Abstract
Preeclampsia is an important syndrome complicating pregnancy. While the pathogenesis of preeclampsia is not entirely known, poor placental perfusion leading to widespread maternal endothelial dysfunction is accepted as a major mechanism. It has been suggested that altered placental expression of matrix metalloproteinases (MMPs) may cause shallow cytotrophoblastic invasion and incomplete remodeling of the spiral arteries. MMPs are also thought to link placental ischemia to the cardiovascular alterations of preeclampsia. In fact, MMPs may promote vasoconstriction and surface receptors cleavage affecting the vasculature. Therefore, the overall goal of this review article is to provide an overview of the pathophisiology of preeclampsia, more specifically regarding the role of MMPs in the pathogenesis of preeclampsia and the potential of MMP inhibitors as therapeutic options.
Keywords: Hypertension, hypertensive disorders, matrix metalloproteinases, preeclampsia, pregnancy, therapy
1. INTRODUCTION
Hypertensive disorders affect up to 10% of pregnancies worldwide [1], being one of the major causes of maternal death in developed countries (~16%) and in Latin America (~26%) [2]. The National High Blood Pressure Education Program (NHBPEP) categorizes hypertension during pregnancy as follows [3]:
Preeclampsia-eclampsia: new-onset hypertension (>140 mmHg systolic or >90 mmHg diastolic blood pressure) and proteinuria (>0.3 g in 24 h) after 20 weeks of gestation in a previously normotensive women. If seizures also occur, the disease is called eclampsia;
Chronic hypertension: hypertension present before pregnancy or first diagnosed before 20 weeks of gestation;
Chronic hypertension superimposed on preeclampsia: new-onset or acutely worse proteinuria, sudden increase in blood pressure, thrombocytopenia, or elevated liver enzymes after 20 weeks of gestation in women with preexisting hypertension;
Gestational hypertension: hypertension first diagnosed after 20 weeks of gestation, not accompanied by proteinuria. If blood pressure returns to normal by 12 weeks post-partum it is called transient hypertension, otherwise it is considered as chronic hypertension.
Besides increasing the risk of maternal mortality and morbidity, most fetal adverse events (i.e., intra-uterine grow restriction, preterm birth, low birth weight, and perinatal death) are attributable directly to preeclampsia [4]. In addition, recent studies have suggested that preeclamptic women [5–7] and their offspring [8] are at increased risk of cardiovascular and renal diseases later in life. Although its pathophysiology is not entirely known, there are several recognized risk factors for the development of preeclampsia, such as primiparity, multiple gestation, ethnicity, preexisting medical conditions (hypertension, diabetes), and obesity [9–11]. A higher incidence of these risk factors in industrialized countries over the last decades is a most likely cause for an increased rate of preeclampsia [1]. While the only definitive cure for preeclampsia is still delivery of the fetus and placenta [12], unraveling the etiologic mechanisms of preeclampsia may provide better approaches for treatment and ultimately prevention [13]. Therefore, the overall goal of this review is to provide an overview of the pathophisiology of preeclampsia, more specifically regarding the role of matrix metalloproteinases (MMPs), and the potential of MMP inhibitors as therapeutic options.
2. CARDIOVASCULAR ADAPTATIONS DURING NORMAL PREGNANCY
Several functional and anatomical alterations occur in the cardiovascular system of the pregnant woman to ensure nutrient supply to the fetus. During normal gestation, there is an expansion of 40–50% in blood volume [14–16], due to a greater increase in plasma volume than an increase in red blood cell mass, resulting in the physiologic anemia of pregnancy [17, 18]. An elevation in heart rate and stroke volume leads to an increase of 30–50% in cardiac output [14, 15, 19]. Additionally, there is a widespread vasodilatation, with increased arterial compliance and reduced peripheral vascular resistance [14, 15, 19], enhancing the blood flow especially in the uteroplacental circulation [20–22]. There is also an increase in renal blood flow (RBF) by 60–80% and in glomerular filtration rate (GFR) up to 50% [14, 23]. As a result of systemic vasodilatation and renal hyperfiltration, there is a decrease in systolic and diastolic blood pressure of about 5–10 mmHg [14, 15, 19, 24]. Moreover, these physiological alterations in preload and afterload enlarge the cardiac chambers, particularly the left ventricle which undergoes remodeling with increased wall thickness and mass [19, 25, 26]. Interestingly, this hemodynamic shift begins prior to placentation, reaches a peak in the second trimester of pregnancy, and then remains relatively constant until delivery [14, 15].
The cardiovascular adaptations to gestation are mainly induced by humoral and neural mechanisms, with multiple receptors and effectors interacting to regulate blood pressure [27]. Placental hormones, such as estrogen, progesterone and human chorionic gonadotropin, also exert a significant effect on maternal hemodynamics [28]. These hormones interact with the the renin-angiotensin-aldosterone system (RAAS) [29] and the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway to control blood pressure during pregnancy [30]. Studies in humans and pregnant models have implicated NO [31, 32] and prostacyclin [33, 34] as key vasodilators responsible for the reduced vascular resistance seen in pregnancy [35]. Another important mechanism contributing for the systemic vasodilatation is a decreased vascular responsiveness to angiotensin II [36]. These changes initiate further baroreceptor-mediated neurohormonal events, including activation of the RAAS [14, 37–39], with a subsequent increase in body sodium and water retention. Alterations in cortisol, vasopressin, kallikreins, vascular endothelial growth factor (VEGF), atrial natriuretic peptide, and in the sympathetic nervous system may also mediate cardiovascular adaptations during pregnancy [27, 35]. In addition, relaxin, an ovarian hormone secreted in large amounts by the placenta and decidua during gestation, appears to be an upstream mediator of the increased renal NO synthesis, resulting in an elevated RBF and GFR [40]. However, preeclamptic pregnancies are not accompanied by many of these alterations, being characterized by high vascular resistance, low plasma volume and reduced cardiac output [41–43], which ultimately leads to increased blood pressure in order to guarantee placental and fetal demands for oxygen and nutrients.
3. PATHOPHYSIOLOGY OF PREECLAMPSIA
Experimental studies in animals and humans have implicated placental ischemia and hypoxia as a central causative factor in the etiology of preeclampsia [44–46]. The pathophysiology of preeclampsia is thought to occur in two stages. The first stage is a poorly understood abnormality in the normal placentation process which is maternally asymptomatic. The second symptomatic stage is associated with altered proangiogenic and antiangiogenic factor balance, increased maternal oxidative stress, and immunological dysfunction. There is also widespread activation/dysfunction of the maternal vascular endothelium which results in enhanced formation of endothelin and decreased nitric oxide synthesis and/or bioavailability. These endothelial abnormalities, in turn, cause hypertension by impairing renal function and increasing total peripheral resistance. Recent research into both of these stages has revealed glimpses into the underlying origins of the disease and the mechanisms of the resulting maternal symptoms.
There is now growing awareness that immunological dysfunction is an important factor in the pathogenesis of preeclampsia [47]. While it is well established that pregnancy alone sets off an increased maternal inflammatory response, the secretion of inflammatory cytokines in preeclamptic women is markedly increased. Recent work has demonstrated that the production of tumor Necrosis Factor-α (TNF-α) and other inflammatory cytokines is primed by circulating syncytiotrophoblast microparticles, which are elevated in preeclamptic women [48]. In the reduced uterine perfusion pressure (RUPP) animal model of preeclampsia, circulating levels of interleukin-6 (IL-6) and TNF-α are elevated, and these alterations are consistent with those found in human subjects [49, 50]. Furthermore, infusion of IL-6 or TNF-α to levels consistent with those seen in preeclamptic women leads to gestational hypertension in rats [51]. Moreover, TNF-α blockade by the soluble TNF-α receptor etanerecept in the RUPP model partially attenuates the associated hypertension [51].
Another interesting recent development in our understanding of the role of immunity in the pathophysiology of preeclampsia is the identification of a circulating angiotensin II type-I receptor agonistic autoantibody (AT1-AA) [52–54]. The AT1-AA was found in the circulation of preeclamptic patients, and its epitope eventually mapped to the second extracellular loop of the AT1 receptor [54]. In response to placental ischemia in pregnant rats AT1-AA is produced to levels comparable to those seen in preeclamptic women [53]. Furthermore, chronic administration of the purified autoantibody to normal pregnant rats to levels seen in pregnant women and RUPP rats resulted in concurrent ~20% increases in blood pressure and dramatic increases in tissue expression of ET-1 [52].
Another promising area of research on the etiology of preeclampsia is the interplay between pro- and anti-angiogenic factors [55–58]. Soluble fms-like tyrosine kinase-1 (sFlt-1) is a splice variant of the longer VEGFR-1 cell surface receptor in which the cytoplasmic and transmembrane domains have been post-transcriptionally excised. This molecule, which is produced by placental trophoblasts in its soluble form (sFlt-1), acts as an antagonist for the proangiogenic proteins VEGF and PlGF by sequestering free protein in the plasma, making them unavailable for receptor binding. Furthermore, elevated levels of sFlt-1 in both placenta and plasma were shown in preeclamptic women when compared to women with normal pregnancy [57].
Observations from a number of animal models of preeclampsia also implicate sFlt-1 as an important factor in the pathology of the disorder. Adenovirus vector expression of sFlt-1 in pregnant rats produced a preeclampsia-like phenotype, with increased arterial pressure, glomerular endotheliosis, and proteinuria [57]. Furthermore, placental ischemia induced by reductions in uterine perfusion pressure in rats resulted in increased plasma and placental sFlt-1 concentrations, results confirmed in a non-human primate model of placental ischemia [59, 60]. Chronic infusion of sFlt-1 into rats to circulating levels mimicking those seen in preeclamptic women leads to significant increases in maternal blood pressure, with concomitant decreases in fetal weight and increases in both placental and vascular reactive oxygen species, an important factor in endothelial dysfunction [61, 62].
Human studies indicate that the decidua in preeclampitic women has significantly higher levels of lipid hydroperoxides and free isoprostane, a byproduct of free-radical peroxidation of arachodonic acid. A number of oxidative stress markers have also been reported systemically in preeclamptic women, among these peroxynitrite [63–65]. In the placental ischemia rat (RUPP model) there is also an increase in oxidative stress during gestation, suggesting a link between placental ischemia/hypoxia and the production of reactive oxygen species [66]. The administration of a SOD mimetic drug (tempol) attenuated the hypertensive responses to placental ischemia [66]. In a related study, administration of the NADPH oxidase inhibitor apocynin also significantly attenuated placental ischemia-induced hypertension, suggesting this enzyme as an important player in the pathogenesis of preeclampsia in the RUPP animal model [63–66].
One promising target in the study of preeclampsia pursued by our group in recent years is the potent vasoconstrictor peptide endothelin [67]. The majority of clinical studies which have investigated endothelin showed elevated levels of this peptide in preeclamptic women when compared to healthy controls [68], although this difference has not been universally observed [69]. Interestingly, the hypertension induced by placental ischemia or TNF-α infusion in pregnant rats are completely abrogated by pretreatment with an endothelin receptor type A (ET-A) antagonist [67]. In addition, studies utilizing the AT1-AA infusion in a rat model of preeclampsia demonstrated elevated tissue endothelin production [67]. Administration of ET-A specific antagonists blunted the hypertension associated with this model [67]. Finally, increased cortical endothelin transcription was found in the animal model of preeclampsia induced by sFlt-1 infusion, and the ET-A receptor blockade normalized blood pressure [60]. The elevation in endothelin concentrations in response to these varied models of preeclampsia argues for an important role of this peptide in the pathophysiology of preeclampsia.
4. ROLE OF MMPS IN THE PATHOGENESIS OF PREECLAMPSIA
Trophoblasts are important precursor cells from the human placenta which exert critical roles to promote a healthy gestation including embryo implantation, hormone production, fetal immune protection, and placental vascularization. In the first trimester of a normal pregnancy, cytotrophoblastic cells invade the uterine tissue and migrate against the bloodstream into the maternal spiral arteries, where they undergo differentiation into cells with endothelial phenotype. The trophoblastic invasion of maternal vessels results in extracellular matrix remodeling, which gives rise to high uteroplacental vessel distensibility to accommodate the increased blood flow [70–72]. In preeclampsia, however, trophoblastic invasion is reduced, leading to incomplete modification of maternal spiral arteries and therefore to decreases in placental perfusion [46, 73–75].
A prerequisite for the trophoblastic invasion success, angiogenesis and embryogenesis is the degradation and remodeling of the uterine extracellular matrix (ECM) [76, 77]. Remodeling of the umbilical cord vessels may also contribute to decreased blood flow to the fetus of women with preeclampsia.
Matrix metalloproteinases (MMPs) are a family of structurally related, zinc-dependent enzymes with multiple functions and tissue distribution [78]. Their activity target extracellular matrix components during development and morphogenesis. Specifically, MMP-2 and MMP-9 are involved in remodeling of placental and uterine arteries [79, 80], and abnormal expression of these MMPs has been reported in hypertensive disorders of pregnancy. Indeed, there is now evidence that MMPs may affect the vascular function and play a role in the vascular alterations found in preeclampsia and in other cardiovascular diseases [81–86].
Under normal circumstances, MMP activity is regulated at the level of transcription, activation of latent forms, and inhibition by endogenous MMP inhibitors (tissue inhibitors of metalloproteinase; TIMPs) [87]. Interestingly, functional genetic polymorphisms apparently modify MMP-2 transcriptional levels and may contribute to disease conditions by affecting MMP-2 transcriptional levels [88]. While MMP-9 activity is regulated at different levels including activation of MMP-9 latent forms, by interaction with TIMPs, especially TIMP-1, it is also regulated at the transcriptional level [89]. Again, genetic polymorphisms in the MMP-9 gene were also shown to affect MMP-9 transcription [90] and disease susceptibility [91, 92].
The ischemic placenta releases vasopressors into the maternal circulation that modify endothelial function by altering the balance between vasodilators (nitric oxide, prostacyclin) and vasoconstrictors (endothelin-1, increased response to angiotensin II) [13, 93–95]. The endothelial dysfunction affects multiple maternal organs, and the impaired control of vascular function contributes to hypertension and increased glomerular vascular permeability, thus leading to proteinuria, an important feature of preeclampsia.
Although unproven in the particular context of preeclampsia, it is possible that upregulated MMP activity in preeclampsia promotes increased concentrations of vasoconstrictors including endothelin-1-related peptides, and reduced concentrations of vasodilators including adrenomedullin and calcitonin gene related-peptide, as previously suggested [96–99]. These previous studies suggest that imbalanced MMP activity apparently generates vasoconstrictors and degrades vasodilators, promoting vasoconstriction and hypertension [96–99]. In addition, the release of the proform of TNF-α from its membrane-bound site is an MMP-dependent process [100, 101]. Conversely, TNF-α indirectly stimulates the proteolytic activity of MMPs [102], especially during the implantation process [103, 104], suggesting that abnormal MMPs and inflammatory mediators may interact contributing to the features of this syndrome.
Moreover, endothelial dysfunction in preeclampsia may result of oxidative stress and reduced nitric oxide bioavailability [62–65, 82]. Indeed, increased concentrations of reactive oxygen species including superoxide may enhance vascular concentrations of peroxynitrite, a powerful oxidizing agent that contributes to the pathogenesis of many cardiovascular including preeclampsia [64]. This agent may directly activate MMPs [84], although this mechanism has not been clearly shown in preeclampsia.
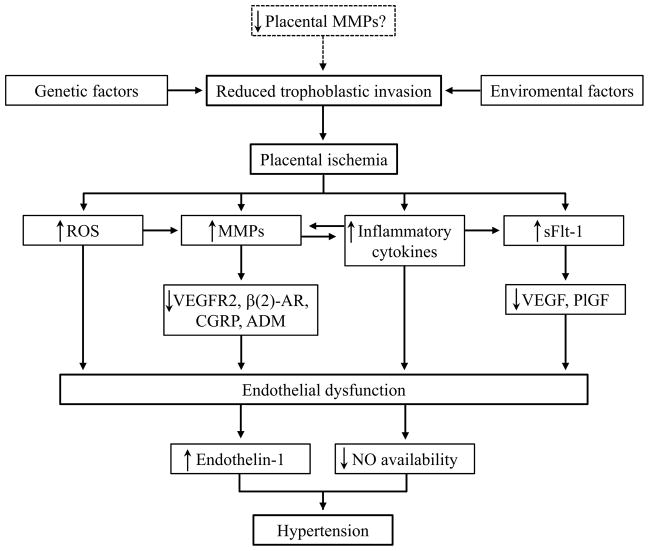
Activated MMPs may also contribute to cardiovascular dysfunction in preeclampsia through proteolysis of cell surface receptors, such as VEGFR-2 and β(2)-adrenergic receptor, as previously shown in other animal models of cardiovascular diseases (Fig. 1) [105–109]. However, these suggestions remain to be proved in preeclampsia.
Fig. 1.
Possible involvement of MMPs in the pathogenesis of preeclampsia. MMP inhibitors such as doxycycline may prevent the alterations associated with imbalanced MMP activity in preeclampsia. MMP, matrix metalloproteinase; ROS, reactive oxygen species; sFlt-1, soluble fms-like tyrosine kinase-1; VEGFR2, vascular endothelial growth factor receptor-2; β(2)-AR, β(2)-adrenergic receptor; CGRP, calcitonin gene related-peptide; ADM, adrenomedullin; VEGF, vascular endothelial growth factor; PlGF, placental growth factor; NO, nitric oxide.
5. CLINICAL FINDINGS SHOWING MMP ALTERATIONS IN PREECLAMPSIA
Although MMPs have an important function in tissue formation and remodeling during pregnancy, only few studies have evaluated the role of MMP-2 and MMP-9 in the pathophysiology of preeclampsia. Huisman et al. studied MMP-2 and MMP-9 in placental bed biopsies as early as 10–12 weeks of gestation, but their levels were not different when uncomplicated pregnancies were compared with pregnancies complicated by preeclampsia/HELLP syndrome [110]. These findings are not in agreement those reported by Kolben et al. [111] and Shokry et al. [112], who observed reduced immunologically defined MMP-9 levels in preeclamptic placental tissues collected at delivery. In addition, Galewska et al. used distinct techniques to show that preeclamptic umbilical cord tissues (artery and vein) had lower MMP-2 and MMP-9 levels than healthy tissues [113]. However, they found increased MMP-9 levels in plasma obtained from umbilical cord blood samples from preeclamptic newborns, and no significant differences in MMP-2, TIMP-1 and TIMP-2 compared with those measured in healthy pregnancies [114]. Furthermore, Lavee et al. demonstrated increased MMP-2 (by ELISA) and TIMP-2 (by western blotting) levels in aminiotic fluid of women who subsequently develop preeclampsia [115]. Interestingly, they determined pro-MMP-9 levels in normal amniotic fluid, but zymogram wells loaded with preeclamptic amniotic fluid did not present any MMP-9 bands [115].
Regarding circulating MMP-9 and TIMP-1, Kolben et al. found no significant differences in immunoreactive plasma MMP-9 concentrations between preeclamptic/eclamptic patients and healthy pregnant women [111]. While we have used zymography to show no differences in pro-MMP-9 levels [116], ELISA assays revealed that plasma MMP-9 concentrations may be increased in preeclampsia [117]. Moreover, we found elevated plasma TIMP-1 concentrations in preeclampsia, but no differences in MMP-9/TIMP-1 ratios [116, 117]. Montagnana et al. also observed no differences in serum immunoreactive MMP-9 and increased TIMP-1 concentrations in preeclampsia [118]. Conversely, Myers et al. found that western blotting defined plasma TIMP-1 levels were not altered in preeclampsia, although they reported decreased levels in the same patients before diagnosis [119]. Interestingly, Poon et al. showed that plasma MMP-9 concentrations are increased in women prior to presentation of preeclampsia. However, this biomarker did not contribute significantly to prediction of disease [120].
With respect to circulating MMP-2 and TIMP-2 levels, Davidge’s group has used zymographic techniques to show higher plasma MMP-2 levels in preeclamptic patients [121] and in women who subsequently develop preeclampsia [119] compared to healthy pregnant. Although plasma pro-MMP-2 (by zimography) and TIMP-2 levels were not statistically different in our small cohort with 32 weeks of gestation [116], MMP-2 (by ELISA) and TIMP-2 concentrations were elevated in preeclampsia when we studied a larger number of patients at 36 weeks of gestation [122]. None of our studies revealed differences in MMP-2/TIMP-2 ratios [116, 122]. Additionally, Montagnana et al. observed increased serum immunoreactive MMP-2 concentrations, but no significant differences in TIMP-2, in preeclamptic patients compared with healthy pregnant women [118].
Genetic reports have also been divergent regarding the association of MMP-2 and MMP-9 polymorphisms with preeclampsia. Coolman et al. observed a reduced prevalence of the rare T allele of the MMP-9 C−1562T polymorphism in preeclampsia [123]. Intriguingly, in vitro studies have showed that the “C” to “T” substitution at −1562 position of the MMP-9 promoter increases MMP-9 gene expression [90]. Therefore, the lower frequency of the 1562T allele in preeclamptic patients suggests that they may have decreased MMP-9 levels, which might predispose them to maladaptation of the spiral arteries and decreased degradation of the decidua. However, MMP-9 polymorphisms were not linked to preeclampsia in Fraser et al. [124] and our studies [117, 125]. In addition, we did not find significant associations between MMP-2 polymorphisms and preeclampsia [122, 126], although genetic variations of the C−1306T and C−735T polymorphisms were associated with altered plasma MMP-2 and TIMP-2 concentrations in preeclamptic patients [122]. Taken together, these findings suggest that altered MMPs and TIMPs levels may contribute to preeclampsia. However, further studies are warranted to establish how imbalanced MMP activity may contribute to the pathogenesis of preeclampsia.
6. EVIDENCE SUPPORTING THE USE OF MMP INHIBITORS IN PREECLAMPSIA
Although antihypertensive drugs do not reverse the pathogenic processes in preeclampsia, they are used to prevent and treat severe hypertension, and to extend pregnancy for as long as possible. A major challenging issue is to decide what blood pressure levels should be targeted to minimize maternal and neonatal complications and avoid fetal distress and toxicity [127]. Currently, options of anti-hypertensive drugs for preeclampsia are limited, and only few drugs have been adequately evaluated in pregnant women. Methyldopa is the drug of choice based on its well documented first trimester safety and long follow-up in neonates. Second-line agents include nifedipine, hydralazine and labetalol, which are commonly used when monotherapy with methyldopa is insufficient or in cases of methyldopa intolerance [3, 12, 127, 128].
As highlighted previously, many complex mechanisms mediate the widespread endothelial dysfunction seen in preeclampsia, leading to diverse clinical features, such as hypertension, proteinuria, edema, and cerebral and hepatic disturbances. The key role of MMPs in these cardiovascular alterations has been demonstrated in different animal models of hypertension [129–132]. Collectively, these reports have found increased MMP-2 or MMP-9 activity in different tissues, and treatment with doxycycline (a nonspecific MMP inhibitor) ameliorated hypertension, vascular dysfunction and artery/cardiac remodeling associated with this condition. Moreover, another broad-spectrum MMP inhibitor markedly blunted the age-associated increases in arterial pressure via retardation of age-associated proinflammatory signaling, preservation of intact elastin fibers, and reduction of collagen deposition [133].
To our knowledge, only one study has tested MMP inhibitors in experimental models of preeclampsia [134]. Verlohren et al. showed that doxycycline treatment from gestational day 12 to 18 resulted in lighter placentas and intrauterine growth restriction in both control and preeclamptic pregnant rats. Additionally, doxycycline reduced trophoblastic invasion and placental perfusion only in the preeclamptic group. However, they did not evaluate the effect of doxycycline on the development of pregnancy-induced hypertension [134]. Although, previous studies have also reported adverse effects of MMP inhibitors on pregnant animals [135–138], such effects were not reported by others [139–141]. Indeed, doxycycline is the only MMP inhibitor currently approved by the U.S. Food and Drug Administration (FDA) [142] and, when prescribed as an antibiotic during pregnancy, it is classified as a potentially teratogenic medication (class D: potential benefits from the use of the drug in pregnant women may be acceptable despite its potential risks) [143]. However, human studies have led to the conclusion that the teratogenic risk of doxycycline is unlikely [144–146]. Moreover, since it is used as an MMP inhibitor at sub-antimicrobial doses [147], we would expect that doxycycline causes minimal or no fetal adverse reactions when used at this dosage. Nevertheless, its use should be carefully monitored during pregnancy.
It is now clear that some antihypertensive drugs may have important effects on circulating MMP concentrations [148]. Among the drugs usually prescribed to patients with preeclampsia, clinical studies have evaluated only the effect of calcium channel blockers on MMP levels [149–151]. For example, lercanidipine therapy for 15 days decreased plasma MMP-9 activity, whereas MMP-2 and TIMP-1 levels remained unaltered [151]. Conversely, treatment of hypertensive patients with amlodipine for 6 months increased plasma MMP-9 levels back to the concentrations seen in controls [150]. Treatment with felodipine increased plasma MMP-2 concentrations, whereas diltiazem had no effects on circulating MMPs [149]. In addition, studies in rodent hypertensive models suggest similar effects of different calcium channel blockers (lercanidipine, nifedipine, nimodipine, and amlodipine) on vascular MMP-2 activity, which inhibited cardiovascular remodeling [152–154].
Since some antihypertensive drugs affect cardiovascular MMP activity, we examined whether circulating MMP-2 and MMP-9 levels are different in preeclamptic women who respond to antihypertensive therapy as compared with those who do not respond to therapy. We found lower plasma MMP-9 concentrations and MMP-9/TIMP-1 ratio (an index of net MMP-9 activity) in non-responsive patients compared to responsive patients, thus suggesting that the most severe cases of the disease may have undergone abnormal remodeling of placental and uterine tissues [117]. Conversely, we observed higher plasma MMP-2 concentrations and MMP-2/TIMP-2 ratio (an index of net MMP-2 activity) in non-responsive patients compared to responsive patients [126], thus suggesting that antihypertensive drugs may ameliorate preeclamptic symptoms by decreasing net MMP-2 activity.
We have also examined whether MMP-2 and MMP-9 polymorphisms affect the responses to antihypertensive therapy in preeclampsia. While a MMP-9 haplotype (the combination of T and H alleles of the C−1562T and −90(CA)13-25 polymorphisms, respectively) was associated with lack of responsiveness [117], MMP-2 polymorphisms were not linked to variability in responsiveness [126]. In view of these findings, we might speculate that preeclamptic patients would benefit from the use of MMPs inhibitors, at least during the systemic phase of the disease.
Finally, low-dose aspirin has been suggested as a pharmacological treatment to prevent the development of preeclampsia [155, 156] especially in high risk patients [157–159]. Since, aspirin may also affect MMPs [160–163], the association of doxycycline with aspirin may have synergic action in this syndrome. However, despite the growing evidence indicating that MMPs are pharmacological targets in cardiovascular diseases [142, 164, 165], it remains to be determined whether the use of MMPs inhibitors would improve maternal and fetal outcomes in preeclampsia.
CONCLUSIONS AND FUTURE PERSPECTIVES
The primary event in preeclampsia is proposed to be poor placental perfusion which leads to widespread maternal endothelial dysfunction. However, the causes of the reduction in the placental blood flow have yet to be fully elucidated. It has been suggested that decreased placental MMP expression may cause shallow cytotrophoblastic invasion and incomplete remodeling of the spiral arteries. MMPs are also thought to act by linking placental ischemia and cardiovascular dysfunction. Supporting a potential role of MMPs in preeclampsia are findings that placental and amniotic liquid MMP-9 levels are decreased, and plasma concentrations of MMP-2 and MMP-9 are elevated in preeclamptic women, even before the appearance of clinical symptoms. MMP-2 can stimulate vasoconstriction through the cleavage of different peptides. Moreover, MMP-9 cleaves surface receptors involved in angiogenesis and vasodilatation processes. Nevertheless, MMP-9 haplotypes have been associated with lack of responsiveness to antihypertensive drugs in preeclampsia. Collectively, these findings suggest that MMP-2 and MMP-9 may play a role in causing hypertension during pregnancy through multiple complex pathways.
The determination of plasma MMP-2 and MMP-9 concentrations and genotypes for MMP-9 polymorphisms may be valuable tools to predict which patients are at increased risk of developing preeclampsia, and which will respond to antihypertensive therapy, respectively. Such patients could benefit from the use of MMPs inhibitors such as doxycycline. However, the quantitative importance of MMPs in mediating high blood pressure and other features of preeclampsia remains unclear, and new basic and clinical studies are required, especially those designed to examine MMP inhibitors as adjuvant therapy of preeclampsia.
Acknowledgments
This study was supported by Fundacao de Amparo a Pesquisa do Estado de Sao Paulo, Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, and the National Institutes of Health grants HL51971, HL108618, and HL109763.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 3.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 4.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–30. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008;359:800–9. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 8.Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–61. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 9.Broughton Pipkin F. Risk factors for preeclampsia. N Engl J Med. 2001;344:925–6. doi: 10.1056/NEJM200103223441209. [DOI] [PubMed] [Google Scholar]
- 10.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Bmj. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trogstad L, Magnus P, Stoltenberg C. Pre-eclampsia: Risk factors and causal models. Best Pract Res Clin Obstet Gynaecol. 2011;25:329–42. doi: 10.1016/j.bpobgyn.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008;2:484–94. doi: 10.1016/j.jash.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 13.VanWijk MJ, Kublickiene K, Boer K, VanBavel E. Vascular function in preeclampsia. Cardiovasc Res. 2000;47:38–48. doi: 10.1016/s0008-6363(00)00087-0. [DOI] [PubMed] [Google Scholar]
- 14.Chapman AB, Abraham WT, Zamudio S, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–63. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 15.Clapp JF, 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80:1469–73. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 16.Pirani BB, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw. 1973;80:884–7. doi: 10.1111/j.1471-0528.1973.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 17.Scott DE. Anemia in pregnancy. Obstet Gynecol Annu. 1972;1:219–44. [PubMed] [Google Scholar]
- 18.Taylor DJ, Lind T. Red cell mass during and after normal pregnancy. Br J Obstet Gynaecol. 1979;86:364–70. doi: 10.1111/j.1471-0528.1979.tb10611.x. [DOI] [PubMed] [Google Scholar]
- 19.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–5. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 20.Flo K, Wilsgaard T, Vartun A, Acharya G. A longitudinal study of the relationship between maternal cardiac output measured by impedance cardiography and uterine artery blood flow in the second half of pregnancy. Bjog. 2010;117:837–44. doi: 10.1111/j.1471-0528.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 21.Jurkovic D, Jauniaux E, Kurjak A, Hustin J, Campbell S, Nicolaides KH. Transvaginal color Doppler assessment of the uteroplacental circulation in early pregnancy. Obstet Gynecol. 1991;77:365–9. [PubMed] [Google Scholar]
- 22.Thaler I, Manor D, Itskovitz J, Rottem S, et al. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162:121–5. doi: 10.1016/0002-9378(90)90834-t. [DOI] [PubMed] [Google Scholar]
- 23.Davison JM. Kidney function in pregnant women. Am J Kidney Dis. 1987;9:248–52. doi: 10.1016/s0272-6386(87)80117-8. [DOI] [PubMed] [Google Scholar]
- 24.Grindheim G, Estensen ME, Langesaeter E, Rosseland LA, Toska K. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens. 2012;30:342–50. doi: 10.1097/HJH.0b013e32834f0b1c. [DOI] [PubMed] [Google Scholar]
- 25.Mone SM, Sanders SP, Colan SD. Control mechanisms for physiological hypertrophy of pregnancy. Circulation. 1996;94:667–72. doi: 10.1161/01.cir.94.4.667. [DOI] [PubMed] [Google Scholar]
- 26.Valensise H, Novelli GP, Vasapollo B, et al. Maternal cardiac systolic and diastolic function: relationship with uteroplacental resistances. A Doppler and echocardiographic longitudinal study. Ultrasound Obstet Gynecol. 2000;15:487–97. doi: 10.1046/j.1469-0705.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 27.Ganzevoort W, Rep A, Bonsel GJ, de Vries JI, Wolf H. Plasma volume and blood pressure regulation in hypertensive pregnancy. J Hypertens. 2004;22:1235–42. doi: 10.1097/01.hjh.0000125436.28861.09. [DOI] [PubMed] [Google Scholar]
- 28.Longo LD. Maternal blood volume and cardiac output during pregnancy: a hypothesis of endocrinologic control. Am J Physiol. 1983;245:R720–9. doi: 10.1152/ajpregu.1983.245.5.R720. [DOI] [PubMed] [Google Scholar]
- 29.Oelkers WK. Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids. 1996;61:166–71. doi: 10.1016/0039-128x(96)00007-4. [DOI] [PubMed] [Google Scholar]
- 30.Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol. 1997;21:367–80. doi: 10.1016/s0146-0005(97)80003-1. [DOI] [PubMed] [Google Scholar]
- 31.Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol. 1997;272:H748–52. doi: 10.1152/ajpheart.1997.272.2.H748. [DOI] [PubMed] [Google Scholar]
- 32.Conrad KP, Joffe GM, Kruszyna H, et al. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993;7:566–71. [PubMed] [Google Scholar]
- 33.Goodman RP, Killam AP, Brash AR, Branch RA. Prostacyclin production during pregnancy: comparison of production during normal pregnancy and pregnancy complicated by hypertension. Am J Obstet Gynecol. 1982;142:817–22. doi: 10.1016/s0002-9378(16)32525-x. [DOI] [PubMed] [Google Scholar]
- 34.Greer IA, Walker JJ, McLaren M, et al. Immunoreactive prostacyclin and thromboxane metabolites in normal pregnancy and the puerperium. Br J Obstet Gynaecol. 1985;92:581–5. doi: 10.1111/j.1471-0528.1985.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 35.Valdes G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol. 2009;7:79. doi: 10.1186/1477-7827-7-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gant NF, Worley RJ, Everett RB, MacDonald PC. Control of vascular responsiveness during human pregnancy. Kidney Int. 1980;18:253–8. doi: 10.1038/ki.1980.133. [DOI] [PubMed] [Google Scholar]
- 37.Daniels CR, Eisen V, Slater JD. The renin-angiotensinogen reaction during pregnancy and oral contraception: estimation of kinetic parameters by an autologous plasma renin assay. J Endocrinol. 1987;112:465–72. doi: 10.1677/joe.0.1120465. [DOI] [PubMed] [Google Scholar]
- 38.Sealey JE, Itskovitz-Eldor J, Rubattu S, et al. Estradiol- and progesterone-related increases in the renin-aldosterone system: studies during ovarian stimulation and early pregnancy. J Clin Endocrinol Metab. 1994;79:258–64. doi: 10.1210/jcem.79.1.8027239. [DOI] [PubMed] [Google Scholar]
- 39.Valdes G, Germain AM, Corthorn J, et al. Urinary vasodilator and vasoconstrictor angiotensins during menstrual cycle, pregnancy, and lactation. Endocrine. 2001;16:117–22. doi: 10.1385/ENDO:16:2:117. [DOI] [PubMed] [Google Scholar]
- 40.Conrad KP. Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Investig. 2004;11:438–48. doi: 10.1016/j.jsgi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:978–84. doi: 10.1016/s0029-7844(99)00430-5. [DOI] [PubMed] [Google Scholar]
- 42.Rang S, Wolf H, van Montfrans GA, Karemaker JM. Serial assessment of cardiovascular control shows early signs of developing pre-eclampsia. J Hypertens. 2004;22:369–76. doi: 10.1097/00004872-200402000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47:203–8. doi: 10.1161/01.HYP.0000200042.64517.19. [DOI] [PubMed] [Google Scholar]
- 44.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–60. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 45.Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2:543–9. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- 46.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–9. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 47.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 48.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–56. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 49.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48:711–6. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 50.LaMarca B, Speed J, Fournier L, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–7. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaMarca BD, Gilbert J, Granger JP. Recent progress toward the understanding of the pathophysiology of hypertension during preeclampsia. Hypertension. 2008;51:982–8. doi: 10.1161/HYPERTENSIONAHA.107.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaMarca B, Parrish M, Ray LF, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–9. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor alpha in pregnant rats. Hypertension. 2008;52:1168–72. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallukat G, Homuth V, Fischer T, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–52. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 56.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 57.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142–7. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 60.Murphy SR, LaMarca BB, Cockrell K, Granger JP. Role of endothelin in mediating soluble fms-like tyrosine kinase 1-induced hypertension in pregnant rats. Hypertension. 2010;55:394–8. doi: 10.1161/HYPERTENSIONAHA.109.141473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bridges JP, Gilbert JS, Colson D, et al. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22:564–8. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 63.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Roggensack AM, Zhang Y, Davidge ST. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension. 1999;33:83–9. doi: 10.1161/01.hyp.33.1.83. [DOI] [PubMed] [Google Scholar]
- 65.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 66.Sedeek M, Gilbert JS, LaMarca BB, et al. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–6. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LaMarca BD, Alexander BT, Gilbert JS, et al. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gend Med. 2008;5 (Suppl A):S133–8. doi: 10.1016/j.genm.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–7. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 69.Benigni A, Orisio S, Gaspari F, Frusca T, Amuso G, Remuzzi G. Evidence against a pathogenetic role for endothelin in pre-eclampsia. Br J Obstet Gynaecol. 1992;99:798–802. doi: 10.1111/j.1471-0528.1992.tb14409.x. [DOI] [PubMed] [Google Scholar]
- 70.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89:210–22. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damsky CH, Librach C, Lim KH, et al. Integrin switching regulates normal trophoblast invasion. Development. 1994;120:3657–66. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 72.Fisher SJ, Damsky CH. Human cytotrophoblast invasion. Semin Cell Biol. 1993;4:183–8. doi: 10.1006/scel.1993.1022. [DOI] [PubMed] [Google Scholar]
- 73.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–91. [PubMed] [Google Scholar]
- 74.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–74. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 75.Pridjian G, Puschett JB. Preeclampsia. Part 1: clinical and pathophysiologic considerations. Obstet Gynecol Surv. 2002;57:598–618. doi: 10.1097/00006254-200209000-00023. [DOI] [PubMed] [Google Scholar]
- 76.Roy R, Zhang B, Moses MA. Making the cut: protease-mediated regulation of angiogenesis. Exp Cell Res. 2006;312:608–22. doi: 10.1016/j.yexcr.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 77.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–33. doi: 10.1101/gad.815400. [DOI] [PubMed] [Google Scholar]
- 78.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 79.Isaka K, Usuda S, Ito H, et al. Expression and activity of matrix metalloproteinase 2 and 9 in human trophoblasts. Placenta. 2003;24:53–64. doi: 10.1053/plac.2002.0867. [DOI] [PubMed] [Google Scholar]
- 80.Shimonovitz S, Hurwitz A, Dushnik M, Anteby E, Geva-Eldar T, Yagel S. Developmental regulation of the expression of 72 and 92 kd type IV collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am J Obstet Gynecol. 1994;171:832–8. doi: 10.1016/0002-9378(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 81.Lalu MM, Xu H, Davidge ST. Matrix metalloproteinases: control of vascular function and their potential role in preeclampsia. Front Biosci. 2007;12:2484–93. doi: 10.2741/2249. [DOI] [PubMed] [Google Scholar]
- 82.Sankaralingam S, Arenas IA, Lalu MM, Davidge ST. Preeclampsia: current understanding of the molecular basis of vascular dysfunction. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010465. [DOI] [PubMed] [Google Scholar]
- 83.Karthikeyan VJ, Lane DA, Beevers DG, Lip GY, Blann AD. Matrix metalloproteinases and their tissue inhibitors in hypertension-related pregnancy complications. J Hum Hypertens. 2013;27(2):72–8. doi: 10.1038/jhh.2012.8. [DOI] [PubMed] [Google Scholar]
- 84.Chow AK, Cena J, Schulz R. Acute actions and novel targets of matrix metalloproteinases in the heart and vasculature. Br J Pharmacol. 2007;152:189–205. doi: 10.1038/sj.bjp.0707344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadler-Olsen E, Fadnes B, Sylte I, Uhlin-Hansen L, Winberg JO. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011;278:28–45. doi: 10.1111/j.1742-4658.2010.07920.x. [DOI] [PubMed] [Google Scholar]
- 86.Donnelly R, Collinson DJ, Manning G. Hypertension, matrix metalloproteinases and target organ damage. J Hypertens. 2003;21:1627–30. doi: 10.1097/00004872-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 87.Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–98. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Price SJ, Greaves DR, Watkins H. Identification of novel, functional genetic variants in the human matrix metalloproteinase-2 gene: role of Sp1 in allele-specific transcriptional regulation. J Biol Chem. 2001;276:7549–58. doi: 10.1074/jbc.M010242200. [DOI] [PubMed] [Google Scholar]
- 89.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 90.Zhang B, Ye S, Herrmann SM, et al. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation. 1999;99:1788–94. doi: 10.1161/01.cir.99.14.1788. [DOI] [PubMed] [Google Scholar]
- 91.Peters DG, Kassam A, St Jean PL, Yonas H, Ferrell RE. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke. 1999;30:2612–6. doi: 10.1161/01.str.30.12.2612. [DOI] [PubMed] [Google Scholar]
- 92.Shimajiri S, Arima N, Tanimoto A, et al. Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene. FEBS Lett. 1999;455:70–4. doi: 10.1016/s0014-5793(99)00863-7. [DOI] [PubMed] [Google Scholar]
- 93.Myers J, Mires G, Macleod M, Baker P. In preeclampsia, the circulating factors capable of altering in vitro endothelial function precede clinical disease. Hypertension. 2005;45:258–63. doi: 10.1161/01.HYP.0000153461.58298.a4. [DOI] [PubMed] [Google Scholar]
- 94.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–8. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 95.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Pre-eclampsia: a disorders of the endothelial cells? Gynakologe. 1992;25:2–6. [PubMed] [Google Scholar]
- 96.Fernandez-Patron C, Stewart KG, Zhang Y, Koivunen E, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ Res. 2000;87:670–6. doi: 10.1161/01.res.87.8.670. [DOI] [PubMed] [Google Scholar]
- 97.Martinez A, Oh HR, Unsworth EJ, et al. Matrix metalloproteinase-2 cleavage of adrenomedullin produces a vasoconstrictor out of a vasodilator. Biochem J. 2004;383:413–8. doi: 10.1042/BJ20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–11. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 99.Fernandez-Patron C, Zouki C, Whittal R, Chan JS, Davidge ST, Filep JG. Matrix metalloproteinases regulate neutrophil-endothelial cell adhesion through generation of endothelin-1[1-32] FASEB J. 2001;15:2230–40. doi: 10.1096/fj.01-0178com. [DOI] [PubMed] [Google Scholar]
- 100.Gearing AJ, Beckett P, Christodoulou M, et al. Matrix metalloproteinases and processing of pro-TNF-alpha. J Leukoc Biol. 1995;57:774–7. doi: 10.1002/jlb.57.5.774. [DOI] [PubMed] [Google Scholar]
- 101.Chandler S, Miller KM, Clements JM, et al. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–61. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 102.Vaday GG, Franitza S, Schor H, et al. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J Leukoc Biol. 2001;69:885–92. [PubMed] [Google Scholar]
- 103.Bischof P, Meisser A, Campana A. Mechanisms of endometrial control of trophoblast invasion. J Reprod Fertil Suppl. 2000;55:65–71. [PubMed] [Google Scholar]
- 104.Staun-Ram E, Shalev E. Human trophoblast function during the implantation process. Reprod Biol Endocrinol. 2005;3:56. doi: 10.1186/1477-7827-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodrigues SF, Tran ED, Fortes ZB, Schmid-Schonbein GW. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299:H25–35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmid-Schonbein GW. Matrix metalloproteinases activities in hypertension: emerging opportunities. Hypertension. 2011;57:24–5. doi: 10.1161/HYPERTENSIONAHA.110.162032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tran ED, DeLano FA, Schmid-Schonbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47:423–31. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tran ED, Yang M, Chen A, Delano FA, Murfee WL, Schmid-Schonbein GW. Matrix metalloproteinase activity causes VEGFR-2 cleavage and microvascular rarefaction in rat mesentery. Microcirculation. 2011;18:228–37. doi: 10.1111/j.1549-8719.2011.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu KI, Schmid-Schonbein GW. Nuclear factor kappa B and matrix metalloproteinase induced receptor cleavage in the spontaneously hypertensive rat. Hypertension. 2011;57:261–8. doi: 10.1161/HYPERTENSIONAHA.110.158709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huisman MA, Timmer A, Zeinstra M, et al. Matrix-metalloproteinase activity in first trimester placental bed biopsies in further complicated and uncomplicated pregnancies. Placenta. 2004;25:253–8. doi: 10.1016/j.placenta.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Kolben M, Lopens A, Blaser J, et al. Proteases and their inhibitors are indicative in gestational disease. Eur J Obstet Gynecol Reprod Biol. 1996;68:59–65. doi: 10.1016/0301-2115(96)02484-0. [DOI] [PubMed] [Google Scholar]
- 112.Shokry M, Omran OM, Hassan HI, Elsedfy GO, Hussein MR. Expression of matrix metalloproteinases 2 and 9 in human trophoblasts of normal and preeclamptic placentas: preliminary findings. Exp Mol Pathol. 2009;87:219–25. doi: 10.1016/j.yexmp.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 113.Romanowicz L, Galewska Z. Extracellular Matrix Remodeling of the Umbilical Cord in Pre-eclampsia as a Risk Factor for Fetal Hypertension. J Pregnancy. 2011;2011:542695. doi: 10.1155/2011/542695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galewska Z, Romanowicz L, Jaworski S, Bankowski E. Gelatinase matrix metalloproteinase (MMP)-2 and MMP-9 of the umbilical cord blood in preeclampsia. Clin Chem Lab Med. 2008;46:517–22. doi: 10.1515/CCLM.2008.083. [DOI] [PubMed] [Google Scholar]
- 115.Lavee M, Goldman S, Daniel-Spiegel E, Shalev E. Matrix metalloproteinase-2 is elevated in midtrimester amniotic fluid prior to the development of preeclampsia. Reprod Biol Endocrinol. 2009;7:85. doi: 10.1186/1477-7827-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Palei AC, Sandrim VC, Cavalli RC, Tanus-Santos JE. Comparative assessment of matrix metalloproteinase (MMP)-2 and MMP-9, and their inhibitors, tissue inhibitors of metalloproteinase (TIMP)-1 and TIMP-2 in preeclampsia and gestational hypertension. Clin Biochem. 2008;41:875–80. doi: 10.1016/j.clinbiochem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 117.Palei AC, Sandrim VC, Amaral LM, et al. Matrix metalloproteinase-9 polymorphisms affect plasma MMP-9 levels and antihypertensive therapy responsiveness in hypertensive disorders of pregnancy. Pharmacogenomics J. 2011 doi: 10.1038/tpj.2011.31. [DOI] [PubMed] [Google Scholar]
- 118.Montagnana M, Lippi G, Albiero A, et al. Evaluation of metalloproteinases 2 and 9 and their inhibitors in physiologic and pre-eclamptic pregnancy. J Clin Lab Anal. 2009;23:88–92. doi: 10.1002/jcla.20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myers JE, Merchant SJ, Macleod M, Mires GJ, Baker PN, Davidge ST. MMP-2 levels are elevated in the plasma of women who subsequently develop preeclampsia. Hypertens Pregnancy. 2005;24:103–15. doi: 10.1081/PRG-200059836. [DOI] [PubMed] [Google Scholar]
- 120.Poon LC, Nekrasova E, Anastassopoulos P, Livanos P, Nicolaides KH. First-trimester maternal serum matrix metalloproteinase-9 (MMP-9) and adverse pregnancy outcome. Prenat Diagn. 2009;29:553–9. doi: 10.1002/pd.2234. [DOI] [PubMed] [Google Scholar]
- 121.Narumiya H, Zhang Y, Fernandez-Patron C, Guilbert LJ, Davidge ST. Matrix metalloproteinase-2 is elevated in the plasma of women with preeclampsia. Hypertens Pregnancy. 2001;20:185–94. doi: 10.1081/PRG-100106968. [DOI] [PubMed] [Google Scholar]
- 122.Palei AC, Sandrim VC, Amaral LM, et al. Association between matrix metalloproteinase (MMP)-2 polymorphisms and MMP-2 levels in hypertensive disorders of pregnancy. Exp Mol Pathol. 2012;92:217–21. doi: 10.1016/j.yexmp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Coolman M, de Maat M, Van Heerde WL, et al. Matrix metalloproteinase-9 gene −1562C/T polymorphism mitigates preeclampsia. Placenta. 2007;28:709–13. doi: 10.1016/j.placenta.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 124.Fraser R, Walker JJ, Ekbote UV, Martin KL, McShane P, Orsi NM. Interleukin-4 −590 (C>T), toll-like receptor-2 +2258 (G>A) and matrix metalloproteinase-9 −1562 (C>T) polymorphisms in pre-eclampsia. Bjog. 2008;115:1052–6. doi: 10.1111/j.1471-0528.2008.01771.x. discussion 6. [DOI] [PubMed] [Google Scholar]
- 125.Palei AC, Sandrim VC, Duarte G, Cavalli RC, Gerlach RF, Tanus-Santos JE. Matrix metalloproteinase (MMP)-9 genotypes and haplotypes in preeclampsia and gestational hypertension. Clin Chim Acta. 2010;411:874–7. doi: 10.1016/j.cca.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 126.Palei AC, Sandrim VC, Amaral LM, et al. Effects of Matrix Metalloproteinase (MMP)-2 Polymorphisms on Responsiveness to Anti-hypertensive Therapy of Women with Hypertensive Disorders of Pregnancy. Basic Clin Pharmacol Toxicol. 2012 doi: 10.1111/j.1742-7843.2012.00905.x. [DOI] [PubMed] [Google Scholar]
- 127.Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–9. doi: 10.1161/HYPERTENSIONAHA.106.075895. [DOI] [PubMed] [Google Scholar]
- 128.James PR, Nelson-Piercy C. Management of hypertension before, during, and after pregnancy. Heart. 2004;90:1499–504. doi: 10.1136/hrt.2004.035444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castro MM, Rizzi E, Figueiredo-Lopes L, et al. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198:320–31. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 130.Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29:194–201. doi: 10.1016/j.matbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 131.Rizzi E, Castro MM, Prado CM, et al. Matrix metalloproteinase inhibition improves cardiac dysfunction and remodeling in 2-kidney, 1-clip hypertension. J Card Fail. 2010;16:599–608. doi: 10.1016/j.cardfail.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 132.Bouvet C, Gilbert LA, Girardot D, deBlois D, Moreau P. Different involvement of extracellular matrix components in small and large arteries during chronic NO synthase inhibition. Hypertension. 2005;45:432–7. doi: 10.1161/01.HYP.0000154680.44184.01. [DOI] [PubMed] [Google Scholar]
- 133.Wang M, Zhang J, Telljohann R, et al. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–66. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Verlohren S, Geusens N, Morton J, et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension. 2010;56:304–10. doi: 10.1161/HYPERTENSIONAHA.110.153163. [DOI] [PubMed] [Google Scholar]
- 135.Claussen U, Breuer HW. The teratogenic effects in rabbits of doxycycline, dissolved in polyvinylpyrrolidone, injected into the yolk sac. Teratology. 1975;12:297–301. doi: 10.1002/tera.1420120312. [DOI] [PubMed] [Google Scholar]
- 136.Rechtman MP, Zhang J, Salamonsen LA. Effect of inhibition of matrix metalloproteinases on endometrial decidualization and implantation in mated rats. J Reprod Fertil. 1999;117:169–77. doi: 10.1530/jrf.0.1170169. [DOI] [PubMed] [Google Scholar]
- 137.Siddiqui MA, Janjua MZ. Effect of prenatal doxycycline administration on skeletal differentiation in long bones of Albino rat. J Pak Med Assoc. 2002;52:211–4. [PubMed] [Google Scholar]
- 138.Younis HS, Jessen BA, Wu EY, Stevens GJ. Inhibiting matrix metalloproteinases with prinomastat produces abnormalities in fetal growth and development in rats. Birth Defects Res B Dev Reprod Toxicol. 2006;77:95–103. doi: 10.1002/bdrb.20073. [DOI] [PubMed] [Google Scholar]
- 139.Bastianini L, Felisati D. [Studies on gravidic and fetal toxicity of alpha-6-deoxy-5-oxytetracycline (doxycycline) in mouse and rabbit]. Antibiotica. 1970;8:161–78. [PubMed] [Google Scholar]
- 140.Cahen RL, Fave A. Absence of teratogenic effect of 6-alpha-deoxy-5 oxytetracycline. Fed Proc Fed Am Soc Exp Biol. 1972:238. [Google Scholar]
- 141.Delahunt CS, Jacobs RT, Stebbins RB, Reiser N. Toxicology of vibramycin. Toxicol Appl Pharmacol. 1967:402. [Google Scholar]
- 142.Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–79. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwarz EB, Maselli J, Norton M, Gonzales R. Prescription of teratogenic medications in United States ambulatory practices. Am J Med. 2005;118:1240–9. doi: 10.1016/j.amjmed.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 144.Czeizel AE, Rockenbauer M. Teratogenic study of doxycycline. Obstet Gynecol. 1997;89:524–8. doi: 10.1016/S0029-7844(97)00005-7. [DOI] [PubMed] [Google Scholar]
- 145.Horne HW, Jr, Kundsin RB. The role of mycoplasma among 81 consecutive pregnancies: a prospective study. Int J Fertil. 1980;25:315–7. [PubMed] [Google Scholar]
- 146.Nahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: what is and is not known about teratogenic and toxic risks. Obstet Gynecol. 2006;107:1120–38. doi: 10.1097/01.AOG.0000216197.26783.b5. [DOI] [PubMed] [Google Scholar]
- 147.Guimaraes DA, Rizzi E, Ceron CS, et al. Doxycycline dose-dependently inhibits MMP-2-mediated vascular changes in 2K1C hypertension. Basic Clin Pharmacol Toxicol. 2011;108:318–25. doi: 10.1111/j.1742-7843.2010.00656.x. [DOI] [PubMed] [Google Scholar]
- 148.Fontana V, Silva PS, Gerlach RF, Tanus-Santos JE. Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin Chim Acta. 2012;413:656–62. doi: 10.1016/j.cca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 149.Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+ channel antagonists on plasma concentrations of matrix metalloproteinase-2 and -9 in essential hypertension. Am J Hypertens. 2004;17:273–6. doi: 10.1016/j.amjhyper.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 150.Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment. J Hum Hypertens. 2003;17:119–24. doi: 10.1038/sj.jhh.1001518. [DOI] [PubMed] [Google Scholar]
- 151.Martinez ML, Lopes LF, Coelho EB, et al. Lercanidipine reduces matrix metalloproteinase-9 activity in patients with hypertension. J Cardiovasc Pharmacol. 2006;47:117–22. doi: 10.1097/01.fjc.0000196241.96759.71. [DOI] [PubMed] [Google Scholar]
- 152.Marcal DM, Rizzi E, Martins-Oliveira A, et al. Comparative study on antioxidant effects and vascular matrix metalloproteinase-2 downregulation by dihydropyridines in renovascular hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:35–44. doi: 10.1007/s00210-010-0573-y. [DOI] [PubMed] [Google Scholar]
- 153.Martinez ML, Castro MM, Rizzi E, et al. Lercanidipine reduces matrix metalloproteinase-2 activity and reverses vascular dysfunction in renovascular hypertensive rats. Eur J Pharmacol. 2008;591:224–30. doi: 10.1016/j.ejphar.2008.06.096. [DOI] [PubMed] [Google Scholar]
- 154.Yamada T, Nagata K, Cheng XW, et al. Long-term administration of nifedipine attenuates cardiac remodeling and diastolic heart failure in hypertensive rats. Eur J Pharmacol. 2009;615:163–70. doi: 10.1016/j.ejphar.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 155.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–8. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 156.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007:CD004659. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 157.Bujold E, Morency AM, Roberge S, Lacasse Y, Forest JC, Giguere Y. Acetylsalicylic acid for the prevention of preeclampsia and intra-uterine growth restriction in women with abnormal uterine artery Doppler: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2009;31:818–26. doi: 10.1016/S1701-2163(16)34300-6. [DOI] [PubMed] [Google Scholar]
- 158.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–14. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 159.Ruano R, Fontes RS, Zugaib M. Prevention of preeclampsia with low-dose aspirin -- a systematic review and meta-analysis of the main randomized controlled trials. Clinics (Sao Paulo) 2005;60:407–14. doi: 10.1590/s1807-59322005000500010. [DOI] [PubMed] [Google Scholar]
- 160.Lu L, Liu H, Peng J, et al. Regulations of the key mediators in inflammation and atherosclerosis by aspirin in human macrophages. Lipids Health Dis. 2010;9:16. doi: 10.1186/1476-511X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yiqin Y, Meilin X, Jie X, Keping Z. Aspirin inhibits MMP-2 and MMP-9 expression and activity through PPARalpha/gamma and TIMP-1-mediated mechanisms in cultured mouse celiac macrophages. Inflammation. 2009;32:233–41. doi: 10.1007/s10753-009-9125-3. [DOI] [PubMed] [Google Scholar]
- 162.Hua Y, Xue J, Sun F, Zhu L, Xie M. Aspirin inhibits MMP-2 and MMP-9 expressions and activities through upregulation of PPARalpha/gamma and TIMP gene expressions in ox-LDL-stimulated macrophages derived from human monocytes. Pharmacology. 2009;83:18–25. doi: 10.1159/000166183. [DOI] [PubMed] [Google Scholar]
- 163.Nicolae M, Tircol M, Alexandru D. Inhibitory effect of acetylsalicylic acid on matrix metalloproteinase - 2 activity in human endothelial cells exposed to high glucose. J Cell Mol Med. 2005;9:953–60. doi: 10.1111/j.1582-4934.2005.tb00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castro MM, Kandasamy AD, Youssef N, Schulz R. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64:551–60. doi: 10.1016/j.phrs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 165.Castro MM, Tanus-Santos JE, Gerlach RF. Matrix metalloproteinases: targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol Res. 2011;64:567–72. doi: 10.1016/j.phrs.2011.04.002. [DOI] [PubMed] [Google Scholar]