Abstract
Active amyloid-β (Aβ) immunotherapy is under investigation to prevent or treat early Alzheimer's disease (AD). In 2002, a Phase II clinical trial (AN1792) was halted due to meningoencephalitis in ∼6% of the AD patients, possibly caused by a T-cell-mediated immunological response. Thus, generating a vaccine that safely generates high anti-Aβ antibody levels in the elderly is required. In this study, MER5101, a novel conjugate of Aβ1-15 peptide (a B-cell epitope fragment) conjugated to an immunogenic carrier protein, diphtheria toxoid (DT), and formulated in a nanoparticular emulsion-based adjuvant, was administered to 10-month-old APPswe/PS1ΔE9 transgenic (Tg) and wild-type (Wt) mice. High anti-Aβ antibody levels were observed in both vaccinated APPswe/PS1ΔE9 Tg and Wt mice. Antibody isotypes were mainly IgG1 and IgG2b, suggesting a Th2-biased response. Restimulation of splenocytes with the Aβ1-15:DT conjugate resulted in a strong proliferative response, whereas proliferation was absent after restimulation with Aβ1-15 or Aβ1-40/42 peptides, indicating a cellular immune response against DT while avoiding an Aβ-specific T-cell response. Moreover, significant reductions in cerebral Aβ plaque burden, accompanied by attenuated microglial activation and increased synaptic density, were observed in MER5101-vaccinated APPswe/PS1ΔE9 Tg mice compared with Tg adjuvant controls. Last, MER5101-immunized APPswe/PS1ΔE9 Tg mice showed improvement of cognitive deficits in both contextual fear conditioning and the Morris water maze. Our novel, highly immunogenic Aβ conjugate vaccine, MER5101, shows promise for improving Aβ vaccine safety and efficacy and therefore, may be useful for preventing and/or treating early AD.
Introduction
Alzheimer's disease (AD) is the most common form of dementia. AD is characterized pathologically by the aggregation and deposition of cerebral amyloid β (Aβ), neuritic plaques, gliosis, neuron loss, and neurofibrillary tangles (Selkoe, 2001). Aβ is thought to play a critical role in AD pathogenesis (Golde, 2003), suggesting that therapies to inhibit its production, enhance its degradation, or improve its clearance from the brain would benefit AD patients. Schenk first reported Aβ lowering by active Aβ1-42 immunization in PDAPP transgenic (Tg) mice (Schenk et al., 1999). Thereafter, many Aβ immunization studies have reported reduced cerebral Aβ levels and/or improved cognition in mice (Bard et al., 2000; 2003; Janus et al., 2000; Lemere et al., 2000; Morgan et al., 2000; Weiner et al., 2000), nonhuman primates (Gandy et al., 2004; Lemere et al., 2004), and to some extent, humans (Nicoll et al., 2003; Bayer et al., 2005). The AN1792 human clinical trial was halted in 2002 due to meningoencephalitis in 6% of immunized AD patients (Orgogozo et al., 2003; Ferrer et al., 2004; Gilman et al., 2005), possibly due to T-cell recognition of the self-antigen, Aβ1-42, in combination with a strong, Th1-based adjuvant, QS21 (Cribbs et al., 2003). Although the dosing ended prematurely, only 19.7% of immunized patients developed an antibody response (Gilman et al., 2005).
Whereas B-cell epitope resides within Aβ1-15 in humans (Geylis et al., 2005), monkeys (Lemere et al., 2004), and mice (Lemere et al., 2000; McLaurin et al., 2002; Agadjanyan et al., 2005), T-cell epitopes reside within Aβ16-42 (Cribbs et al., 2003; Monsonego et al., 2003). Immunization with Aβ N-terminal derivatives generated antibodies, lowered Aβ (Bard et al., 2003; Maier et al., 2006; Seabrook et al., 2006, 2007), and protected cognition (Sigurdsson et al., 2004; Maier et al., 2006) in AD-like mouse models. A Th2-biased adjuvant may help safely induce effective immune responses in the absence of Th1-mediated events (Lemere et al., 2000, 2002; Maier et al., 2005; Asuni et al., 2006; Ghochikyan et al., 2006).
In this study, we used a novel Aβ B-cell epitope vaccine, MER5101, composed of Aβ1-15 conjugated via 7 aa to a carrier protein, diphtheria toxoid (DT), and formulated in a Th2-biased adjuvant, MAS-1. MAS-1 adjuvant is a water-in-oil (w/o) nanoparticle emulsion that differs from incomplete Freund's adjuvant (IFA) and uses metabolizable components that are better tolerated than the mineral oil-based IFA. MAS-1 enhances antibody responses to protein antigens up to 50-fold compared with alum and >10-fold compared with oil-in-water adjuvants (unpublished results). Self-antigen conjugate vaccines adjuvanted with MAS-1 targeting gastrin and gonadotrophin-releasing hormone have been tested clinically in ∼1500 cancer patients, including gastrointestinal adenocarcinoma and prostate cancer (Simms et al., 2000; Smith et al., 2000; Brett et al., 2002; Gilliam et al., 2004; Ajani et al., 2006). These patients included elderly and those further immunocompromised by late stage cancer and chemotherapy; nevertheless, immune response rates reached 80% across all groups. Here, we assessed the efficacy of MER5101 in AD-like Tg mice.
Materials and Methods
Animals and treatment.
Immunization treatment was performed in 10-month-old APPswe/PS1ΔE9 Tg mice (original breeders obtained from Jackson Laboratories) on a mixed C57BL/6 × DBA/2 (B6D2F1) background and B6D2F1 wild-type (Wt) mice. APPswe/PS1ΔE9 Tg mice harbor the Swedish APP (K594N/M595L) and PS1ΔE9 (deletion of exon 9) human transgenes under a mouse prion protein promoter (Jankowsky et al., 2004). Tg breeders were originally obtained from The Jackson Laboratory and bred in our colony by crossing a APPswe/PS1ΔE9 mice on a C57BL/6 background with DBA/2 Wt mice. APPswe/PS1ΔE9 mice begin to exhibit cognitive deficits starting at 6 months, which are exacerbated with age (Puoliväli et al. 2002; Park et al. 2006; Gimbel et al. 2010). All animal use was approved by the Harvard Standing Committee for Animal Use and was in compliance with all state and federal regulations.
The MER5101 vaccine is composed of multiple copies of Aβ1-15 peptide conjugated to DT using a 7 aa spacer to enhance presentation of self-epitopes and immunogenicity, and formulated in Mercia's Th2-biased adjuvant, MAS-1 (Mercia Pharma), a stable w/o emulsion. Conjugate antigen was prepared by linking Aβ1-15 peptide to DT with the heterobifunctional cross-linker ε-maleimidocaproic acid N-hydroxysuccinimide ester, yielding conjugate with a molar substitution ratio of 18.6 peptides per DT by amino acid analysis of conjugate. MER5101 vaccine formulations were prepared on each injection day by combining aqueous phase-containing antigen with MAS-1 in a 30:70 w/o ratio by weight, vortexing to prepare a pre-emulsion, then emulsifying by repeated passage between two syringes connected with a plastic stopcock. The same volume of sterile PBS solution was administered to the MAS-1 adjuvant (vehicle) controls. Vaccinated APPswe/PS1ΔE9 Tg (n = 5; 2F, 3M) and Wt mice (n = 6; 2F, 4M) received five subcutaneous injections of 100 μg Aβ1-15:DT conjugate in an injection volume of 0.1 ml MER5101 per dose. Vehicle control Tg (n = 6; 2F, 4M) and Wt (n = 5; 2F, 3M) mice received five subcutaneous injections of 0.1 ml/dose of MER5101 placebo, comprising PBS (no antigen) emulsified with MAS-1. The first two injections were administered biweekly, followed by three additional injections given 4 weeks apart. Mice underwent cognitive testing at 14 months of age, followed by euthanasia.
Plasma and tissue collection.
Plasma was collected from the tail vein as previously described (Maier et al., 2005). Ten days after the final immunization, mice were killed by CO2 inhalation and transcardially perfused with 20 ml PBS. The brain was removed and divided sagittally. One hemibrain was fixed for 2 h in 10% buffered formalin and embedded in paraffin as previously described (Leverone et al., 2003; Maier et al., 2005); the other hemibrain was snap frozen in liquid nitrogen and stored at −80°C for biochemical analysis. Tris-buffered saline (TBS)-soluble and 5 m guanidine-soluble (i.e., TBS-insoluble) brain homogenates were prepared as previously reported (Weiner et al., 2000).
Anti-Aβ antibody ELISA and Aβ ELISA.
Low pH dissociation treatment for plasma samples was conducted as previously described (Li et al., 2007) with some modification. Briefly, plasma was diluted 1:100 with dissociation buffer (1.5% bovine serum albumin and 0.2 m glycine-acetate in PBS) at pH 3.5, and incubated at room temperature (RT) for 20 min. The plasma was then transferred into the sample reservoir of a centrifugal filter device, YM-30 (30,000 MW cutoff; Millipore) and centrifuged at 8000 × g for 20 min at RT. The solution remaining in the sample reservoir was collected and adjusted to pH 7.0 with 1 m Tris buffer, pH 9.0. Anti-Aβ antibodies in predissociated and postdissociated plasma samples were measured by ELISA as previously described (Spooner et al., 2002). ELISAs for antibody isotypes and epitope mapping were performed as previously reported (Lemere et al., 2002). The standards for the IgG1 and IgG2b isotype ELISAs were purchased from Southern Biotechnology Associates, while the standards for the IgG2a and IgM ELISAs were purchased from Invitrogen. The following overlapping Aβ fragment peptides were used for Aβ epitope-mapping by ELISA: Aβ1-15, Aβ1-7, Aβ3-9, Aβ3-13, Aβ7-12, Aβ11-25, Aβ26-42, Aβ1-40 (Biopolymer Laboratory, UCLA, Los Angeles, CA).
Levels of Aβx-40 and Aβx-42 in brain homogenates (TBS soluble and guanidine soluble) and in plasma were measured by chemiluminescent BetaMark x-40 and x-42 ELISA kits (Covance), respectively. ELISA for Aβ1-total level in brain homogenates and plasma was performed as described previously (Peng et al., 2010).
Immunoprecipitation and Western blotting.
Conditioned media from Chinese hamster ovary (CHO) cells stably transfected to express mutant human APP (cell line 7PA2; a kind gift from Dr. Dennis Selkoe, Brigham and Women's Hospital, Boston, MA) or nontransfected CHO cells was centrifuged to remove cellular debris. The conditioned media was then incubated with plasma (1:50) from immunized (adjusted to 1 mg/ml of anti-Aβ antibody) or control mice. The anti-Aβ monoclonal antibody (mAb) 6E10 (1:100) (Covance Laboratories) served as a positive control. A standard immunoprecipitation (IP) procedure using Protein G beads (Pierce) was performed, with the products being electrophoresed on 12% Bis-Tris gels (Invitrogen) before being transferred to 0.2 μm nitrocellulose membranes. The general anti-Aβ polyclonal antibody (pAb), R1282 (a gift from Dr. Dennis Selkoe, Brigham and Women's Hospital, Boston, MA), was used to probe the blots, and was visualized using a LI-COR scanner (LI-COR Biosciences).
Splenocyte proliferation assay.
All cell culture reagents were obtained from Invitrogen. Spleens of mice were pooled within each treatment group. Splenocytes were isolated, cultured, and restimulated as previously described (Maier et al., 2005). Aβ1-15:DT, Aβ1-15, and Aβ1-40/42 (3:1 ratio) peptides were added to splenocytes at final concentrations of 0, 0.5, 5, or 50 μg/ml in triplicate wells. To measure proliferation, 1 μCi of [3H]-thymidine was added to cells at 72 h. Eighteen hours later, the cells were harvested and thymidine incorporation was measured using a 1450 Microbeta liquid scintillation counter (PerkinElmer). The stimulation index (SI) was calculated using the following formula: counts per minute (CPM) of well with antigen/CPM with no antigen. An SI index >3 indicates a proliferative cellular immune response of the splenocytes to the peptide.
Cytokine ELISA.
Splenocyte cell culture supernatants were collected at 72 h, just before the addition of [3H]-thymidine and stored at −80°C for cytokine assays. The Mouse Pro-Inflammatory TH1/TH2 9-plex from MesoScale Discovery was used, following the manufacturer's protocol, to measure cytokine release. In brief, cell culture supernatants from the following restimulation conditions were loaded undiluted in duplicate: 50 μg/ml Aβ1-40/42; 50 μg/ml Aβ1-15:DT; media alone (negative control), and 5 μg/ml concavalin A (ConA; Sigma-Aldrich) (positive control). The Multi-Spot ELISA plate was precoated with antibodies specific for the following cytokines: IFN-γ, IL-1β, IL-10, IL-12 total, IL-2, IL-4, IL-5, KC, and TNF-α and detected with the appropriate SULFO-TAG detection antibodies. Light emitted upon electrochemical stimulation was read using a SECTOR Imager 2400A.
Immunohistochemistry, immunofluorescent staining, and quantification.
Immunohistochemistry (IHC) was performed on 10 μm paraffin sections of human AD brain and mouse brain as reported previously (Lemere et al., 2000) using Vector Elite ABC kits (Vector Laboratories). The following antibodies were used for neuropathological analysis: anti-Aβ R1282 pAb (raised against Aβ1-40; 1:1000), anti-Aβ 6E10 mAb (raised against Aβ1-16 peptide, 1:1000; Covance), anti-Iba-1 pAb (a marker for both resting and activated microglia, 1:1000; Wako Chemicals), anti-CD45 mAb (a marker for activated microglia, 1:5000; Serotec), anti-GFAP mAb (a marker for astrocytes, 1:500; Dako), anti-synaptophysin mAb (SYP; a presynaptic protein marker, 1:200; Sigma-Aldrich), anti-postsynaptic density protein-95 mAb (PSD-95; a postsynaptic protein marker, 1:200; EMD Millipore), and anti-mouse CD5 mAb (a marker for T-lymphocytes, 1:200; BD PharMingen/Biosciences). One percent aqueous Thioflavin S (Thio S; Sigma-Aldrich) was used to visualize fibrillar amyloid in plaques and blood vessels. Hemosiderin staining using 2% ferrocyanide (Sigma-Aldrich) in 2% hydrochloric acid was used to detect microhemorrhages. Quantification of total R1282 immunoreactivity (IR) and Thio S staining was conducted using BIOQUANT image analysis. The threshold of detection was held constant during the entire analysis. The percentage area occupied by R1282 IR and Thio S staining was quantified for three equidistant sagittal planes, 300 μm apart per mouse in the following regions: hippocampus (HC), frontal cortex (CTX), and cerebellum (CB). Thio S-positive vascular amyloid in CB was evaluated semiquantitatively for three equidistant sagittal planes 300 μm apart per brain region per mouse using the following criteria: 0 = no Thio S-positive blood vessels; 1 = 1–10 Thio S-positive blood vessels; 2 = 11–20 Thio S-positive blood vessels, and 3 = 21 or more Thio S-positive blood vessels.
The mouse-monoclonal anti-APP antibody, 22C11 (1:1000; EMD Millipore Corp), was applied to paraffin sections followed by Alexa Fluor 488 goat anti-mouse IgG secondary antibody (1:100; Invitrogen) for immunofluorescent labeling of dystrophic neurites within neuritic plaques.
Behavioral testing.
Cognitive efficacy of MER5101 was evaluated using contextual fear conditioning (CFC) and Morris water maze (MWM) testing. The operator was blinded to treatment group and genotype throughout all behavioral testing. The CFC test was performed over 2 d. On day 1, the mouse was placed in a chamber with visible cues on the sidewall for 3 min and then given a 30 s sound signal followed by a 0.7 mA electric stimulation on the floor of the chamber. Fifteen seconds after the electric shock, the mouse was moved back to its home cage. On day 2, the mouse was placed in the same chamber with the same environment but without the shock for 3 min. During this period, mouse freezing behavior was checked every 10 s and the number of freezings recorded.
MWM test was conducted as previously described (Peng et al., 2010). Spatial learning was evaluated by the escape latency for each mouse to reach and climb onto the platform in five daily hidden platform trials. Spatial memory was evaluated by the analysis of the dwelling time in each quadrant of the pool and the analysis of an annulus-crossing index (ACI) in two probe trials, which were performed 2 and 24 h after the last hidden platform trial on day 5. The ACI represents the number of crosses over the platform site in the target quadrant (quadrant with escape platform) adjusted for crosses over the platform sites in alternative quadrants. A visible cue test was performed to confirm the visual abilities of each mouse.
Data analysis.
Prism 4 software (GraphPad) was used to analyze the data. All data were expressed as mean ± the SEM. Kruskal–Wallis nonparametric one-way ANOVA analysis was used with the treatment as a between-subject factor, and training days as a within-subject factor for CFC and the hidden platform trial of MWM. The Mann–Whitney U nonparametric analysis was used to compare ACI and percentage dwelling time values from the probe trials in MWM, percentage area of IR in IHC and histology, and Aβ levels by ELISA. A value of p < 0.05 was considered significant for all statistical tests.
Results
MER5101, an Aβ1-15:DT conjugate vaccine, generates high anti-Aβ antibody levels in mouse plasma
Five subcutaneous immunizations were administered to 10-month-old mice on days 0, 14, 42, 84, and 112. Plasma was collected at 1 d before the first immunization (baseline) and on days 28, 56, 96, and 121. Anti-Aβ antibody levels in mouse plasma samples were measured using ELISA. Anti-Aβ antibodies were detected in both APPswe/PS1ΔE9 Tg and Wt mice immunized with Aβ1-15:DT in MAS-1 adjuvant by day 28 (Fig. 1A). Peak antibody levels reached 978.6 ± 242.3 and 1893.8 ± 95.6 μg/ml in APPswe/PS1ΔE9 Tg and Wt mice, respectively, by day 56 and declined thereafter. Vehicle-treated (MAS-1 alone) mice did not produce detectable anti-Aβ antibodies. Low pH dissociation of antibodies from their antigens in plasma (Li et al., 2007) revealed that anti-Aβ antibody levels in immunized Tg mice were maintained at high levels (1070.0 ± 416.8 and 1008.7 ± 205.2 μg/ml at days 96 and 121, respectively), whereas the antibody levels in immunized Wt mice were unchanged by low pH dissociation (Fig. 1B).
Figure 1.
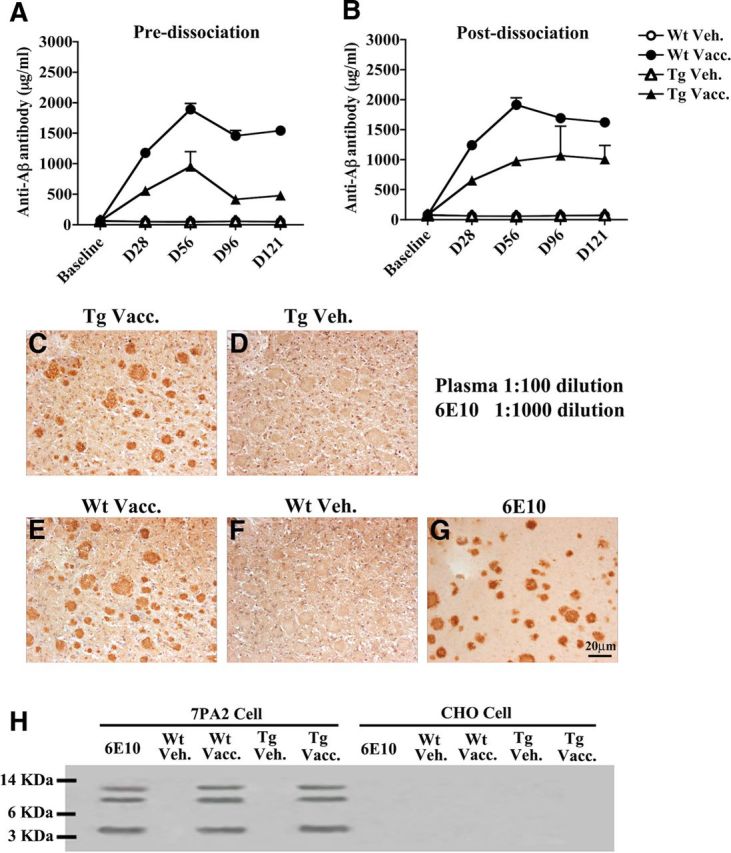
Plasma anti-Aβ antibody levels with and without low pH dissociation, and binding to human Aβ. A, Immunization with Aβ1-15:DT conjugate generated detectable plasma anti-Aβ antibody levels in both Wt and APPswe/PS1ΔE9 Tg mice by day 28. B, Low pH dissociation of antibodies and antigens in APPswe/PS1ΔE9 Tg mouse plasma resulted in enhanced detection of anti-Aβ antibodies at days 96 and 121 relative to levels detected before dissociation. C–F, Serial human AD brain sections were stained using plasma (1:10 dilution) from a MER5101-vaccinated APPswe/PS1ΔE9 Tg mouse (C), a Tg vehicle-control mouse (D), a MER5101-vaccinated Wt mouse (E), or a Wt vehicle-control mouse (F). Both vaccinated Wt and Tg mouse plasma, but not that of the vehicle-treated mice, immunolabeled Aβ plaques. G, A human AD brain section was stained using an anti-human Aβ monoclonal antibody, 6E10, as a positive control. H, Both monomeric and oligomeric Aβ in 7PA2 condition media were recognized by plasma antibodies from MER5101-vaccinated Wt and APPswe/PS1ΔE9 Tg mice but not from vehicle controls; 6E10 was used as a positive control. CHO media was used as negative control for all groups in the IP assay.
Plasma antibodies from vaccinated Tg and Wt mice bound Aβ plaques in human AD cortical brain sections (Fig. 1C,E) while plaques were not labeled by plasma from vehicle controls (Fig. 1D,F). To further confirm the plasma antibody specificity for recognizing Aβ, conditioned media from 7PA2 cells, known to produce naturally secreted Aβ monomers and oligomers but not aggregates (Walsh et al., 2005), was examined by IP. Plasma anti-Aβ antibodies from vaccinated mice bound Aβ monomers and oligomers (Fig. 1H).
MER5101 generates Th2 IgG isotypes and antibodies against the Aβ N terminus
Ig isotyping of anti-Aβ antibodies was performed on the final bleed sample (day 121) using isotype-specific ELISAs (Fig. 2A). IgG1, a noninflammatory Th2 Ig, was the most predominant Ig isotype in vaccinated APPswe/PS1ΔE9 Tg and Wt mice, followed by IgG2b (Th2), which was about three times higher than the level of the pro-inflammatory IgG2a isotype (Th1). Low levels of IgM were detected in all four groups of animals.
Figure 2.
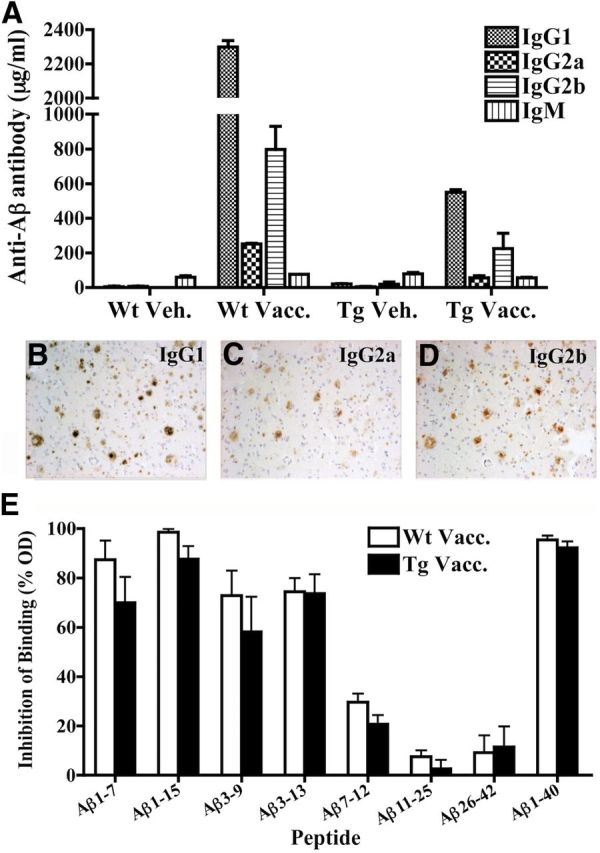
Ig isotype and epitope mapping of plasma anti-Aβ antibodies. Ig isotyping of IgG1, IgG2a, IgG2b, and IgM anti-Aβ antibodies was measured by isotype-specific ELISA of plasma collected on day 121, the final bleed. A, IgG1 anti-Aβ antibodies were predominant in both vaccinated groups, followed by IgG2b anti-Aβ antibodies. Few IgG2a or IgM anti-Aβ antibodies were detected. B–D, Diluted plasma (1:100) from an immunized Wt mouse was used in combination with different isotype-specific secondary antibodies including goat-anti-mouse IgG1 (B), IgG2a (C), and IgG2b (D), respectively, to detect Aβ plaques on formalin-fixed paraffin-embedded AD brain tissues. Plasma with mouse IgG1 secondary antibody showed the clearest and most abundant staining of Aβ plaques (B). E, Epitope-mapping of antibodies was performed by pre-incubating mouse plasma overnight with short Aβ peptide fragments before using an ELISA to measure the ability of the antibodies to bind the plate-bound immunogen. Pre-incubation with Aβ1-7, Aβ1-15, Aβ3-9, and Aβ3-13 inhibited binding to the plate-bound immunogen, indicating that the vast majority of antibodies was directed against the Aβ1-15 sequence. The optical density (OD) levels are shown relative to the absence of peptide as a negative control. Data are shown as mean ± SEM.
To further confirm the isotype specificity, plasma from immunized mice was used for immunostaining of human AD brain sections in combination with IgG-specific biotinylated secondary antibodies (including IgG1, IgG2b, and IgG2a). Consistent with the ELISA results, plaque staining with immunized mouse plasma was strongest using the IgG1 secondary antibody (Fig. 2B), with slightly less intense staining with the IgG2b secondary antibody (Fig. 2C), and little or no detectable staining with the IgG2a secondary antibody (Fig. 2D).
Pre-incubation of the immunized mouse plasma with short Aβ peptides before ELISA indicated that the majority of antibodies recognized a B-cell epitope within the Aβ1-15 region, but did not recognize epitopes within the Aβ mid-region or C terminus (Fig. 2E).
MER5101 generates a strong cellular immune response against the conjugate immunogen in the absence of an Aβ-specific T-cell-mediated immune response
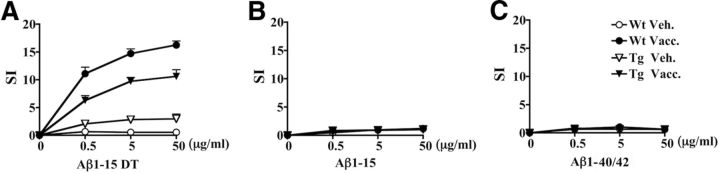
To characterize the cellular immune response, splenocytes were isolated and pooled within each group of immunized animals and restimulated with various concentrations of Aβ1-15:DT conjugate, unconjugated Aβ1-15 peptide, or full-length Aβ1-40/42 (3:1 ratio). Proliferation (i.e., SI) was highest for splenocytes from MER5101 immunized Wt mice restimulated with the Aβ1-15:DT conjugate, whereas restimulation with the same conjugate induced moderate proliferation in splenocytes from MER5101 immunized APPswe/PS1ΔE9 Tg (Fig. 3A). The proliferative response was enhanced with increasing amounts of stimulating peptide, indicating a dose-dependent cellular immune response. No proliferation was observed after restimulation with either Aβ1-15 peptide (Fig. 3B) or full-length Aβ1-40/42 peptide (Fig. 3C) in any mouse group, which is consistent with DT providing carrier function for the antigen construct and the maintenance of self-tolerance to Aβ in the Aβ1-15:DT immunized Tg mice.
Figure 3.
MER5101 induced a cellular immune response specific for the conjugate but not Aβ1-15 or Aβ1-40/42 peptides. Splenocytes from vehicle-treated Wt and APPswe/PS1ΔE9 Tg mice as well as MER5101-immunized Wt and APPswe/PS1ΔE9 Tg mice were isolated, pooled within each treatment group, and cultured in the presence of 0, 0.5, 5, or 50 μg/ml Aβ1-15:DT (A), Aβ1-15 (B), or Aβ1-40/42 (C) for 72 h, after which [3H]-thymidine was added for 18 h. The CPM was determined and the SI was calculated. Splenocytes isolated from MER5101-vaccinated Wt (line with solid circle) and APPswe/PS1ΔE9 (line with solid triangle) Tg mice showed a high SI in response to restimulation with the immunizing conjugate peptide, Aβ1-15:DT, but did not proliferate upon restimulation either Aβ1-15 or Aβ1-40/42 peptides. No proliferation of splenocytes from vehicle-treated Wt (line with open circle) or APPswe/PS1ΔE9 Tg mice (line with open triangle) was observed in response to any peptides.
Cytokine analysis of cell supernatants from the Wt mice demonstrated elevated levels of IFN-γ, IL-10, and IL-2, and IL-4, IL-5, and KC to lesser levels, in Aβ1-15:DT compared with Aβ1-40/42 restimulated cells from subcutaneous immunized mice (Table 1). Compared with the Wt mouse cells, and in accord with lower proliferation levels seen in the Tg versus Wt mouse cells, supernatants from cells taken from the Tg mice showed smaller cytokine effects in response to stimulation with Aβ1-15:DT, including elevations in IFN-γ and KC, and reduced production of IL-12. The Wt mouse cell data could indicate the induction of regulatory T (Treg) cells; fluorescence-activated cell sorting analysis of the in vitro stimulated splenocytes indicated that MER5101 specifically induced T-cells with the CD4+/CD25+/Foxp3 regulatory phenotype responsive to Aβ1–15:DT, but not Aβ1-15 epitope or intact Aβ1-40/42 (Table 2). Treg induction could have therapeutic implications for vaccine safety, as IL-10 and IFN-γ production by Treg cells has been shown to relate to therapeutic anti-inflammatory activity in non-obese diabetic mice immunized subcutaneously with Insulin B chain 9–23 in IFA (Fousteri, 2010).
Table 1.
Cytokines secreted into splenocyte supernatants upon restimulation
| Groups | Restim peptide | IFN-γ (pg/ml) | IL-1β (pg/ml) | IL-10 (pg/ml) | IL-12 (pg/ml) | IL-2 (pg/ml) | IL-4 (pg/ml) | IL-5 (pg/ml) | KC (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|---|---|---|---|---|---|
| Wt Vacc. | Aβ1-40/42 | 0.2 | 0.7 | 30.9 | 135.1 | 17 | 0.8 | 0.4 | 4.2 | 0 |
| Aβ1-15:DT | 116 | 1 | 62.2 | 187.2 | 244.9 | 3.8 | 4.5 | 9.7 | 1.3 | |
| ConA | 5864.3 | 19 | 1947.1 | 732.7 | 2948.4 | 362.9 | 186.4 | 42.7 | 342.9 | |
| Neg Cont. | 0.4 | 0.7 | 24.4 | 124.4 | 18.1 | 0.6 | 0.1 | 2.4 | 0 | |
| Wt Veh. | Aβ1-40/42 | 0.1 | 0.5 | 25.1 | 115.5 | 14.0 | 0.3 | 0.2 | 2.4 | 0 |
| Aβ1-15:DT | 0.1 | 0.3 | 29.7 | 118.9 | 20.7 | 0.2 | 0.2 | 3.5 | 0 | |
| ConA | 6234.1 | 18.9 | 1404.4 | 832.0 | 2598.0 | 241.6 | 162.2 | 37.5 | 305.8 | |
| Neg Cont. | 0.7 | 1.0 | 31.8 | 126.9 | 17.0 | 1.0 | 0.9 | 3.0 | 0.5 | |
| Tg Vacc. | Aβ1-40/42 | 0.1 | 0.9 | 11.4 | 80.4 | 2.0 | 0.8 | 0.5 | 2.5 | 0.1 |
| Aβ1-15:DT | 9.2 | 0.7 | 36.6 | 99.3 | 62.6 | 1.3 | 0.9 | 8.3 | 0.0 | |
| ConA | 3773.2 | 12.7 | 512.1 | 389.5 | 1929.8 | 173.5 | 121.2 | 83.4 | 74.1 | |
| Neg Cont. | 0.2 | 0.5 | 10.0 | 68.1 | 2.6 | 0.9 | 0.3 | 2.6 | 0.0 | |
| Tg Veh. | Aβ1-40/42 | 0.1 | 0.7 | 31.4 | 147.6 | 11.9 | 0.6 | 0.2 | 2.7 | 0.0 |
| Aβ1-15:DT | 2.5 | 0.5 | 34.4 | 162.0 | 51.6 | 1.4 | 0.6 | 3.3 | 0.0 | |
| ConA | 6486.7 | 21.8 | 2029.0 | 837.8 | 2416.4 | 327.1 | 190.8 | 39.6 | 499.3 | |
| Neg Cont. | 0.2 | 0.7 | 12.6 | 160.4 | 12.1 | 0.7 | 0.0 | 2.3 | 0.0 |
Table 2.
Cellular immune response to MER5101 in APP/PS1ΔE9 Tg mice–in vitro stimulation assay
| Immunization | Con A | Stimulating antigen |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aβ1-40/42 (μg/ml) |
Aβ1-15 (μg/ml) |
Aβ1-15:DT (μg/ml) |
||||||||
| 0.5 | 5.0 | 50.0 | 0.5 | 5.0 | 50.0 | 0.5 | 5.0 | 50.0 | ||
| % CD4+/CD25+/Foxp3 + regulatory T-cells by FACS | ||||||||||
| Wild type | ||||||||||
| MAS-1 vehicle | 17.90% | 3.53% | 4.23% | 3.52% | 4.95% | 4.41% | 4.70% | 4.43% | 3.27% | 2.86% |
| MER5101 | 13.20% | 5.11% | 4.36% | 4.62% | 6.27% | 3.82% | 5.37% | 5.12% | 8.86% | 8.30% |
APP/PS1 E9 Tg E9 Tg |
||||||||||
| MAS-1 vehicle | 15.10% | 4.62% | 5.51% | 5.61% | 5.34% | 6.84% | 6.79% | 8.53% | 5.03% | 6.61% |
| MER5101 | 26.40% | 4.96% | 3.32% | 5.31% | 4.71% | 4.60% | 4.61% | 5.91% | 6.85% | 6.25% |
| Within-group % change in antigen-specific T regulatory cells | ||||||||||
| Wild type | ||||||||||
| MAS-1 vehicle | −19% | 0.2% | −19% | 21% | 5% | 14% | 5% | −23% | −32% | |
| MER5101 | 5% | −13.5% | −7% | 35% | −26% | 11% | 4% | 80% | 69% | |
APP/PS1 E9 Tg E9 Tg
|
||||||||||
| MAS-1 vehicle | −23% | −5.7% | −4% | −9% | 23% | 22% | 47% | −13% | 14% | |
| MER5101 | 10% | −31.4% | 20% | 3% | 0% | 1% | 29% | 49% | 36% | |
Aβ deposition was reduced in APPswe/PS1ΔE9 Tg mouse brain after MER5101 immunization
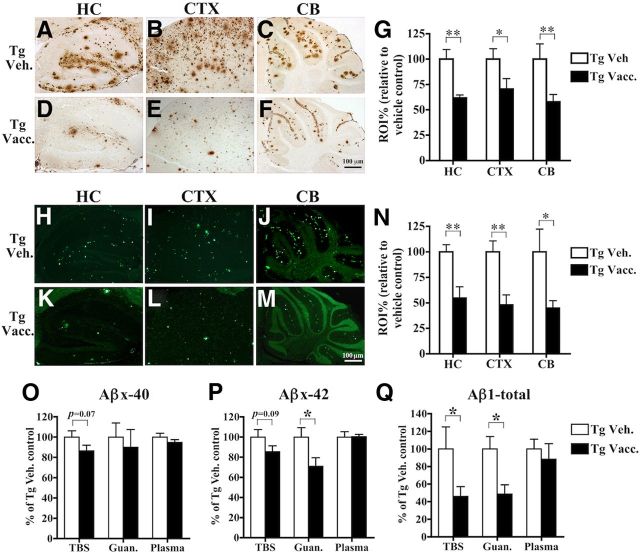
Plaque deposition in APPswe/PS1ΔE9 Tg mice begins in CTX at ∼5–6 months of age, after which, the plaque burden increases and extends to HC and CB with aging (Garcia-Alloza et al., 2006). MER5101 vaccination significantly reduced total Aβ IR detected by R1282, a general Aβ polyclonal antibody, in HC by 38.3% (p = 0.0089), CTX by 29.5% (p = 0.041), and in CB by 42.1% (p = 0.0087) in APPswe/PS1ΔE9 Tg mice versus Tg vehicle controls (Fig. 4A–G). Aβ fibrillar plaques (Thio S positive) were also markedly diminished in MER5101-immunized mice by 45.4% (p = 0.0079), 52.1% (p = 0.0069), and 55.2% (p = 0.028) in HC, CTX, and CB, respectively (Fig. 4H–N).
Figure 4.
Cerebral Aβ protein load was significantly reduced in immunized APPswe/PS1ΔE9 Tg mice. A–F, Representative staining is shown for R1282-positive plaques in HC, frontal CTX, and CB in vehicle-treated APPswe/PS1ΔE9 Tg mice (A–C) and MER5101-vaccinated Tg mice (D–F). G, Quantitative image analysis demonstrated a significant reduction in R1282-positive Aβ plaque burden after MER5101 vaccination. H–M, Representative staining is shown for Thio S-positive fibrillar plaques in HC, frontal CTX, and CB in both vehicle-treated Tg (H–J) and MER5101-vaccinated Tg mice (K–M). N, Quantitative image analysis revealed that cerebral Thio S-positive plaques were significantly decreased in MER5101-vaccinated APPswe/PS1ΔE9 Tg mice versus vehicle-treated Tg controls. O–Q, The levels of Aβx-40 (O), Aβx-42 (P), and Aβ1-total (Q) in TBS-soluble and guanidine-soluble (TBS-insoluble) fractions of brain homogenates and plasma were measured by Aβ ELISA. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01.
Vascular amyloid is most prominent in the leptomeningeal blood vessels in the CB in APPswe/PS1ΔE9 Tg mice. Therefore, we semiquantitatively scored the amount of vascular amyloid in MER5101-vaccinated and vehicle-control Tg mice. We observed no significant differences (p = 0.16) in Thio S-positive vascular amyloid between MER5101-vaccinated and vehicle Tg mice (data not shown).
Biochemical analysis of cerebral Aβ levels by ELISA showed that TBS-soluble and insoluble (guanidine HCl-soluble) levels of Aβx-40 were slightly but not significantly decreased by 13.6% (p = 0.069) and 10.1% (p = 0.33), respectively, in vaccinated Tg mice compared with Tg vehicle controls (Fig. 4O). Soluble Aβx-42 was modestly reduced by 14.6% (p = 0.089), while insoluble Aβx-42 was significantly decreased by 29.0% (p = 0.041; Fig. 4P) in immunized Tg mice versus Tg vehicle controls. In addition, soluble and insoluble levels of Aβ1-total were lowered by 45.0% (p = 0.028) and 42.8% (p = 0.026), respectively (Fig. 4Q), in the vaccinated Tg mice. Plasma levels of Aβx-40 (Fig. 4O), Aβx-42 (Fig. 4P), and Aβ1-total (Fig. 4Q) were unchanged by MER5101 vaccination.
MER5101 vaccination results in attenuation of gliosis and restored synaptic density in HC
Plaque-associated microgliosis and astrogliosis have been reported previously in human AD brains (Itagaki et al., 1989; Mattiace et al., 1990) and in AD-like animal models (Frautschy et al., 1998; Gordon et al., 2002). In this study, immunoreactivities for Aβ (Fig. 5A,B), Iba-1 (Fig. 5C,D), a marker for both resting and activated microglia, and CD45 (Fig. 5E,F), a marker for activated microglia, were reduced in the HC of MER5101-immunized APPswe/PS1ΔE9 mice compared with Tg vehicle controls. Changes in GFAP-positive astrocytes were less obvious between Tg vehicle controls and MER5101-vaccinated Tg mice (Fig. 5G,H).
Figure 5.
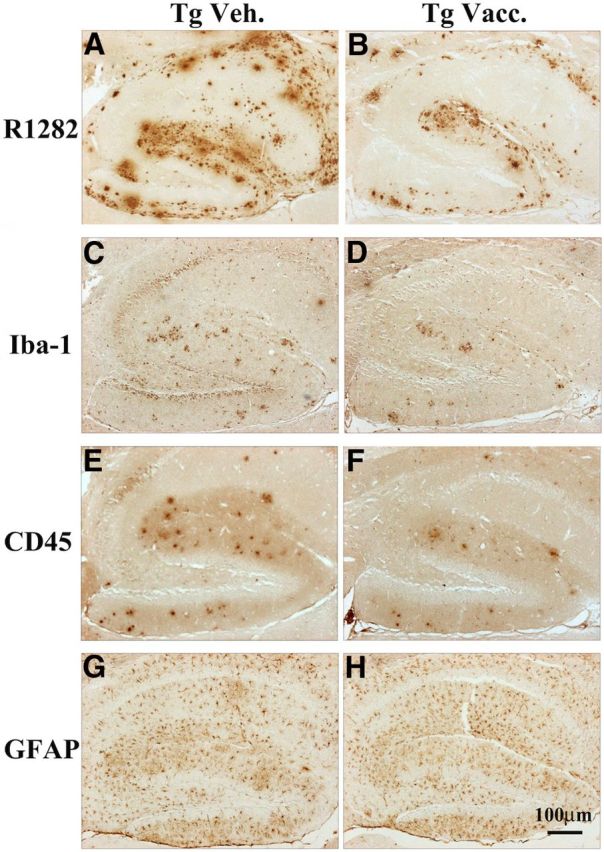
Plaque-associated microglial activation but not astrocyte reactivity was decreased in the HC of MER5101-vaccinated APPswe/PS1ΔE9 Tg mice. R1282 Aβ (A, B), Iba-1 (C, D), CD45 (E, F), and GFAP (G, H) immunoreactivities were detected on brain sections of immunized and vehicle-treated APPswe/PS1dE9 Tg mice. The number of Iba-1- and CD45-immunopositive microglia was reduced in MER5101-vaccinated APPswe/PS1ΔE9 Tg mice (D and E, respectively). GFAP IR was similar between vaccinated and vehicle-treated Tg mice, with perhaps more clustering of astrocytes around plaques in the HC of vehicle Tg controls.
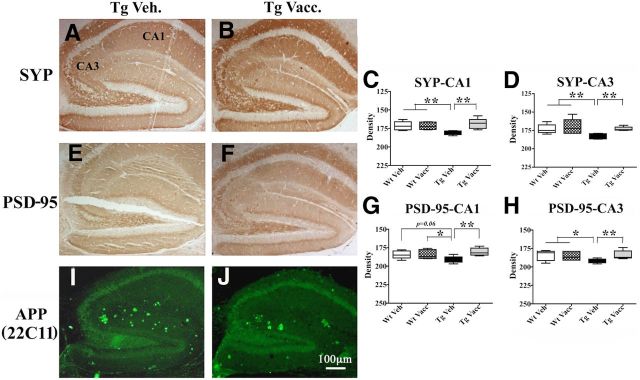
Synaptic immunostaining was performed using a presynaptic marker, SYP, and a postsynaptic marker, PSD-95. APPswe/PS1ΔE9 Tg mice showed reduced synaptic density in HC compared with age- and gender-matched Wt control mice (Fig. 6). MER510-vaccinated Tg mice showed significantly higher densities of both SYP (Fig. 6B–D) and PSD-95 (Fig. 6F–H) in hippocampal CA1 and CA3 regions compared with Tg vehicle controls (Fig. 6A,E), suggesting that hippocampal synaptic density, thought to be reduced in APPswe/PS1ΔE9 mice due to Aβ toxicity (Blanchard et al., 2003; Trinchese et al., 2004), was restored by MER5101 immunization. Neuritic plaques labeled with an APP monoclonal antibody, 22C11, were reduced in HC in immunized Tg mice (Fig. 6J) compared with Tg vehicle controls (Fig. 6I). In addition, neither CD5-positive T-cells nor hemosiderin-positive microhemorrhages (Pfeifer et al., 2002) were observed in the brain parenchyma in any of the mice (data not shown).
Figure 6.
Enhanced synaptic IR and reduced neuritic plaques were observed in the HC of MER5101-vaccinated APPswe/PS1ΔE9 Tg mice compared with vehicle Tg controls. The synaptic density was evaluated by staining with a presynaptic marker, SYP (A, B), and a postsynaptic marker, PSD-95 (E, F). Both SYP and PSD-95 immunoreactivities were increased in vaccinated APPswe/PS1ΔE9 Tg mice (B, F) compared with vehicle Tg controls (A, E). The optical density of SYP (C, D) and PSD-95 (G, H) IR was quantified in both the CA1 and CA3 regions of mouse HC for all treatment groups. Vehicle-treated APPswe/PS1ΔE9 Tg mice had significantly less SYP- and PSD-95-positive synaptic staining in HC compared with Wt mice. However, MER5101 vaccination restored synaptic staining close to Wt levels. Neuritic plaques, labeled by anti-human APP mAb, 22C11, were decreased in the HC of vaccinated APPswe/PS1ΔE9 Tg mice compared with vehicle controls. *p < 0.05; **p < 0.01.
MER5101 attenuated cognitive deficits in APPswe/PS1ΔE9 Tg mice
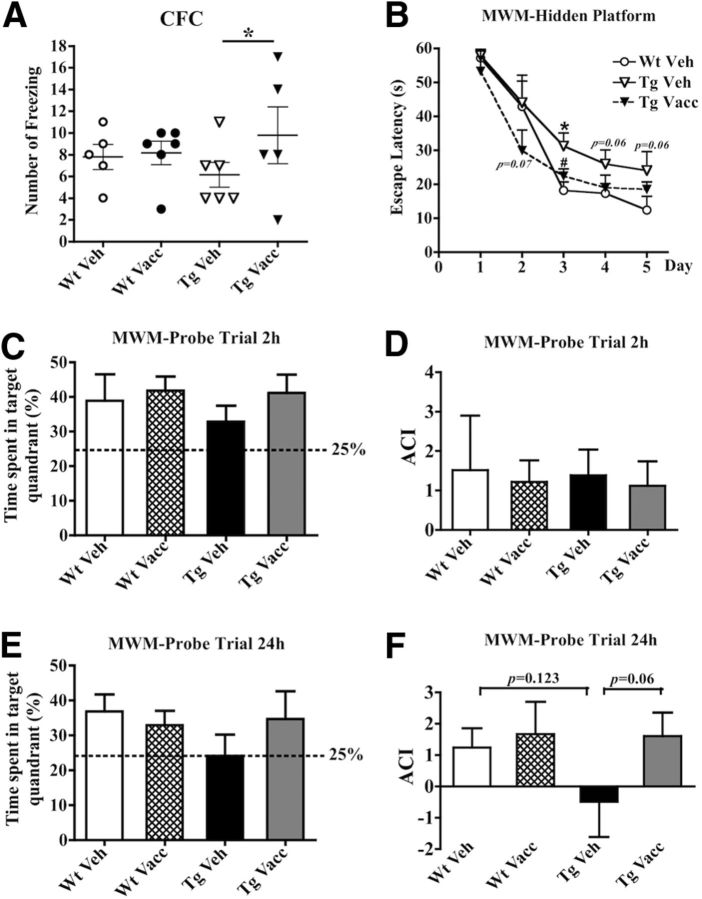
Cognitive efficacy of MER5101 in APPswe/PS1ΔE9 Tg mice was determined by CFC and MWM. In the CFC task (Fig. 7A), Wt mice treated with either MER5101 (solid circles) or vehicle (open circles) showed similar freezing frequency (7.8 ± 1.2 and 8.2 ± 1.1, respectively). Vehicle-treated APPswe/PS1ΔE9 mice (open triangles) exhibited a modest reduction in the number of freezings (6.2 ± 1.2; p = 0.16), implying a potentially weak recall for the stimulation by environmental conditions. However, MER5101 vaccination led to a significant increase in freezing frequency (9.8 ± 2.6; p = 0.023) in APPswe/PS1ΔE9 Tg mice (solid triangles).
Figure 7.
MER5101 vaccination resulted in attenuation of cognitive deficits in APPswe/PS1ΔE9 Tg mice. A, CFC showed that the vehicle-treated APPswe/PS1ΔE9 Tg mice froze less often than either vaccinated or vehicle-treated Wt mice, whereas MER5101-vaccinated APPswe/PS1ΔE9 Tg mice showed significantly more freezing behavior than the Tg vehicle controls (p = 0.04), indicating an improvement in fear conditioned memory in the vaccinated Tg mice. B, In the MWM, vehicle-treated Tg mice showed slower learning acquisition during days 3–5 of the hidden platform trials compared with Wt vehicle controls, indicating a spatial learning deficit. However, MER5101-vaccinated Tg mice showed faster learning acquisition during days 2–5 compared with vehicle-treated Tg mice. C, D, No group differences were observed in the first probe trial for short-term spatial memory (2 h after the last hidden platform trial on day 5). E, F, The second probe trial (24 h after the last hidden platform trial on day 5) revealed that vehicle-treated Tg mice had no preference for exploring the target quadrant (E), and had a lower and negative ACI (defined as average frequency of swims over sites in other quadrants of the pool; F) compared with the other groups of mice. However, MER5101-vaccinated Tg mice spent more time in the target quadrant searching for the platform (strong trend; p < 0.06), similar to the two groups of Wt mice and a positive ACI, indicating beneficial effects of MER5101 vaccination on spatial memory. *p < 0.05.
Interestingly, we have found that Aβ deposition in HC was inversely correlated (linear regression p = 0.084, r2 = 0.68, strong trend) with the number of freezings in the CFC in the MER5101-vaccinated Tg mice, but not in the vehicle-control Tg mice. Although there were no overall significant correlations between anti-Aβ antibody levels, Aβ deposition in HC, or CFC freezing, within the vaccinated Tg group, the two mice with the highest number of freezings also had the highest anti-Aβ antibody levels. In addition, the hippocampal Aβ plaque burden for each of these two mice (ranked lowest and third lowest in the vaccinated Tg mice) was lower than that of any of the vehicle-control Tg mice. Similarly, within the vehicle-treated Tg group, the mouse that had the highest number of freezings also had the lowest hippocampal Aβ level. Thus, while there are no significant correlations, there are some trends for correlations between CFC task, plaque level and antibody levels.
In the MWM task, a 5 d hidden platform trial followed by two probe trials, which were conducted 2 and 24 h, respectively, after the last hidden platform trial on day 5 were performed to evaluate both spatial learning acquisition and spatial memory in APPswe/PS1ΔE9 Tg and Wt mice. Overall, vehicle-treated APPswe/PS1ΔE9 Tg mice showed slower learning acquisition during days 3–5 compared with vehicle-treated Wt mice; however, MER5101-vaccinated APPswe/PS1ΔE9 Tg mice displayed improved learning acquisition during days 2–5 versus adjuvant-treated Tg controls, comparable to the performance of age-matched Wt mice (Fig. 7B).
The results of the first probe trial (2 h post the last hidden platform trial on day 5) indicated that all groups of mice retained short-term memory for the platform location and preferentially dwelled in the target quadrant (Fig. 7C,D). In the second probe trial (24 h post the last hidden platform trial on day 5), immunized and vehicle-treated Wt mice (36.8 ± 4.8 and 32.9 ± 4.2%, respectively) as well as MER5101-vaccinated APPswe/PS1ΔE9 Tg mice (34.7 ± 7.9%), still showed preferential memory for the target quadrant and spent comparable time on platform exploration; however, vehicle-treated APPswe/PS1ΔE9 Tg mice (24.1 ± 6.1%) showed no preference for target quadrant (Fig. 7E). Consistently, the vehicle-treated APPswe/PS1ΔE9 Tg mice showed a negative ACI, indicating that they continued to search for the platform in a all quadrants of the pool, whereas the MER5101-vaccinated APPswe/PS1ΔE9 Tg mice showed a positive ACI, similar to the ACI of Wt mice, suggesting that 4 months of immunization with Aβ1-15:DT/MAS-1 improved spatial memory deficits in APPswe/PS1ΔE9 Tg mice. The two groups of APPswe/PS1ΔE9 Tg mice (vehicle and vaccinated) were not significantly different from each other in the second probe trial, possibly due to small group sizes and high variability; however, a strong trend for improved spatial memory was observed in the vaccinated Tg mice.
Discussion
The objective of this study was to test a novel Aβ B-cell epitope immunogen conjugate (Aβ1-15:DT) with a Th2-biased adjuvant (MAS-1), MER5101, in Wt and APPswe/PS1ΔE9 Tg mice for its immunopotency and its subsequent effects on Aβ-related pathologies and cognition. Previously, we reported that Aβ1-15 alone was not an effective primary immunogen (Leverone et al., 2003), but priming by injection of full-length Aβ followed by intranasal boosting with either Aβ1-15 peptide or dendrimeric Aβ1-15 (16 copies of Aβ1-15 on a lysine tree; dAβ1-15) formulated in a mucosal adjuvant, Escherichia coli LT(R192G), resulted in high anti-Aβ antibody levels and reduced cerebral amyloid plaque burden in J20 APP Tg mice (Leverone et al., 2003; Seabrook et al., 2006). Intranasal delivery of dAβ1-15/LT(R192G) without a priming injection also induced robust anti-Aβ levels and lowered cerebral Aβ levels and plaques in these mice while avoiding an Aβ-specific cellular immune response (Seabrook et al., 2007). Intranasal administration of 2xAβ1-15 (tandem repeat of Aβ1-15 linked by two lysine residues) had similar effects and improved cognition in the MWM (Maier et al., 2006).
In the present study, we coupled Aβ1-15 to the immunogenic carrier protein, DT, via a 7 aa spacer, to provide T-cell help for Ig production and immunological memory. DT has long been approved for use in childhood and adult vaccines and is available as a GMP-compliant component (Audibert et al., 1982; Wenger et al., 1991; Lambert et al., 1998). The adjuvant used in this study, MAS-1, is a 30:70 w/o nanoparticular emulsion-based delivery system with a median globule size of 300 nm. MAS-1-based self-antigen conjugated vaccines have been reported to be safe and well tolerated, uniquely producing robust Th2 type neutralizing antibody responses while avoiding Th1 cell-mediated cytotoxicity or breaking immune self-tolerance of the native antigen (Gilliam et al., 2004; Simms et al., 2000).
We first tested MER5101 in young DBA/2 (6-week-old) and aged C57BL/6 (12-month-old) mice. After four immunizations, significant anti-Aβ antibody levels were observed in both mouse strains (unpublished data). Importantly, the vaccine induced predominantly Th2 antibody isotypes against the N-terminal region of Aβ1-42. We then tested MER5101 vaccination in APPswe/PS1ΔE9 Tg mice and found that it was effective in stimulating a strong humoral immune response with Th2-biased antibody isotypes in the absence an Aβ-specific cellular immune response, T-cell infiltration, or microhemorrhage in the brain.
Notably, plasma antibody levels decreased sharply after day 56 in immunized APPswe/PS1ΔE9 Tg mice, which may have been due to tolerance of the immunogen or by the antibodies binding with circulating Aβ in plasma. The reduction of antibody levels in plasma of vaccinated APPswe/PS1ΔE9 Tg mice at days 96 and 121 was restored after low pH dissociation treatment, whereas the antibody levels in immunized Wt mice or adjuvant control mice were not affected, implying that peripheral anti-Aβ antibody levels were maintained and suggesting that a portion of them were bound to Aβ in plasma.
The potent immune response induced by MER5101 effectively lowered cerebral AD pathologies in APPswe/PS1ΔE9 Tg mice. High cerebral levels of Aβ42 have been reported to be associated with plaque load and cognitive status in AD patients (Mann et al., 1996; Cataldo et al., 2004; Cosentino et al., 2010) and AD-like Tg mice (Radde et al., 2006; Murphy et al., 2007). Aβx-42 is more hydrophobic and aggregates more readily than Aβx-40. The ratio of Aβ42/40 is increased in AD patients, and soluble Aβ42 oligomers are considered extremely toxic to neurons, thereby contributing to cognitive decline in AD (Lambert et al., 1998; Fukuda et al., 1999). In this study, Aβ plaques were attenuated in immunized APPswe/PS1ΔE9 Tg mice, which correlated with reduced insoluble Aβ extracted from brain, similar to the results of our previous studies (Maier et al., 2006; Seabrook et al., 2007). MER5101 lowered mainly insoluble Aβx-42 and both soluble/insoluble Aβ1-total but had no effect on vascular amyloid. Fibrillar Aβ plaques have been shown to correlate with microglial activation in many AD-like Tg mouse models (Frautschy et al., 1998; Gordon et al., 2002; Kitazawa et al., 2005). Here, MER5101-immunized Tg mice had fewer Iba-1- and CD45-immunopositive microglia in HC compared with Tg vehicle controls, perhaps due to the lower plaque burden. MER5101 also lowered Aβ in the TBS-soluble brain fraction, which presumably contains Aβ oligomers with toxicities that are thought to affect neuron viability and lead to cognition decline (Shankar et al., 2008; Tomic et al., 2009). MER5101-vaccinated APPswe/PS1ΔE9 Tg mice had higher synaptic density and fewer neuritic plaques, which together, may have contributed to their superior cognitive performance compared with the vehicle-treated Tg mice.
The exact mechanism by which MER5101 vaccination mediates cerebral Aβ clearance is not clear yet; however, different mechanisms of Aβ removal by antibodies have been proposed. These include (1) Fc receptor (FcR)-dependent (Bard et al., 2000, 2003; Nicoll et al., 2003; Ferrer et al., 2004) or -independent (Bacskai et al., 2002; Das et al., 2003; Tamura et al., 2005) microglia-mediated phagocytosis, (2) antibody-mediated Aβ disaggregation and neutralization of Aβ toxicity (Solomon et al., 1997; Frenkel et al., 2000; Bacskai et al., 2001; Oddo et al., 2006), (3) a shift in efflux of Aβ from brain to periphery known as “the peripheral sink hypothesis” (DeMattos et al., 2001; Sigurdsson et al., 2001, 2002; Lemere et al., 2003), and/or (4) intracerebral sequestration of monomeric Aβ (Yamada et al., 2009). In our prior immunization studies (Leverone et al., 2003; Maier et al., 2006), we observed a simultaneous lowering of cerebral soluble Aβ and an increase in plasma Aβ levels in vaccinated mice that generated high anti-Aβ antibody levels, suggesting a potential antibody-driven shift of soluble Aβ from the brain to the periphery and leading to enhanced catabolism of Aβ, consistent with the peripheral sink hypothesis (DeMattos et al., 2001). In the present study, while cerebral-soluble Aβ levels were reduced in brain homogenates of MER5101-vaccinated APPswe/PS1ΔE9 Tg mice, there was no change in plasma Aβ levels before or after low pH dissociation of the antigen/antibody complexes compared with Tg vehicle controls. Plasma Aβ was undetectable post-dissociation due to dilution in the dissociation buffer. Nevertheless, the dissociation treatment resulted in an overt unmasking of anti-Aβ antibody levels in plasma samples collected from MER5101-vaccinated Tg mice at the end of treatment, suggesting that some portion of plasma Aβ may have been bound by antibodies before dissociation by low pH. Thus, the peripheral sink hypothesis may be one of the mechanisms for Aβ clearance in this study, although other aforementioned mechanisms may have contributed, too.
Clearance of fibrillar Aβ may require antibodies to across the blood–brain barrier, bind with cerebral Aβ, and recruit activated microglia for phagocytosis through an FcR-dependent (Bard et al., 2000, 2003; Nicoll et al., 2003; Ferrer et al., 2004) or -independent manner (Bacskai et al., 2002; Das et al., 2003; Tamura et al., 2005). In this study, the levels of insoluble Aβ (guanidine-soluble fraction) in brain homogenates as well as fibrillar Aβ plaques (Thio S labeled) were significantly lower in APPswe/PS1ΔE9 Tg mice after MER5101 vaccination, suggesting that in addition to the peripheral sink, the anti-Aβ antibodies may have directly interacted with compacted Aβ assemblies in brain and induced phagocytosis by microglia. Although microglial activation was not enhanced but attenuated in immunized Tg mouse brains at the end of this study, it is possible that FcR-mediated Aβ clearance may have occurred early and had subsided by the end of the study due to the removal of plaques. Interestingly, activated microglia surrounded the remaining plaques, suggesting an ongoing phagocytotic process.
Together, our data demonstrate that MER5101, a novel conjugate vaccine composed of the B-cell epitope, Aβ1-15, and an immunological carrier protein, DT, in a Th2-type adjuvant, MAS-1, is sufficient for eliciting a robust humoral immune response, resulting in predominantly Th2-biased immunoglobulins without Aβ-specific T-cell reactivity in APPswe/PS1ΔE9 Tg mice, and is effective in lowering Aβ-correlated pathologies and improving cognitive deficits. Prior results using the MER platform in ∼1500 patients for vaccines targeting self-hormones gastrin-17 (G17) and gonadotrophin-releasing hormone (GnRH) in the elderly and immunocompromised individuals elicited robust antibody responses specific to the self hormones. Systemic toxicities were not reported, nor were toxicities in organs producing the targeted hormones or bearing receptors for these hormones, including gastrointestinal organs in patients immunized against G17 and hypothalamus/pituitary tissues in patients immunized against GnRH, suggesting that Th1 type cell-mediated toxicities were avoided. These observations with vaccines comprising self-peptide-to-DT conjugates formulated with MAS-1 adjuvant, along with the responses to MER5101 described here, suggest that MER5101 may be safe and effective in generating antibody titers in the elderly and lowering cerebral Aβ deposition before cognitive changes or in early stages of AD. We believe that our novel Aβ immunogen conjugate together with the Th2-type adjuvant, MAS-1, is a promising candidate for a second-generation vaccine leading to an effective immunotherapy for AD while avoiding a potentially deleterious pro-inflammatory cellular immune response.
Footnotes
P.B. is the President and Founder and S.G. is a Principal Scientist at Mercia Pharma, Inc. All other authors declare no competing interests.
Funding provided by National Institutes of Health RO1 AG20159 (and ARRA Supplement) to C.A.L.
References
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- Ajani JA, Hecht JR, Ho L, Baker J, Oortgiesen M, Eduljee A, Michaeli D. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106:1908–1916. doi: 10.1002/cncr.21814. [DOI] [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages. Eur J Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert F, Jolivet M, Chedid L, Arnon R, Sela M. Successful immunization with a totally synthetic diphtheria vaccine. Proc Natl Acad Sci U S A. 1982;79:5042–5046. doi: 10.1073/pnas.79.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci U S A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Blanchard V, Moussaoui S, Czech C, Touchet N, Bonici B, Planche M, Canton T, Jedidi I, Gohin M, Wirths O, Bayer TA, Langui D, Duyckaerts C, Tremp G, Pradier L. Time sequence of maturation of dystrophic neurites associated with Abeta deposits in APP/PS1 transgenic mice. Exp Neurol. 2003;184:247–263. doi: 10.1016/S0014-4886(03)00252-8. [DOI] [PubMed] [Google Scholar]
- Brett BT, Smith SC, Bouvier CV, Michaeli D, Hochhauser D, Davidson BR, Kurzawinski TR, Watkinson AF, Van Someren N, Pounder RE, Caplin ME. Phase II study of anti-gastrin-17 antibodies, raised to G17DT, in advanced pancreatic cancer. J Clin Oncol. 2002;20:4225–4231. doi: 10.1200/JCO.2002.11.151. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Terio NB, Peterhoff CM, Durham R, Mercken M, Mehta PD, Buxbaum J, Haroutunian V, Nixon RA. Abeta localization in abnormal endosomes: association with earliest Abeta elevations in AD and Down syndrome. Neurobiol Aging. 2004;25:1263–1272. doi: 10.1016/j.neurobiolaging.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Cosentino SA, Stern Y, Sokolov E, Scarmeas N, Manly JJ, Tang MX, Schupf N, Mayeux RP. Plasma {beta}-amyloid and cognitive decline. Arch Neurol. 2010;67:1485–1490. doi: 10.1001/archneurol.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma-/- knock-out mice. J Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sánchez Guerra ML, Rey MJ, Costa-Jussá F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri G, Dave A, Bot A, Juntti T, Omid S, von Herrath M. Subcutaneous insulin B:9–23/IFA immunisation induces Tregs that control late-stage prediabetes in NOD mice through IL-10 and IFNgamma. Diabetologia. 2010;53:1958–1970. doi: 10.1007/s00125-010-1777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsiao K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Katz O, Solomon B. Immunization against Alzheimer's beta-amyloid plaques via EFRH phage administration. Proc Natl Acad Sci U S A. 2000;97:11455–11459. doi: 10.1073/pnas.97.21.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Shimizu T, Nakajima M, Mori H, Shirasawa T. Synthesis, aggregation, and neurotoxicity of the Alzheimer's Abeta1–42 amyloid peptide and its isoaspartyl isomers. Bioorg Med Chem Lett. 1999;9:953–956. doi: 10.1016/S0960-894X(99)00121-3. [DOI] [PubMed] [Google Scholar]
- Gandy S, DeMattos RB, Lemere CA, Heppner FL, Leverone J, Aguzzi A, Ershler WB, Dai J, Fraser P, St George Hyslop P, Holtzman DM, Walker LC, Keller ET. Alzheimer's Abeta vaccination of rhesus monkeys (Macaca mulatta) Mech Ageing Dev. 2004;125:149–151. doi: 10.1016/j.mad.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Geylis V, Kourilov V, Meiner Z, Nennesmo I, Bogdanovic N, Steinitz M. Human monoclonal antibodies against amyloid-beta from healthy adults. Neurobiol Aging. 2005;26:597–606. doi: 10.1016/j.neurobiolaging.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Ghochikyan A, Mkrtichyan M, Petrushina I, Movsesyan N, Karapetyan A, Cribbs DH, Agadjanyan MG. Prototype Alzheimer's disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. doi: 10.1016/j.vaccine.2005.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam AD, Watson SA, Henwood M, McKenzie AJ, Humphreys JE, Elder J, Iftikhar SY, Welch N, Fielding J, Broome P, Michaeli D. A phase II study of G17DT in gastric carcinoma. Eur J Surg Oncol. 2004;30:536–543. doi: 10.1016/j.ejso.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Laurén J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE. Alzheimer disease therapy: can the amyloid cascade be halted? J Clin Invest. 2003;111:11–18. doi: 10.1172/JCI17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MN, Holcomb LA, Jantzen PT, DiCarlo G, Wilcock D, Boyett KW, Connor K, Melachrino J, O'Callaghan JP, Morgan D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp Neurol. 2002;173:183–195. doi: 10.1006/exnr.2001.7754. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-X. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, Hancock WW, Weiner HL, Selkoe DJ. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Ann N Y Acad Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Spooner ET, Leverone JF, Mori C, Clements JD. Intranasal immunotherapy for the treatment of Alzheimer's disease: Escherichia coli LT and LT(R192G) as mucosal adjuvants. Neurobiol Aging. 2002;23:991–1000. doi: 10.1016/S0197-4580(02)00127-6. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, Matsuoka Y, Taylor JW, DeMattos RB, Holtzman DM, Clements JD, Selkoe DJ, Duff KE. Evidence for peripheral clearance of cerebral Abeta protein following chronic, active Abeta immunization in PSAPP mice. Neurobiol Dis. 2003;14:10–18. doi: 10.1016/S0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer's disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/S0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CA. Abeta1–15 is less immunogenic than Abeta1–40/42 for intranasal immunization of wild-type mice but may be effective for “boosting.”. Vaccine. 2003;21:2197–2206. doi: 10.1016/S0264-410X(02)00754-5. [DOI] [PubMed] [Google Scholar]
- Li Q, Gordon M, Cao C, Ugen KE, Morgan D. Improvement of a low pH antigen-antibody dissociation procedure for ELISA measurement of circulating anti-Abeta antibodies. BMC Neurosci. 2007;8:22. doi: 10.1186/1471-2202-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lemere CA. Modulation of the humoral and cellular immune response in Abeta immunotherapy by the adjuvants monophosphoryl lipid A (MPL), cholera toxin B subunit (CTB) and E. coli enterotoxin LT(R192G) Vaccine. 2005;23:5149–5159. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Iwatsubo T, Cairns NJ, Lantos PL, Nochlin D, Sumi SM, Bird TD, Poorkaj P, Hardy J, Hutton M, Prihar G, Crook R, Rossor MN, Haltia M. Amyloid beta protein (Abeta) deposition in chromosome 14-linked Alzheimer's disease: predominance of Abeta42(43) Ann Neurol. 1996;40:149–156. doi: 10.1002/ana.410400205. [DOI] [PubMed] [Google Scholar]
- Mattiace LA, Davies P, Yen SH, Dickson DW. Microglia in cerebellar plaques in Alzheimer's disease. Acta Neuropathol. 1990;80:493–498. doi: 10.1007/BF00294609. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI200318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J Biol Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.WNL.0000073623.84147.A8. [DOI] [PubMed] [Google Scholar]
- Park JH, Widi GA, Gimbel DA, Harel NY, Lee DH, Strittmatter SM. Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer's transgenic mice. J Neurosci. 2006;26:13279–13286. doi: 10.1523/JNEUROSCI.4504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Sun J, Hon S, Nylander AN, Xia W, Feng Y, Wang X, Lemere CA. L-3-n-butylphthalide improves cognitive impairment and reduces amyloid-beta in a transgenic model of Alzheimer's disease. J Neurosci. 2010;30:8180–8189. doi: 10.1523/JNEUROSCI.0340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Puoliväli J, Wang J, Heikkinen T, Heikkilä M, Tapiola T, van Groen T, Tanila H. Hippocampal A beta 42 levels correlate with spatial memory deficit in APP and PS1 double transgenic mice. Neurobiol Dis. 2002;9:339–347. doi: 10.1006/nbdi.2002.0481. [DOI] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Jiang L, Thomas K, Lemere CA. Boosting with intranasal dendrimeric Abeta1-15 but not Abeta1-15 peptide leads to an effective immune response following a single injection of Abeta1-40/42 in APP-tg mice. J Neuroinflammation. 2006;3:14. doi: 10.1186/1742-2094-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, Bitan G, Lemere CA. Dendrimeric Abeta1–15 is an effective immunogen in wildtype and APP-tg mice. Neurobiol Aging. 2007;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer's disease-associated pathology in transgenic mice. Am J Pathol. 2001;159:439–447. doi: 10.1016/S0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson EM, Wisniewski T, Frangione B. A safer vaccine for Alzheimer's disease? Neurobiol Aging. 2002;23:1001–1008. doi: 10.1016/S0197-4580(02)00124-0. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T. An attenuated immune response is sufficient to enhance cognition in an Alzheimer's disease mouse model immunized with amyloid-beta derivatives. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms MS, Scholfield DP, Jacobs E, Michaeli D, Broome P, Humphreys JE, Bishop MC. Anti-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br J Cancer. 2000;83:443–446. doi: 10.1054/bjoc.2000.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Justin T, Michaeli D, Watson SA. Phase I/II study of G17-DT, an anti-gastrin immunogen, in advanced colorectal cancer. Clin Cancer Res. 2000;6:4719–4724. [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci U S A. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner ET, Desai RV, Mori C, Leverone JF, Lemere CA. The generation and characterization of potentially therapeutic Abeta antibodies in mice: differences according to strain and immunization protocol. Vaccine. 2002;21:290–297. doi: 10.1016/S0264-410X(02)00464-4. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Hamajima K, Matsui K, Yanoma S, Narita M, Tajima N, Xin KQ, Klinman D, Okuda K. The F(ab)′2 fragment of an Abeta-specific monoclonal antibody reduces Abeta deposits in the brain. Neurobiol Dis. 2005;20:541–549. doi: 10.1016/j.nbd.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer's disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–358. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer's disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer's disease. Ann Neurol. 2000;48:567–579. doi: 10.1002/1531-8249(200010)48:4<567::AID-ANA3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Wenger JD, Pierce R, Deaver KA, Plikaytis BD, Facklam RR, Broome CV. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in US children aged 18–59 months. Haemophilus Influenzae Vaccine Efficacy Study Group. Lancet. 1991;338:395–398. [PubMed] [Google Scholar]
- Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, Terasaki T, Hashimoto T, Iwatsubo T. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci. 2009;29:11393–11398. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]