Abstract
Introduction
Of the presigmoid approaches, the translabyrinthine approach is often used when a large exposure is needed to the cerebello-pontine angle, but hearing preservation is not a concern. At our institution, this approach is performed in collboration with ENT/Otolaryngology for temporal bone drilling and exposure.
Methods
In this video and paper, we demonstrate the use of the translabyrinthine approach for resection of a large cystic vestibular schwannoma, delineating the steps of positioning, opening, temporal bone drilling, tumor resection and closure.
Results
Gross total resection was obtained in this patient. His postoperative facial function was House-Brackmann grade II on the side ipsilateral to the tumor, although this improved with time.
Conclusion
The translabyrinthine approach to the cerebello-pontine angle is an excellent approach for masses that extend toward the midline or anterior to the pons. Although hearing is sacrificed, facial nerve function is generally spared.
Keywords: Translabyrinthine, Vestibular Schwannoma, Surgical Technique, Video
INTRODUCTION
Although the translabyrinthine approach was described by Panse in 1904 and first used to resect a cerebellopontine angle tumor by Quix in 1912, it was not until House published 47 resections with no mortalities in 1964 that the approach was truly popularized1. Since that time it has been well described in the literature as a useful approach for resection of vestibular schwannomas in cases where hearing preservation is not a concern. Additionally Morrison and King have described a modified use of this approach in combination with a transtentorial component for the resection of vestibular schwannomas and other lesions of the cerebellopontine angle and proximate anatomy4.
Surgical series of translabyrinthine resections often include meningiomas of the cerebellopontine angle as well as the internal acoustic meatus, schwannomas of the facial and trigeminal nerves, and cholesteatomas, neurinomas, and chordomas – illustrating the multiple uses of this approach3. In this paper, the translabyrinthine approach for resection of a large, cystic vestibular schwannoma (figure 1) is illustrated in a stepwise manner using a narrated video and still images.
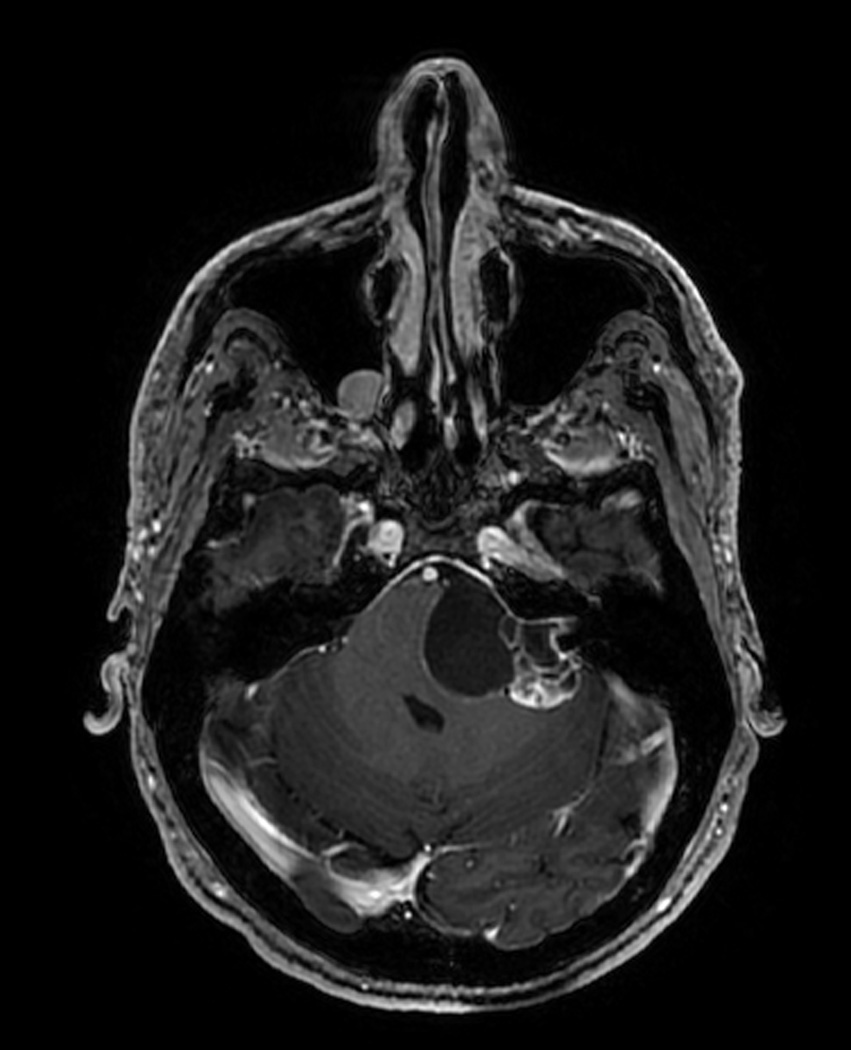
Figure 1.
Preoperative axial T1 MRI image with contrast. This slice is at the level of the internal auditory canal and demonstrates the patient’s large left cystic vestibular schwannoma.
SURGICAL INDICATIONS
Although case series exist describing hearing preservation in the setting of partial labyrinthectomy, the main indication of this approach remains resection of vestibular schwannomas in patients who have absent or non-serviceable hearing preoperatively2. When hearing preservation is a goal of surgery, or when a smaller lesion is being resected, retrosigmoid craniotomy may be appropriate. In the case of the largest of vestibular schwannomas, a combined translabyrinthine-transtentorial approach may be indicated4.
POSITIONING AND INCISION
The patient is positioned supine with the head turned to the patient’s contralateral side. This must be done to the point that the zygomatic arch is almost parallel to the floor. If the patient’s neck is inflexible, a shoulder roll may be required for positioning. The chin must also be assessed, and should be 2 fingerbreadths away from the sternum – flexion should not be severe enough to compromise venous return from the head. Distance should be kept between the planned incision and the patient’s ipsilateral shoulder, which will otherwise limit the surgeon’s range of motion later in the case.
The head is placed in pins in the Mayfield skull clamp and the navigational frame is anchored to this. The neuronavigation system is registered and used for incisional planning and later to aid in tumor resection. Facial EMG needles are placed for 7th nerve monitoring to be performed later in the case. At the authors’ institution, the otolaryngology team prefers to position the patient facing away from anesthesia, with the surgeon sitting between the patient and the anesthesiologist.
A C-shaped incision is planned behind the ear as needed to expose the mastoid tip, sigmoid sinus and transverse-sigmoid junction, and be as high as the floor of the middle cranial fossa (figure 2). The floor of the middle fossa is marked by the level of the zygomatic arch, but neuronavigation can assist in determining this as well as the location of the junction of the transverse and sigmoid sinuses. As a final check before prepping and draping, the table should be airplaned toward and away from anesthesia. This ensures that the patient will stable later in the case when the table will need to be manipulated to facilitate dissection distally along the 7th nerve and in the internal acoustic meatus. When prepping, it must be kept in mind that an abdominal field and potentially a lateral thigh field should be prepped for fat graft harvest and potential fascial harvest.
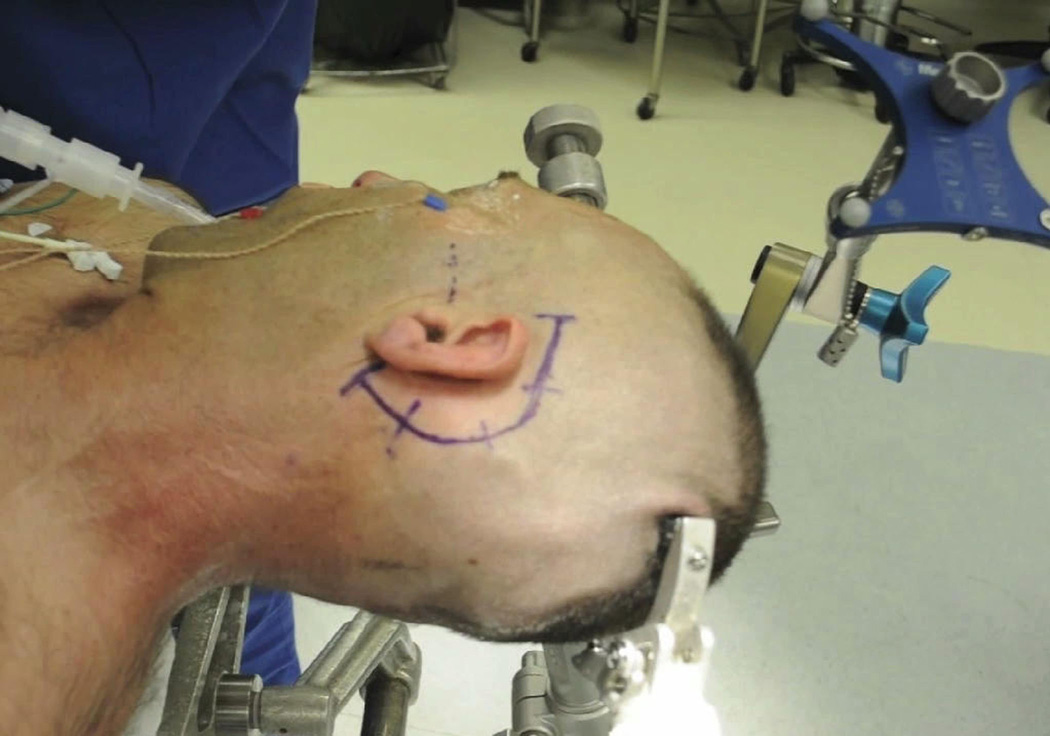
Figure 2.
Photograph depicting the patient’s head positioned and secured in pins with EMG needles in place and a navigational frame attached. The zygomatic arch and planned incision are marked.
OPENING AND APPROACH
The skin is divided sharply and electrocautery is used to divide the muscle, leaving the periosteum intact for the time being. The periosteal layer will be used as an additional layer and protection against CSF leak at the time of closure. Skin edges are undermined anteriorly and posteriorly both to benefit exposure and to enable the surgeon to harvest a fascial graft from the temporalis muscle. In the event that this is not possible, fascial graft can be harvested either from the rectus muscle when the abdominal fat graft is taken, or from the tensor fascia lata.
The periosteum is then elevated. As emissary veins are encountered, the periosteum around them should is cleared completely from bone and the veins waxed. As the periosteum is elevated, the limits of the exposure are the mastoid tip inferiorly, 1–2 cm behind the sigmoid sinus posteriorly and the spine of Henle anteriorly. Once this is accomplished, the mastoid drilling may begin.
At the authors’ institution, drilling starts with a large cutting burr, and the suction irrigator. Standard mastoidectomy begins with skeletonization of the tegmen, the sigmoid sinus and the posterior border of the external auditory canal. The drill is then used to enter the mastoid antrum and identify the incus and the lateral semicircular canal. A diamond bit can then be used to remove the remainder of bone off of the sigmoid sinus and 1–2 cm of the posterior fossa dura. The middle fossa dura is exposed by removing the tegmenand the presigmoid dura is also exposed by finishing the boney removal there – the endolymphatic duct will likely be transected during this process. This boney removal is accomplished with a small rongeur once the bone has been sufficiently thinned with the drill. The rongeur will also be used at this point to remove the petrous ridge over the superior petrosal sinus heading medially (figure 3).
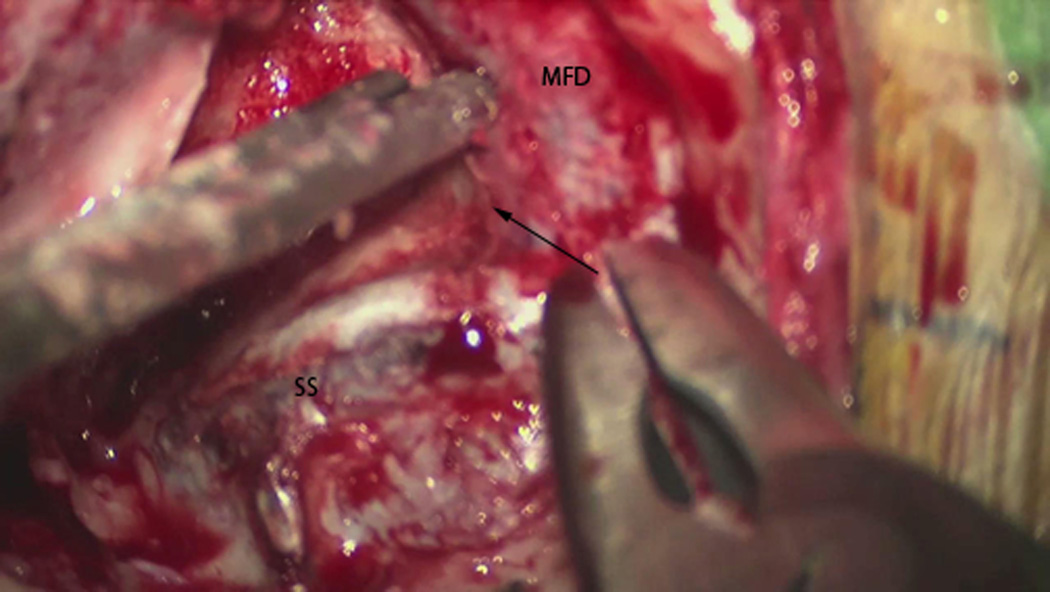
Figure 3.
Microscope still photo illustrating the dural exposure partway through the temporal bone drilling. SS = sigmoid sinus, MFD = middle fossa dura, Arrow demonstrates the direction of the superior petrosal sinus along the petrous ridge.
At this time, further drilling with the diamond bit allows the surgeon to identify the vertical segment of the facial nerve in the proximal mastoid. This is followed toward the stylomastoid foramen leaving a thin shell of bone over the nerve in the fallopian canal. Labyrinthectomy begins by entering the lumen of the lateral semicircular canal and then the posterior semicircular canal. The vestibule is then exposed and the lateral semicircular canal is removed. The subarcuate artery will often be encountered at this point in the foramen of the superior semicircular canal.
The sigmoid sinus can then be traced toward the jugular bulb, which is defined with the drill. Variations in this anatomy may limit exposure in the setting of a high-riding jugular bulb, which can be directly inferior to the internal auditory canal (IAC) in some patients. This limits the trough which will later be drilled below the IAC, as the inferior limit of this trough is the jugular bulb. Once the jugular bulb is identified, drilling can commence to uncover the dura of the IAC. Then, troughs are drilled superior and inferior to the IAC with the goal being to expose the IAC 270 degrees around its circumference. The superior trough is delimited by the superior edge of the IAC and the middle fossa dura. At this point, the only bone on the IAC dura should be along its anterior aspect (figure 4).
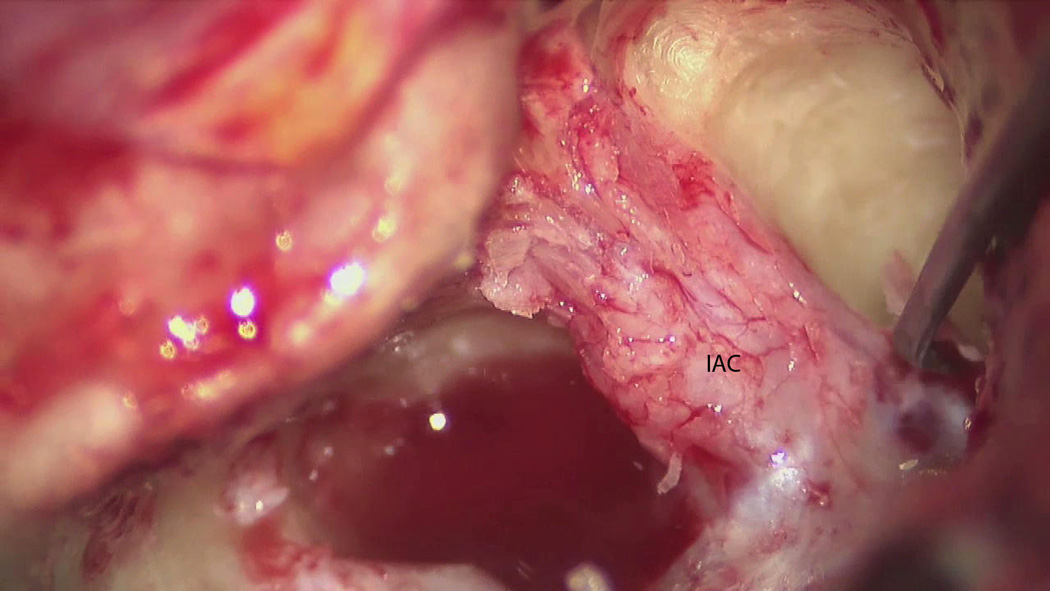
Figure 4.
Microscope still photo showing the dura of the internal auditory canal exposed with troughs drilled superiorly and inferiorly. IAC = internal auditory canal.
The falciform crest should be identified at this point, in order to identify the superior vestibular nerve, which sits superior to the falciform crest. This is the nerve from which most vestibular schwannomas arise, and it can be transected with little morbidity. Most frequently its transection results in a transient vertiginous symptoms. The dura of the IAC is then opened, proceeding medially and a plane can be developed between the tumor and the facial nerve. The dura is then opened further laterally and then inferiorly and superiorly along the presigmoid posterior fossa dura. This is best done with a micro-surgical blade followed by scissors.
TUMOR REMOVAL
While the first portion of the tumor in the IAC may be dissected partially off of the facial nerve, the majority of this portion of the dissection is left for the end of the resection. It is rather the cisternal portion of the tumor that is resected first. Once enough general dissection has been achieved to visualize the tumor, it is debulked internally to improve its mobility. This allows the surgeon to then dissect the mass partially away from the brainstem and begin attempting to identify cranial nerves. The goal is ultimately to achieve circumferential dissection around the tumor. To debulk internally, the surface of the lesion is coagulated and cut with scissors. This allows the surgeon access to the center of the tumor, which can be resected with pituitary rongeurs or an ultrasonic aspirator. If the course of the facial nerve has not already been established, the surface of the tumor must be stimulated to determine that the facial nerve does not have twigs running through the area which is about to be coagulated and cut. The facial nerve most commonly runs along the anterior surface of the tumor, and is also commonly found along the anterior-superior or anterior-inferior surface. Although the surface of the tumor is coagulated with the bipolar electrocautery, care is taken not to use this instrument near the brainstem or any cranial nerves.
In the standard translabyrinthine approach, where hearing is sacrificed, the 8th nerve can be identified at the brainstem, coagulated and transected (figure 5). This is first stimulated to verify that it is not the facial nerve. In cases where the facial nerve has been damaged by the tumor, a threshold for stimulation of the facial nerve must be established before concluding that a nerve does not stimulate and therefore must not be the facial nerve. It should be noted that the cochlear nerve in the IAC is not taken at this time. It is left for the end of the resection, so that the facial nerve is given some stability to be able to handle the dissection of the adherent tumor. Therefore the cochlear nerve in the IAC is taken only as the last portions of the tumor are resected (figure 6).
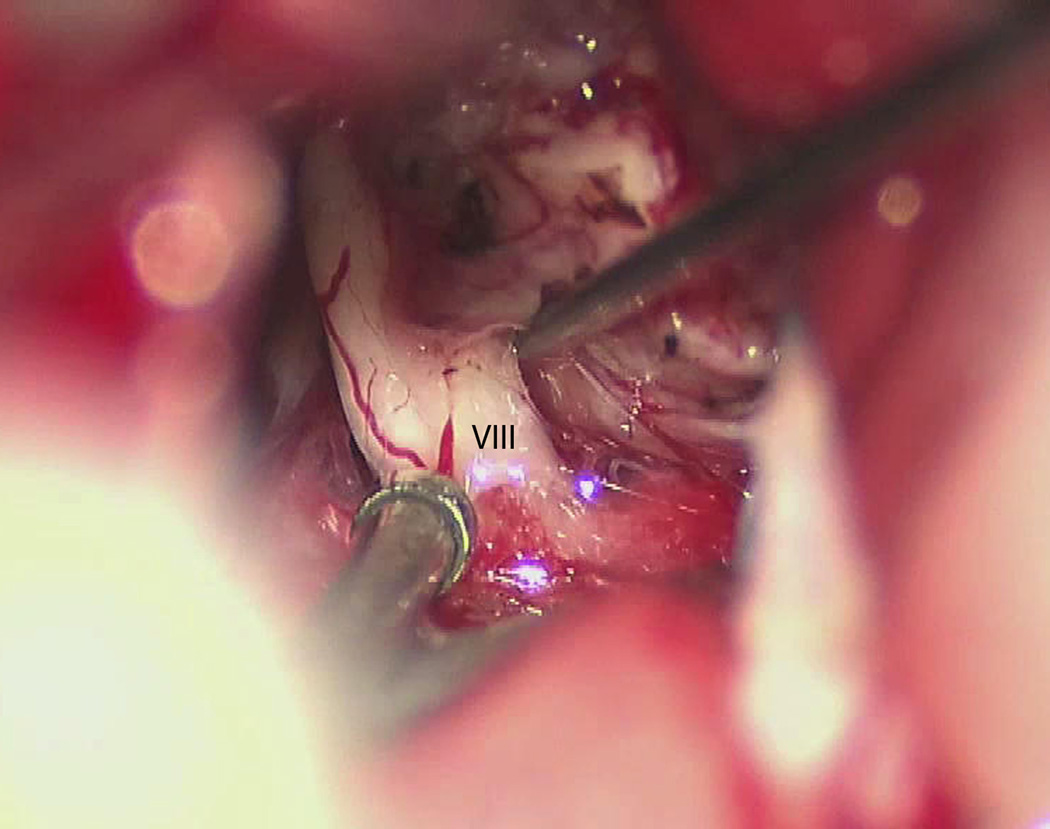
Figure 5.
Microscope still photo. Cranial nerve eight identified along the posterior edge of the tumor at its entry zone along the brainstem. VIII = cranial nerve eight.
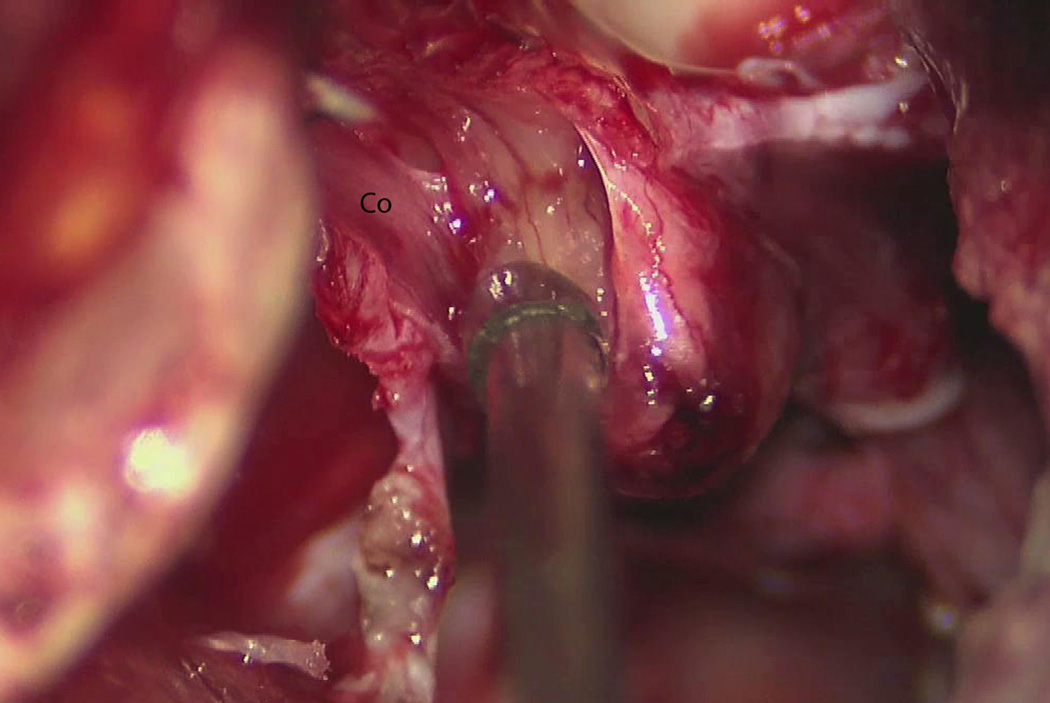
Figure 6.
Microscope still photo showing a small remnant of tumor adherent to the facial nerve, which is not well visualized. At this stage in the resection, the cochlear nerve is about to be divided in the IAC. Co = cochlear nerve.
Transection of the 8th nerve allows for further dissection of the mass off of the brainstem and therefore further debulking of the mass. As this back-and-forth process of debulking and dissection continues, the tumor will eventually be a husk that has been separated from the brainstem. At this time the shell of the tumor is resected with curved microscissors, leaving only the outer portion of the tumor which is adherent to the facial nerve. This last fragment is dissected from the facial nerve with sharp dissection techniques and the diamond and arachnoid knives.
In large masses, anatomy is quite distorted and a systematic approach to identification of landmarks is needed. A helpful strategy in this case is first to identify the inferior pole of the tumor. From this point cranial nerves can be identified as the tumor is rolled from inferior to superior, starting with cranial nerves 9, 10 and 11. From here, flocculus is identified along with choroid plexus in the foramen of Luschka. Anterior to this point, the surgeon should find the 8th nerve and the 7th nerve just ventral to it. The anterior inferior cerebellar artery should have a loop between these two nerves at the brainstem. Whichever strategy the surgeon takes, the arteries and veins of the posterior fossa should be respected, and every effort should be made not to take the superior petrosal veins.
CLOSURE
Once tumor resection is complete and hemostasis has been achieved, the wound is packed closed. The dura that has been opened cannot be closed primarily, and a fat graft harvested from the abdomen is used. One large piece of fat may be harvested, but strips will be cut and inserted into the surgical cavity to the level of the dura. It is important not to prolapse these fat strips past the level of the dura into the cerebellopontine angle or pack to aggressively, as cranial nerve deficits may result. Fascial graft, as mentioned above, may be taken during the opening from the temporalis muscle. Alternatively it may be harvested from the rectus muscle via the fat graft incision or from the tensor fascia lata of the thigh. This fascial graft is simply draped over the mastoid antrum, mastoid and tympanic facial nerve and all air cells of the facial recess and zygomatic groove. At this point in the case, a synthetic fibrin glue or other suitable epoxy can be interspersed among the fat strips and fascial graft to guard against CSF leak.
The periosteum, muscle and skin are closed in layers. Skin closure is often with a running locked stitch, or interrupted vertical mattress sutures in redo cases or when poor wound healing is a concern secondary to radiation, etc. A mastoid dressing is then applied and will be left in place for several days.
POSTOPERATIVE MANAGEMENT
If there is evidence of hydrocephalus or some other reason to be concerned for wound healing, a lumbar drain is placed at the outset of surgery, although this is uncommon. The patient is admitted to the neuro ICU and the lumbar drain is drained hourly. At the author’s institution, the drainage is typically begun at a rate of 5 mL/hour and increased as long as the patient does not suffer intolerable headaches and nausea. Drainage is often increased to a rate of 15 mL/hour, and occasionally higher. The duration of drainage is dependent on the surgeon’s level of concern for CSF leak. Usually the lumbar drain can be clamped for 24 hours at the conclusion of the healing process to ensure that no CSF leak is present, and then the drain is discontinued.
In the majority of cases, however, no such cerebrospinal fluid drainage is required. The patient spends their first postoperative night in the ICU and has an MRI on postop day one (figure 7). After this, and barring any specific concerns, the patient can be transferred to the floor. Although particular attention is paid to the posterior fossa on the MRI, these patients will often have a small filling defect in the sigmoid sinus, representing clot. This is generally treated by keeping the patient well hydrated and is not treated with specific anticoagulation. As long as there is no significant edema, the patient can be weaned quickly from decadron. Other postoperative care is not specific to this procedure.
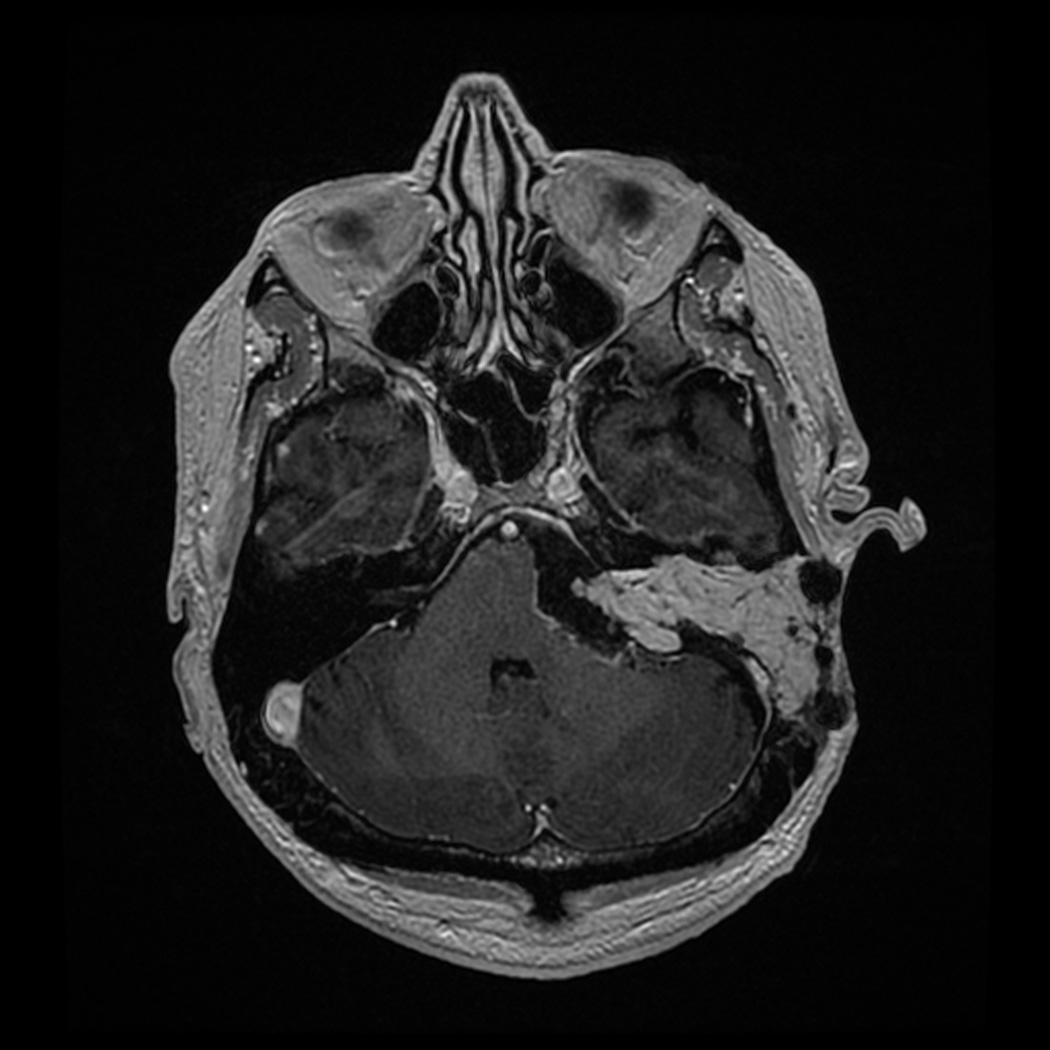
Figure 7.
Postoperative axial T1 MRI image with contrast. This slice illustrates the extent of resection of the tumor and the placement of the fat strips into the surgical cavity to the level of the dural defect.
Supplementary Material
Acknowledgements
Author SG is supported by NIH-NICHD P30HD03352 and the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
No portion of this work has been previously presented.
YouTube video link: http://www.youtube.com/watch?v=dVJagFJcJsA&feature=youtu.be
DISCLOSURE
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
REFERENCES
- 1.Doig JA. Surgical treatment of acoustic neuroma. The translabyrinthine approach. Proceedings of the Royal Society of Medicine. 1970;63:775. [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch BE, Cass SP, Sekhar LN, Wright DC. Translabyrinthine approach to skull base tumors with hearing preservation. Am J Otol. 1993;14:533–543. [PubMed] [Google Scholar]
- 3.Morrison AW. Translabyrinthine surgical approach to the internal acoustic meatus. Journal of the Royal Society of Medicine. 1978;71:269. doi: 10.1177/014107687807100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison AW, King TT. Experiences with a translabyrinthine-transtentorial approach to the cerebellopontine angle. 1973 doi: 10.3171/jns.1973.38.3.0382. http://dx.doi.org/10.3171/jns.1973.38.3.0382. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.