Abstract
Staphylococcus aureus is a leading cause of ventricular assist device–related infections. This study evaluated the protective effect against S. aureus infection of active and passive immunization that targeted 3 proteins involved in bacterial attachment to a murine intra-aortic polyurethane patch. Active immunization of mice with a combination of the A domains of clumping factor A (ClfA), fibronectin-binding protein A (FnBPA) and fibronectin-binding protein B or passive immunization with monoclonal antibodies against ClfA and FnBPA resulted in a higher level of protection than that obtained by vaccination with either protein or antibody alone. The combination of antibodies or protein antigens appears to provide enhanced protection against prosthetic-device infection.
Ventricular assist devices (VADs) are an important form of therapy for patients with end-stage congestive heart failure. However, a high incidence of VAD-related infections, often caused by Staphylococcus aureus, has been a serious limitation to the long-term use of VADs [1]. Eradication of infection usually requires removal of the device, posing a major threat to survival [1, 2]. The VAD surface undergoes a number of changes over time as host components adhere and form a dynamic neointimal layer [3]. The first step in the pathogenesis of VAD-related infections involves bacterial adhesion to the prosthetic neointimal surface. For this process, S. aureus possesses an array of surface proteins that adhere to both cellular and extracellular host components [4].
Via their fibrinogen-binding domains, 3 adhesins—clumping factor A (ClfA), fibronectin-binding protein A (FnBPA), and fibronectin-binding protein B (FnBPB)—mediate in vitro adherence of S. aureus to explanted VAD membranes, as well as in vivo binding to an implanted aortic patch in mice [5]. In this study, we investigated the potential role of the A domains of ClfA, FnBPA, and FnBPB (rAClfA, rAFnBPA, and rAFnBPB, respectively) as protective antigens against S. aureus infection in an aortic patch murine model by both active and passive immunization.
Materials and methods
S. aureus strain LS-1 [6] was grown at 37°C in tryptic soy broth or mannitol salt agar (BD Biosciences). Escherichia coli strain XL-1 Blue (Stratagene) was grown at 37°C in Luria Bertani broth (BD Biosciences), with or without 1% agar, supplemented with ampicillin (100 µg/mL). Oligonucleotides used to generate the appropriate DNA fragments that code for the A domains of ClfA, FnBPA, and FnBPB were described elsewhere [7, 8]. DNA fragments were amplified from S. aureus strain LS-1 using Platinum PCR Supermix High Fidelity (Invitrogen). The fragments were digested, ligated to pQE-30 (Qiagen) by use of the appropriate restriction enzymes (New England Biolabs), and introduced into E. coli strain XL1-Blue. Recombinant polyhistidine-tagged proteins were purified using HisTrap HP columns (GE Healthcare) in accordance with the manufacturer’s instructions and dialyzed extensively against phosphate-buffered saline (PBS). Protein concentrations were determined using the Bio-Rad Protein Assay.
Monoclonal antibody (MAb) 12-9 (anti-rAClfA) was raised against rAClfA from S. aureus strain Newman and has been described elsewhere [5]. MAb 43-677.2 (anti-rAFnBPA) recognizes the peptide-spanning repeats N2 and N3 of FnBPA from S. aureus strain 8325-4 and inhibits rAFnBPA binding to fibrinogen (kindly provided by John Vernachio [Inhibitex]). Between 3 and 6 h after implantation of the aortic patch, C57BL/6J mice were immunized by intraperitoneal injection with 1 mg of the corresponding antibodies unless otherwise stated. Murine IgG1 (Sigma) was used as an isotypic control antibody. For active immunization experiments, C57BL/6J mice were immunized using a previously described protocol [9], and each mouse received 30 µg of protein per injection. Bovine serum albumin (BSA [Sigma]) was used as the antigen control. The first subcutaneous immunization was performed on day −21, followed by a booster injection on day −10. Aortic patch implantation was performed on day 0. Blood samples were obtained by retro-orbital bleeding on day 0 for measurement of antibody concentrations.
Implantation of polyurethane patches and infection of mice were performed using our previously described model with minor modifications [5]. Briefly, S. aureus strain LS-1 was cultured overnight to stationary phase, diluted into fresh medium and allowed to reach logarithmic growth phase. Cells were harvested and resuspended in PBS to an OD600 of 0.15. Sterile polyurethane patches (size, 1 × 4 mm [Thoratec Corporation]) were implanted in the abdominal aorta. Twenty-four hours later, all mice were challenged by tail vein injection with an identical bacterial inoculum (7 × 106–10 × 106 colony-forming units [CFU]). This inoculum had been previously shown to achieve an infection rate of 100%. A blood sample was obtained 30 min after inoculation to confirm bacteremia. Twenty-four hours after inoculation, animals were anesthetized, and polyurethane patches were excised and processed as previously described [5]. Bacterial loads were calculated by serial dilution of homogenized samples, plating onto tryptic soy agar, and incubation for 24 h at 37°C.
Serum antibody concentrations from individual mice were determined by enzyme-linked immunosorbent assay (ELISA). Triplicate microtiter wells were coated with 0.25 µg of the A domains of ClfA, FnBPA, or FnBPB. Wells were blocked with 2% skim milk in PBS and incubated with sera from immunized mice (dilution, 1:1000 in PBS), followed by incubation with anti-mouse IgG horseradish peroxidase– conjugated antibody (Sigma). Peroxidase activity was measured using 1-Step Ultra-TMB-ELISA (Pierce), according to the manufacturer’s instructions. Statistical evaluation was done by analysis of variance.
Whole-cell dot immunoblot was performed as follows: serial dilutions of a bacterial suspension with an OD600 of 50 were prepared in PBS, and 1-µL drops were adsorbed to nitrocellulose filters, air dried, blocked with 5% skim milk in PBS supplemented with 0.05% Tween-20 (PBS-T), and incubated with anti-rAFnBPA (dilution, 1:5000 in PBS-T), followed by anti-mouse IgG horseradish peroxidase– conjugated antibody (dilution, 1:5000 in PBS-T) and Immobilon Western substrate (Millipore), according to the manufacturer’s instructions.
The Institutional Animal Care and Use Committee of Columbia University approved this study.
Results
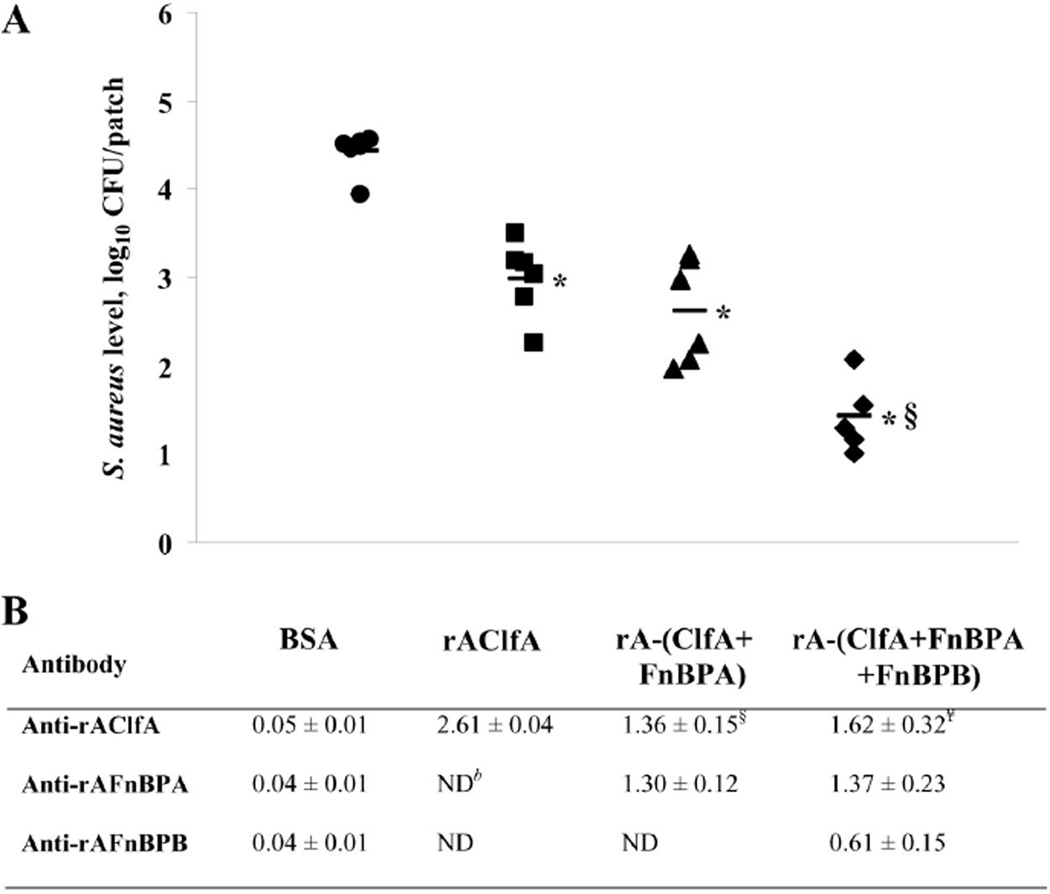
Patches from mice actively immunized with rAClfA had significantly lower levels of S. aureus than patches explanted from control mice immunized with BSA (P < .01), suggesting that an immune response against rAClfA was able to partially protect against S. aureus colonization of the aortic patch. Subsequently, mice were vaccinated with combinations of 2 or 3 antigens. Vaccination with a combination of rAClfA and rAFnBPA elicited levels of protection against patch infection by S. aureus that were similar to those obtained by vaccination with rAClfA alone (P = .54). However, a mixture of all 3 antigens previously shown to mediate in vivo colonization of aortic patches yielded a significantly higher level of protection against S. aureus attachment to the implanted material than did immunization with rAClfA alone (P < .01) (figure 1).
Figure 1.
Levels of Staphylococcus aureus recovered from intra-aortic patches in actively immunized mice (A), and immune responses (expressed as mean absorbance450 ± SEM) in serum specimens from individual mice in each immunization group (B). Mice were immunized with bovine serum albumin (BSA; circles), the A domain of clumping factor A (rAClfA; squares), rAClfA and the A domain of fibronectin-binding protein A (rAFnBPA; triangles), or rAClfA, rAFnBPA, and the A domain of fibronectin-binding protein B (rAFnBPB; diamonds). Horizontal black bars, mean values. CFU, colony-forming units; ND, not determined. *P < .01, compared with the control (BSA); §P < .01, compared with rAClfA; ¥P < .01, compared with antibody response in mice immunized with rAClfA alone.
The titers of specific antibodies from actively immunized mice against the different staphylococcal surface proteins were determined. The presence of specific antibodies was consistent with the pattern of immunogens used in each group of mice. Interestingly, the immune response to rAClfA was significantly higher in mice immunized with rAClfA alone than in those animals vaccinated with combinations of 2 or 3 staphylococcal immunogens (P < .01). In the 2 groups that received multiple immunogens, the immune response was divided among the corresponding immunogens, although the total anti-MSCRAMM IgG levels remained at comparable levels (figure 1).
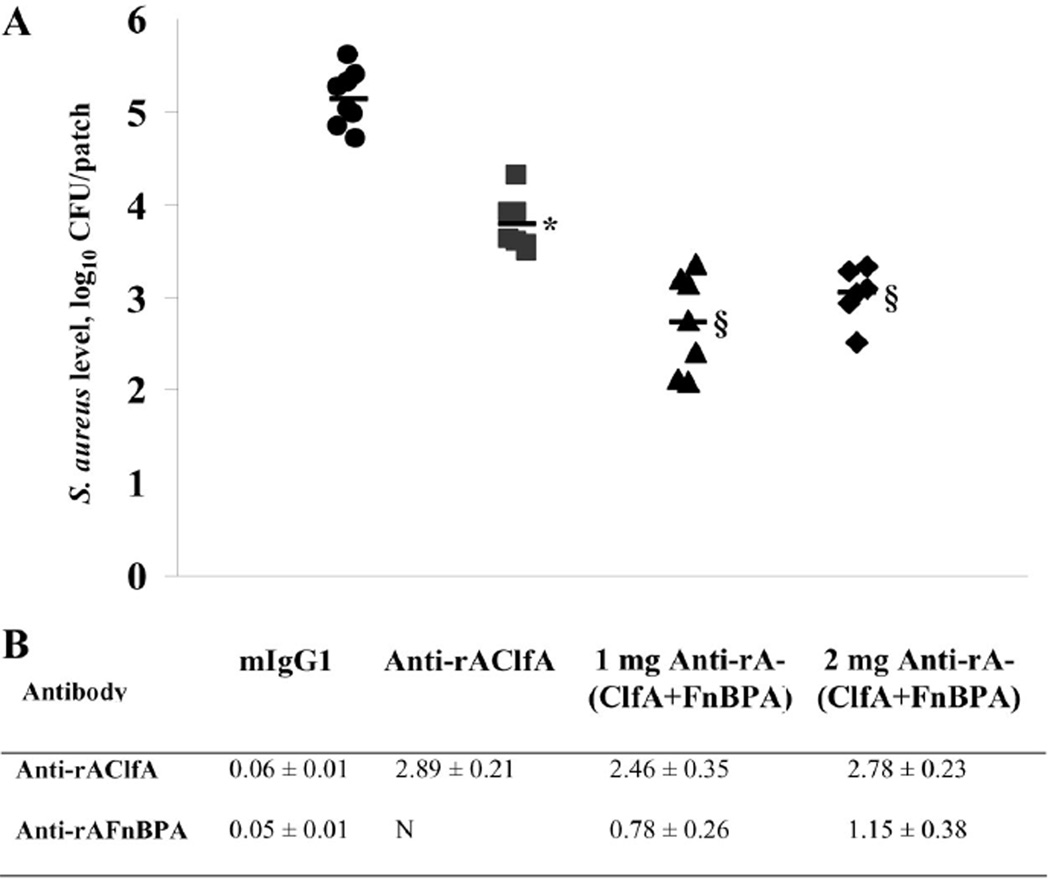
Mice were passively immunized with monoclonal antibodies directed against the fibrinogen-binding domains of ClfA and FnBPA. Monoclonal antibodies against rAFnBPB were not available during these studies. Figure 2 shows that passive immunization with 1 mg of anti-rAClfA antibodies significantly reduced levels of S. aureus, compared with immunization with isotypic control antibodies (P < .01). Subsequently, mice were immunized with 1 mg of a 1:1 mixture of murine IgG1 anti-rAClfA and anti-rAFnBPA. A further reduction in the level of S. aureus strain LS-1 colonization of aortic patches was observed (P < .01) (figure 2). Finally, to determine whether this observed protective effect could be enhanced by increasing the amount of IgG1 per animal, we measured S. aureus infection of aortic patches from mice previously immunized with 2 mg of the 1:1 mixture of anti-rAClfA and anti-rAFnBPA. No significant difference in S. aureus colonization of patches was observed when the dose of antibodies was doubled (figure 2).
Figure 2.
Levels of Staphylococcus aureus recovered from intra-aortic patches in passively immunized mice (A), and antibody titers (expressed as mean absorbance450 ± SEM) that remained in serum specimens from individual mice in each immunization group (B). Mice were immunized with 1 mg of isotypic control murine IgG1 (circles), 1 mg of antibody to the A domain of clumping factor A (anti-rAClfA; squares), 0.5 mg of anti-rAClfA plus 0.5 mg of antibody to the A domain of fibronectin-binding protein A (anti-rAFnBPA; triangles), or 1 mg of anti-rAClfA plus 1 mg of anti-rAFnBPA (diamonds). Horizontal black bars, mean values. CFU, colony-forming units; ND, not determined. *P < .01, compared with the isotopic control; §P < .01, compared with anti-rAClfA.
Anti-rAClfA and anti-rAFnBPA levels that remained in the blood were determined (figure 2). Both types of single-targeted immunization rendered comparable levels of anti-rAClfA titers (P = .025). Similarly, mice immunized against rAClfA and rAFnBPA showed high levels of antibodies irrespective of the type of immunization used (figures 1 and 2).
In light of the reported allelic variation in S. aureus FnBPA, which might affect the efficacy of an immunization strategy, we used whole-cell dot immunoblot titration to compare the reactivity of anti-rAFnBPA against unique clinical isolates of S. aureus. The MAb used in this study recognized FnBPA on the cell surfaces of S. aureus strains LS-1 and 8325–4 and of 6 of 7 S. aureus clinical isolates tested with similar efficiency. The remaining clinical isolate was recognized by anti-rAFnBPA with a lower efficiency (data not shown).
Discussion
We previously demonstrated that the fibrinogen-binding domains of ClfA, FnBPA, and FnBPB are important virulence factors in S. aureus infection of murine intra-aortic polyurethane patches [5]. Similarly, ClfA and FnBPA have also been shown to constitute virulence determinants in the development of other staphylococcal infections, including arthritis and endocarditis [9–11].
In this study we showed that immunization with purified rAClfA was partially protective against staphylococcal attachment to implanted aortic polyurethane patches. Moreover, a comparable result was obtained when mice were passively immunized with a MAb that recognized the fibrinogen-binding domain of ClfA. Although protection was not complete with either type of immunization, the bacterial inoculum in the clinical setting is likely to be much lower than the 106–107 CFU used in this model.
Antibodies that recognize the A domain of ClfA on the S. aureus surface may reduce the risk of patch infection and enhance survival by blocking S. aureus from binding to the implanted material, a process facilitated in vivo by rAClfA [5]. In addition, anti-rAClfA IgGs could enhance bacterial clearance by opsonization of S. aureus, thereby facilitating phagocytosis of the bacteria by neutrophils. Elsewhere, it was shown that vaccination with the fibrinogen-binding domain of ClfA protected mice against S. aureus arthritis [9]. Likewise, passive immunization with anti-rAClfA rat IgGs protected mice against septic death [9]. This study demonstrates a similar finding for prosthetic material.
We hypothesized that the redundancy in fibrinogen-binding proteins previously shown to mediate the binding of S. aureus to implanted aortic patches might hamper the efficiency of our strategy to block this event. A multiple-target approach might therefore provide enhanced protection against patch colonization by S. aureus. Vaccination with a combination of rAClfA and rAFnBPA elicited a level of protection against patch colonization that was similar to the level previously observed with rAClfA alone. However, when mice were immunized with all 3 fibrinogen-binding domains (i.e., rAClfA, rAFnBPA and rAFnBPB), significantly better protection was obtained. Fixed amounts of each antigen (60 µg per animal) were used throughout these active immunization experiments. Titers of the corresponding specific antibodies in blood were at comparable levels in all 3 groups of mice irrespective of the formulation of the immunogen mixture used. Also, rAFnBPB appeared to be less immunogenic than rAClfA and rAFnBPA but still improved protection against S. aureus colonization. This suggests that the combination of antigenic targets plays an important role in protecting against S. aureus colonization of the implanted patch.
A combination of 2 different MAbs during passive immunization also appears to elicit better protection against patch colonization than a single-MAb immunization strategy. This suggests that antibodies directed against fibrinogen-binding domains can effectively block adherence. It has been previously shown that immunization with fusion proteins encompassing fibronectin-binding D-domains from FnBPA resulted in the production of antibodies able to block adherence to fibronectin and in protection against S. aureus in endocarditis and mastitis animal models [12, 13].
A limitation of the study was the variability of the results, especially those between the actively and passively immunized groups. The difficulty of performing the surgical procedure limited the number of mice that could be studied at one time and thus increased the variability of results. In addition, differences in preinfection procedures precluded comparison between the actively and passively immunized groups.
The variety of virulence factors in S. aureus suggests that the development of an effective vaccine will most likely require multiple immunologic targets. It is increasingly accepted that multitarget vaccines are the best approach towards a successful vaccine [14]. It has been proposed that these targets should span a variety of activities, ranging from staphylococcal attachment to host components and cell penetration to immune system evasion [14]. Recently, other authors have shown enhanced protection against S. aureus infection by an immunization approach that targets proteins involved in different steps of the pathogenic process [15]. However, to our knowledge, this is the first study demonstrating that vaccination with a combination of antigens directed against a single process (i.e., bacterial attachment to a prosthetic material) can also result in increased protection against S. aureus infection. Allelic variation, as observed with the fibronectin-binding proteins, will need to be considered in selecting candidate vaccine antigens. This approach, therefore, has potential relevance for the prevention of prosthetic device infections in general.
Acknowledgments
We thank Peter Vavagiakis for his valuable help with the statistical analysis and Inhibitex for kindly providing the antibodies used in this study.
Financial support: NHCBI Specialized Center for Clinically Oriented Research (grant HL077096).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.Holman WL, Park SJ, Long JW, et al. Infection in permanent circulatory support: experience from the REMATCH trial. J Heart Lung Transplant. 2004;23:1359–1365. doi: 10.1016/j.healun.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Gordon RJ, Quagliarello B, Lowy FD. Ventricular assist device-related infections. Lancet Infect Dis. 2006;6:426–437. doi: 10.1016/S1473-3099(06)70522-9. [DOI] [PubMed] [Google Scholar]
- 3.Spanier TB, Chen JM, Oz MC, Stern DM, Rose EA, Schmidt AM. Time-dependent cellular population of textured-surface left ventricular assist devices contributes to the development of a biphasic systemic procoagulant response. J Thorac Cardiovasc Surg. 1999;118:404–413. doi: 10.1016/S0022-5223(99)70176-5. [DOI] [PubMed] [Google Scholar]
- 4.Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 5.Arrecubieta C, Asai T, Bayern M, et al. The role ofStaphylococcus aureus adhesins in the pathogenesis of ventricular assist device-related infections. J Infect Dis. 2006;193:1109–1119. doi: 10.1086/501366. [DOI] [PubMed] [Google Scholar]
- 6.Bremell T, Lange S, Svensson L, et al. Outbreak of spontaneous staphylococcal arthritis and osteitis in mice. Arthritis Rheum. 1990;33:1739–1744. doi: 10.1002/art.1780331120. [DOI] [PubMed] [Google Scholar]
- 7.O’Connell DP, Nanavaty T, McDevitt D, Gurusiddappa S, Hook M, Foster TJ. The fibrinogen-binding MSCRAMM (clumping factor) of Staphylococcus aureus has a Ca2+-dependent inhibitory site. J Biol Chem. 1998;273:6821–6829. doi: 10.1074/jbc.273.12.6821. [DOI] [PubMed] [Google Scholar]
- 8.Roche FM, Downer R, Keane F, Speziale P, Park PW, Foster TJ. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J Biol Chem. 2004;279:38433–38440. doi: 10.1074/jbc.M402122200. [DOI] [PubMed] [Google Scholar]
- 9.Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis. 2001;184:1572–1580. doi: 10.1086/324430. [DOI] [PubMed] [Google Scholar]
- 10.Que YA, Haefliger JA, Piroth L, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med. 2005;201:1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreillon P, Entenza JM, Francioli P, et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schennings T, Heimdahl A, Coster K, Flock JI. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb Pathog. 1993;15:227–236. doi: 10.1006/mpat.1993.1073. [DOI] [PubMed] [Google Scholar]
- 13.Mamo W, Jonsson P, Flock JI, et al. Vaccination against Staphylococcus aureus mastitis: immunological response of mice vaccinated with fibronectin-binding protein (FnBP-A) to challenge with S. aureus. Vaccine. 1994;12:988–992. doi: 10.1016/0264-410x(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 14.Shinefield HR, Black S. Prospects for active and passive immunization against Staphylococcus aureus. Pediatr Infect Dis J. 2006;25:167–168. doi: 10.1097/01.inf.0000199887.18267.9a. [DOI] [PubMed] [Google Scholar]
- 15.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]