Abstract
Background
Thyroid cancer incidence has risen steadily over the last few decades in most of the developed world, but information on incidence trends in developing countries is limited. Sao Paulo, Brazil, has one of the highest rates of thyroid cancer worldwide, higher than in the United States. We examined thyroid cancer incidence patterns using data from the Sao Paulo Cancer Registry (SPCR) in Brazil and the National Cancer Institute's Surveillance Epidemiology End Results (SEER) program in the United States.
Methods
Data on thyroid cancer cases diagnosed during 1997–2008 were obtained from SPCR (n=15,892) and SEER (n=42,717). Age-adjusted and age-specific rates were calculated by sex and histology and temporal patterns were compared between the two populations.
Results
Overall incidence rates increased over time in both populations and were higher in Sao Paulo than in the United States among females (SPCR/SEER incidence rate ratio [IRR]=1.65) and males (IRR=1.23). Papillary was the most common histology in both populations, followed by follicular and medullary carcinomas. Incidence rates by histology were consistently higher in Sao Paulo than in the United States, with the greatest differences for follicular (IRR=2.44) and medullary (IRR=3.29) carcinomas among females. The overall female/male IRR was higher in Sao Paulo (IRR=4.17) than in SEER (IRR=3.10) and did not change over time. Papillary rates rose over time more rapidly in Sao Paulo (annual percentage change=10.3% among females and 9.6% among males) than in the United States (6.9% and 5.7%, respectively). Regardless of sex, rates rose faster among younger people (<50 years) in Sao Paulo, but among older people (≥50 years) in the United States. The papillary to follicular carcinoma ratio rose from <3 to >8 among both Sao Paulo males and females, in contrast to increases from 9 to 12 and from 6 to 7 among U.S.males and females, respectively.
Conclusions
Increased diagnostic activity may be contributing to the notable rise in incidence, mainly for papillary type, in both populations, but it is not likely to be the only reason. Differences in iodine nutrition status between Sao Paulo and the U.S. SEER population might have affected the observed incidence patterns.
Introduction
Thyroid cancer incidence rates vary internationally and have been rising steadily over the last few decades in most of the developed world (1–13), but information on incidence trends in developing countries is limited. Sao Paulo, Brazil, has one of the highest rates of thyroid cancer worldwide (14.9 cases per 100,000 females and 3.9 cases per 100,000 males during 1998–2002, age-adjusted using the world standard) (1); and the thyroid is the fourth most common cancer site among females, just after breast, skin, and colon/rectum cancer (2). The Sao Paulo thyroid cancer rates were considerably higher than U.S rates of 9.8 and 3.0 among females and males, respectively, in the Surveillance, Epidemiology, and End Results (SEER) registries during 1998–2002 (1). Approximately 1000 new thyroid cancer cases are diagnosed each year in Sao Paulo, which is one third the annual number of thyroid cancer cases diagnosed in SEER.
In the United States incidence rates have increased among both males and females of all ages, with increases particularly pronounced for papillary carcinomas, and not only for small and localized tumors but also larger size and regional stage tumors (3,4). These findings suggest that the implementation of more sensitive diagnostic procedures cannot completely explain the observed increases in thyroid cancer incidence over time, and the reasons for the rising rates are not completely understood.
To our knowledge, no study has attempted to compare the thyroid cancer incidence patterns observed in the United States with those in Brazil. Such comparisons may provide some exploratory clues to the reasons for the geographic variation and rising rates around the world. Sao Paulo cancer incidence data, which are now available through 2008, provide the opportunity to compare thyroid cancer patterns with those in the National Cancer Institute's SEER program, which also may help to elucidate reasons for the high rates in Sao Paulo.
Methods
Study population
Data on thyroid cancer cases diagnosed during 1997–2008 in Sao Paulo, Brazil, were obtained from the Sao Paulo Cancer Registry (SPCR), which covers the geographic area of the city of Sao Paulo, the capital of the State of Sao Paulo. This is the largest cancer registry in Brazil, covering ∼11 million inhabitants in 2008 and accounting for 6% of the Brazilian population. Thyroid cancer cases for the U.S. population for the same time period were obtained from the National Cancer Institute's SEER 13 Registries Database, November 2010 submission (5), which includes data from Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah, Los Angeles, San Jose-Monterey, rural Georgia, and the Alaska Native tumor registry, covering ∼40 million inhabitants in 2008 or ∼14% of the U.S. population.
Case definition and tumor characteristics
We abstracted demographic data, including age at diagnosis and sex, as well as tumor information, such as histological type and mode of diagnosis, for each case diagnosed in SPCR and SEER. Information on ethnicity, tumor size, or stage of the disease was not available in SPCR and could not be used in this analysis. We included all cases, regardless of ethnicity, in the SEER dataset.
A total of 15,955 thyroid cancer cases were diagnosed in Sao Paulo from 1997 to 2008. Of these, 1834 (11%) were not microscopically confirmed, 1579 (10%) had an unknown age at diagnosis, 60 (0.4%) were identified by death certificate only, and three (0.02%) were diagnosed at autopsy. We excluded the 63 cases that were identified only by death certificate or diagnosed at autopsy. Due to the large proportion of cases in SPCR with unknown age at diagnosis and without microscopic confirmation, we assumed that these cases had a similar age and histology distribution as cases with known age at diagnosis and that were microscopically confirmed. We reallocated cases with unknown age at diagnosis into 5-year age groups, according to the age-specific proportion by sex and histological type for three time periods (1997–2000, 2001–2004, and 2005–2008). Cases without microscopic confirmation were reallocated proportionally to the major histological types, specific for time period, sex, and age group. Among the 14,135 cases with histological confirmation, 11% had a poorly specified histology.
In SEER, 369 cases (0.8%) were identified by death certificate only or diagnosed at autopsy and were excluded from the analysis. Since cases without microscopic confirmation (127 cases) and with missing age at diagnosis (three cases) in SEER were <1% of the total number of cases, they were omitted from the analysis. A total of 42,717 patients were diagnosed with malignant thyroid tumors that were microscopically confirmed and were not diagnosed at autopsy or identified only by death certificate. Cases with poorly specified histology represented only 1% of the cases that were microscopically confirmed.
All thyroid cancer cases were classified according to the recommendations of the International Association of Cancer Research as used in Cancer Incidence in Five Continents, volume IX (1), using the World Health Organization's International Classification of Diseases for Oncology, 3rd edition (6). Cases were subdivided into the major histological subtypes: papillary carcinoma (ICD-O-3 codes 8050, 8260, 8340–8344, 8350, 8450–8460), follicular carcinoma (ICD-O-3 codes 8290, 8330–8335), medullary carcinoma (8345, 8510–8513), anaplastic carcinoma (8020–8035), other specified (8036–8046, 8051, 8052, 8070–8124, 8130,8131, 8140–8255, 8261–8280, 8300–8225, 8337, 8346, 8347, 8370–8443, 8461–8507, 8514–9589) or poorly specified (8000–8005, 8010–8015) carcinomas.
Age-adjusted and age-specific incidence rates
Age-specific and age-adjusted [to the World Standard Population (1)] rates per 100,000 person-years were calculated using case data from SPCR and SEER and population data from the Brazilian Vital Statistics System (7) and SEER. We examined the incidence rates by sex, age groupings (10-year groups and <50 years versus ≥50 years), and histology. Rates were calculated for the time periods 1997–2000, 2001–2004, and 2005–2008. Incidence rate ratios (IRRs) were used to compare rates among females and males in Sao Paulo and SEER. The Joinpoint Regression Program (8) was used to analyze the temporal trends in the age-adjusted rates over the 12-year period. The annual percentage change (APC) was calculated for each sex, for those histological types with at least one case diagnosed in each year, assuming a constant rate of increase or decrease over the period considered. The APC was tested to determine whether it was significantly different from the null hypothesis of no change (0%) (two-sided, p≤0.05) (9).
We also examined thyroid cancer mortality trends in Sao Paulo and the SEER population. The number of deaths from thyroid cancer during 1997–2008 and the corresponding person-years at risk were obtained from the Brazilian Vital Statistics System and the SEER database, which also provides information on the underlying cause of death. In order to assess if the high thyroid cancer incidence rate in Sao Paulo could be attributed to better access to diagnostic procedures among users of private health plans, we also abstracted data on type of source of diagnosis (public or private) from the cancer registry.
Results
Thyroid cancer by sex and type
Table 1 presents the overall and histology-specific thyroid cancer incidence rates for the Sao Paulo and the United States (13 SEER areas) populations for the study period 1997 to 2008. Incidence rates were higher in Sao Paulo than in the United States; the overall IRRs were 1.65 among females and 1.23 among males. Papillary thyroid cancer was the most common histology, accounting for 72% of all thyroid cancers in Sao Paulo and 86% in SEER, followed by follicular, medullary, anaplastic, and other specified histologies. Incidence rates for each of these histologic groups were consistently higher in Sao Paulo than in SEER. The greatest differences were for other specified and medullary carcinomas among females, for which rates in Sao Paulo were 3.00 to 3.29 times those in the United States, respectively. Many more cases in Sao Paulo than in SEER did not have the histologic type specified; the Sao Paulo/SEER IRR was almost 30 among females and more than 10 among males. The female predominance was higher in Sao Paulo than in SEER, with overall F/M IRRs of 4.17 and 3.10, respectively. The F/M IRRs were larger for papillary and follicular than the other types in both countries. The greatest difference in F/M IRRs between Sao Paulo and the United States was for medullary carcinoma (IRR=3.73 and 1.31, respectively). Overall F/M IRRs were virtually the same across the three study periods (1997–2000, 2001–2004, and 2005–2008) in the United States (2.97, 3.13, and 3.18, respectively) and Sao Paulo (4.07. 4.17, and 4.21, respectively; not shown).
Table 1.
Thyroid Cancer Incidence Rates and Incidence Rate Ratios in Sao Paulo, Brazil, and the U.S. SEER 13 Registries During the Years 1997–2008
| |
Sao Paulo (n =15,892) |
SEER (n =42,717) |
Sao Paulo/SEER IRR |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Female |
Male |
|
Female |
Male |
|
|
|
||||
| Cases | Rate | Cases | Rate | F/M IRR | Cases | Rate | Cases | Rate | F/M IRR | Female | Male | |
| Overall | 13,313 | 18.43 | 2579 | 4.42 | 4.17 | 32,562 | 11.17 | 10,155 | 3.60 | 3.10 | 1.65 | 1.23 |
| Papillary | 9607 | 13.22 | 1818 | 3.05 | 4.33 | 28,414 | 9.85 | 8219 | 2.92 | 3.37 | 1.34 | 1.04 |
| Follicular | 1650 | 2.32 | 299 | 0.54 | 4.30 | 2879 | 0.95 | 1128 | 0.40 | 2.38 | 2.44 | 1.35 |
| Medullary | 390 | 0.56 | 85 | 0.15 | 3.73 | 506 | 0.17 | 354 | 0.13 | 1.31 | 3.29 | 1.15 |
| Anaplastic | 93 | 0.13 | 33 | 0.07 | 1.86 | 279 | 0.06 | 187 | 0.06 | 1.00 | 2.17 | 1.17 |
| Others specified | 143 | 0.21 | 59 | 0.11 | 1.91 | 242 | 0.07 | 151 | 0.05 | 1.40 | 3.00 | 2.20 |
| Poorly specified | 1429 | 2.00 | 285 | 0.50 | 4.00 | 242 | 0.07 | 116 | 0.04 | 1.75 | 28.57 | 12.50 |
Values are age-adjusted rates (world population) per 100,000 person-years.
IRR, incidence rate ratios; F/M, female/male.
Age-adjusted thyroid cancer incidence trends
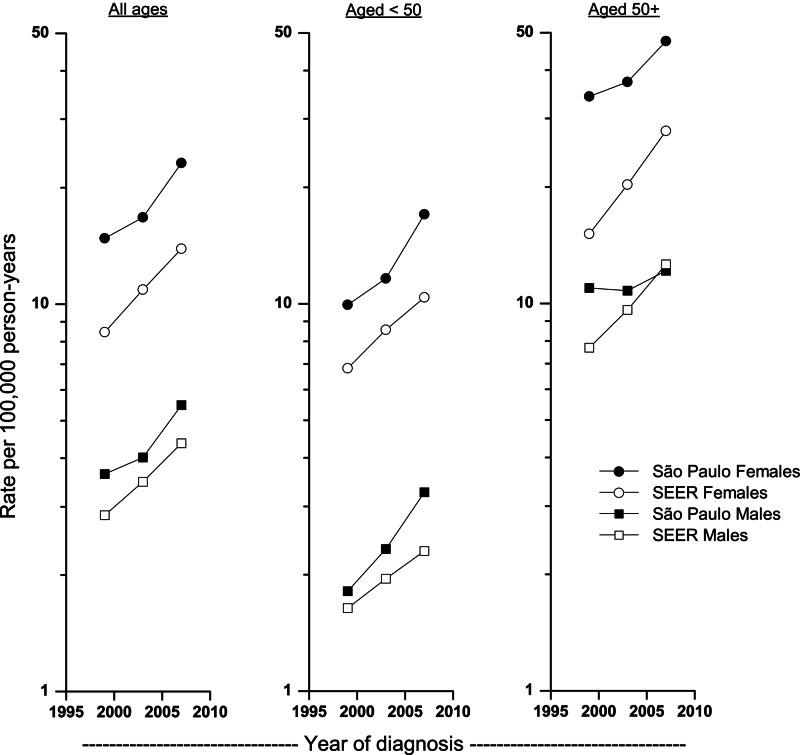
Between the periods 1997–2000 and 2005–2008, overall incidence rates increased significantly at 5.2% per year among Sao Paulo females and males, 5.3% per year among SEER males, and more rapidly among SEER females (APC=6.3%; Fig. 1 and Table 2). In Sao Paulo, the rates rose more rapidly among people who were younger than 50 (APC=6.5% and 7.5% for female and males, respectively) than for people 50 years or older (APC=3.7% and 3.5% for females and males, respectively). In contrast, rates in SEER increased more rapidly among people who were 50 years or older (APC=7.7% and 6.1% in females and males, respectively) than among those younger than 50 years of age (APC=5.5% and 4.3% in females and males, respectively). All the age-specific increases were significant except among those ages 50+ years in Sao Paulo.
FIG. 1.
Thyroid cancer incidence rates among females and males at all ages, under 50 and older than 50 years, Sao Paulo, Brazil, and the U.S. Surveillance, Epidemiology, and End Results (SEER) 13 registries, 1997–2008.
Table 2.
Thyroid Cancer Incidence Rates by Histology in Sao Paulo, Brazil, and the U.S. SEER 13 Registries During the Years 1997–2008
| |
Sao Paulo (n=15,892) |
SEER (n=42,717) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
1997–2000 |
2001–2004 |
2005–2008 |
|
1997–2000 |
2001–2004 |
2005–2008 |
|
||||||
| Sex; age at diagnosis | Cases | Rate | Cases | Rate | Cases | Rate | APC | Cases | Rate | Cases | Rate | Cases | Rate | APC |
| Overall | ||||||||||||||
| Female | ||||||||||||||
| All ages | 3250 | 14.80 | 4034 | 16.76 | 6029 | 23.11 | 5.21a | 7858 | 8.47 | 10,585 | 10.9 | 14,119 | 13.88 | 6.32a |
| <50 | 1950 | 9.94 | 2468 | 11.62 | 3753 | 17 | 6.54a | 4870 | 6.81 | 6275 | 8.56 | 7642 | 10.38 | 5.48a |
| 50+ | 1300 | 34.22 | 1566 | 37.33 | 2276 | 47.55 | 3.67 | 2988 | 15.11 | 4310 | 20.25 | 6477 | 27.86 | 7.74a |
| Male | ||||||||||||||
| All ages | 632 | 3.64 | 782 | 4.02 | 1165 | 5.49 | 5.24a | 2503 | 2.85 | 3268 | 3.48 | 4384 | 4.37 | 5.32a |
| <50 | 319 | 1.81 | 435 | 2.33 | 645 | 3.26 | 7.52a | 1199 | 1.64 | 1478 | 1.95 | 1754 | 2.3 | 4.25a |
| 50+ | 313 | 10.96 | 347 | 10.78 | 520 | 12.15 | 3.48 | 1304 | 7.68 | 1790 | 9.62 | 2630 | 12.62 | 6.13a |
| Papillary | ||||||||||||||
| Female | ||||||||||||||
| All ages | 1870 | 8.36 | 2812 | 11.64 | 4925 | 18.81 | 10.26a | 6690 | 7.3 | 9190 | 9.55 | 12,534 | 12.43 | 6.85a |
| <50 | 1255 | 6.29 | 1784 | 8.39 | 3181 | 14.36 | 10.54a | 4341 | 6.06 | 5601 | 7.65 | 6987 | 9.49 | 5.85a |
| 50+ | 615 | 16.63 | 1028 | 24.68 | 1744 | 36.64 | 9.69a | 2349 | 12.26 | 3589 | 17.19 | 5547 | 24.21 | 8.60a |
| Male | ||||||||||||||
| All ages | 382 | 2.15 | 512 | 2.53 | 924 | 4.31 | 9.64a | 1981 | 2.26 | 2668 | 2.85 | 3570 | 3.58 | 5.72a |
| <50 | 214 | 1.21 | 316 | 1.65 | 534 | 2.69 | 10.65a | 1025 | 1.4 | 1281 | 1.69 | 1524 | 2.00 | 4.46a |
| 50+ | 168 | 5.9 | 196 | 6.03 | 390 | 10.82 | 8.59a | 956 | 5.68 | 1387 | 7.51 | 2046 | 9.91 | 6.84a |
| Follicular | ||||||||||||||
| Female; all ages | 695 | 3.22 | 392 | 1.64 | 564 | 2.2 | −1.94 | 782 | 0.8 | 984 | 0.97 | 1113 | 1.06 | 3.35a |
| Male; all ages | 131 | 0.79 | 63 | 0.34 | 105 | 0.51 | −4.88 | 309 | 0.35 | 331 | 0.35 | 488 | 0.48 | 3.96a |
| Medullary | ||||||||||||||
| Female; all ages | 173 | 0.83 | 143 | 0.35 | 74 | 0.22 | −11.21 | 160 | 0.17 | 162 | 0.17 | 184 | 0.17 | −0.07 |
| Male; all ages | 28 | 0.16 | 26 | 0.15 | 33 | 0.16 | −0.05 | 104 | 0.12 | 108 | 0.12 | 142 | 0.14 | 2.85 |
| Anaplastic | ||||||||||||||
| Female; all ages | 25 | 0.12 | 47 | 0.17 | 21 | 0.07 | −5.81 | 100 | 0.07 | 88 | 0.07 | 91 | 0.06 | −2.03 |
| Male; all ages | 11 | 0.06 | 17 | 0.09 | 5 | NA | NA | 47 | 0.05 | 64 | 0.06 | 76 | 0.07 | 2.29 |
| Other specified | ||||||||||||||
| Female; all ages | 39 | 0.31 | 60 | 0.47 | 43 | 0.25 | −3.06 | 46 | 0.05 | 91 | 0.08 | 105 | 0.09 | 9.77a |
| Male; all ages | 14 | 0.20 | 29 | 0.30 | 16 | 0.18 | NA | 34 | 0.04 | 57 | 0.06 | 60 | 0.06 | 3.14 |
| Poorly specified | ||||||||||||||
| Female; all ages | 448 | 2.07 | 581 | 2.41 | 401 | 1.55 | −5.84 | 80 | 0.07 | 70 | 0.06 | 92 | 0.07 | −0.7 |
| Male; all ages | 66 | 0.38 | 139 | 0.73 | 80 | 0.39 | 2.66 | 28 | 0.03 | 40 | 0.04 | 48 | 0.04 | 2.9 |
| Mortality (overall) | ||||||||||||||
| Female; all ages | 107 | 0.53 | 116 | 0.49 | 89 | 0.37 | −5.29a | 2981 | 0.27 | 3190 | 0.27 | 3584 | 0.28 | 0.14 |
| Male; all ages | 56 | 0.37 | 53 | 0.34 | 37 | 0.30 | −2.21 | 1993 | 0.26 | 2252 | 0.28 | 2607 | 0.29 | 1.42 |
Values are total number of cases in each 4-year period, and age-adjusted rates (world population) per 100,000 person-years.
The annual percentage change is significantly different from zero (p≤0.05).
APC, annual percent change, based on annual age-adjusted rates using Joinpoint Regression Program (7); NA, not applicable (rate based on <10 cases and/or APC could not be calculated due to zero cases for ≥1 year).
Papillary thyroid cancer rates increased more rapidly than the overall rates in both populations and both sexes, more rapidly among females than males in both populations, and in Sao Paulo (APC=10.3% among females and 9.6% among males) than in the United States (APC=6.9% and 5.7%, respectively).
Rates in Sao Paulo for follicular, medullary, anaplastic, other specified, and poorly specified carcinomas did not change significantly over time. Rates for follicular carcinomas in SEER increased annually at 3.4% and 4.0% among females and males, respectively, whereas rates for medullary and anaplastic carcinomas did not change greatly. Thyroid cancer mortality rates have been extremely low in both areas. Rates in Sao Paulo were higher and decreasing, while in SEER they were lower and relatively stable over time.
Examining data on medical source of diagnosis (public or private), we found that most of the cases from the SPCR were diagnosed in private clinics, hospitals, and laboratories (75%), while 13% of the cases were diagnosed in the public health system, and 12% were diagnosed in medical centers that use both systems (private and public). The proportion of cases diagnosed in the public health system significantly decreased from 1997–2000 (14.7%) to 2005–2008 (11.2%) (p value<0.01), while the proportion of cases diagnosed in the private health system increased significantly (74.3% during 1997–2000 and 76.1% during 2005–2008, p value=0.04). No sex disparity by clinic type was apparent.
Age-specific incidence rates
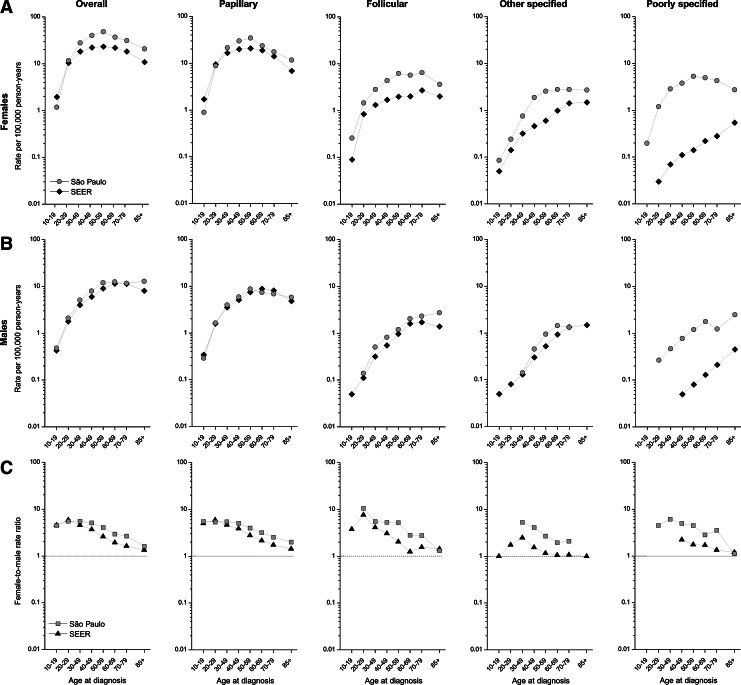
Figure 2 presents the age-specific rates by sex and the female-to-male rate ratios overall and for papillary, follicular, other specified, and poorly specified thyroid cancers for Sao Paulo and SEER. The overall and papillary age-specific incidence rates among females in Sao Paulo rose rapidly until age 50–59 years and then progressively decreased at older ages, whereas rates among females in SEER increased until age 40–49 years, flattened until age 60–69 years, and then declined. In both countries, the overall rates among men rose more slowly, peaking at ages 60–69 and then declining at older ages. Rates for follicular and other carcinomas rose more consistently with age among both males and females in both areas. The pattern among females in Sao Paulo for the poorly specified histology was similar to that observed for papillary histology, increasing steadily with increasing age until age 50–69, when rates started to decline. Among females, incidence rates for all histological categories were consistently higher in Sao Paulo than in SEER at each age group except the youngest. Among males, age-specific incidence rates were similar in both geographic areas, except for poorly specified carcinomas, for which rates were notably higher in Sao Paulo than SEER.
FIG. 2.
Thyroid cancer age-specific incidence rates among (A) females and (B) males and (C) female/male rate ratios, Sao Paulo, Brazil, and SEER 13 registries, 1997–2008.
The F/M IRRs generally decreased with age for each of the types in both of the areas, although the female-to-male rate ratios across all age groups were consistently larger in Sao Paulo than in the U.S., regardless of histology (Fig. 2C). Overall, both the Sao Paulo and SEER rate ratios were >5 at ages <50 years and steadily declined with advancing age to <2 at age 80+ years.
Discussion
To our knowledge, this report is the largest study on thyroid cancer patterns in Brazil and the first one to compare the incidence in Brazil and the United States. Our results revealed that thyroid cancer incidence is on the rise in Sao Paulo, Brazil, following a similar trend observed in many other developed countries (10). Moreover, we found that thyroid cancer incidence rates were consistently higher in Sao Paulo than in the United States across the entire study period, particularly among females. Papillary thyroid cancer was the most common histology, accounting for 72% of all thyroid cancers in Sao Paulo and 86% in the United States. The Sao Paulo/SEER IRRs were the largest for other specified and medullary carcinomas among females, for which rates in Sao Paulo were 3 to 3.3 times those in the United States, respectively. The F/M IRRs were consistently higher in Sao Paulo than SEER and declined notably with age in both areas and for each type. Thyroid cancer incidence rose steadily over the study period in both populations, and this rise was largely limited to the papillary subtype, especially in Sao Paulo. In contrast, mortality rates decreased in Sao Paulo for both sexes whereas they tended to be constant in the United States during the study period. Thyroid cancer mortality is also greater in Sao Paulo than in the United States for both sexes. While thyroid cancer mortality rates are nearly equal between males and females in the United States, females have a 40% greater mortality than males in Sao Paulo. The decrease in mortality in Sao Paulo may in part reflect the fact that elderly males and females have a relatively low proportion of undifferentiated carcinomas and/or an increase in the diagnosis of less aggressive tumors. It also may reflect improvements in survival in Sao Paulo during the study period that may have already occurred in the United States before our study began.
Papillary cancer rates are rising much more rapidly in Sao Paulo than in the SEER population among both sexes and age groups, which may be interpreted as possibly resulting from significant differences in an unknown risk factor between the SEER and Sao Paulo populations, as well as potentially improving specificity of assigning the histologic type in Sao Paulo.
Very little data on trends in thyroid cancer incidence have been reported for South American countries. A recent report from Puerto Rico indicated that thyroid cancer incidence increased significantly from 1985 to 2004, mostly due to an increase of papillary cancer (11). Kilfoy et al. (12) examined incidence data from Cancer Incidence in Five Continents over the 30-year period 1973–2002 from 19 populations in the Americas, Asia, Europe, and Oceania. The authors reported increases in thyroid cancer incidence for most of the populations, including Cali, Colombia, which was the only South American registry included in this evaluation. In Brazil, Reis et al. (13) have investigated thyroid cancer trends by sex, histology, and tumor stage using data from the Goiania Cancer Registry from 1988 to 2002. Although the authors reported a rising thyroid cancer incidence rate among both males and females over the study period, they suggested that this increase might be related to an improvement in diagnosis because they also observed an increase in the proportion of tumors that were localized over the study period (28% in 1988–1992 to 73% in 1998–2002). However, the proportion of tumors with unknown stage of disease decreased from 60% to 10% over the same time period, which limited the authors' conclusions.
Thyroid cancer incidence may reflect socioeconomic differences since lack of access to health care would limit screening efforts among segments of the population. A prior report (14) found similar age-specific patterns and lack of geographical variation across SEER racial/ethnic groups in the United States, indicating that a detection effect cannot completely explain the observed increases in thyroid cancer incidence given that the amount or quality of healthcare may vary by ethnicity, sex, and age. We were unable to perform such an investigation in Sao Paulo, due to the lack of information on ethnicity in the SPCR. Nevertheless, the marked socioeconomic differences among segments of the population in Brazil are indeed a great limitation to health care access. Brazil has both a public and privately-funded health care system, and the dependency on the public health system is related to the income level, which varies greatly within regions, states, and cities. Only 20% of the Brazilian population is covered by private health plans, mainly concentrated in the southeast region of Brazil. Sao Paulo presents the highest coverage of the southeast region, with 42% of the population covered by private health care in 2008, ranging from 17.3% among the lowest to 83.4% among highest income levels (15). Our finding that 75% of thyroid cancer cases were diagnosed in the private health system is suggestive of greater medical surveillance among users of private health plans. However, the proportion with health insurance coverage in Sao Paulo did not seem to increase over the study period (15), concurrent with the rising thyroid cancer rates. Sao Paulo is one of the most developed cities in Brazil and presents the highest thyroid cancer incidence rate among Brazilian cancer registries, which indeed may be related to better access to medical care. Nevertheless, it is unlikely that the quality or amount of health care provided in Sao Paulo would be greater than that provided in the United States. Furthermore, the overall increases in thyroid cancer in Sao Paulo were mainly due to rising papillary cancer rates, while the rates of other histologic types decreased over time. If a significant diagnostic effect played a role in the increased thyroid cancer rate, one might expect similar increases for the other histologic types.
Along with medical surveillance, other factors that vary with time may also have contributed to the rising incidence of thyroid cancer in both populations. Increased radiation exposure in childhood due to the greater use of pediatric computed tomography scanning has been suggested as potentially relating to the increasing rates in the recent decades (3). Other factors potentially contributing to the temporal trends and geographic variations include obesity (16–18), iodine intake (10,19), environmental exposure to polychlorinated biphenyls and dioxins (20), use of fertility drugs (21), changes in reproductive patterns (22,23), insulin resistance syndrome (24), and others. Apart from obesity and iodine intake, little is known regarding the prevalence of these potential risk factors in Brazil and how they might have affected the incidence patterns in both populations.
Low iodine intake has been firmly established as a risk factor for goiter and benign thyroid nodules, whereas high iodine in the diet has been associated with thyroiditis, hypothyroidism, and hyperthyroidism. However, the role of iodine in the pathogenesis of thyroid cancer is still controversial, and differences in risk according to tumor histology have been observed (10,25–29). Chronic iodine deficiency has been associated with increased risk of follicular and anaplastic carcinoma, whereas iodine excess increased risk of papillary and decreased risk of follicular carcinoma (30–32). Several studies conducted in areas with previous iodine deficiency have reported a progressive increase in the ratio of papillary to follicular carcinoma after iodine prophylaxis (25,33–36).
Brazil changed from a state of chronic iodine deficiency in the 19th century to a recent history of excessive iodine intake from 1998 to 2003 (37). In brief, a salt iodination program in Brazil started in 1953, but only in areas with endemic goiter. From 1956 to 1992, the program was extended to all the Brazilian population, although it was not very effective due to the lack of regulatory control and cooperation of the salt industry. From 1992 to 1995, most of the salt industries stopped adding iodine to the salt due to a legal dispute between the salt industries and the Brazilian government. In 1995, a new law was approved, and limits for salt iodination were set at 40–60 mg iodine/kg of salt. In order to provide a broader range limit, iodine concentration in table salt was increased to 40–100 mg/kg, in 1998 resulting in an excessive iodine intake among the Brazilian population from 1998 to 2003. Thereafter, iodination of table salt in Brazil was lowered to 20–60 mg/kg of salt, which is currently the limit in use.
Dietary iodine intake appears to differ between Sao Paulo and the United States. The World Health Organization (WHO) defines nutritional iodine sufficiency according to urinary iodine concentrations as follows: excessive iodine intake, >300 μg/L; more than adequate intake, 200–299 μg/L; adequate intake, 100–199 μg/L; mild iodine deficiency, 50–90 μg/L; moderate iodine deficiency, 20–49 μg/L; and severe iodine deficiency, <20 μg/L (38). Two population studies conducted during 2000–2003 in Brazil, after reintroducing systematic salt iodination, reported that ∼70% of the examined subjects had elevated iodine urinary excretion (>300 μg/L) (39,40). An increased prevalence of chronic autoimmune thyroiditis and hypothyroidism in the Sao Paulo population associated with the excessive nutritional iodine intake was reported in 2004 (41). A national survey of schoolchildren is currently underway to evaluate the adequacy of iodine intake levels after the reduction of the iodine concentration in table salt in 2003 (37).
Reports from the U.S. Health and Nutrition Examination Survey (NHANES) have indicated that the dietary iodine intake of the U.S. general population has remained at an adequate and stable level since 2000 (42–45). In addition, the 2007–2008 NHANES indicated that children aged 6–11 years have more than adequate levels of iodine in their diets, with a median iodine urinary excretion of 215 μg/L (44).
The differences in iodine intake between Sao Paulo and the United States may help explaining the different trends in the papillary-to-follicular carcinoma rate ratios. In Sao Paulo, the ratio of papillary to follicular carcinoma increased from 2.6 to 8.5 (229%) among females and from 2.7 to 8.6 (211%) among males in 1997–2000 to 2005–2008, respectively. In SEER, the increase in ratio of papillary to follicular was less pronounced, rising from 9.1 to 11.7 (29%) among females and from 6.5 to 7.5 (16%) among males over the same time period. Considering a latency period for the clinical expression of past phases of iodine nutrition in Sao Paulo, we can assume that the low ratio of papillary to follicular carcinomas in the first period of the study (1997–2000) probably reflects the iodine-deficiency period in Sao Paulo (until 1998), whereas the increase in the ratio of papillary to follicular in the later period (2005–2008) would reflect the period of excessive iodine intake (1998–2003).
Consistent with the literature, it can be suggested that differences in iodine nutrition status between the Sao Paulo and SEER populations may account for the observed thyroid cancer incidence patterns between both populations. As long as iodine intake remains adequate in the Brazilian population, a decrease in ratio of papillary to follicular cancer might be expected in the coming years in the Sao Paulo population.
Regarding obesity, the prevalence of overweight and obesity increased dramatically over the recent decades in Brazil (46) and also in the United States (47). Nonetheless, estimates of obesity in the United States tend to be higher than in other countries. A recent population-based study in Sao Paulo among children and adolescents aged 7–18 years reported a prevalence of obesity of 8.9% among boys and 4.3% among girls (48). These totals are still lower than recent estimates for the United States (49), which reported an obesity prevalence among boys and girls aged 6–19 years of 19.8% and 16.5%, respectively. Therefore, obesity prevalence would not explain the higher thyroid cancer rates in Sao Paulo than in SEER, especially among females.
A female preponderance was observed in Sao Paulo for all histologic types, as in SEER, with the F/M IRRs consistently higher in Sao Paulo than SEER. Of note, the F/M IRR for medullary carcinoma was 3.73, much higher than the 1.31 in SEER. The higher medullary rate among females in Sao Paulo than in SEER, in contrast to similar rates among males, is also notable, especially because a genetic origin has been suggested for these tumors (10,50). Further research is required to investigate a possible genetic susceptibility in Sao Paulo females.
Strengths of this study include the large number of thyroid cancer cases available for analysis and the ability to evaluate thyroid cancer patterns in a developed and developing country, especially from a Latin American population. Sao Paulo is one of the most densely populated cities in the world (6897 habitants/km2) (1), resulting in a large number of cases diagnosed each year, comparable to those obtained from small countries in Europe.
However, certain data limitations should be considered. Changes in registration data quality may affect time trends. This is especially important when using cancer registry data from different countries in order to make comparison across populations and time. Nevertheless, both SEER and SPCR have fulfilled the IARC indices of data quality used for cancer registration (1), presenting a low proportion of registration from death certificates that did not change over time, suggesting a high degree of completeness of case ascertainment across the studied period. Some delays in case ascertainment and reporting to the cancer registries may have occurred, but this was minimized by using 4-year time periods to estimate the incidence rates. Also, the high thyroid cancer rates in SPCR do not appear to be related to underestimation of the population at risk because rates for other common cancers were not uniformly elevated in Sao Paulo in comparison to SEER (1), as would be expected if the Sao Paulo population was underestimated. Moreover, similarities in the female-to-male IRRs and the age-specific incidence patterns (mainly among males) in both geographies also argued against a possible bias caused by the underestimation of the population at risk.
Since information on size and stage of tumors is not available in the SPCR, we also were unable to assess the role of an increased detection effect in this trend (i.e., whether the trends in Sao Paulo varied according to these tumor characteristics).
The proportion of cases with unknown age at diagnosis and without microscopic confirmation varied over the study period, but these variations appear to have been random because no pattern could be identified. Since we reallocated these cases by assuming that they had a similar age and histology distribution as patients with known age at diagnosis, and that were microscopically confirmed, the trends were quite similar whether we included or excluded the reallocated subjects. Excluding the reallocated cases did reduce the Sao Paulo rates and the SPCR/SEER IRRs, but the patterns were generally similar.
The accuracy and completeness of information on histology may improve over time among cases that were microscopically confirmed, resulting in lower frequencies of subjects with poorly specified tumor histology in later years. Despite the high proportion of cases with poorly specified histology in Sao Paulo (13%) as compared with the United States (1%), poorly specified histology rates in Sao Paulo decreased 25% during the study period. The reason for this relatively high proportion of poorly specified histology in Sao Paulo is unknown and is being investigated by the SPCR. It is important to note that age-specific incidence rates for poorly specified histology among females in Sao Paulo presented the same hook pattern observed for the age-specific incidence rates for papillary (i.e., rapidly increasing during reproductive ages and then declining at older ages). This similarity may be suggestive of misclassification of papillary carcinoma cases as poorly specified histology. Also, changes in diagnostic criteria for histological classification of thyroid cancer cannot explain the observed trends in the incidence of papillary and follicular carcinomas since changes in classification occurred nearly a decade before our study period.
Other histopathological misclassification cannot be ruled out, especially for the uncommon histologies. Nevertheless, if misclassification occurred it should not be sex related. The higher medullary and follicular rates among females in Sao Paulo than in SEER contrast with the similar rates observed among males, suggesting that misclassification is not playing an important role for these histologies. Therefore, we do not have any evidence that the observed differences in histology-specific rates in Sao Paulo versus SEER would be simply related to misclassification. Moreover, if it occurred, it would not affect the observed increase in overall thyroid cancer incidence.
In summary, our study revealed striking differences and similarities in thyroid cancer incidence patterns in Sao Paulo and the United States. The female-to-male IRR patterns were very similar in the two populations, although the ratios across all age groups were consistently greater in Sao Paulo than in the United States. Age-specific incidence patterns by sex differed by geographic region for females, but not for males, such that rates peaked more sharply and at older ages among females in Sao Paulo (50–59 years) than in the United States (40–49 years), which may reflect a detection effect and/or effect of certain age-related exposures. Striking differences include the fact that the rise in thyroid cancer incidence in Sao Paulo has been limited to the papillary subtype, with decreasing rates for other histologies, mainly for follicular carcinomas. In the United States, incidence rates rose for other histologies. The rapidly increasing ratios of papillary to follicular carcinomas observed in Sao Paulo, but not in the United States, suggest that differences in iodine nutrition status between the Sao Paulo and SEER populations might have affected the observed incidence patterns.
In conclusion, it is not possible to identify the exact cause or causes of this notable rise in incidence, mainly for papillary carcinomas, in both populations. Increased diagnostic activity may play a role, but is not likely to be the only reason because incidence continues to rise rather than leveling off at some point in time. Future studies in Sao Paulo should attempt to retrieve tumor stage and size in order to determine the role of advances in diagnostic accuracy and to evaluate the impact of iodine prophylaxis on the papillary and follicular carcinoma incidence patterns.
Acknowledgments
The authors are grateful to Fernanda Alessandra Silva and Aryane Simon (coordinators of the Sao Paulo Cancer Registry, Brazil) for providing the data for analysis and David Check (National Cancer Institute) for figure development. We also would like to honor the late Dr. Elaine Ron who inspired and initiated this work. This research was supported in part by the Intramural Research Program of the National Institutes of Health National Cancer Institute Division of Cancer Epidemiology and Genetics.
Author Disclosure Statement
The authors declare no conflicts of interest
References
- 1.Cancer incidence in five continents. In: Curado M, editor; Edwards B, editor; Shin HR, editor; Storm H, editor; Ferlay J, editor; Heanue M, editor; Boyle P, editor. IX. IARC Scientific Publications No. 160. IARC; Lyon, France: 2007. [Google Scholar]
- 2.Michels FAS. Simon AS. Sconza AC. Veneziano DB. Latorre MRDO. Registro de Câncer de Sao Paulo; Sao Paulo: 2011. Cancer in Sao Paulo 1997–2008: incidence, mortality, cancer trend in Sao Paulo municipality. [Google Scholar]
- 3.Enewold L. Zhu K. Ron E. Marrogi AJ. Stojadinovic A. Peoples GE. Devesa SS. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen AY. Jemal A. Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 5.Public-Use Database (1992–2008) Surveillance, Epidemiology, End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; Bethesda, Maryland: Released April 2011 (updated October 28, 2011), based on the November 2010 submission. [Google Scholar]
- 6.Fritz A. Jack A. Parkin DM. Percy C. Shanmugarathan S. Sobin L. Whelan S. International Classification of Diseases for Oncology. 3rd. World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 7.Ministry of Health., DATASUS: Database of the Unified Health System. http://www2.datasus.gov.br/DATASUS/index.php?area=02. [Sep;2010 ]. http://www2.datasus.gov.br/DATASUS/index.php?area=02 in Portuguese.
- 8.National Cancer Institute 2011 Joinpoint Regression Program, Version 3.5.0. Statistical Research and Applications Branch, National Cancer Institute; Bethesda, Maryalnd: [Google Scholar]
- 9.Kim HJ. Fay MP. Feuer EJ. Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Ron E. Schneider A. Thyroid cancer. In: Schottenfeld D, editor; Fraumeni JF Jr, editor. Cancer Epidemiology and Prevention. 3rd. Oxford University Press, Inc.; New York: 2006. pp. 975–994. [Google Scholar]
- 11.Ramirez-Vick M. Nieves-Rodriguez M. Lugaro-Gomez A. Perez-Irizarry J. Increasing incidence of thyroid cancer in Puerto Rico, 1985–2004. P R Health Sci J. 2011;30:109–115. [PubMed] [Google Scholar]
- 12.Kilfoy BA. Zheng T. Holford TR. Han X. Ward MH. Sjodin A. Zhang Y. Bai Y. Zhu C. Guo GL. Rothman N. International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20:525–531. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis DSM. Morihisa IA. Medeiros KC. Fernandes LM. Martins E. Curado MP. Oliveira JC. Thyroid cancer in Goiania: descriptive analysis on the population based from 1988 to 2003. Rev Bras Cir Cabeça Pescoço. 2008;37:4. [Google Scholar]
- 14.Aschebrook-Kilfoy B. Ward MH. Sabra MM. Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21:125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pessoto UC. Heimann LS. Boaretto RC. Castro IE. Kayano J. Ibanhes LC. Junqueira V. da Rocha JL. Barboza R. Cortizo CT. Martins Lda C. Luiz Odo C. [Health care services utilization and access inequalities in the Sao Paulo Metropolitan Region] Cien Saude Colet. 2007;12:351–362. doi: 10.1590/s1413-81232007000200011. [DOI] [PubMed] [Google Scholar]
- 16.Kitahara CM. Platz EA. Beane Freeman L. Hsing AW. Linet MS. Park Y. Schairer C. Schatzkin A. Shikany J. Berrington de Gonzalez A. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of 5 prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–472. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clero E. Leux C. Brindel P. Truong T. Anger A. Teinturier C. Diallo I. Doyon F. Guenel P. de Vathaire F. Pooled analysis of two case-control studies in New Caledonia and French Polynesia of body mass index and differentiated thyroid cancer: the importance of body surface area. Thyroid. 2010;20:1285–1293. doi: 10.1089/thy.2009.0456. [DOI] [PubMed] [Google Scholar]
- 18.Engeland A. Tretli S. Akslen LA. Bjorge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95:366–370. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harach HR. Ceballos GA. Thyroid cancer, thyroiditis and dietary iodine: a review based on the Salta, Argentina model. Endocr Pathol. 2008;19:209–220. doi: 10.1007/s12022-008-9038-y. [DOI] [PubMed] [Google Scholar]
- 20.Boas M. Feldt-Rasmussen U. Skakkebaek NE. Main KM. Environmental chemicals and thyroid function. Eur J Endocrinol. 2006;154:599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- 21.Hannibal CG. Jensen A. Sharif H. Kjaer SK. Risk of thyroid cancer after exposure to fertility drugs: results from a large Danish cohort study. Hum Reprod. 2008;23:451–456. doi: 10.1093/humrep/dem381. [DOI] [PubMed] [Google Scholar]
- 22.Brindel P. Doyon F. Rachedi F. Boissin JL. Sebbag J. Shan L. Chungue V. Sun LY. Bost-Bezeaud F. Petitdidier P. Paoaafaite J. Teuri J. de Vathaire F. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case-control study. Am J Epidemiol. 2008;167:219–229. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- 23.Negri E. Dal Maso L. Ron E. La Vecchia C. Mark SD. Preston-Martin S. McTiernan A. Kolonel L. Yoshimoto Y. Jin F. Wingren G. Rosaria Galanti M. Hardell L. Glattre E. Lund E. Levi F. Linos D. Braga C. Franceschi S. A pooled analysis of case-control studies of thyroid cancer., II. Menstrual and reproductive factors. Cancer Causes Control. 1999;10:143–155. doi: 10.1023/a:1008880429862. [DOI] [PubMed] [Google Scholar]
- 24.Rezzonico J. Rezzonico M. Pusiol E. Pitoia F. Niepomniszcze H. Introducing the thyroid gland as another victim of the insulin resistance syndrome. Thyroid. 2008;18:461–464. doi: 10.1089/thy.2007.0223. [DOI] [PubMed] [Google Scholar]
- 25.Harach HR. Williams ED. Thyroid cancer and thyroiditis in the goitrous region of Salta, Argentina, before and after iodine prophylaxis. Clin Endocrinol (Oxf) 1995;43:701–706. doi: 10.1111/j.1365-2265.1995.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 26.Bagchi N. Brown TR. Parish RF. Thyroid dysfunction in adults over age 55 years. A study in an urban US community. Arch Intern Med. 1990;150:785–787. [PubMed] [Google Scholar]
- 27.Diez JJ. Iglesias P. Spontaneous subclinical hypothyroidism in patients older than 55 years: an analysis of natural course and risk factors for the development of overt thyroid failure. J Clin Endocrinol Metab. 2004;89:4890–4897. doi: 10.1210/jc.2003-032061. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen IB. Laurberg P. Knudsen N. Jorgensen T. Perrild H. Ovesen L. Rasmussen LB. An increased incidence of overt hypothyroidism after iodine fortification of salt in Denmark: a prospective population study. J Clin Endocrinol Metab. 2007;92:3122–3127. doi: 10.1210/jc.2007-0732. [DOI] [PubMed] [Google Scholar]
- 29.Szabolcs I. Podoba J. Feldkamp J. Dohan O. Farkas I. Sajgo M. Takats KI. Goth M. Kovacs L. Kressinszky K. Hnilica P. Szilagyi G. Comparative screening for thyroid disorders in old age in areas of iodine deficiency, long-term iodine prophylaxis and abundant iodine intake. Clin Endocrinol (Oxf) 1997;47:87–92. doi: 10.1046/j.1365-2265.1997.2271040.x. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson B. Adami HO. Wilander E. Coleman MP. Trends in thyroid cancer incidence in Sweden, 1958–1981, by histopathologic type. Int J Cancer. 1991;48:28–33. doi: 10.1002/ijc.2910480106. [DOI] [PubMed] [Google Scholar]
- 31.Bacher-Stier C. Riccabona G. Totsch M. Kemmler G. Oberaigner W. Moncayo R. Incidence and clinical characteristics of thyroid carcinoma after iodine prophylaxis in an endemic goiter country. Thyroid. 1997;7:733–741. doi: 10.1089/thy.1997.7.733. [DOI] [PubMed] [Google Scholar]
- 32.Burgess JR. Dwyer T. McArdle K. Tucker P. Shugg D. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978–1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metab. 2000;85:1513–1517. doi: 10.1210/jcem.85.4.6554. [DOI] [PubMed] [Google Scholar]
- 33.Farahati J. Geling M. Mader U. Mortl M. Luster M. Muller JG. Flentje M. Reiners C. Changing trends of incidence and prognosis of thyroid carcinoma in Lower Franconia, Germany, from 1981–1995. Thyroid. 2004;14:141–147. doi: 10.1089/105072504322880382. [DOI] [PubMed] [Google Scholar]
- 34.Gomez Segovia I. Gallowitsch HJ. Kresnik E. Kumnig G. Igerc I. Matschnig S. Stronegger WJ. Lind P. Descriptive epidemiology of thyroid carcinoma in Carinthia, Austria: 1984–2001. Histopathologic features and tumor classification of 734 cases under elevated general iodination of table salt since 1990: population-based age-stratified analysis on thyroid carcinoma incidence. Thyroid. 2004;14:277–286. doi: 10.1089/105072504323030933. [DOI] [PubMed] [Google Scholar]
- 35.Lind P. Kumnig G. Heinisch M. Igerc I. Mikosch P. Gallowitsch HJ. Kresnik E. Gomez I. Unterweger O. Aigner H. Iodine supplementation in Austria: methods and results. Thyroid. 2002;12:903–907. doi: 10.1089/105072502761016539. [DOI] [PubMed] [Google Scholar]
- 36.Langsteger W. Koltringer P. Wolf G. Dominik K. Buchinger W. Binter G. Lax S. Eber O. The impact of geographical, clinical, dietary and radiation-induced features in epidemiology of thyroid cancer. Eur J Cancer. 1993;29A:1547–1553. doi: 10.1016/0959-8049(93)90292-n. [DOI] [PubMed] [Google Scholar]
- 37.Medeiros-Neto G. Iodine nutrition in Brazil: where do we stand? Arq Bras Endocrinol Metabol. 2009;53:470–474. doi: 10.1590/s0004-27302009000400014. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (WHO) Iodine Status Wordwide: WHO global database on iodine deficiency. WHO; Geneva, Switzerland: 2004. [Google Scholar]
- 39.Pretell EA. Delange F. Hostalek U. Corigliano S. Barreda L. Higa AM. Altschuler N. Barragan D. Cevallos JL. Gonzales O. Jara JA. Medeiros-Neto G. Montes JA. Muzzo S. Pacheco VM. Cordero L. Iodine nutrition improves in Latin America. Thyroid. 2004;14:590–599. doi: 10.1089/1050725041692909. [DOI] [PubMed] [Google Scholar]
- 40.Duarte GC. Tomimori EK. Boriolli RA. Ferreira JE. Catarino RM. Camargo RY. Medeiros-Neto G. [Echographic evaluation of the thyroid gland and urinary iodine concentration in school children from various regions of the State of Sao Paulo, Brazil] Arq Bras Endocrinol Metabol. 2004;48:842–848. doi: 10.1590/s0004-27302004000600010. [DOI] [PubMed] [Google Scholar]
- 41.Camargo RY. Tomimori EK. Neves SC. I GSR. Galrao AL. Knobel M. Medeiros-Neto G. Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo, Brazil. Eur J Endocrinol. 2008;159:293–299. doi: 10.1530/EJE-08-0192. [DOI] [PubMed] [Google Scholar]
- 42.Caldwell KL. Jones R. Hollowell JG. Urinary iodine concentration: United States National Health And Nutrition Examination Survey 2001–2002. Thyroid. 2005;15:692–699. doi: 10.1089/thy.2005.15.692. [DOI] [PubMed] [Google Scholar]
- 43.Caldwell KL. Miller GA. Wang RY. Jain RB. Jones RL. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003–2004. Thyroid. 2008;18:1207–1214. doi: 10.1089/thy.2008.0161. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell KL. Makhmudov A. Ely E. Jones RL. Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid. 2011;21:419–427. doi: 10.1089/thy.2010.0077. [DOI] [PubMed] [Google Scholar]
- 45.Hollowell JG. Staehling NW. Hannon WH. Flanders DW. Gunter EW. Maberly GF. Braverman LE. Pino S. Miller DT. Garbe PL. DeLozier DM. Jackson RJ. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994) J Clin Endocrinol Metab. 1998;83:3401–3408. doi: 10.1210/jcem.83.10.5168. [DOI] [PubMed] [Google Scholar]
- 46.Sichieri R. Coitinho DC. Leao MM. Recine E. Everhart JE. High temporal, geographic, and income variation in body mass index among adults in Brazil. Am J Public Health. 1994;84:793–798. doi: 10.2105/ajph.84.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogden CL. Carroll MD. Curtin LR. McDowell MA. Tabak CJ. Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 48.Duncan S. Duncan EK. Fernandes RA. Buonani C. Bastos KD. Segatto AF. Codogno JS. Gomes IC. Freitas IF., Jr Modifiable risk factors for overweight and obesity in children and adolescents from Sao Paulo, Brazil. BMC Public Health. 2011;11:585. doi: 10.1186/1471-2458-11-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogden CL. Carroll MD. Kit BK. Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Block MA. Horn RC., Jr Miller JM. Barrett JL. Brush BE. Familial medullary carcinoma of the thyroid. Ann Surg. 1967;166:403–412. doi: 10.1097/00000658-196709000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]