Abstract
Simvastatin (SIM), a drug commonly administered for the treatment of hypercholesterolemia, has been recently reported to induce bone regeneration/formation. In this study, we investigated the properties of hydrogel composed of gelatin–poly(ethylene glycol)–tyramine (GPT) as an efficient SIM delivery vehicle that can trigger osteogenic differentiation. Sustained delivery of SIM was achieved through its encapsulation in an injectable, biodegradable GPT-hydrogel. Cross-linking of the gelatin-based GPT-hydrogel was induced by the reaction of horse radish peroxidase and H2O2. GPT-hydrogels of three different matrix stiffness, 1,800 (GPT-hydrogel1), 5,800 (GPT-hydrogel2), and 8,400 Pa (GPT-hydrogel3) were used. The gelation/degradation time and SIM release profiles of hydrogels loaded with two different concentrations of SIM, 1 and 3 mg/ml, were also evaluated. Maximum swelling times of GPT-hydrogel1, GPT-hydrogel2, and GPT-hydrogel3 were observed to be 6, 12, and 20 days, respectively. All GPT-hydrogels showed complete degradation within 55 days. The in vitro SIM release profiles, investigated in PBS buffer (pH 7.4) at 37°C, exhibited typical biphasic release patterns with the initial burst being more rapid with GPT-hydrogel1 compared with GPT-hydrogel3. Substantial increase in matrix metalloproteinase-13, osteocalcin expression levels, and mineralization were seen in osteogenic differentiation system using MC3T3-E1 cells cultured with GPT-hydrogels loaded with SIM in a dose-dependent manner. This study demonstrated that controlled release of SIM from a biodegradable, injectable GPT-hydrogel had a promising role for long-term treatment of chronic degenerative diseases such as disc degenerative disease.
KEY WORDS: hydrogel, MC3T3-E1, MMP-3, osteocalcin, osteogenesis, simvastatin
INTRODUCTION
Degenerative bone and cartilaginous diseases such as rheumatoid arthritis, degenerative disc disease (DDD), and osteoporosis have been gradually increasing in prevalence (1). DDD is estimated to affect approximately 80% of all adults at some point during their lifetime; thus, there is obvious need for new therapeutics to restore degenerated disc tissue (1). The primary treatment of DDD is with nonoperative administration of nonsteroidal anti-inflammatory medications. When nonoperative medical management fails to control the pain, surgical intervention becomes the option. Biological treatments (i.e., with protein growth factors or gene therapy) to promote bone matrix repair and restoration of physiological function have also been explored (2,3). Although many treatments are available, a definitive therapy for DDD at the cellular level has yet to be developed.
Simvastatin (SIM), a hydroxymethylglutaryl-coenzyme A reductase inhibitor, has been commonly used to treat hyperlipidemic patients (4). It has also been reported that SIM can efficiently induce osteogenic differentiation by enhancing bone morphogenic proteins (BMPs), alkaline phosphatase, and osteocalcin (OC) expression (5,6).
Although many reports have described the promising role of SIM for degenerative bone disease treatment (7–9), this has yet to be realized in practice. A significant challenge has been the development of formulations that provide sufficient long-term controllable release kinetics of the drug while simultaneously also providing mechanical support to the diseased joint.
Hydrogel systems have attracted increasing attention as tissue engineering scaffolds and for the sustained release of drugs (10–12). In particular, the use of hydrogel as scaffolds for tissue engineering is promising because of similarities in physical properties with natural tissues (13–15). Most hydrogel, however, require surgical implantation which often results in tissue irritation and damage (16). The development of injectable in situ gel-forming polymeric hydrogel systems, therefore, is of great benefits. Advantages of injectable hydrogel systems for biomedical application include: (1) high biocompatibility; (2) easy loading of bioactive drugs and chemicals by simple mixing; (3) easy implantation through a facile, minimally invasive process; and (4) the ability to fill irregular defect sites with drug loaded hydrogel (17–21). Various hydrogels formed through in situ crosslinking by chemical or enzymatic reactions have been reported and used (22,23). Regardless of mechanism, it is essential that the hydrogel form within a narrow range of physiologically acceptable temperatures and at a sufficiently rapid rate at the implant site within the body (24,25). Delivery of SIM by a hyaluronic acid (HA)-based hydrogel has been recently introduced (11). Nevertheless, HA-based hydrogel still showed limitations, as well as weak mechanical properties. Hence, the need for developing a hydrogel system that possesses proper mechanical characteristics and controllable release kinetics to achieve delivery of the most optimal concentration of SIM continues.
We previously described the development of a novel gelatin-based, in situ crosslinkable gelatin-poly(ethylene glycol)-tyramine (GPT) hydrogel as an injectable material for tissue regeneration (26). Gelation was found to occur rapidly through an enzymatic oxidative coupling reaction—catalyzed by the reaction of horse radish peroxidase (HRP) and hydrogen peroxide (H2O2) (26). This enzymatic reaction was a well-known method for the preparation of biocompatible in situ forming hydrogels for tissue engineering and efficient drug delivery (24,27). In addition, the physicochemical characteristics, gelation time, swelling time, and degradation time were readily controllable. Furthermore, this hydrogel possessed high biocompatibility and bioactivities. These characteristics implied that this in situ GPT-hydrogel could be an ideal system for SIM delivery and tissue engineering for the purpose of bone/disc regeneration.
In our previous work, we demonstrated 2 weeks after a single intradiscal administration of the SIM formulation to the stab injury-induced degenerated interverbral disc area of a rat, significant osteogenic effects were observed (28). Based on these results, we hypothesized that by placing a biodegradable hydrogel containing sustain-released SIM to the injured disc area, does not necessarily relates to the duration of drug release, would significantly augment the drug-induced disc regeneration. At the same time, the hydrogel drug carrier would gradually degrade thereby not affecting the disc healing process. Hence, the primary purpose and objective of the current research was to synthesize such a SIM delivery system, characterize this system in vitro under relevant cell culture environment, and then select the most optimum system for the next phase in vivo animal studies.
Long-term treatment of DDD by injection of GPT-hydrogel into the disc area, which is filled with cerebrospinal fluid (CSF), however, requires a hydrogel that possesses a degradation profile longer than 2 weeks accompanied with a sustained SIM release. The CSF is a clear solution that contains ions (Na+, K+, Cl−, and HCO3−), white cells (0–5 cells/mm3), proteins (<0.5 g/l), and glucose (2.2–4.4 mmol/l) (29). In terms of the composition of ions, proteins, and cytokines, CSF is very similar to an in vitro solution that contains both PBS and serum. In line with this consideration, we investigated the in vitro release characteristics of the GPT-loaded hydrogel in PBS buffer and serum condition.
In this report, we investigated the potential for osteogenic differentiation through the sustained delivery of SIM from GPT-hydrogel. The biocompatibility and physicochemical properties of SIM-loaded GPT-hydrogels compared with nonloaded GPT-hydrogels were evaluated both under PBS and serum condition. The effects of delivered SIM on osteogenic differentiation were determined by RT-PCR and Western blot analysis to confirm the expression of fully differentiated osteoblast markers.
MATERIALS AND METHODS
Materials
SIM was purchased from Cayman (Ann Arbor, MI). Alrizarin red-S, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT), and bicinchoninic acid (BCA) protein assay kit were purchased from Sigma (St. Louis, MO). Antibody against matrix metalloproteinase-13 (MMP-13) was purchased from Aviva Systems Biology Corp. (San Diego, CA) and OC and actin antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Enhanced chemiluminescence reagent was purchased from Amersham Biosciences, Inc. (Newbrunswick, NJ). MC3T3-E1 cells were purchased from ATCC (Rockville, MD). Fetal bovine serum (FBS), α-minimal essential medium (α-MEM), trypsin/EDTA, penicillin–streptomycin, trizol reagent, Moloney murine leukemia virus reverse transcriptase (M-MLV RT), oligo(dT)15, and rTaq DNA polymerase were obtained from Gibco-BRL (Carlsbad, CA). Radio-immunoprecipitation assay (RIPA) buffer and Protease Inhibitor Cocktail™ were purchased from Roche Molecular Biochemicals (Indianapolis, IN).
In situ GPT-Hydrogel Formation, Swelling, and Degradation Time
In situ hydrogel was rapidly formed by the enzymatic reaction of HRP with H2O2, as previously reported (30) gelatin–poly(ethylene glycol)–tyramine (GPT) conjugates were kindly provided by Professor KD Park at Ajou University (Suwon, Korea). The same procedure reported previously was followed for in situ GPT-hydrogel formation (30). In brief, a constant polymer concentration of 3 wt.% was used to prepare all GPT-hydrogel in this study. One milliliter of HRP and H2O2 solution were added to two separate tubes each containing 30 mg of GPT polymer. Stock solutions of HRP and H2O2 were prepared in 0.01 M PBS (pH 7.4) at concentrations of 0.01 mg/ml and 0.0125 wt.%, respectively. GPT-hydrogels were prepared at room temperature by gently mixing 200 μL of GPT/HRP solutions (at concentrations of 0.5, 1, 1.25, 2, and 10 μg/ml) and 200 μl of GPT/H2O2 solutions (at weight percent of 0.0038, 0.075, and 0.0125 for GPT-hydrogel1, GPT-hydrogel2, and GPT-hydrogel3, respectively). The mechanical stiffness of the GPT-hydrogels were found to be 1,800 (30% = GPT-hydrogel1), 5,800 (60% = GPT-hydrogel2), and 8,400 Pa (100% of crosslinking = GPT-hydrogel3) depending on the H2O2 concentration.
After GPT-hydrogels formation, 2 ml of 0.01 M PBS (pH 7.4) were added and the stability of GPT-hydrogel determined by weighing after incubation at 37°C for different time period during the first day (0, 1, 2 ,4, 12, and 24 h) and then daily until complete degradation was observed. The supernatant solution was removed at each time point and analyzed for SIM release. Swelling and degradation ratios of GPT-hydrogels were determined according to a previously established method and calculated using the equation below (30):
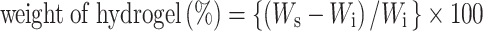 |
where Ws and Wi are the weight of the test and initial GPT-hydrogel samples, respectively. After weighing, fresh PBS solution was added to the GPT-hydrogel samples. All experiments were conducted in triplicate with three independent sets.
In Vitro GPT-Hydrogel Swelling and Degradation Time with SIM Loading
To check the SIM effects on the swelling and gelation time, two different concentrations of SIM (1 and 3 mg/ml) were mixed with GPT/HRP solution first and then mixed with GPT/H2O2 to form SIM-loaded GPT-hydrogel (SIM-GPT-hydrogel). GPT-hydrogel2 was employed to retain the constant gel stiffness, and 30 s was chosen as the gelation time. The gel weight in the vial was measured according to the previously described procedure.
Cytotoxicity of SIM on MC3T3-E1 Cells
MC3T3-E1 cells were maintained in α-MEM containing 10% (v/v) FBS, 100 units of penicillin–streptomycin at 37°C under 5% (v/v) CO2 in humidified atmosphere. To determine the concentration of SIM that did not induce cytotoxicity, MC3T3-E1 cells were cultured with SIM-GPT-hydrogel for 7 days. The concentrations of SIM loaded onto GPT-hydrogel were 0, 0.05, 0.1, 0.5, 1, 3, 5, and 10 mg/ml. After 7 days of treatment, cell proliferation assay was quantified using the MTT assay (30). Briefly, after seeding cells in a 96-well plate and incubating with GPT-hydrogel supernatant for 24 h at 37°C, aliquots containing 100 μl MTT solution (5 mg/ml in DMEM) were added, followed by incubation for another 5 h. MTT-containing medium was then removed, and 200 μl of dimethyl sulfoxide was added to dissolve the formazan of crystals by live cells. Absorbance was measured at 570 nm, and the cell viability (in percent) was calculated according to the following equation (31):
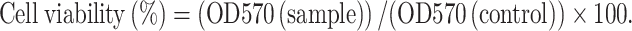 |
Controlled Release Kinetics of SIM
PBS (2 ml) was added to GPT-hydrogel containing SIM (1 and 3 mg/ml) in 4-ml glass vials, and the mixture was incubated at 37°C. Aliquots were removed at designated sampling intervals for analysis, were replaced, and the same amount of fresh PBS was added to the samples to maintain sink conditions. SIM concentration was determined by measuring the absorbance at 262 nm. Data were presented as the percentage of release.
Osteogenic Differentiation of MC3T3-E1 Cells Cultured with GPT-Hydrogel-SIM
The effect of SIM released from GPT-hydrogel-SIM on the cells under osteogenic differentiation was determined using a multiwell insert system (transwell chamber system), as shown in Fig. 1a. Cells were seeded onto the 24-well plates at a density of 3.5 × 105 cells/well. For sustained release of SIM during osteogenic differentiation (14 days), GPT-hydrogel2 was chosen for osteogenic differentiation experiments. A plated insert chamber containing SIM-loaded GPT-hydrogel2 polymer solution (400 μl of GPT-hydrogel2 + 3 mg of SIM) was then added inside a 24-multiwell insert system. Cells were grown for 14 days with osteogenic differentiation medium (DM) being changed every other day. Osteogenic differentiation was induced by culturing MC3T3-E1 pre-osteoblast cells with DM that contains ascorbic acid (50 μg/ml) and β-glycerophosphate (10 mM), which was added after the cells reached confluence for about 14 days (31,32).
Fig. 1.
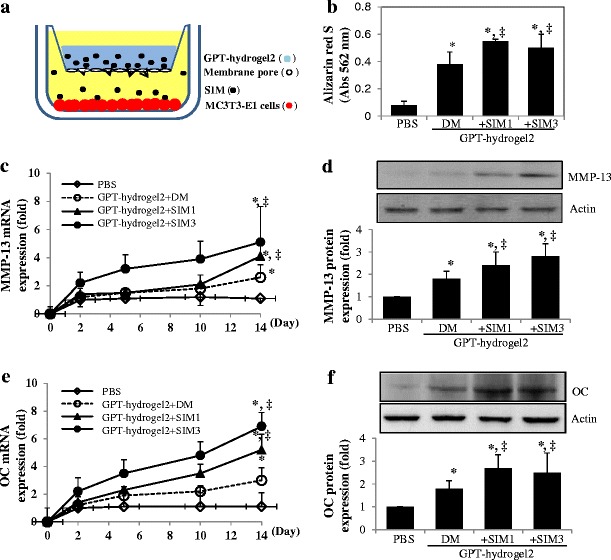
Mineralization and osteogenic marker expression in MC3T3-E1 cells cultured with SIM-loaded GPT-hydrogel2 system (GPT-hydrogel2-SIM). a Experimental scheme of MC3T3-E1 cell culture system using SIM-loaded GPT-hydrogel under osteogenic differentiation. b Bone mineralization effect of SIM released from GPT-hydrogel was evaluated by using the Alizarin red S assay. Expressions of MMP-13 (c, d), OC (e), and mRNA (f) and protein expressions were examined in MC3T3-E1 cells cultured with GPT-hydrogel2 containing two concentrations of SIM (1 and 3 mg/ml) under osteogenic differentiation process using RT-PCR and Western blot analysis, respectively. All the experiments were performed duplicate under three independent sets. *, ‡p < 0.05, the difference was statistically significant, when comparing the experimental groups with PBS-treated control cells (asterisk) or with cells cultured with GPT-hydrogel2 in differentiation medium (DM) without SIM (double dagger; GPT-hydrogel+DM vs. GPT-hydrogel2+SIM1 or GPT-hydrogel2+SIM3). All the experiments were performed in triplicate
This cell culture system containing GPT-hydrogel was subject to subsequent in vitro experiments.
Alizarin Red S Assay
To confirm the mineralization in differentiated MC3T3-E1 cells, 2% alizarin red S was prepared in distilled water and the pH was adjusted to 4.1–4.3 using 0.5% ammonium hydroxide. Differentiated MC3T3-E1 cells were fixed with 10% formalin (15 min), washed, and stained with alizarin red S for 10–15 min. After removal of un-reacted dye, the mineralized nodules were identified as red spots. The red dye was extracted using cetylpyridinium chloride method and then measured for absorbance at 550 nm according to a previously established protocol (33).
Gene and Protein Expressions of MMP-13 and OC in MC3T3-E1 Cells
Total RNA was extracted from MC3T3-E1 cells cultured in GPT-hydrogel containing multi-well insert system (Fig. 1a) using Trizol solution, according to the instructions from manufacturer. First-strand cDNA synthesis was performed by using 1 μg of RNA, oligo(dT)15, and M-MLV RT. Semi-quantitative PCR amplification of cDNAs encoding MMP-13, OC, and actin were carried out using rTaqDNA polymerase. The primer sequences for MMP-13 (494 bp) were established as follows: forward 5′-GCCAAGACCTGAAACTCTGC-3′, and reverse 5′-CTTGCCCCACTTACCAGTGT-3′; for OC (405 bp), forward 5′- CAGGGGCAGACACTGAAAAT-3, and reverse 5′-CCTCCAGCATCCAGTAGCAT-3′. The amplification of actin cDNA fragments (500 bp) was performed in a separate tube to enable semi-quantitative normalization with the following primers: forward 5′-ACC ACA GTC CAT GCC ATC AC-3′, reverse 5′-TCC ACC ACC TGT TGC TGT A-3′. The amplified fragments were analyzed on a 1% agarose gel.
Total protein in MC3T3-E1 cells was extracted using RIPA buffer containing Protease Inhibitor Cocktail™. Protein concentrations were determined with a BCA protein assay kit. Equal quantities of proteins (30 μg) were separated on a sodium dodecyl sulfate-polyacrylamide gel under reducing condition, and were then electrophoretically transferred onto a nitrocellulose membrane. The blots were probed by utilizing the appropriate amount of antibody directed against MMP-13 (1:1,000), OC (1:1,000), and actin (1:3,000), and then following with the corresponding secondary antibody. The level of actin protein expression was used as an internal control. All the band images from RT-PCR and Western blot were quantified using Image J software (National Institutes of Health, Bethesda, MD).
Cytotoxic Effect of the By-Products Derived from GPT-Hydrogel Incubation
MC3T3-E1 cell line was used to study the cytoxicity of the by-products arising from GPT-hydrogel incubation. Aliquots (1 ml) were withdrawn from the incubated GPT-hydrogel2 solution at different time points for a total of 53 days until complete degradation of GPT-hydrogel was observed, and were then added to a 24-well plate containing MC3T3-E1 cells culture. After incubation, cell cytotoxicity was measured using the MTT assay described previously.
Statistical Analysis
All statistical analyses of the experimental results were performed using an SPSS (Version 11.0), and all data were expressed as mean ± SD. Statistically significant difference between the test groups were assessed using the Student’s t test. The level of significance was represented as p < 0.05 (*, ‡) and p < 0.01 (**).
RESULTS
Gelation Time and Swelling Time of GPT-Hydrogels
The gelation time, determined by the vial tilting method, was shown to vary with the HRP and H2O2 concentrations (Fig. 2a). As seen for GPT-hydrogel1, the gelation time decreased from 127 to 6 s as the HRP concentration increased from 0.01 to 10 mg/ml when the polymer concentration (3 wt.%) and H2O2 concentration (0.0038 wt.%), were controlled constant. On the other hand, at HRP concentrations below 2 mg/ml, the gelation time was seen to decrease when increasing the H2O2 concentration from 0.0038 wt.% in GPT-hydrogel1 to 0.0125 wt.% in GPT-hydrogel3 (Fig. 2a).
Fig. 2.
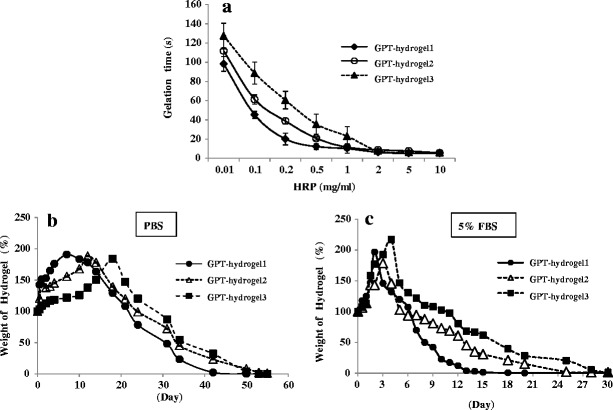
Gelation and swelling time of GPT-hydrogel. In situ GPT hydrogel was rapidly formed via the enzymatic oxidative reaction of HRP with H2O2. a Gelation time was measured in accordance to HRP concentrations at three different concentrations of H2O2 (GPT-hydrogel1, 0.0038 wt.%; GPT-hydrogel2, 0.0075 wt.%; and GPT-hydrogel3, 0.0125 wt.%). b The degree of swelling and degradation time of GPT-hydrogels under PBS condition. Experiments were carried out until a complete degradation of GPT-hydrogels was observed. c The degree of swelling and degradation profile of GPT-hydrogels in the presence of 5% FBS. Experiments were carried out until a complete degradation of GPT-hydrogels was observed. All experiments were conducted in duplicate with three independent sets
The swelling rate and degradation time under different concentrations of HRP and H2O2 in PBS buffer condition were shown in Fig. 2b. The swelling time was estimated by measuring the increase in GPT-hydrogel weight due to water absorption. GPT-hydrogel1, GPT-hydrogel2 and GPT-hydrogel3 reached a maximum swelling ratio, which ranged from 150% to 200%, after 7, 12, and 18 days of incubation, respectively. These polymers, however, were completely degraded after 42, 49, and 53 days of incubation, due to decomposition of crosslinkages within GPT-hydrogel. Obviously, GPT-hydrogel formed with a lower HRP concentration degraded faster than that with a higher HRP concentration (Fig. 2b). Although the time to reach the maximum swelling ratio increased as a function of H2O2 concentration, all GPT-hydrogels displayed a similar degradation pattern.
We examined the degradation profile of GPT-hydrogel under serum condition using 5% FBS solution (Fig. 2c). To determine the maximum concentration of serum that would not interfere with the spectrometric quantification of SIM released from the GPT-hydrogel, we examined the system in PBS buffer containing various concentrations (i.e., 5%, 10%, and 20%) of FBS. Among these serum concentrations, PBS containing 5% FBS was determined to be the maximum concentration providing a sufficient low background absorbance (data not shown).
The degradation of GPT-hydrogels was more rapid in the presence of 5% FBS compared with hydrogels placed in PBS buffer without serum (Fig. 2b). Samples of GPT-hydrogel1, GPT-hydrogel2 and GPT-hydrogel3 reached maximum swelling ratios ranging from 150% to 200% following 2, 3, and 4 days of incubation, respectively, in an FBS environment (Fig. 2c). All three GPT-hydrogels were completely degraded after 14, 24, and 28 days of incubation, due to decomposition of the crosslinks within the hydrogel. It agreed well with our anticipation that the GPT-hydrogel system would have an accelerated degradation rate in buffer containing serum.
Cytotoxic Effect of SIM on MC3T3-E1 Cell Proliferation
To determine the optimum SIM loading for osteogenic differentiation, we cultured the MC3T3-E1 cells with GPT-hydrogel formulations containing varying concentrations of SIM (0, 0.05, 0.1, 0.5, 1, 3, 5, and 10 mg/ml) for 7 days. Morphologies of these cells at different SIM loadings were presented in Fig. 3a. As seen in Fig. 3b, cells treated with 1 and 3 mg/ml SIM displayed significant cytotoxic effect, resulting in a decrease of cell viability ranging from 75% to 68% as well as cell morphological changes (Fig. 3a), when compared with the nontreated control cells (p < 0.05). In addition, this cytotoxic effect was augmented by 34% and 12% over the control cells, when increase the SIM concentration from 5 to 10 mg/ml, respectively.
Fig. 3.
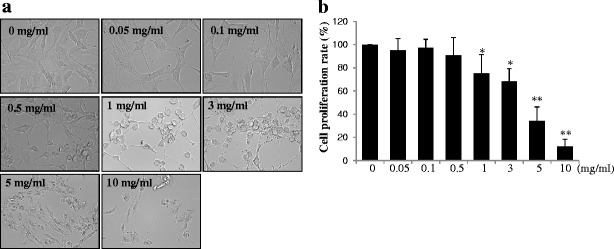
Cell cytotoxicity of SIM released from GPT-hydrogel2. To determine the optimum SIM concentration without cytotoxicity, varying concentrations of SIM (0, 0.05, 0.1, 0.5, 1, 3, 5, and 10 mg/ml) were employed to treat MC3T3-E1 cells after culturing them with GPT-hydrogel2 for 7 days. Cell cytotoxicity was determined by the MTT assay. a Morphological changes of the MC3T3-E1cells under different SIM concentrations. b Cell proliferation was measured by the MTT assay. *p < 0.05; **p < 0.01—represented that the differences was statistically significant, when comparing the results with the initial SIM concentration (0 mg/ml)
The concentrations of SIM (i.e., 1 and 3 mg/ml) that will be loaded into the GPT-hydrogel for further experiments were determined by the MTT assay result (Fig. 3b). These concentrations were comparable to those of another report presenting the regenerating effect of SIM (0.1–2.2 mg loaded in methylcellulose gels) on mandibular defects in rats (34). Another study reported murine cranial bone apposition with the injection of gels containing 2.2 mg SIM (35). In line with these, the SIM concentrations used in the current study can be further applied for long-term delivery for in vivo bone regeneration.
Release Kinetics of SIM
To examine the feasibility in achieving controlled SIM delivery from the hydrogels, we loaded two different concentrations of SIM (1 mg/ml and 3 mg/ml) into all of the three GPT-hydrogels (GPT-hydrogel1, GPT-hydrogel2, and GPT-hydrogel3) (Fig. 4a, b). Each concentration of SIM was mixed with the HRP polymer solution prior to gelation of the SIM-loaded GPT-hydrogels. As shown in Fig. 4, the release kinetics of SIM was dependent on its loading concentration. A significant initial burst release was observed for GPT-hydrogel1 at both concentrations of SIM (1 and 3 mg/ml) after 1 day of incubation at 37°C (Fig. 4a, b). In contrast, GPT-hydrogel2 and GPT-hydrogel3 exhibited more sustained release kinetics, as the accumulated SIM release reached the maximum after 14 and 20 days of incubation for GPT-hydrogel2 and GPT-hydrogel3, respectively, compared with the duration of 5 days for GPT-hydrogel1. As expected, the stronger mechanical strength of the hydrogel, the lower initial burst and more sustained release of SIM (GPT-hydrogel3).
Fig. 4.
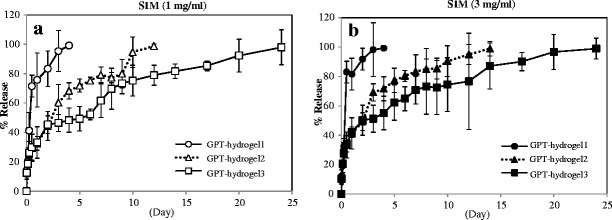
Cumulative percent release of SIM from GPT-hydrogel. GPT-hydrogels containing two concentrations of SIM (1 and 3 mg/ml) were prepared. The amount of released SIM into the PBS sink buffer was determined by measuring the absorbance at 262 nm. Cumulative release of SIM1 was expressed as a relative percentage from GPT-hydrogel containing a 1 and b 3 mg/ml of SIM. All experiments were conducted in duplicate with three independent sets
Bone Mineralization Assay
To examine the osteogenic effect of SIM, MC3T3-E1 cells were cultured in the osteogenic DM for 14 days with GPT-hydrogel2 containing two different SIM loadings (1 and 3 mg/ml, SIM1 and SIM3, respectively; Fig. 1a). The medium used in our in vitro experiments to induce osteogenic differentiation, DM, contained 50 μg/ml ascorbic acid and 10 mM β-glycerophosphate. It is a widely employed standard medium in osteogenic differentiation studies and has been reported to possess osteogenic activities (31,32). Because of its necessary involvement in the cell culture studies, we also examined its osteogenic effects and used DM treatment as a background to assess the actual osteogenic activities by the released SIM. As shown in Fig. 1b, SIM1 treatment yielded a 1.4-fold increase in cell mineralization over that of the DM-treated cells.
In Vitro MMP-13 mRNA Expression and OC Protein Expression
To examine the effect of SIM on osteogenic differentiation, MC3T3-E1 cells were grown with SIM-loaded GPT-hydrogel2 for 14 days, followed by measuring the specific marker for mature osteoblasts, MMP-13, and OC mRNA and protein expression levels, using the RT-PCR and Western blot analysis. While DM-treated cells displayed a 2.6-fold increase in MMP-13 mRNA expression over the PBS-treated control cells, treatment with GPT-hydrogel2-SIM1 and GPT-hydrogel2-SIM3 further induced a significantly magnified MMP-13 mRNA expression, by 1.6- and 2.0-fold over the DM-treated cells and 4.1- and 5.1-fold over the PBS-treated cells, respectively (Fig. 1c). In addition, GPT-hydrogel-SIM1- and GPT-hydrogel-SIM3-treated cells also produced a markedly enhanced MMP-13 protein expression over the PBS-treated nondifferentiated cells (2.4- and 2.8-fold increase, respectively), as well as another 30–40% increase over that of the DM-treated cells (Fig. 1d). All of these changes were statistically very significant (p < 0.05).
In addition, GPT-hydrogel2-SIM1 and GPT-hydrogel2-SIM3 also showed 1.7- and 2.3-fold of increase in OC mRNA expression compared with DM only treated cells after complete osteogenic differentiation, day 14 (Fig. 1e). OC protein expression during osteogenic differentiation was significantly increased in the culture with SIM-loaded GPT-hydrogels (GPT-hydrogel-SIM1 and GPT-hydrogel-SIM3, 2.7- and 2.5-fold increase over PBS-treated cells, Fig. 1f). In comparison with DM-treated cells, SIM-treated cells (GPT-hydrogel-SIM) also showed significant increase in OC expression (Fig. 1f). Based on results, SIM released from GPT-hydrogel appeared to trigger significant osteogenic differentiation.
Cytotoxicity from Hydrogel By-products
For prolonged use of the SIM-loaded GPT-hydrogels, we also examined the cytotoxic effect of the by-products derived from GPT-hydrogel degradation after long-term (53 days) incubation. Results showed that after long-term incubation, ranging from 1 to 53 days, PBS solution containing the GPT-hydrogel by-products did not exhibit significant cytotoxic effect on the cells, showing over 80% of cell proliferation rate (Fig. 5). It therefore implies that GPT-hydrogels can be further employed in in vivo implantation without much cytotoxicity concern.
Fig. 5.
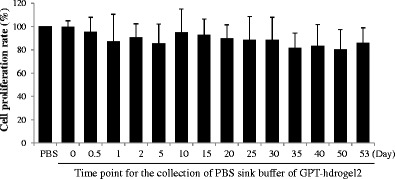
Cytotoxicity of GPT-hydrogel by-products. MC3T3-E1 cell was used to study the cytotoxicity of the GPT-hydrogel by-products. The PBS sink buffer of GPT-hydrogel that contains by-products of GPT-hydrogel was treated with MC3T3-E1 cells for 1 day. Cell cytotoxicity was measured using the MTT assay. All the experiments were performed in triplicate
DISCUSSION
Hydrogel systems have been extensively used and evaluated for controlled release of drugs to treat diseases in the biomedical field (18). Many injectable and in situ forming hydrogel approaches have been investigated, including those that made by enzyme-catalyzed crosslinking reactions (36,37).
Here, we report the development of GPT-hydrogel formed via the in situ enzymatic reaction of HRP and H2O2, and examine its biodegradability and biocompatibility in triggering the osteogenic differentiation. The gelation time was regulated between 5 and 127 s through variation of reactant concentrations, mainly HRP. It should be noted that control of gelation time could enable greater versatility for this system in biomedical applications. For example, rapid gelation time could limit the burst release of drugs from GPT-hydrogel. In contrast, a slower gelation time would enable the injectable scaffold to flow and localize onto both regular and irregular defect sites (36).
The GPT-hydrogel was found to yield great biocompatibility, as the MTT assay displayed almost no cytotoxicity (Fig. 3). Furthermore, the by-products and degradation products from the GPT-hydrogel also exhibited no specific cytotoxicity (Fig. 5). These results imply that the GPT-hydrogel is unlikely to generate side-effects after in vivo implantation. Although H2O2 is known to be a cytotoxic agents due to its reactive oxidation reaction, the H2O2 concentrations employed in this work (i.e., 0.0038 (GPT-hydrogel1), 0.0075 (GPT-hydrogel2), and 0.0125 wt.% (GPT-hydrogel3)) were below the 0.063% threshold reported previously for potential cytotoxic effects (30). Moreover, any excessive H2O2 was also converted to water through its decomposition by HRP.
The in vitro degradability of GPT-hydrogel depends primarily on the concentrations of H2O2 (Fig. 2b). The weight of GPT-hydrogels formed by the reaction of HRP and H2O2 gradually decreased over a 2-month period. Hence, it could be recognized that the degradability of GPT-hydrogel was controlled by the degree of crosslinking of the hydrogel polymer. Based on this finding, we could assess the most ideal condition in achieving optimal drug therapy and tissue regeneration, of which controlling the degradability of GPT-hydrogel was critical.
We previously examined the stability of the GPT-hydrogel system under different incubation conditions and reported the occurrence of proteolytic degradation in buffer containing 0.02 wt.% collagenase (30). The tetramine-tyramine hydrogel (GPT-hydrogel2), for example, showed 60% degradation in 3 days through proteolytic decomposition of the peptide chains. In addition, degradation was found to be dependent on the gelatin-hydroxyphenyl propionic acid or H2O2 concentrations, when they were used during the formation of the hydrogels (30).
Literature reports indicated that degradation of the gelatin-based hydrogel is faster in phosphate buffer than in distilled water due to the presence of various charged species that act as a catalyst in promoting gel degradation (36). Results from the current study revealed that the GPT-hydrogels are completely degraded between 42 and 53 days (see Fig. 2b), indicating that a single application of GPT-hydrogel to treat DDD could last for one period of about 1 month.
Degradation rate of GPT-hydrogels in buffer containing 5% FBS produced an accelerated hydrogel degradation, from 49 to 53 days in PBS buffer (Fig. 2b) to 24 and 28 days in serum for GPT-hydrogel2 and GPT-hydrogel3, respectively (Fig. 2c). Even with such accelerated degradation rates, these SIM-releasing hydrogels are still suitable to be applied to the injured disc area in achieving a long-lasting SIM release and subsequently significantly enhanced disc regeneration, when comparing with the previous system of utilizing a single intradiscal SIM administration (28).
The CSF is the fluid that fills the discal area, and comprises of PBS, plasma proteins and cytokines. Compared with the composition of normal complete serum (100%) that contains 6.0–7.8 g/100 ml (i.e., 60–78 mg/ml) of proteins (29), CSF contains merely <0.5 mg/ml of proteins (38,39), 6- to 8-fold lower than the protein content in 5% FBS (i.e., 3.0–3.9 mg/ml of proteins). Actually, hydrogel degradation in CSF is expected to be far slower than that in 5% FBS. Rather, it should be closer to that in ∼1% serum, according to the concentrations of proteins. Based on the measured degradation rates of 24 and 28 days in 5% FBS for GPT-hydrogel2 and GPT-hydrogel3, respectively, these hydrogels with slow SIM release functions deem to be adequately suited for the next phase in vivo rat studies.
SIM is well known to reduce blood cholesterol concentration and stimulate bone formation (5,6). Recently, hydrophobic statins have been shown to provide a dramatic upregulation effect on BMP2 and bone matrix mineralization (3,6,28,34,35). As a result, researchers are now investigating the possibility of incorporating statins into scaffolds for local delivery in treatment of bone defect (40–42) and human mesenchymal stem cell differentiation (43). However, the right delivery method has yet to be determined.
According to our findings presented in Fig. 2b, the GPT-hydrogel1, 2, and 3 samples degraded in about 42, 49, and 53 days under PBS condition, respectively. Assuming the GPT-hydrogels would be completely degraded in CSF in about 24–28 days, as shown by our data in 5% FBS (Fig. 2c), and assuming the loaded SIM would be released in accordance with the release profiles shown in Fig. 4, we made a proposition that one application of the GPT-hydrogel for treatment of the DDD could probably last for a 1-month period; based on assumption that CSF contained less amount of proteases than 5% FBS and thus hydrogel degradation in CSF should be slower than that in 5% FBS.
With regards to determination of osteogenic differentiation, we adopted an MC3T3-E1 pre-osteoblast cell system. It has been reported that it would take a period of 2 weeks to achieve a complete osteogenic differentiation in medium containing l-ascorbic acid and β-glycerophosphate (31,32). Using this in vitro system, it was found that SIM released from the hydrogels maintained its osteogenic effect, as evidenced by the expression of the osteogenic mRNA gene and protein (MMP-13 and OC; Fig. 1c–f). Lee et al. reported that local SIM application presented a major therapeutic advantage by delivering a higher drug concentration locally and preventing systemic side effects (44). Local application of statins has been shown to effectively induce new bone formation (44). However, a single injection of SIM produced limited bone formation due to the inability to maintain an optimum drug concentration, whereas multiple dose injections yielded an additive, or synergistic effect in promoting enhanced bone regeneration (44). Another report by Bae et al. indicated that sustained release of SIM from photo-cured hyaluronic acid hydrogel was able to regulate MC3T3-E1 cells for 7 days, under 0.1 and 1 mg/ml concentrations (11). These investigators also suggested that the loading of SIM could significantly influence cell viability.
Here, we demonstrated that incubation of 1 and 3 mg/ml SIM-loaded GPT-hydrogel with cultured MC3T3-E1 cells produced no detectable cytotoxicity yet provided a complete osteogenic differentiation for 14 days. As known, MMP-13 was considered to be an important factor in bone matrix turnover during bone formation/remodeling and also a marker for mature osteoblasts (45). Therefore, the observed elevation in MMP-13 expression, fibronectin, and procollagen gene activation could foster an increased collagen synthesis and osteoblast recruitment associated with local SIM-induced bone growth (46,47). Also known was that another osteogenic marker, OC, played a pivotal role in late stages of osteoblast differentiation (11).
Our results of SIM release profiles showed that the SIM could have sustained and controlled release kinetics for almost 2 months to yield efficient bone formation evaluated by using the in vitro system (Fig. 1).
To this regard, the current research successfully accomplished our purpose and goal. Both GPT-hydrogel2 and GPT-hydrogel3 seemed suitable for in vivo applications, as they displayed reasonable durations for degradation in buffer containing 5% FBS of 24 and 28 days (Fig. 2c), respectively, as well as a complete drug release profile of over 14 days and as long as 30 days (Fig. 4), respectively. In theory, any duration time of SIM release would be beneficial in terms of augmenting the disc regeneration; although if given the chance, one would wish to have a longer duration time than a shorter one in order to enable a more complete healing of injured disc.
In line with this, we conclude that the gelatin-based GPT-hydrogel can be a desirable delivery vehicle for SIM in achieving osteogenic differentiation.
CONCLUSIONS
A simple, nontoxic and injectable in situ GPT-hydrogel system was developed using a novel enzymatic oxidative coupling reaction. The ease-of-injection and biocompatibility of this in situ GPT-hydrogel system provide significant benefits for local SIM delivery and bone/disc tissue regeneration.
Based on the current findings, we believe that the consistent release of the SIM can promote bone regeneration at the disc space and ultimately resolve mechanical pain associated with spinal disc disorders. An alternative research investigation is to look at whether the SIM-contained compound can promote intervertebral disc regeneration with a developed degenerative disc rat model over a 3- to 6-month period. Further animal studies of the SIM-loaded GPT-hydrogel in bone tissue engineering using the injection implantation method in an appropriately established bone degenerative animal model as well as the effect of SIM-loaded hydrogel on local regulation of pro-inflammatory cytokines are currently underway in our laboratories at the University of Michigan.
Acknowledgments
The authors gratefully acknowledge funding support provided by the National Institutes of Health. The project described was supported by Grant Number R01 AR056649 from NIAMS/NIH. This work was also supported in part by NIH R01 Grant CA114612 and partially sponsored by Grant R31-2008-000-10103-01 from the World Class University project of the MEST and NRF of South Korea. Victor C. Yang is currently a Participating Faculty in the Department of Molecular Medicine and Biopharmaceutical Sciences, College of Medicine, and College of Pharmacy, Seoul National University, South Korea.
Abbreviations
- SIM
Simvastatin
- HRP
Horse radish peroxidase
- GPT
Gelatin–poly(ethylene glycol)–tyramine
- MMP-13
Matrix metalloproteinase-13
- OC
Osteocalcin
References
- 1.Hicks GE, Morone N, Weiner DK. Degenerative lumbar disc and facet disease in older adults: prevalence and clinical correlates. Spine (Phila Pa 1976) 2009;34(12):1301–6. doi: 10.1097/BRS.0b013e3181a18263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hassett G, Hart DJ, Manek NJ, Doyle DV, Spector TD. Risk factors for progression of lumbar spine disc degeneration: the Chingford study. Arthritis Rheum. 2003;48(11):3112–7. doi: 10.1002/art.11321. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Lin CY. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine (Philadelphia, Pa 1976) 2008;33(16):E525–31. doi: 10.1097/BRS.0b013e31817c561b. [DOI] [PubMed] [Google Scholar]
- 4.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–4. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 5.Montagnani A, Gonnelli S, Cepollaro C, Pacini S, Campagna MS, Franci MB, et al. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone. 2003;32(4):427–33. doi: 10.1016/S8756-3282(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 6.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 7.Hatano H, Maruo A, Bolander ME, Sarkar G. Statin stimulates bone morphogenetic protein-2, aggrecan, and type 2 collagen gene expression and proteoglycan synthesis in rat chondrocytes. J Orthop Sci. 2003;8(6):842–8. doi: 10.1007/s00776-003-0724-9. [DOI] [PubMed] [Google Scholar]
- 8.Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280(3):874–7. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- 9.Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H, et al. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun. 2003;308(3):458–62. doi: 10.1016/S0006-291X(03)01408-6. [DOI] [PubMed] [Google Scholar]
- 10.Garbern JC, Hoffman AS, Stayton PS. Injectable pH- and temperature-responsive poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for delivery of angiogenic growth factors. Biomacromolecules. 2010;11(7):1833–9. doi: 10.1021/bm100318z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bae MS, Yang DH, Lee JB, Heo DN, Kwon YD, Youn IC, et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials. 2011;32(32):8161–71. doi: 10.1016/j.biomaterials.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XZ, Jo Lewis P, Chu CC. Fabrication and characterization of a smart drug delivery system: microsphere in hydrogel. Biomaterials. 2005;26(16):3299–309. doi: 10.1016/j.biomaterials.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Adv Drug Deliv Rev. 2002;54(1):53–77. doi: 10.1016/S0169-409X(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 14.Kimura M, Fukumoto K, Watanabe J, Ishihara K. Hydrogen-bonding-driven spontaneous gelation of water-soluble phospholipid polymers in aqueous medium. J Biomater Sci Polym Ed. 2004;15(5):631–44. doi: 10.1163/156856204323046898. [DOI] [PubMed] [Google Scholar]
- 15.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53(3):321–39. doi: 10.1016/S0169-409X(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 16.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388(6645):860–2. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 17.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164–71. doi: 10.1002/(SICI)1097-4636(200008)51:2<164::AID-JBM4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Hiemstra C, Zhong Z, Li L, Dijkstra PJ, Feijen J. In-situ formation of biodegradable hydrogels by stereocomplexation of PEG-(PLLA)8 and PEG-(PDLA)8 star block copolymers. Biomacromolecules. 2006;7(10):2790–5. doi: 10.1021/bm060630e. [DOI] [PubMed] [Google Scholar]
- 19.Jung JP, Gasiorowski JZ, Collier JH. Fibrillar peptide gels in biotechnology and biomedicine. Biopolymers. 2010;94(1):49–59. doi: 10.1002/bip.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan-pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009;5(6):1956–65. doi: 10.1016/j.actbio.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355(1–2):1–18. doi: 10.1016/j.ijpharm.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80(1–3):9–28. doi: 10.1016/S0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 23.Langer R. New methods of drug delivery. Science. 1990;249(4976):1527–33. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 24.Kurisawa M, Chung JE, Yang YY, Gao SJ, Uyama H. Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering. Chem Commun (Camb). 2005;(34):4312–4. doi: 10.1039/b506989k. [DOI] [PubMed]
- 25.Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25(7–8):1339–48. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Park KM, Shin YM, Joung YK, Shin H, Park KD. In situ forming hydrogels based on tyramine conjugated 4-Arm-PPO-PEO via enzymatic oxidative reaction. Biomacromolecules. 2010;11(3):706–12. doi: 10.1021/bm9012875. [DOI] [PubMed] [Google Scholar]
- 27.Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30(20):3371–7. doi: 10.1016/j.biomaterials.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Wang L, Park JB, Park P, Yang VC, Hollister SJ, et al. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res Ther. 2009;11(6):R172. doi: 10.1186/ar2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker HJ, Lindsay JR, Weisbroth SH. The laboratory rat, volume I: biology and diseases. New York: Academic; 1979. p. 115. [Google Scholar]
- 30.Park KM, Lee Y, Son JY, Oh DH, Lee JS, Park KD. Synthesis and characterizations of in situ cross-linkable gelatin and 4-arm-PPO-PEO hybrid hydrogels via enzymatic reaction for tissue regenerative medicine. Biomacromolecules. 2012;13(3):604–11. doi: 10.1021/bm201712z. [DOI] [PubMed] [Google Scholar]
- 31.Park JB, Zhang H, Lin CY, Chung CP, Byun Y, Park YS, et al. Simvastatin maintains osteoblastic viability while promoting differentiation by partially regulating the expressions of estrogen receptors α. J Surg Res. 2012;174(2):278–83. doi: 10.1016/j.jss.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 32.Khatiwala CB, Peyton SR, Metzke M, Putnam AJ. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J Cell Physiol. 2007;211(3):661–72. doi: 10.1002/jcp.20974. [DOI] [PubMed] [Google Scholar]
- 33.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, et al. Local simvastatin effects on mandibular bone growth and inflammation. J Periodontol. 2005;76(11):1861–70. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, et al. Effects of simvastatin gels on murine calvarial bone. J Periodontol. 2002;73(10):1141–8. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 36.Gutowska A, Jeong B, Jasionowski M. Injectable gels for tissue engineering. Anat Rec. 2001;263(4):342–9. doi: 10.1002/ar.1115. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37(8):1473–81. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 38.Wright BL, Lai JT, Sinclair AJ. Cerebrospinal fluid and lumbar puncture: a practical review. J Neurol. 2012;259(8):1530–45. doi: 10.1007/s00415-012-6413-x. [DOI] [PubMed] [Google Scholar]
- 39.Clarke C, Howard R, Rossor M, Shorvon S. Neurology: a queen aquare textbook. 1. Chichester: Wiley; 2009. [Google Scholar]
- 40.Cartmell S. Controlled release scaffolds for bone tissue engineering. J Pharm Sci. 2009;98(2):430–41. doi: 10.1002/jps.21431. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, An HS, Tannoury C, Thonar EJ, Freedman MK, Anderson DG. Biological treatment for degenerative disc disease: implications for the field of physical medicine and rehabilitation. Am J Phys Med Rehabil. 2008;87(9):694–702. doi: 10.1097/PHM.0b013e31817c1945. [DOI] [PubMed] [Google Scholar]
- 42.Adah F, Benghuzzi H, Tucci M, Russell G, England B. Cholesterol production inhibitor (statin) increased bone healing in surgically created femoral defect in an animal model. Biomed Sci Instrum. 2007;43:95–103. [PubMed] [Google Scholar]
- 43.Benoit DS, Nuttelman CR, Collins SD, Anseth KS. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials. 2006;27(36):6102–10. doi: 10.1016/j.biomaterials.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y, Schmid MJ, Marx DB, Beatty MW, Cullen DM, Collins ME, et al. The effect of local simvastatin delivery strategies on mandibular bone formation in vivo. Biomaterials. 2008;29(12):1940–9. doi: 10.1016/j.biomaterials.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayami T, Kapila YL, Kapila S. Divergent upstream osteogenic events contribute to the differential modulation of MG63 cell osteoblast differentiation by MMP-1 (collagenase-1) and MMP-13 (collagenase-3) Matrix Biol. 2011;30(4):281–9. doi: 10.1016/j.matbio.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura H, Sato G, Hirata A, Yamamoto T. Immunolocalization of matrix metalloproteinase-13 on bone surface under osteoclasts in rat tibia. Bone. 2004;34(1):48–56. doi: 10.1016/j.bone.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann N Y Acad Sci. 2003;995:109–16. doi: 10.1111/j.1749-6632.2003.tb03214.x. [DOI] [PubMed] [Google Scholar]


