Abstract
Background
The aim of this study was to determine the maternal serum concentrations of eNOS, ADMA, and homocysteine in preeclamptic pregnancies.
Material/Methods
The study was carried out on 62 patients with pregnancy complicated by early onset and 53 patients with late onset preeclampsia. The control group consisted of 65 healthy normotensive pregnant patients. The serum eNOS, ADMA and homocysteine concentrations were determined using ELISA assays.
Results
Our study revealed elevated levels of homocysteine and ADMA in the serum of women with preeclampsia. The highest levels were observed in patients with early onset preeclampsia, but the differences between both groups of preeclamptic patients with early and late onset of preeclampsia were not statistically significant. Both groups of preeclamptic women had slightly lower levels of maternal serum endothelial nitric oxide synthase than in normotensive pregnant women, but these differences were not statistically significant.
Conclusions
The higher levels of homocysteine and ADMA observed in patients with early onset preeclampsia may suggest that higher levels of maternal serum homocysteine and ADMA correlate with the severity, and may determine the earlier clinical onset of the disease. The elevated levels of ADMA and the unchanged levels of eNOS in preeclamptic pregnancies suggest that NO deficiency in this pregnancy disorder results not from a reduced level or activity of eNOS, but from elevated levels of ADMA, an endogenous eNOS inhibitor. The lowering of increased levels of homocysteine and ADMA may be helpful in therapy of vascular disturbances occurring in preeclampsia.
Keywords: preeclampsia, homocysteine, ADMA, eNOS
Background
Preeclampsia, a unique human pregnancy disorder, is characterized by the development of new hypertension and proteinuria after 20 weeks of gestation in patients free from any clinical disease. But it is not only hypertension and proteinuria; preeclampsia is a multiorgan disease in which the target organs are the endothelium, brain, liver, kidneys (glomerular endotheliosis) and the coagulation system. Delivery still remains the only curative treatment in cases of severe preeclampsia, but is not always advantageous for the fetus. Management decisions concerning patients with preeclampsia must be individualized and should balance the maternal risks of continued pregnancy against the fetal risks associated with induced preterm delivery [1,2].
Preeclampsia is a major cause of maternal and fetal mortality and morbidity, and remains amongst the biggest challenges in obstetrics, but its precise etiopathogenesis is still unclear [3,4]. It has been suggested that the root cause of preeclampsia is the placenta [5,6]. The placenta, as the interface between the mother and fetus, regulates fetal growth and development. Its functions are determined by vascular development and blood flow, which depend on proper trophoblast growth and differentiation [6,7]. According to the most recent hypothesis, preeclampsia results from impaired placentation early in the beginning of the pregnancy, leading to placental hypoxia and dysfunction [3,7,8]. There are differences between the placental findings in early and late onset preeclampsia, but it is difficult to determine whether these are qualitative, indicating different diseases, or simply quantitative differences within the same disease [5]. It has been suggested that the onset, clinical manifestations, severity, and progression of preeclampsia are affected by the maternal response to placentally derived antiangiogenic factors that lead to the imbalance between angiogenic and antiangiogenic factors [3,4]. Nitric oxide (NO) regulates the placental blood flow and actively participates in trophoblast invasion and placental development [9]. One theory (of many) suggests that clinical manifestations of preeclampsia caused by failure of the placental vasculature and endothelial malfunction, including insufficient nitric oxide synthesis or NO bioavailability, may contribute to increased blood pressure, systemic vascular resistance, and sensitivity to the pressors [4,10–13].
Nitric oxide, initially described as an endothelium-derived relaxant factor (EDRF) is the smallest biologically active molecule produced by endothelial cells, and plays many important functions in basic life processes [14]. Nitric oxide is the key transmitter for the endothelium-dependent regulation of the vascular tone and it regulates blood pressure, abolishes the toxic activity of superoxide ions, inhibits the adhesion and activation of platelet aggregation, and acts as an anticoagulant and antiatherogenic substance [10,14]. Nitric oxide contributes to the vasodilatation of blood vessels and to the decrease in vascular resistance observed during normal pregnancy [10,13,15]. Nitric oxide is produced in intact endothelial cells by endothelial NO synthase (eNOS) as the key enzyme from L-arginine [14,15]. Preeclampsia is associated with impaired uteroplacental adaptations during pregnancy and abnormalities in the endothelial nitric oxide synthase (eNOS)-nitric oxide pathway. However, the mechanism associated with the alteration of nitric oxide formation in pregnancies complicated by preeclampsia is not well understood. It is also unknown whether eNOS deficiency plays a causal role in preeclampsia [16]. Animal studies suggest that hyperhomocysteinemia affects the blood vessel wall and causes a change in the endothelium and smooth muscle proliferation [17]. Hyperhomocysteinemia is a risk factor of cardiovascular diseases and vasculopathy. It may be a cause of changes and lesions in endothelial cells due to vascular fibrosis, which results in the activation of thrombogenesis, alterations in the coagulation system, and enhanced platelet activation – changes that are noted in preeclampsia [17]. It has also been postulated that hyperhomocysteinemia may contribute to the development of placental microvascular diseases and preeclampsia, which adversely affect the endothelium [18]. Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of endothelial NO synthase (NOS), has been linked to endothelial dysfunction [11,19,20]. In addition, preliminary evidence has suggested that hyperhomocysteinemia leads to endothelial dysfunction and an accumulation of ADMA [21,22]. These observations were the inspiration for the present study.
The aim of the present study was to evaluate the alterations of maternal serum concentrations of endothelial nitric oxide synthase (eNOS), asymmetric dimethylarginine (ADMA), and homocysteine in women with pregnancies complicated by early and late onset severe preeclampsia in comparison to healthy normotensive pregnant women.
Material and Methods
In total, 115 pregnant patients diagnosed with severe pre-eclampsia according to the criteria published in the National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy (ACOG 2002) [23], and 65 healthy normotensive women with uncomplicated pregnancies in their 3rd trimester were included in the study. Preeclamptic patients were divided into early onset preeclampsia (before 34+0 weeks of gestation; the ePRE group, n=62) and late onset preeclampsia (after 34+0 weeks of gestation; the lPRE group, n=53) groups.
Preeclampsia was diagnosed by an increased blood pressure of >140 mmHg systolic and >90 mmHg diastolic in women who were normotensive before 20 weeks of gestation accompanied by proteinuria, defined as the urinary excretion of >0.3 g protein in a 24-h specimen. Severe preeclampsia was diagnosed based on the following criteria: systolic blood pressure >160 mmHg, diastolic blood pressure >110 mmHg, and proteinuria >5 g/in a 24-h period. In addition, patients were considered to have severe preeclampsia if they had 1 or more of the following clinical manifestations: renal abnormalities (oliguria), hematologic abnormalities (thrombocytopenia and microangiopathic hemolysis), HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count and right-upper quadrant pain), or neurologic symptoms (headache, visual disturbances and seizures). None of the pregnant patients with preeclampsia were affected by chronic hypertension or renal disorders and/or proteinuria before pregnancy, and all were normotensive before the 20th week of pregnancy. Preeclamptic patients were admitted to the Department of Obstetrics and Perinatology in the University Hospital in Lublin because of the symptoms of the disease, but without signs of labor.
The control group consisted of 65 healthy normotensive pregnant patients with singleton uncomplicated pregnancies, without any renal, cardiac, or vascular diseases, with normal laboratory tests and appropriate-for-gestational-age weight infants (the Control group).
All arterial blood pressure measurements in the Control group of pregnant patients were normal and did not exceed 135/85mmHg, and none of the patients had proteinuria.
No participants smoked, used caffeine or alcohol, or had a history of endocrinological disease, diabetes, pre-pregnancy cardiovascular disease, or hypertension. Pregnant women with multiple pregnancies were also excluded from this study.
The blood pressure (BP) of all the participants was measured at rest. The body mass index (BMI) of the patients was calculated as kg/m2. Serum samples were collected from patients immediately after the diagnosis, before administering any medication, and from controls at their routine visits. The research protocol, including the consent form, was approved by the institutional Ethics Committee for the Protection of Human Subjects from the Medical University in Lublin, and informed consent for peripheral blood sampling was obtained from all participants.
Homocysteine endothelial nitric oxide synthase (eNOS) and asymmetric dimethylarginine (ADMA) determination
Blood samples collected from the patients were allowed to clot and then were centrifuged at 1500×g for 15 min and the serum samples were stored at −70°C until assayed. Commercially available enzyme-linked immunosorbent assay (ELISA) system kits (Human Sandwich ELISA kit, Axis-Shield Diagnostics Ltd, UK) were used according to the manufacturer’s recommendations, to determine the maternal serum homocysteine concentrations. The endothelial NOS3 levels were measured in the maternal serum samples using a commercially available ELISA kit according to the manufacturer’s instructions (Human Endothelial Nitric Oxide Synthase 3 kit made by USC Life Science Inc., Wuhan, China). The asymmetric dimethylarginine levels from maternal serum were evaluated using a sandwich ELISA assay according to the manufacturer’s instructions (Human ADMA Sandwich ELISA kit, Immundiagnostik AG, Stubenwald-Allee Ba, Bensheim).
Statistical analysis
Data were expressed as mean ±SD. All calculations were carried out using Statistica v.8 PL software. Analysis of variance (ANOVA) tests were used to test differences between the 3 independent groups. A statistically significant effect in ANOVA was followed up with post-hoc Tukey’s test in order to assess differences between groups. The level of statistical significance was established as p<0.05. Data are expressed as mean ±SD.
Results
There were no statistically significant differences in parity, maternal age, weight, and height in patient profiles between the groups. Creatinine and urea levels were normal in all patients.
Maternal BMI values were higher in both groups of patients with pregnancy complicated by preeclampsia than in the control group, but these differences were not statistically significant. Systolic and diastolic blood pressure and mean arterial blood pressure were higher in both study groups of pregnant women with early and late onset preeclampsia than in the control group. These differences were statistically significant (p<0.000001). The mean systolic blood pressure values were 167.43±16.69 mmHg in the ePRE group, 169.13±18.25 mmHg in the lPRE group, and 113.56±9.60 mmHg in the control group. The mean diastolic blood pressure values were 111.67±10.60 mmHg in the ePRE group, 109.06±9.14 mmHg in the lPRE, and 72.24±9.43 mmHg in the healthy controls. The mean arterial pressure values were 130.22±11.64 mmHg in group of early onset preeclampsia patients, 129.06±10.92 mmHg in patients with late onset preeclampsia, and 85.66±9.63 mmHg in the healthy controls. The characteristics of the study groups are presented in Table 1.
Table 1.
The basic characteristics of the pregnant women enrolled in the present study.
| Parameters | Early onset preeclampsia group (the ePRE group) (n=62) | Late onset preeclampsia group (the lPre group) (n=53) | The Control group (the C group) (n=65) | ANOVA test (p value; * statistical significance) | Post hoc test (* statistical significance) |
|---|---|---|---|---|---|
| Age (years) | 30.71±5.72 | 28.83±4.88 | 29.21±4.42 | p=0.081492 | |
| Gravidity | 1.97±1.48 | 1.59±0.94 | 1.41±0.68 | p=0.009280* | ePRE/C* |
| Parity | 1.74±1.40 | 1.46±0.80 | 1.34±0.55 | p=0.051795 | |
| Height (cm) | 163.11±5.61 | 164.27±6.14 | 164.70±5.54 | p=0.294651 | |
| Weight (kg) | 80.40±13.97 | 83.28±15.45 | 78.26±12.36 | p=0.216629 | |
| BMI (kg/m2) | 30.07±4.40 | 30.76±4.95 | 28.85±4.35 | p=0.113296 | |
| SBD (mmHg) | 167.43±16.69 | 169.13±18.25 | 113.56±9.60 | p<.000001* | ePRE/C*; lPRE/C* |
| DBP (mmHg) | 111.67±10.60 | 109.06±9.14 | 72.24±9.43 | p<.000001* | ePRE/C*; lPRE/C* |
| MAP (mmHg) | 130.22±11.64 | 129.06±10.92 | 85.66±9.63 | p<.000001* | ePRE/C*; lPRE/C* |
| Age of gestation (weeks) | 30.56±2.52 | 37.23±2.0 | 38.13±1.95 | p<.000001* | ePRE/lPRE*, ePRE/C* |
| Homocysteine levels (μmol/L) | 11.428±4.158 | 10.046±2.795 | 7.835±2.482 | p<.000001* | ePRE/C*, lPRE/C* |
| ADMA levels (μmol/L) | 0.586±0.163 | 0.555±0.165 | 0.488±0.111 | p=0.002146* | ePRE/C*; lPRE/C* |
| eNOS levels (U/ml) | 154.327±155.308 | 156.247±127.019 | 217.744±265.114 | p=0.646878 |
Data presented as a mean ±SD.
Statistical significance (p<0.05). Groups of studied pregnant women: C group – healthy normotensive pregnant women; ePRE group – preeclamptic women with early onset of preeclampsia; lPRE group – preeclamptic women with late onset of preeclampsia; SBP – systolic blood pressure; DBP – diastolic blood pressure; MAP – mean arterial blood pressure.
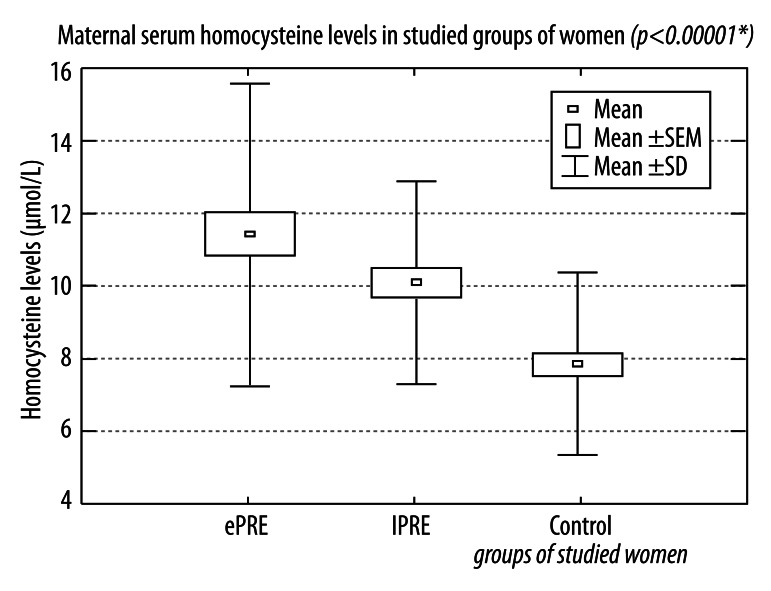
Our results show that the serum concentrations of homocysteine and ADMA were increased in both groups of women with preeclamptic pregnancies. The highest levels were observed in the patients with early onset preeclampsia, but the differences between groups of preeclamptic patients with early and late onset of preeclampsia were not statistically significant. The mean values of maternal serum homocysteine were 11.428±4.158 μmol/L in the ePRE group, 10.046±2.795 μmol/L in lPRE group, and 7.835±2.482 μmol/L in the control group (Figure 1).
Figure 1.
Maternal serum homocysteine levels in studied groups of women with pregnancies complicated by early and late onset preeclampsia and in healthy normotensive pregnant women.
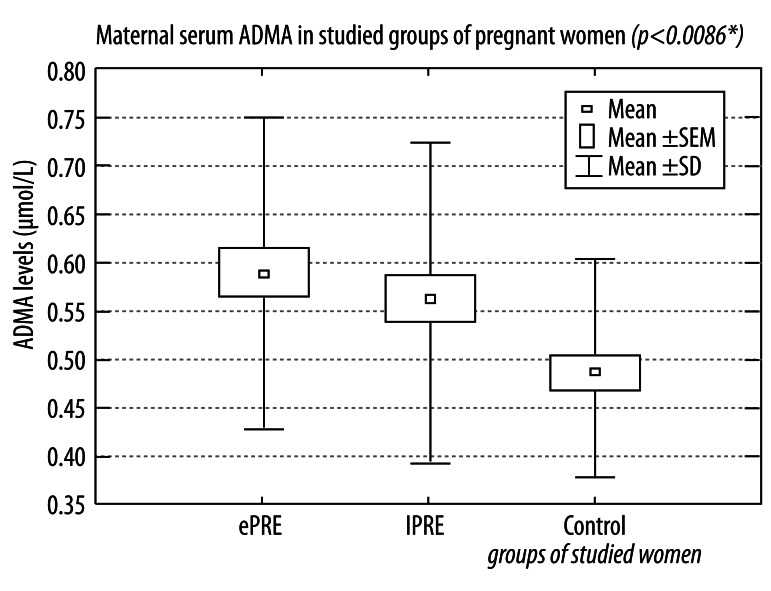
The mean values of maternal serum asymmetric dimethylarginine were 0.583±0.163 μmol/L in the group of early onset of preeclampsia, 0.555±0.165 μmol/L in the group of late onset of preeclampsia, and 0.488±0.111 μmol/L in the control group (Figure 2).
Figure 2.
Maternal serum asymmetric dimethylarginine in studied groups of women with pregnancies complicated by early and late onset preeclampsia and in healthy normotensive pregnant women.
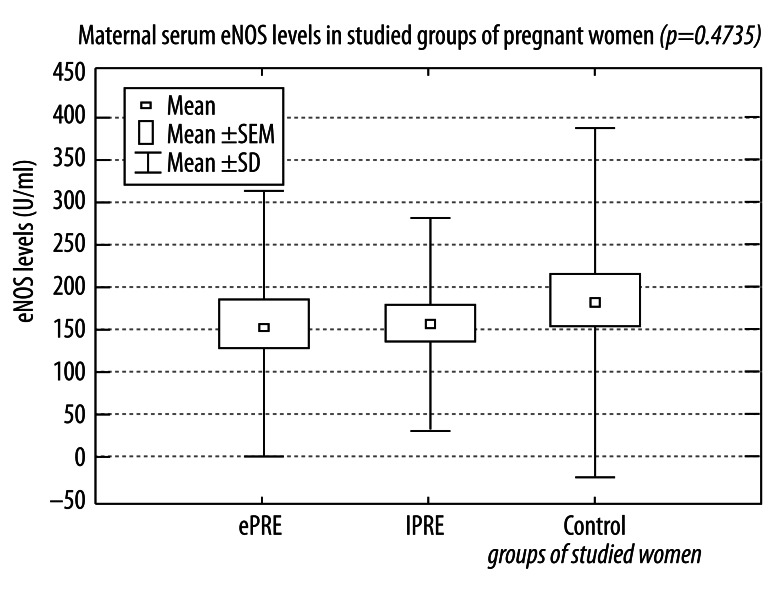
One important conclusion of our study is that we did not find a statistically significant decrease of eNOS concentrations in either group of preeclamptic women compared to the healthy women with uncomplicated pregnancies from the control group. The preeclamptic women had slightly lower levels of maternal serum endothelial nitric oxide synthase than in normotensive pregnant women, but these differences were not statistically significant. The mean values of maternal serum eNOS were 154.327±155.308 U/mL in women with pregnancies complicated by early onset preeclampsia, 156.247±127.019 U/mL in patients with late onset preeclampsia, and 217.744±265.114 U/mL in the healthy pregnant controls (Figure 3).
Figure 3.
Maternal serum endothelial nitric oxide synthase (eNOS) in studied groups of women with pregnancies complicated by early and late onset preeclampsia and in healthy normotensive pregnant women.
Discussion
During a normal pregnancy, spiral artery remodelling reduces maternal blood flow resistance and increases uteroplacental perfusion to meet the requirements of the fetus. It has also been observed that eNOS in the mother and in the fetus contribute to uteroplacental vascular changes and increased uterine arterial blood flow [16].
Whereas preeclampsia is associated with impaired uteroplacental adaptations during pregnancy and abnormalities in the endothelial NO synthase (eNOS)-NO pathway, it is unknown whether eNOS deficiency plays a causal role there [16]. It has also been suggested that disturbances in the homocysteine-ADMA-NO pathway may be at least partly responsible for the etiology of preeclampsia and could be regarded as markers for the severity of the disease [24]. Homocysteine inhibits the expression and activity of dimethylamino dimethyl hydrolase (DDAH), the enzyme hydrolyzing and degrading ADMA to citrulline and dimethylamine [10,21,25,26]. Because of this metabolic relation, it has been suggested that ADMA is a mediator of endothelial dysfunction in hyperhomocysteinemia [25].
In the present study we found significantly increased maternal serum concentrations of homocysteine and asymmetric dimethylarginine in pregnancies complicated by early and late onset preeclampsia compared to uncomplicated pregnancies. The higher levels of homocysteine and ADMA in patients with early onset preeclampsia may suggest a relationship between the levels of these factors and the time of the clinical manifestation of preeclampsia. They may also suggest that higher levels of maternal serum homocysteine and ADMA correlate with the severity, and may determine the earlier clinical onset of the disease. In contrast, endothelial nitric oxide synthase did not show any significant differences between normal and preeclamptic pregnant women.
Our results regarding the elevated ADMA and homocysteine levels in preeclamptic pregnancies compared to uncomplicated pregnancies are in agreement with several other studies. Rizos et al. [27] observed significantly elevated ADMA concentrations in the second trimester in pregnancies that later developed preeclampsia. López-Quesada et al. [28] found significantly higher homocysteine levels in preeclamptic women. Similar findings were observed by Wang et al. [29], who demonstrated elevated levels of maternal plasma homocysteine in preeclamptic pregnancies and in pregnancies with a suspected fetal compromise and umbilical or placental vascular disease. They concluded that elevated plasma homocysteine plays a role in the pathogenesis of the vascular disease in the uteroplacental circulation in placental insufficiency, and it in turn may suggest vascular lesions in the maternal uteroplacental bed in preeclampsia and fetal growth restriction.
Similar results concerning higher homocysteine levels in preeclamptic women and their positive correlation with asymmetric dimethylarginine concentrations were presented by Mao et al. [24]. These authors suggested that the altered homocysteine-ADMA-NO signalling pathway may be responsible for the etiology of preeclampsia [24].
Our findings are in disagreement with results from the study by Siroen et al. [30], who observed similar levels of ADMA in women with preeclampsia compared to normotensive pregnant women. However, they observed higher ADMA levels in women with a clinical worsening of preeclampsia with impaired condition of liver and kidneys, which are organs responsible for the elimination of ADMA. Siroen et al. [30] reported increased levels of ADMA in relationship to the laboratory parameters of liver and kidney dysfunction and the clinical picture of systolic and diastolic blood pressure, the birth weight of infants, and the weight of the placenta. On the basis of these studies, Siroen et al drew far-reaching conclusions, suggesting a causal role of ADMA in the development of renal failure and liver and placental insufficiency [30].
Holden et al. [19] showed that lowering blood pressure in early pregnancy is accompanied by a significant decrease in the plasma concentrations of asymmetric dimethylarginine. This phenomenon was not observed in women who developed preeclampsia later on. However, there was an increase in circulating blood levels of ADMA, and they reached higher levels than in non-pregnant subjects [30]. Holden et al confirmed the role of both asymmetric dimethylarginine and nitric oxide in the sequence of changes in blood pressure observed in both normal and preeclamptic pregnancies [19].
Our data are similar to those of Stühlinger et al. [21], who showed that the homocysteine induced increase in ADMA is associated with a reduction in DDAH activity, and that ADMA accumulation is associated with a temporally related decline in DDAH activity. These authors observed a reduced release of NO by endothelial cells in hyperhomocysteinemia, and suggested that impairment of the eNOS pathway by DDAH inhibition could have biological implications beyond the vasculature. Yucel et al. [31] suggested that hyperhomocysteinemia is a risk factor for atherosclerosis, and is associated with endothelial dysfunction.
However, it has been observed that hyperhomocysteinemia may also impair endothelial function through a mechanism largely independent of the pathway of ADMA/DDAH, and without elevating ADMA, probably through the inhibition of endothelial nitric oxide synthase activity by protein kinase C or oxidative inactivation of NO, induced by dysregulation of renal cellular antioxidant enzymes [24]. Dayal et al. found that hyperhomocysteinemia causes tissue-specific decreases in DDAH expression without altering plasma ADMA levels in mice, but with endothelial dysfunction [32].
Hyperhomocysteinemia may result in vasomotor dysfunction, because the amended structure and biomechanics of blood vessels and enhanced thrombosis are considered to be independent risk factors for metabolic and cardiovascular disease [26]. The mechanism of vascular damage by homocysteine has not been fully explained, but the importance of vascular smooth muscle cell proliferation and vascular remodelling leading to thrombosis and atherosclerosis should be considered. During normal pregnancy, physiological homocysteine levels are reduced secondary to hormonal changes and kidney, liver, and placental metabolism.
According to De Falco et al. [33], hyperhomocysteinemia during pregnancy could be responsible for placental abnormalities, which may be the cause of these very serious pregnancy complications. Steegers-Thenissen et al. [34] suggested that hyperhomocysteinemia was associated with an approximately 2- to 3-fold increased risk of pregnancy-induced hypertension, abruption of the placenta, and intrauterine growth restriction.
Elevated levels of ADMA and unchanged levels of eNOS in pregnancies complicated by severe preeclampsia suggest that the nitric oxide deficiency in this pregnancy disorder results not from a reduced level or activity of eNOS, but from elevated levels of asymmetric dimethylarginine, an endogenous eNOS inhibitor.
The results of the studies of eNOS activity were inconclusive. Myatt et al. [35] observed the primary location of eNOS in the syncytiotrophoblast of preeclamptic placenta. These authors also noted the lack of eNOS expression in vascular terminal villi and a weak expression in the endothelial cells of villous vessels in placenta from normal pregnancy. This location showed intense expression of eNOS in both types of vessels in placentas from pregnancies complicated by preeclampsia.
In contrast, Beinder et al. [36] observed similar placental level of eNOS activity in patients with pregnancy complicated by preeclampsia and healthy pregnant women with pathological and normal blood flow in the umbilical cord. However, they observed a lower activity of endothelial nitric oxide synthase where the uterine and placental vessels meet and increased uterine artery resistance in preeclamptic women compared to healthy pregnant women. Nasiell et al and Schiessl et al found significantly increased placental expression of endothelial nitric oxide synthase in pregnancies complicated by preeclampsia [37,38].
Our findings are also in disagreement with the results of Kim et al., who found lower expression of eNOS in the syncytiotrophoblast, reduced concentrations of L-arginine, and unchanged ADMA in the serum of women with pregnancies complicated by preeclampsia [39]. NO synthase plays a very important role in the physiology and pathology of the placental circulation; nitric oxide produced by endothelial nitric oxide synthase is an important regulator of cardiovascular physiology [39,40]. NO appears to be an antiatherogenic agent, thus ADMA may be a common mediator of endothelial dysfunction. In addition, studies have shown that ADMA is not only a risk factor for atherosclerosis and a marker of endothelial injury, but it can also play an important role in the progression of renal damage [41–43]. Homocysteine has an inhibitory effect on ADMA metabolism, leading to increased ADMA concentrations in hyperhomocysteinemia in preeclamptic women. Finally, this study suggests that lowering the increased homocysteine levels may be helpful in the therapy of vascular disturbances in preeclampsia and may be associated with the down-regulation and impaired bioavailability of NO that results from higher levels of ADMA, an endogenous endothelial nitric oxide synthase inhibitor. Larger scale prospective studies are needed to determine the impact of therapies aimed at decreasing serum homocysteine and ADMA levels.
Conclusions
Our results confirm the key role of elevated levels of homocysteine and asymmetric dimethylarginine in the development of preeclampsia. The higher levels of homocysteine and ADMA observed in patients with early onset preeclampsia may suggest a relationship between the levels of these factors and the time of clinical manifestation of preeclampsia. They may also suggest that higher levels of maternal serum homocysteine and ADMA correlate with the severity, and may determine the earlier clinical onset of the disease.
Elevated levels of ADMA and the unchanged levels of eNOS in pregnancies complicated by severe preeclampsia lead to the conclusion that the nitric oxide deficiency in this pregnancy disorder result not from a reduced level or activity of eNOS, but rather from elevated levels of asymmetric dimethylarginine, an endogenous eNOS inhibitor.
Our results also suggest that ADMA and homocysteine reduction may be a goal in the prevention and treatment of preeclampsia, but expanded studies are needed to develop new perspectives into this topic. Studies with larger populations will yield more informative results for understanding the etiological determinants of preeclampsia.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Source of support: Departmental sources
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Uzan J, Carbonnel M, Piconne O, et al. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–74. doi: 10.2147/VHRM.S20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–75. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 4.Rytlewski K, Huras H, Kusmierska-Urban K, et al. Lepton and interferon-gamma as possibile predictors of cesarean section among women with hypertensive disorders of pregnancy. Med Sci Monit. 2012;18(8):CR506–11. doi: 10.12659/MSM.883271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts JM, Escudero C. The placenta in Preeclampsia. Pregnancy Hypertens. 2012;2(2):72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31(Suppl):S66–69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mislanova C, Martsenyuk O, Huppertz B, Obolenskaya M. Placental markers of folate-related metabolism in preeclampsia. Reproduction. 2011;142(3):467–76. doi: 10.1530/REP-10-0484. [DOI] [PubMed] [Google Scholar]
- 9.Huang LT, Hsieh CS, Chang KA, Tain YL. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int J Mol Sci. 2012;13(11):14606–22. doi: 10.3390/ijms131114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demir B, Demir S, Pasa S, et al. The role of homocysteine, asymmetric dimethylarginine and nitric oxide in pre-eclampsia. J Obstet Gynaecol. 2012;32(6):525–28. doi: 10.3109/01443615.2012.693985. [DOI] [PubMed] [Google Scholar]
- 11.Fickling SA, Williams D, Vallance P, et al. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet. 1993;342:242–43. doi: 10.1016/0140-6736(93)92335-q. [DOI] [PubMed] [Google Scholar]
- 12.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 13.Speer PD, Powers RW, Frank MP, et al. Elevated asymmetric dimethylarginine concentrations precede clinical preeclampsia, but not pregnancies with small-for-gestational-age infants. Am J Obstet Gynecol. 2008;198:112 e111–e117. doi: 10.1016/j.ajog.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gielen S, Sandri M, Erbs S, Adams V. Exercise-induced modulation of endothelial nitric oxide production. Curr Pharm Biotechnol. 2011;12(9):1375–84. doi: 10.2174/138920111798281063. [DOI] [PubMed] [Google Scholar]
- 15.Stefano GB, Kream RM. Reciprocal regulation of cellular nitric oxide formation by nitric oxide synthase and nitrite reductases. Med Sci Monit. 2011;17(10):RA221–26. doi: 10.12659/MSM.881972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulandavelu S, Whiteley KJ, Qu D, et al. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension. 2012;60(1):231–38. doi: 10.1161/HYPERTENSIONAHA.111.187559. [DOI] [PubMed] [Google Scholar]
- 17.Aubard Y, Darodes N, Cantaloube M. Hyperhomocysteinemia and pregnancy – review of our present understanding and therapeutic implications. Eur J Obstet Gynecol Reprod Biol. 2000;93:157–65. doi: 10.1016/s0301-2115(00)00282-7. [DOI] [PubMed] [Google Scholar]
- 18.Sarandol E, Safak O, Dirican M, Uncu G. Oxidizability of apolipoprotein B-containing lipoproteins and serum paraoxonase/arylesterase activities in preeclampsia. Clin Biochem. 2004;37:990–96. doi: 10.1016/j.clinbiochem.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Holden DP, Fickling SA, Whitley GS, Nussey SS. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am J Obstet Gynecol. 1998;178:551–56. doi: 10.1016/s0002-9378(98)70437-5. [DOI] [PubMed] [Google Scholar]
- 20.Pettersson A, Hedner T, Milsom I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in pre-eclampsia. Acta Obstet Gynecol Scand. 1998;77(8):808–13. [PubMed] [Google Scholar]
- 21.Stuhlinger MC, Tsao PS, Her JH, et al. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569–75. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann W, Isber S, Obeid R, et al. Concentrations of homocysteine, related metabolites and asymmetric dimethylarginine in pre-eclamptic women with poor nutritional status. Clin Chem Lab Med. 2005;43(10):1139–46. doi: 10.1515/CCLM.2005.198. [DOI] [PubMed] [Google Scholar]
- 23.ACOG. Practice Bulletin No. 33: Diagnosis and management of preeclampsia and eclampsia. Obstetrics and Gynecology. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 24.Mao D, Che J, Li K, et al. Association of homocysteine, asymmetric dimethylarginine, and nitric oxide with preeclampsia. Arch Gynecol Obstet. 2010;282(4):371–75. doi: 10.1007/s00404-009-1234-6. [DOI] [PubMed] [Google Scholar]
- 25.Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: A systematic review. Placenta. 1999;20(7):519–29. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- 26.Lentz SR. Mechanisms of homocysteine-induced atherothrombosis. J Thromb Haemost. 2005;3(8):1646–54. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 27.Rizos D, Eleftheriades M, Batakis E, et al. Levels of asymmetric dimethylarginine throughout normal pregnancy and in pregnancies complicated with preeclampsia or had a small for gestational age baby. J Matern Fetal Neonatal Med. 2012;25(8):1311–15. doi: 10.3109/14767058.2011.632037. [DOI] [PubMed] [Google Scholar]
- 28.López-Quesada E, Also-Razo E, Vilaseca MA. Hyperhomocysteinemia during pregnancy as a risk factor of preeclampsia. Med Clin. 2003;121(9):350–55. doi: 10.1016/s0025-7753(03)73944-x. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Trudinger BJ, Duarte N, et al. Elevated circulating homocysteine levels in placental vascular disease and associated pre-eclampsia. Br J Obstet Gynaecol. 2000;107:935–38. doi: 10.1111/j.1471-0528.2000.tb11095.x. [DOI] [PubMed] [Google Scholar]
- 30.Siroen MPC, Teerlink T, Bolte AC, et al. No compensatory upregulation of placental dimethylarginine dimethylaminohydrolase activity in preeclampsia. Gynecol Obstet Invest. 2006;62:7–1331. doi: 10.1159/000091752. [DOI] [PubMed] [Google Scholar]
- 31.Yucel H, Ozaydin M, Dogan A, et al. Plasma concentrations of asymmetric dimethylarginine, nitric oxide and homocysteine in patients with slow coronary flow. Scand J Clin Lab Invest. 2012;72(6):495–500. doi: 10.3109/00365513.2012.699637. [DOI] [PubMed] [Google Scholar]
- 32.Dayal S, Rodionov RN, Arning E, et al. Tissue-specific downregulation of dimethylarginine dimethylaminohydrolase in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295(2):H816–25. doi: 10.1152/ajpheart.01348.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Falco M, Pollio F, Scaramelino M, et al. Homocysteinemia during pregnancy and placental disease. Clin Exp Obstet Gynecol. 2000;27:188–90. [PubMed] [Google Scholar]
- 34.Steegers-Theunissen RP, Van Iersel CA, Peer PG, et al. Hyperhomocysteinemia, pregnancy complications, and the timing of investigation. Obstet Gynecol. 2004;104:336–43. doi: 10.1097/01.AOG.0000129955.47943.2a. [DOI] [PubMed] [Google Scholar]
- 35.Myatt L, Eis AL, Brockman DE, et al. Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum Reprod. 1997;12(1):167–72. doi: 10.1093/humrep/12.1.167. [DOI] [PubMed] [Google Scholar]
- 36.Beinder E, Mohaupt MG, Schlembach D, et al. Nitric oxide synthase activity and Doppler parameters in the fetoplacental and uteroplacental circulation in preeclampsia. Hypertens Pregnancy. 1999;18(2):115–27. doi: 10.3109/10641959909023071. [DOI] [PubMed] [Google Scholar]
- 37.Nasiell J, Nisell H, Blanck A, et al. Placental expression of endothelial constitutive nitric oxide synthase mRNA in pregnancy complicated by preeclampsia. Acta Obstet Gynecol Scand. 1998;77(5):492–96. [PubMed] [Google Scholar]
- 38.Schiessl B, Mylonas I, Hantschmann P, et al. Expression of endothelial NO synthase, inducible NO synthase, and estrogen receptors alpha and beta in placental tissue of normal, preeclamptic, and intrauterine growth-restricted pregnancies. J Histochem Cytochem. 2005;53(12):1441–49. doi: 10.1369/jhc.4A6480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YJ, Park HS, Lee HY, et al. Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Placenta. 2006;27(4–5):438–44. doi: 10.1016/j.placenta.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 40.McCormick ME, Goel R, Fulton D, et al. Platelet-endothelial cell adhesion molecule-1 regulates endothelial NO synthase activity and localization through signal transducers and activators of transcription 3-dependent NOSTRIN expression. Arterioscler Thromb Vasc Biol. 2011;31(3):643–49. doi: 10.1161/ATVBAHA.110.216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Napora M, Graczykowska A, Próchniewska K. Relationship between serum asymmetric dimethylarginine and left ventricular structure and function in patients with end stage renal disease treated with hemodialysis. Pol Arch Med Wewn. 2012;122(5):226–34. doi: 10.20452/pamw.1222. [DOI] [PubMed] [Google Scholar]
- 42.Hörl WH. Uremic toxins: new aspects. J Nephrol. 2000;13(Suppl 3):S83–88. [PubMed] [Google Scholar]
- 43.Vanholder R, De Smet R, Glorieux G, et al. European Uremic Toxin Work Group (EUTox) Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 2003;63(5):1934–43. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]