Abstract
Background
Insulin-degrading enzyme (IDE, insulysin, insulinase; EC 3.4.22.11), a thiol metalloendopeptidase, is involved in intracellular degradation of insulin, thereby inhibiting its translocation and accumulation to the nucleus. Recently, protein expression of IDE has been demonstrated in the epithelial ducts of normal breast and in breast cancer tissue (Radulescu et al., Int J Oncol 30:73; 2007).
Materials and Methods
Utilizing four different antibodies generated against different epitopes of the IDE molecule, we performed western blot analysis and immunohistochemical staining on several normal human tissues, on a plethora of tumor cell lines of different tissue origin, and on malignant breast and ovarian tissue.
Results
Applying the four IDE-directed antibodies, we demonstrate IDE expression at the protein level, both by means of immunoblotting and immunocytochemistry, in all of the tumor cell lines analyzed. Besides, IDE protein expression was found in normal tissues of the kidney, liver, lung, brain, breast and skeletal muscle, as well as in breast and ovarian cancer tissues. Immunohistochemical visualization of IDE indicated cytoplasmic localization of IDE in all of the cell lines and tissues assessed.
Conclusions
We performed for the first time a wide-ranging survey on IDE protein expression in normal and malignant tissues and cells and thus extend knowledge about cellular and tissue distribution of IDE, an enzyme which so far has mainly been studied in connection with Alzheimer’s disease and diabetes but not in cancer.
Keywords: Insulin-degrading enzyme (IDE), tumor cell lines, normal human tissues, breast cancer, ovarian cancer
INTRODUCTION
Insulin’s major effect on protein metabolism is inhibition of protein degradation. This is via inhibition of proteasome activity via an interaction with insulin-degrading enzyme (IDE) (1). IDE (insulysin, insulinase; EC 3.4.22.11, MW=110 kD; M16.002) is a highly conserved, neutral zinc- and thiol-dependent metallopeptidase. It belongs to the M16 (pitrilysin) family of zinc-metalloendopeptidases, namely inverzincins, characterized by the inverted zinc binding motif HXXEH (2). IDE is present in human beings, animals, fungi, and plants (2-4; http://merops.sanger.ac.uk/, see distribution within family M16.002: insulysin); and is reported to be expressed in the liver, adipocytes, muscle cells, erythrocytes, and kidney (3-5) but also in cell types not responsive to insulin (6; www.genecards.org/cgi-bin/carddisp.pl?gene=IDE). Despite its predominant presence in the cytosol, IDE is also found in small but significant amounts in subcellular compartments, e.g. the plasma membrane, endosomes, peroxisomes, and mitochondria (7-15).
IDE exhibits a preference for basic (arginine, lysine) or bulky hydrophobic residues (phenylalanine, leucine, tyrosine) at the P1 site of the target protein (16). This preference for cleavage at hydrophobic and basic residues supports the assumption that substrate recognition by IDE rather depends on the tertiary peptide conformation than on the amino acid sequence (17).
IDE is reported to cleave small proteins of diverse sequence, several of which have in common to form β-pleated sheet-rich amyloid fibrils, i.e. insulin, amyloid β-protein, amylin, glucagon, atrial natriuretic factor, and calcitonin (16-20), although IDE is also known to be a significant enzyme responsible for the degradation of insulin-like growth factors I and II (21) and transforming growth factor α (22, 23). IDE knockout mice show significantly elevated levels of blood insulin, brain beta-amyloid, and brain amyloid-precursor protein intracellular domain, providing key in vivo evidence that IDE degrades both extracellular and intracellular peptides (24).
Conducting its proteolytic activity, insulin-degrading enzyme regulates translocation of insulin from the cytoplasm to the nucleus (25), preventing insulin from binding to and inactivating the nuclear tumor suppressor retinoblastoma protein (RB) (26).
Based on this previous notion, implying an underlying role of IDE not only in diabetes (27, 28) and Alzheimer disease (29) but also in tumor progression, and on the recently published data of Radulescu et al. (30) presenting immunohistochemical expression of IDE in normal and malignant human breast tissue, we now extend our study on analysis of IDE expression in various normal tissues, in breast and ovarian cancer tissues, and in tumor cell lines of different tissue origin by means of immunohistochemistry and western blotting, employing various antibodies generated against different epitopes of IDE.
MATERIALS AND METHODS
Tumor cell lines
The following human cell lines, cultivated in DMEM-10 % fetal calf serum-0.2 % arginine/asparagine / 1 % HEPES were used in the study: CAL 27 (squamous cell carcinoma of the tongue; German Collection of Microorganisms and Cell Cultures, DSMZ, Braunschweig, Germany), FaDu (esophageal squamous cell carcinoma of the hypopharynx; M. Baumann, Dresden), OVMZ-6 (epithelial ovarian cancer; V. Moebus, Frankfurt, Germany), HeLa (epithelial cervical cancer; ATCC-CCL-2, American Type Culture Collection (ATCC), Manassas, USA), Caco-2 (epithelial colon adenocarcinoma ATCC-HTB-37; K.P. Janssen, Munich), HT-1080 (fibrosarcoma ATCC-CCL-121; American Type Culture Collection), and HaCaT (spontaneously transformed (immortalized) keratinocyte cell line; M. Kotzsch, Dresden, Germany). The following cell lines were cultivated in RPMI–10 % fetal calf serum–1 % glutamine: U-937 (myelomonocytic histiocytic lymphoma cell line ATCC-CRL-1593.2; American Type Culture Collection) and SK-BR-3 (epithelial breast adenocarcinoma of metastatic origin (pleural effusion) ATCC-HTB-30; American Type Culture Collection).
Tissue microarray construction
The following formalin-fixed (buffered) paraffin-embedded tissues obtained from adult human individuals were selected at random from the archives of the Institute of Pathology, Technical University Munich: skeletal muscle, lung, brain, liver, kidney, and breast. Tissue microarrays were prepared using a 1 mm punch core needle instrument (MTA I Personal Tissue Arrayer, Beecher Instruments, USA) as described (31). In order to compensate for individual differences in tissue heterogeneity and staining intensity, specimens from three different individuals were sampled per organ.
Cell microarray construction
Cultured cells (3 × 107) were washed twice in 3 ml phosphate-buffered saline, PBS, (20 °C) and centrifuged (300 × g, 5 min, 20 °C). Then the cell pellet was resuspended in 5 ml of 10 % formalin in PBS, (30 min, 20 °C), centrifuged (800 × g, 5 min, 20 °C), washed with Tris-buffered saline, TBS, (20 °C), followed by an additional wash in PBS and then centrifuged again. Subsequently, 150 μl thrombin (10 U / ml H2Odist.; Sigma-Aldrich, Taufkirchen, Germany), 750 μl casein (Sigma-Aldrich; 10 mg / ml 0.04 M Tris-HCl, pH 8.0) and 600 μl fibrinogen (25 mg / ml H2Odist.; Sigma) were added to the cell pellet and left overnight at 4 °C to solidify before paraffin-embedding. Since cells are formalin-fixed and paraffin-embedded, the procedure followed for construction of the CMAs is identical to the way tissue microarrays are prepared (31).
Cell and tissue extracts
Cell extracts were prepared by lysing the cells with the non-ionic detergent Triton X-100 (1 % w/v in TBS; 12 h, 4 °C) and the supernatant containing the IDE protein harvested by high-speed centrifugation (25,000 × g, 10 min, 4 °C). For the preparation of tissue extracts, fresh-frozen tissue specimens after storage in liquid nitrogen were pulverized in the still frozen state by use of the Mikro-Dismembrator S laboratory ball mill (Sartorius, Göttingen, Germany) and then the powder subjected to 1 % Triton X-100 (1 % w/v in TBS; 12 h, 4 °C) for protein extraction, followed by high-speed centrifugation (32). Extracts were aliquoted and stored in liquid nitrogen until further use.
Immunoblotting analysis of recombinant and cellular IDE
Cell line extracts (20 μg/lane) and tissue extracts (25 μg/lane) as well as recombinant 6His-tagged IDE (20 ng/lane) (Figure 1; generated by Dr. W. Tang, Ben May Institute for Cancer Research, Center for Integrative Sciences, Chicago, IL, USA (33)), were heated and reduced (5 min, 95 °C) in the presence of 2 % (w/v) SDS and 5 % (v/v) 2-mercaptoethanol. Samples were immediately subjected to electrophoresis on 10 % SDS-polyacrylamide gels and separated proteins transferred onto a polyvinylidene fluoride membrane (PVDF, PALL; Dreieich, Germany; 3 h at 75 mA/membrane), applying a semi-dry transfer device (Whatman Biometra; Göttingen, Germany). After transfer, membranes were blocked with 5 % skimmed milk powder in PBS-0.1 % Tween-20 (pH 7.4, 1 h, RT). Subsequently, blots were incubated overnight at 4 °C with four different primary antibodies diluted in incubation buffer (Table I), and then washed three times (10 min, RT) with PBS-0.1 % Tween-20. Antigen-antibody complexes were visualized using HRP-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch; #111-035-003) or HRP-conjugated rabbit anti-mouse IgG (Dianova; #315-035-045), diluted 1:10,000 in 5 % skimmed milk powder in PBS-0.1 % Tween-20, followed by chemiluminescent reaction (ECL, Amersham Biosciences; Little Chalfont, UK). For assessment of the relative molecular mass of the separated proteins, a prestained Protein IV-Marker set (PeqLab; Erlangen, Germany) was used. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. The same blots were incubated with mouse anti-GAPDH (#MAB374; Chemicon, Billerica, MA, USA), after stripping the membranes in 1.5 % w/v glycine, 0.1 % w/v SDS, and 1 % Tween-20; pH 2.2 for 1 h at RT.
Figure 1. Reaction pattern (western blot) of 6His-tagged full-length IDE protein with four different antibodies to IDE.
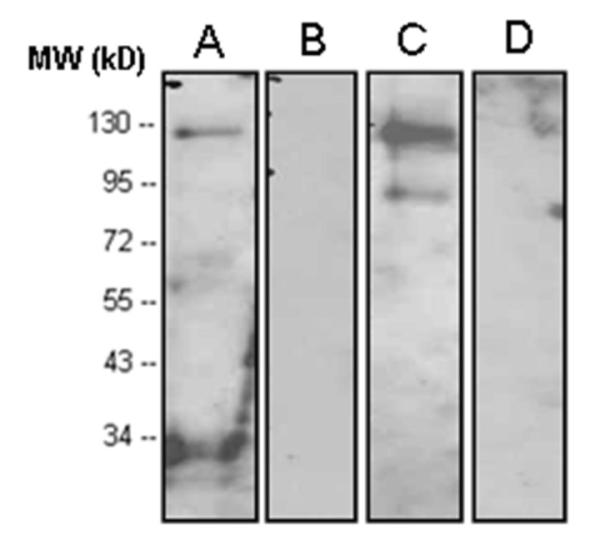
A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda.
Table I.
Antibodies used, raised against different epitopes of insulin-degrading enzyme (IDE)
| Antibody/Source | Immunogen | Host animal |
|---|---|---|
| UCG 43/6, University of Chicago, USA (33, 34) |
6His-tagged full length IDE | Rabbit, polyclonal, antiserum |
| #MMS-282R, Covance, clone 9B12, Berkley, CA, USA |
IDE purified from human erythrocytes |
Mouse, monoclonal IgG1, ascites |
| #PRB-282C, Covance, lot BC-2, Berkley, CA, USA |
Recombinant IDE-GST-fusion protein, containing aa 97-273 of rat IDE |
Rabbit, polyclonal, antiserum |
| anti-IDE-p15, Pineda, Berlin, Germany |
IDE-peptide p15 encompassing aa 940-953 of rat IDE |
Rabbit, polyclonal; antiserum purified by IDE-aa 940-953 affinity chromatography |
Immunohistochemical analysis of IDE (insulin-degrading enzyme) in human tissues and tumor cell lines
For immunohistochemical analyses, tissue microarray sections and cell microarray sections (2 μm thick) were deparaffinized and rehydrated by passing them through xylene twice (10 min), followed by a descending series of graded ethanol (100 %, 96 % and 70 % ethanol, 5 min for each step). At this point, no antigen retrieval procedure was employed. After a 5-min washing step in TBS at RT including buffer change, endogenous peroxidase activity was blocked by incubating the tissue sections with 3 % H2O2 (K33354110, Merck, Darmstadt, Germany) in H2Odist (20 min, RT). Subsequent to a 5-min washing step in TBS at RT including buffer change, sections were incubated at 4 °C overnight with primary antibodies (directed to different epitopes of human IDE; Table I) diluted in antibody diluent (S2022, Dako).
The procedure resumed the next day with a 5-min washing step in TBS at RT including buffer change. Then a peroxidase-labeled streptavidin-biotin detection kit (K5003, Dako, Hamburg, Germany) was applied, whereby secondary biotinylated goat-anti-rabbit/anti-mouse IgG was added to the tissue sections at RT for 30 min, followed by a 5-min washing step and then a by 30 min incubation period with streptavidin-peroxidase at RT as recommended by the manufacturer. After an additional washing step, the chromogenic reaction was carried out by incubating the tissue sections with peroxidase substrate AEC (3-amino-9-ethylcarbazole; K3464, Dako) (RT, 10 min). After a final washing step, nuclei were counterstained with Mayer’s acid hematoxylin solution for 10 sec. Finally, the slides were rinsed under running tap water, transferred to H2Odist and mounted with Kaiser’s glycerol gelatine (Merck, Darmstadt, Germany). Stained sections were photographed using a SONY 3CCD COLOR video camera attached to a Zeiss Axioplan 2 microscope and employing the Zeiss AxioVision software 4.5 SP1.
RESULTS
Tumor cell lines
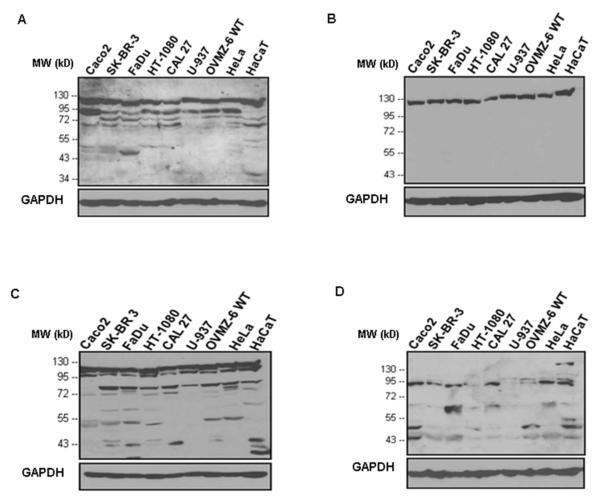
To analyze the expression of IDE in various human normal and malignant tissues as well as in several human tumor cell lines of different tissue origin, we utilized four antibodies raised against different epitopes of the target molecule (described in Table I). As demonstrated by immunoblotting (Figure 1), two of the antibodies employed (polyclonal antibody UCG 43/6 to 6His-tagged full length IDE and polyclonal antibody #PRB-282C to recombinant IDE-GST-fusion protein containing aa 97-273 of rat IDE) but neither monoclonal antibody #MMS-282R to human erythrocyte IDE nor polyclonal antibody to peptide p15 (aa 940-953 of rat IDE) detected IDE at the expected position of ~110 kDa.
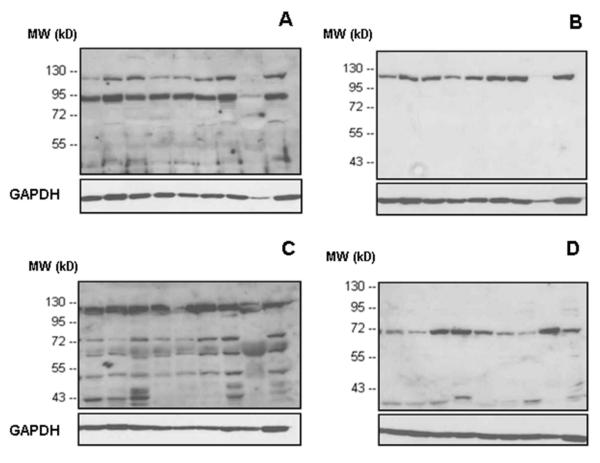
Concerning the cell lines assessed (Figure 2; Caco-2, SK-BR-3, FaDu, HT-1080, CAL 27, U-937, OVMZ-6, HeLa, HaCaT), three of the antibodies, but not anti-p15-IDE, interacted with the main IDE-band whereas for some of the cell lines anti-p15-IDE reacted with polypeptide bands located at ~90 kD or lower molecular weight positions (Figure 2D). Additional polypeptide bands other than the main IDE-protein were also detected by antibody UCG 43/6 and antibody #PRB-282C, both staining a prominent band at ~80 kDa (Figure 2A,C). Withstanding that, the monoclonal mouse #MMS-282R antibody, generated against IDE purified from human erythrocytes, detected very specifically the 110 kD IDE-band, only, in any of the cell lines tested (Figure 2B), not showing any reaction with other IDE-originating polypeptide bands.
Figure 2. Western blot analysis of IDE protein in detergent extracts obtained from various cell lines applying different antibodies to IDE.
Expression patterns of IDE-protein in cell extracts visualized by four different antibodies: A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda. GAPDH (38 kDa) is used as an internal control (bottom line).
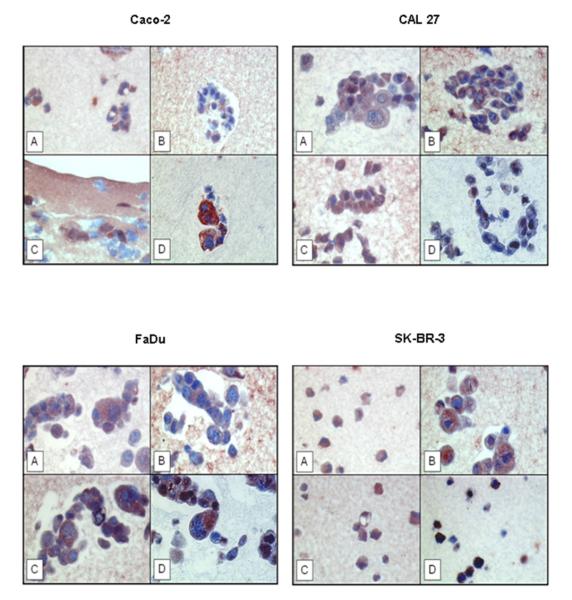
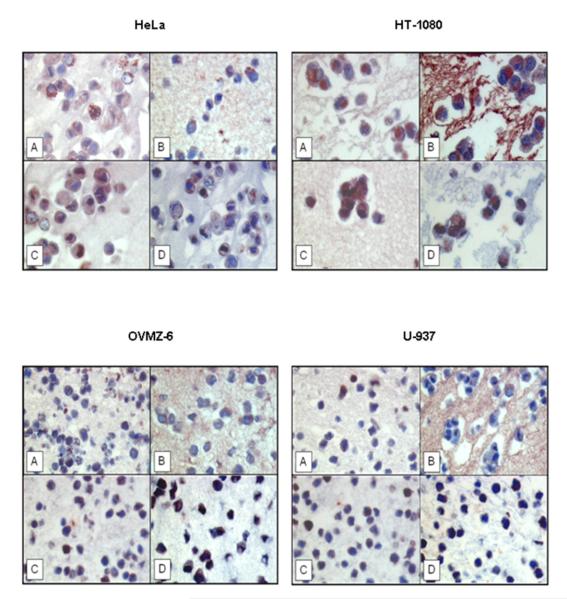
Protein expression of IDE was verified by immunohistochemical staining as well, employing the same four antibodies directed to IDE (Figure 3). IDE protein was detected by all of the antibodies in all of the cell lines tested, irrespective of their western blot reaction pattern, but with different degree of intensity. IDE is predominantly localized to the cytoplasmic portion of the cells. We also noted that antibodies #MMS-282R and #PRB-282C were also cross-reacting with artificial-clot elements, consisting of fibrin(ogen), casein, and thrombin (Figure 3B,C). Such crossreaction was not noted for the other two IDE-directed antibodies (Figure 3A,D). Omission of the primary antibody yielded no staining results (data not shown).
Figure 3. Immunohistochemical localization of IDE protein in paraffin-embedded cell lines applying different antibodies to IDE.
A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda. Red color: IDE staining. Blue color: staining of nuclei. Magnification x 400.
Normal tissues
Alike the tumor cell lines tested (Figure 2,3), IDE protein was found to be expressed in a variety of normal human tissues (e.g. kidney, liver, lung, brain, skeletal muscle, breast) (Figure 4,5). Similar to the cell lines tested, by western blot analysis, antibody #MMS-282R is detecting the 110 kDa band only while the other three antibodies detect the 110 kDa band as well, plus additional polypeptide bands of lower molecular weight.
Figure 4. Western blot analysis of IDE protein expression in detergent-released extracts of normal tissues, applying different antibodies to IDE.
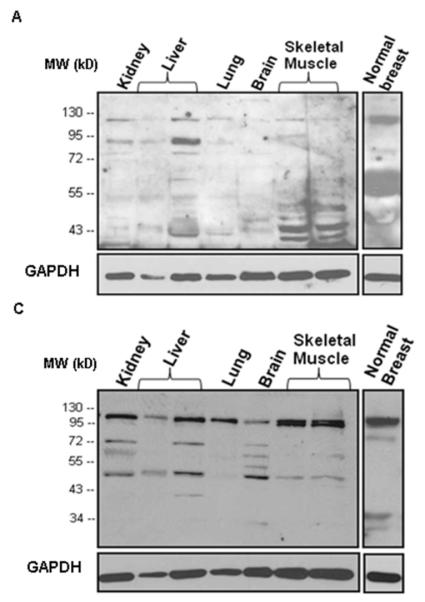
A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda. GAPDH (38 kDa) is used as an internal control (bottom line).
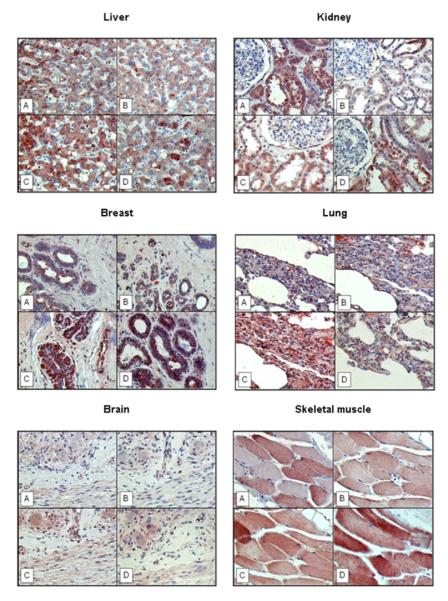
Figure 5. Immunohistochemical visualization of cytosolic IDE protein in paraffin-embedded human normal tissues applying different antibodies to IDE.
A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda. Red color: IDE staining. Blue color: staining of nuclei. Magnification x 200.
Employing the same four antibodies in assessing normal tissues by immunohistochemical staining, IDE protein expression was detected in all of the normal human tissues tested, such as that of the liver, kidney, breast, lung, brain, and skeletal muscle (Figure 5). In the liver, IDE-directed immunoreactivity was confined to hepatocytes; kidney specimens showed staining for IDE in tubular cells; occasionally, scattered stromal cells within the glomeruli were stained as well. IDE protein expression was also detected in the duct epithelia of the mammary gland, in the respiratory epithelia of the lung, in ganglions of the brain, and skeletal muscle. Similar to the tumor cell lines investigated, IDE-directed immunoreactivity was localized to the cytoplasmic compartment. It is worth mentioning that all four IDE-directed antibodies reacted equally but displayed certain variations in staining intensity. Furthermore, in some tissues (e.g. breast, brain), weak to moderate staining of the extracellular matrix by all four antibodies was observed. Omission of the primary antibody yielded no staining results (data not shown).
Breast and ovarian cancer tissues
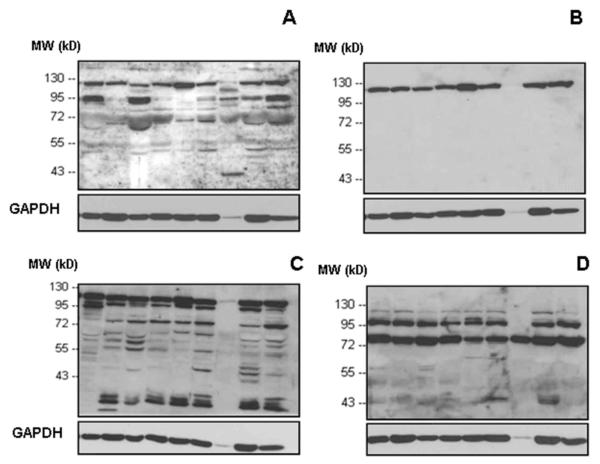
Apart from normal human tissues, we extended our investigations on the presence and tissue distribution of IDE protein to breast and ovarian cancer tissues, both by western blot analysis (Figure 6,7) and immunohistochemistry (Figure 8). Applying the same set of antibodies as described before, IDE was detected in detergent-extracted breast by western blotting (Figure 6). Regarding all nine breast cancer tissue specimens investigated, antibody #MMS-282R generated to erythrocyte IDE reacted with the 110 kDa IDE band only (Figure 6B), a pattern seen before with normal tissues and tumor cell lines. Likewise, rabbit antibody #PRB-282C reacted with the same IDE-linked 110 kDa band, but showing reaction with lower polypeptide bands as well, especially at ~50, ~60, and ~70 kDa (Figure 6C). Antibody UCG 43/6 also detected the 110 kDa band, but in addition to this, a prominent band at ~90 kDa stained with the antibody, too (Figure 6A), which is not present in normal breast tissue extracts (Figure 4). Different from the reaction pattern seen in Figure 6A,B, anti IDE-p15 reacted mainly with a ~70 kDa band (Figure 6D); a band also reactive with antibody #PRB-282C (Figure 6C).
Figure 6. Western blot analysis of IDE protein expression in detergent-released extracts of breast cancer tumor tissues (n=9) applying different antibodies to IDE.
A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda. GAPDH (38 kDa) is used as an internal control (bottom line).
Figure 7. Western blot analysis of IDE protein expression in detergent-released extracts of ovarian cancer tumor tissues (n=9) applying different antibodies to IDE.
A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda. GAPDH (38 kDa) is used as an internal control (bottom line).
Figure 8. IDE protein expression in breast (upper panel) and ovarian cancer (lower panel) tissue specimens applying different antibodies to IDE.
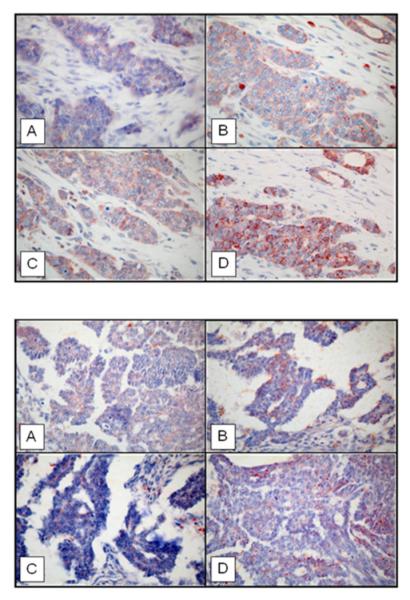
(Magnification x 400). A) rabbit antibody UCG 43/6, University of Chicago, B) mouse antibody #MMS-282R, Covance, C) rabbit antibody #PRB-282C, Covance, and D) rabbit antibody to IDE-peptide p15, Pineda.
Concerning all of the nine ovarian cancer tissue specimens investigated, antibody #MMS-282R (Figure 7B) reacted with the 110 kDa IDE band only, a pattern seen before with normal tissues, tumor cell lines, and breast cancer tissue extracts. Antibody #PRB-282C reacted with the 110 kDa band as well, but showing reaction with lower polypeptide bands, too (Figure 7C), with intense reaction at ~90 kDa. Antibody UCG 43/6 detected the 110 kDa band as well, but in addition to this, in some cases, a prominent band at ~90 kDa (Figure 7A), such as in breast cancer extracts. Anti IDE-p15 reacted with two main bands at positions ~70 kDa (such as in breast cancer) and ~90 kDa (Figure 7D).
Regarding IDE protein expression in breast cancer (Figure 8, upper panel) as assessed by immunohistochemical staining applying the four different IDE-directed antibodies, like in normal breast ductal epithelium, IDE protein is expressed in epithelial cells with scattered abundance in non-tumor cells present in the extracellular matrix confirming our initial observation reported by Radulescu et al. (30). Similar to epithelial cells in ducts of the normal breast, IDE is found in the cytosolic part of the tumor cells, with heterogeneous expression, as shown for all of the four antibodies applied. Although different polypeptide bands are stained with the antibodies when analyzing tumor tissue extracts by immunoblotting (Figure 6), no qualitative differences were observed regarding the various antibodies and the resulting staining patterns (Figure 8, upper panel). Similar findings were obtained when assessing ovarian cancer tumor tissue specimens (Figure 7,8 lower panel).
DISCUSSION
Expression and function of the metalloprotease insulin-degrading enzyme (IDE), an enzyme known to be involved in the degradation of neurotoxic amyloid peptides and insulin, is usually impaired in Alzheimer’s disease and diabetes (27-29, 35). Yet, reports about its involvement in malignancy are scarce (25, 30, 36). IDE was initially purified from human red blood cells (37) but is also present in a wide range of nucleated cells (www.genecards.org/cgi-bin/carddisp.pl?gene=IDE). Depending on the type of cell, IDE may be located in the cytosol (38, 39), peroxisomes (13, 40), endosomes (12, 41), mitochondria (15), or on the cell membrane (7-11, 42).
Particularly, after binding to its plasma membrane receptor, insulin succeeds its internalization into the cell (43), where it is released from endosomes into the cytosol and follows its pathway to the nucleus. IDE is found to be mostly active in the cytosol, therefore it seems to take part in the regulation of insulin’s translocation to the nucleus. By degrading insulin in a thiol-, zinc-dependent manner, IDE hinders the hormone to be involved in nuclear processes such as cell growth and gene expression (44).
The way IDE interacts with its hormone substrate, insulin, together with the fact that so far IDE activity has only been related to pathological conditions like Alzheimer’s disease (45) and diabetes mellitus type 2 (46) prompted us to study IDE expression in normal human organs, but also in malignant tumor tissues and tumor cell lines, by techniques utilizing antibodies such as western blotting and immunohistochemistry.
We confirm the initial findings of Chang et al. (36) and Bai et al. (47), reporting the presence of IDE in the human colon adenocarcinoma cell line Caco-2, and there more specifically in the cytosol of the cells, responsible for the majority of insulin clearance. Furthermore, we have demonstrated protein expression of IDE in so far not studied cell lines of different tissue origin by immunoblotting, such as SK-BR-3, FaDu, HT-1080, CAL 27, U-937, OVMZ-6, HeLa and HaCaT, by employing four different antibodies generated against different epitopes of the enzyme. Regarding these cell lines, all four antibodies displayed different patterns of IDE expression, whereby antibody #MMS-282R, generated by immunizing mice with purified IDE enriched from human erythrocytes, is only reactive with the prominent 110 kDa band. Interestingly, anti-IDE-p15 did not detect the 110 kDa band but instead a ~90 kDa band, although it has been described previously to recognize full length IDE extracted from rat tissue (48, 49). Antibody UCG 43/6 directed to full length IDE also detected this IDE band, plus a variety of other bands in the low molecular weight range. Still, at the light microscopic level, all of the antibodies examined did localize IDE to the cytoplasm of the tumor cell lines investigated.
This feature was also seen in healthy and diseased tissues. As demonstrated by western blot analysis and immunohistochemistry tissues like kidney and liver, which comprise the primary sites of insulin clearance, or skeletal muscle, an insulin-sensitive tissue, expressed IDE, and so did other types of tissues (lung, brain, breast). In accordance with previous own results (30), we show expression of IDE in tumor cells of breast cancer tissue. By extending these findings, we now demonstrate that IDE is also present in tumor cells in ovarian cancer tissue.
Yet, acknowledging the fact that this is the first wide-ranging study reporting the expression of IDE on the background of malignancy, one may speculate about a regulatory role of IDE in tumor progression and metastasis. To prove this, functional in vitro and vivo tests will have to be performed at molecular and cellular level.
ACKNOWLEDGEMENTS
This work was supported by a grant to M. Schmitt and C. Giersig provided by the Federal Institute for Drugs and Medical Devices (BfArM), Bonn. The authors thank Daniela Hellmann for expert technical assistance.
REFERENCES
- 1.Fawcett J, Permana PA, Levy JL, Duckworth WC. Regulation of protein degradation by insulin-degrading enzyme: analysis by small interfering RNA-mediated gene silencing. Arch Biochem Biophys. 2007;468:128–133. doi: 10.1016/j.abb.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2008;36:D320–D325. doi: 10.1093/nar/gkm954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirsky IA, Broth-Kahn RH. The inactivation of insulin by tissue extracts. I. The distribution and properties of insulin inactivating extracts (insulinase) Arch. Biochem. 1949;20:1–9. [PubMed] [Google Scholar]
- 4.Duckworth WC. Insulin degradation: mechanisms, products, and significance. Endocr Rev. 1988;9:319–345. doi: 10.1210/edrv-9-3-319. [DOI] [PubMed] [Google Scholar]
- 5.Duckworth WC. Insulin degrading enzyme. In: Cuatrecasas P, Jacobs S, editors. Insulin. Springer-Verlag; Berlin Heidelberg, Germany: 1990. [Google Scholar]
- 6.Duckworth WC, Bennett RG, Hamel FG. Insulin Degradation: Progress and Potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 7.Duckworth WC. Insulin and glucagons binding and degradation by the kidney cell membrane. Endocrinology. 1978;102:1766–1774. doi: 10.1210/endo-102-6-1766. [DOI] [PubMed] [Google Scholar]
- 8.Duckworth WC. Insulin degradation by liver cell membranes. Endocrinology. 1979;104:1758–1764. doi: 10.1210/endo-104-6-1758. [DOI] [PubMed] [Google Scholar]
- 9.Yokono K, Imamura Y, Sakai H, Bab S. Insulin degrading activity of plasma membranes from rat skeletal muscle: its isolation, characterization, and biologic significance. Diabetes. 1979;28:810–817. doi: 10.2337/diab.28.9.810. [DOI] [PubMed] [Google Scholar]
- 10.Yokono K, Roth RA, Baba S. Identification of insulin-degrading enzyme on the surface of cultured human lymphocytes, rat hepatoma cells, and primary cultures of rat hepatocytes. Endocrinology. 1982;111:1102–1108. doi: 10.1210/endo-111-4-1102. [DOI] [PubMed] [Google Scholar]
- 11.Goldfine ID, Williams JA, Bailey AC, Wong KY, Iwamoto Y, Yokono K, Baba S, Roth RA. Degradation of insulin by isolated mouse pancreatic acini: evidence for cell surface protease activity. Diabetes. 1984;33:64–72. doi: 10.2337/diab.33.1.64. [DOI] [PubMed] [Google Scholar]
- 12.Hamel FG, Mahoney MJ, Duckworth WC. Degradation of intraendosomal insulin by insulin degrading enzyme without acidification. Diabetes. 1991;40:436–443. doi: 10.2337/diab.40.4.436. [DOI] [PubMed] [Google Scholar]
- 13.Authier F, Bergeron JJ, Ou WJ, Rachubinski RA, Posner BI, Walton PA. Degradation of the cleaved leader peptide of thiolase by a peroxisomal proteinase. Proc Natl Acad Sci USA. 1995;92:3859–3863. doi: 10.1073/pnas.92.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabkin R, Birn H, Shi J, Roth R, Christensen E. Insulin degrading enzyme (IDE) in renal proximal tubules. Diabetes. 1992;41:15A. [Google Scholar]
- 15.Leissring MA, Farris W, Wu X, Christodoulou DC, Haigis MC, Guarente L, Selkoe DJ. Alternative translation initiation generates a novel isoform of insulin-degrading enzyme targeted to mitochondria. Biochem J. 2004;383(Pt. 3):439–446. doi: 10.1042/BJ20041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Authier F, Posner BI, Bergeron JJM. Insulin-degrading enzyme. Clin Investig Med. 1996;19:149–160. [PubMed] [Google Scholar]
- 17.Kurochkin IV. Amyloidogenic determinant as a substrate recognition motif of insulin-degrading enzyme. FEBS Lett. 1998;427:153–156. doi: 10.1016/s0014-5793(98)00422-0. [DOI] [PubMed] [Google Scholar]
- 18.Bennett RG, Duckworth WC, Hamel FG. Degradation of amylin by insulin-degrading enzyme. J Biol Chem. 2000;275:36621–36625. doi: 10.1074/jbc.M006170200. [DOI] [PubMed] [Google Scholar]
- 19.Kurochkin IV. Insulin-degrading enzyme: embarking on amyloid destruction. Trends Biochem Sci. 2001;26:421–425. doi: 10.1016/s0968-0004(01)01876-x. [DOI] [PubMed] [Google Scholar]
- 20.Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003;22:487–493. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- 21.Bennett RG, Hamel FG, Duckworth WC. Characterization of the insulin inhibition of the peptidolytic activities of the insulin-degrading enzyme-proteasome complex. Diabetes. 1997;46:197–203. doi: 10.2337/diab.46.2.197. [DOI] [PubMed] [Google Scholar]
- 22.Gehm BD, Rosner MR. Regulation of insulin, epidermal growth factor, and transforming growth factor-α levels by growth factor-degrading enzymes. Endocrinology. 1991;128:1603–1610. doi: 10.1210/endo-128-3-1603. [DOI] [PubMed] [Google Scholar]
- 23.Hamel FG, Gehm BD, Rosner MR, Duckworth WC. Identification of the cleavage sites of transforming growth factor alpha by insulin-degrading enzymes. Biochim Biophys Acta. 1997;1338:207–214. doi: 10.1016/s0167-4838(96)00202-6. [DOI] [PubMed] [Google Scholar]
- 24.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harada S, Smith RM, Smith JA, Jarett L. Inhibition of insulin-degrading enzyme increases translocation of insulin to the nucleus in H35 rat hepatoma cells-evidence of a cytosolic pathway. Endocrinology. 1993;132:2293–2298. doi: 10.1210/endo.132.6.8504733. [DOI] [PubMed] [Google Scholar]
- 26.Radulescu RT, Doklea ED, Kehe K, Mückter H. Nuclear colocalization and complex formation of insulin with retinoblastoma protein in HepG2 human hepatoma cells. J Endocrinol. 2000;166:R1–4. doi: 10.1677/joe.0.166r001. [DOI] [PubMed] [Google Scholar]
- 27.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 28.Kwak SH, Cho YM, Moon MK, Kim JH, Park BL, Cheong HS, Shin HD, Jang HC, Kim SY, Lee HK, Park KS. Association of polymorphisms in the insulin-degrading enzyme gene with type 2 diabetes in the Korean population. Diabetes Res Clin Pract. 2008;79:284–290. doi: 10.1016/j.diabres.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 29.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 30.Radulescu RT, Hufnagel C, Luppa P, Hellebrand H, Kuo WL, Rosner MR, Harbeck N, Giersig C, Meindl A, Schmitt M, Weirich G. Immunohistochemical demonstration of the zinc metalloprotease insulin-degrading enzyme in normal and malignant human breast: Correlation with tissue insulin levels. International Journal of Oncology. 2007;30:73–80. [PubMed] [Google Scholar]
- 31.Simon R, Mirlacher M, Sauter G. Tissue microarrays in cancer diagnosis. Expert Rev Mol Diagn. 2003;3:421–430. doi: 10.1586/14737159.3.4.421. [DOI] [PubMed] [Google Scholar]
- 32.Schmitt M, Mengele K, Schueren E, Sweep FC, Foekens JA, Brünner N, Laabs J, Malik A, Harbeck N. European Organisation for Research and Treatment of Cancer (EORTC) Pathobiology Group standard operating procedure for the preparation of human tumour tissue extracts suited for the quantitative analysis of tissue-associated biomarkers. Eur J Cancer. 2007;43:835–844. doi: 10.1016/j.ejca.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006;443(7113):870–874. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chesneau V, Rosner MR. Functional human insulin-degrading enzyme can be expressed in bacteria. Protein Expr Purif. 2000;19:91–98. doi: 10.1006/prep.2000.1217. [DOI] [PubMed] [Google Scholar]
- 35.Sun MK, Alkon DL. Links between Alzheimer’s disease and diabetes. Drugs Today (Barc) 2006;42:481–489. doi: 10.1358/dot.2006.42.7.973588. [DOI] [PubMed] [Google Scholar]
- 36.Chang LL, Stout LE, Wong WD, Buls JG, Rothenberger DA, Shier WT, Sorenson RL, Bai JP. Immunohistochemical localization of insulin-degrading enzyme along the rat intestine, in the human colon adenocarcinoma cell line (Caco-2), and in human ileum. J Pharm Sci. 1997;86:116–119. doi: 10.1021/js960035q. [DOI] [PubMed] [Google Scholar]
- 37.Shii K, Yokono K, Baba S, Roth RA. Purification and characterization of insulin-degrading enzyme from human erythrocytes. Diabetes. 1986;35:675–683. doi: 10.2337/diab.35.6.675. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz A, Schneider A, Kummer MP, Herzog V. Endoplasmic reticulum-localized amyloid beta-peptide is degraded in the cytosol by two distinct degradation pathways. Traffic. 2004;5:89–101. doi: 10.1111/j.1600-0854.2004.00159.x. [DOI] [PubMed] [Google Scholar]
- 39.Bai JP, Hong HJ, Rothenberger DA, Wong WD, Buls JG. The presence of insulin-degrading enzyme in human ileal and colonic mucosal cells. J Pharm Pharmacol. 1996;48:1180–1184. doi: 10.1111/j.2042-7158.1996.tb03917.x. [DOI] [PubMed] [Google Scholar]
- 40.Morita M, Kurochkin IV, Motojima K, Goto S, Takano T, Okamura S, Sato R, Yokota S, Imanaka T. Insulin-degrading enzyme exists inside of rat liver peroxisomes and degrades oxidized proteins. Cell Struct Funct. 2000;25:309–315. doi: 10.1247/csf.25.309. [DOI] [PubMed] [Google Scholar]
- 41.Duckworth WC, Hamel FG. Cellular and endosomal insulin degradation. Adv Second Messenger Phosphoprotein Res. 1990;24:521–528. [PubMed] [Google Scholar]
- 42.Lynch JA, George AM, Eisenhauer PB, Conn K, Gao W, Carreras I, Wells JM, McKee A, Ullman MD, Fine RE. Insulin degrading enzyme is localized predominantly at the cell surface of polarized and unpolarized human cerebrovascular endothelial cell cultures. J Neurosci Res. 2006;83:1262–1270. doi: 10.1002/jnr.20809. [DOI] [PubMed] [Google Scholar]
- 43.Exton JH. Some thoughts on the mechanism of action of insulin. Diabetes. 1991;40:521–526. doi: 10.2337/diab.40.5.521. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien RM, Granner DK. Regulation of gene expression by insulin. Biochem J. 1991;278(Pt 3):609–619. doi: 10.1042/bj2780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE. Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science. 2000;290:2302–2303. doi: 10.1126/science.290.5500.2302. [DOI] [PubMed] [Google Scholar]
- 46.Fakhrai-Rad H, Nikoshkov A, Kamel A, Fernström M, Zierath JR, Norgren S, Luthman H, Galli J. Insulin-degrading enzyme identified as a candidate diabetes susceptibility gene in GK rats. Hum Mol Genet. 2000;9:2149–2158. doi: 10.1093/hmg/9.14.2149. [DOI] [PubMed] [Google Scholar]
- 47.Bai JP, Hsu MJ, Shier WT. Insulin-degrading enzyme in a human colon adenocarcinoma cell line (Caco-2) Pharm Res. 1995;12:513–517. doi: 10.1023/a:1016241610649. [DOI] [PubMed] [Google Scholar]
- 48.Camberos MC, Pérez AA, Udrisar DP, Wanderley MI, Cresto JC. ATP inhibits insulin-degrading enzyme activity. Exp Biol Med. 2001;226:334–341. doi: 10.1177/153537020122600411. [DOI] [PubMed] [Google Scholar]
- 49.Udrisar DP, Wanderley MI, Porto RC, Cardoso CL, Barbosa MC, Camberos MC, Cresto JC. Androgen- and estrogen-dependent regulation of insulin-degrading enzyme in subcellular fractions of rat prostate and uterus. Exp Biol Med. 2005;230:479–486. doi: 10.1177/153537020523000706. [DOI] [PubMed] [Google Scholar]