Abstract
Based on its lower Log P value relative to metoprolol, a marker for the low/high-permeability (Peff) class boundary, pseudoephedrine was provisionally classified as BCS low-permeability compound. On the other hand, following oral administration, pseudoephedrine fraction dose absorbed (Fabs) and systemic bioavailability approaches 100%. This represents a challenge to the generally recognized Peff–Fabs correlation. The purpose of this study was to elucidate the underlying mechanisms behind the confusion in pseudoephedrine’s BCS classification. Pseudoephedrine’s BCS solubility class was determined, and its physicochemical properties and intestinal permeability were thoroughly investigated, both in vitro and in vivo in rats, considering the complexity of the whole of the small intestine. Pseudoephedrine was found to be unequivocally a high-solubility compound. All of the permeability studies revealed similar phenomenon; at any given intestinal segment/pH, the permeability of metoprolol was higher than that of pseudoephedrine, however, as the intestinal region becomes progressively distal, and the pH gradually increases, pseudoephedrine’s permeability rises above that of metoprolol in the former segment. This unique permeability pattern likely explains pseudoephedrine’s complete absorption. In conclusion, pseudoephedrine is a BCS Class I compound; no discrepancy between Peff and Fabs is involved in its absorption. Rather, it reflects the complexity behind Peff when considering the whole of the intestine. We propose to allow high-permeability classification to drugs with Peff that matches/exceeds the low/high class benchmark anywhere throughout the intestinal tract and not restricted necessarily to the jejunum.
KEY WORDS: BCS classification, biowaiver, intestinal absorption, regioselective absorption, segmental-dependent permeability
INTRODUCTION
Amidon et al. (1) revealed that the two key parameters governing the fraction of dose absorbed (Fabs) following oral administration are the permeability (Peff) through the gastrointestinal (GI) wall and the solubility/dissolution of the drug dose in the GI milieu. Based on extensive research, it was determined that an excellent correlation exists between the human jejunal Peff measured using intestinal perfusion and the Fabs obtained from pharmacokinetic or mass-balance studies in humans (2–7). Indeed, drug regulatory agencies worldwide, including the American Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have been using Peff as a surrogate for Fabs, and implemented this Peff–Fabs correlation in BCS-based biowaiver decisions.
The commonly used decongestant pseudoephedrine is a sympathomimetic agent that acts predominantly on the α-adrenergic receptors. Based on its significantly lower Log P value relative to metoprolol, a widely used marker for the low/high-permeability class boundary, pseudoephedrine was provisionally classified as a low-permeability, BCS Class III compound (8). On the other hand, following oral administration, pseudoephedrine Fabs and systemic bioavailability approaches 100% (9). Pseudoephedrine undergoes no presystemic metabolism, less than 1% of the dose is metabolized by the liver (9), and up to 96% of the dose is excreted unchanged in the urine (10). Hence, considering its high solubility, it was classified as Class III also according to the biopharmaceutics drug disposition classification system (BDDCS) (11). As noted above, a match is expected between high Fabs and high Peff; the FDA and EMA definitions for high permeability is Fabs > 90% and Fabs > 85%, respectively. It is generally recognized, therefore, that a high Fabs drug must exhibit high Peff in the human intestine, even though actual permeability data may not be available. The intestinal absorption of pseudoephedrine, however, allegedly represents a case of discrepancy between Peff and Fabs. This directly affects its BCS classification, which in turns dictates whether a generic immediate-release (IR) oral drug product containing pseudoephedrine may be eligible for a BCS-based biowaiver. This is therefore a question of considerable scientific and financial importance that involves public health policy aspects as well (12). This alleged Peff–Fabs disparity emphasizes that the intestinal permeability, considering the whole of the intestine, is more complex than generally recognized, as will be further discussed in this paper (13).
The purpose of this study was to elucidate the underlying mechanisms behind the confusion in pseudoephedrine’s BCS classification. We have determined the solubility class of pseudoephedrine, and thoroughly investigated its physicochemical properties and intestinal permeability, both in vitro and in vivo in rats, taking into consideration the complexity of the whole of the small intestine. The results were compared with metoprolol, the FDA reference drug for the low/high-permeability class boundary. We then performed a thorough theoretical physicochemical analysis of pseudoephedrine vs. metoprolol, to further clarify the mechanistic explanation behind the experimental data. Overall, this study points out a unique intestinal absorption pattern through elucidation of the solubility and permeability class membership of pseudoephedrine and highlights the underlying complexity and the care that must be taken when interpreting intestinal permeability data.
MATERIALS AND METHODS
Materials
Pseudoephedrine hydrochloride, metoprolol tartrate, phenol red, potassium chloride, potassium phosphate monobasic, sodium chloride, octanol, hexadecane, and trifluoroacetic acid (TFA) were purchased from Sigma Chemical Co. (St. Louis, MO). Acetonitrile, methanol and water (Merck KGaA, Darmstadt, Germany) were ultra-performance liquid chromatography (UPLC) grade. All other chemicals were of analytical reagent grade.
Solubility Studies
The solubility class membership of pseudoephedrine was determined according to the FDA’s BCS guidance for industry (14). The equilibrium solubility of the drug was determined at both 37°C and at room temperature (25°C), in phosphate buffer at pH 7.5, acetate buffer at pH 4.5, and maleate buffer at pH 1.0, using the shake-flask method, as previously reported (15–17). Briefly, excess amounts of pseudoephedrine were added to glass vials containing the different buffers. Solution pH was verified after addition of the drug to the buffer. The vials were tightly closed and placed in a shaking (100 rpm) water bath at 37°C or 25°C. Establishment of equilibrium was confirmed by comparison of 48- and 72-h samples. Prior to sampling, the vials were centrifuged (10,000 rpm for 10 min) and the supernatant was carefully withdrawn and immediately assayed by UPLC.
Determination of Octanol–Buffer Partition Coefficients
Experimental octanol–buffer partition coefficients, Log D, for pseudoephedrine and metoprolol at pH 6.5, 7.0, and 7.5 were determined using the traditional shake-flask method (18,19). Briefly, solutions of pseudoephedrine or metoprolol were prepared in octanol-saturated phosphate buffers with pH values of 6.5, 7.0, and 7.5. These aqueous solutions were then equilibrated at room temperature with an equivalent volume of buffer saturated octanol for 48 h. The octanol and aqueous phases were then separated by centrifugation, and the drug concentration in the aqueous phase was determined by UPLC. The drug concentration in the octanol phase was obtained by mass balance. From these data, the apparent octanol/buffer partition coefficient was determined. Experimental octanol–buffer partition coefficients of the unionized form, Log P, for pseudoephedrine and metoprolol were determined at pH 13 solution, using the same method described above.
Parallel Artificial Membrane Permeability Assay
Permeability studies through artificial membrane were carried out in two different methods: the hexadecane-based parallel artificial membrane permeability assay (PAMPA) and the pre-coated PAMPA assay (BD Gentest™).
The hexadecane-based permeability studies were carried out as previously described with minor modifications (20,21). Briefly, three solutions of pseudoephedrine or metoprolol were prepared with different ratios of potassium phosphate monobasic and sodium phosphate dibasic, to give pH values of 6.5, 7.0, and 7.5. Osmolality (290 mOsm/L) and ionic strength were similar in all buffers. Millipore (Danvers, MA) 96-well MultiScreen-Permeability filter plates with 0.3 cm2 polycarbonate filter support (0.45 μm) were used. The filter supports were impregnated with 15 μl of a 5% hexadecane in hexane solution, and were then allowed to dry for 1 h, during which the hexane was completely evaporated resulting in a uniform layer of hexadecane. Then, the donor wells were filled with the different pseudoephedrine solutions (200 μl), the receiver wells were filled with blank buffers (300 μl), and the PAMPA sandwich was incubated at room temperature. Receiver plates were collected hourly for 4 h.
The pre-coated PAMPA experiments (BD Gentest™, BD Biosciences, Bedford, MA) were carried out according to the manufacturer instructions, with the addition of tracking the transport rate, as described for the hexadecane-based PAMPA. Receiver plates were collected hourly for 5 h.
Apparent permeability coefficient (Papp) values for both PAMPA methods were calculated from the linear plot of drug accumulated in the acceptor side vs. time using the equation:
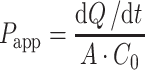 |
where dQ/dt is the steady-state appearance rate of pseudoephedrine/metoprolol on the receiver side, C0 is the initial concentration of the drug in the donor side (250 μM in all experiments), and A is the membrane surface area (0.048 cm2). Linear regression was carried out to obtain the steady-state appearance rate of the drugs on the receiver side.
Rat Intestinal Perfusions
The in situ effective permeability coefficient (Peff) of pseudoephedrine vs. metoprolol was determined using the single-pass rat intestinal perfusion model. All animal experiments were conducted using protocols approved by the Ben-Gurion University of the Negev Animal Use and Care Committee (Protocol IL-60-11-2010). The animals (male Wistar rats weighing 270–300 g, Harlan, Israel) were housed and handled according to the Ben-Gurion University of the Negev Unit for Laboratory Animal Medicine Guidelines.
The experimental procedure followed previous reports (22,23). Briefly, anesthetized rats were placed on a heated (37°C) surface (Harvard Apparatus Inc., Holliston, MA), and a midline abdominal incision of 3–4 cm was made. To account for the complexity of the whole of the small intestine, permeability was determined in three different 10-cm segments; a proximal jejunal segment (starting 2 cm below the ligament of Treitz), mid-small intestinal segment (isolated between the end of the upper and the beginning of the lower segments), and a distal ileal segment (ending 2 cm above the cecum). Each intestinal segment (approximately 10 cm) was cannulated on two ends, and was rinsed with blank perfusion buffer. All solutions were incubated in a 37°C water bath.
Three perfusion buffers containing pseudoephedrine, metoprolol and phenol red (a non-absorbable marker for water flux measurements) were prepared with different ratios of potassium phosphate monobasic and sodium phosphate dibasic, to give pH values of 6.5, 7.0, and 7.5, while osmolality (290 mOsm/L) and ionic strength were similar in all buffers. The permeability in each intestinal segment was measured at the pH that corresponds to the physiological pH of that region: (1) proximal jejunum, pH 6.5; (2) mid-small intestine, pH 7.0; and (3) distal ileum, pH 7.5 (24,25). The perfusion buffer was first perfused for 1 h, to ensure steady-state conditions, followed by additional 1 h of perfusion with samples taken every 10 min. The pH of the collected samples was measured at the outlet, to verify that there was no pH change throughout the perfusion. All samples were immediately assayed by UPLC. At the end of the experiment, the length of each perfused intestinal segment was accurately measured.
The effective permeability (Peff; in centimeters per second) through the rat gut wall was determined according to the following equation:
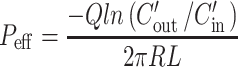 |
where Q is the perfusion buffer flow rate (0.2 mL/min), 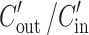 is the ratio of the outlet and the inlet concentration of drug that has been adjusted for water transport (26–28), R is the radius of the intestinal segment (set to 0.2 cm), and L is the length of the perfused intestinal segment.
is the ratio of the outlet and the inlet concentration of drug that has been adjusted for water transport (26–28), R is the radius of the intestinal segment (set to 0.2 cm), and L is the length of the perfused intestinal segment.
Physicochemical Analysis
The theoretical fraction extracted into octanol (fe) was calculated using the following equation from Winne (29) and Wagner and Sedman (30):
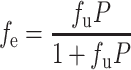 |
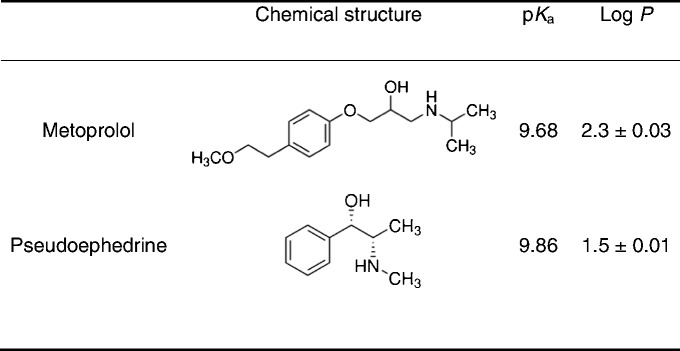
where P is the octanol–water partition coefficient of the unionized form of the drug and fu is the fraction unionized of the drug at a given pH. The fuvs. pH was plotted according to the Henderson–Hasselbalch equation, using the following literature pKa values: 9.68 for metoprolol (31) and 9.86 for pseudoephedrine (32).
Ultra-Performance Liquid Chromatography
UPLC experiments were performed on a Waters (Milford, MA) Acquity UPLC H-Class system equipped with photodiode array detector and Empower software. The simultaneous determination of pseudoephedrine, metoprolol and phenol red was achieved using a Waters (Milford, MA) Acquity UPLC BEH C18 1.7-μm 2.1 × 100 mm column. The mobile phase consisted of 90:10 going to 20:80 (v/v) water:acetonitrile (both with 0.1% TFA) over 5 min (flow rate, 0.5 mL/min). The detection wavelengths and retention times for pseudoephedrine, metoprolol and phenol red were 256, 275, and 285 nm and 2.5, 3.1, and 3.6 min, respectively. Injection volumes for all UPLC analyses ranged from 2 to 50 μL.
Statistical Analysis
Log D and Log P determinations were performed in triplicates. All other in vitro experiments were replicated with n = 4, and all animal experiments were replicated with n = 5. Values are expressed as means ± standard deviation (SD). To determine statistically significant differences among the experimental groups, the nonparametric Kruskal–Wallis test was used for multiple comparisons and the two-tailed nonparametric Mann–Whitney U test for two-group comparison where appropriate. A p value of less than 0.05 was termed significant.
RESULTS
Solubility Studies
The solubility of pseudoephedrine in the three pH values of 1.0, 4.5, and 7.5, at both 37°C and at room temperature, is presented in Table I. The data indicate that pseudoephedrine is unequivocally a high-solubility compound; taking 60 mg as the highest single unit dose strength, the minimal dose number (D0) at 37°C is 0.00034, indicating a BCS high-solubility class membership.
Table I.
The Solubility (in Milligrams per Milliliter) of Pseudoephedrine in the Three pH Values 1.0, 4.5, and 7.5, at 37°C and at Room Temperature (25°C)
| pH 1.0 | pH 4.5 | pH 7.5 | |
|---|---|---|---|
| 37°C | 835 ± 33 | 743 ± 18 | 700 ± 9 |
| 25°C | 428 ± 2 | 250 ± 1 | 213 ± 1 |
Data presented as mean ± SD; n = 3
Octanol–Buffer Partition Coefficients
The Log D values for pseudoephedrine and metoprolol at the three pH values of 6.5, 7.0, and 7.5, representing the conditions throughout the small intestine, are presented in Fig. 1. It can be seen that at any given pH, the octanol–buffer partition coefficient of metoprolol is higher than that of pseudoephedrine. However, pseudoephedrine’s Log D value at 7.5 approximately equals that of metoprolol at 6.5. The octanol–buffer partition coefficient values of the unionized form, Log P, for pseudoephedrine and metoprolol were determined to be 1.5 and 2.3, respectively (Table II).
Fig. 1.
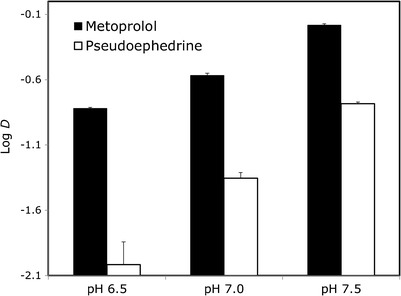
The octanol–buffer partition coefficients, Log D, for pseudoephedrine and metoprolol at the three pH values 6.5, 7.0, and 7.5. Data are presented as the mean ± SD; n = 3 in each experimental group
Table II.
Pseudoephedrine and Metoprolol Physicochemical Properties Used for the Fraction Unionized (f u) and Fraction Extracted into Octanol (f e) Analyses
Permeability Studies
The accumulated amount transported vs. time plots of pseudoephedrine and metoprolol in the hexadecane-based PAMPA and the Pre-coated PAMPA assay, at the three pH values of 6.5, 7.0, and 7.5, are presented in Figs. 2 and 3, respectively. The in situ effective permeability coefficient (Peff) values of pseudoephedrine vs. metoprolol determined using the single-pass rat intestinal perfusion model, in the three small intestinal segments the proximal jejunum (pH 6.5), mid-small intestine (pH 7.0), and the distal ileum (pH 7.5), are presented in Fig. 4. It can be seen that all of these permeability studies revealed a similar phenomenon; at any given intestinal segment/pH, the permeability of metoprolol was higher than that of pseudoephedrine, however, as the intestinal region was progressively more distal, and the pH of that region was gradually increasing, pseudoephedrine’s permeability rose above that of metoprolol in the former segment. This results in the unique phenomenon in which the permeability of pseudoephedrine at pH 7.0 was higher than that of metoprolol at pH 6.5, and the permeability of pseudoephedrine at pH 7.5 was higher than that of metoprolol at pH 7.0.
Fig. 2.
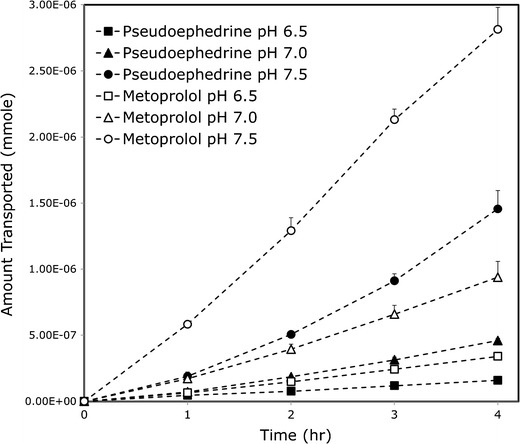
The mass transfer of pseudoephedrine and metoprolol at the three pH values 6.5, 7.0, and 7.5 in the hexadecane-based PAMPA assay. Data are presented as the mean ± SD; n = 4 in each experimental group
Fig. 3.
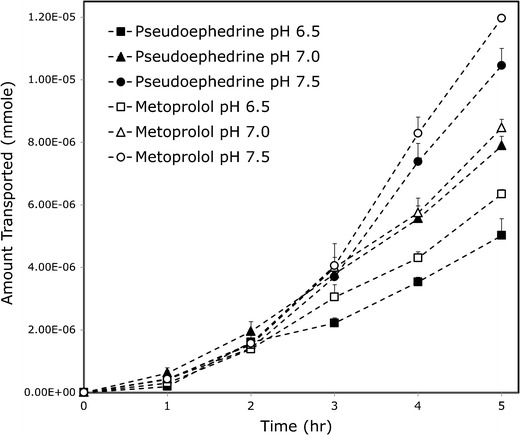
The mass transfer of pseudoephedrine and metoprolol at the three pH values 6.5, 7.0, and 7.5 in the pre-coated PAMPA experiments (BD Gentest™). Data are presented as the mean ± SD; n = 4 in each experimental group
Fig. 4.
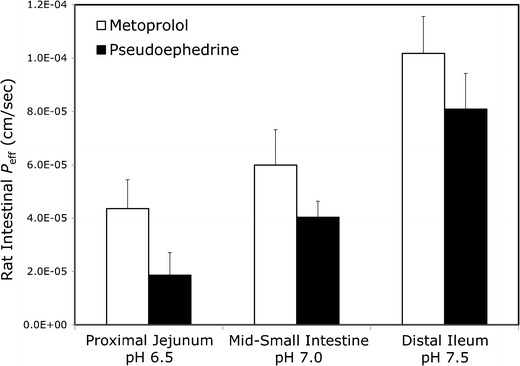
Effective permeability values (P eff; in centimeters per second) obtained for pseudoephedrine and metoprolol after in situ single pass perfusion to the rat proximal jejunum at pH 6.5, mid-small intestine at pH 7.0, and to the distal ileum at pH 7.5. Data are presented as the mean ± SD; n = 5 in each experimental group
Physicochemical Analysis
The theoretical fu and fe plots as a function of pH for pseudoephedrine and metoprolol are presented in Fig. 5. The physicochemical properties used for these analyses are summarized in Table II. The fu of the basic compounds pseudoephedrine and metoprolol is negligible at low pH, and increases as the pH rises, resulting in the classic sigmoidal shape. For both drugs, the fevs. pH plot follows a similar pattern, but with a shift to the left (lower pH values). The shift magnitude equals to Log(P-1) at the midpoint of the fe and fu sigmoidal curves (29,30). Both drugs have approximately similar pKa; however, because of pseudoephedrine’s lower Log P the rise in its fe occurs at higher pH values in comparison to metoprolol. Experimental octanol–buffer partitioning of the drugs at the three pH values of 6.5, 7.0, and 7.5 are also presented in Fig. 5 and were in good agreement with the theoretical plots.
Fig. 5.
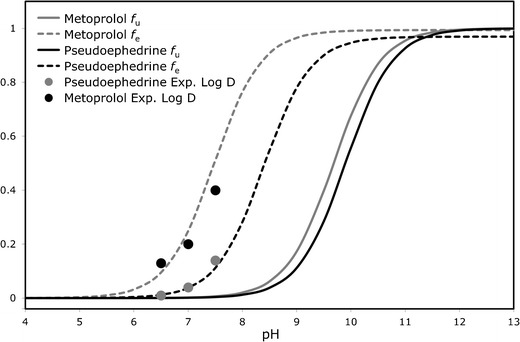
The theoretical fraction unionized (f u) and fraction extracted into octanol (f e) plots as a function of pH for pseudoephedrine and metoprolol, and experimental buffer–octanol partitioning of the drugs in the three pH values 6.5, 7.0, and 7.5 (plotted as fraction extracted into octanol)
DISCUSSION
While in the FDA guidance for industry regarding BCS solubility classification there is an explicit recognition of the changes in this parameter along the GI tract, demonstrated in the requirement to show that the drug dose is dissolved in 250 mL of aqueous media in all luminal conditions, that is pH 1–7.5 (14), the permeability classification does not consider the changes along the intestine and requires only the value in the jejunum. This policy misses the complexity behind the permeability measure, considering the whole of the intestine; permeability is location dependent, and pertaining to each point throughout the GI tract. In fact, even the Peff values we report here for the different segment, proximal jejunum, mid-small intestine, and distal ileum, are average values; these values were calculated based on perfusion through a 10-cm intestinal segment, over which the permeability may change locally at any point, and hence the overall value represents the average permeability in this 10-cm segment. Within a 10-cm intestinal segment with approximately similar characteristics the local Peff changes may be small, but when considering the many parameters that vary significantly from one region to another throughout the entire intestine, looking merely at one segment can lead to misjudgment regarding Peff and Fabs, potentially impacting decisions regarding drug discovery, formulation design, drug development and regulation. The comparison of pseudoephedrine and metoprolol presented in this paper emphasizes this point and its consequences.
When evaluating Peff data, one must first define the borderline for the low/high-permeability class membership; the completely absorbed β-blocker metoprolol is a widely used and acceptable marker for this purpose (3,33,34). However, as can be seen in Fig. 4, metoprolol’s permeability increases significantly as the drug travels along the small intestine and the pH rises; the next question is, therefore, which Peff value of metoprolol should be taken as the low/high class benchmark, the proximal jejunal value, or the higher value at the distal ileum? A careful examination of the oral absorption of IR metoprolol reveals that 80–90% of the dose is absorbed from the upper 50 cm, i.e., the proximal jejunum, in both human (35) and rats (36). It follows, hence, that metoprolol’s Peff in the proximal jejunum is the value that allows its high absorption, and that the values in more distal segments are in fact not relevant for the absorption of an IR dose of metoprolol. This analysis establishes metoprolol’s Peff in the proximal jejunum as the marker for the low/high-permeability class boundary. In light of this point, the results we present here for the intestinal permeability of pseudoephedrine become critical; although showing low permeability in the upper small intestine, pseudoephedrine’s Peff values in the subsequent small intestinal regions are comparable/higher than the benchmark of metoprolol’s permeability in the proximal jejunum. As a result, it is evident that an IR oral dose of pseudoephedrine is under high-permeability conditions throughout the majority of the small intestinal transit time, which allows for its complete absorption. It should be noted that this phenomenon was revealed by all of the experimental methods used in this study (Log D, hexadecane-based PAMPA, pre-coated PAMPA, in vivo rat intestinal perfusion, and the theoretical physicochemical analysis). It can be concluded, therefore, that pseudoephedrine is a BCS Class I compound, and that no discrepancy between Peff and Fabs is involved in its intestinal absorption; rather, the confusion in its BCS classification was a reflection of the complexity behind the permeability measure when considering the whole of the intestinal tract. When making a case for a BCS classification, looking solely at the jejunum can be misleading, for the solubility class, as explicitly recognized by the FDA guidelines, but also for the permeability class assignment, as evident by the data presented here. We therefore suggest the following extension to the current high-permeability criterion: setting metoprolol’s Peff in the proximal jejunum as the low/high-permeability class boundary, and, if the permeability of the tested compound matches/exceeds this benchmark anywhere throughout the intestinal tract, i.e., not necessarily in the jejunum, then this compound should be classified as high permeability. This extension may allow a scientifically justified Class I classification for compounds that according to the current BCS guideline fail to meet the high-permeability criterion due to segmental-dependent permeability. Naturally, regulatory changes cannot be based on animal studies only, and additional human data are needed.
To a large extent, the regional-permeability is determined by the interplay between the physicochemical properties of the drug and the function of the membrane (4,22,37–40). Intestinal segments may differ from each other in many aspects, including the total surface area of the membrane, tight junctional resistance, enzyme activities, amount and capacity of transporters, unstirred water layer, capillary blood flow, etc. In the pseudoephedrine case presented in this paper, the segmental-dependent permeability is attributable mainly to the pH changes along the intestine, as evident by the high correlation between the PAMPA and the in vivo studies, by the good agreement of the experimental and the theoretical analyses (Fig. 5), and by previous reported studies (41–43). The increasing pH along the intestine may result in this unique absorption pattern for a whole class of molecular entities; we have previously shown that the β-blocker sotalol follows a similar permeability pattern, starting as low-permeability in the proximal GI sections, increasing gradually with the pH increase throughout the small intestine, and becomes a high-permeability compound as the pH rises above 7.5 in the ileum, which allows for complete absorption of this drug (24). While pseudoephedrine includes the basic secondary amine as a sole ionizable center, sotalol is an amphoteric molecule that contains the acidic methanesulfonamide functionality and the basic secondary amine. Sotalol’s isoelectric point is, however, beyond the pH range relevant to the small intestine, and hence it behaves essentially as a weak base, leading to the observed absorption pattern. It can be concluded, hence, that a molecule must contain a basic functionality to present with this phenomenon, but it does not necessarily have to be the sole ionizable center.
The study reported in this paper emphasizes the underlying complexity and the care that must be taken with interpretation of intestinal permeability data. While the wrong impression of low permeability may be received for pseudoephedrine when looking merely at the proximal jejunum, the correct high-permeability classification is revealed when the permeability is more thoroughly assessed. Overall, therefore, the scientifically justified BCS classification for this compound is Class I. As noted above, pseudoephedrine undergoes no presystemic metabolism, less than 1% of the dose is metabolized by the liver (9), and up to 96% of the dose is excreted unchanged in the urine (10). Therefore, its BDDCS classification would be Class III (11). Pseudoephedrine hence represents a case of disagreement between the BCS and the BDDCS, and a deviation from the Peff–Fmet (fraction metabolized) correlation proposed by the BDDCS. Thus, while the extent of drug metabolism may be useful in supporting permeability classification under certain circumstances, the data presented in this paper demonstrate the care that must be taken with intestinal permeability/extent of absorption determinations (12,24,44).
CONCLUSIONS
In conclusion, this study provides evidence to indicate that pseudoephedrine is a BCS Class I compound. No discrepancy between Peff and Fabs is involved in its absorption, rather, it reflects the complexity behind Peff when considering the whole of the intestinal tract. As an extension to the current high-permeability criterion, we propose to allow high-permeability classification to drugs with Peff that matches/exceeds the low/high class benchmark anywhere throughout the intestinal tract, i.e., not necessarily only in the jejunum.
REFERENCES
- 1.Amidon GL, Lennernas H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12(3):413–420. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Amidon GL, Sinko PJ, Fleisher D. Estimating human oral fraction dose absorbed: a correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm Res. 1988;5(10):651–654. doi: 10.1023/A:1015927004752. [DOI] [PubMed] [Google Scholar]
- 3.Dahan A, Miller J, Amidon G. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J. 2009;11(4):740–746. doi: 10.1208/s12248-009-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennernäs H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37(10–11):1015–1051. doi: 10.1080/00498250701704819. [DOI] [PubMed] [Google Scholar]
- 5.Lennernäs H, Crison J, Amidon G. Permeability and clearance views of drug absorption: a commentary. J Pharmacokinet Pharmacodyn. 1995;23(3):333–337. doi: 10.1007/BF02354289. [DOI] [PubMed] [Google Scholar]
- 6.Sinko PJ, Leesman GD, Amidon GL. Predicting fraction dose absorbed in humans using a macroscopic mass balance approach. Pharm Res. 1991;8(8):979–988. doi: 10.1023/A:1015892621261. [DOI] [PubMed] [Google Scholar]
- 7.Lennernäs H. Human intestinal permeability. J Pharm Sci. 1998;87(4):403–410. doi: 10.1021/js970332a. [DOI] [PubMed] [Google Scholar]
- 8.Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3(6):631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 9.Kanfer I, Dowse R, Vuma V. Pharmacokinetics of oral decongestants. Pharmacother J Hum Pharmacol Drug Ther. 1993;13(6P2):116S–128S. [PubMed] [Google Scholar]
- 10.Hardman J, Limbird LE. Goodman & Gilman’s: the pharmacological basis of therapeutics. 10. New York: McGraw-Hill; 2001. [Google Scholar]
- 11.Benet L, Broccatelli F, Oprea T. BDDCS applied to over 900 drugs. AAPS J. 2011;13(4):519–547. doi: 10.1208/s12248-011-9290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amidon KS, Langguth P, Lennernas H, Yu L, Amidon GL. Bioequivalence of oral products and the biopharmaceutics classification system: science, regulation, and public policy. Clin Pharmacol Ther. 2011;90(3):467–470. doi: 10.1038/clpt.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahan A, Lennernäs H, Amidon GL. The fraction dose absorbed, in humans, and high jejunal human permeability relationship. Mol Pharm. 2012;9(6):1847–1851. doi: 10.1021/mp300140h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDER/FDA. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate release dosage forms based on a biopharmaceutical slassification system. Center for Drug Evaluation and Research; 2000.
- 15.Miller JM, Beig A, Carr RA, Spence JK, Dahan A. A win–win solution in oral delivery of lipophilic drugs: supersaturation via amorphous solid dispersions increases apparent solubility without sacrifice of intestinal membrane permeability. Mol Pharm. 2012;9(7):2009–2016. doi: 10.1021/mp300104s. [DOI] [PubMed] [Google Scholar]
- 16.Miller JM, Beig A, Carr RA, Webster GK, Dahan A. The solubility–permeability interplay when using cosolvents for solubilization: revising the way we use solubility-enabling formulations. Mol Pharm. 2012;9(3):581–590. doi: 10.1021/mp200460u. [DOI] [PubMed] [Google Scholar]
- 17.Miller JM, Beig A, Krieg BJ, Carr RA, Borchardt TB, Amidon GE, et al. The solubility–permeability interplay: mechanistic modeling and predictive application of the impact of micellar solubilization on intestinal permeation. Mol Pharm. 2011;8(5):1848–1856. doi: 10.1021/mp200181v. [DOI] [PubMed] [Google Scholar]
- 18.Miller JM, Dahan A, Gupta D, Varghese S, Amidon GL. Quasi-equilibrium analysis of the ion-pair mediated membrane transport of low-permeability drugs. J Control Release. 2009;137(1):31–37. doi: 10.1016/j.jconrel.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Miller JM, Dahan A, Gupta D, Varghese S, Amidon GL. Enabling the intestinal absorption of highly polar antiviral agents: ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir. Mol Pharm. 2010;7(4):1223–1234. doi: 10.1021/mp100050d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci. 2010;99(6):2739–2749. doi: 10.1002/jps.22033. [DOI] [PubMed] [Google Scholar]
- 21.Wohnsland F, Faller B. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J Med Chem. 2001;44(6):923–930. doi: 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]
- 22.Dahan A, Amidon GL. Segmental dependent transport of low permeability compounds along the small intestine due to P-gp: the role of efflux transport in the oral absorption of BCS class III drugs. Mol Pharm. 2009;6(1):19–28. doi: 10.1021/mp800088f. [DOI] [PubMed] [Google Scholar]
- 23.Dahan A, Amidon GL. Grapefruit juice and its constituents augment colchicine intestinal absorption: potential hazardous interaction and the role of P-glycoprotein. Pharm Res. 2009;26(4):883–892. doi: 10.1007/s11095-008-9789-7. [DOI] [PubMed] [Google Scholar]
- 24.Dahan A, Miller JM, Hilfinger JM, Yamashita S, Yu LX, Lennernäs H, et al. High-permeability criterion for BCS classification: segmental/pH dependent permeability considerations. Mol Pharm. 2010;7(5):1827–1834. doi: 10.1021/mp100175a. [DOI] [PubMed] [Google Scholar]
- 25.Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–1405. doi: 10.1021/mp100149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahan A, Amidon GL. Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. Am J Physiol Gastrointest Liver Physiol. 2009;297(2):G371–G377. doi: 10.1152/ajpgi.00102.2009. [DOI] [PubMed] [Google Scholar]
- 27.Dahan A, Sabit H, Amidon GL. Multiple efflux pumps are involved in the transepithelial transport of colchicine: combined effect of P-gp and MRP2 leads to decreased intestinal absorption throughout the entire small intestine. Drug Metab Dispos. 2009;37(10):2028–2036. doi: 10.1124/dmd.109.028282. [DOI] [PubMed] [Google Scholar]
- 28.Dahan A, Amidon GL. MRP2 mediated drug–drug interaction: indomethacin increases sulfasalazine absorption in the small intestine, potentially decreasing its colonic targeting. Int J Pharm. 2010;386(1–2):216–220. doi: 10.1016/j.ijpharm.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Winne D. Shift of pH-absorption curves. J Pharmacokinet Biopharm. 1977;5(1):53–94. doi: 10.1007/BF01064809. [DOI] [PubMed] [Google Scholar]
- 30.Wagner JG, Sedman AJ. Quantitaton of rate of gastrointestinal and buccal absorption of acidic and basic drugs based on extraction theory. J Pharmacokinet Pharmacodyn. 1973;1(1):23–50. doi: 10.1007/BF01060026. [DOI] [Google Scholar]
- 31.Teksin ZS, Hom K, Balakrishnan A, Polli JE. Ion pair-mediated transport of metoprolol across a three lipid-component PAMPA system. J Control Release. 2006;116(1):50–57. doi: 10.1016/j.jconrel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Vree T, Muskens A, van Rossum J. Some physico-chemical properties of amphetamine and related drugs. J Pharm Pharmacol. 1969;21(11):774–775. doi: 10.1111/j.2042-7158.1969.tb08168.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Mitchell S, Kijek P, Tsume Y, Hilfinger J, Amidon GL. The suitability of an in situ perfusion model for permeability determinations: utility for BCS class I biowaiver requests. Mol Pharm. 2006;3(6):686–694. doi: 10.1021/mp060042f. [DOI] [PubMed] [Google Scholar]
- 34.Polli J, Abrahamsson B, Yu L, Amidon G, Baldoni J, Cook J, et al. Summary workshop report: bioequivalence, biopharmaceutics classification system, and beyond. AAPS J. 2008;10(2):373–379. doi: 10.1208/s12248-008-9040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jobin G, Cortot A, Godbillon J, Duval M, Schoeller J, Hirtz J, et al. Investigation of drug absorption from the gastrointestinal tract of man. I. Metoprolol in the stomach, duodenum and jejunum. Br J Clin Pharmacol. 1985;19(Suppl 2):97S–105S. doi: 10.1111/j.1365-2125.1985.tb02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masaoka Y, Tanaka Y, Kataoka M, Sakuma S, Yamashita S. Site of drug absorption after oral administration: assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. Eur J Pharm Sci. 2006;29(3–4):240–250. doi: 10.1016/j.ejps.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Dahan A, West BT, Amidon GL. Segmental-dependent membrane permeability along the intestine following oral drug administration: evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. Eur J Pharm Sci. 2009;36(2–3):320–329. doi: 10.1016/j.ejps.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Li LY, Amidon GL, Kim JS, Heimbach T, Kesisoglou F, Topliss JT, et al. Intestinal metabolism promotes regional differences in apical uptake of indinavir: coupled effect of P-glycoprotein and cytochrome P450 3A on indinavir membrane permeability in rat. J Pharmacol Exp Ther. 2002;301(2):586–593. doi: 10.1124/jpet.301.2.586. [DOI] [PubMed] [Google Scholar]
- 39.Lindahl A, Sjoberg A, Bredberg U, Toreson H, Ungell A, Lennernas H. Regional intestinal absorption and biliary excretion of fluvastatin in the rat: possible involvement of mrp2. Mol Pharm. 2004;1(5):347–356. doi: 10.1021/mp0499297. [DOI] [PubMed] [Google Scholar]
- 40.Ungell AL, Nylander S, Bergstrand S, Sjoberg A, Lennernas H. Membrane transport of drugs in different regions of the intestinal tract of the rat. J Pharm Sci. 1998;87(3):360–366. doi: 10.1021/js970218s. [DOI] [PubMed] [Google Scholar]
- 41.Pade V, Stavchansky S. Estimation of the relative contribution of the transcellular and paracellular pathway to the transport of passively absorbed drugs in the Caco-2 cell culture model. Pharm Res. 1997;14(9):1210–1215. doi: 10.1023/A:1012111008617. [DOI] [PubMed] [Google Scholar]
- 42.Pade V, Stavchansky S. Link between drug absorption solubility and permeability measurements in Caco-2 cells. J Pharm Sci. 1998;87(12):1604–1607. doi: 10.1021/js980111k. [DOI] [PubMed] [Google Scholar]
- 43.Palamanda J, Mei H, Morrison R, McLeod R, McCormick K, Corboz M, et al. Pharmacokinetics of pseudoephedrine in rats, dogs, monkeys and its pharmacokinetic-pharmacodynamic relationship in a feline model of nasal congestion. Drug Metab Lett. 2010;4(2):56–61. doi: 10.2174/187231210791292762. [DOI] [PubMed] [Google Scholar]
- 44.Chen M-L, Yu L. The use of drug metabolism for prediction of intestinal permeability. Mol Pharm. 2009;6(1):74–81. doi: 10.1021/mp8001864. [DOI] [PubMed] [Google Scholar]


