Purines are heterocyclic aromatic molecules that are among the oldest and most influential biochemical compounds in evolutionary history.1 The purine nucleotide adenosine triphosphate (ATP) is the universal energy currency of intracellular biologic reactions on which mammalian life is based. Here we examine the roles that purines play as extracellular signaling molecules, with a particular focus on ATP, adenosine diphosphate (ADP), and adenosine.
The first reports of purinergic signaling date back to 1929, when scientists intravenously injected extracts from cardiac tissues into intact animals and observed transient slowing of the heart rate; they subsequently identified the biologic agent as an “adenine compound.”2 Transient heart block induced by intravenous injection of adenosine remains an important clinical application of purinergic signaling (Table 1).
Table 1.
Clinical Therapeutic Agents Targeting Purinergic Signaling.
| Drug | Target | Indication | Mechanism of Action | Potential Side Effects |
|---|---|---|---|---|
| Clopidogrel | P2Y12 nucleotide receptors | Acute coronary syndrome | Inhibition of P2Y12; direct and irreversible inhibition of platelet function | Hemorrhage, neutropenia, thrombotic thrombocytopenic purpura (rare) |
| Adenosine | Cardiac A1 adenosine receptors | Supraventricular tachycardia, pharmacologic stress echocardiography | Adenosine receptor agonist; induction of transient complete heart block (agonist on A1 adenosine receptors), coronary vasodilatation (agonist on A2A adenosine receptors) | Prolonged heart block, metallic taste |
| Caffeine | Adenosine receptors | Headache, fatigue | Inhibition of cerebral adenosine A2A receptors | CNS excitation, caffeine intoxication |
| Theophylline | Adenosine receptors | Bronchodilatation | Unclear (phosphodiesterase inhibition or antagonism of adenosine receptors) | Narrow therapeutic index, CNS excitation, seizures |
| Methotrexate | Enhanced CD73-dependent adenosine production | Rheumatoid arthritis, IBD, autoimmune diseases | Enhancement of adenosine-mediated inhibition of inflammation | Ulcerative stomatitis, low white-cell count, high teratogenicity |
| Sulfasalazine | Enhanced CD73-dependent adenosine production | Rheumatoid arthritis; IBD, autoimmune diseases | Inhibition of 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside transformylase, enhancement of adenosine release at an inflamed site, and diminished inflammation through adenosine occupancy of A2 receptors on inflammatory cells | Gastrointestinal distress, headache, dizziness |
| Dipyridamole | Equilibrative nucleoside transporters ENT1 and ENT2 | Platelet inhibition, coronary vasodilatation during stress echocardiography | Inhibition of ENT1 and ENT2; increased extracellular adenosine levels due to inhibition of adenosine uptake | Oral drug: bleeding tendency; intravenous drug: chest pain, angina in patients with coronary artery disease |
| Regadenoson | Adenosine A2A receptors | Stress echocardiography | Agonist on A2A adenosine receptors; adenosine A2A–receptor–mediated coronary vasodilatation | Dyspnea, headache |
CNS denotes central nervous system, and IBD inflammatory bowel disease.
Additional research in recent decades has led to the discovery of many biologic effects of ATP, ADP, and adenosine signaling. These mediators function through the activation of G-protein–coupled or ligand-gated ion-channel receptors. Adenosine is derived from ATP and ADP through the actions of enzymes on cellular surfaces, which also express the receptors for these mediators. Since the receptors of ATP and ADP and the receptors of adenosine often transduce opposite effects, the resulting cellular response is attributable to both the ratio of ADP and ATP to adenosine concentrations and the relative levels of expression and signaling intensity of their receptors.3 Here we discuss the functional role of purinergic signaling in inflammatory diseases and the contribution of disordered purinergic signaling to the mechanisms of acute and chronic disease.
EXTRACELLULAR NUCLEOTIDE RELEASE AND SIGNALING
In their physiologic state, mammalian cells contain high concentrations of ATP (5 to 8 mM). Pathologic conditions such as inflammation or ischemia cause the release of ATP. Cellular necrosis is associated with ATP egress from intracellular stores.4 During apoptosis, controlled ATP release can occur through pannexin hemichannels, where ATP functions as a chemotactic signal for phagocytes.5 Endothelial cells or activated inflammatory cells such as polymorphonuclear neutrophils release ATP through connexin hemichannels.6–8 Platelets release purines in the form of ADP from intracellular granules (Fig. 1).4
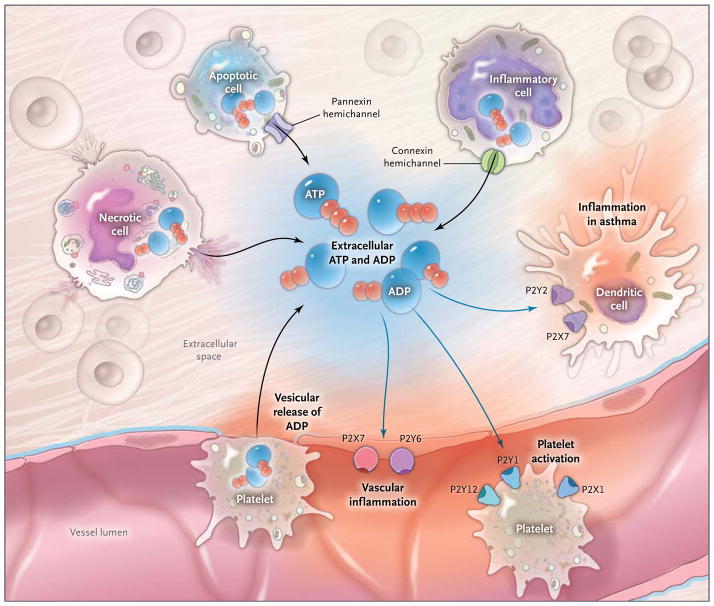
Figure 1. Release of Extracellular Adenosine Triphosphate (ATP) and Adenosine Diphosphate (ADP) and Activation of ATP (P2) Receptors during Inflammation.
During inflammatory conditions that occur in vascular thrombosis, hypoxia, ischemia, inflammatory bowel disease, and acute lung injury, multiple cell types release nucleotides, typically in the form of ATP or ADP, from the intracellular compartment into the extracellular space. The release of nucleotides includes release of ATP from necrotic cells, pannexin-hemichannel– dependent release of ATP during apoptosis, and release of ATP through connexin hemichannels from activated inflammatory cells such as polymorphonuclear granulocytes (neutrophils).4,5,8 In addition, release of extracellular ATP has been shown to occur through vesicular exocytosis or connexin hemichannels from endothelial7 and urothelial cells, osteoblasts, and astrocytes, as well as nerves (not shown).3 An additional source of extracellular nucleotides in inflammatory conditions is provided by activated platelets, which release ATP and ADP through the release of granules and exocytosis. In the extracellular space, these nucleotides function as signaling molecules that can activate P2Y receptors (G-protein– coupled receptors) or P2X receptors (ligand-gated ion channels). Examples of nucleotide-receptor signaling in inflammatory conditions include P2Y6- or P2X7-receptor signaling, which mediates vascular inflammation,9,10 and P2Y1-, P2X1-, and P2Y12-receptor signaling, which mediates platelet activation. Activation of P2 receptors of the P2Y2 and P2X7 family that are expressed on dendritic cells is thought to play a role in promoting lung inflammation in chronic lung diseases such as asthma.11
Two decades ago, Burnstock described the existence of ATP receptors that he referred to as “P2” receptors.3 Subsequently, they were divided on the basis of their chemical properties into P2X receptors (ligand-gated ion channels) and P2Y receptors (G-protein–coupled receptors). Gene-targeted mice with deleted P2 receptors are typically viable, indicating redundancy of the signaling system in physiologic conditions. However, in pathologic conditions such as asthma, vascular inflammation, or graft-versus-host disease, P2-receptor knockout mice are protected from inflammatory diseases. 9,11,12 Genetic studies directly implicate ATP signaling in human inflammatory or neoplastic diseases. For example, in genetic studies involving humans, mutations in the P2X7-receptor gene have been linked to susceptibility to tuberculosis13 and the clinical outcomes of chronic lymphocytic leukemia,14 and genetic abnormalities in T-cell–dependent P2Y11 signaling have been implicated in narcolepsy.15 Pharmacologic P2-receptor antagonists inhibit inflammation such as that which occurs in inflammatory bowel disease (IBD), lung inflammation, and ischemia and reperfusion.4,11,16
EXTRACELLULAR CONVERSION OF ATP AND ADP TO ADENOSINE
In the extracellular space, ATP and ADP are rapidly metabolized to adenosine monophosphate (AMP), which in turn is metabolized to adenosine. This nucleotide phosphohydrolysis involves a two-step enzymatic process regulated by ectoenzymes. In the first step, ATP and ADP are both converted to AMP through the ectonucleoside triphosphate diphosphohydrolase 1 (CD39). Mice with genetic deletion of Cd39 are viable, indicating that CD39-dependent phosphohydrolysis is not vital under physiologic conditions. Elevations in extracellular ATP and ADP levels in conjunction with attenuated adenosine levels account for increased susceptibility to the development of pathologic inflammation during disease states in Cd39−/− mice.17–20 In humans, polymorphisms of noncoding regions of CD39 decrease ectonucleotidase expression, leading to increased susceptibility to IBD and multiple sclerosis.21,22
In the second step of the extracellular generation of adenosine, ecto-5′-nucleotidase (CD73) converts extracellular AMP to adenosine. Mice with a genetic deletion of Cd73 are viable, indicating that CD73-dependent phosphohydrolysis of AMP is not vital under physiologic conditions. However, a recent study involving humans identified loss-of-function mutations of the gene encoding CD73 as the genetic basis of familial peripheral-artery calcifications.23 During pathophysiological conditions, Cd73−/− mice have attenuated adenosine signaling, whereas extracellular ATP and ADP levels remain almost unchanged. The lack of extracellular adenosine signaling in Cd73−/− mice causes susceptibility to hypoxia-induced inflammation24 and barrier dysfunction of the vasculature and the intestine.25,26 The antiinflammatory actions of methotrexate, as well as sulfasalazine, are mediated at least in part through CD73-dependent production of adenosine.27,28 Pharmacologic studies in a laboratory or a clinical setting using pharmacologic compounds that increase extracellular ATP and ADP conversion to adenosine show therapeutic effects (Table 1) in patients with inflammatory disease or ischemia.17,29–32
EXTRACELLULAR ADENOSINE SIGNALING
Adenosine can signal through four distinct G-protein– coupled receptors: the adenosine A1 receptor (ADORA1), the adenosine A2A receptor (ADORA2A), the adenosine A2B receptor (ADORA2B), and the adenosine A3 receptor (ADORA3) (Fig. 2).40–42 Adenosine-receptor subtypes are distributed differently on each adenosine target cell. For example, ADORA2B is highly expressed on vascular endothelial cells,36,43 whereas ADORA2A is highly expressed on immune cells such as neutrophils33 and lymphocytes.34 Specific human disease states relating to defects or mutations of any of the four defined adenosine receptors are currently unknown. Adenosine-receptor–knockout mice are viable, indicating redundancy of the system under physiologic conditions.
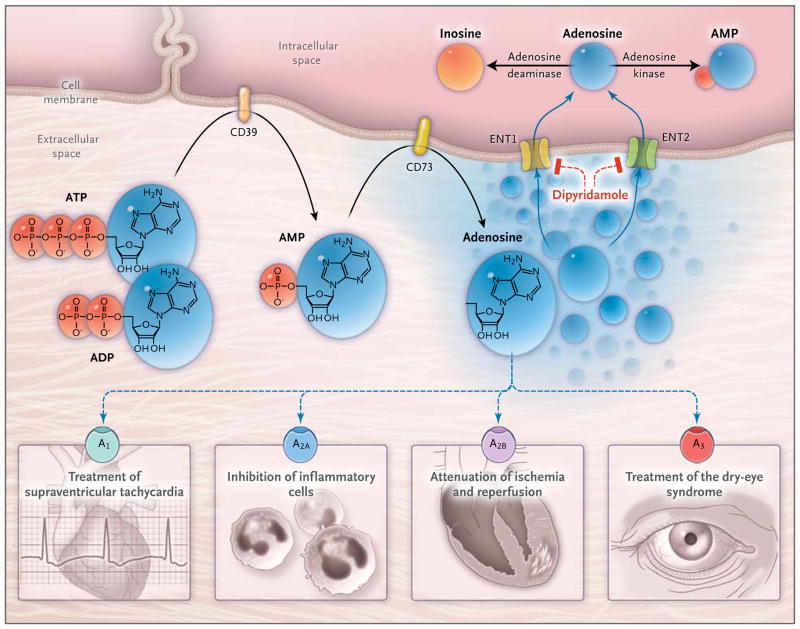
Figure 2. Extracellular Adenosine Signaling and Its Termination.
In inflammatory conditions, extracellular adenosine is derived predominantly from the enzymatic conversion of the precursor nucleotides ATP and ADP to AMP through the enzymatic activity of the ectonucleoside triphosphate diphosphohydrolase 1 (CD39) and the subsequent conversion of AMP to adenosine through ecto-5′-nucleotidase (CD73). Extracellular adenosine can signal through four distinct adenosine receptors: ADORA1 (A1), ADORA2A (A2A), ADORA2B (A2B), and ADORA3 (A3). An example of the functional role of extracellular adenosine signaling is A1-receptor activation during intravenous administration of adenosine for the treatment of supraventricular tachycardia. In addition, experimental studies implicate activation of A2A that is expressed on inflammatory cells such as neutrophils33 or lymphocytes in the attenuation of inflammation.34,35 Other experimental studies provide evidence of signaling events through A2B in tissue adaptation to hypoxia and attenuation of ischemia and reperfusion.36–38 A clinical trial has shown that an oral agonist of the A3 adenosine receptor may be useful in the treatment of the dry-eye syndrome.39 Adenosine signaling is terminated by adenosine uptake from the extracellular space toward the intracellular space, predominantly through equilibrative nucleoside transporter 1 (ENT1) and equilibrative nucleoside transporter 2 (ENT2), followed by metabolism of adenosine to AMP through the adenosine kinase or to inosine through the adenosine deaminase. Blockade of equilibrative nucleoside transporters by dipyridamole is associated with increased extracellular adenosine concentrations and signaling (e.g., during pharmacologic stress echocardiography or in protection of tissue from ischemia).
In contrast, many specific biologic and cell-specific functions have been identified for each receptor under pathologic conditions, and a few select examples are discussed below. The chronotropic effects of adenosine that are critical in the treatment of supraventricular tachycardia are dependent on ADORA1.44 Pharmacologic studies show antiinflammatory functions of ADORA2A signaling in human neutrophils33; ADORA2A plays a critical role in attenuating inflammatory-cell activation at multiple tissue sites.33,45 Pharmacologic studies show therapeutic benefits of ADORA2A antagonists in Parkinson’s disease.46 ADORA2B plays a role in tissue adaptation to conditions of hypoxia, inflammation, or ischemia, 32,47–49 whereas ADORA3A a has been linked to chloride transport in the production of aqueous humor by the nonpigmented epithelium of the eye.50 A recent randomized clinical trial showed salutary effects of an ADORA3 agonist in the treatment of the dry-eye syndrome.39
TERMINATION OF ADENOSINE SIGNALING
During the termination of signaling, adenosine is transported from the extracellular space to the intracellular compartment (Fig. 2). This transport involves equilibrative nucleoside transporters — diffusion-limited channels that allow adenosine to freely cross the cellular membrane according to its concentration gradient.43,51 Mice with genetic deletion of equilibrative nucleoside transporters are viable, but they have elevated adenosine levels during disease states which help to protect them during organ ischemia.43 Pharmacologic inhibition of equilibrative nucleoside transporters with dipyridamole and concomitant elevations of extracellular adenosine levels can be used to induce coronary-artery vasodilatation during stress echocardiography (thereby revealing coronary lesions),52 to prevent recurrent stroke by inhibiting platelet aggregation,53 and to conserve the patency of hemodialysis grafts.54
Within the intracellular compartment, adenosine is rapidly metabolized to inosine through adenosine deaminase (ADA)55 or to AMP through adenosine kinase.56 Inhibition of adenosine kinase by cyclosporine — leading to enhanced extracellular adenosine concentrations — may contribute, at least in part, to the antiinflammatory effects of cyclosporine.57 Studies in mice with genetic deletion of Ada show elevated extracellular adenosine levels, leading to severe lung inflammation.58 Cytotoxic effects of adenosine metabolites on lymphocytes in patients with a defect in the gene encoding ADA cause severe combined immunodeficiency (SCID). Gene therapy with autologous CD34+ bone marrow cells transduced with a retroviral vector containing the ADA gene has been used for the treatment of patients with ADA-associated SCID.59
PURINERGIC SIGNALING AS A REGULATOR OF PLATELET FUNCTION
Inflammation is closely linked to thrombosis, and platelets are critical mediators in this process. ATP and ADP signaling plays an essential role during platelet activation (Fig. 3). ADP initiates platelet aggregation by simultaneous activation of P2Y1 and P2Y12. Activation of P2Y1, in turn, activates phospholipase C and triggers shape changes, whereas P2Y12 couples to the G protein Gi to reduce adenylyl cyclase activity and inactivate the fibrinogen-receptor glycoprotein IIb/IIIa (GPIIb/IIIa) receptor, which is critical for platelet aggregation.62 Thienopyridine drugs such as ticlopidine and clopidogrel exert their antithrombotic effect through inhibition of the platelet P2Y12 receptor.63,64 Patients with a defect in the P2Y12-receptor gene have a congenital bleeding disorder. 65 In contrast, gain-of-function mutations of the P2Y12 gene are associated with increased ADP-induced platelet aggregation, and a case–control study suggested that persons with such mutations have an increased risk of arteriosclerosis. 66,67 However, a large clinical trial that examined genetic determinants of the response to clopidogrel and cardiovascular events showed that gene mutations influencing drug absorption and metabolic activation, rather than P2Y12 mutations, are more clinically relevant for pharmacologic effects. 68 Platelets also express P2X1 receptors, which alter platelet aggregation and shape change with ADP and ATP activation in vivo.61
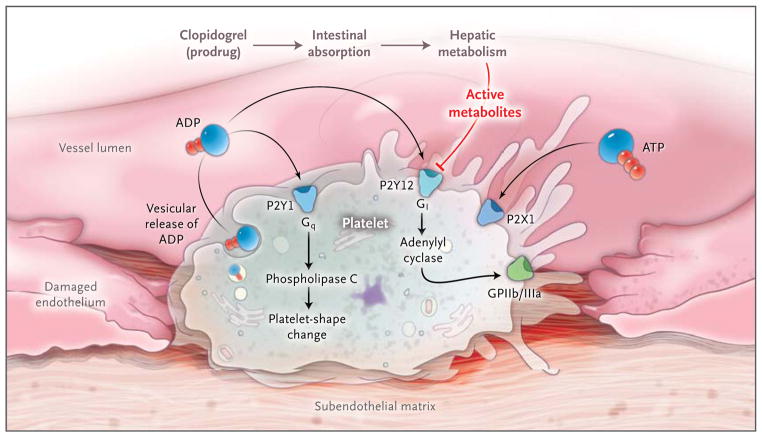
Figure 3. Purinergic Signaling in Platelet Function and Thrombosis.
Purinergic signaling is an important link among platelet activation, vascular thrombosis, and inflammation. Platelets mediate primary hemostasis through adhesion, aggregation, and subsequent thrombus formation. Extracellular nucleotides are continuously released from cells associated with the blood. When the vessel wall is injured, platelets roll and become tethered to the subendothelial matrix. These interactions cause changes in the shape of platelets and vesicular release of ADP. Platelet responses to ADP require the coordinated activation of P2Y1 and P2Y12, which function as guanosine triphosphate–binding protein (G protein)-coupled receptors.60 ADP-dependent activation of the P2Y1 receptor causes activation of phospholipase C mediated through the G protein Gq, leading to subsequent changes in the shape of platelets. ADP also activates P2Y12 receptors, with subsequent activation of the G protein Gi, thereby contributing to fibrinogen-receptor activation (the glycoprotein IIb/IIIa [GPIIb/IIIa] receptor) and platelet aggregation. The platelet inhibitor clopidogrel has pharmacologic effects on the P2Y12 receptor once it has been absorbed and converted from a prodrug to active metabolites. Both intestinal absorption of the prodrug and hepatic metabolism are controlled by specific gene products. Active clopidogrel metabolites irreversibly antagonize the P2Y12 receptor, which in turn inactivates the GPIIb/IIIa-fibrinogen receptor. Clinical trials that examined genetic determinants of the response to clopidogrel and cardiovascular events indicate that gene mutations affecting drug absorption and metabolic activation, rather than P2Y12 mutations, account for the pharmacologic effects of clopidogrel. Platelets also express P2X1 receptors, which mediate modulation of platelet aggregation and shape changes after activation by ADP or ATP in vivo.61
Experimental studies involving mutant mice with induced defects in the extracellular conversion of ATP and ADP to adenosine have shown that termination of nucleotide signaling and concomitant generation of adenosine inhibit platelet activation. Thus, Cd39−/− mice had increased cardiac ischemic injury,20 increased cerebral infarct volumes, and reduced postischemic perfusion after experimental stroke induction, whereas treatment with soluble ectonucleotidases corrected the phenotype and was therapeutic in wild-type mice.69
Ex vivo treatment of human blood with soluble nucleotidase — which enhances the conversion of AMP to adenosine — is associated with elevated adenosine levels and inhibition of platelet aggregation. 70 Signaling events through ADORA2A71 and ADORA2B72 inhibit platelet aggregation. Similarly, the adenosine uptake inhibitor dipyridamole is used as a platelet inhibitor for the prevention of recurrent stroke53 or to improve the patency of hemodialysis grafts in patients.54 Moreover, the P2Y12-receptor antagonist ticagrelor inhibits platelet function — at least in part — by inhibiting adenosine uptake, thereby elevating extracellular adenosine levels.73
EXTRACELLULAR ATP AND ADENOSINE DURING ISCHEMIA AND REPERFUSION
Ischemia–reperfusion injury is linked to important clinical conditions such as organ transplantation and cardiovascular disease characterized by the activation of inflammatory pathways.4,74 Extracellular ATP release can elicit an immune response during ischemia and reperfusion, acting as a chemotactic signal for phagocytes,5 activating the NLRP3 inflammasome,75 and have chemotactic effects on inflammatory cells.76 Pharmacologic strategies to block ATP release or P2-receptor signaling are thought to be useful in attenuating sterile inflammation during ischemia and reperfusion.4
In contrast, extracellular adenosine has been referred to as a “safety signal” that dampens hypoxia-induced inflammation during ischemia and reperfusion.77 Extracellular conversion of ATP to adenosine has a central role in attenuating sterile inflammation during ischemia–reperfusion injury. Experimental studies have shown that pharmacologic strategies to increase the breakdown of ATP to adenosine are effective in attenuating tissue injury and sterile inflammation during ischemia and reperfusion.17,19,20,31,32,78,79 In addition, several experimental studies provide evidence of a protective role of adenosine signaling in models of ischemia and reperfusion (e.g., through activation of ADORA2A on inflammatory cells33,34,45 or activation of ADORA2B on vascular endothelium, epithelium, or cardiac myocytes32,36,37,80). For example, a recent study has identified a regulatory circuit in the heart through which ADORA2B signaling controls expression of the circadian protein PER2, which stabilizes the transcription factor hypoxia-inducible factor (HIF) 1 (Hif-1), promotes glycolytic metabolism, and has cardioprotective effects.38 Exposure of mice to intense light stabilized Per2 in the heart and reduced cardiac injury after myocardial ischemia. 38 Activation of Adora2a on T cells attenuates ischemia and reperfusion in experimental models of sickle cell disease.34
P1- AND P2-RECEPTOR SIGNALING DURING ACUTE LUNG INJURY
Acute lung injury is among the leading causes of morbidity and mortality associated with critical illness. Experimental studies show elevated levels of pulmonary ATP after exposure to lung injury81 and indicate that activation of P2 receptors such as P2Y6 or P2X7 result in enhanced inflammation and vascular leakage.9,10 Studies of lung injury in mice with genetic defects in Cd39 or Cd73 have shown increased levels of pulmonary edema, lung inflammation, and attenuated gas exchange.29,30 Genetic and pharmacologic studies in mice point to the potential role of adenosine-receptor signaling in the treatment of acute lung injury.45 For example, the Adora2b agonist BAY 60-6583 increases clearance of alveolar fluid and attenuates capillary–alveolar leakage during lung injury induced by mechanical ventilation in mice.47
Other experimental studies suggest that iatrogenic hyperoxia — used for the treatment of patients with acute lung injury and shock like states — may suppress protective adenosine-signaling effects. One of us has noted that mice exposed to acute lung injury while breathing air with an oxygen concentration of 60 to 100% — thereby mimicking therapeutic oxygenation — have higher mortality than mice kept at sea level while breathing ambient air with an oxygen concentration of 21%. Inhalation of ADORA2A agonists compensates for the oxygenation-associated loss of generation of endogenous adenosine and preserves ADORA2A-mediated tissue protection while allowing the oxygenation of hypoxic tissues.82
PURINERGIC SIGNALING IN IBD
IBD is associated with excessive inflammation of the bowel, and purinergic signaling has been implicated in IBD. Intestinal inflammation is associated with a severe shift in metabolic supply and demand for oxygen, resulting in profound hypoxia of the inflamed mucosa.83 This metabolic alteration is associated with the post-translational stabilization of hypoxia-dependent transcription factors such as HIF — the key transcription factor for adaptation to hypoxia.84 Studies have shown that hypoxia signaling transcriptionally induces CD39 and CD73 during intestinal inflammation, thereby shifting the balance from ATP to adenosine signaling.16–18 Moreover, pharmacologic studies in mice suggest that HIF activators can attenuate intestinal inflammation85,86 and that this protection involves enhancement of extracellular adenosine production and signaling (Fig. 4).91
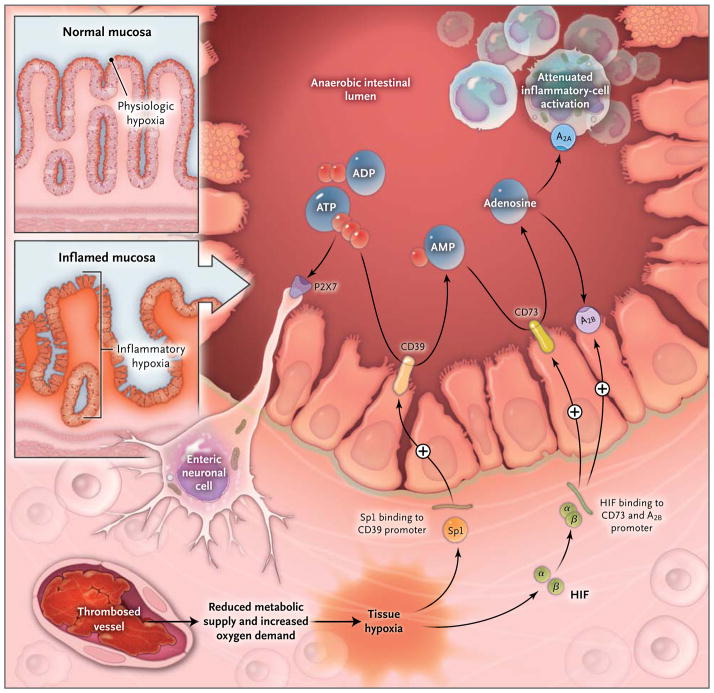
Figure 4. Hypoxia Control of Extracellular Adenosine Generation and Signaling in Intestinal Inflammation.
Histologic staining of intestinal sections for hypoxia shows that hypoxia is present within the apical surface of the intestinal mucosa (orange area in upper insert). This presence is most likely due to the fact that the intestinal lumen is anaerobic, which results in a steep oxygen gradient across the epithelial monolayer. In patients with intestinal inflammation such as that which occurs in the course of inflammatory bowel disease, a decrease in metabolic supply (e.g., due to thrombosed vessels) and profound increases in oxygen demand result in an imbalance in oxygen availability. This imbalance causes severe hypoxia of the inflamed mucosa, as indicated by histologic staining for tissue hypoxia (as shown in the lower insert, the orange staining that extends from the apical aspects of the mucosa into the crypts and submucosal tissues indicates severe tissue hypoxia).83 Release of ATP or ADP from inflammatory cells, platelets, or epithelial cells results in the activation of P2 receptors such as the P2X7 receptor, expressed on enteric neurons, thereby promoting tissue inflammation and injury.87 Hypoxia causes the activation of transcriptional programs that result in an Sp1-dependent induction of CD3917 and a hypoxia-inducible factor (HIF)–dependent induction of CD7325 and the ADORA2B (A2B) adenosine receptor.88 These transcriptional changes lead to an increased rate of turnover of the extracellular nucleotides ATP and ADP to AMP (through CD39) and subsequently to adenosine (through CD73). Experimental studies indicate that adenosine-receptor activation — particularly through Adora2a (A2A)89 and A2B 90 — dampens intestinal inflammation and promotes epithelial integrity during intestinal inflammation.
ATP signaling has been implicated in long-term gut dysmotility and enteric-nerve injury in IBD, findings that are consistent with a proinflammatory role of P2-receptor signaling in this disorder. P2X7, pannexin-1 channels, the Asc adapter protein, and caspases are all involved in the ATP-induced signaling pathways that drive enteric-nerve death during intestinal inflammation.87 In addition, regulatory T cells — a subset of CD4+ T lymphocytes that are critical in suppressing experimentally induced intestinal inflammation92 — require both CD39- and CD73-dependent production of extracellular adenosine for their suppressor functions in mouse models.93 In general, mice in which Cd3922 or Cd7394 has been knocked out have a more severe course of experimentally induced intestinal inflammation than do normal mice, a finding that is consistent with this notion. Adenosine-receptor signaling appears to have antiinflammatory and barrier-protective effects during experimentally induced intestinal inflammation, through adenosine signaling events involving Adora2a or Adora2b receptors. 89,90,95 Moreover, the antiinflammatory effects of sulfasalazine and methotrexate — both commonly used to treat IBD — involve the release of extracellular adenosine.27,28 Together, these findings highlight the therapeutic potential of pharmacologic strategies that shift the balance from proinflammatory activation of P2 receptors to antiinflammatory activation of adenosine receptors (particularly Adora2a and Adora2b)89,90 for the treatment of IBD. For example, such a shift can be achieved with the use of HIF activators, soluble forms of apyrase (which converts ATP and ADP to AMP) or ectonucleotidases (which convert AMP to adenosine), or adenosine-receptor agonists.
IMMUNOTHERAPY OF CANCER
Although studies have implicated purinergic signaling in the rate of cancer-cell growth,96 purinergic signaling has immunologic consequences in patients with neoplastic disease. Recent advances in cancer treatment have included adoptive T-cell therapy, pharmacologic use of antibodies, and boosting of innate immune responses, with encouraging results in lymphoproliferative disease and “immune-responsive tumors” such as renal-cell cancer and melanoma. However, effective immunotherapeutic targeting of other common solid cancers remains elusive.
Damage-associated molecular pattern molecules are released by injured tissue and by cancer cells to initiate adaptive and innate immune responses. These molecules include purines such as ATP, which mediates inflammasome activation75 and activation of dendritic cells, thereby potentiating tumor antigen presentation and tumor clearance. Enhancement of ATP-mediated effects on the immune system may constitute a new and effective means of inducing anticancer activity. Indeed, ATP conversion to adenosine is immunosuppressive. For example, a subset of type 17 helper T cells express CD39 and CD73, thereby leading to adenosine release, regulatory functions, and the subsequent suppression of CD4+ and CD8+ T-cell effector functions.97 T-cell signaling through ADORA2A generates immunosuppressive mechanisms, limiting tissue injury.45,98 In contrast, these processes preclude autoimmunity and provide protection for malignant tumors in hypoxic environments characterized by high levels of adenosine.99 On the basis of these observations, induction of ectonucleotidases and consequent enhancement of adenosine-dependent Adora2a signaling has been thought to play a role in the pathophysiological inhibition of T lymphocytes. For example, genetic deletion or pharmacologic inhibition of ADORA2A or CD39 strongly augments tumor immune injury and rejection by T cells.100,101 Strategies for manipulating the tumor microenvironment with adenosine-receptor antagonists or inhibition of ectonucleotidases warrant investigation as potential cancer treatment.
CONCLUSIONS
Purinergic signaling is an important regulatory mechanism in a wide range of inflammatory diseases. There are many instances in which signaling events initiated by adenosine P1 receptors and those initiated by nucleotide P2 receptors have opposing effects in biologic systems, and shifting the balance between purinergic P1 and P2 signaling is an emerging therapeutic concept in efforts to dampen pathologic inflammation and promote healing. Several drugs that affect purinergic signaling — such as adenosine, caffeine, clopidogrel, and dipyridamole — are already used in patients. Increasing developments in this arena will open up several new avenues for the treatment of patients with inflammatory diseases.
Acknowledgments
We thank Shelley A. Eltzschig for artwork during preparation of an earlier draft of the manuscript and our colleagues for their advice and help over the years.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Miller SL, Urey HC. Organic compound synthesis on the primitive earth. Science. 1959;130:245–51. doi: 10.1126/science.130.3370.245. [DOI] [PubMed] [Google Scholar]
- 2.Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–37. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Eckle T. Ischemia and reperfusion — from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35:445–55. doi: 10.1016/j.immuni.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103–7. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One. 2008;3(7):e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–8. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 9.Riegel AK, Faigle M, Zug S, et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117:2548–55. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiao CW, Tostes RC, Webb RC. P2X7 receptor activation amplifies lipopolysaccharide-induced vascular hyporeactivity via interleukin-1 beta release. J Pharmacol Exp Ther. 2008;326:864–70. doi: 10.1124/jpet.107.135350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idzko M, Hammad H, van Nimwegen M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–9. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 12.Wilhelm K, Ganesan J, Müller T, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434–8. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 13.Fernando SL, Saunders BM, Sluyter R, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med. 2007;175:360–6. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 14.Thunberg U, Tobin G, Johnson A, et al. Polymorphism in the P2X7 receptor gene and survival in chronic lymphocytic leukaemia. Lancet. 2002;360:1935–9. doi: 10.1016/S0140-6736(02)11917-9. [DOI] [PubMed] [Google Scholar]
- 15.Kornum BR, Kawashima M, Faraco J, et al. Common variants in P2RY11 are associated with narcolepsy. Nat Genet. 2011;43:66–71. doi: 10.1038/ng.734. [Erratum, Nat Genet 2011;43: 1040.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:153–75. doi: 10.1146/annurev-physiol-020911-153230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart ML, Gorzolla IC, Schittenhelm J, Robson SC, Eltzschig HK. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–24. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–32. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grenz A, Zhang H, Hermes M, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–73. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 20.Köhler D, Eckle T, Faigle M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–94. doi: 10.1161/CIRCULATIONAHA.107.690180. [Erratum, Circulation 2007;116(18):e514.] [DOI] [PubMed] [Google Scholar]
- 21.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–10. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 22.Friedman DJ, Kunzli BM, A-Rahim YI, et al. CD39 Deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–93. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Hilaire C, Ziegler SG, Markello TC, et al. NT5E mutations and arterial calcifications. N Engl J Med. 2011;364:432–42. doi: 10.1056/NEJMoa0912923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Thompson LF, Karhausen J, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–92. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 25.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson LF, Eltzschig HK, Ibla JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cronstein BN, Montesinos MC, Weissmann G. Salicylates and sulfasalazine, but not glucocorticoids, inhibit leukocyte accumulation by an adenosine-dependent mechanism that is independent of inhibition of prostaglandin synthesis and p105 of NFkappaB. Proc Natl Acad Sci U S A. 1999;96:6377–81. doi: 10.1073/pnas.96.11.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morabito L, Montesinos MC, Schreibman DM, et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest. 1998;101:295–300. doi: 10.1172/JCI1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reutershan J, Vollmer I, Stark S, Wagner R, Ngamsri KC, Eltzschig HK. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–82. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 30.Eckle T, Füllbier L, Wehrmann M, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;1(78):8127–37. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 31.Hart ML, Much C, Gorzolla IC, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135(5):1739.e3–1750.e3. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 32.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 33.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–7. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace KL, Linden J. Adenosine A2A receptors induced on iNKT and NK cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–20. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Day YJ, Toufektsian MC, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–7. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 36.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5(6):e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–35. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–82. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avni I, Garzozi HJ, Barequet IS, et al. Treatment of dry eye syndrome with orally administered CF101: data from a phase 2 clinical trial. Ophthalmology. 2010;117:1287–93. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–15. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors — an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grenz A, Bauerle JD, Dalton JH, et al. Equilibrative nucleoside transporter 1 (ENT1) regulates postischemic blood flow during acute kidney injury in mice. J Clin Invest. 2012;122:693–710. doi: 10.1172/JCI60214. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4(8):e6784. doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–20. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 46.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–82. doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–15. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckle T, Köhler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberger P, Schwab JM, Mirakaj V, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell CH, Peterson-Yantorno K, Carré DA, et al. A3 adenosine receptors regulate Cl-channels of nonpigmented ciliary epithelial cells. Am J Physiol. 1999;276:C659–C666. doi: 10.1152/ajpcell.1999.276.3.C659. [DOI] [PubMed] [Google Scholar]
- 51.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–18. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Löffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–13. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 53.Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–51. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dixon BS, Beck GJ, Vazquez MA, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eltzschig HK, Faigle M, Knapp S, et al. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–10. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–80. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 57.Spychala J, Mitchell BS. Cyclosporin A and FK506 decrease adenosine kinase activity and adenosine uptake in T-lymphocytes. J Lab Clin Med. 2002;140:84–91. doi: 10.1067/mlc.2002.125798. [DOI] [PubMed] [Google Scholar]
- 58.Blackburn MR, Volmer JB, Thrasher JL, et al. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med. 2000;192:159–70. doi: 10.1084/jem.192.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–58. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 60.Woulfe D, Yang J, Brass L. ADP and platelets: the end of the beginning. J Clin Invest. 2001;107:1503–5. doi: 10.1172/JCI13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grenegård M, Vretenbrant-Oberg K, Nylander M, et al. The ATP-gated P2X1 receptor plays a pivotal role in activation of aspirin-treated platelets by thrombin and epinephrine. J Biol Chem. 2008;283:18493–504. doi: 10.1074/jbc.M800358200. [DOI] [PubMed] [Google Scholar]
- 62.Goto S, Tamura N, Ishida H, Ruggeri ZM. Dependence of platelet thrombus stability on sustained glycoprotein IIb/IIIa activation through adenosine 5′-diphosphate receptor stimulation and cyclic calcium signaling. J Am Coll Cardiol. 2006;47:155–62. doi: 10.1016/j.jacc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 63.Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–8. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–7. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 65.Nurden P, Savi P, Heilmann E, et al. An inherited bleeding disorder linked to a defective interaction between ADP and its receptor on platelets: its influence on glycoprotein IIb-IIIa complex function. J Clin Invest. 1995;95:1612–22. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108:989–95. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 67.Fontana P, Gaussem P, Aiach M, Fiessinger JN, Emmerich J, Reny JL. P2Y12 H2 haplotype is associated with peripheral arterial disease: a case-control study. Circulation. 2003;108:2971–3. doi: 10.1161/01.CIR.0000106904.80795.35. [DOI] [PubMed] [Google Scholar]
- 68.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 69.Pinsky DJ, Broekman MJ, Peschon JJ, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109:1031–40. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hart ML, Köhler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK. Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 2008;28:1477–83. doi: 10.1161/ATVBAHA.108.169219. [DOI] [PubMed] [Google Scholar]
- 71.Ledent C, Vaugeois JM, Schiffmann SN, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–8. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- 72.Yang D, Chen H, Koupenova M, et al. A new role for the A2b adenosine receptor in regulating platelet function. J Thromb Haemost. 2010;8:817–27. doi: 10.1111/j.1538-7836.2010.03769.x. [DOI] [PubMed] [Google Scholar]
- 73.van Giezen JJ, Sidaway J, Glaves P, Kirk I, Bjorkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther. 2012;17:164–72. doi: 10.1177/1074248411410883. [DOI] [PubMed] [Google Scholar]
- 74.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [Erratum, Br Med Bull 2005;73–74:139.] [DOI] [PubMed] [Google Scholar]
- 75.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–6. doi: 10.1126/science.1195491. [Erratum, Science 2011;331:1517.] [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Corriden R, Inoue Y, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 77.Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxidants Redox Signal. 2011;15:2221–34. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–45. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 79.Hart ML, Henn M, Köhler D, et al. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–97. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–8. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 81.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 82.Thiel M, Chouker A, Ohta A, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3(6):e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–55. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyl-oxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–65. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Gulbransen BD, Bashashati M, Hirota SA, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–4. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–50. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 89.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–9. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 90.Frick JS, MacManus CF, Scully M, Glover LE, Eltzschig HK, Colgan SP. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–64. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK. Hypoxia-inducible factor-1α-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–74. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Groux H, O’Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 93.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Louis NA, Robinson AM, MacManus CF, Karhausen J, Scully M, Colgan SP. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–55. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 95.Aherne CM, Collins CB, Masterson JC, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61:695–705. doi: 10.1136/gutjnl-2011-300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–7. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 97.Chalmin F, Mignot G, Bruchard M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–73. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–82. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 99.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–21. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 100.Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun X, Wu Y, Gao W, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–40. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]