issues to Consider on mAb Cassette Dosing
There are three major issues for cassette-dosing of small-molecule drugs that may lead to inaccurate data and wrong rank ordering (9,10,15,20). Here, we discuss those issues as well as specific considerations of cassette-dosing of mAb biotherapeutics:
-
Drug–drug interaction (DDI). Unknown reactions of the small-molecule candidates with the CYP450 enzymes and transporters may result in different PK parameters with cassette-dosing than those of discrete dosing. DDI can be minimized by limiting the cassette size to five or less (10,13,21) or eliminated by cassette pooling instead of dosing (19).
The PK behavior of mAb is unlikely to be affected by CYP450 enzymes or transporters and the risk of DDI in cassette dosing is expected to be low. However, the interaction of the mAbs toward a specific target may be affected by the other candidates that react with the same target. Drug–target interaction is expected for candidates that follow the non-linear target-mediated drug disposition at non-saturating doses (22).
Solubility of combined dosing formulation. Many small-molecule candidates are hydrophobic with low solubility. It would be difficult to prepare a combined dosing formulation for these molecules. For mAbs, this should not be a major issue due to their hydrophilic nature and relative ease in dosing solution preparation for injection.
Excessive time to set up LC-MS/MS method for large cassette size and cross-talk of candidate ions in MS. This issue has been greatly diminished with the advances in high-resolution MS instruments that offer superior selectivity and sensitivity (23–26). It is not an issue with mAbs with proper choice of signature peptides as illustrated in the method presented here, and the set up of even complex LC-MS/MS is relatively rapid compared with the generation, selection, and characterization of specific reagents required for ligand binding reagents.
Immunogenicity. Data from animals with ADA should not be used for PK comparison due to the unknown amount of ADA and their effect on CL. The experiment described in this paper was heavily affected by ADA; future cassette dosing experiments would need to reduce the incidence of ADA to manageable levels, for example, by intravenous dosing and use of appropriate species. An important question is whether one immunogenic candidate in the cassette may cause immune response to an otherwise non-immunogenic candidate. Such complication could occur with cassette-dosing. Immunogenicity will be an important issue to be investigated in future experiments with combined dosing of novel biologic compounds. This could be further investigated by dosing a highly immunogenic antigen and one that is relatively non-immunogenic in the preclinical species.
CONCLUSIONS
We successfully applied a strategy for simultaneous analysis of multiple mAbs by selecting the surrogate peptides that are representative and specific to each of the individual mAb. The unique signature peptide of four mAbs and the SIL-IS were chosen from a similar locale at the CDR with good MS/MS signals. The on-bead tryptic digestion minimized the variability in enzyme hydrolysis. The multi-analyte LC-MS/MS method efficiently provided accurate and precise concentration data for each analyte as well as the total sum of mAbs. Applicability was demonstrated for samples from rats simultaneously dosed with four mAbs. The data were compared with those of discrete dosing. We have considered the issues of cassette-dosing that are special for mAb compounds. The pros and cons of cassette-dosing should be further studied with other mAbs and species. The multiple-analyte LC-MS/MS method can also be applied to other mAb-related constructs development, such as the quantification of intact and metabolites of fusion proteins and functional regions of bispecific mAbs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 192 kb)
Acknowledgment
We thank Agi Hamburger and Richele Bruno on their contribution on the preparation of the whole molecule SIL-IS; Marcus Soto and Kevin Salyers for conducting the preclinical PK study; and Gary Skiles and Mark Rose for their encouragement and support on this research project. We also thank Gary Skiles and Michael Hall for critical review of this manuscript.
References
- 1.Dubois M, Becher F, Herbet A, Ezan E. Immuno-mass spectrometry assay of EPI-HNE4, a recombinant protein inhibitor of human elastase. Rapid Comm Mass Spectrom. 2007;21(3):352–358. doi: 10.1002/rcm.2844. [DOI] [PubMed] [Google Scholar]
- 2.Dubois M, Fenaille F, Clement G, Lechmann M, Tabet JC, Ezan E, et al. Immunopurification and mass spectrometric quantification of the active form of a chimeric therapeutic antibody in human serum. Anal Chem. 2008;80(5):1737–1745. doi: 10.1021/ac7021234. [DOI] [PubMed] [Google Scholar]
- 3.Ezan E, Dubois M, Becher F. Bioanalysis of recombinant proteins and antibodies by mass spectrometry. Analyst. 2009;134(5):825–834. doi: 10.1039/b819706g. [DOI] [PubMed] [Google Scholar]
- 4.Hagman C, Ricke D, Ewert S, Bek S, Falchetto R, Bitsch F. Absolute quantification of monoclonal antibodies in biofluids by liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(4):1290–1296. doi: 10.1021/ac702115b. [DOI] [PubMed] [Google Scholar]
- 5.Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K, Lehmann N, et al. Towards absolute quantification of therapeutic monoclonal antibody in serum by LC-MS/MS using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal Chem. 2008;80(11):4200–4207. doi: 10.1021/ac800205s. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Manuilov AV, Chumsae C, Babineau ML, Tarcsa E. Quantitation of a recombinant monoclonal antibody in monkey serum by liquid chromatography-mass spectrometry. Anal Biochem. 2011;414(1):147–153. doi: 10.1016/j.ab.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Ortiz R, Tran L, Hall M, Spahr C, Walker K, et al. General LC-MS/MS method approach to quantify therapeutic monoclonal antibodies using a common whole antibody internal standard with application to preclinical studies. Anal Chem. 2012;84(3):1267–1273. doi: 10.1021/ac202792n. [DOI] [PubMed] [Google Scholar]
- 8.Hall MP, Gegg C, Walker K, Spahr C, Ortiz R, Patel V, et al. Ligand-binding mass spectrometry to study biotransformation of fusion protein drugs and guide immunoassay development: strategic approach and application to peptibodies targeting the thrombopoietin receptor. AAPS J. 2010;12(4):576–585. doi: 10.1208/s12248-010-9218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayliss MK, Frick LW. High-throughput pharmacokinetics: cassette dosing. Curr Opin Drug Discov Dev. 1999;2(1):20–25. [PubMed] [Google Scholar]
- 10.Manitpisitkul P, White RE. Whatever happened to cassette-dosing pharmacokinetics? Drug discov today. 2004;9(15):652–658. doi: 10.1016/S1359-6446(04)03137-X. [DOI] [PubMed] [Google Scholar]
- 11.Korfmacher WA, Cox KA, Ng KJ, Veals J, Hsieh Y, Wainhaus S, et al. Cassette-accelerated rapid rat screen: a systematic procedure for the dosing and liquid chromatography/atmospheric pressure ionization tandem mass spectrometric analysis of new chemical entities as part of new drug discovery. Rapid Comm Mass Spectrom. 2001;15(5):335–340. doi: 10.1002/rcm.235. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Chang J, Gordon WP, Isbell J, Zhou Y, Tuntland T. Snapshot PK: a rapid rodent in vivo preclinical screening approach. Drug discov today. 2008;13(7–8):360–367. doi: 10.1016/j.drudis.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Nagilla R, Nord M, McAtee JJ, Jolivette LJ. Cassette dosing for pharmacokinetic screening in drug discovery: comparison of clearance, volume of distribution, half-life, mean residence time, and oral bioavailability obtained by cassette and discrete dosing in rats. J Pharm Sci. 2011;100(9):3862–3874. doi: 10.1002/jps.22525. [DOI] [PubMed] [Google Scholar]
- 14.Smith NF, Raynaud FI, Workman P. The application of cassette dosing for pharmacokinetic screening in small-molecule cancer drug discovery. Mol Cancer Ther. 2007;6(2):428–440. doi: 10.1158/1535-7163.MCT-06-0324. [DOI] [PubMed] [Google Scholar]
- 15.White RE, Manitpisitkul P. Pharmacokinetic theory of cassette dosing in drug discovery screening. Drug Metabol Dispos. 2001;29(7):957–966. [PubMed] [Google Scholar]
- 16.Bautista AC, Salimi-Moosavi H, Jawa V. Universal immunoassay applied during early development of large molecules to understand impact of immunogenicity on biotherapeutic exposure. AAPS J. 2012 Sep 1;14(4)843-849. [DOI] [PMC free article] [PubMed]
- 17.Ponce R, Abad L, Amaravadi L, Gelzleichter T, Gore E, Green J, et al. Immunogenicity of biologically-derived therapeutics: assessment and interpretation of nonclinical safety studies. Regul Toxicol Pharmacol. 2009;54(2):164–182. doi: 10.1016/j.yrtph.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: impact on PK/PD and efficacy. AAPS J. 2012;14(2):296–302. doi: 10.1208/s12248-012-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hop CE, Wang Z, Chen Q, Kwei G. Plasma-pooling methods to increase throughput for in vivo pharmacokinetic screening. J Pharm Sci. 1998;87(7):901–903. doi: 10.1021/js970486q. [DOI] [PubMed] [Google Scholar]
- 20.Christ DD. Cassette dosing pharmacokinetics: valuable tool or flawed science? Drug Metabol Dispos. 2001;29(7):935. [PubMed] [Google Scholar]
- 21.He K, Qian M, Wong H, Bai SA, He B, Brogdon B, et al. N-in-1 dosing pharmacokinetics in drug discovery: experience, theoretical and practical considerations. J Pharm Sci. 2008;97(7):2568–2580. doi: 10.1002/jps.21196. [DOI] [PubMed] [Google Scholar]
- 22.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–532. doi: 10.1023/A:1014414520282. [DOI] [PubMed] [Google Scholar]
- 23.Hager JW, Le Blanc JC. High-performance liquid chromatography-tandem mass spectrometry with a new quadrupole/linear ion trap instrument. J Chrom. 2003;1020(1):3–9. doi: 10.1016/S0021-9673(03)00426-6. [DOI] [PubMed] [Google Scholar]
- 24.Hopfgartner G, Varesio E, Tschappat V, Grivet C, Bourgogne E, Leuthold LA. Triple quadrupole linear ion trap mass spectrometer for the analysis of small molecules and macromolecules. J Mass Spectrom. 2004;39(8):845–855. doi: 10.1002/jms.659. [DOI] [PubMed] [Google Scholar]
- 25.Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Graham Cooks R. The Orbitrap: a new mass spectrometer. J Mass Spectrom. 2005;40(4):430–443. doi: 10.1002/jms.856. [DOI] [PubMed] [Google Scholar]
- 26.Kay RG, Barton C, Velloso CP, Brown PR, Bartlett C, Blazevich AJ, et al. High-throughput ultra-high-performance liquid chromatography/tandem mass spectrometry quantitation of insulin-like growth factor-I and leucine-rich alpha-2-glycoprotein in serum as biomarkers of recombinant human growth hormone administration. Rapid Comm Mass Spectrom. 2009;23(19):3173–3182. doi: 10.1002/rcm.4237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 192 kb)


