Abstract
Modernization has increased longevity and decreased fertility in many human populations, but it is not well understood how or to what extent these demographic transitions have altered patterns of natural selection. I integrate individual-based multivariate phenotypic selection approaches with evolutionary demographic methods to demonstrate how a demographic transition in 19th century female populations of Utah altered relationships between fitness and age-specific survival and fertility. Coincident with this demographic transition, natural selection for fitness, as measured by the opportunity for selection, increased by 13–20% over 65 years. Proportional contributions of age-specific survival to total selection (the complement to age-specific fertility) diminished from approximately 1/3 to 1/7 following a marked increase in infant survival. Despite dramatic reductions in age-specific fertility variance at all ages, the absolute magnitude of selection for fitness explained by age-specific fertility increased by approximately 45%. I show that increases in the adaptive potential of fertility traits followed directly from decreased population growth rates. These results suggest that this demographic transition has increased the adaptive potential of the Utah population, intensified selection on reproductive traits, and de-emphasized selection on survival-related traits.
Keywords: Demography, Senescence, Fecundity, Fitness, Life-History Evolution, Selection–Natural
Introduction
“In traditional societies, fertility and mortality are high. In modern societies, fertility and mortality are low. In between, there is a demographic transition.” – Demeny 1968
As human populations have modernized, especially since the late 18th century, mortality and reproductive rates have declined (Notestein 1945; Demeny 1968). These demographic transitions are viewed by sociologists and anthropologist as evolutionary puzzles, as they seem to violate our intuitive sense that improved environmental conditions should increase fertility rates. Three evolutionary models have been proposed to explain this phenomenon (Borgerhoff Mulder 1998). First, fitness optima for family size may have changed with environmental change and caused natural selection to favor smaller families over time. Second, reproductive behaviors of individuals may have changed to mimic changes they observed in others, and this has caused a cultural evolution towards decreased family size. Third, low fertility may be maladaptive, and humans are pursuing poor fitness maximization strategies because we do not properly incorporate modern environmental cues into our reproductive decisions. Much theoretical and empirical attention has focused on understanding the relative roles of each possible evolutionary cause for demographic transitions (e.g., Kaplan 1996). However, the evolutionary consequences of transitions have not been studied in much detail. As demographic transitions are still ongoing (Cohen 1998; Morgan 2003), an understanding of any such evolutionary changes wrought in the past are relevant to predicting change in the future.
By definition, survival and reproduction at different ages (collectively termed vital rates) change throughout these transitions. However, evolutionary theory predicts that vital rates selection is determined by the population means of all vital rates (Medawar 1952; Williams 1957; Hamilton 1966; Charlesworth 1994). Thus, demographic transitions generate changes in selection that contribute to the evolution of demographic structure if there is heritable variation for vital rates. This component of change will act in synergy or in antagonism with other determinants of a demographic transition. While the direction and the magnitude of this feedback is not obvious, it is clearly worth understanding if natural selection generally favors ever-accelerating demographic transitions or if it provides resistance against further demographic change.
The evolutionary change of a trait is a function of natural selection acting on that trait (phenotypic selection), the fidelity with which traits are transmitted from an ancestral to a descendent population (e.g., heritability), and any bias associated with that transmission (e.g., mutation or environmental changes) (Price 1970, 1972). While the influence of any or all of these factors may have changed with demographic transitions, I focus on understanding the influence of demographic change on selection. There are three fundamental and quantitative ways to ask how selection changes over time: 1) how has the strength of selection on specific traits changed; 2) how has the strength of total selection acting on all traits changed; and 3) how has the potential for specific traits to affect adaptive change changed over time? Each of these questions can be applied quantitatively to study how the adaptive roles of vital rates have changed with a demographic transition.
Selection differentials and gradients
Natural selection samples phenotypes from an ancestral population, and fitness defines the weighting of these samples in a descendent population. The difference between the trait mean of a generation before and after selection is the selection differential, the among-individual covariance between relative fitness (the ratio of absolute fitness to population mean fitness) and value of the trait. An evolutionary change in the mean (the response to selection) is the product of this covariance and the slope of the regression of offspring on parent trait values (narrow-sense heritability). Equivalently, it is the product of the slope of the regression of relative fitness on trait values (the selection differential) and the covariance between ancestral and descendent phenotypes (additive genetic variance) (Robertson 1966; Price 1970, 1972; Lande 1980; Arnold and Wade 1984; Brodie et al. 1995). When multiple traits determine fitness, then a multivariate response to selection is the product of a matrix of additive genetic variances and covariances and a vector of selection gradients (Lande and Arnold 1983). Because selection gradients clearly connect to the response to selection and gradients are (in principle) easy to measure, they are a popular comparative metric for studies of natural selection (Hoekstra et al. 2001; Kingsolver et al. 2001).
Two interpretive caveats exist, however, in the general case. First, selection gradients quantify a causal model of fitness, which is defined by the investigator and may be incomplete. For example, if unmeasured fitness traits correlate with measured traits, then selection gradients may present a misleading perspective of causality (Wade and Kalisz 1990). Second, selection gradients do not directly reflect all the adaptive significance of a trait in the sense that they do not indicate how much selection for fitness is generated by trait variation. I will show how both issues can be resolved when one considers relationships between fitness and vital rates.
The opportunity for selection
Variation in relative fitness, the covariance between relative fitness and itself, is a fundamentally important quantity for describing the adaptive potential of a population (Crow 1958; O’Donald 1970a, b; Wade 1979; Wade and Arnold 1980). This variance, also known as the opportunity for selection, or I, summarizes selection on all traits, measured or not, defines selection on relative fitness, and places an upper-limit on the evolutionary change for any trait. Fitness change from one generation to the next owing to selection is , where is the narrow-sense heritability of fitness. I has been measured in many human populations to assess their adaptive capacities (e.g., Brown et al. 2009; Moorad et al. 2011; Courtiol et al. 2012) and to evaluate how these potentials may change over time (Terrenato et al. 1979; Hed 1987; Reddy and Chopra 1990; Korpelainen 2003; Alfonso-Sanchez et al. 2004; Gautam 2009; Moorad et al. 2011).
Components of I
Recently, Moorad and Wade (2012) derived the relationship between selection gradients and I. They showed how a causal model of fitness explains some measurable fraction R2 of the total opportunity for selection. More usefully, they demonstrated how the fitness variance explained by the causal model, R2I, can be decomposed into additive components attributable to each trait. Each component is a function of the relevant selection differential, and each defines the capacity for a population to evolve greater fitness through selection on identified traits. Crow (1958) suggested a crude method to partition I into components that derive separately from differential juvenile mortality and realized total lifetime reproductive variance (conditioned on juvenile survival). This method is employed by most analyses of human demographic transitions (Terrenato et al. 1979; Hed 1987; Reddy and Chopra 1990; Korpelainen 2003; Alfonso-Sanchez et al. 2004; Gautam 2009). Unfortunately, this conflates adult mortality with fertility, which may inflate or deflate contributions of mortality and reproduction to I, and this approach does not always define fitness properly for populations with overlapping generations. Nor does it allow a fine-scale perspective on the contributions of specific vital rates. These issues are resolved here.
Fitness
These methods require that fitness be measured precisely. In simple cases, fitness can be equated with the number of offspring, or total lifetime reproductive success (R0). When generations overlap and the population size changes over time, then the relationship between R0 and fitness becomes a complicated function of reproductive timing. In these cases, the need for a definition for individual fitness is often avoided by employing demographic and population ecology methods that equate population intrinsic growth rates (λ) with fitness (Hamilton 1966; Lande 1982; Charlesworth 1994; Caswell 2001). These approaches yield ‘sensitivities’ for vital rates that have been regarded as synonymous with a set of partial regression coefficients (selection gradients) that collectively define a model of fitness using all relevant vital rates (see Methods).
While sensitivities are valuable metrics for understanding selection for vital rates, their relationship to the opportunity for selection is unclear. The relative fitness variance in among individuals would seem to require measurements of individual fitness. Here I use Fisher’s concept of reproductive value to define individual fitness. The reproductive value quantitatively addresses the question, “To what extent will persons of this age, on the average, contribute to the ancestry of future generations?” (p.27, Fisher 1958), I apply the concept to the individual at birth (see equation [1] in Methods). This strategy is not without precedent: this definition of fitness has been applied to specific phenotypes (Lande 1982) and genotypes (Charlesworth 1994; Tatar and Promislow 1997), and Crow and Kimura (1970) applied the concept of reproductive value to individuals. This definition used here is fully consistent with Goodman’s observation (1982) that “Reproductive value is the central construct in life history optimization.”
Goals of this study
This study has two primary goals: 1) to show how multiple regression can be used to describe selection on phenotypes, and vital rates in particular, in populations with overlapping generations and 2) to illustrate how a demographic transition can alter patterns of natural selection for fitness and for vital rates. The study system is a female population of humans born between 1830 and 1894 who lived in what is now the state of Utah in the western USA. I begin by describing how a demographic transition in this population decreased age-specific mortality and fertility rates and lowered the intrinsic population growth rate λ. Second, I apply multiple regressions to longitudinal individual records of longevity and reproduction to show how these demographic trends changed selection differentials for vital rates. Third, I demonstrate how the opportunity for selection changed over time, and I partition I into vital rate-specific contributions to identify the main causes of its change over time. Finally, I explore the consequences of neglecting population growth by using R0 to measure natural selection.
Methods
Data
I used two sets of data from the Utah Population Database (UPDB) that were kindly provided by Dr. Ken Smith at the University of Utah. The first set contained birth and death dates of 70,889 unique females known to have reproduced born between 1830 and 1894 (mothers). The second set contained birth dates, sex, and parental identification for 630,410 individuals (children). Of these, 489,988 were identified to have been children of the ‘mothers’. Using ‘mother’ and ‘children’ datasets, individual longitudinal records of birth, death, and reproductive timing were constructed for all mothers. Mothers were subdivided into 65 birth-year-specific populations (cohorts) and treated as independent populations.
Mothers that produced no children were considered to be nulliparous. Note, however, that the UPDB is a descendent-based genealogy and some non-reproductives females may have not been included in the database. In addition, incomplete records may mean that some apparently nulliparous females may have reproduced. Moorad et al (2011) attempted to correct for these issues by including life table information derived from a US Census report. I used the estimated fractions of true nulliparity from Moorad et al (2011) to obtain a cohort-specific weighting factor that I applied to apparently nulliparous females. These weightings were used in all calculations.
Individual vital rates were derived from longitudinal records. Age-specific survival rates px were recorded for each of the first 99 years of life. Individuals that survived a transition from one year to the next were scored a value of ‘1’. Individuals that died were scored with a ‘0’. If a female did not survive to some age y, then her survival at that age was imputed from the population mean of the surviving subset (see Moorad and Wade (2012) for a discussion about imputing from the mean for non-existing data classes). For example, the first six survival rates for a female that died at age three would be {1,1,1,0, p̄5, p̄6}. Age-specific fertility rates mx were recorded for each of the first 100 years of life. As with survival rates, fertility rates at ages after death were imputed from the surviving population’s mean. Vital rates that exhibited no variance within a birth cohort (e.g., age-specific fertility at post-reproductive ages) were excluded from the analysis of selection for that cohort, and the relevant selection gradients and phenotypic (co)variances were defined as zero. Following the usual convention in evolutionary demographic studies (Bronikowski and Promislow 2005), age-specific survival rates are reported using the natural logarithm of mortality, ln(μx) = ln(− ln(p̄x)), where μx is the mortality rate at some age x. Age-specific reproductive rates are presented on their natural scale.
For any individual i born in cohort j, its fitness was is reproductive value at birth (Fisher 1958),
| (1) |
where lx is cumulative survival of the individual, it is the product of individual survival rates (included imputed values) from birth to age x (individuals have cumulative survival rates of ‘1’ for ages up to death and then ‘0’ thereafter). As the mean reproductive value at birth is one (Fisher 1958; Crow 2002), (1) expresses relative fitness. The intrinsic population growth rate λj is calculated from cohort-mean vital rates using population projection methods (Caswell 2001). For X consecutive age classes, an X-degree square matrix is constructed with mean age-specific fertility rates along the first row and mean age-specific survival probabilities along the subdiagonal. The dominant eigenvalue of this ‘Leslie’ Matrix (Leslie 1945) is λ. Joint distributions of vital rates and fitness (means, variances, and covariances) were calculated using values from mothers and appropriately weighted values from nulliparous females.
I calculated cohort-specific selection gradients for all vital rates (βwz(x)) by multiple regression, the total opportunity for selection (I) by calculating relative fitness variance, and trait-specific contributions to the opportunity for selection ( Iz(x)) using a method recently developed by Moorad and Wade (2012): the component of I that is explained by variation for some trait z is
| (2) |
where βwz and bwz are slopes of partial and simple regressions of relative fitness on the trait z, qz is the fraction of the population with a trait value, and is the phenotypic variance exhibited by this fraction. Partial and simple regressions are applied to all individuals in the population; trait values that are logically precluded by circumstance (and not by missing or incomplete observation) are imputed using the trait means of the individuals with meaningful values. Imputation will not affect the slopes of the regression, but the variance after imputation is equal to . Applied to a vital rate that is expressed at some age x, equation (2) becomes
| (3) |
Note that the sum of Iz taken over all vital rates is equal to I because the definition of relative fitness ensures that all fitness variance must derive from vital rate variance (R2 = 1). There are no unmeasured fitness traits, and, so long as fitness is defined properly, linear selection gradients accurately reflect causality. I discuss some implications of this property in the discussion.
Relationship between regression approach and life history models
Traditional demographic approaches calculate ‘sensitivities’ of intrinsic growth rates on population-mean vital rates; each of these means are conditioned on cumulative survival. These approaches also tend to quantify selection using time scales that are typically much briefer than generations (e.g., one year for humans). Following Lande (1982) and Charlesworth (1994), time scales can be reconciled by multiplying sensitivities by the number of time units in a generation. Applied to Charlesworth’s (1994) expressions for vital rate sensitivities gives, multiplication yields
| (4a) |
| (4b) |
A major strength of this approach is that vital rate means, frequently available in published life tables, can be used to describe selection. Sensitivities can also be calculating by re-sampling individual data (Coulson et al. 2006).
Lande (1982) and Caswell (2001) equate sensitivities with selection gradients. Viewed from this perspective, the cumulative survival terms within equations (4a–4b) discount selection gradients by the dead fraction of the cohort at age x (note that lx is a component of the ly term in [4a]). Multiple regression applied to mean-imputed non-existent data discounts the variance by the same amount. For the purposes of defining the strength of selection for a some vital rate, both methods are fundamentally equivalent: . For the remainder of this paper, I will refer to partial regression coefficients arising directly from multiple regressions as selection gradients and products of these with lx as sensitivities. As expected, sensitivities calculated in this analysis by multiple regression and by traditional methods (4a–b) were identical (e.g., see Fig. A1).
Contributions of specific vital rates to I change over time. To understand better why these changes occur, I partition each component of (3) into multiplicative elements. Note that differences between partial and simple regression coefficients (βwz(x) and bwz(x), respectively) can arise only through correlations between vital rates. In general, negative correlations (e.g., fitness trade-offs) will tend to make bwz(x) < βwz(x) and positive correlations (e.g., demographic heterogeneity sensu Vaupel et al (1979; 1998)) will cause bwz(x) > βwz(x). The ratio bwz(x)/βwz(x) can be thought of as an index of phenotypic integration across vital rates. Equation (3) can be re-stated as
| (5) |
which is a multiplicative function of measured values that can change with time: selection differentials, an index of vital rate integration, cumulative survival rates of the population, and trait variance among survivors.
Interpretation of the term is especially clear for age-specific reproduction: following (4b) and relationship between selection differentials and sensitivities, this term is equal to λ−2x, and (5) can be rewritten as
| (6) |
It would be useful if (6) could be used to predict how a demographic transition might affect I generated by age-specific fertility variance. For this, it is necessary to predict the effect of a transition on each of the four multiplicative components in (6). First, there appears to be no obvious relationship between demographic transitions and the ratio of simple to partial regression coefficients. Second, selection gradients for age-specific fertility, λ−x, strengthen as λ decreases, as might be expected if fertility rates decline, and the effect of this change on I is squared. Third, increases in survival will increase lx. Lastly, decreases in fertility will decrease in humans whenever time intervals are short because there is a strong association between the mean and variance of age-specific reproduction. Demographic transitions may affect the multiplicative components in equation (6) in different directions; meaning that no a priori predictions relating to changes in (6) can be made safely for all transitions. Nevertheless, these multiplicative components can each be measured repeatedly over time to quantify the effects of a specific demographic transition. I do this for each of the 65 female birth year cohorts.
The consequence of a demographic transition on the contribution of age-specific survival to I is less clear. From (4a), the selection gradient for age-specific survival is a function of λ and vital rates at older ages. Re-arranging this to reflect selection gradients,
| (7) |
Increased survival will increase the product of sequence term, but it will decrease the weighting of this term by increasing λ. Decreased age-specific reproduction will decrease the growth rate and increase λ−y. Thus, no obvious relationship emerges that would suggest that all demographic transitions must have the same qualitative effects on selection gradients for age-specific survival. The effects of demographic change on the opportunity for selection generated by age-specific survival (the square of this selection gradient placed into equation [5]) are even less predictable. I measure multiplicative components of (5) to assess the effects of the 19th century Utah demographic transition on each component Ip(x).
Lastly, I evaluated R0 as a measure of fitness by defining relative fitness for an individual i in cohort j as wij = R0,ij/R̄0,i. I re-analyzed the data in three ways. First, I estimated I in each cohort, essentially repeating the female analysis performed by Moorad et al (2011). Next, I correlated these new values for individual fitness to the original values separately for each cohort. Then, I recalculated specific vital rate contributions towards I using the new fitness values.
Results
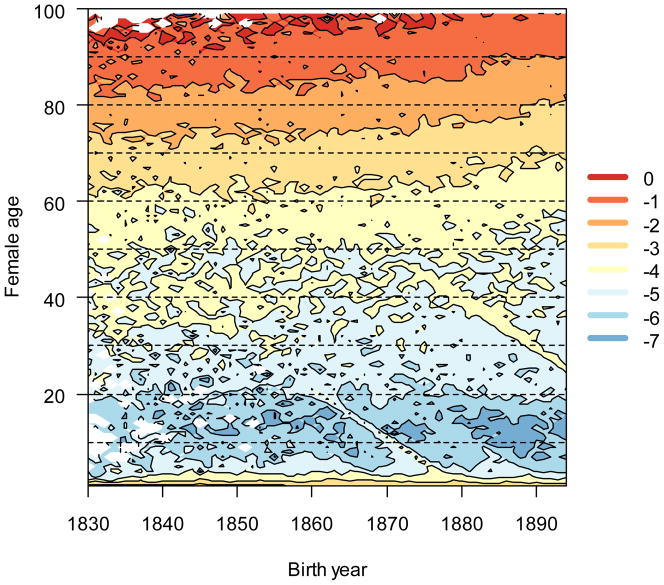
A demographic transition in the Utah population is indicated in three ways: decreases in age-specific mortality (Fig. 1), decreases in age-specific fertility (Fig. 2), and decreases in λ and R0 (Fig. 3). I found survival rates changed little in the ages before the early-twenties. Three notable exceptions are: 1) elevated first year mortality between the cohorts born between 1830 and the mid-1850s; 2) slightly suppressed mortality two-year-old in the first ten years of the study; and 3) an ephemeral burst of child and early-adult mortality in the late 1870s. The westward migration of the Mormons from previously settled areas where infant mortality was relatively high (Bean et al. 2002) began in 1846. The initial decline in infant mortality may reflect a change in population density. The mortality increase in the 1870s was likely caused by a diphtheria epidemic (Bean et al. 2002). Another brief increase in mortality co-occurred with the Spanish Flu outbreak of 1918, although the study period ended before the mortality effects on the very young can be observed here. Age-specific adult mortality rates decreased over the study period.
Figure 1.
Age-specific (log-scaled) mortality decreased over time. Dark shades (warm colors online) indicate low mortality rates, and light shades (cool colors) indicate high mortality rates. Reductions in age-specific mortality with time (a mortality transition) are visualized as isoclines that increase simultaneously along the x (birth year) and y (age) axes. White values represent ages where no deaths occurred (no mortality). Ridges that run diagonally down and to the right indicate brief (one to two years) periods of elevated mortality that is somewhat age-independent.
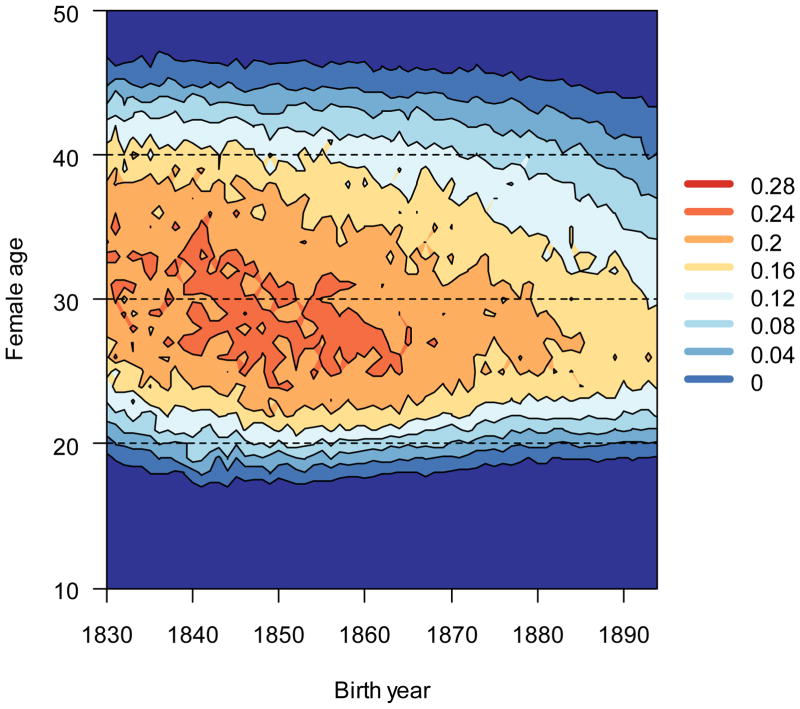
Figure 2.
Age-specific reproductive rates declined over time. Dark shades (warm) indicate high reproductive rates, and light shades (cool) indicate low reproductive rates. Reductions in age-specific reproduction with time (a fertility transition) are visualized as isoclines that decrease on the y axis (age) with increased birth year x.
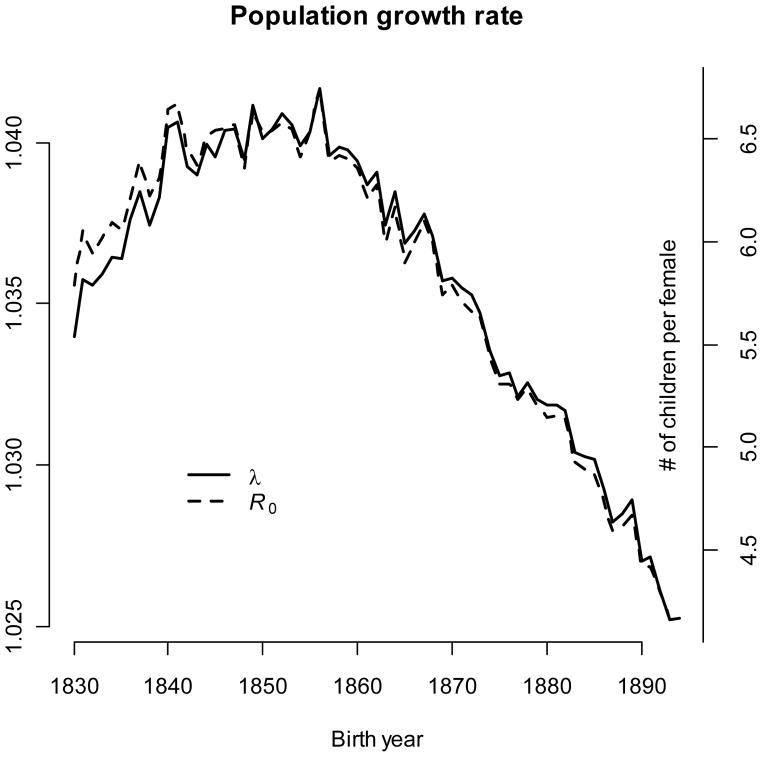
Figure 3.
Population growth rate and mean R0 changed over time. The left axis references the per-year growth rate (λ), and the right axis references the per-generation number of children produced by females.
There was a dramatic reduction in mean fertility at every age from 1850 onwards (Fig. 2). Mean fertility decreases were most precipitous at older ages. Late teenage reproduction decreased slightly, but teenagers never represented much of the total reproductive output of any cohort. The mode of the age-fertility function decreased from approximately 30 years of age in 1830–1855 to the mid-twenties over the next twenty-five years.
Intrinsic population growth rates and mean R0 tracked each other very closely over the study despite enormous changes to both (Fig. 3). The growth rate increased slightly from 1.035 to approximately 1.040/year over the first 25 years, after which it declined rapidly to 1.025/year. Mean R0 dropped from 6.4 to 4.2 children per female over the last 35 years.
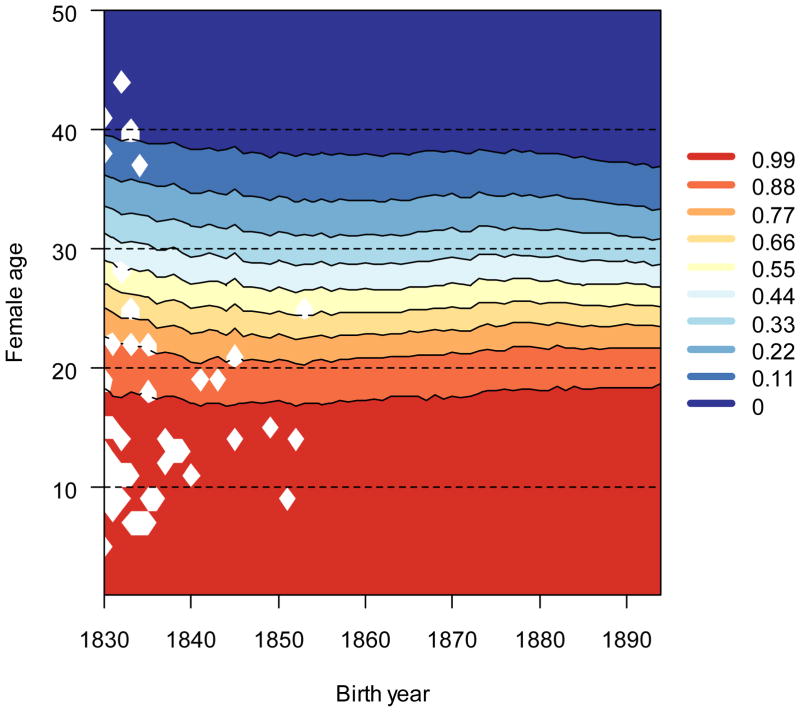
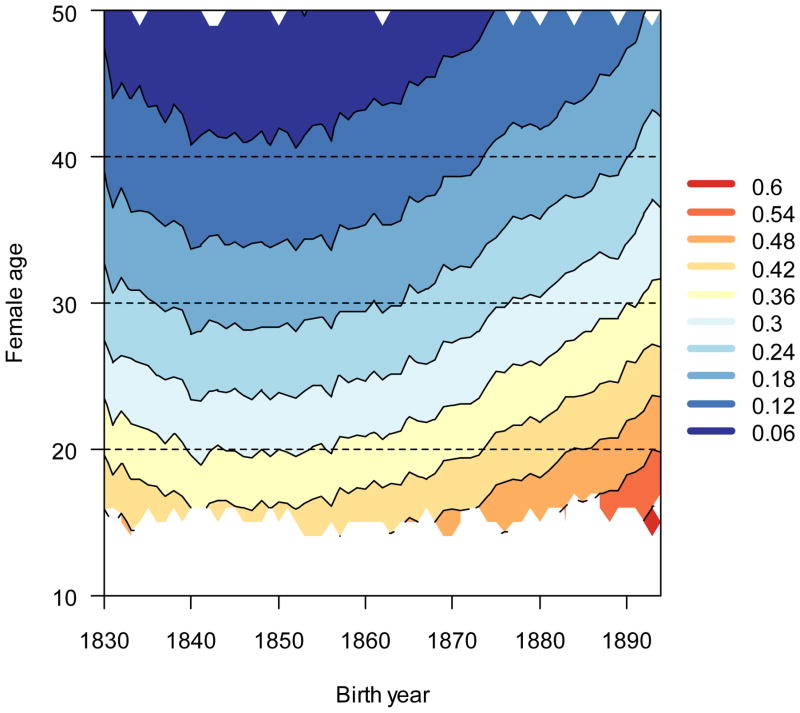
As measured by sensitivities, selection for age-specific survival changed slightly (Fig. 4) and selection for age-specific fertility changed dramatically (Fig. 5). Age-related decreases in survival sensitivities were slightly compressed in the later cohorts as compared to earlier cohorts; the age at which selection started to decline was delayed slightly, but the age-related declines in sensitivities were more precipitous. Survival sensitivities in the mid- and late-thirties were less for the later born cohorts than for the early cohorts. In contrast to selection trends for survival, sensitivities for age-specific reproduction (Fig. 5) increased greatly at every age from the 1840s to the 1890s.
Figure 4.
Selection for age-specific survival changed slightly over time. Sensitivities, the product of the selection gradients derived from multivariate regression and cumulative survival rates, are presented here. Dark shades (warm) indicate high sensitivities, and light shades (cool) indicate low sensitivities. White values represent ages at which no deaths were recorded.
Figure 5.
Selection (presented in terms of sensitivities) for age-specific reproduction increased over time. Dark shades (warm) indicate high sensitivities, and light shades (cool) indicate low sensitivities.
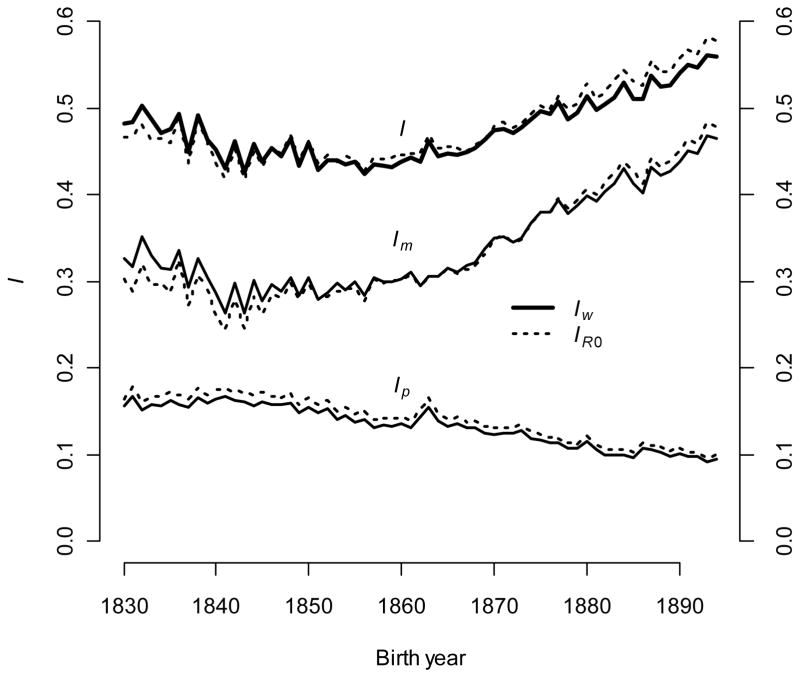
The total opportunity for selection decreased slightly from approximately 0.48 in the 1830s to 0.44 in the 1850s (Fig. 6). From this time onwards, I increased rapidly to 0.55 in the 1890s, an increase of approximately 20%. Because components of I combine additively, the total opportunity for selection was partitioned into complementary components of survival, IΣ(p), and fertility, IΣ(m). The trend over time clearly indicates that IΣ(m) increased and IΣ(p) decreased over absolute and relative scales. Approximately 67% of I was explained by IΣ(m) at the beginning of the study period, but that fraction increased to roughly 83% (corresponding to a 45% increase in the absolute contribution). At the end of the 19th century, the portion of I caused by IΣ(m) exceeded the total opportunity for selection in the middle of the century. The absolute influence of mortality on I was reduced by 37% between the first and last decade of the study.
Figure 6.
The opportunity for selection changed over time. The total opportunity for selection, I, is the sum of: 1) the opportunity derived from survival (over all ages), IΣ(p), and 2) the opportunity derived from reproduction (over all ages), IΣ(m). Opportunities are calculated using individual reproductive value at birth (sold line) and R0 (dotted line).
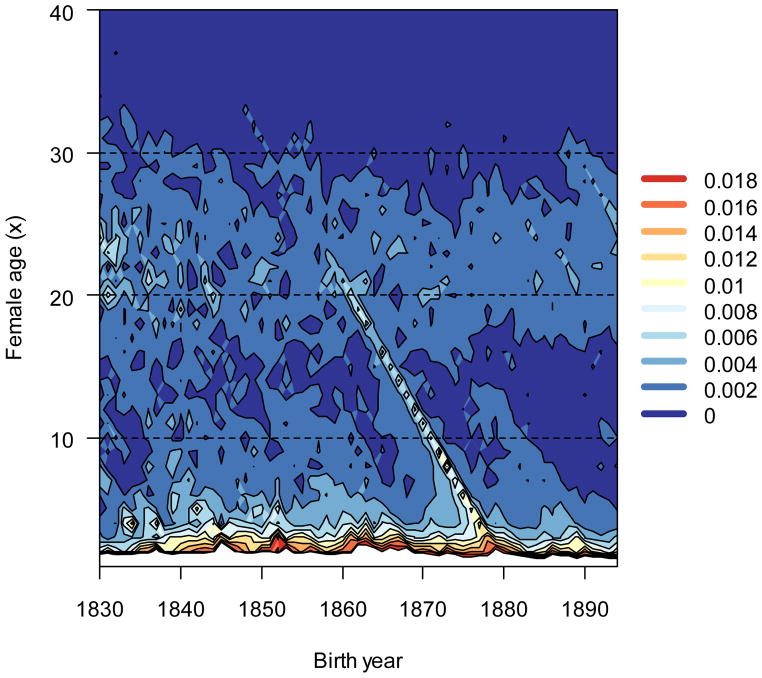
A finer-scale perspective of the relative fitness variance shows how age-specific components caused IΣ(p) to decline. After age three, there was no clear trend for changes in Ip(x) over time (Fig. 7). In general, there was a bimodal relationship between age x and Ip(x) that was preserved over the study period, with global maxima at the youngest ages, minima in the early teenage years, and local maxima in the early twenties. A ridge connecting the two maxima between the 1860 and 1878 cohorts reflects a brief, but intense, increase in Ip(x) caused by excess mortality that was likely associated with the diphtheria outbreak. I caused by survival variance in the first year of life, Ip(1), decreased by slightly more than half (Fig. 8). Survival variance over the next two years of age contributed more to the total opportunity for selection than any other age except year one, but these contributions were stable over time.
Figure 7.
The effect of age-specific survival for ages over three years of age on I changed little with time. Dark shades (warm) indicate relatively high contributions from age-specific survival, and light shades (cool) indicate low contributions. White areas represent ages (from birth to the third year) that generated too much variance in relative fitness to present graphically here. The contributions from survival in the first three years are presented separately in Fig. 8.
Figure 8.
The opportunity for selection derived from age-specific survival in the first three years. The contribution of survival in the first year of life to I declined quickly over time. Dark shades (warm) indicate relatively high contributions from age-specific survival, and light shades (cool) indicate low contributions.
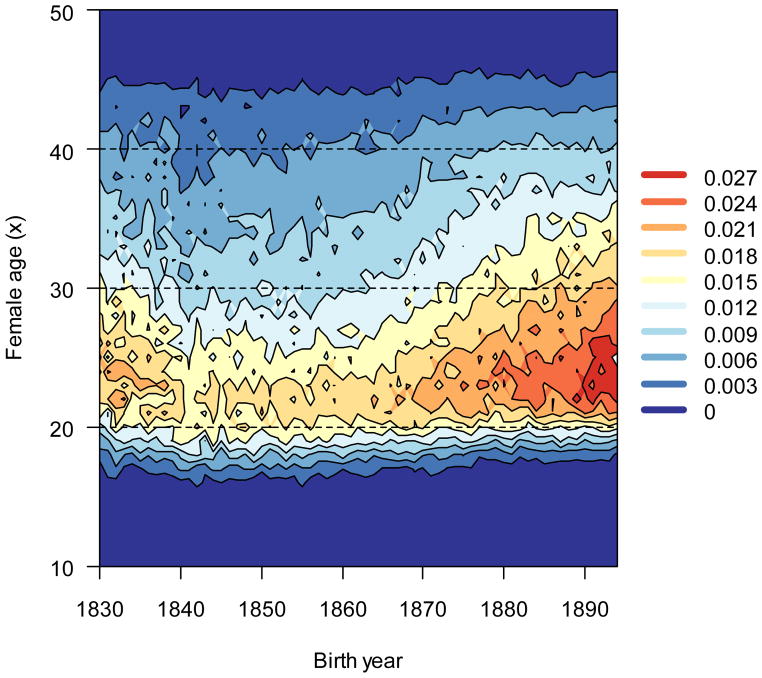
Age-specific fertility variance generated more I in later-born cohorts than in early-born cohorts (Fig. 9). Ages in the mid-twenties contributed most. Late teenage years contributed slightly more in the early part of the study period, but these differences were slight and their contributions were an order of magnitude smaller than at peak reproductive ages. Interestingly, fertility variation in the mid-thirties generated more fitness variance in later cohorts despite far greater fertility variance in the early cohorts (compare Figs. 2 and 9).
Figure 9.
Age-specific fertility at nearly all ages contributed more to I over time. Dark shades (warm) indicate relatively high contributions from age-specific reproduction, and light shades (cool) indicate low contributions.
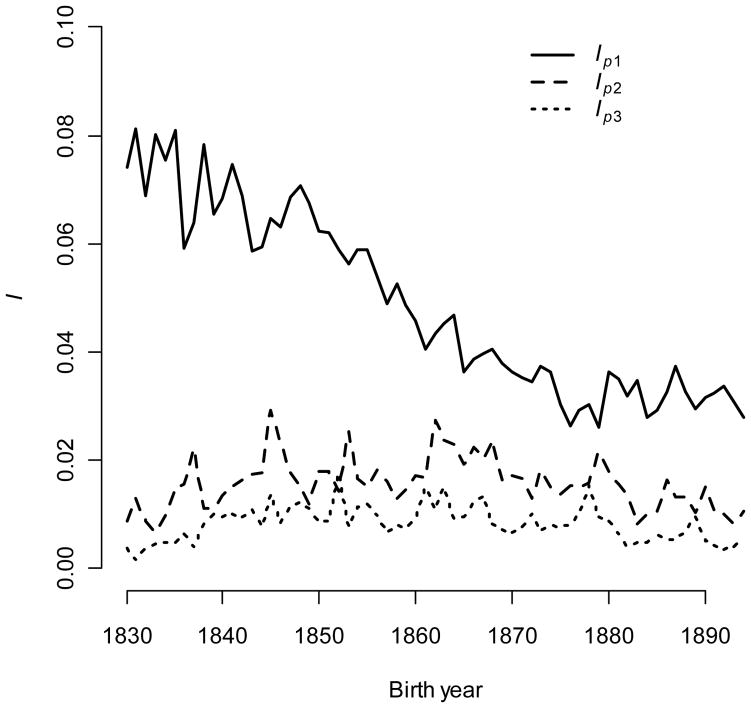
To summarize, I detected secular trends in vital rate contributions to the opportunity for selection. Contributions from infant survival and teenage fertility became less important, and contributions from fertility at all other ages became more important. I identified Ip(1), Im(18), Im(24), Im(30), and Im(36) as vital rates that best exemplified the consequences of the transition on the magnitude of I. I dissected each of these into four multiplicative components to identify the cause of change over time (Fig. A2). From (6), these are: 1) the squared selection gradient; 2) a measure of trait integration arising from correlations among vital rates; 3) cumulative survival; and 4) phenotypic variance. For year one, cumulative survival is constrained to be one. No correlations exist between p1 and other vital rates, and bwp(1) = βwp(1). The squared selection gradient decreased slightly, but this was not enough to explain large declines in Ip(1); change followed from declining mean first-year mortality (the variance of a frequency is the product of the mean and its complement).
The opportunity for selection caused by reproductive variance at 18 years of age increased quickly over the first few years of the study into the mid-1840s before steadily declining. Trait integration and cumulative survival changed little, but reproductive variance closely tracked changes in Im(18). Because changes in Im(18) and the squared selection gradient were in opposite directions, I infer that Im(18) change was driven primarily by reduced reproductive variance caused by reduced mean reproductive.
Changes in trait variances altered the relatively minor sources of If arising from early reproduction, but changes from later reproduction were driven by decreased intrinsic population growth rates. Furthermore, the proportional influence of declining population growth rates on Im(x) increased with increased age. This is expected as the selection gradient is λ−2x. Changing cumulative survival and inter-trait correlations had little effect on I. The contribution of reproductive rate variances decreased, as one would expect from declines in mean age-specific reproduction, but these decreases mitigated only slightly the influence of declining λ.
The relationship between R0 and w
There appeared to be very good agreement between I measured using w and R0 (Fig. 6). Because R0 is truly individual fitness when λ = 1 (see equation [1]), one might expect that IR0 and Iw values converged as population growth decreased over the study period. This did not happen. Survival rate variance created slightly more IR0 than Iw in all cohorts. Fertility rate variance created more Iw than IR0 in the early cohorts, but this relationship reversed in the later cohorts, when the rankings of total Iw and total IR0 changed.
Correlations between R0 and fitness were nearly perfect within cohorts (between 0.978 and 0.992 – see Fig. A3). Other studies have compared joint distributions of individual population growth rates (another proposed definition of individual fitness) with R0 and quantified the strength of association between the two variables by using a regression slope instead of a correlation (McGraw and Caswell 1996; Brommer et al. 2002), but this interpretation is problematic because the variables can be scaled differently. For example, the slope of the regression of fitness on R0 in the 1843 cohort is 0.156 and the correlation is 0.978.
Given the extremely high correlation between w and R0, one might be tempted to use the latter measure and ignore population growth entirely. However, this will inaccurately identify from what traits the selection for fitness is coming. I calculated a Im(x) surface using R0 for fitness (Fig. A4) to compare to the surface calculated using w (Fig. 9). It is clear from a comparison of these two surfaces that R0 distorts the contributions to I by underestimating the importance of early reproductive variance and overestimating the importance of later reproductive variance. These biases likely contributed to the slight disagreements between IR0 and Iw.
Discussion
In principle, and irrespective of the mechanism for its origin, demographic transitions can change selection for fitness for vital rates by changing population growth rates, cumulative survival rates, and the variances and covariances of age-specific survival and reproduction. In the 19th century Utah demographic transition, the most important effect is to increase the total opportunity for selection by increasing contributions through age-specific reproductive rates, caused by decreased population intrinsic growth rates. In the general case, selection gradients for age-specific reproduction diminish with increased growth rates, and the influence of this change on contributions to the variance in relative fitness is squared. Because mortality rates declined in this population, reduced fertility must have decreased λ. Small changes in cumulative survival rates over the birth rate cohorts appeared to have little effect on I. The ultimate cause for enhanced selection on age-specific fertility and in the increased adaptive potential of fertility variation was a decline in fertility itself (a fertility transition). The direct effect of reduced fertility variance appeared to be very weak.
Empirical results and (6) suggest that all demographic transitions that decrease λ and increase survival rates should enhance the fitness variance caused by fertility variance. While it would seem that lowered fertility and λ would go hand-in-hand, it is possible that simultaneous large increases in survival and small decreases in reproductive rates could increase population growth rates. In fact, as decreased mortality has been invoked as a cause, or at least a predictor, of fertility transitions (Kirk 1996), it may be possible that demographic transitions are occasionally associated briefly with increased λ. In such cases, fertility selection and selection for fitness through fertility variance may be relaxed. Unfortunately, I am unaware of any other selection study that documents a transition in such ecological terms.
The major implication of these interpretations is that fertility transitions likely cause selection to act more strongly to resist further reductions in reproductive rates. Fertility transitions may generate negative evolutionary feedback: the more extreme the transition, the stronger the selection to oppose further transition. As the response to selection depends on selection and inheritance, however, any complete evolutionary theory of demographic transitions requires an understanding of heritable variation for fitness. Some evidence exists to suggest that demographic transitions can cause fitness heritability to change over time; Kohler et al (2002) reported that heritability for female fertility in Denmark increased over a fertility transition occurring between 1870 and 1968. A follow-up heritability study of fitness in the Utah population will help clarify this issue.
This Utah population suffered less mortality than most contemporaneous populations, especially at early ages (Bean et al. 2002). Large differences between early mortality rates among human populations can contribute to large differences in I (compare, for example, Moorad et al (2011) and Courtiol et al (2012)). A study of vital rates-specific contributions to I in a population with higher mortalities would likely find greater contributions from age-specific survival variance. The influence of mortality on the change on I would likely be more pronounced as there would be more survival variance to lose over the transition. Not surprisingly, large reductions in the influence of mortality upon I have been consistently observed in other studies of demographic transitions in populations with high early mortality (Terrenato et al. 1979; Hed 1987; Reddy and Chopra 1990; Korpelainen 2003; Alfonso-Sanchez et al. 2004; Gautam 2009). Note, however, that these changes are limited to mortality at subjectively defined juvenile age ranges and not changes caused by shifts in mortality at all ages. For example, these studies attribute the impact of changed survival in the 20s to effects caused by changed fertility.
Compared to females in these other populations, Utah females produced far more children, especially during the early frontier years of the Utah Territory. Notably, this was the only population to show a clear, sustained increase in I over a fertility transition. Some studies (Terrenato et al. 1979; Korpelainen 2003; Alfonso-Sanchez et al. 2004) show slight increases over some portions of fertility transitions, while others show little or no effects at all (Reddy and Chopra 1990; Gautam 2009). Hed (1987) showed a change in the opposite direction. With the exception of Korpelainen (2003), the effects of juvenile mortality variance overwhelmed the effects of fertility variance and caused I to decline persistently over time in all of these studies. These differences may reflect real differences among these demographic transitions (the Utah study clearly misses a large mortality transition, for example, and begins with much lower initial mortality levels). However, the current study and that of Korpelainen (2003) are the only attempts to correct for population growth in fitness measures (although our methods differ considerably). As I have shown here, ignoring this demographic detail can cause components of I to be biased. Combined with fertility-survival correlations that are ignored by Crow’s decomposition of I, these biases may cause the fitness effects of mortality and fertility variance to be conflated in unpredictable ways. As I show in this study, Moorad and Wade’s method (Moorad and Wade 2012) allows one to apportion variance components correctly and in an intuitively clear manner. The strategy introduced here to measure vital rate contributions to I yields more statistically sound and conceptually transparent comparative metrics for describing relevant changes in natural selection over time.
Selection gradients represent a different sort of comparative metric because they describe effects of hypothetical variation on fitness. Declines in intrinsic growth rates caused selection gradients for age-specific reproduction to increase, and this may have had profound effects on the evolution of senescence, which is intrinsically bound to selection for vital rates (Hamilton 1966; Charlesworth 1994; Caswell 2001; Baudisch 2005; Moorad and Promislow 2011). Two classes of evolution models for aging are mutation accumulation (Medawar 1952), which assumes some statistical independence between deleterious effects from mutations expressed at early and at late ages, and antagonistic pleiotropy (Williams 1957), which considers the consequences of mutations with beneficial early effects and deleterious late effects on vital rates. Population genetic models illustrate conditions under which senescence, manifested as age-related declines in survival or fertility, can evolve through either mechanism (Charlesworth 1994; Charlesworth and Hughes 1996; Charlesworth 2001). However, by varying selection for vital rates, demographic transitions may alter the calculus of these models and may change evolutionary trajectories in two ways. First, shorter lifespans may become more favored (or less unfavored) if genetic trade-offs between early-age fertility and late-age survival are important. Second, prolonged lifespan will be favored if genes for fertility predict greater lifespan.
At a finer scale of perspective, the effects of demographic transitions on the evolutionary fate of specific disease genes can be projected. Consider, for example, the BRCA1/2 mutation known to segregate in the Utah population. This mutation has been linked to elevated risks for developing breast and ovarian cancers (Easton et al. 1995; Friedman et al. 2006). Recently, Smith et al (2012) showed elevated fertility for BRCA1/2 carriers born in the early 20th century, and they interpreted this as evidence for a fitness/longevity trade-off. One can expect from the results of the current study that, all else equal, fitness benefits gained from carrying the BRCA1/2 genes are greater after the demographic transition than before. In other words, if BRCA1/2 still enhances fertility, it may promote the evolution of aging more strongly now than it did during the highly fertile early years of the Utah population. However, genetic screening may now increase selection against the gene by affecting family planning (Smith et al. 2004; Smith et al. 2012) and overwhelm, or even reverse, any change in selection caused by a fertility transition.
Multiple regression is a powerful and popular tool to understanding the evolution of phenotypes, but this is the first study to apply these methods to measure selection for age-specific survival and reproduction while allowing for population growth. This approach differs from traditional sensitivity-based methods for measuring this selection (e.g., Hamilton 1966; Charlesworth 1994; Caswell 2001), in part because it requires a definition for the fitness of individuals. However, both approaches make at least two assumptions that are violated in most sets of real data. First, they assume that vital rates are sex-independent. Sensitivity-based approaches have been proposed to relax this assumption (Charlesworth 1994; Tuljapurkar et al. 2007; Caswell 2008). In the companion paper, I relax the assumption by applying a two-sex definition of individual reproductive value at birth (Moorad 2012). Given sexual dimorphisms in vital rates in this population, it is perhaps safest to interpret the patterns of selection described in the present paper as pertaining only to the females. The second assumption is that vital rates are stable over time; this is clearly violated here, as it must be when a demographic transition is studied. There is a large literature dedicated to the analysis of selection when environmental variation causes vital rates to change (Tuljapurkar 1989; Tuljapurkar 1990; Andersen 1994; Caswell 2001). For the regression-based approach taken here, the concern is that changing vital rates alter the interpretation of reproductive values (Price and Smith 1972), and this change might cause individual reproductive values at birth to incorrectly identify individual relative fitness. Further investigation into this issue will be useful for refining this regression-based approach.
Others have employed R0 as absolute fitness (e.g., Clutton-Brock 1988) in populations with overlapping generations. However, as I have shown here, this practice can cause selection analyses to over-emphasize the importance of selection acting on (or through) late-acting traits when populations are growing. Some have advocated using intrinsic growth rates applied to individuals as measures of individual fitness (McGraw and Caswell 1996; Brommer et al. 2002; Korpelainen 2003), but others have argued that these measures are necessarily biased (Lenski and Service 1982). Partitioning covariances and variances that involve individual intrinsic growth rates consequentially may be fraught with conceptual problems, such as disagreements between the means of populations of growth rates and growth rates of populations. In addition, there is no acceptable definition for the long-term intrinsic growth rate of an individual that never reproduces (in principle, any value will do because the population/individual will reach size zero in the long term, and the product of any growth rate and zero is zero).
Individual intrinsic growth rates have been used to illustrate severe mismatches between R0 and putative fitness (McGraw and Caswell 1996; Brommer et al. 2002), but by defining individual reproductive value at birth as fitness, I find that reproductive output correlates extremely well with fitness. While R0 does not identify well the ages at which selection works best, it does quantify the relative fitness variance very well in this population. I see little evidence to question estimates of I from other studies of human populations simply because they ignore intrinsic growth, although I do note that the longer reproductive tenure in males will tend to intensify whatever problems might arise by ignoring intrinsic growth rates. A two-sex generalization of the age-structured model of individual fitness will help clarify this issue. In any case, if estimates of λ exist for a studied population, or if they can be obtained, then there is no reason not to use them to define a more appropriate measure of individual fitness.
I have shown that regressions on individual reproductive value at birth yields the same selection gradients for vital rates as those obtained using life table or population projection methods. I interpret this as a validation of the fitness definition and the approach, but does multiple regression offer any advantages over traditional methods? One advantage is that multiple regression clarifies the interdependence between fitness and vital rates in an important way. Note that from (1), fitness is defined as a linear function of vital rates. There is no error term, meaning that R2 = 1 (this can be verified by summing over (3) for all vital rates). When all vital rates are considered, all selection is directional and there is no opportunity for non-linear selection (e.g., I arising from stabilizing or correlational selection). In other words, the causal model of fitness defined by individual reproductive values and vital rates requires that these nonlinear selection differentials are irrelevant for predicting the phenotypic evolution of vital rates. This is in apparent disagreement with some interpretations of non-linear sensitivities that arise in some demographic analyses (e.g., Caswell 2001). I note, however, it is well-known that demographic analyses based upon population projection methods can yield large sensitivities for vital rates that are understood to be evolutionary irrelevant, such as fertility rates at pre-reproductive ages or age transitions that are logically impossible (such as skipping or reducing ages) (Caswell 2001).
Another advantage of multiple regression is that it allows us to reduce our causal model of fitness to assess the relationship between fitness and some character without holding all vital rates constant. Moorad and Wade (2012) measured the strength of directional and stabilizing selection on mating success in males of the Utah population, while controlling for death before the age of 15 to mitigate the effects of correlations between mating success and childhood death (R0 was defined as fitness in that paper). In principle, they could have included age-specific survival at all ages in their causal model of fitness to assess the strength of sexual selection that acted only through reproductive variance. While intentionally reducing the ability of a model to explain fitness may seem strange (i.e., R2 is decreased), it may be appreciated that adding traits (such as mating success) to a model with all relevant vital rates will add no explanatory power, and selection on the new traits will appear to be zero. The proper way to interpret this phenomenon is to appreciate that selection can act on other traits, but only by causing vital rate variance (which may or may not be included in the model).
Surprisingly, multiple regressions are not needed to estimate the components of I in age-structured populations. In fact, previous demographic methods can serve if individual-based data are available. Given the relationship between selection gradients, cumulative survival, and sensitivities, equation (3) can be rewritten as
| (7) |
where is the van Tienderen’s ‘integrated sensitivity’ (van Tienderen 1995; Caswell 2001). Note that ‘∂’ indicates that a partial regression is taken, and that ‘d’ indicates that a simple regression is taken. It should also be noted that because integrated sensitivities require a phenotypic correlation matrix to be measured, a complete description of the population means, variances, and covariances of all vital rates is required. In other words, the same information is needed to estimate components of the opportunity for selection regardless of whether multiple regression or equation (3) is used.
By way of conclusion, I suggest that researchers consider the multiple regression approach as a viable, simple, and flexible alternative to sensitivity analyses for describing the strength of selection acting on vital rates whenever individual-based data is available. I also recommend that selection studies include estimates of the opportunity for selection and its components to gain a valuable additional perspective on natural selection in age-structured populations.
Supplementary Material
Figure A1: Sensitivities by life table and multiple regression methods yield the same values. Life table values (lines) follow from eqs [4a–4b]. Measurements come from the 1862 cohort. This cohort was chosen because it was at the middle of the study period.
Figure A2: The determinants of vital rate contributions to I changed over time. Each of these determinants are standardized by the simple mean of the factor taken over the 65-year study period (unweighted by cohort size).
Figure A3: The correlations between individual reproductive values at birth and R0 are very high and change slightly over time. Changes are opposite of those observed from population growth rates (Fig. 3).
Figure A4: The opportunity for selection IR(0) derived from age-specific reproduction overestimates the contribution of late reproductive variance and underestimates the contribution of early reproductive variance. Warm shades indicate relatively high contributions from age-specific reproduction, and cool shades indicate low contributions.
Acknowledgments
The work was funded by NIH grant T32AG000139. This paper was improved by the thoughtful commentary of Susan Alberts, Monique Borgerhoff Mulder, Jean-Michel Gaillard, Charles Goodnight, Daniel Promislow, Ken Smith, Michael Wade, and one anonymous reviewer as well as helpful discussions with Brian Charlesworth, Alex Courtiol, and Shripad Tuljapurkar. I thank the Pedigree and Population Resource (funded by the Huntsman Cancer Foundation) for its role in the ongoing collection, maintenance and support of the Utah Population Database. I also acknowledge Ken Smith, Geraldine Mineau and Alison Fraser, for their careful management of and assistance with the data used for this study. The study is solely my responsibility and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Literature Cited
- Alfonso-Sanchez MA, Calderon R, Pena JA. Opportunity for natural selection in a Basque population and its secular trend: Evolutionary implications of epidemic mortality. Human Biology. 2004;76:361–381. [PubMed] [Google Scholar]
- Andersen M. Stochastic models of age-structured populations. Comments Theoretical Biology. 1994;3:365–395. [Google Scholar]
- Arnold SJ, Wade MJ. On the measurement of natural and sexual selection -theory. Evolution. 1984;38:709–719. doi: 10.1111/j.1558-5646.1984.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Baudisch A. Hamilton’s indicators of the force of selection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8263–8268. doi: 10.1073/pnas.0502155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean LL, Smith KR, Mineau GP, Fraser A. Infant deaths in Utah, 1850–1939. Utah Historical Quarterly. 2002;70:158–173. [Google Scholar]
- Borgerhoff Mulder M. The demographic transition: are we any closer to an evolutionary explanation? Trends in Ecology & Evolution. 1998;13:266–270. doi: 10.1016/s0169-5347(98)01357-3. [DOI] [PubMed] [Google Scholar]
- Brodie ED, Moore AJ, Janzen FJ. Visualizing and quantifying natural selection. Trends in Ecology & Evolution. 1995;10:313–318. doi: 10.1016/s0169-5347(00)89117-x. [DOI] [PubMed] [Google Scholar]
- Brommer JE, Merila J, Kokko H. Reproductive timing and individual fitness. Ecological Letters. 2002;5 [Google Scholar]
- Bronikowski AM, Promislow DEL. Testing evolutionary theories of aging in wild populations. Trends in Ecology & Evolution. 2005;20:271–273. doi: 10.1016/j.tree.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Brown GR, Laland KN, Borgerhoff Mulder M. Bateman’s principles and human sex roles. Trends in Ecology & Evolution. 2009;24:297–304. doi: 10.1016/j.tree.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell H. Matrix population models: construction, analysis, and interpretation. Sinauer Associates; Sunderland, Mass: 2001. [Google Scholar]
- Caswell H. Perturbation analysis of nonlinear matrix population models. Demographic Research. 2008;18:59–116. [Google Scholar]
- Charlesworth B. Evolution in Age-structured Populations. Cambridge University Press; Cambridge, UK: 1994. [Google Scholar]
- Charlesworth B. Patterns of age-specific means and genetic variances of mortality rates predicted by the mutation-accumulation theory of ageing. Journal of theoretical biology. 2001;210:47–65. doi: 10.1006/jtbi.2001.2296. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Hughes KA. Age-specific inbreeding depression and components of genetic variance in relation to the evolution of senescence. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6140–6145. doi: 10.1073/pnas.93.12.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. Reproductive success. University of Chicago; Chicago: 1988. [Google Scholar]
- Cohen B. The emerging fertility transition in sub-Saharan Africa. World Dev. 1998;26:1431–1461. [Google Scholar]
- Coulson T, Benton TG, Lundberg P, Dall SRX, Kendall BE, Gaillard JM. Estimating individual contributions to population growth: evolutionary fitness in ecological time. P Roy Soc B-Biol Sci. 2006;273:547–555. doi: 10.1098/rspb.2005.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtiol A, Pettay JE, Jokela M, Rotkirch A, Lumma V. Natural and sexual selection in a monogamous historical human population. Proceedings of the National Academy of Science. 2012;109:8044–8049. doi: 10.1073/pnas.1118174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JF. Some possibilities for measuring selection intensities in man. Human Biology. 1958;30:1–13. [PubMed] [Google Scholar]
- Crow JF. Perspective: Here’s to Fisher, additive genetic variance, and the fundamental theorem of natural selection. Evolution. 2002;56:1313–1316. [PubMed] [Google Scholar]
- Crow JF, Kimura M. Alpha Editions. Edina, MN: 1970. An Introduction to Population Genetic Theory. [Google Scholar]
- Demeny P. Early fertility decline in Austria-Hungary: a lesson in demographic transition. Daedalus. 1968;97:502–522. [PubMed] [Google Scholar]
- Easton DF, Ford D, Bishop DT, Haites N, Milner B, Allan L, Easton DF, Ponder BAJ, Peto J, Smith S, Ford D, Stratton M, Narod SA, Lenoir GM, Feunteun J, Lynch H, Arason A, Barkdardottir R, Egilsson DV, Bishop DT, Black DM, Kelsell D, Spurr NK, Devilee P, Cornelisse CJ, Varsen H, Birch JM, Santibanezkoref MS, Teare MD, Steel M, Porter D, Cohen BB, Carothers A, Smyth E, Weber B, Boehnke M, Collins FS, Cannonalbright LA, Goldgar D, Skolnick M. Breast and ovarian cancer incidence in BRCA1-mutation carriers. American journal of human genetics. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. The Genetical Theory of Natural Selection. Oxford University Press; Oxford, UK: 1958. [Google Scholar]
- Friedman E, Kotsopoulos J, Lubinski J, Lynch HT, Ghadirian P, Neuhausen SL, Isaacs C, Weber B, Foulkes WD, Moller P, Rosen B, Kim-Sing C, Gershoni-Baruch R, Ainsworth P, Daly M, Tung N, Eisen A, Olopade OI, Karlan B, Saal HM, Garber JE, Rennert G, Gilchrist D, Eng C, Offit K, Osborne M, Sun P, Narod SA, Study HBCC. Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res. 2006;8:R15. doi: 10.1186/bcr1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam RK. Opportunity for natural selection among the Indian population: secular trend, covariates and implications. J Biosoc Sci. 2009;41:705–745. doi: 10.1017/S0021932009990095. [DOI] [PubMed] [Google Scholar]
- Goodman D. Optimal life histories, optimal notation, and the value of reproductive value. American Naturalist. 1982;119:803–823. [Google Scholar]
- Hamilton WD. Moulding of senescence by natural selection. Journal of theoretical biology. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hed HM. Trends in opportunity for natural selection in the Swedish population during the period 1650–1980. Hum Biol. 1987;59:785–797. [PubMed] [Google Scholar]
- Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hoang A, Hill CE, Beerli P, Kingsolver JG. Strength and tempo of directional selection in the wild. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9157–9160. doi: 10.1073/pnas.161281098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. A theory of fertility and parental investment in traditional and modern human societies. Yearb Phys Anthropol. 1996;39:91–135. [Google Scholar]
- Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, Hill CE, Hoang A, Gibert P, Beerli P. The strength of phenotypic selection in natural populations. American Naturalist. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Kirk D. Demographic transition theory. Population Studies. 1996;50:361–387. doi: 10.1080/0032472031000149536. [DOI] [PubMed] [Google Scholar]
- Kohler HP, Rodgers JL, Christensen K. Between nurture and nature: the shifting determinants of female fertility in Danish twin cohorts. Soc Biol. 2002;49:218–248. doi: 10.1080/19485565.2002.9989060. [DOI] [PubMed] [Google Scholar]
- Korpelainen H. Human life histories and the demographic transition: a case study from Finland, 1870–1949. American journal of physical anthropology. 2003;120:384–390. doi: 10.1002/ajpa.10191. [DOI] [PubMed] [Google Scholar]
- Lande R. The genetic covariance between characters maintained by pleiotropic mutations. Genetics. 1980;94:203–215. doi: 10.1093/genetics/94.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. A quantitative genetic theory of life-history evolution. Ecology. 1982;63:607–615. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Lenski RE, Service PM. The statistical analysis of population growth rates calculated from schedules of survivorship and fecundity. Ecology. 1982;63:655–662. [Google Scholar]
- Leslie PH. On the use of matrices in certain population mathematics. Biometrika. 1945;33:183–212. doi: 10.1093/biomet/33.3.183. [DOI] [PubMed] [Google Scholar]
- McGraw JB, Caswell H. Estimation of individual fitness from life-history data. American Naturalist. 1996;147:47–64. [Google Scholar]
- Medawar PB. An Unsolved Problem of Biology. H.K. Lewis; London: 1952. [Google Scholar]
- Moorad JA. Multi-level sexual selection: Individual and family-level selection for mating success in a recent human population. Evolution Accepted for publication. 2012 doi: 10.1111/evo.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorad JA, Promislow DE. Evolutionary demography and quantitative genetics: age-specific survival as a threshold trait. Proceedings. 2011;278:144–151. doi: 10.1098/rspb.2010.0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorad JA, Promislow DEL, Smith KR, Wade MJ. Mating system change reduces the strength of sexual selection in an American frontier population of the 19th century. Evolution and Human Behavior. 2011;32:147–155. doi: 10.1016/j.evolhumbehav.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorad JA, Wade MJ. Selection gradients, the opportunity for selection, and the coefficient of determination. American Naturalist Accepted for publication. 2012 doi: 10.1086/669158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SP. Is low fertility a twenty-first-century demographic crisis? Demography. 2003;40:589–603. doi: 10.1353/dem.2003.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notestein F. Population: The long view. In: Schultz TW, editor. Food for the World. University of Chicago Press; Chicago: 1945. pp. 36–57. [Google Scholar]
- O’Donald P. Change of fitness by selection for a quantitative character. Theoretical population biology. 1970a;1:219–232. doi: 10.1016/0040-5809(70)90036-5. [DOI] [PubMed] [Google Scholar]
- O’Donald P. Measuring the change of population fitness by natural selection. Nature. 1970b;227:307–308. doi: 10.1038/227307a0. [DOI] [PubMed] [Google Scholar]
- Price GR. Selection and covariance. Nature. 1970;227:520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- Price GR. Extension of covariance selection mathematics. Annals of Human Genetics. 1972;35:485–490. doi: 10.1111/j.1469-1809.1957.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Price GR, Smith CA. Fisher’s Malthusian parameter and reproductive value. Annals of Human Genetics. 1972;36:1–7. doi: 10.1111/j.1469-1809.1972.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Reddy BM, V, Chopra P. Opportunity for natural selection among the Indian populations. American journal of physical anthropology. 1990;83:281–296. doi: 10.1002/ajpa.1330830303. [DOI] [PubMed] [Google Scholar]
- Robertson A. A mathematical model of culling process in dairy cattle. Animal Production. 1966;8:95–108. [Google Scholar]
- Smith KR, Ellington L, Chan AY, Croyle RT, Botkin JR. Fertility intentions following testing for a BRCA1 gene mutation. Cancer Epidem Biomar. 2004;13:733–740. [PubMed] [Google Scholar]
- Smith KR, Hanson HA, Mineau GP, Buys SS. Effects of BRCA1 and BRCA2 mutations on female fertility. P Roy Soc B-Biol Sci. 2012;279:1389–1395. doi: 10.1098/rspb.2011.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Promislow DEL. Fitness costs of female reproduction. Evolution. 1997;51:1323–1326. doi: 10.1111/j.1558-5646.1997.tb03980.x. [DOI] [PubMed] [Google Scholar]
- Terrenato L, Ulizzi L, San Martini A. The effects of demographic transition on the opportunity for selection: changes during the last century in Italy. Ann Hum Genet. 1979;42:391–399. doi: 10.1111/j.1469-1809.1979.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar S. An uncertain life: demography in in random environments. Theoretical population biology. 1989;32:227–294. doi: 10.1016/0040-5809(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Tuljapurkar S. Population dynamics in variable environments. Springer-Verlag; New York: 1990. [Google Scholar]
- Tuljapurkar S, Puleston CO, Gurven MD. Why men matter: mating patterns drive evolution of human lifespan. PLoS ONE. 2007;2:e785. doi: 10.1371/journal.pone.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tienderen PH. Life-cycle trade-offs in matrix population models. Ecology. 1995;76:2482–2489. [Google Scholar]
- Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, Iachine IA, Kannisto V, Khazaeli AA, Liedo P, Longo VD, Zeng Y, Manton KG, Curtsinger JW. Biodemographic trajectories of longevity. Science (New York, NY. 1998;280:855–860. doi: 10.1126/science.280.5365.855. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Wade MJ. Sexual selection and variance in reproductive success. American Naturalist. 1979;114:742–747. doi: 10.1086/424531. [DOI] [PubMed] [Google Scholar]
- Wade MJ, Arnold SJ. The intensity of sexual selection in relation to male sexual behavior, female choice, and sperm precedence. Animal Behaviour. 1980;28:446–461. [Google Scholar]
- Wade MJ, Kalisz S. The causes of natural selection. Evolution. 1990;44:1947–1955. doi: 10.1111/j.1558-5646.1990.tb04301.x. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A1: Sensitivities by life table and multiple regression methods yield the same values. Life table values (lines) follow from eqs [4a–4b]. Measurements come from the 1862 cohort. This cohort was chosen because it was at the middle of the study period.
Figure A2: The determinants of vital rate contributions to I changed over time. Each of these determinants are standardized by the simple mean of the factor taken over the 65-year study period (unweighted by cohort size).
Figure A3: The correlations between individual reproductive values at birth and R0 are very high and change slightly over time. Changes are opposite of those observed from population growth rates (Fig. 3).
Figure A4: The opportunity for selection IR(0) derived from age-specific reproduction overestimates the contribution of late reproductive variance and underestimates the contribution of early reproductive variance. Warm shades indicate relatively high contributions from age-specific reproduction, and cool shades indicate low contributions.