Abstract
Background
Population-based estimates of the prevalence of thyrotoxicosis (TTX), the frequency of antithyroid drug (ATD) use, and risk of adverse events in pregnant women and their infants are lacking. Therefore, our objective was to obtain epidemiologic estimates of these parameters within a large population-based sample of pregnant women with TTX.
Methods
A retrospective claims analysis was performed from the MarketScan Commercial Claims and Encounters health insurance database for the period 2005–2009. Women aged 15–44 years, enrolled for at least 2 years, and who had a pregnancy during the study period were included. Diagnosis of TTX was based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes using narrow (TTX-1=ICD 242.0) and broad (TTX-2=ICD 242.0 or 242.9) definitions. ATD use was based on prescriptions filled for propylthiouracil (PTU) or methimazole (MMI). Adverse events in mothers and infants were determined from the ICD-9-CM diagnosis codes recorded on submitted claims.
Results
The database contained 904,497 eligible women. The average yearly prevalence per 1000 pregnant women was 2.46 for TTX-1 and 5.88 for TTX-2. Thirty-nine percent used ATD at any time during the study period. Compared to women without a TTX diagnosis, there was more than a twofold increase for liver disease among women with TTX (odds ratio [OR]=2.08, p<0.001) and a 13% increased risk for congenital anomalies (OR=1.13, p=0.014), but no association was observed with ATD use. The rates of congenital defects (per 1000 infants) associated with ATD use were 55.6 for MMI, 72.1 for PTU, and 65.8 for untreated women with TTX, compared to 58.8 among women without TTX.
Conclusions
There was some indication of an elevated risk of liver disease and congenital anomalies in women with TTX, but the risk did not appear to be related to the ATD use. There seems to be a higher pregnancy termination rate for women with TTX on MMI, which likely reflects elective pregnancy terminations.
Introduction
Thyrotoxicosis (TTX) is a common disorder in women, with Graves' disease being the most common cause (1,2). It is estimated that there are 30,000 women of child-bearing age with TTX in the United States, and that 4000–8000 women are treated for TTX during pregnancy annually (3–6).
Antithyroid drug (ATD) therapy is the recommended treatment for TTX during pregnancy (4,5). Based on the observations of birth defects (aplasia cutis and choanal atresia) in offspring of mothers treated with methimazole (MMI) during pregnancy (7–13) and the absence of such reports related to propylthiouracil (PTU) use, PTU has been recommended as the drug of choice for pregnant women with TTX (5). However, our preliminary review using the U.S. Food and Drug Administration Adverse Event Reporting System data (14) found a number of reported major birth defects in the offspring of women treated with PTU during pregnancy. From an internal review of International Clearinghouse for Birth Defects Surveillance and Research data, we observed birth defects with PTU (congenital heart defects) and MMI (choanal atresia and omphalocele) use during pregnancy.
In April of 2009, Rivkees and Mattison (15) alerted healthcare professionals of the risk of serious liver injury with the use of PTU in pediatric and adult patients. In April 2010, a black-box warning was added to the labeling of PTU (16). While a shift has been seen toward an increased MMI usage in the overall TTX population (17), it was noted that the current recommendations for the treatment of TTX during pregnancy with ATDs may not be optimal, and further studies of this issue were needed.
To address the limitations in this area, we examined the prevalence of TTX among pregnant women in a large U.S. study population and examined the frequency of use of antithyroid therapies (drug and surgical). In addition, the risk of adverse events during pregnancy and adverse pregnancy outcomes and birth defects was also examined.
Materials and Methods
A retrospective claims analysis was performed using the data from the MarketScan® Commercial Claims and Encounters database (18) for the period 2005–2009. The MarketScan database has been used extensively to conduct research across a wide range of medical issues to understand disease progression, treatment patterns, and health outcomes, with >500 articles using MarketScan data published in peer-reviewed journals. These data cover health insurance claims across the continuum of care, including inpatient, outpatient, and outpatient pharmacy claims, as well as enrollment data from large employers and health plans across the United States. This administrative claims database includes fee for service, preferred provider organizations, and capitated health plans. These data represent the paid medical claims of insured employees and their dependents for active employees, early retirees, COBRA* continues, and their dependents insured by employer-sponsored plans.
Our analyses included women aged 15–44 years who were enrolled for at least 24 months with prescription drug benefits and had at least two pregnancy-related medical service claims between 2005 and 2009. We also included the linked infant records of women with delivered pregnancies.
Definition of TTX
Two definitions for identifying women with TTX were used to maximize case ascertainment. The designation TTX-1 was a specific definition that required an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) (19) diagnosis code 242.0x (toxic diffuse goiter, including Graves' disease), where x denotes any value. The TTX-1 definition required at least one inpatient claim of 242.0x, or at least two outpatient claims of 242.0x that were at least 30 days apart.
The second definition, TTX-2, was broader than TTX-1 and required either ICD-9-CM diagnosis code 242.0x or 242.9x (TTX without mention of goiter or other cause). In addition, the TTX-2 definition required at least one inpatient claim of 242.0x or 242.9x; or at least two outpatient claims of 242.0x or 242.9x at least 30 days apart; or at least one outpatient claim of 242.0x or 242.9x and at least one prescription claim for the antithyroid medications PTU or MMI. Applying this definition, women with TTX-1 are also included in the TTX-2 group. Some analyses were based on mutually exclusive categories of women with TTX-1 versus those with TTX-2, but not TTX-1.
Prevalence of TTX among pregnant women
Calculations of TTX prevalence among pregnant women were calendar-year specific. Separate TTX prevalence estimates were computed for 2005, 2006, 2007, 2008, and 2009. To be included in the prevalence analysis in Year X (the denominator of the prevalence calculation), the women must have been continuously enrolled during Year X (i.e., enrolled for at least one day in each of the 12 months), and had to have two or more claims with a pregnancy-related code (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy) in Year X. To be counted as a case of TTX (the numerator of the prevalence calculation) generally required a medical claim with an ICD-9-CM code of 242.0x (for TTX-1) or 242.9 (for TTX-2) in Year X. Additional details of the case-defining algorithm are given in the Appendix.
Antithyroid therapy
Prescription drug claims were used to determine ATD therapy. For medications such as MMI and PTU, a claim signifies that a prescription for the drug was dispensed. Prescriptions filled within 6 months before or during the pregnancy were used for analyses that focused on acute adverse events potentially associated with a specific pregnancy. Prescription claims for thyroid hormone were used as a proxy for prior history of thyroidectomy or radioactive iodine (RAI), while American Medical Association Current Procedural Terminology (CPT) and ICD-9 procedure codes (19,20) were used to identify current RAI therapy and thyroidectomy.
Risk of adverse events in women
Our analyses focused on adverse events related to liver diseases. Adverse events were identified using the diagnosis (ICD-9-CM) codes from outpatient and inpatient claims (listed in Table 1). The events and medication use may have occurred at any time during the study period (before, during, or after pregnancy). For analyses, examining the association between drug exposure and adverse events, however, only drug use that occurred before the date of diagnosis of an adverse event was included.
Table 1.
Number and Prevalence (per 1000) of Women with Liver Disease by Drug Exposure Category
| |
Drug exposure categorya |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
MMI and/or PTUb |
No MMI or PTU |
|
|
||||||||
| |
MMI only |
PTU only |
MMI and PTU |
TTX |
No TTX |
Total |
||||||
| |
N=1201 |
N=1533 |
N=590 |
N=4902 |
N=801,375 |
N=809,601 |
||||||
| Liver disease (ICD-9-CM code) | n | Prev.c | n | Prev. | n | Prev. | n | Prev. | n | Prev. | n | Prev. |
| Acute and subacute necrosis (570) | 1 | 0.83 | 1 | 0.65 | 0 | 0.00 | 4 | 0.82 | 143 | 0.18 | 149 | 0.18 |
| Chronic liver disease and cirrhosis (571) | 1 | 0.83 | 2 | 1.30 | 2 | 3.40 | 23 | 4.69 | 1591 | 1.99 | 1619 | 2.00 |
| Liver abscess and sequelae (572) | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 5 | 1.02 | 99 | 0.12 | 104 | 0.13 |
| Other disorders of liver (573) | 4 | 3.34 | 8 | 5.23 | 0 | 0.00 | 44 | 8.96 | 2014 | 2.51 | 2070 | 2.56 |
| Livers disorders in pregnancy (646.7) | 0 | 0.00 | 1 | 0.65 | 0 | 0.00 | 9 | 1.84 | 642 | 0.80 | 652 | 0.81 |
| Any of the above | 6 | 5.03 | 10 | 6.54 | 2 | 3.40 | 70 | 14.24 | 4002 | 4.99 | 4090 | 5.05 |
| Odds ratiod | 0.96 | 1.24 | 0.66 | 2.70 | ||||||||
| 95% confidence intervald | [0.43, 2.15] | [0.67, 2.31] | [0.17, 2.65] | [2.13, 3.42] | ||||||||
| p-Valued | 0.924 | 0.500 | 0.559 | <0.001 | Ref. | |||||||
For women with the liver disease diagnosis, drug use is at any time before the diagnosis; for women without the liver disease diagnosis, drug use is at any time during the study. Therefore, sample sizes may vary slightly across disease categories due to the time censoring of the event.
MMI and/or PTU categories contain 176 women with a prescription claim for MMI and/or PTU, but without a claim with a thyrotoxicosis diagnosis (ICD-9-CM 242.0 or 242.9).
Prevalence per 1000 women.
Odds ratio, 95% confidence interval, and p-value for “Any of the above” with “No TTX” as the reference group from logistic regression model adjusting for mother's age.
MMI, methimazole; PTU, propylthiouracil; TTX, thyrotoxicosis; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; Prev., prevalence; Ref., used as reference for statistical comparisons.
Risk of adverse pregnancy outcomes and birth defects
We identified pregnancies and imputed gestational age at pregnancy outcome based on the diagnosis code associated with the pregnancy outcome using an algorithm adopted from Hornbrook et al. (21) (Supplementary Table S1). The list of ICD-9-CM codes used to define each congenital anomaly category is given in Supplementary Table S2. We included congenital anomalies diagnosed within 12 months of birth.
Statistical analysis
Descriptive statistical analyses included calculations of prevalence, percentages, and 95% confidence intervals (CIs). Odds ratios (ORs), CIs, and p-values were obtained from logistic regression models that adjusted for the mother's age. Statistical software SAS v. 9.2 (Cary, NC) was used for all analyses.
Results
Characteristics of the study population
The database contained records for 904,497 women between the ages of 15 and 44 with pregnancy-related claims during 2005–2009. Our study population comprised 809,601 women who represented 984,945 pregnancies with sufficient information to impute pregnancy start and end dates. The age distribution of pregnant women was consistent between the years 2005 and 2009, and the regional distribution of our study population reflected the U.S. population. Matched infant records were linked to 634,803 pregnancies, for a total of 641,939 infants.
Prevalence of TTX during pregnancy
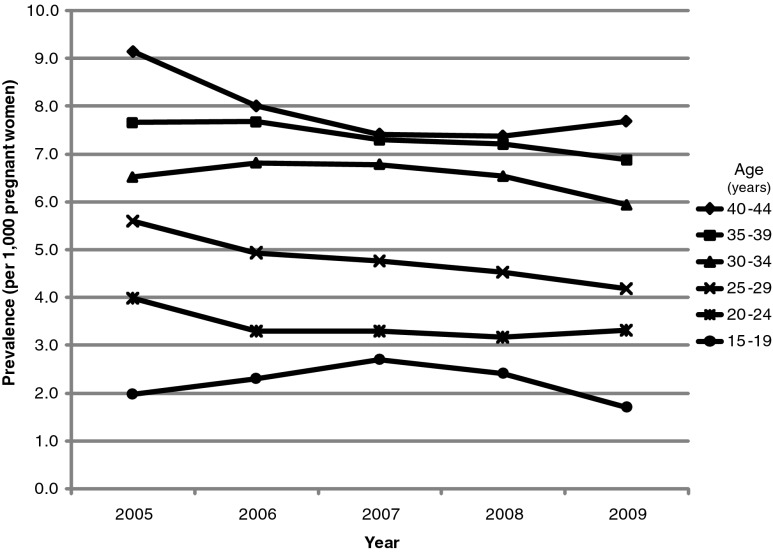
The average yearly prevalence of TTX-2 (the broader definition of 242.0x or 242.9x) among pregnant women was 5.9 per 1000 women, whereas the prevalence of TTX-1 (the narrower definition requiring 242.0x) was 2.5 per 1000 women. For both TTX definitions, the prevalence showed a slight decline from 2005 to 2009, and increased with age. The prevalence for TTX-2 by age and over calendar time is shown in Figure 1. The prevalence of TTX-1 and TTX-2 did not vary greatly by geographic region, although the highest prevalence was observed in the Northeast and the lowest prevalence was in the Midwest (data not shown).
FIG. 1.
Prevalence of thyrotoxicosis (per 1000 pregnant women)—Broad Definition [TTX-2; requires ICD-9-CM 242.0x or 242.9x]) by year and age. TTX, thyrotoxicosis; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification.
Use of ATD therapy
ATD use at any time during the study period is reported for three groups: TTX-1, TTX-2 (but not TTX-1), and those without a TTX diagnosis (Table 2). Women in the TTX-1 category were more likely to use MMI or PTU than women with TTX-2. However, 47% of women in the TTX-1 category did not use MMI or PTU during the study period, compared to 71% of women in the TTX-2 category. The percentages of women in the TTX-1 and TTX-2 categories who had thyroid hormone replacement therapy were 43% and 31%, respectively. For those women without a TTX diagnosis, the use of MMI or PTU was very low (<0.1%), and 5.7% received thyroid hormone replacement therapy.
Table 2.
Number (Percent) of Women on Antithyroid Therapy by Thyrotoxicosis Category
| |
Thyrotoxicosis category |
|
|
|||||
|---|---|---|---|---|---|---|---|---|
| |
TTX-1a(N=3304) |
TTX-2 (but not TTX-1)b(N=4746) |
No TTX (N=801,551) |
Total (N=809,601) |
||||
| Therapy used by women at any time during the study period | n | (%) | n | (%) | n | (%) | n | (%) |
| MMI | 1021 | (30.9) | 683 | (14.4) | 87 | (0.01) | 1791 | (0.22) |
| PTU | 1166 | (35.3) | 854 | (18.0) | 103 | (0.01) | 2123 | (0.26) |
| MMI or PTU | 1758 | (53.2) | 1390 | (29.3) | 176 | (0.02) | 3324 | (0.41) |
| MMI and PTU | 429 | (13.0) | 147 | (3.1) | 14 | (<0.01) | 590 | (0.07) |
| MMI only (no PTU) | 592 | (17.9) | 536 | (11.3) | 73 | (0.01) | 1201 | (0.15) |
| PTU only (no MMI) | 737 | (22.3) | 707 | (14.9) | 89 | (0.01) | 1533 | (0.19) |
| Neither MMI nor PTU | 1546 | (46.8) | 3356 | (70.7) | 801,375 | (>99.9) | 806,277 | (99.6) |
| THRT | 1433 | (43.4) | 1491 | (31.4) | 45,900 | (5.73) | 48,824 | (6.03) |
| RAI | 5 | (0.15) | 8 | (0.17) | 87 | (0.01) | 100 | (0.01) |
| Thyroidectomy | 103 | (3.12) | 94 | (1.98) | 1011 | (0.13) | 1208 | (0.15) |
TTX-1 applies to women with an ICD-9-CM diagnosis code of 242.0x at any time during study period.
TTX-2 (but not TTX-1) applies to women who do not have an ICD-9-CM diagnosis code of 242.0x, but do have a code of 242.9 at any time during study period.
THRT, thyroid hormone replacement therapy; RAI, radioactive iodine.
There were variations in the type of ATD therapies used within 6 months before, during, and after pregnancy among women with TTX. The use of MMI (only) decreased from 3.6% before the pregnancy to 0.9% during the pregnancy, and increased to 8.2% after the pregnancy. By contrast, the use of PTU (only) increased from 5.4% before the pregnancy to 10.7% during the pregnancy, and the PTU use remained at that level (10.0%) after the pregnancy.
We also examined drug use over the periods of embryogenesis, when teratogen exposure can lead to birth defects or adverse pregnancy outcomes. During pregnancy or within 6 months before pregnancy, 222 (2.2%) of the pregnant women had MMI (only) exposure, and 1204 women (11.8%) had PTU (only) exposure. Approximately 84% of women with TTX did not have a prescription filled for MMI or PTU during pregnancy or within 6 months before pregnancy.
Risk of adverse events in women
The prevalence of liver diseases for the two TTX categories was similar; thus, the two categories were combined. Women with a diagnosis of TTX were more likely to have liver disease than women without a diagnosis for several specific types of liver disease, as well as more than a twofold increased risk for any liver disease (OR=2.1 [CI 1.7, 2.6], p<0.001). When further examining the association between specific ATD use and liver disease, statistical comparisons were made only for the outcome “any liver disease” due to the small number of events for specific liver disease diagnosis codes (Table 1). We did not observe that women taking MMI and/or PTU were at an increased risk of liver disease. However, women with a TTX diagnosis who did not use MMI or PTU (i.e., no prescription claims submitted) had more than a twofold increase risk of liver disease (OR=2.7 [CI 2.1, 3.4], p<0.001).
Risk of adverse pregnancy outcomes and birth defects
Approximately 16% of all pregnancies ended at 8–10 weeks of gestation [based on an imputed gestational age using an algorithm adopted from Hornbrook et al. (21)]. Among pregnancies associated with MMI use within 6 months before or during pregnancy, the percentage of early terminations was 31%, compared with 9% among pregnancies where PTU was prescribed, and 16% among pregnancies where no ATD was prescribed or in women who did not have TTX. Among pregnancies that were not terminated at 8–10 weeks, ∼94% went to 32–40 weeks of gestation regardless of drug exposure or diagnosis of TTX.
The numbers and percentages of congenital anomalies diagnosed within 12 months of birth among the 641,939 infants who were linked to their mothers' pregnancy were computed. The overall prevalence of any congenital defect was similar in infants whose mothers were found to have TTX before or during pregnancy (65.7 per 1000) and those whose TTX was diagnosed after pregnancy (68.6 per 1000). Therefore, these two groups were combined and compared to infants of mothers who were not found to have TTX. Although the number of specific congenital defects was modest, there was a trend toward a higher prevalence of several specific types of anomalies among infants whose mothers had a TTX diagnosis. Overall, there was a 13% increased risk of any congenital anomaly among infants of mothers with a TTX diagnosis (OR [CI]=1.13 [1.03, 1.24]; p=0.014).
The prevalence of congenital anomalies was further examined according to the mothers' ATD exposure over the 6 months before or during the pregnancy (Table 3). The prevalence of any congenital anomalies in infants of women without TTX was 58.8 per 1000, which was similar to the prevalence among those exposed to MMI (OR=0.9 [CI 0.4, 2.1], p=0.85). For women in the PTU-only group, the OR was 1.2 ([CI 0.95, 1.6], p=0.12), and for those with exposure to both MMI and PTU, the OR was 2.0 ([CI 1.1, 3.5], p=0.02).
Table 3.
Number and Prevalence (per 1000) of Infants with Congenital Anomalies by Mother's Drug Exposure Category
| |
Mother's drug exposure |
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
MMI and/or PTUb |
No MMI or PTU |
|
|
||||||||||
| |
MMI only |
PTU only |
MMI and PTU |
TTXcbefore/during pregnancy |
TTX after pregnancy |
No TTX |
Total |
|||||||
| |
N=108 |
N=915 |
N=126 |
N=3236 |
N=2696 |
N=634,858 |
N=641,939 |
|||||||
| Congenital anomalya | N | Prev.d | N | Prev. | N | Prev. | N | Prev. | N | Prev. | N | Prev. | N | Prev. |
| Congenital anomalies of eye | 1 | 9.26 | 1 | 1.09 | 0 | 0.00 | 8 | 2.47 | 9 | 3.34 | 1540 | 2.43 | 1559 | 2.43 |
| Complex congenital heart disease | 0 | 0.00 | 13 | 14.21 | 3 | 23.81 | 26 | 8.03 | 21 | 7.79 | 3832 | 6.04 | 3895 | 6.07 |
| Atrial or ventricular septal heart defects (ASD/VSD) | 1 | 9.26 | 13 | 14.21 | 2 | 15.87 | 42 | 12.98 | 39 | 14.47 | 7970 | 12.55 | 8067 | 12.57 |
| Congenital anomalies of respiratory system | 0 | 0.00 | 5 | 5.46 | 1 | 7.94 | 8 | 2.47 | 6 | 2.23 | 1714 | 2.70 | 1734 | 2.70 |
| Congenital anomalies of genital organs | 0 | 0.00 | 10 | 10.93 | 3 | 23.81 | 30 | 9.27 | 31 | 11.50 | 6161 | 9.70 | 6235 | 9.71 |
| Congenital anomalies of urinary system | 0 | 0.00 | 7 | 7.65 | 2 | 15.87 | 16 | 4.94 | 20 | 7.42 | 2780 | 4.38 | 2825 | 4.40 |
| Certain congenital musculoskeletal deformities | 1 | 9.26 | 10 | 10.93 | 3 | 23.81 | 35 | 10.82 | 40 | 14.84 | 7432 | 11.71 | 7521 | 11.72 |
| Any congenital defecte | 6 | 55.56 | 66 | 72.13 | 14 | 111.11 | 205 | 63.35 | 185 | 68.62 | 37,351 | 58.83 | 37,827 | 58.93 |
| Odds ratiof | 0.93 | 1.22 | 1.98 | 1.05 | 1.18 | |||||||||
| 95% confidence intervalf | [0.41, 2.11] | [0.95, 1.57] | [1.14, 3.46] | [0.91, 1.21] | [1.01, 1.37] | |||||||||
| p-Valuef | 0.852 | 0.121 | 0.016 | 0.476 | 0.035 | Ref. | ||||||||
Congenital anomaly diagnosed within 12 months of birth.
Congenital anomaly categories with at least 1,000 infants in the total group.
Drug use within 6 months before the pregnancy start date or during pregnancy.
Thyrotoxicosis (ICD-9-CM 242.0x or 242.9x).
Prevalence per 1000 infants.
ICD-9-CM codes within 740–759. See Supplementary Table S2 for complete listing of congenital anomalies codes.
Odds ratio, 95% confidence interval, and p-value for “Any congenital defect” with “No TTX” as the reference group from logistic regression model adjusting for mother's age.
Discussion
The optimal medical management of TTX and Graves' disease in women during pregnancy is controversial, in part due to the absence of epidemiological studies that have examined the risks of antithyroid medications in this vulnerable population. In 2007, a consensus group recommended PTU as the ATD of choice during pregnancy because of potential concerns about MMI teratogenic effects (5). When a hepatotoxicity safety signal was unmasked in 2009 (15), it was suggested that the PTU use be restricted to the first trimester, and MMI be used in the remainder of pregnancy to minimize potential risks to the fetus and mother (22). In those recommendations, it was recognized that there were insufficient clinical and basic teratogenicity data to fully address this issue. To address these limitations, we examined the prevalence of TTX, the frequency of ATD use, and adverse events based on health insurance claims records of nearly one million pregnant women in the United States during 2005–2009.
Our nationwide study population included more than 800,000 women, representing one million pregnancies during the 5-year study period. The analysis of this study population produced precise estimates of the yearly prevalence of TTX among pregnant women. We applied two definitions of TTX using a narrow criterion (TTX-1, if a woman had a claim with an ICD-9-CM code of 242.0x) and a broader criterion (TTX-2, if a woman had a claim with an ICD-9-CM code of 242.0x or 242.9x). The average annual prevalence of TTX-2 in pregnant women (5.9 per 1000) was more than twice compared to TTX-1 (2.5 per 1000). For both definitions, we observed a slight decrease in the overall prevalence during this time period, a higher prevalence with increasing age, highest prevalence in the Northeast, and lowest prevalence in the Midwest.
The MMI and PTU use was more common among women with the more narrow definition of TTX, confirming the higher specificity expected with TTX-1. Interestingly, 47% of women in the TTX-1 group and 71% of women in the TTX-2 (but not TTX-1) group did not have a prescription filled for either MMI or PTU during our study period. In addition, 66% of women with a diagnosis of TTX had no evidence of ATD use or other related therapies during pregnancy.
MMI and PTU use was very specific to TTX. Only 0.2% of women without a TTX diagnosis had a prescription filled for MMI or PTU, and 95% of women who had a claim for MMI or PTU had a diagnosis of TTX. The exclusive use of MMI decreased from 3.6% before a pregnancy to 0.9% during pregnancy, and increased to 8.2% after pregnancy. These rates compare to the before, during, and after pregnancy use of exclusive use of PTU of 5.4%, 10.7%, and 10.0%, respectively. This pattern likely reflects recommendations that MMI be avoided during pregnancy (5).
The occurrence of liver disease and other adverse events associated with antithyroid medications was more common in women with TTX than those who did not have a diagnosis of TTX. However, when associations with specific drug exposures were examined, the highest rates of liver abnormalities were observed among women with TTX who did not have prescription claims for either MMI or PTU. There were no appreciable differences in the event rate between women prescribed MMI and those prescribed PTU, nor among those prescribed MMI or PTU and those without TTX. It is notable that women with TTX diagnoses but not treated with ATDs had higher rates of liver dysfunction than those treated. These observations may reflect the association between untreated or inactive hyperthyroidism and the elevation of liver transaminases, or the association between autoimmune disease and liver problems. There may be women with gestational TTX not receiving treatment, and abnormalities in liver function tests in these women are not uncommon, particularly in women with hyperemesis gravidarum. It is also possible that physicians avoided prescribing ATDs to women with known liver problems.
The percentage of early-terminated pregnancies (8–10 weeks of gestation) was significantly greater among women prescribed with MMI (31%) than those prescribed with PTU (9%). Considering the current warnings that MMI may be associated with an increased risk of birth defects, it is possible that women who had been taking MMI were encouraged to terminate their pregnancies. However, it is not possible to distinguish from our data whether early pregnancy terminations are due to elective abortions or are a consequence of an adverse medical condition in the mother or fetus. At present, it is important to note that there are no data to support the notion that pregnancies be terminated due to MMI exposure in gestation.
There was a slight trend toward an increased risk of congenital anomalies in infants whose mothers had a diagnosis of TTX. When examining exposures to specific ATDs, however, we did not observe an increased risk of congenital anomalies associated with MMI use, as only six infants with this exposure had any congenital defect, nor did we find a statistically significant association with PTU use. The lack of an association between MMI and congenital defects may be confounded by the fact that a higher percentage of pregnancies where the women were prescribed MMI was terminated early and did not result in a birth compared to PTU or no ATD therapy.
We recognize that retrospective claims analyses, such as this one, are subject to several limitations. Analyses are based exclusively on submitted health insurance claims without supplemental information or confirmation from medical charts. For example, we did not have laboratory test results or clinical notes to use as part of our identification of cases of TTX. We addressed these limitations by using two definitions of TTX (narrow and broad, based on ICD-9-CM codes) and requiring either one inpatient claim or two outpatient claims for visits at least 30 days apart. Medication use was based on the prescription fill date on a submitted claim. While the insurance claim indicates that the patient not only was prescribed but also obtained the drug, it is not a definitive record that the patient actually took the medication. Nevertheless, we found a very high correlation between our algorithms for identifying cases of TTX and use of MMI and PTU. We also recognize that despite the large size of our overall study population, the number of women and infants with specific rare adverse events (especially from women with TTX who used antithyroid medications) was modest. Therefore, we formed composite measures (e.g., any liver disease; any congenital anomaly) to increase the number of observed events and power (resulting in >80% power to detect at least a 2.5-fold increased risk). Nevertheless, it is possible that there are maternal risks associated with ATDs that occur too infrequently to be seen in this study.
Our data provide important population-based estimates of prevalence, medication use, and health-related outcomes that are relevant to the care of pregnant women with TTX. However, definitive answers to a number of questions remain unknown. After completion of basic science and other human epidemiology studies, new evidence-based recommendations should be developed for the treatment in women with TTX during pregnancies.
Supplementary Material
Appendix
Prevalence of Thyrotoxicosis Definitions
Calculations of thyrotoxicosis (TTX) prevalence among pregnant women were calendar year-specific (i.e., separate TTX prevalence estimates were computed for 2005, 2006, 2007, 2008, and 2009).
To be included in the population for the prevalence estimate in Year X (the denominator of the prevalence calculation), the women must have been continuously enrolled during Year X (i.e., enrolled for at least one day in each of the 12 months), and had to have two or more claims with a pregnancy-related code (Supplementary Table S1) in Year X.
To be included as a case of TTX-1 in Year X (the numerator of the prevalence calculation), a woman was counted (once) if she met any of the following conditions:
One or more inpatient claims of 242.0x in Year X
At least one outpatient claim of 242.0x in Year X and another outpatient claim of 242.0x in any year at least 30 days apart from any claim of 242.0x in Year X
At least one outpatient claim of 242.0x in Year X and an inpatient claim of 242.0x at any time (in any year)
For the prevalence of TTX-2 in Year X, a woman was counted (once) in the numerator if she met any of the following conditions (note: 242.0/9x means a claim for 242.0x or 242.9x):
Meets any of the conditions for TTX-1
One or more inpatient claims of 242.0/9x in Year X
At least one outpatient claim of 242.0/9x in Year X and another outpatient claim of 242.0/9x in any year at least 30 days apart from any claim of 242.0/9x in Year X
At least one outpatient claim of 242.0/9x in Year X and an inpatient claim of 242.0/9x at any time (in any year)
At least one outpatient claim of 242.0/9x in Year X and a claim for methimazole (MMI) or propylthiouracil (PTU) at any time (in any year)
At least one medication claim for MMI or PTU in Year X and an inpatient or outpatient claim of 242.0/9x at any time (in any year)
The prevalence was then computed as the numerator divided by the denominator, as defined above, and expressed as the number of cases per 1000 pregnant women.
Footnotes
The Consolidated Omnibus Budget Reconciliation Act of 1985 (COBRA) gives U.S. workers and their families who lose their health insurance benefits the option to extend their group health plan coverage for limited periods of time under circumstances such as voluntary or involuntary job loss, reduction in work hours, death, divorce, and other life events (www.dol.gov).
Acknowledgment
Support for this work was provided by NIH Grant R01HD65200.
Disclosure Statement
No competing financial interests exist.
References
- 1.Weetman AP. Graves' disease. N Engl J Med. 2000;343:1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- 2.Franklyn JA. Boelaert K. Thyrotoxicosis. Lancet. 2012;379:1155–1166. doi: 10.1016/S0140-6736(11)60782-4. [DOI] [PubMed] [Google Scholar]
- 3.Rivkees SA. Mandel S. Thyroid disease in pregnancy. Horm Res Paediatr. 2011;76(Suppl 1):91–96. doi: 10.1159/000329186. [DOI] [PubMed] [Google Scholar]
- 4.Chan GW. Mandel SJ. Therapy insight: management of Graves' disease during pregnancy. Nat Clin Pract Endocrinol Metab. 2007;3:470–478. doi: 10.1038/ncpendmet0508. [DOI] [PubMed] [Google Scholar]
- 5.Abalovich M. Amino N. Barbour LA. Cobin RH. De Groot LJ. Glinoer D. Mandel SJ. Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 6.Metsman JH. Hyperthyroidism in pregnancy. Best Pract Res Clin Endocrinol Metab. 2004;18:267–288. doi: 10.1016/j.beem.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Barbero P. Valdez R. Rodriguez H. Tiscornia C. Mansilla E. Allons A. Coll S. Liascovich R. Choanal atresia associated with maternal hyperthyroidism treated with methimazole: a case-control study. Am J Med Genet A. 2008;146A:2390–2395. doi: 10.1002/ajmg.a.32497. [DOI] [PubMed] [Google Scholar]
- 8.Valdez RM. Barbero PM. Liascovich RC. De Rosa LF. Aguirre MA. Alba LG. Methimazole embryopathy: a contribution to defining the phenotype. Reprod Toxicol. 2007;23:253–255. doi: 10.1016/j.reprotox.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Chattaway JM. Klepser TB. Propylthiouracil versus methimazole in treatment of Graves' disease during pregnancy. Ann Pharmacother. 2007;41:1018–1022. doi: 10.1345/aph.1H535. [DOI] [PubMed] [Google Scholar]
- 10.Wing DA. Millar LK. Koonings PP. Montoro MN. Mestman JH. A comparison of propylthiouracil versus methimazole in the treatment of hyperthyroidism in pregnancy. Am J Obstet Gynecol. 1994;170:90–95. doi: 10.1016/s0002-9378(94)70390-6. [DOI] [PubMed] [Google Scholar]
- 11.Mandel SJ. Brent GA. Larsen PR. Review of antithyroid drug use during pregnancy and report of a case of aplasia cutis. Thyroid. 1994;4:129–133. doi: 10.1089/thy.1994.4.129. [DOI] [PubMed] [Google Scholar]
- 12.Van Dijke CP. Heydendael RJ. De Kleine MJ. Methimazole, carbimazole, and congenital skin defects. Ann Intern Med. 1987;106:60–61. doi: 10.7326/0003-4819-106-1-60. [DOI] [PubMed] [Google Scholar]
- 13.Bachrach LK. Burrow GN. Aplasia cutis congenita and methimazole. Can Med Assoc J. 1984;130:1264. [PMC free article] [PubMed] [Google Scholar]
- 14.U.S., Food and Drug Administration Adverse Event Reporting System. www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/adversedrugeffects/default.htm. [Apr 20;2013 ]. www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/adversedrugeffects/default.htm
- 15.Rivkees S. Mattison D. Propylthiouracil (PTU) hepatoxicity in children and recommendations for discontinuation of use. Int J Pediatr Endocrinol. 2009;2009:132041. doi: 10.1155/2009/132041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA 2010 FDA Drug Safety Communication: New Boxed Warning on Severe Liver Injury with Propylthiouracil. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm209023.htm. [Apr 20;2013 ]. www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm209023.htm
- 17.Emiliano AB. Governale L. Parks M. Cooper DS. Shifts in propylthiouracil and methimazole prescribing practices: antithyroid drug use in the United States from 1991 to 2008. J Clin Endocrinol Metab. 2010;95:2227–2233. doi: 10.1210/jc.2009-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MarketScan® Commercial Claims and Encounters database. www.truvenhealth.com/your_healthcare_focus/pharmaceutical_and_medical_device/data_databases_and_online_tools.aspx. [Apr 20;2013 ]. www.truvenhealth.com/your_healthcare_focus/pharmaceutical_and_medical_device/data_databases_and_online_tools.aspx
- 19.International Classification of Diseases, Ninth Revision, Clinical Modification and Procedure Codes. http://icd9cm.chrisendres.com/index.php. [Apr 20;2013 ]. http://icd9cm.chrisendres.com/index.php
- 20.American Medical Association Current Procedural Terminology procedure codes. www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/cpt.page. [Apr 20;2013 ]. www.ama-assn.org/ama/pub/physician-resources/solutions-managing-your-practice/coding-billing-insurance/cpt.page
- 21.Hornbrook MC. Whitlock EP. Berg CJ. Callaghan WM. Bachman DJ. Gold R. Bruce FC. Dietz PM. Williams SB. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res. 2007;42:908–927. doi: 10.1111/j.1475-6773.2006.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahn R. Burch H. Cooper D. Garber J. Greenlee C. Klein IL. Laurberg P. McDougall IR. Rivkees SA. Douglas R. Sosa JA. Stan MN. The role of propylthiouracil in the management of Graves' disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid. 2009;19:673–674. doi: 10.1089/thy.2009.0169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.