OVERVIEW OF REGULATED GLUCOCORTICOID PRODUCTION
Clinical observations indicate that diverse disorders disrupt hypothalamic-corticotropic-adrenal (HPA) homeostasis. Prominent examples include depression, mania, dementia, posttraumatic stress disorder, chronic fatigue syndrome, alcoholism, visceral adiposity, diabetes mellitus, polycystic ovarian disease, acute illness, systemic disease, and multiorgan failure.1–19 Age, gender, and sex steroids are salient physiologic factors that determine the magnitude and duration of stress-adaptive cortisol production, albeit via mechanisms that are essentially unknown in the human.20–34
Understanding the regulation of normal HPA outflow is significant, because chronically increased glucocorticoid concentrations correlate with metabolic features of syndrome X (visceral adiposity, insulin resistance, low high-density lipoprotein levels, high blood pressure, increased triglyceride levels), physical frailty (reduced bone and muscle mass, decreased aerobic capacity), immune suppression, hypogonadism, growth hormone (GH), and insulinlike growth factor 1 deficiency and impaired memory and spatial cognition (Fig. 1).15,35–44 Aging itself is associated with similar changes (Box 1). Accordingly, there is an ongoing scientific need to elucidate the basic mechanisms that govern cortisol homeostasis in the aging human.22,26,27,32,45–51 Inferable effects of aging on HPA function may be confounded by multiple factors, including gender, stress, and genetics (Box 2).
Fig. 1.
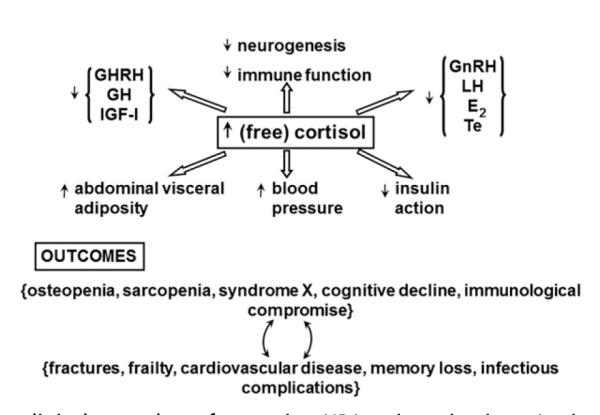
Putative clinical sequelae of excessive HPA axis activation. An increase in (free) cortisol availability is associated with adverse outcomes in diverse body systems.
Box 1. Similar changes occur in aging as in HPA hyperactivity.
↓ physical performance if ↑ evening cortisol
↓ walking speed
↓ bone-mineral density in postmenopausal women
↑ functional disabilities
↓ immune responses
↑ syndrome X (visceral adiposity, ↑ blood pressure, ↓ insulin action)
↓ cognitive function ↓ memory
↑ depression
↑ blood pressure
↓ lean muscle mass
↑ insulin resistance ↓ glucose tolerance
Abbreviations: ↑, increase; ↓, decrease.
Box 2. Confounding factors in HPA evaluation with age.
Gender differences90
Sex-steroid milieu184
Cumulative glucocorticoid exposure185
Dementia or depression186
Genetic polymorphisms in HPA genes187
Inflammation, obesity, weight loss, medication use, illness130,188
Type of stress (psychosocial, physical, metabolic, cognitive)189
Onset versus recovery of stress response190
Structural brain changes191
Cigarette smoking63
Impaired sleep192
Socioeconomic insecurity63
Ethnicity, psychological traits193
↑, ↓ or unchanged serum total cortisol (ages 19–89 years)125,130,194–198
↓ urinary free cortisol in centenarians65
↓ circannual free cortisol rhythm197
DIURNAL ADRENOCORTICOTROPIC HORMONE AND CORTISOL RHYTHMS
In principle, age could modulate mean hormone concentrations, secretion rates, elimination kinetics, pulse size (amplitude) or number (frequency), pattern regularity, or circadian (approximately 24-hour) rhythms. Nycthemeral (night-day) cortisol rhythms are consistently altered in aging individuals (Box 3). Most clinical studies report a phase-advanced acrophase (clock time of maximal adrenocorticotropic hormone (ACTH) or cortisol concentrations within the 24-hour day), eg, 06:30 am (older) vis-à-vis 09:00 am (young). Concomitantly, there is an increased circadian nadir (lowest 24-hour concentration) in the late evening and through midnight.52–54 The higher nadir blunts the overall 24-hour increase in cortisol levels. Possible relevance of these findings is that certain target-tissue effects of cortisol, such as reduced lymphocyte subtype populations, share in the phase shift.55,56
Box 3. Circadian cortisol changes with age.
Sleep disruption (reduced deep sleep or early awakening) occurs in many older people.57–59 The degree to which these alterations reflect or cause aging-associated changes in functional disability, anxiety, depression, social support, caloric intake, and lifestyle modifications is not clear.60–65 However, structural alterations in the hippocampus, suprachiasmatic nuclei, hypothalamus, adrenal gland, and possibly the autonomic nervous system can accompany aging in animals (Box 4).66–68 A confounding unresolved issue is the extent to which memory or cognitive decline in older adults results from (is caused by) versus elicits (causes) increased cortisol secretion in the late day.69–71 Available data do not exclude bidirectional effects.72–74
Box 4. Age modifies selective components of HPA axis in animals and humans.
| Component of Axis | Age Effect |
|---|---|
| Hippocampus | ↓ GR and ↓ MR |
| Suprachiasmatic nucleus (circadian) | ↓ VIP (older men only)202 |
| Hypothalamus (paraventricular nucleus) | ↑ CRH ↑ AVPa,203 |
| Pituitary gland | No change in ACTH |
| Adrenal gland zona reticularis | ↓ DHEAa,204,205 |
| Autonomic nervous system | ↓ NE outflowa |
| Plasma cortisol-binding globulin | No changea,130 |
| Monocytes | ↓ GR (both sexes)206 |
Abbreviations: AVP, arginine vasopressin (ADH); DHEA, dehydroepiandrosterone; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; NE, norepinephrine; VIP, vasoactive intestinal polypeptide.
Human data.
HPA ALTERATIONS IN AGED ANIMALS
Significant functional changes occur in the HPA axis of aged laboratory animals (Box 5). A consistent adaptation is reduction in brain corticosteroid receptors, type I (MR) and type II (GR).75 Both mRNA and protein levels of MR and GR decline in the aged male Fischer rat. This model shows increased hypothalamopituitary portal venous CRH, consistent with a functional decrement in corticosteroid negative feedback. However, species and strain differences limit the consistency of laboratory animal models.
Box 5. Aged animals: HPA alterations.
↓ hippocampal MR and GR in Fischer-344 rat207
↑ portal venous CRH (Fischer)208
↓ portal venous AVP (Fischer)208
↑ corticosterone (Long-Evans rat)209,210
↓ hippocampal MR but not GR209
↑ evening cortisol (female Rhesus monkey)211
↓ cortisol escape after dexamethasone (DEX)211
EXPERIMENTAL INSIGHTS INTO AND CLINICAL INFERENCES REGARDING SEX-STEROID REGULATION OF GLUCOCORTICOID AVAILABILITY
Experimental Insights
Sex steroids direct key regulatory mechanisms within the HPA axis of several mammalian species (ie, rat,76–84 mouse,85 sheep,86,87 monkey88,89 and human46,90–92). How gonadal steroids regulate ACTH and cortisol secretion is well articulated in the young adult rat, as highlighted in Fig. 2. Sex differences in HPA regulation in the rodent arise from both neuronal imprinting during early development and distinct actions of estradiol (E2) and testosterone (Te) in adulthood.93–97 In the young adult animal, exposure to E2 typically potentiates stress-induced ACTH secretion by: (1) attenuating negative feedback in the hypothalamus, hippocampus, amygdala, and pituitary gland98,99; (2) inducing AVP, CRH, and CRH-R1 gene expression in the paraventricular nucleus (PVN)77,93,100–103; (3) enhancing adrenal responsiveness to ACTH104–107; (4) muting hippocampal and bed nucleus of the stria terminalis–directed inhibition of PVN neurons108; and (5) blunting homologous downregulation of limbic GR.76 Conversely, Te and 5α-dihydrotestosterone (5α-DHT) generally exert opposing effects on the fore-going mechanisms, resulting in diminished stress-stimulated ACTH secretion (see Fig. 2).6,81,84,109–114 What remains unclear is how aging per se modulates the sex-steroid effects.
Fig. 2.
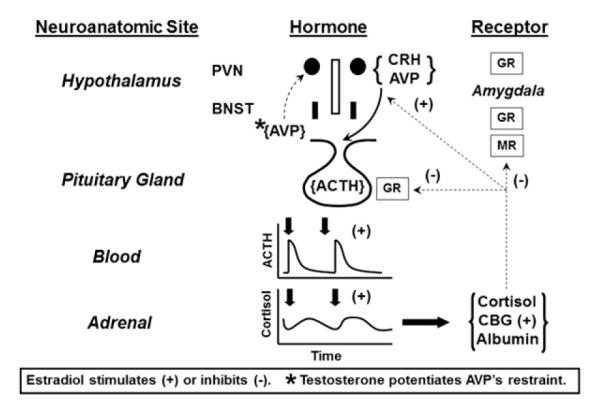
Experimentally based schema of loci of sex-steroid (estradiol and testosterone) control of HPA axis outflow. The + and − signs denote stimulation and inhibition, respectively.
The impact of gonadal steroids in the rat is not expressly dichotomous, because E2 may augment certain actions of 5α-DHT, whereas 5α-DHT can impede other effects of E2115–117. In addition, in some studies, low concentrations of estrogen diminish rather than amplify stress-induced ACTH and glucocorticoid secretion in the ovariprival state.82,118,119
Clinical Inferences
Certain HPA modulators and stressors affect ACTH and cortisol homeostasis, with strong age dependence and female or male predominance (Box 6).21,22,46,120–122 Modulators in this category include CRH, AVP, DEX, somatostatin, social stress, cognitive stress, and pharmacologic probes. Most often, greater responses occur in aging, especially in women. The precise bases for age-associated gender selectively in these clinical settings is not established. Investigations in the human are limited in scope and discrepant in inference. Critical review shows the following fragmentary and disparate observations:
Daily cortisol secretion rates are higher in men,123,124 greater in older women,125 comparable in women and men,126–130 and increased in young women in the luteal compared with follicular phase of the menstrual cycle131,132;
Maximal ACTH-stimulated cortisol secretion is greater in women,33,49,133 similar by gender,126 accentuated by Te administration in women,134 or unaffected by estrogen in men31,34,135;
Maximal CRH-stimulated ACTH or cortisol secretion is equivalent in the 2 sexes,26,27,46 higher in men,136 greater in women,32,42,48,136–139 not affected by E2 in women,140 in augmented by E2 in men141 or suppressed by Te men142;
AVP-induced ACTH secretion is comparable in the 2 genders47 or greater in women48;
Synergy between CRH and AVP is greater in men than women or comparable by gender46;
Stress-induced elevations in ACTH and cortisol concentrations are greater in men,22,23,25,143–146 greater in women,28,145,147 comparable by gender,26,32 repressed by E2 in women, and dependent on age22,25,26,28,122,144 or independent of age32;
Delayed (integral) glucocorticoid negative feedback is either muted in women32,48,148 or accentuated in women148,149 compared with men;
Rapid cortisol-mediated negative feedback has been studied in men,149 but responses have not been compared in women and men; and i. The metabolic clearance rate of cortisol is the same in the 2 sexes,126,130 decreased in women150 or increased in women.91
Box 6. Gender-related and age-related distinctions in human HPA responsiveness.
↑ or unchanged plasma ACTH with age212–214
↑ stimulation of CRH of ACTH and cortisol secretiona,48
↑ AVP/CRH (combined) stimulationa,46
↓ glucocorticoid suppression of effect of basal ACTH and CRH48,56,74,148,149,213,215
↑ paradoxic ACTH/cortisol response to somatostatin216
↑ social stress effecta,22
↑ response to cognitive stressa,217
↑ stimulation by 5HT-1A agonist (ipsapirone)a,218
↑ response to hypothermic stress218
↑ effect of physostigminea,219
↑ cortisol response to naltrexone (opiate blocker)a,220
↑ ACTH/cortisol response to MR blocker221,222
a More prominent age contrast in women than men.
The foregoing inconsistencies and the lack of precise data on body compositional effects preclude definitive inferences regarding putatively joint interactions among age, gender, sex steroids and body-fat distribution in regulating glucocorticoid availability in humans.
Glucocorticoid Negative Feedback Studies in the Human: Confounding by Gender and Age
Increased glucocorticoid concentrations and impaired suppression of ACTH and cortisol concentration by DEX tend to correlate with increased age and decreased cognitive function.15,38,151,152 Although such observations could signify that aging increases mean cortisol levels and reduces glucocorticoid negative feedback, clinical data are controversial. For example, awakening morning salivary cortisol seems to decrease with age, especially in women (Box 7). In contrast, cortisol responses to glucose less than 50 mg/dL are preserved in aging.153 In 1 study, feedback inhibition of ACTH and cortisol concentrations by graded doses of DEX did not differ in older and young men.29 In other investigations, intravenous (IV) infusion of cortisol suppressed ACTH concentrations less in older than young men, and more in postmenopausal women than elderly men.149,154,155 Analogously, DEX administration lowered cortisol concentrations more in 106 women than 203 men aged 66 to 78 years.156 Assessments of rapid negative feedback have typically used pharmacologic doses of cortisol (5–50 mg IV) without evaluating age and gender effects.149,157,158 Experimental data indicate that feedback effects and loci of inhibition differ between synthetic glucocorticoids and cortisol.159 Box 7 presents several other age (and gender) effects on human HPA dynamics, showing variously no effect, diminution, and accentuation of ACTH/cortisol responses to distinct stressors in aging humans. Age does not seem to alter responses to infusions of insulin, naloxone, or ACTH. Aging attenuates ACTH/cortisol responses to speech stress, low socioeconomic status, deep sleep, psychosocial stress, and MR stimulation with 9α-fludrocortisone, and potentiating HPA responses to an MR antagonist and a selective serotonin uptake inhibitor.
Box 7. Age-related changes in human HPA dynamics.
| 1. Various stressors |
| a. Depression |
| i. ↑ cortisol with age in both sexes196 |
| ii. ↑ post-DEX/CRH cortisol with age223 |
| b. Memory impairment |
| i. ↑ basal and post-DEX cortisol in older women with (vs without) memory impairment69 |
| c. Awakening |
| i. ↑ salivary cortisol after meal224 |
| ii. ↓ salivary cortisol in older (vs young) adults especially women60,224–227 |
| iii. Salivary cortisol predicts: |
| 1. executive function positively in older adults228 |
| 2. working memory positively in older men and declarative memory negatively (both sexes)229 |
| d. Hypoglycemia |
| i. Normal cortisol response to ↓ glucose of 50 mg/dL with age153 |
| e. Speech task |
| i. ↓ (salivary) cortisol response in older men only230 |
| f. Socioeconomic status |
| i. ↓ (salivary) cortisol with age in socioeconomically matched women only227 |
| g. Sleep |
| i. ↓ deep sleep and amplitude of 24-hour cortisol rhythm with age (men)58,192 |
| h. Abdominal visceral adiposity in older women |
| i. ↓ estimated ACTH efficacy231 |
| i. Psychosocial stress |
| i. ↓ ACTH increment after DEX/CRH in older (vs young) men22 |
| j. Mineralocorticoid agonist (9a-fludrocortisone) |
| i. ↓ ACTH/cortisol suppression in older (vs young) men232 |
| k. Mineralocorticoid antagonist (spironolactone) |
| i. ↑ cortisol more in older (than young) adults222 |
| 2. Adrenal feedback |
| a. Hydrocortisone-imposed negative feedback (25-mg IV bolus) |
| i. ↓ ACTH suppression in older men149 |
| b. DEX (0.25–3 mg orally) |
| i. ↓ ACTH/cortisol suppression in: |
| 1. older volunteers48,233,234 |
| 2. and in luteal-phase women235 |
| 3. Pharmacologic interventions |
| a. ACTH infusion |
| i. No age effect on maximal cortisol response234 |
| b. Naloxone infusion (stimulus to ACTH/cortisol) |
| i. No effect of age on cortisol response in men236 |
| c. Selective serotonin reuptake inhibitor (citalopram) |
| i. ↑ ACTH secretion with age in men237 |
| 4. Sex-steroid interactions |
| a. Short-term (2-week–8-week) E2 treatment |
| i. ↓ cortisol hyperresponsiveness after DEX/CRH in older women treated with estrogen32,118,238 |
| ii. Progesterone potentiates cortisol release to exercise239–241 |
| iii. E2 has no effect (vs hypogonadism) on exercise response in young women240 |
UNRESOLVED ISSUES
Delineating Relevant in Vivo Cortisol Feedback Signals
Free (protein-unbound), cortisol-binding globulin (CBG), and albumin-bound cortisol concentrations constitute respectively 6%, 80%, and 14% of total plasma cortisol in the human.126,160 Indirect evidence points to greater biological relevance of free than total glucocorticoid concentration in mediating certain tissue-specific effects, such as hippocampal GR occupancy, negative feedback efficacy, stimulation of glycogen synthesis, fractional hepatic extraction, and uptake into cerebrospinal fluid.161–164 Clinical studies are needed to quantify negative feedback control of CRH and AVP secretion and stimulation of ACTH secretion by each of free, CBG-bound, and albumin-bound cortisol in young and older women and men exposed to E2, Te, and GR or MR antagonists.
Estimating Condition-Specific and Subject-Specific Kinetics of Cortisol and ACTH Elimination
In the human, the nominal half-life of total cortisol is 35 to 65 minutes.126,127,165,166 Longer half-lives occur with higher CBG concentrations in estrogen-rich young women than Te-predominant men.167,168 Model-based analyses of the fate of cortisol in plasma in middle-aged adults predict gender-independent half-lives of free cortisol diffusion of 1.8 minutes and of free and total cortisol elimination of 4.1 minutes and 48 minutes, respectively.126 Such calculations also forecast higher free and albumin-bound cortisol concentrations in the morning than evening and thereby more rapid clearance, as observed clinically.169 The half-life of human ACTH is reportedly 8 to 25 minutes by bioassay and immunoassay.126,165,170,171 Rapid ACTH disappearance imposes a requirement for frequent blood sampling to monitor pulsatile secretion.172–174
Noninvasive Analyses of Multisignal Regulation of ACTH Secretion
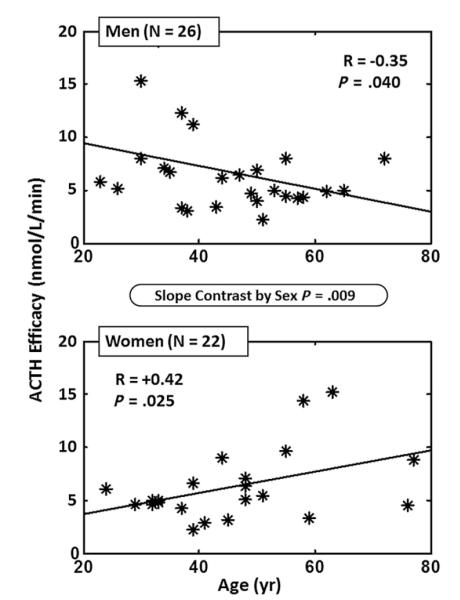
New analytical procedures are needed to reconstruct feedforward and feedback dose-response properties using only paired measurements of linked hormones. The objective is to obviate the previous long-standing necessity to inject agonists, antagonists, or marked molecules.126,175,176 Implementation of 1 such methodology in 32 healthy adults ages 26 to 79 years permitted estimation of unstressed ACTH feed-forward efficacy (maximal cortisol secretion), potency (one-half maximally effective concentration, [EC50]) and adrenal sensitivity (absolute slope of effector-response relationship).126 A computed EC50 (potency) of endogenous ACTH of 24 ± 3.3 ng/L was associated with a mean plasma ACTH concentration in the same cohort of 19 ± 6.2 ng/L. Empirical validation of the equation set was by frequent (5-minute), extended (4-hour–12-hour) direct sampling of hypothalamopituitary portal and internal-jugular blood to measure CRH, AVP, ACTH, and cortisol as well as gonadotropin-releasing hormone, luteinizing hormone, and Te concentration in the awake unrestrained horse and sheep.175,176 Statistical verification was by direct mathematical proof of maximum-likelihood estimation of all parameter asymptotes.176 An extension of this concept is to reconstruct the 3-parameter relationship among CRH concentration, AVP concentration, and ACTH secretion at a given steady-state cortisol concentration using data from pituitary portal vessels (Fig. 3).177 Based on these methods, estimated ACTH efficacy decreases in men and increases in women with age (Fig. 4).178 In addition, age and body mass index together determine adrenal sensitivity to endogenous ACTH (Fig. 5).
Fig. 3.
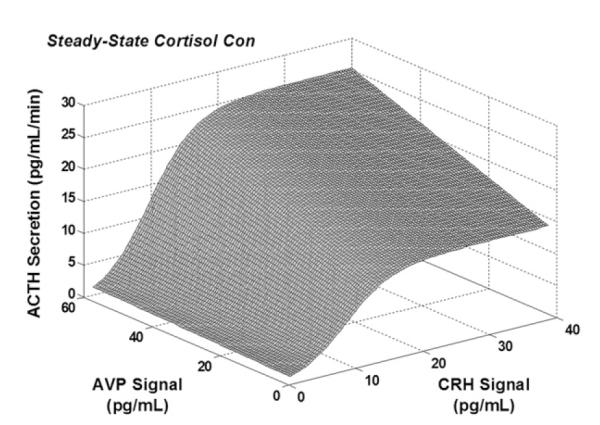
Joint AVP and CRH drive of ACTH secretion under fixed cortisol concentration (con) estimated from hypothalamopituitary portal venous sampling in the horse.
Fig. 4.
Opposite impact of age (independent variable) on ACTH efficacy (asymptotically maximal ACTH-stimulated pulsatile cortisol secretory rate) in men (N = 26, top) and women (N = 22, bottom). Pearson’s product-moment correlation coefficients are shown for the regressions. The P value in the ellipse denotes the gender difference in slopes. (Adapted from Keenan DM, Roelfsema F, Carroll BJ, et al. Sex defines the age dependence of endogenous ACTH-cortisol dose responsiveness. Am J Physiol Regul Integr Comp Physiol 2009;297(2):R515–23; with permission.)
Fig. 5.
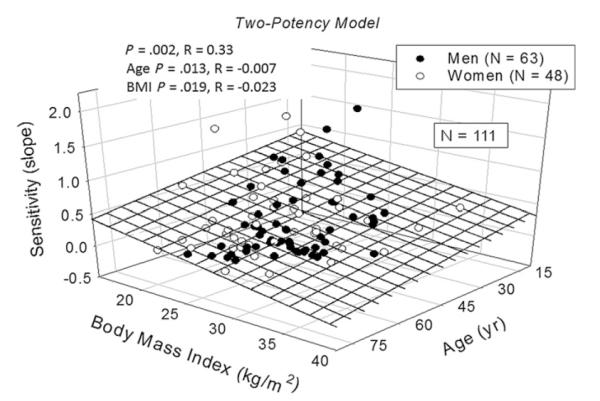
Age and body mass image (BMI) jointly attenuate adrenal sensitivity (maximal slope of ACTH-cortisol dose-response function) in a cohort of 111 healthy adults (overall P = .0017). Sensitivity was negatively associated with both age (P = .014) and BMI (P = .019), indicating reduced adrenal cortisol secretory responsiveness per unit increase in ACTH concentrations. R values are the correlation coefficients. (Adapted from Veldhuis JD, Iranmanesh A, Roelfsema F, et al. Tripartite control of dynamic ACTH-cortisol dose-responsiveness by age, body mass index and gender in 111 healthy adults. J Clin Endocrinol Metab 2011;96(9):2874–81; with permission.)
Other Diurnal Rhythms in Aging
Melatonin, core body temperature, DHEA, T-helper cells, slow-wave sleep, and other nycthemeral rhythms are also flattened with age (Box 8). The relationship between these and HPA changes is not known. GH secretagogues also drive ACTH/cortisol secretion, but the mechanism is not defined.179
Box 8. Other diurnal rhythms affected in aging.
↓ melatonin secretion at night194,213
↓ core body temperature rhythm242
↓ DHEA-sulfate levels201
↓ CD4 T-helper cell rhythm243
↓ δ (slow-wave) [and rapid-eye-movement] sleep57,59
↓ salivary amylase on awakening62
↓ free Te peak and phase advance (earlier)201
↓ thyrotropin (centenarians)65
SUMMARY
Age, gender, and sex steroids represent key modulators of physiologically incremental and pathologically overt adaptations in the ensemble regulation of CRH, AVP, ACTH, and cortisol output.126,165,175,180,181 Challenges in this arena include development and application of new safe rational approaches to identify, prevent, and treat deficient or excessive cortisol production. Further clinical investigations should aid in clarifying mechanistic HPA changes in aging and comorbid states (eg, increased visceral adiposity), with the goal of obviating HPA-associated morbidity, mortality, disability, and impaired quality of life in aging women and men.181–183
KEY POINTS.
• Aging increases adrenocorticotropic hormone (ACTH)/cortisol responses to corticotropin-releasing hormone (CRH) (especially in women) and to vasopressin/CRH (especially in men).
• Estrogen treatment reduces hyperresponsiveness in postmenopausal women.
• Age decreases glucocorticoid feedback (inhibition), leading to slow ACTH recovery after stress.
• There is no age effect on corticosteroid-binding globulin.
• Cortisol responses to hypoglycemia (insulin tolerance test) are preserved in aging.
• How body composition interacts with age in hypothalamic-corticotropic-adrenal regulation is not known.
ACKNOWLEDGMENTS
We thank Jill Smith for support with manuscript preparation.
Supported in part via R01 DK073148 and DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 1UL1 RR024150-01.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- 1.Mortola JF, Liu JH, Gillen JC, et al. Pulsatile rhythms of adrenocorticotropin (ACTH) and cortisol in women with endogenous depression: evidence for increased ACTH pulse frequency. J Clin Endocrinol Metab. 1987;65:962–8. doi: 10.1210/jcem-65-5-962. [DOI] [PubMed] [Google Scholar]
- 2.Huizenga NA, Koper JW, De Lange P, et al. A polymorphism in the glucocorticoid receptor gene may be associated with an increased sensitivity to glucocorticoids in vivo. J Clin Endocrinol Metab. 1998;83(1):144–51. doi: 10.1210/jcem.83.1.4490. [DOI] [PubMed] [Google Scholar]
- 3.Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43(1):1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 4.Fries E, Hesse J, Hellhammer J, et al. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–6. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Vamvakopoulos NC, Chrousos GP. Hormonal regulation of human corticotropin-releasing hormone gene expression: implications for the stress response and immune/inflammatory reaction. Endocr Rev. 1994;15(4):409–20. doi: 10.1210/edrv-15-4-409. [DOI] [PubMed] [Google Scholar]
- 6.Spinedi E, Suescun MO, Hadid R, et al. Effects of gonadectomy and sex hormone therapy on the endotoxin-stimulated hypothalamo-pituitary-adrenal axis: evidence for a neuroendocrine-immunological sexual dimorphism. Endocrinology. 1992;131(5):2430–6. doi: 10.1210/endo.131.5.1330501. [DOI] [PubMed] [Google Scholar]
- 7.Cameron OG, Kronfol Z, Greden JF, et al. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41(11):1090–5. doi: 10.1001/archpsyc.1983.01790220080013. [DOI] [PubMed] [Google Scholar]
- 8.Karanth S, Linthorst AC, Stalla GK, et al. Hypothalamic-pituitary-adrenocortical axis changes in a transgenic mouse with impaired glucocorticoid receptor function. Endocrinology. 1997;138(8):3476–85. doi: 10.1210/endo.138.8.5331. [DOI] [PubMed] [Google Scholar]
- 9.Invitti C, De Martin M, Delitala G, et al. Altered morning and nighttime pulsatile corticotropin and cortisol release in polycystic ovary syndrome. Metabolism. 1998;47(2):143–8. doi: 10.1016/s0026-0495(98)90210-4. [DOI] [PubMed] [Google Scholar]
- 10.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136(8):3299–309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 11.Spinedi E, Salas M, Chisari A, et al. Sex differences in the hypothalamo-pituitary-adrenal axis response to inflammatory and neuroendocrine stressors. Evidence for a pituitary defect in the autoimmune disease-susceptible female Lewis rat. Neuroendocrinology. 1994;60(6):609–17. doi: 10.1159/000126804. [DOI] [PubMed] [Google Scholar]
- 12.Rasmusson AM, Lipschitz DS, Wang S, et al. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):965–77. doi: 10.1016/s0006-3223(01)01264-1. [DOI] [PubMed] [Google Scholar]
- 13.McCormick CM, Smythe JW, Sharma S, et al. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain gluco-corticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84(1):55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- 14.Hudson JI, Hudson MS, Rothschild AJ, et al. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41(11):1086–9. doi: 10.1001/archpsyc.1983.01790220076012. [DOI] [PubMed] [Google Scholar]
- 15.Fonda SJ, Bertrand R, O’Donnell A, et al. Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60(3):385–90. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 16.Pfohl B, Sherman B, Schlechte J, et al. Differences in plasma ACTH and cortisol between depressed patients and normal controls. Biol Psychiatry. 1985;20(10):1055–72. doi: 10.1016/0006-3223(85)90004-6. [DOI] [PubMed] [Google Scholar]
- 17.Fliers E, Swaab DF, Pool CW, et al. The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus; changes with aging and in senile dementia. Brain Res. 1985;342(1):45–53. doi: 10.1016/0006-8993(85)91351-4. [DOI] [PubMed] [Google Scholar]
- 18.Gotthardt U, Schweiger U, Fahrenberg J, et al. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol. 1995;268(4 Pt 2):R865–73. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- 19.Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. Am J Psychiatry. 2005;162(6):1192–9. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundberg U. Stress hormones in health and illness: the roles of work and gender. Psychoneuroendocrinology. 2005;30(10):1017–21. doi: 10.1016/j.psyneuen.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 22.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, et al. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 23.Kudielka BM, Hellhammer J, Hellhammer DH, et al. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J Clin Endocrinol Metab. 1998;83(5):1756–61. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- 24.Weiss JM, McEwen BS, Silva MT, et al. Pituitary-adrenal influences on fear responding. Science. 1969;163(863):197–9. doi: 10.1126/science.163.3863.197. [DOI] [PubMed] [Google Scholar]
- 25.Collins A, Frankenhaeuser M. Stress responses in male and female engineering students. J Human Stress. 1978;4(2):43–8. doi: 10.1080/0097840X.1978.9934986. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54(6):648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ohashi M, Fujio N, Kato K, et al. Aging is without effect on the pituitary-adrenal axis in men. Gerontology. 1986;32(6):335–9. doi: 10.1159/000212812. [DOI] [PubMed] [Google Scholar]
- 28.Traustadottir T, Bosch PR, Cantu T, et al. Hypothalamic-pituitary-adrenal axis response and recovery from high-intensity exercise in women: effects of aging and fitness. J Clin Endocrinol Metab. 2004;89(7):3248–54. doi: 10.1210/jc.2003-031713. [DOI] [PubMed] [Google Scholar]
- 29.Waltman C, Blackman MR, Chrousos GP, et al. Spontaneous and glucocorticoid-inhibited adrenocorticotropin hormone and cortisol secretion are similar in healthy young and old men. J Clin Endocrinol Metab. 1991;73:495–502. doi: 10.1210/jcem-73-3-495. [DOI] [PubMed] [Google Scholar]
- 30.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. J Psychiatr Res. 1994;28(4):341–56. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 31.Ohashi M, Kato K, Nawata H, et al. Adrenocortical responsiveness to graded ACTH infusions in normal young and elderly human subjects. Gerontology. 1986;32(1):43–51. doi: 10.1159/000212764. [DOI] [PubMed] [Google Scholar]
- 32.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, et al. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70(6):422–30. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- 33.Parker CR, Jr, Slayden SM, Azziz R, et al. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85(1):48–54. doi: 10.1210/jcem.85.1.6265. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen A, Deslypere JP, Schelfhout W, et al. Adrenocortical function in old age: response to acute adrenocorticotropin stimulation. J Clin Endocrinol Metab. 1982;54(1):187–91. doi: 10.1210/jcem-54-1-187. [DOI] [PubMed] [Google Scholar]
- 35.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19(6):717–97. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 36.Montaron MF, Drapeau E, Dupret D, et al. Lifelong corticosterone level determines age-related decline in neurogenesis and memory. Neurobiol Aging. 2006;27(4):645–54. doi: 10.1016/j.neurobiolaging.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Bjorntorp P. Stress and cardiovascular disease. Acta Physiol Scand Suppl. 1997;640:144–8. [PubMed] [Google Scholar]
- 38.Wright CE, Kunz-Ebrecht SR, Iliffe S, et al. Physiological correlates of cognitive functioning in an elderly population. Psychoneuroendocrinology. 2005;30(9):826–38. doi: 10.1016/j.psyneuen.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83(6):1853–9. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34(2):271–92. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 41.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 42.Burke HM, Davis MC, Otte C, et al. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–56. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Watts LM, Manchem VP, Leedom TA, et al. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes. 2005;54(6):1846–53. doi: 10.2337/diabetes.54.6.1846. [DOI] [PubMed] [Google Scholar]
- 44.Smith GD, Ben Shlomo Y, Beswick A, et al. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005;112(3):332–40. doi: 10.1161/CIRCULATIONAHA.104.489088. [DOI] [PubMed] [Google Scholar]
- 45.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14(6):506–13. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 46.Born J, Ditschuneit I, Schreiber M, et al. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol. 1995;132(6):705–11. doi: 10.1530/eje.0.1320705. [DOI] [PubMed] [Google Scholar]
- 47.Rubin RT, Sekula LK, O’Toole S, et al. Pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration in normal women and men. Neuropsychopharmacology. 1999;20(5):434–46. doi: 10.1016/S0893-133X(98)00077-3. [DOI] [PubMed] [Google Scholar]
- 48.Heuser IJ, Gotthardt U, Schweiger U, et al. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging. 1994;15(2):227–31. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 49.Roelfsema F, van den Berg G, Frolich M, et al. Sex-dependent alteration in cortisol response to endogenous adrenocorticotropin. J Clin Endocrinol Metab. 1993;77:234–40. doi: 10.1210/jcem.77.1.8392084. [DOI] [PubMed] [Google Scholar]
- 50.Horrocks PM, Jones AF, Ratcliffe WA, et al. Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf) 1990;32(1):127–34. doi: 10.1111/j.1365-2265.1990.tb03758.x. [DOI] [PubMed] [Google Scholar]
- 51.Kirschbaum C, Kudielka BM, Gaab J, et al. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–62. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Heaney JL, Phillips AC, Carroll D. Ageing, physical function, and the diurnal rhythms of cortisol and dehydroepiandrosterone. Psychoneuroendocrinology. 2012;37(3):341–9. doi: 10.1016/j.psyneuen.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Van Cauter E, Plat L, Leproult R, et al. Alterations of circadian rhythmicity and sleep in aging: endocrine consequences. Horm Res. 1998;49(3–4):147–52. doi: 10.1159/000023162. [DOI] [PubMed] [Google Scholar]
- 54.Deuschle M, Gotthardt U, Schweiger U, et al. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61(22):2239–46. doi: 10.1016/s0024-3205(97)00926-0. [DOI] [PubMed] [Google Scholar]
- 55.Mazzoccoli G, Vendemiale G, La VM, et al. Circadian variations of cortisol, melatonin and lymphocyte subpopulations in geriatric age. Int J Immunopathol Pharmacol. 2010;23(1):289–96. doi: 10.1177/039463201002300127. [DOI] [PubMed] [Google Scholar]
- 56.Struder HK, Hollmann W, Platen P, et al. Neuroendocrine system and mental function in sedentary and endurance-trained elderly males. Int J Sports Med. 1999;20(3):159–66. doi: 10.1055/s-2007-971111. [DOI] [PubMed] [Google Scholar]
- 57.Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- 58.Espiritu JR. Aging-related sleep changes. Clin Geriatr Med. 2008;24(1):1–14. doi: 10.1016/j.cger.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88(5):2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 60.Heaney JL, Phillips AC, Carroll D. Ageing, depression, anxiety, social support and the diurnal rhythm and awakening response of salivary cortisol. Int J Psychophysiol. 2010;78(3):201–8. doi: 10.1016/j.ijpsycho.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Lai JC, Chong AM, Siu OT, et al. Humor attenuates the cortisol awakening response in healthy older men. Biol Psychol. 2010;84(2):375–80. doi: 10.1016/j.biopsycho.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Strahler J, Berndt C, Kirschbaum C, et al. Aging diurnal rhythms and chronic stress: distinct alteration of diurnal rhythmicity of salivary alpha-amylase and cortisol. Biol Psychol. 2010;84(2):248–56. doi: 10.1016/j.biopsycho.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 63.Kumari M, Badrick E, Chandola T, et al. Measures of social position and cortisol secretion in an aging population: findings from the Whitehall II study. Psychosom Med. 2010;72(1):27–34. doi: 10.1097/PSY.0b013e3181c85712. [DOI] [PubMed] [Google Scholar]
- 64.Wrosch C, Miller GE, Schulz R. Cortisol secretion and functional disabilities in old age: importance of using adaptive control strategies. Psychosom Med. 2009;71(9):996–1003. doi: 10.1097/PSY.0b013e3181ba6cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrari E, Cravello L, Falvo F, et al. Neuroendocrine features in extreme longevity. Exp Gerontol. 2008;43(2):88–94. doi: 10.1016/j.exger.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Kripke DF, Elliott JA, Youngstedt SD, et al. Circadian phase response curves to light in older and young women and men. J Circadian Rhythms. 2007;5:4. doi: 10.1186/1740-3391-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lavoie HB, Marsh EE, Hall JE. Absence of apparent circadian rhythms of gonadotropins and free alpha-subunit in postmenopausal women: evidencefor distinct regulation relative to other hormonal rhythms. J Biol Rhythms. 2006;21(1):58–67. doi: 10.1177/0748730405283244. [DOI] [PubMed] [Google Scholar]
- 68.Copinschi G, van Reeth O, Van Cauter E. Biologic rhythms. Effect of aging on the desynchronization of endogenous rhythmicity and environmental conditions. Presse Med. 1999;28(17):942–6. [in French] [PubMed] [Google Scholar]
- 69.Wolf OT, Dziobek I, McHugh P, et al. Subjective memory complaints in aging are associated with elevated cortisol levels. Neurobiol Aging. 2005;26(10):1357–63. doi: 10.1016/j.neurobiolaging.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 70.Buckley TM, Schatzberg AF. Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. Am J Geriatr Psychiatry. 2005;13(5):344–52. doi: 10.1176/appi.ajgp.13.5.344. [DOI] [PubMed] [Google Scholar]
- 71.Yehuda R, Golier JA, Harvey PD, et al. Relationship between cortisol and age-related memory impairments in Holocaust survivors with PTSD. Psychoneuroendocrinology. 2005;30(7):678–87. doi: 10.1016/j.psyneuen.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Ferrari E, Cravello L, Muzzoni B, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. Eur J Endocrinol. 2001;144(4):319–29. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- 73.Magri F, Locatelli M, Balza G, et al. Changes in endocrine circadian rhythms as markers of physiological and pathological brain aging. Chronobiol Int. 1997;14(4):385–96. doi: 10.3109/07420529709001459. [DOI] [PubMed] [Google Scholar]
- 74.Ferrari E, Magri F, Locatelli M, et al. Chrono-neuroendocrine markers of the aging brain. Aging (Milano) 1996;8(5):320–7. doi: 10.1007/BF03339588. [DOI] [PubMed] [Google Scholar]
- 75.Agarwal AK, Simha V, Oral EA, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab. 2003;88(10):4840–7. doi: 10.1210/jc.2003-030855. [DOI] [PubMed] [Google Scholar]
- 76.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131(3):1261–9. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 77.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 78.Bowman RE, MacLusky NJ, Sarmiento Y, et al. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses and central neurotransmitters. Endocrinology. 2004;145(8):3778–87. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 79.Patchev VK, Almeida OF. Gender specificity in the neural regulation of the response to stress: new leads from classical paradigms. Mol Neurobiol. 1998;16(1):63–77. doi: 10.1007/BF02740603. [DOI] [PubMed] [Google Scholar]
- 80.Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13(5):442–52. doi: 10.1046/j.1365-2826.2001.00653.x. [DOI] [PubMed] [Google Scholar]
- 81.Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16(5):1866–76. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young EA, Altemus M, Parkison V, et al. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25(6):881–91. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 83.Le Mevel JC, Abitbol S, Beraud G, et al. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology. 1979;105(3):812–7. doi: 10.1210/endo-105-3-812. [DOI] [PubMed] [Google Scholar]
- 84.Bingaman EW, Magnuson DJ, Gray TS, et al. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59(3):228–34. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- 85.Lee HC, Chang DE, Yeom M, et al. Gene expression profiling in hypothalamus of immobilization-stressed mouse using cDNA microarray. Brain Res Mol Brain Res. 2005;135(1–2):293–300. doi: 10.1016/j.molbrainres.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Canny BJ, O’Farrell KA, Clarke IJ, et al. The influence of sex and gonadectomy on the hypothalamo-pituitary-adrenal axis of the sheep. J Endocrinol. 1999;162(2):215–25. doi: 10.1677/joe.0.1620215. [DOI] [PubMed] [Google Scholar]
- 87.Turner AI, Canny BJ, Hobbs RJ, et al. Influence of sex and gonadal status of sheep on cortisol secretion in response to ACTH and on cortisol and LH secretion in response to stress: importance of different stressors. J Endocrinol. 2002;173(1):113–22. doi: 10.1677/joe.0.1730113. [DOI] [PubMed] [Google Scholar]
- 88.Lado-Abeal J, Robert-McComb JJ, Qian XP, et al. Sex differences in the neuroendocrine response to short-term fasting in rhesus macaques. J Neuroendocrinol. 2005;17(7):435–44. doi: 10.1111/j.1365-2826.2005.01323.x. [DOI] [PubMed] [Google Scholar]
- 89.Roy BN, Reid RL, van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140(5):2191–8. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- 90.Young EA. The role of gonadal steroids in hypothalamic-pituitary-adrenal axis regulation. Crit Rev Neurobiol. 1995;9(4):371–81. [PubMed] [Google Scholar]
- 91.Raven PW, Taylor NF. Sex differences in the human metabolism of cortisol. Endocr Res. 1996;22(4):751–5. doi: 10.1080/07435809609043772. [DOI] [PubMed] [Google Scholar]
- 92.Pomara N, Willoughby LM, Ritchie JC, et al. Sex-related elevation in cortisol during chronic treatment with alprazolam associated with enhanced cognitive performance. Psychopharmacology (Berl) 2005;2:1–6. doi: 10.1007/s00213-005-0088-2. [DOI] [PubMed] [Google Scholar]
- 93.Carey MP, Deterd CH, De Koning J, et al. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144(2):311–21. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 94.Wagner CK, Morrell JI. Distribution and steroid hormone regulation of aromatase mRNA expression in the forebrain of adult male and female rats: a cellular-level analysis using in situ hybridization. J Comp Neurol. 1996;370(1):71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 95.Arai Y, Gorski RA. Critical exposure time for androgenization of the developing hypothalamus in the female rat. Endocrinology. 1968;82(5):1010–4. doi: 10.1210/endo-82-5-1010. [DOI] [PubMed] [Google Scholar]
- 96.Seale JV, Wood SA, Atkinson HC, et al. Organisational role for testosterone and estrogen on adult HPA axis activity in the male rat. Endocrinology. 2005;146(4):1973–82. doi: 10.1210/en.2004-1201. [DOI] [PubMed] [Google Scholar]
- 97.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimorphism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peiffer A, Lapointe B, Barden N. Hormonal regulation of type II glucocorticoid receptor messenger ribonucleic acid in rat brain. Endocrinology. 1991;129(4):2166–74. doi: 10.1210/endo-129-4-2166. [DOI] [PubMed] [Google Scholar]
- 99.Burgess LH, Handa RJ. Estrogen-induced alterations in the regulation of mineralcorticorticoid and glucocorticoid receptor messenger RNA expression in the female rat anterior pituitary gland and brain. Mol Cell Neurosci. 1993;4:191–8. doi: 10.1006/mcne.1993.1023. [DOI] [PubMed] [Google Scholar]
- 100.Paulmyer-Lacroix O, Hery M, Pugeat M, et al. The modulatory role of estrogens on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of ovariectomized rats: role of the adrenal gland. J Neuroendocrinol. 1996;8(7):515–9. doi: 10.1046/j.1365-2826.1996.04835.x. [DOI] [PubMed] [Google Scholar]
- 101.Patchev VK, Hayashi S, Orikasa C, et al. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J. 1995;9(5):419–23. doi: 10.1096/fasebj.9.5.7896013. [DOI] [PubMed] [Google Scholar]
- 102.Nappi RE, Rivest S. Ovulatory cycle influences the stimulatory effect of stress on the expression of corticotropin-releasing factor receptor messenger ribonucleic acid in the paraventricular nucleus of the female rat hypothalamus. Endocrinology. 1995;136(9):4073–83. doi: 10.1210/endo.136.9.7649116. [DOI] [PubMed] [Google Scholar]
- 103.Bohler HC, Jr, Zoeller RT, King JC, et al. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res Mol Brain Res. 1990;8(3):259–62. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- 104.Ellison ET, Burch JC. The effect of estrogenic substances upon the pituitary, adrenals and ovaries. Endocrinology. 1936;20:746–52. [Google Scholar]
- 105.Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–24. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 106.Critchlow V, Liebelt RA, Bar-Sela M, et al. Sex difference in resting pituitaryadrenal function in the rat. Am J Physiol. 1963;205(5):807–15. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 107.Gompertz D. The effect of sex hormones on the adrenal gland of the male rat. Endocrinology. 1958;17:107–13. doi: 10.1677/joe.0.0170107. [DOI] [PubMed] [Google Scholar]
- 108.Adan RA, Burbach JP. Regulation of vasopressin and oxytocin gene expression by estrogen and thyroid hormone. Prog Brain Res. 1992;92:127–36. [PubMed] [Google Scholar]
- 109.Viau V, Chu A, Soriano L, et al. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19(15):6684–93. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lund TD, Munson DJ, Haldy ME, et al. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16(3):272–8. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- 111.Miller MA, Vician L, Clifton DK, et al. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10(3):615–9. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- 112.DeLeon KR, Grimes JM, Melloni RH., Jr Repeated anabolic-androgenic steroid treatment during adolescence increases vasopressin V(1A) receptor binding in Syrian hamsters: correlation with offensive aggression. Horm Behav. 2002;42(2):182–91. doi: 10.1006/hbeh.2002.1802. [DOI] [PubMed] [Google Scholar]
- 113.Handa RJ, Nunley KM, Lorens SA, et al. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55(1):117–24. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 114.Miller MA, Urban JH, Dorsa DM. Steroid dependency of vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Endocrinology. 1989;125(5):2335–40. doi: 10.1210/endo-125-5-2335. [DOI] [PubMed] [Google Scholar]
- 115.De Vries GJ, Duetz W, Buijs RM, et al. Effects of androgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399(2):296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- 116.Moore RJ, Gazak JM, Wilson JD. Regulation of cytoplasmic dihydrotestosterone binding in dog prostate by 17 beta-estradiol. J Clin Invest. 1979;63(3):351–7. doi: 10.1172/JCI109310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rance NE, Max SR. Modulation of the cytosolic androgen receptor in striated muscle by sex steroids. Endocrinology. 1984;115(3):862–6. doi: 10.1210/endo-115-3-862. [DOI] [PubMed] [Google Scholar]
- 118.Lindheim SR, Legro RS, Bernstein L, et al. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167(6):1831–6. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 119.Dayas CV, Xu Y, Buller KM, et al. Effects of chronic oestrogen replacement on stress-induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol. 2000;12(8):784–94. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- 120.Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997;154(12):1641–7. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 121.Jenkins R. Sex differences in depression. Br J Hosp Med. 1987;38(5):485–6. [PubMed] [Google Scholar]
- 122.Seeman TE, Singer B, Wilkinson CW, et al. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26(3):225–40. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 123.Vierhapper H, Nowotny P, Waldhausl W. Sex-specific differences in cortisol production rates in humans. Metabolism. 1998;47(8):974–6. doi: 10.1016/s0026-0495(98)90353-5. [DOI] [PubMed] [Google Scholar]
- 124.Vierhapper H, Nowotny P, Waldhausl W. Production rates of cortisol in men with hypogonadism. Metabolism. 2004;53(9):1174–6. doi: 10.1016/j.metabol.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 125.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468–73. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 126.Keenan DM, Roelfsema F, Veldhuis JD. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am J Physiol Endocrinol Metab. 2004;287(4):E652–61. doi: 10.1152/ajpendo.00167.2004. [DOI] [PubMed] [Google Scholar]
- 127.Kraan GP, Dullaart RP, Pratt JJ, et al. The daily cortisol production reinvestigated in healthy men. The serum and urinary cortisol production rates are not significantly different. J Clin Endocrinol Metab. 1998;83(4):1247–52. doi: 10.1210/jcem.83.4.4694. [DOI] [PubMed] [Google Scholar]
- 128.Baumann G. Estrogens and the hypothalamo-pituitary-adrenal axis in man: evidence for normal feedback regulation by corticosteroids. J Clin Endocrinol Metab. 1983;57(6):1193–7. doi: 10.1210/jcem-57-6-1193. [DOI] [PubMed] [Google Scholar]
- 129.Mizuno TM, Kleopoulos SP, Bergen HT, et al. Hypothalamic proopiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47(2):294–7. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 130.Purnell JQ, Brandon DD, Isabelle LM, et al. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89(1):281–7. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- 131.Genazzani AR, Lemarchand-Beraud T, Aubert ML, et al. Pattern of plasma ACTH, hGH, and cortisol during menstrual cycle. J Clin Endocrinol Metab. 1975;41(3):431–7. doi: 10.1210/jcem-41-3-431. [DOI] [PubMed] [Google Scholar]
- 132.Marinari KT, Leshner AI, Doyle MP. Menstrual cycle status and adrenocortical reactivity to psychological stress. Psychoneuroendocrinology. 1976;1(3):213–8. doi: 10.1016/0306-4530(76)90011-1. [DOI] [PubMed] [Google Scholar]
- 133.Atkinson HC, Waddell BJ. The hypothalamic-pituitary-adrenal axis in rat pregnancy and lactation: circadian variation and interrelationship of plasma adrenocorticotropin and corticosterone. Endocrinology. 1995;136(2):512–20. doi: 10.1210/endo.136.2.7835284. [DOI] [PubMed] [Google Scholar]
- 134.Polderman KH, Gooren LJ, Van der Veen EA. Testosterone administration increases adrenal response to adrenocorticotrophin. Clin Endocrinol (Oxf) 1994;40(5):595–601. doi: 10.1111/j.1365-2265.1994.tb03010.x. [DOI] [PubMed] [Google Scholar]
- 135.Arvat E, Di Vito L, Lanfranco F, et al. Stimulatory effect of adrenocorticotropin on cortisol, aldosterone, and dehydroepiandrosterone secretion in normal humans: dose-response study. J Clin Endocrinol Metab. 2000;85(9):3141–6. doi: 10.1210/jcem.85.9.6784. [DOI] [PubMed] [Google Scholar]
- 136.Greenspan SL, Rowe JW, Maitland LA, et al. The pituitary-adrenal glucocorticoid response is altered by gender and disease. J Gerontol. 1993;48(3):M72–7. doi: 10.1093/geronj/48.3.m72. [DOI] [PubMed] [Google Scholar]
- 137.Luisi S, Tonetti A, Bernardi F, et al. Effect of acute corticotropin releasing factor on pituitary-adrenocortical responsiveness in elderly women and men. J Endocrinol Invest. 1998;21(7):449–53. doi: 10.1007/BF03347325. [DOI] [PubMed] [Google Scholar]
- 138.Gallucci WT, Baum A, Laue L, et al. Sex differences in sensitivity of the hypothalamic-pituitary-adrenal axis. Health Psychol. 1993;12(5):420–5. doi: 10.1037//0278-6133.12.5.420. [DOI] [PubMed] [Google Scholar]
- 139.Bloch M, Rubinow DR, Schmidt PJ, et al. Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J Clin Endocrinol Metab. 2005;90(2):695–9. doi: 10.1210/jc.2004-1388. [DOI] [PubMed] [Google Scholar]
- 140.Liu JH, Rasmussen DD, Rivier J, et al. Pituitary responses to synthetic corticotropin-releasing hormone: absence of modulatory effects by estrogen and progestin. Am J Obstet Gynecol. 1987;157(6):1387–91. doi: 10.1016/s0002-9378(87)80229-6. [DOI] [PubMed] [Google Scholar]
- 141.Kirschbaum C, Schommer N, Federenko I, et al. Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. J Clin Endocrinol Metab. 1996;81(10):3639–43. doi: 10.1210/jcem.81.10.8855815. [DOI] [PubMed] [Google Scholar]
- 142.Rubinow DR, Roca CA, Schmidt PJ, et al. Testosterone suppression of CRH-stimulated cortisol in men. Neuropsychopharmacology. 2005;30(10):1906–12. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Diamond MP, Jones T, Caprio S, et al. Gender influences counterregulatory hormone responses to hypoglycemia. Metabolism. 1993;42:1568–72. doi: 10.1016/0026-0495(93)90152-e. [DOI] [PubMed] [Google Scholar]
- 144.Rubin RT, Rhodes ME, O’Toole S, et al. Sexual diergism of hypothalamo-pituitary-adrenal cortical responses to low-dose physotigmine in elderly vs. young women and men. Neuropsychopharmacology. 2002;26(5):672–81. doi: 10.1016/S0893-133X(01)00376-1. [DOI] [PubMed] [Google Scholar]
- 145.Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol Psychiatry. 2002;52(4):318–27. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- 146.Earle TL, Linden W, Weinberg J. Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. J Psychosom Res. 1999;46(2):125–41. doi: 10.1016/s0022-3999(98)00075-0. [DOI] [PubMed] [Google Scholar]
- 147.Deuster PA, Petrides JS, Singh A, et al. High intensity exercise promotes escape of adrenocorticotropin and cortisol from suppression by dexamethasone: sexually dimorphic responses. J Clin Endocrinol Metab. 1998;83(9):3332–8. doi: 10.1210/jcem.83.9.5110. [DOI] [PubMed] [Google Scholar]
- 148.Wilkinson CW, Petrie EC, Murray SR, et al. Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. J Clin Endocrinol Metab. 2001;86(2):545–50. doi: 10.1210/jcem.86.2.7232. [DOI] [PubMed] [Google Scholar]
- 149.Boscaro M, Paoletta A, Scarpa E, et al. Age-related changes in glucocorticoid fast feedback inhibition of adrenocorticotropin in man. J Clin Endocrinol Metab. 1998;83(4):1380–3. doi: 10.1210/jcem.83.4.4745. [DOI] [PubMed] [Google Scholar]
- 150.Veldhuis JD, Keenan DM, Roelfsema F, et al. Aging-related adaptations in the corticotropic axis: modulation by gender. Endocrinol Metab Clin North Am. 2005;34:993–1014. doi: 10.1016/j.ecl.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 151.O’Brien JT, Schweitzer I, Ames D, et al. Cortisol suppression by dexamethasone in the healthy elderly: effects of age, dexamethasone levels, and cognitive function. Biol Psychiatry. 1994;36(6):389–94. doi: 10.1016/0006-3223(94)91214-9. [DOI] [PubMed] [Google Scholar]
- 152.Oxenkrug GF, Pomara N, McIntyre IM, et al. Aging and cortisol resistance to suppression by dexamethasone: a positive correlation. Psychiatry Res. 1983;10(2):125–30. doi: 10.1016/0165-1781(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 153.Ortiz-Alonso FJ, Galecki A, Herman WH, et al. Hypoglycemia counterregulation in elderly humans: relationship to glucose levels. Am J Physiol. 1994;267(4 Pt 1):E497–506. doi: 10.1152/ajpendo.1994.267.4.E497. [DOI] [PubMed] [Google Scholar]
- 154.Pavlov EP, Harman SM, Chrousos GP, et al. Responses of plasma adrenocorticotropin, cortisol, and dehydroepiandrosterone to ovine corticotropin-releasing hormone in healthy aging men. J Clin Endocrinol Metab. 1986;62(4):767–72. doi: 10.1210/jcem-62-4-767. [DOI] [PubMed] [Google Scholar]
- 155.Wilkinson CW, Peskind ER, Raskind MA. Decreased hypothalamic-pituitary-adrenal axis sensitivity to cortisol feedback inhibition in human aging. Neuroendocrinology. 1997;65(1):79–90. doi: 10.1159/000127167. [DOI] [PubMed] [Google Scholar]
- 156.Reynolds RM, Walker BR, Syddall HE, et al. Is there a gender difference in the associations of birthweight and adult hypothalamic-pituitary-adrenal axis activity? Eur J Endocrinol. 2005;152(2):249–53. doi: 10.1530/eje.1.01846. [DOI] [PubMed] [Google Scholar]
- 157.Won JG, Jap TS, Chang SC, et al. Evidence for a delayed, integral, and proportional phase of glucocorticoid feedback on ACTH secretion in normal human volunteers. Metabolism. 1996;35:254–9. doi: 10.1016/0026-0495(86)90210-6. [DOI] [PubMed] [Google Scholar]
- 158.Reader SC, Alaghband-Zadeh J, Daly JR, et al. Negative rate-sensitive feedback effects on adrenocorticotrophin secretion by cortisol in normal subjects. J Endocrinol. 1982;92(3):443–8. doi: 10.1677/joe.0.0920443. [DOI] [PubMed] [Google Scholar]
- 159.Mason BL, Pariante CM, Thomas SA. A revised role for P-glycoprotein in the brain distribution of dexamethasone, cortisol, and corticosterone in wild type and ABCB1A/B-deficient mice. Endocrinology. 2008;149(10):5244–53. doi: 10.1210/en.2008-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lentjes EG, Romijn F, Maassen RJ, et al. Free cortisol in serum assayed by temperature-controlled ultrafiltration before fluorescence polarization immunoassay. Clin Chem. 1993;39(12):2518–21. [PubMed] [Google Scholar]
- 161.Slaunwhite WR, Jr, Lockle GN, Back N, et al. Inactivity in vivo of transcortin-bound cortisol. Science. 1962;135:1062–3. doi: 10.1126/science.135.3508.1062. [DOI] [PubMed] [Google Scholar]
- 162.Schwarz S, Pohl P. Steroid hormones and steroid hormone binding globulins in cerebrospinal fluid studied in individuals with intact and with disturbed blood-cerebrospinal fluid barrier. Neuroendocrinology. 1992;55(2):174–82. doi: 10.1159/000126112. [DOI] [PubMed] [Google Scholar]
- 163.Viau V, Sharma S, Meaney MJ. Changes in plasma adrenocorticotropin, corticosterone, corticosteroid-binding globulin, and hippocampal glucocorticoid receptor occupancy/translocation in rat pups in response to stress. J Neuroendocrinol. 1996;8(1):1–8. doi: 10.1111/j.1365-2826.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 164.Kawai A, Yates FE. Interference with feedback inhibition of adrenocorticotropin release by protein binding of corticosterone. Endocrinology. 1966;79(6):1040–6. doi: 10.1210/endo-79-6-1040. [DOI] [PubMed] [Google Scholar]
- 165.Keenan DM, Veldhuis JD. Cortisol feedback state governs adrenocorticotropin secretory-burst shape, frequency and mass in a dual-waveform construct: time-of-day dependent regulation. Am J Physiol. 2003;285(5):R950–61. doi: 10.1152/ajpregu.00299.2003. [DOI] [PubMed] [Google Scholar]
- 166.Veldhuis JD, Iranmanesh A, Lizarralde G, et al. Amplitude modulation of a burst-like mode of cortisol secretion subserves the circadian glucocorticoid rhythm in man. Am J Physiol. 1989;257:E6–14. doi: 10.1152/ajpendo.1989.257.1.E6. [DOI] [PubMed] [Google Scholar]
- 167.Bright GM. Corticosteroid-binding globulin influences kinetic parameters of plasma cortisol transport and clearance. J Clin Endocrinol Metab. 1995;80(3):770–5. doi: 10.1210/jcem.80.3.7883829. [DOI] [PubMed] [Google Scholar]
- 168.Fernandez-Real JM, Pugeat M, Grasa M, et al. Serum corticosteroid-binding globulin concentration and insulin resistance syndrome: a population study. J Clin Endocrinol Metab. 2002;87(10):4686–90. doi: 10.1210/jc.2001-011843. [DOI] [PubMed] [Google Scholar]
- 169.de Lacerda L, Kowarski A, Migeon CJ. Diurnal variation of the metabolic clearance rate of cortisol. Effect on measurement of cortisol production rate. J Clin Endocrinol Metab. 1973;36(6):1043–9. doi: 10.1210/jcem-36-6-1043. [DOI] [PubMed] [Google Scholar]
- 170.Besser GM, Orth DN, Nicholson WE, et al. Dissociation of the disappearance of bioactive and radioimmunoreactive ACTH from plasma in man. J Clin Endocrinol Metab. 1971;32:595–603. doi: 10.1210/jcem-32-5-595. [DOI] [PubMed] [Google Scholar]
- 171.Veldhuis JD, Iranmanesh A, Naftolowitz D, et al. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab. 2001;86(11):5554–63. doi: 10.1210/jcem.86.11.8046. [DOI] [PubMed] [Google Scholar]
- 172.Iranmanesh A, Lizarralde G, Veldhuis JD. Coordinate activation of the corticotropic axis by insulin-induced hypoglycemia: simultaneous estimates of B-endorphin, ACTH, and cortisol secretion and disappearance in normal men. Acta Endocrinol (Copenh) 1993;128:521–8. doi: 10.1530/acta.0.1280521. [DOI] [PubMed] [Google Scholar]
- 173.Iranmanesh A, Short D, Lizarralde G, et al. Intensive venous sampling paradigms disclose high-frequency ACTH release episodes in normal men. J Clin Endocrinol Metab. 1990;71:1276–83. doi: 10.1210/jcem-71-5-1276. [DOI] [PubMed] [Google Scholar]
- 174.Veldhuis JD, Iranmanesh A, Johnson ML, et al. Amplitude, but not frequency, modulation of ACTH secretory bursts gives rise to the nyctohemeral rhythm of the corticotropic axis in man. J Clin Endocrinol Metab. 1990;71:452–63. doi: 10.1210/jcem-71-2-452. [DOI] [PubMed] [Google Scholar]
- 175.Keenan DM, Licinio J, Veldhuis JD. A feedback-controlled ensemble model of the stress-responsive hypothalamo-pituitary-adrenal axis. Proc Natl Acad Sci U S A. 2001;98(7):4028–33. doi: 10.1073/pnas.051624198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Keenan DM, Alexander SL, Irvine CH, et al. Reconstruction of in vivo time-evolving neuroendocrine dose-response properties unveils admixed deterministic and stochastic elements. Proc Natl Acad Sci U S A. 2004;101(17):6740–5. doi: 10.1073/pnas.0300619101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Keenan DM, Alexander S, Irvine CH, et al. Quantifying nonlinear interactions within the hypothalamo-pituitary-adrenal axis in the conscious horse. Endocrinology. 2009;150:1941–51. doi: 10.1210/en.2008-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Keenan DM, Roelfsema F, Carroll BJ, et al. Sex defines the age dependence of endogenous ACTH-cortisol dose responsiveness. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R515–23. doi: 10.1152/ajpregu.00200.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Veldhuis JD, Roemmich JN, Richmond EJ, et al. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27(2):101–40. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 180.Milad MA, Jusko WJ. Pharmacodynamic interpretation of adrenocorticotropin stimulation tests. Ann Pharmacother. 1993;27(10):1195–7. doi: 10.1177/106002809302701008. [DOI] [PubMed] [Google Scholar]
- 181.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22(4):502–48. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 182.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214(4521):685–7. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 183.Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46(11):1461–71. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 184.Liu J, Bisschop PH, Eggels L, et al. Intrahypothalamic estradiol modulates hypothalamus-pituitary-adrenal-axis activity in female rats. Endocrinology. 2012;153(7):3337–44. doi: 10.1210/en.2011-2176. [DOI] [PubMed] [Google Scholar]
- 185.Workel JO, Oitzl MS, Fluttert M, et al. Differential and age-dependent effects of maternal deprivation on the hypothalamic-pituitary-adrenal axis of brown Norway rats from youth to senescence. J Neuroendocrinol. 2001;13(7):569–80. doi: 10.1046/j.1365-2826.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- 186.O’Brien JT, Lloyd A, McKeith I, et al. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161(11):2081–90. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 187.Berghard A, Hagglund AC, Bohm S, et al. Lhx2-dependent specification of olfactory sensory neurons is required for successful integration of olfactory, vomeronasal, and GnRH neurons. FASEB J. 2012;26(8):3464–72. doi: 10.1096/fj.12-206193. [DOI] [PubMed] [Google Scholar]
- 188.Marin P, Darin N, Amemiya T, et al. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41(8):882–6. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 189.Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. 2013;43:449–69. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- 190.Cocchi D. Age-related alterations in gonadotropin, adrenocorticotropin and growth hormone secretion. Aging (Milano) 1992;4(2):103–13. doi: 10.1007/BF03324075. [DOI] [PubMed] [Google Scholar]
- 191.Swaab DF, Bao AM. (Re-)activation of neurons in aging and dementia: lessons from the hypothalamus. Exp Gerontol. 2011;46:178–84. doi: 10.1016/j.exger.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 192.van Coevorden A, Mockel J, Laurent E, et al. Neuroendocrine rhythms and sleep in aging men. Am J Physiol. 1991;260:E651–61. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- 193.Martin CG, Bruce J, Fisher PA. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: the role of parental psychosocial risk and monitoring. Horm Behav. 2012;61(5):661–8. doi: 10.1016/j.yhbeh.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sharma M, Palacios-Bois J, Schwartz G, et al. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25(3):305–19. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- 195.Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 1985;61(3):439–43. doi: 10.1210/jcem-61-3-439. [DOI] [PubMed] [Google Scholar]
- 196.Halbreich U, Asnis GM, Zumoff B, et al. Effect of age and sex on cortisol secretion in depressives and normals. Psychiatry Res. 1984;13(3):221–9. doi: 10.1016/0165-1781(84)90037-4. [DOI] [PubMed] [Google Scholar]
- 197.Touitou Y, Sulon J, Bogdan A, et al. Adrenocortical hormones, ageing and mental condition: seasonal and circadian rhythms of plasma 18-hydroxy-11-deoxycorticosterone, total and free cortisol and urinary corticosteroids. J Endocrinol. 1983;96(1):53–64. doi: 10.1677/joe.0.0960053. [DOI] [PubMed] [Google Scholar]
- 198.Milcu SM, Bogdan C, Nicolau GY, et al. Cortisol circadian rhythm in 70–100-year-old subjects. Endocrinologie. 1978;16(1):29–39. [PubMed] [Google Scholar]
- 199.Deuschle M, Weber B, Colla M, et al. Effects of major depression, aging and gender upon calculated diurnal free plasma cortisol concentrations: a re-evaluation study. Stress. 1998;2(4):281–7. doi: 10.3109/10253899809167292. [DOI] [PubMed] [Google Scholar]
- 200.Bergendahl M, Iranmanesh A, Mulligan T, et al. Impact of age on cortisol secretory dynamics basally and as driven by nutrient-withdrawal stress. J Clin Endocrinol Metab. 2000;85(6):2203–14. doi: 10.1210/jcem.85.6.6628. [DOI] [PubMed] [Google Scholar]
- 201.Montanini V, Simoni M, Chiossi G, et al. Age-related changes in plasma dehydroepiandrosterone sulphate, cortisol, testosterone and free testosterone circadian rhythms in adult men. Horm Res. 1988;29(1):1–6. doi: 10.1159/000180956. [DOI] [PubMed] [Google Scholar]
- 202.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging. 1995;16(4):571–6. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 203.Zhou JN, Swaab DF. Activation and degeneration during aging: a morphometric study of the human hypothalamus. Microsc Res Tech. 1999;44(1):36–48. doi: 10.1002/(SICI)1097-0029(19990101)44:1<36::AID-JEMT5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 204.Aiba M, Fujibayashi M. Alteration of subcapsular adrenocortical zonation in humans with aging: the progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. J Histochem Cytochem. 2011;59(5):557–64. doi: 10.1369/0022155411404071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ferrari M, Mantero F. Male aging and hormones: the adrenal cortex. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):92–5. [PubMed] [Google Scholar]
- 206.Tanaka H, Akama H, Ichikawa Y, et al. Glucocorticoid receptors in normal leukocytes: effects of age, gender, season, and plasma cortisol concentrations. Clin Chem. 1991;37(10 Pt 1):1715–9. [PubMed] [Google Scholar]
- 207.Morano MI, Vazquez DM, Akil H. The role of the hippocampal mineralocorticoid and glucocorticoid receptors in the hypothalamo-pituitary-adrenal axis of the aged Fisher rat. Mol Cell Neurosci. 1994;5(5):400–12. doi: 10.1006/mcne.1994.1050. [DOI] [PubMed] [Google Scholar]
- 208.Hauger RL, Thrivikraman KV, Plotsky PM. Age-related alterations of hypothalamic-pituitary-adrenal axis function in male Fischer 344 rats. Endocrinology. 1997;134:1528–36. doi: 10.1210/endo.134.3.8119195. [DOI] [PubMed] [Google Scholar]
- 209.Meaney MJ, Aitken DH, Sharma S, et al. Basal ACTH, corticosterone and corticosterone-binding globulin levels over the diurnal cycle, and age-relatedchanges in hippocampal type I and type II corticosteroid receptor binding capacity in young and aged, handled and nonhandled rats. Neuroendocrinology. 1992;55(2):204–13. doi: 10.1159/000126116. [DOI] [PubMed] [Google Scholar]
- 210.Issa AM, Rowe W, Gauthier S, et al. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10(10):3247–54. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gust DA, Wilson ME, Stocker T, et al. Activity of the hypothalamic-pituitary-adrenal axis is altered by aging and exposure to social stress in female rhesus monkeys. J Clin Endocrinol Metab. 2000;85(7):2556–63. doi: 10.1210/jcem.85.7.6696. [DOI] [PubMed] [Google Scholar]
- 212.Haus E, Nicolau G, Lakatua DJ, et al. Circadian rhythm parameters of endocrine functions in elderly subjects during the seventh to the ninth decade of life. Chronobiologia. 1989;16(4):331–52. [PubMed] [Google Scholar]
- 213.Ferrari E, Magri F, Dori D, et al. Neuroendocrine correlates of the aging brain in humans. Neuroendocrinology. 1995;61(4):464–70. doi: 10.1159/000126869. [DOI] [PubMed] [Google Scholar]
- 214.Blichert-Toft M, Hummer L. Serum immunoreactive corticotrophin and response to metyrapone in old age in man. Gerontology. 1977;23:236–43. doi: 10.1159/000212192. [DOI] [PubMed] [Google Scholar]
- 215.da Silva RM, Pinto E, Goldman SM, et al. Children with Cushing’s syndrome: primary pigmented nodular adrenocortical disease should always be suspected. Pituitary. 2011;14(1):61–7. doi: 10.1007/s11102-010-0260-5. [DOI] [PubMed] [Google Scholar]
- 216.Ambrosio MR, Campo M, Zatelli MC, et al. Unexpected activation of pituitary-adrenal axis in healthy young and elderly subjects during somatostatin infusion. Neuroendocrinology. 1998;68(2):123–8. doi: 10.1159/000054358. [DOI] [PubMed] [Google Scholar]
- 217.Seeman TE, Singer B, Charpentier P. Gender differences in patterns of HPA axis response to challenge. Macarthur studies of successful aging. Psychoneuroendocrinology. 1995;20(7):711–25. doi: 10.1016/0306-4530(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 218.Gelfin Y, Lerer B, Lesch KP, et al. Complex effects of age and gender on hypothermic, adrenocorticotrophic hormone and cortisol responses to ipsapirone challenge in normal subjects. Psychopharmacology. 1995;120(3):356–64. doi: 10.1007/BF02311184. [DOI] [PubMed] [Google Scholar]
- 219.Peskind ER, Raskind MA, Wingerson D, et al. Hypothalamic-pituitary-adrenocortical axis responses to physostigmine: effects of Alzheimer’s disease and gender. Biol Psychiatry. 1996;40(1):61–8. doi: 10.1016/0006-3223(95)00318-5. [DOI] [PubMed] [Google Scholar]
- 220.Lovallo WR, King AC, Farag NH, et al. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37:1922–8. doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Giordano R, Bo M, Pellegrino M, et al. Hypothalamus-pituitary-adrenal hyperactivity in human aging is partially refractory to stimulation by mineralocorticoid receptor blockade. J Clin Endocrinol Metab. 2005;90(10):5656–62. doi: 10.1210/jc.2005-0105. [DOI] [PubMed] [Google Scholar]
- 222.Heuser I, Deuschle M, Weber A, et al. The role of mineralocorticoid receptors in the circadian activity of the human hypothalamus-pituitary-adrenal system: effect of age. Neurobiol Aging. 2000;21(4):585–9. doi: 10.1016/s0197-4580(00)00145-7. [DOI] [PubMed] [Google Scholar]
- 223.von Bardeleben U, Holsboer F. Effect of age on the cortisol response to human corticotropin-releasing hormone in depressed patients pretreated with dexamethasone. Biol Psychiatry. 1991;29(10):1042–50. doi: 10.1016/0006-3223(91)90360-x. [DOI] [PubMed] [Google Scholar]
- 224.Martens EA, Lemmens SG, Adam TC, et al. Sex differences in HPA axis activity in response to a meal. Physiol Behav. 2012;106(2):272–7. doi: 10.1016/j.physbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 225.Heaney JL, Phillips AC, Carroll D. Aging, health behaviors, and the diurnal rhythm and awakening response of salivary cortisol. Exp Aging Res. 2012;38(3):295–314. doi: 10.1080/0361073X.2012.672134. [DOI] [PubMed] [Google Scholar]
- 226.Olbrich D, Dittmar M. The cortisol awakening response is related with PERIOD1 clock gene expression in older women. Exp Gerontol. 2012;47(7):527–33. doi: 10.1016/j.exger.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 227.Brandtstadter J, Baltes-Gotz B, Kirschbaum C, et al. Developmental and personality correlates of adrenocortical activity as indexed by salivary cortisol: observations in the age range of 35 to 65 years. J Psychosom Res. 1991;35(2–3):173–85. doi: 10.1016/0022-3999(91)90072-v. [DOI] [PubMed] [Google Scholar]
- 228.Evans P, Hucklebridge F, Loveday C, et al. The cortisol awakening response is related to executive function in older age. Int J Psychophysiol. 2012;84(2):201–4. doi: 10.1016/j.ijpsycho.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 229.Almela M, van der Meij L, Hidalgo V, et al. The cortisol awakening response and memory performance in older men and women. Psychoneuroendocrinology. 2012;37:1929–40. doi: 10.1016/j.psyneuen.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 230.Nicolson N, Storms C, Ponds R, et al. Salivary cortisol levels and stress reactivity in human aging. J Gerontol A Biol Sci Med Sci. 1997;52(2):M68–75. doi: 10.1093/gerona/52a.2.m68. [DOI] [PubMed] [Google Scholar]
- 231.Roelfsema F, Pijl H, Keenan DM, et al. Diminished adrenal sensitivity and adrenocorticotropin efficacy in obese premenopausal women. Eur J Endocrinol. 2012;167:633–42. doi: 10.1530/EJE-12-0592. [DOI] [PubMed] [Google Scholar]
- 232.Otte C, Yassouridis A, Jahn H, et al. Mineralocorticoid receptor-mediated inhibition of the hypothalamic-pituitary-adrenal axis in aged humans. J Gerontol A Biol Sci Med Sci. 2003;58(10):B900–5. doi: 10.1093/gerona/58.10.b900. [DOI] [PubMed] [Google Scholar]
- 233.Hatzinger M, Brand S, Herzig N, et al. In healthy young and elderly adults, hypothalamic-pituitary-adrenocortical axis reactivity (HPA AR) varies with increasing pharmacological challenge and with age, but not with gender. J Psychiatr Res. 2011;45(10):1373–80. doi: 10.1016/j.jpsychires.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 234.Bjorntorp P. Alterations in the ageing corticotropic stress-response axis. Novartis Found Symp. 2002;242:46–58. [PubMed] [Google Scholar]
- 235.Altemus M, Redwine L, Leong YM, et al. Reduced sensitivity to glucocorticoid feedback and reduced glucocorticoid receptor mRNA expression in the luteal phase of the menstrual cycle. Neuropsychopharmacology. 1997;17(2):100–9. doi: 10.1016/S0893-133X(97)00039-0. [DOI] [PubMed] [Google Scholar]
- 236.Coiro V, Passeri M, Volpi R, et al. Different effects of aging on the opioid mechanisms controlling gonadotropin and cortisol secretion in man. Horm Res. 1989;32(4):119–23. doi: 10.1159/000181272. [DOI] [PubMed] [Google Scholar]
- 237.Berardelli R, Margarito E, Ghiggia F, et al. Neuroendocrine effects of citalopram, a selective serotonin re-uptake inhibitor, during lifespan in humans. J Endocrinol Invest. 2010;33(9):657–62. doi: 10.1007/BF03346666. [DOI] [PubMed] [Google Scholar]
- 238.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84(2):606–10. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- 239.Lavoie JM, Dionne N, Helie R, et al. Menstrual cycle phase dissociation of blood glucose homeostasis during exercise. J Appl Physiol. 1987;62(3):1084–9. doi: 10.1152/jappl.1987.62.3.1084. [DOI] [PubMed] [Google Scholar]
- 240.Roca CA, Schmidt PJ, Altemus M, et al. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 2003;88(7):3057–63. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- 241.Altemus M, Roca C, Galliven E, et al. Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J Clin Endocrinol Metab. 2001;86(6):2525–30. doi: 10.1210/jcem.86.6.7596. [DOI] [PubMed] [Google Scholar]
- 242.Kripke DF, Youngstedt SD, Elliott JA, et al. Circadian phase in adults of contrasting ages. Chronobiol Int. 2005;22(4):695–709. doi: 10.1080/07420520500180439. [DOI] [PubMed] [Google Scholar]
- 243.Mazzoccoli G, Carughi S, Sperandeo M, et al. Alteration of circadian rhythmicity of CD31CD41 lymphocyte subpopulation in healthy aging. J Biol Regul Homeost Agents. 2011;25(3):405–16. [PubMed] [Google Scholar]
- 244.Prinz PN, Bailey SL, Woods DL. Sleep impairments in healthy seniors: roles of stress, cortisol, and interleukin-1 beta. Chronobiol Int. 2000;17(3):391–404. doi: 10.1081/cbi-100101053. [DOI] [PubMed] [Google Scholar]