Abstract
Over the last decade, neuromuscular ultrasound has emerged as a useful tool for the diagnosis of peripheral nerve disorders. This article reviews sonographic findings of normal nerves including key quantitative ultrasound measurements that are helpful in the evaluation of focal and possibly generalized peripheral neuropathies. It also discusses several recent papers outlining the evidence base for the use of this technology, as well as new findings in compressive, traumatic, and generalized neuropathies. Ultrasound is well suited for use in electrodiagnostic laboratories where physicians, experienced in both the clinical evaluation of patients and the application of hands-on technology, can integrate findings from the patient’s history, physical examination, electrophysiological studies, and imaging for diagnosis and management.
Keywords: Ultrasound, Peripheral nerve, Carpal tunnel syndrome, Traumatic neuropathies, Ulnar neuropathies
Introduction
The history of ultrasound began with Spallanzani’s discovery that bats navigate in the dark through echolocation. Since then, ultrasound has transformed into an advanced imaging tool used throughout medicine, and Table 1 reviews the history of ultrasound, with a particular focus on its use in the field of neurology [1–9]. Use of ultrasound as a medical diagnostic tool began in the late 1940s and 1950s when doctors attempted to assess the heart and fetus, and the first reports of using ultrasound to evaluate peripheral nerve were published in the late 1980s [2, 10].
Table 1.
A short history of the development of ultrasound
| Year | Discovery | Study |
|---|---|---|
| 1794 | Bats navigate by echolocation | Spallanzani.* |
| 1877 | Discovery of piezoelectricity | Curie.* |
| First mathematical description of sound waves | Raleigh.* | |
| 1917 | Development of underwater sonar instrument | Langevin.* |
| 1930 | Therapeutic uses (thermal applications) of ultrasound | Wood, Harvey, Loomis.* |
| 1949 | Detection of gallstones and description of the mean speed of sound in the human tissue | Ludwig.* |
| 1953 | Cardiac ultrasound | Elder.* |
| 1959 | Imaging of fetal skull in utero | Donald.* |
| 1969 | First world congress on ultrasonic diagnostics in medicine | |
| 1980 | Real-time muscle ultrasound in DMD | Heckmatt et al. [1] |
| 1987 | Ultrasound of normal nerves | Fornage. [2] |
| 1991 | Description of median nerve in CTS | Buchberger et al. [3] |
| Ultrasound-assisted sciatic nerve block | Hullander et al. [4] | |
| 1992 | Dynamic ultrasound for median nerve movement | Nakamichi et al. [5] |
| 1999 | Ultrasound of demyelinating neuropathy | Heinemeyer et al. [6] |
| 2002 | Ultrasound of peroneal nerve | Pedrazzini et al. [7] |
| 2007 | Reference values for nerve size | Cartwright et al. [8] |
| 2008 | Swelling ratio measurements | Hobson-Webb et al. [9] |
| 2012 | First international congress of neuromuscular ultrasound |
http://www.ob-ultrasound.net/history1.html. Accessed June 2012. DMD; Duchenne Muscular Dystrophy, CTS; carpal tunnel syndrome
Over time, clinical experience with ultrasound and improvements in technology have made it helpful for the evaluation of peripheral nerve disease. In fact, developments in transducer materials, enhanced software, and the emergence of transducers of 15 MHz and higher have made it possible to routinely image small sensory nerves such as the digital nerves in the fingers or the palmar cutaneous branch of the median nerve. Moreover, improvements in Doppler sensitivity and power Doppler have made it possible to assess vascular changes within major nerve segments.
Ultrasound addresses two key limitations of electrodiagnostic testing, the inability to provide anatomic detail and discomfort. Ultrasound is painless, is among the least invasive methods of medical diagnostic testing, and provides a view of the anatomy of nerve as well as of surrounding structures. While magnetic resonance imaging offers superior differentiation of imaged tissues, it has lower edge-to-edge resolution and is neither portable nor inexpensive. Like electrodiagnosis, ultrasound provides an assessment of focal areas of individual nerves in real-time, making it an attractive companion tool.
This review will first focus on some of the basic elements of nerve imaging, and then address some of the more recent studies that are advancing the technique.
Ultrasonography of Normal Nerves
Although visualization of some nerves is still limited, high-resolution ultrasound has made it possible to image almost all the main peripheral nerves on a routine basis [11]. Virtually all segments of the median, ulnar, and radial nerves of the upper extremity and many segments of the femoral, sciatic, peroneal (fibular), and tibial nerves of the lower extremity can be imaged with instruments at a cost similar to the equipment found in most electrodiagnostic laboratories. Ultrasound can assess the trunks of the brachial plexus in the area between the spine and clavicle, and can image some portions of it between the clavicle and axilla. The axillary and musculocutaneous nerves can be imaged in the upper extremity along portions of their courses. Although their smaller branches are difficult to image, the sural, saphenous, lateral femoral cutaneous, and even digital nerves can be assessed. Of the cranial nerves, the optic, facial, vagus, and spinal accessory nerves can be imaged at some points along their courses. In cases of pathological conditions with enlargement of the nerve, ultrasound can even evaluate small nerves that are usually very difficult to visualize, such as the dorsal scapular nerve off the upper trunk of the brachial plexus.
Distinguishing nerves from other tissues
Nerves are cable-like structures and have a distinct architecture consisting of fascicles and surrounding epineurium (Figure). In the transverse plane, the echo pattern is described as a “honeycomb” in appearance because of the dark punctuate areas (fascicle groups) distributed throughout a hyperechoic background (perineurium) [12]. In the longitudinal plane, nerves present as long, slim structures with a mixture of parallel hypoechoic and hyperechoic lines.
Figure 1.
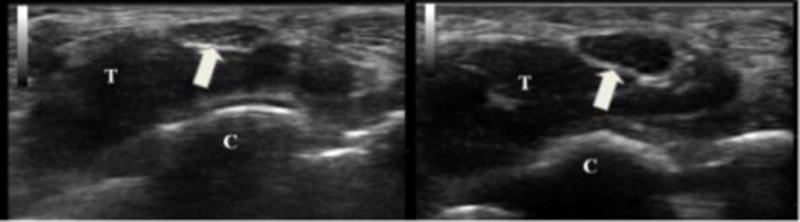
On the left is a cross-sectional view of the median nerve (arrow) at the distal wrist crease in a healthy volunteer. The cross-sectional area of the nerve is 9 mm2 and there is normal nerve echogenicity. On the right is the same view from an individual with carpal tunnel syndrome. The nerve is 19 mm2 and very hypoechoic. T = flexor tendons and C = carpal bones.
Nerves must be distinguished from other nearby structures to ensure correct identification [13]. Muscles have a unique architectural pattern; they are hypoechoic and interspersed with small hyperechoic foci corresponding to the supporting fibrous tissue, which easily distinguishes muscles from nerves. Tendons, which are sometimes adjacent to nerves, move proportionately with joint movement, and have greater anisotropy than do nerves. Anisotropy refers to the sound reflection properties of tissue; tissues with low anisotropy tend to backscatter sound reflection, and those with high anisotropy tend to reflect sound such that the angle of incidence equals the angle of reflection, making them much brighter with perpendicular insonation. Thus, subtle shifts in the angle of insonation do not alter the appearance of a nerve in the way that they alter the appearance of tendons. Also, tendons are more homogenously hyperechoic than nerves and have a distinctive fibrillar composition. Additionally, tendons, if followed proximally or distally, lead to muscle.
Differentiating nerves from vascular structures is relatively straightforward. Arteries pulsate, a phenomenon enhanced with slight probe pressure. With sufficient force arteries collapse; veins do not pulsate, and collapse with less force than arteries. Color Doppler imaging can be useful for detecting vessels and differentiating between nerves and vessels.
Identifying specific nerves
Knowledge of regional anatomy and topography is needed for the sonographic assessment of peripheral nerves. The following anatomical landmarks are useful: the brachial artery for the median nerve in the upper arm; the superficial and deep flexor muscles for the median nerve in the forearm; the contents of the carpal tunnel for the median nerve at the wrist; the ulnar artery for the ulnar nerve at the wrist; the medial epicondyle for the ulnar nerve at the elbow; the radial groove for the radial nerve; the anterior and middle scalene muscles and the proximal subclavian artery for the brachial plexus; popliteal artery for the distal sciatic nerve; fibular head for the fibular nerve; and the posterior tibial artery for the tibial nerve at the ankle. Once identified at any site, the nerves can be traced continuously in the proximal and distal directions. Although all major nerves are found easily and visualized according to these landmarks, knowledge about the entire courses of different nerves and important surrounding structures is helpful for more accurate assessment, especially for smaller or less commonly imaged nerves and individuals with anatomic variants or tissues altered by trauma or surgery.
Ultrasound measurements
Nerve size
Because nerve enlargement is the most important diagnostic marker of an abnormal nerve, quantification of nerve size is essential [14]. Cross-sectional area and swelling ratio can be measured on transverse images, and diameter can be measured on longitudinal images. For correct measurement, the transducer should be held perpendicular to the nerve, with minimal pressure. Although the site of maximal enlargement should be selected for measurement of nerve size, the minimum cross-sectional area at that site must be found to ensure perpendicular scanning. Variability within a measurement can be reduced by using an average of multiple measures. Measuring just inside the echogenic rim of the nerve is the preferred technique [15, 16•]. For the ulnar nerve, an elbow position between extension and 90-degree flexion has been recommended because elbow position with flexion of more than 90 degrees can decrease cross-sectional area [16•].
The swelling ratio is the ratio between the cross-sectional area of the nerve at the site of maximal enlargement and that at an unaffected site. This measurement may improve diagnostic accuracy for entrapment neuropathy in those with polyneuropathy, which can cause diffuse nerve enlargement [17]. The swelling ratio may also correct for the normal variation of nerve cross-section area among individuals.
Another potentially useful parameter for evaluating focal nerve compression is the flattening ratio, which is defined as the ratio between the largest and smallest diameters of nerves. This can be a useful parameter for entrapment neuropathy because an affected nerve sometimes shows a change from a round or triangular shape to a significantly flattened oval at the site of compression. The absolute value of the cross-sectional area remains one of the most reliable and frequently used methods for the diagnosis of focal neuropathy [15], but the other methods offer advantages in situations in which established reference values are unavailable or not applicable.
Vascularity
Vascularity is assessed by placing the power Doppler box over the nerve and slowly increasing the gain. No color Doppler signal will be observed in the normal nerve. At the highest levels of gain, non-specific color signal (noise) may flash unpredictably in tissue, but only focal color flow signals synchronized with the arterial pulsation are interpreted as evidence of blood flow within nerve tissue. Vascular congestion, presumably due to compression or inflammatory response in various neuropathies, can cause increased intraneural blood flow. Color flow in the nerve indicates hypervascularity, which at this time is graded somewhat subjectively. A recent study introduced an image-processing method for quantifying the degree of intraneural vascularity and found that the degree of measured intraneural vascularity correlated highly with the severity of the condition, according to electrodiagnostic testing [18].
Echogenicity
As mentioned above, normal nerve echogenicity is characterized by a honeycomb pattern with a mixture of dark fascicles interspersed amongst a bright background. In pathological conditions, increased intraneural edema may result in reduced enchogenicity and loss of the fascicular pattern. Similar to vascularity, echogenicity is usually assessed subjectively based on visual inspection of the image. However, subjective judgment can introduce error and bias [19], sometimes related to the relative echointensity of other structures within the image. Some investigators have attempted to assess nerve echogenicity in a quantitative manner by using different automatic methods and have shown the superiority of these methods over subjective judgments [19–21]. Finally, another aspect of echotexture change is the presence of selective enlargement of individual fascicles in some patients, a finding present in some forms of Charcot-Marie-Tooth and in certain nerve tumors [22, 23].
Mobility
Nerve mobility can be routinely assessed for the median nerve at the carpal tunnel and the ulnar nerve at the elbow, but may be of importance in other nerve entrapments as well. To assess median nerve mobility, individuals are asked to simultaneously flex their fingers and wrist while a transducer is positioned over the distal wrist crease. Normal mobility is seen when the median nerve dives deep to the flexor tendons during finger and wrist flexion [24], is reduced when the nerve makes a partial turn but does not dive below the tendons, and is absent when virtually no movement is seen. Although studied less often, the median nerve also slides slightly in the distal and proximal direction with finger flexion and extension [25]. Nerve mobility is reduced in carpal tunnel syndrome (CTS), but it is unknown if this is the result of enlargement of the median nerve or if this reflects anatomic changes that predispose to CTS.
Unlike the median nerve at the wrist, which is confined in location by the carpal tunnel, the ulnar nerve at the elbow can be displaced across the medial epicondyle during elbow flexion. Subluxation is defined as movement of the ulnar nerve to the tip of the medial epicondyle, and dislocation is defined as complete relocation of the ulnar nerve over the medial condyle. The occurrence of subluxation and dislocation is not uncommon in healthy controls (24.3–47%) [16•, 26]. Of interest, the mean cross-sectional area of ulnar nerves that displaced was larger than that of the nerves which held their position [26]. This finding suggests that nerves with repetitive displacement may have a higher risk of microtrauma and development of edema, but the relationship to ulnar neuropathy at the elbow has yet to be demonstrated convincingly.
Compressive neuropathy
Compressive neuropathy is often diagnosed clinically and confirmed with electrodiagnosis. Although sensitive and specific for diagnosing compressive neuropathy, electrodiagnosis also has limitations in that it is uncomfortable and does not directly assess the anatomy of the affected nerve and surrounding structures. The majority of potential sites of compressive neuropathy are well visualized by ultrasound with established reference values for nerve cross-sectional area [8, 27, 28].
Of the many peripheral nerve disorders, ultrasound has been studied most in compressive neuropathy (Table 2), particularly CTS and ulnar neuropathy at the elbow. Fewer studies are available on other nerves, but the findings are quite similar in any affected nerve.
Table 2.
Nerve pathology investigated with ultrasound
| Systematic Studies | Case Series and Reports |
|---|---|
| Carpal tunnel syndrome | Median neuropathy outside the wrist |
| Ulnar neuropathy at the elbow | Ulnar neuropathy at the wrist |
| Fibular neuropathy at the knee | Radial neuropathy at spiral groove |
| Intraneural and extraneural cysts | Posterior interosseous neuropathy |
| Traumatic neuropathy | Anterior interosseous neuropathy |
| Multi-focal motor neuropathy | Lateral femoral cutaneous neuropathy |
| Charcot-Marie-Tooth | Tibial neuropathy at the ankle |
| Leprosy | Sciatic neuropathy |
| Nerve sheath tumors | |
| CIDP and Guillain-Barre Syndrome |
CIDP; chronic inflammatory demyelinating polyneuropathy
Abnormal sonographic findings associated with compressive neuropathy include enlargement of the nerve proximal to the site of compression, decreased echogenicity, and increased vascularity. The mechanism underlying these findings remains unclear. In a somewhat paradoxical fashion, nerve enlargement results from a chronic increase in pressure on the nerve. This leads to both venous stasis and damming of axoplasmic flow [29]. Experimental animal studies have shown proximal nerve enlargement following chronic nerve compression along with inflammation, demyelination, remyelination, fibrosis, and thickening of the connective tissues [30, 31]. Of interest, in CTS, a recent study has shown a significant decrease in the enlarged nerve cross-sectional area within the first week after local steroid injection, suggesting that at least some of the enlargement is related to rapidly reversible causes such as inflammation or edema [32]. A coincident reduction in nerve hypervascularity was seen in these patients providing evidence that edema and perhaps increased static blood volume are at least in part responsible for nerve enlargement in chronic compression, and may also account for some of the decreased echogenicity in nerves, as fluid and blood tend to be hypoechoic on ultrasound.
Nerve enlargement has been the main sonographic parameter for diagnosing compressive neuropathy in part because it is simple and easy to measure. Recently, intraneural vascularity has been proposed as an additional diagnostic parameter in patients with CTS with several studies showing its usefulness. Evidence indicating that measuring both cross-sectional area and hypervascularity can increase sensitivity and specificity compared with measurements of cross-sectional area alone has been reported [33–37]. Hypervascularity may also play a role in the diagnosis of early stage compressive neuropathy because it is the only abnormality in some patients with mild CTS [34, 35]. Further research in this area should be informative..
Carpal tunnel syndrome (CTS) – evidence for ultrasound
The most sensitive diagnostic marker for symptomatic CTS patients is an enlarged cross-sectional area of the median nerve proximal to the edge of the flexor retinaculum [38]. This enlargement is sometimes accompanied by hypoechogenicity, reduced motility, or increased vascularity. To assess the diagnostic usefulness of the ultrasound, a number of studies have compared the accuracy of ultrasound. A meta-analysis found that the sensitivity and specificity of ultrasound in the diagnosis of CTS were 77.6% and 86.8%, respectively [39]. There are only a few studies directly comparing the specificity and sensitivity of ultrasound and electrodiagnostic studies, and there have been mixed results, but the trend has been that nerve conduction studies are more accurate. However, the values reported in this meta-analysis suggest that ultrasound is a potential alternative to nerve conduction studies in diagnosing CTS.
Ultrasound findings may also provide information about the severity of CTS [40–43]. A significant correlation between nerve cross-sectional area and all conventional measures of CTS severity, including clinical scales, hand function, and neurophysiological classification, has been found [43] in some but not all studies. Of interest, a recent study reported that hypervascularity, detected by ultrasound, correlated with the severity of nerve damage, assessed by neurophysiology [36, 37].
Another value of ultrasound is that it can detect causes of median neuropathy at the wrist not apparent by electrodiagnostic testing. Most CTS is idiopathic or associated with systemic causes such as diabetes mellitus, hypothyroidism, or obesity [44, 45]. In a minority of cases, other causes of focal median neuropathy such as space-occupying lesions or structural abnormalities are responsible [46]. Screening for structural abnormalities as causes of CTS is likely to be of higher yield in those with atypical symptoms, purely unilateral, or abrupt-onset findings [47].
Anatomical variants, including those bifid median nerve and persistent median artery, have received recent attention because they are now readily apparent through the use of ultrasound. A bifid median nerve may be an independent risk factor for the development of CTS because it has a relatively higher cross-sectional area than a nonbifid median nerve. However, findings in this regard have been inconsistent [48, 49]; one study showed that a bifid median nerve was frequently found in patients with CTS, whereas another did not. The presence of a persistent median artery or its acute thrombosis and dilatation, may result in CTS [50, 51]. Pre-operative awareness of this variant may also be helpful for determining the best surgical approach and preventing potential complications.
The most robust findings on the use of neuromuscular ultrasound in CTS come from a recently published evidence-based guideline [52••]. There is now a level A recommendation, based on four Class 1 studies, that ultrasonographic measurement of median nerve cross-sectional area at the wrist is accurate and may be offered as a diagnostic test for CTS. In addition, there is a level B recommendation that ultrasound should be considered for screening for structural abnormalities in those with CTS.
The usefulness of sonography for follow-up of carpal tunnel release has also been studied. Significant decreases in cross-sectional area have been noted, and decreased cross-sectional area has been correlated with improvement in symptom scores and electrodiagnostic parameters [53–56]. However, another study found no significant difference in cross-sectional area between patients with favorable and unfavorable outcomes, although cross-sectional area was decreased in both groups [57]. Additional investigations into the reliability of sonographic parameters for predicting outcomes are needed. Postoperative ultrasound can be helpful for identifying the causes of treatment failure in patients who do not improve [56]. Incomplete carpal tunnel release is uncommon but results in persistent symptoms and requires surgical revision [58]. In some cases ultrasound has shown portions of ligaments or fascia that were not released, as well as focal narrowing of the median nerve.
Ulnar neuropathy at the elbow
Following CTS, ulnar neuropathy at the elbow is the second most common compressive neuropathy. However, the sensitivity of electrodiagnostic testing for ulnar neuropathy at the elbow is much lower than it is for CTS [59, 60]. Reasons for false negatives may include improper elbow position and ulnar nerve dislocation resulting in inaccurate nerve-length measurements.
Increased cross-sectional area of the ulnar nerve segment around the elbow (the segment from 4 cm proximal to 4 cm distal to the medial epicondyle) is the most common finding in ulnar neuropathy at the elbow. The sensitivity of an increased cross-sectional area or diameter of the ulnar nerve at the elbow is more than 80% in ulnar neuropathy at the elbow [16•]. Furthermore, ultrasound can sometimes identify the site of entrapment in patients suspected to have ulnar neuropathy at the elbow who present with normal electrodiagnostic testing findings [61]. One study reported that the diagnostic sensitivity of combining electrodiagnosis and ultrasound was 98%, significantly higher than that (78%) of electrodiagnostic testing alone [60]. Ultrasound also can increase diagnostic sensitivity by localizing the lesion to a particular site in patients with non-localizing electrodiagnostic findings [60, 62]. In addition to diagnosis, ultrasound may play a role in severity classification. Cross-sectional area is highly correlated with the severity score obtained via electrodiagnosis [63–65]. As with CTS, ulnar neuropathy at the elbow may be associated with underlying abnormalities that are detectable by static or real-time ultrasound imaging such as subluxation or dislocation of nerves, snapping of the medial head of the triceps, ganglia, osteophytes, or tumors.
Traumatic neuropathy
Traumatic peripheral nerve injuries are not uncommon in clinical practice and electrodiagnosis has been a main tool for their diagnosis and assessment. Determination of the degree and type of nerve damage involved in traumatic neuropathy is important because treatment for different degrees and types can differ. Surgical intervention is necessary in cases of severe nerve injuries (Sunderland classification 5, neurotmesis) and may be required in cases with Sunderland classifications 3 and 4 [66]. Electrodiagnosis however, has a limited ability to determine the precise location and severity of nerve injuries in some settings, particularly in the immediate post-injury period because it takes time for certain electrodiagnostic changes to occur. Ultrasound may be valuable for visualizing features of traumatic nerve injuries, such as discontinuity of the nerve, neuroma, bone callus, bone fragments, foreign bodies, and scar tissue. In cadaveric nerves ultrasound can detect transaction with 89% sensitivity and 95% specificity [67]. One recent study assessed the value of ultrasound for determining the type of traumatic peripheral nerve injury [68•]. Using ultrasound, the authors classified the severity of injuries into seven types and compared their results with surgical findings, and the accuracy of classification via ultrasound was 93.2%. Although ultrasound did not detect abnormal findings in some patients (6%) and resulted in rare misclassification of severity (6.8%), the technique is of value [68•].
Dynamic ultrasound imaging may help in decisions regarding early surgical intervention in trauma cases, even if the nerve is difficult to visualize at the precise site of injury. For example, the maintenance of mobility with flexion and extension of a nearby joint can provide evidence of preserved continuity of the nerve [69].
In a more generic sense, ultrasound can contribute to assessment of focal (compressive and traumatic) neuropathy in a clinical neurophysiology laboratory [70••]. In 42.3% of patients referred for suspected focal neuropathy in this recent study, ultrasound strongly modified the diagnostic and therapeutic path chosen. For example, ultrasound allowed determination of an exact diagnosis in patients with normal electrodiagnostic findings and in patients who refused electrodiagnostic testing, and ultrasound added information useful for therapeutic decisions. In another 40% of patients, ultrasound confirmed the electrodiagnostic findings. Although ultrasound was unable to show abnormalities in 17.7% of patients, there were no cases with incorrect ultrasound findings.
New diagnostic areas
Ultrasound has recently demonstrated an increase in nerve cross-sectional area in certain generalized polyneuropathies, including multifocal motor neuropathy, Charcot–Marie–Tooth disease, diabetes, vasculitis, acromegaly, and chronic inflammatory demyelinating polyneuropathy [20, 71–73]. Enlargement tends to be greater in demyelinating than axonal neuropathies [71]. Of interest, reduction in nerve cross-sectional area may occur in motor nerves in amyotrophic lateral sclerosis and in sensory nerves in post-herpetic neuralgia [74, 75].
Another evolving area relates to directly combining ultrasound and electrodiagnosis. One approach has evaluated the benefits of ultrasound in improving sensory nerve conduction studies in areas of variable nerve anatomy. For example, nerve conduction studies of the lateral femoral cutaneous nerve are helpful in the diagnosis of meralgia paresthetica and can assist in the differentiation of a patient with lumbar radiculopathy versus one with lumbar plexopathy. However, it has been difficult to reliably test this nerve with routine conduction studies. Ultrasound effectively localizes the lateral femoral cutaneous nerve from the inguinal ligament to the upper thigh and can be used to more reliably obtain sensory nerve action potentials [76]. Similarly, ultrasound can help placement of near-nerve electrodes for recording sural sensory responses [77, 78]. Furthermore, ultrasound improves signal to noise ratio when electrodes are placed subdermally for recording sural sensory responses.
Of particular interest, ultrasound has proven useful in evaluating lepromatous neuropathy, a complication of a disease with considerable world-wide prevalence and morbidity. Indeed, the early detection of this disorder is important for preventing life-long disability. A hallmark of lepromatous neuropathy is nerve enlargement, which is sometimes palpable, and inflammation. These characteristics can be assessed by ultrasound and are detected as multiple enlarged and hypervascular nerves [79–81]. One study has suggested the usefulness of hypervascularity for detecting leprosy neuropathy because color Doppler flow signal was observed in all nerves that were hypoechoic and had lost their fascicular pattern [80]. In addition, it has been shown that ultrasound can detect subclinical involvement of the ulnar nerve and abnormalities in patients with normal electrodiagnostic findings [80, 81]. Because of the difficulty of providing sophisticated electrodiagnostic testing to those affected by leprosy, the increasingly widespread availability of ultrasound, and the fact that leprosy and lepromatous neuropathy are treatable, ultrasound may prove to be a particularly valuable tool for both diagnosis and treatment of this ancient scourge.
Conclusion
At this time, ultrasound has proven its usefulness for the diagnosis of compressive neuropathy in CTS, and there is evidence that supports its usefulness in ulnar neuropathy at the elbow. The role of ultrasound in detecting other peripheral neuropathies has been expanding, and now includes other entrapment neuropathies, trauma, and potentially, certain polyneuropathies. With these advances, ultrasound offers a tool that can access pathology in nerves and surrounding structures and supplement electrodiagnostic testing. It is said that electrodiagnosis is an extension of the neurological examination and in like manner, ultrasound is now being used with effect when guided by an examiner’s knowledge of the patient’s history, physical examination, and electrodiagnostic findings. Given its increasing availability and ability to perform rapid non-invasive testing, ultrasound imaging will soon qualify as another bedside technique that extends the reach of the neurological examination.
Acknowledgments
Dr. Cartwright has a grant to study neuromuscular ultrasound from the NIH/NINDS (1K23NS062892).
Footnotes
Disclosure
No potential conflicts of interest relevant to this article were reported.
Papers of particular interest, published recently, have been highlighted as:
Of importance
Of outstanding importance
Contributor Information
Jung Im Suk, Assistant Professor of Neurology, School of Medicine, Catholic University of Daegu, 3056-6, Daemyeong-4-dong, Nam-gu, Daegu, South Korea, jihelpgod@cu.ac.kr.
Francis O. Walker, Professor of Neurology, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Michael S. Cartwright, Associate Professor of Neurology, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA, mcartwri@wakehealth.edu.
References
- 1.Heckmatt JZ, Dubowitz V, Leeman S. Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet. 1980;1:1389–1390. doi: 10.1016/s0140-6736(80)92656-2. [DOI] [PubMed] [Google Scholar]
- 2.Fornage BD. Peripheral nerves of the extremities: imaging with US. Radiology. 1988;167:179–182. doi: 10.1148/radiology.167.1.3279453. [DOI] [PubMed] [Google Scholar]
- 3.Buchberger W, Schön G, Strasser K, Jungwirth W. High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med. 1991;10:531–537. doi: 10.7863/jum.1991.10.10.531. [DOI] [PubMed] [Google Scholar]
- 4.Hullander M, Spillane W, Leivers D, Balsara Z. The use of Doppler ultrasound to assist with sciatic nerve blocks. Reg Anesth. 1991;16:282–284. [PubMed] [Google Scholar]
- 5.Nakamichi K, Tachibana S. Transverse sliding of the median nerve beneath the flexor retinaculum. J Hand Surg Br. 1992;17:213–216. doi: 10.1016/0266-7681(92)90092-g. [DOI] [PubMed] [Google Scholar]
- 6.Heinemeyer O, Reimers CD. Ultrasound of radial, ulnar, median, and sciatic nerves in healthy subjects and patients with hereditary motor and sensory neuropathies. Ultrasound Med Biol. 1999;25:481–485. doi: 10.1016/s0301-5629(98)00187-2. [DOI] [PubMed] [Google Scholar]
- 7.Pedrazzini M, Pogliacomi F, Cusmano F, et al. Bilateral ganglion cyst of the common peroneal nerve. Eur Radiol. 2002;12:2803–2806. doi: 10.1007/s00330-002-1322-5. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright MS, Shin HW, Passmore LV, Walker FO. Ultrasonographic findings of the normal ulnar nerve in adults. Arch Phys Med Rehabil. 2007;88:394–396. doi: 10.1016/j.apmr.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Hobson-Webb LD, Massey JM, Juel VC, Sanders DB. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:1353–1357. doi: 10.1016/j.clinph.2008.01.101. [DOI] [PubMed] [Google Scholar]
- 10.Solbiati L, De Pra L, Ierace T, et al. High-resolution sonography of the recurrent laryngeal nerve: anatomic and pathologic considerations. AJR Am J Roentgenol. 1985;145:989–993. doi: 10.2214/ajr.145.5.989. [DOI] [PubMed] [Google Scholar]
- 11.Gruber H, Kovacs P. Sonographic anatomy of the peripheral nervous system. In: Peer S, Bodner G, editors. High-resolution sonography of the peripheral nervous system. Springer; Berlin, Germany: 2003. pp. 13–36. [Google Scholar]
- 12.Silvestri E, Martinoli C, Derchi LE, et al. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197:291–296. doi: 10.1148/radiology.197.1.7568840. [DOI] [PubMed] [Google Scholar]
- 13.Walker FO. Neuromuscular ultrasound. Neurol Clin. 2004;22:563–590. doi: 10.1016/j.ncl.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;173:681–684. doi: 10.2214/ajr.173.3.10470903. [DOI] [PubMed] [Google Scholar]
- 15.Roll SC, Case-Smith J, Evans KD. Diagnostic accuracy of ultrasonography vs. electromyography in carpal tunnel syndrome: a systematic review of literature. Ultrasound Med Biol. 2011;37:1539–1553. doi: 10.1016/j.ultrasmedbio.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 16•.Beekman R, Visser LH, Verhagen WI. Ultrasonography in ulnar neuropathy at the elbow: a critical review. Muscle Nerve. 2011;43:627–635. doi: 10.1002/mus.22019. The authors reviewed all clinical reports of ultrasound diagnosis of ulnar neuropathy at the elbow, and found 7 of 14 papers that were of high quality. Methodologic issues were identified in many of these papers, and although the findings supported the accuracy of ultrasound, a definitive conclusion was not reached. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JS, Walker FO, Cartwright MS. Ultrasonographic swelling ratio in the diagnosis of ulnar neuropathy at the elbow. Muscle Nerve. 2008;38:1231–1235. doi: 10.1002/mus.21094. [DOI] [PubMed] [Google Scholar]
- 18.Ghasemi-Esfe AR, Khalilzadeh O, Vaziri-Bozorg SM, et al. Color and power Doppler US for diagnosing carpal tunnel syndrome and determining its severity: a quantitative image processing method. Radiology. 2011;261:499–506. doi: 10.1148/radiol.11110150. [DOI] [PubMed] [Google Scholar]
- 19.Boom J, Visser LH. Quantitative assessment of nerve echogenicity: Comparison of methods for evaluating nerve echogenicity in ulnar neuropathy at the elbow. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2011.10.050. In press. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Ito H, Sekine A, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med. 2010;29:697–708. doi: 10.7863/jum.2010.29.5.697. [DOI] [PubMed] [Google Scholar]
- 21.Tagliafico A, Tagliafico G, Martinoli C. Nerve density: a new parameter to evaluate peripheral nerve pathology on ultrasound. Preliminary study. Ultrasound Med Biol. 2010;36:1588–1593. doi: 10.1016/j.ultrasmedbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Martinoli C, Schenone A, Bianchi S, et al. Sonography of the median nerve in Charcot-Marie-Tooth disease. AJR Am J Roentgenol. 2002;178:1553–1556. doi: 10.2214/ajr.178.6.1781553. [DOI] [PubMed] [Google Scholar]
- 23.Gruber H, Glodny B, Bendix N, et al. High-resolution ultrasound of peripheral neurogenic tumors. Eur Radiol. 2007;17:2880–2888. doi: 10.1007/s00330-007-0645-7. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh YH, Shih JT, Lee HM, Ho YJ. Ultrasonography of median nerve mobility in the diagnosis of carpal tunnel syndrome. Formos J Musculoskelet Disord. 2010;1:16–19. [Google Scholar]
- 25.Alexander A. Scientific study of the extent of transverse movement of the median nerve at the wrist during active wrist extension in static positions of the upper limb tension test one. Hand Ther. 2012 Mar;17:2–10. [Google Scholar]
- 26.Ozturk E, Sonmez G, Colak A, et al. Sonographic appearances of the normal ulnar nerve in the cubital tunnel. J Clin Ultrasound. 2008;36:325–329. doi: 10.1002/jcu.20486. [DOI] [PubMed] [Google Scholar]
- 27.Wiesler ER, Chloros GD, Cartwright MS, et al. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31:726–732. doi: 10.1016/j.jhsa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Cartwright MS, Passmore LV, Yoon JS, et al. Cross-sectional area reference values for nerve ultrasonography. Muscle Nerve. 2008;37:566–571. doi: 10.1002/mus.21009. [DOI] [PubMed] [Google Scholar]
- 29.Rempel D, Dahlin L, Lundborg G. Pathophysiology of nerve compression syndromes: response of peripheral nerves to loading. J Bone Joint Surg Am. 1999;81:1600–1610. doi: 10.2106/00004623-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Powell HC, Myers RR. Pathology of experimental nerve compression. Lab Invest. 1986;55:91–100. [PubMed] [Google Scholar]
- 31.Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome”. J Neurol Neurosurg Psychiatry. 1983;46:1119–1124. doi: 10.1136/jnnp.46.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cartwright MS, White DL, Demar S, et al. Median nerve changes following steroid injection for carpal tunnel syndrome. Muscle Nerve. 2011;44:25–29. doi: 10.1002/mus.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghasemi-Esfe AR, Khalilzadeh O, Mazloumi M, et al. Combination of high-resolution and color Doppler ultrasound in diagnosis of carpal tunnel syndrome. Acta Radiol. 2011;52:191–197. doi: 10.1258/ar.2010.100299. [DOI] [PubMed] [Google Scholar]
- 34.Mallouhi A, Pülzl P, Trieb T, et al. Predictors of carpal tunnel syndrome: accuracy of gray-scale and color Doppler sonography. AJR Am J Roentgenol. 2006;186:1240–1245. doi: 10.2214/AJR.04.1715. [DOI] [PubMed] [Google Scholar]
- 35.Joy V, Therimadasamy AK, Chan YC, Wilder-Smith EP. Combined Doppler and B-mode sonography in carpal tunnel syndrome. J Neurol Sci. 2011;308:16–20. doi: 10.1016/j.jns.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 36.Mohammadi A, Ghasemi-Rad M, Mladkova-Suchy N, Ansari S. Correlation between the severity of carpal tunnel syndrome and color Doppler sonography findings. AJR Am J Roentgenol. 2012;198:W181–184. doi: 10.2214/AJR.11.7012. [DOI] [PubMed] [Google Scholar]
- 37.Evans KD, Roll SC, Volz KR, Freimer M. Relationship between intraneural vascular flow measured with sonography and carpal tunnel syndrome diagnosis based on electrodiagnostic testing. J Ultrasound Med. 2012;31:729–736. doi: 10.7863/jum.2012.31.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peer S, Kiechl S, Bodner G. Nerve compression syndrome. In: Peer S, Bodner G, editors. High-resolution sonography of the peripheral nervous system. Berlin, Germany: Springer; 2003. pp. 48–54. [Google Scholar]
- 39.Fowler JR, Gaughan JP, Ilyas AM. The sensitivity and specificity of ultrasound for the diagnosis of carpal tunnel syndrome: a meta-analysis. Clin Orthop Relat Res. 2011;469:1089–1094. doi: 10.1007/s11999-010-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karadağ YS, Karadağ O, Ciçekli E, et al. Severity of Carpal tunnel syndrome assessed with high frequency ultrasonography. Rheumatol Int. 2010;30:761–765. doi: 10.1007/s00296-009-1061-x. [DOI] [PubMed] [Google Scholar]
- 41.Bayrak IK, Bayrak AO, Tilki HE, et al. Ultrasonography in carpal tunnel syndrome: comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007;35:344–348. doi: 10.1002/mus.20698. [DOI] [PubMed] [Google Scholar]
- 42.Lee CH, Kim TK, Yoon ES, Dhong ES. Correlation of high-resolution ultrasonographic findings with the clinical symptoms and electrodiagnostic data in carpal tunnel syndrome. Ann Plast Surg. 2005;54:20–23. doi: 10.1097/01.sap.0000141942.27182.55. [DOI] [PubMed] [Google Scholar]
- 43.Padua L, Pazzaglia C, Caliandro P, et al. Carpal tunnel syndrome: ultrasound, neurophysiology, clinical and patient-oriented assessment. Clin Neurophysiol. 2008;119:2064–2069. doi: 10.1016/j.clinph.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Aroori S, Spence RAJ. Carpal tunnel syndrome. Ulster Med J. 2008;77:6–17. [PMC free article] [PubMed] [Google Scholar]
- 45.Bland JD. Carpal tunnel syndrome. Curr Opin Neurol. 2005;18:581–585. doi: 10.1097/01.wco.0000173142.58068.5a. [DOI] [PubMed] [Google Scholar]
- 46.Kang HJ, Jung SH, Yoon HK, et al. Carpal tunnel syndrome caused by space occupying lesions. Yonsei Med J. 2009;50:257–261. doi: 10.3349/ymj.2009.50.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamichi K, Tachibana S. Unilateral carpal tunnel syndrome and space-occupying lesions. J Hand Surg Br. 1993;18:748–749. doi: 10.1016/0266-7681(93)90236-9. [DOI] [PubMed] [Google Scholar]
- 48.Bayrak IK, Bayrak AO, Kale M, et al. Bifid median nerve in patients with carpal tunnel syndrome. J Ultrasound Med. 2008;27:1129–1136. doi: 10.7863/jum.2008.27.8.1129. [DOI] [PubMed] [Google Scholar]
- 49.Granata G, Caliandro P, Pazzaglia C, et al. Prevalence of bifid median nerve at wrist assessed through ultrasound. Neurol Sci. 2011;32:615–618. doi: 10.1007/s10072-011-0582-8. [DOI] [PubMed] [Google Scholar]
- 50.Khashaba A. Carpal tunnel syndrome from thrombosed persistent median artery. J Emerg Med. 2002;22:55–57. doi: 10.1016/s0736-4679(01)00436-x. [DOI] [PubMed] [Google Scholar]
- 51.Feldkamp MM, Gentili F, Hudson AR, Guha A. A persistent median artery causing carpal tunnel syndrome in a patient with chronic renal failure: case report. Neurosurgery. 1995;37:140–143. doi: 10.1227/00006123-199507000-00023. [DOI] [PubMed] [Google Scholar]
- 52••.Cartwright MS, Hobson-Webb LD, Boon AJ, et al. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012 doi: 10.1002/mus.23389. Muscle and Nerve early view. This paper is an evidence based review that screened 724 papers found with the search terms ultrasound and carpal tunnel syndrome. It found sufficient papers with Class I evidence to conclude that ultrasound measurements of the cross sectional area at the wrist were an accurate diagnostic test for carpal tunnel syndrome. [DOI] [PubMed] [Google Scholar]
- 53.Kim JY, Yoon JS, Kim SJ, et al. Carpal tunnel syndrome: Clinical, electrophysiological, and ultrasonographic ratio after surgery. Muscle Nerve. 2012;45:183–188. doi: 10.1002/mus.22264. [DOI] [PubMed] [Google Scholar]
- 54.Abicalaf CA, de Barros N, Sernik RA, et al. Ultrasound evaluation of patients with carpal tunnel syndrome before and after endoscopic release of the transverse carpal ligament. Clin Radiol. 2007;62:891–894. doi: 10.1016/j.crad.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 55.Smidt MH, Visser LH. Carpal tunnel syndrome: clinical and sonographic follow-up after surgery. Muscle Nerve. 2008;38:987–991. doi: 10.1002/mus.20982. [DOI] [PubMed] [Google Scholar]
- 56.Vögelin E, Nüesch E, Jüni P, et al. Sonographic follow-up of patients with carpal tunnel syndrome undergoing surgical or nonsurgical treatment: prospective cohort study. J Hand Surg Am. 2010;35:1401–1409. doi: 10.1016/j.jhsa.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Naranjo A, Ojeda S, Rúa-Figueroa I, et al. Limited value of ultrasound assessment in patients with poor outcome after carpal tunnel release surgery. Scand J Rheumatol. 2010;39:409–412. doi: 10.3109/03009741003685632. [DOI] [PubMed] [Google Scholar]
- 58.Tan TC, Yeo CJ, Smith EW. High definition ultrasound as diagnostic adjunct for incomplete carpal tunnel release. Hand Surg. 2011;16:289–294. doi: 10.1142/S0218810411005564. [DOI] [PubMed] [Google Scholar]
- 59.American Association of Electrodiagnostic Medicine, American Academy of Neurology, and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25:918–922. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- 60.Beekman R, Van Der Plas JP, Uitdehaag BM, et al. Clinical, electrodiagnostic, and sonographic studies in ulnar neuropathy at the elbow. Muscle Nerve. 2004;30:202–208. doi: 10.1002/mus.20093. [DOI] [PubMed] [Google Scholar]
- 61.Yoon JS, Walker FO, Cartwright MS. Ulnar neuropathy with normal electrodiagnosis and abnormal nerve ultrasound. Arch Phys Med Rehabil. 2010;91:318–320. doi: 10.1016/j.apmr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beekman R, Schoemaker MC, Van Der Plas JP, et al. Diagnostic value of high-resolution sonography in ulnar neuropathy at the elbow. Neurology. 2004;62:767–773. doi: 10.1212/01.wnl.0000113733.62689.0d. [DOI] [PubMed] [Google Scholar]
- 63.Bayrak AO, Bayrak IK, Turker H, et al. Ultrasonography in patients with ulnar neuropathy at the elbow: comparison of cross-sectional area and swelling ratio with electrophysiological severity. Muscle Nerve. 2010;41:661–666. doi: 10.1002/mus.21563. [DOI] [PubMed] [Google Scholar]
- 64.Volpe A, Rossato G, Bottanelli M, et al. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford) 2009;48:1098–1101. doi: 10.1093/rheumatology/kep167. [DOI] [PubMed] [Google Scholar]
- 65.Mondelli M, Filippou G, Frediani B, Aretini A. Ultrasonography in ulnar neuropathy at the elbow: relationships to clinical and electrophysiological findings. Neurophysiol Clin. 2008;38:217–226. doi: 10.1016/j.neucli.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Robinson LR. Traumatic injury to peripheral nerves. Muscle Nerve. 2000;23:863–873. doi: 10.1002/(sici)1097-4598(200006)23:6<863::aid-mus4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 67.Cartwright MS, Chloros GD, Walker FO, et al. Diagnostic ultrasound for nerve transection. Muscle Nerve. 2007;35:796–799. doi: 10.1002/mus.20761. [DOI] [PubMed] [Google Scholar]
- 68•.Zhu J, Liu F, Li D, et al. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21:1097–1101. doi: 10.1007/s00330-010-1992-3. The authors prospectively studied 202 patients with suspected traumatic neuropathy and characterized the findings on ultrasound in terms of nerve, epineurium and surrounding tissues. Overall they were able to accurately classify 92% of the lesions. [DOI] [PubMed] [Google Scholar]
- 69.Cartwright MS, Yoon JS, Lee KH, et al. Diagnostic ultrasound for traumatic radial neuropathy. Am J Phys Med Rehabil. 2011;90:342–343. doi: 10.1097/PHM.0b013e3181e29daa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70••.Padua L, Liotta G, Di Pasquale A, et al. Contribution of ultrasound in the assessment of nerve diseases. Eur J Neurol. 2012;19:47–54. doi: 10.1111/j.1468-1331.2011.03421.x. The authors studied 130 patients with suspected, predominantly focal, neuropathies with both ultrasound and electrodiagnstic testing. Ultrasound showed a robust impact on diagnosis and management, by enhancing or confirming findings in over 80% of patients. [DOI] [PubMed] [Google Scholar]
- 71.Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40:960–966. doi: 10.1002/mus.21431. [DOI] [PubMed] [Google Scholar]
- 72.Beekman R, van den Berg LH, Franssen H, et al. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology. 2005;65:305–307. doi: 10.1212/01.wnl.0000169179.67764.30. [DOI] [PubMed] [Google Scholar]
- 73.Resmini E, Tagliafico A, Nizzo R, et al. Ultrasound of peripheral nerves in acromegaly: changes at 1-year follow-up. Clin Endocrinol (Oxf) 2009;71:220–225. doi: 10.1111/j.1365-2265.2008.03468.x. [DOI] [PubMed] [Google Scholar]
- 74.Cartwright MS, Walker FO, Griffin LP, Caress JB. Peripheral nerve and muscle ultrasound in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:346–351. doi: 10.1002/mus.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Renna R, Erra C, Almeida V, Padua L. Ultrasound study shows nerve atrophy in post herpetic neuralgia. Clin Neurol Neurosurg. 2012 doi: 10.1016/j.clineuro.2012.03.022. In press. [DOI] [PubMed] [Google Scholar]
- 76.Boon AJ, Bailey PW, Smith J, et al. Utility of ultrasound-guided surface electrode placement in lateral femoral cutaneous nerve conduction studies. Muscle Nerve. 2011;44:525–530. doi: 10.1002/mus.22102. [DOI] [PubMed] [Google Scholar]
- 77.Scheidegger O, Küffer AF, Kamm CP, Rösler KM. Reproducibility of sensory nerve conduction studies of the sural nerve using ultrasound-guided needle positioning. Muscle Nerve. 2011;44:873–876. doi: 10.1002/mus.22182. [DOI] [PubMed] [Google Scholar]
- 78.Kamm CP, Scheidegger O, Rösler KM. Ultrasound-guided needle positioning in sensory nerve conduction study of the sural nerve. Clin Neurophysiol. 2009;120:1342–1345. doi: 10.1016/j.clinph.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 79.Jain S, Visser LH, Praveen TL, et al. High-resolution sonography: a new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. 2009;3:e498. doi: 10.1371/journal.pntd.0000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bathala L, Kumar K, Pathapati R, et al. Ulnar neuropathy in hansen disease: clinical, high-resolution ultrasound and electrophysiologic correlations. J Clin Neurophysiol. 2012;29:190–193. doi: 10.1097/WNP.0b013e31824d969c. [DOI] [PubMed] [Google Scholar]
- 81.Elias J, Jr, Nogueira-Barbosa MH, Feltrin LT, et al. Role of ulnar nerve sonography in leprosy neuropathy with electrophysiologic correlation. J Ultrasound Med. 2009;28:1201–1209. doi: 10.7863/jum.2009.28.9.1201. [DOI] [PubMed] [Google Scholar]


