Summary
Background and objectives
The role of thrombophilia in failing arteriovenous fistula (AVF) among patients with ESRD undergoing hemodialysis is not established. This study aimed to assess whether AVF primary patency is associated with thrombophilia and coagulation abnormalities.
Design, setting, participants, & measurements
This observational study screened 219 patients between 2002 and 2004 for thrombophilia before AVF surgery. Thrombophilia included factor V Leiden and prothrombin G20210A mutations, protein C and antithrombin activities, and protein S. Coagulation abnormalities included high factor VIII:C, homocysteine, fibrinogen, and d-dimer levels; presence of antiphospholipid antibodies; and short thrombin time. We reviewed patient charts for comorbid conditions, AVF maturation and interventions, kidney transplantation, and patient survival (mean follow-up duration, 3.6 [range, 2.3–5.8] years). Primary patency from the AVF placement and functional primary patency from the first AVF cannulation were analyzed with Kaplan-Meier and Cox proportional hazards models.
Results
Thrombophilia was present in 9% of the patients, and coagulation abnormalities occurred in 77%. One-year primary patency was 68%; 46% of the AVF failures occurred before the initiation of hemodialysis. Female sex (hazard ratio [HR], 2.6; 95% confidence interval [CI], 1.7–4.1) and thrombophilia (HR, 2.2; 95% CI, 1.2–4.2) were independent risk factors for loss of primary patency. Thrombophilia mutations or low antithrombin level (HR, 3.8), female sex (HR, 2.5), and diabetes (HR, 1.9) were associated with shortened functional primary patency of AVF.
Conclusions
Against the background of frequent coagulation abnormalities, thrombophilia and female sex predispose patients with ESRD to access failure, mostly due to thrombosis or stenosis.
Introduction
Vascular access represents a lifeline for patients undergoing hemodialysis (HD). A failure of vascular access among patients receiving regular HD is associated with increased morbidity, mortality, and costs. In fact, in the United States the annual costs of access failure are approximately $1 billion (1). The main causes of access dysfunction—thrombosis and stenosis—are associated with vascular injury and intimal hyperplasia caused by high-shear-rate conditions in the access (2,3).
The overall prevalence and effect of thrombophilia in patients with ESRD are as yet unclear. Studies on inherited or acquired thrombophilia in access thrombosis are scarce among patients with ESRD, and the results are contradictory. This is due to limited patient numbers, retrospective thrombophilia screening, and differences in definition and inclusion of individual types of thrombophilia (4–11). One large retrospective study of 419 patients undergoing HD found at least one thrombophilic disorder in 43% of patients with ESRD, and any thrombophilic disorder increased the risk for access thrombosis (2).
Our aim was to analyze the prevalence and effect of thrombophilia and coagulation abnormalities in patients with ESRD undergoing native arteriovenous fistula (AVF) surgery. We evaluated the effect of thrombophilia and coagulation abnormalities upon AVF survival (i.e., primary patency and functional primary patency).
Materials and Methods
Study Design
This observational study was carried out at Helsinki University Central Hospital, Finland, which provides vascular surgery for a population of 1.2 million. Between 2002 and 2004, 280 patients with ESRD underwent routine prospective screening for thrombophilia and coagulation abnormalities 1 month before their vascular access surgery. Of those 280 patients, we included 219 consecutive patients who underwent creation of an AVF, without exclusion criteria. Exclusion criteria were lack of preoperative thrombophilia screening, arteriovenous graft surgery, reconstruction of preexisting vascular access, surgical revision of the AV anastomosis, and cases in which the access was not needed for HD during follow-up. Clinical demographic characteristics, cause of kidney disease, comorbid conditions, and medications were retrieved from the patient records. Patients were followed until the end of 2007, and the mean follow-up time was 3.6 (range, 2.3–5.8) years. The study was approved by the institutional review board and ethics committee.
AVF Surveillance
The main objective of the study was to assess primary patency of AVF from the placement of fistula until the need for intervention to maintain patency, fistula thrombosis, or abandonment of the access. Functional primary patency of AVF was recorded from the initial fistula cannulation for HD until the need for the first vascular intervention or abandonment of the AVF.
Preoperative duplex scanning facilitated the choice of vascular access and helped rule out the presence of preexisting postphlebitic venous disease. During the access surveillance, primary patency, presence of thrombosis, or critical stenosis was established by duplex scanning and, subsequently, with angiography when needed. During HD, the AVF function was regularly assessed every week and when any difficulty occurred. The AVF assessment included recording of success of puncture, recirculation, blood flow, online measurements of arterial and venous pressure, and puncture site bleeding. Duplex scanning or angiography or both were performed upon any suspicion of malfunction or insufficient flow volume, even in the absence of clinical signs of insufficient dialysis. Only vascular lesions that required interventions and access abandonment were registered as adverse outcome of primary patency.
Screening and Definitions for Thrombophilia and Coagulation Abnormalities
Thrombophilia Screening.
Thrombophilia screening included laboratory assessment of congenital and acquired coagulation abnormalities: factor V Leiden (R506Q) and prothrombin G20210A mutations (cyclic mini-sequencing), activated protein C resistance (Coatest APC Resistance V; Chromogenix, Milan, Italy), protein C and antithrombin activity (Berichrom Protein C, normal range, 74%–141%; Berichrom Antithrombin III A, normal range, 84%–108%; Siemens, Healthcare Diagnostics, Marburg, Germany), and free protein S antigen (Instrumentation Laboratory, Milan, Italy; normal ranges: men, 66%–150%, women, 50%–137%).
Definition of Thrombophilia.
Thrombophilia was defined as one of the following: the presence of homozygous or heterozygous factor V Leiden or prothrombin mutation, low antithrombin activity (≤60%), and decreased activities of protein C and protein S. Decreased activities were defined at their lowest fifth percentile (protein C <74% and protein S <58% in men) and only in patients without vitamin K antagonists (these agents impair synthesis of protein C and protein S).
Acquired Coagulation Abnormalities.
Acquired coagulation abnormalities included high levels of factor VIII:C, fibrinogen, d-dimer, and homocysteine; shortened thrombin time; and presence of antiphospholipid antibodies. The latter were analyzed separately from thrombophilia because they may not be permanent and may be modified by dialysis. Cutoff values for these were set within the 75th quartiles (i.e., clearly above the references for factor VIII:C [>206%], fibrinogen [>5.9 g/L], d-dimer [>2.0 mg/L], and homocysteine [≥35 µmol/L], and the lowest quartile of thrombin time [<17 seconds]). The tests for antiphospholipid antibodies (lupus anticoagulant, cardiolipin, or β2-glycoprotein I antibodies) included lupus anticoagulant (DVVtest 10 test kit, American Diagnostica, Pfungstadt, Germany; Platelin, HemosIL APTT-SP, Instrumentation Laboratory, Milan, Italy) with plasma cardiolipin and β2-glycoprotein I antibodies (Varelisa, cardiolipin IgG antibodies and β2-glycoprotein I IgG antibodies, Phadia GmbH, Freiburg, Germany; normal range for both <15 U/ml). The latter was available for 88 patients from 2004 in our institution.
We measured prothrombin time and international normalized ratio (Nycotest prothromin time reagent, Axis-Shield PoC AS, Oslo, Norway; normal ranges, 70%–130% and 0.9–1.1, respectively), thrombin time (BC-Thrombin reagent, Siemens; normal range, 17–25 seconds), factor VIII coagulant activity, factor III:C (Pathrombin SL and Coagulation factor VIII-deficient plasma, Siemens; normal range, 52%–148%), fibrinogen (modified Clauss method, Multifibern U, Siemens; normal range, 1.7–4 g/L), d-dimer (immunoturbidimetric assay, Tina-quant, d-dimer, Roche Diagnostics, Mannheim, Germany; normal, <0.5 mg/L), and homocysteine (Homocysteine Liquid Stable reagent, Axis-Shield, Dundee, United Kingdom; normal range, 4–15 µmol/L). Behring Coagulation System-XP (Siemens) was used in automated analyses, and the Evolis analyzer (ELISA microplate system, Bio-Rad, Hemel Hempstead, United Kingdom) for measuring cardiolipin and β2-glycoprotein I antibodies. d-Dimer and homocysteine were analyzed with the Modular analyzer (Roche Diagnostics).
Statistical Analyses
The main endpoints were primary patency (from the fistula placement) and functional primary patency (from the initial cannulation) of AVF until the need of first vascular intervention or abandonment of the access or end of follow-up.
The distribution of age, sex, body mass index, smoking, cause of ESRD, transplantation history, access type and flow after surgery, comorbid conditions, use of antithrombotics, statins, antihypertensive medication, preoperative dialysis, and laboratory variables across categories were analyzed by chi-squared tests (Fisher exact test when necessary) or Mann-Whitney U test for continuous variables. Only factors with P values ≤0.2 in correlation analysis by chi-squared tests were subjected to Kaplan-Meier and Cox regression analyses as independent risk factors for access failure.
In the regression analyses, patients without AVF failure or loss of functional primary patency were censored at 2 months after fistula placement in case of maturation failure without manageable specific lesions, at the time of death, at renal transplantation, or at the end of follow-up. Risk factor differences between the groups in the survival analysis were tested with the Breslow method. All significant factors in AVF survival analyses were further examined by distribution analysis to exclude bias due to censoring times between the groups. Statistical analysis was based on PASW Statistics 18 software (SPSS Inc., Chicago, IL). Significance was set at P<0.05.
Results
Patient Characteristics, Thrombophilia, and Coagulation Abnormalities
Clinical demographic characteristics, cause of kidney disease, medications, and AVF data for the 219 patients with ESRD are presented in Tables 1 and 2. Half of the patients had a history of coronary heart disease, peripheral arterial disease, or stroke. A high proportion (88%) of the patients (Table 2) used an antithrombotic medication, and 19% of the patients were receiving a combination of two antiplatelet agents or an antiplatelet agent and low-molecular-weight heparin at the time of surgery. In all, 9% of the patients presented with thrombophilia (Table 3). Factor V Leiden was encountered in seven (3%) patients and prothrombin mutation in one patient. Low protein C or protein S activity was found in 5% of the patients, but only male patients had low protein S activity. Antithrombin activity was low (≤60%) in four patients.
Table 1.
Clinical characteristics and comorbid conditions of 219 patients with ESRD
| Characteristic | Value |
|---|---|
| Median age (range) (yr) | 57 (16–83) |
| Male sex, n (%) | 146 (67) |
| Median body mass index (range) (kg/m2) | 25 (15–42) |
| Current smoker (%) | 60 (27) |
| Cause of kidney disease, n (%) | |
| Diabetes mellitus | 73 (33) |
| Inflammatory diseasea | 74 (34) |
| Polycystic kidney disease, obstructive nephropathy | 33 (15) |
| Renovascular disease, nephrosclerosis, or other | 39 (18) |
| Comorbid conditions, n (%) | |
| Hypertension on medication | 180 (82) |
| Diabetes mellitus | 84 (38) |
| Coronary heart disease | 71 (32) |
| Peripheral arterial disease | 62 (28) |
| Rheumatoid arthritis | 20 (9) |
| Malignancy | 28 (13) |
| History of venous or arterial thrombosis, n (%) | |
| Deep vein thrombosis/pulmonary embolism | 13 (6) |
| Myocardial infarction | 31 (14) |
| Stroke/transient ischemic attack | 33 (15) |
| Any lower extremity amputation | 11 (5) |
| Previous transplantation, n (%) | 12 (6) |
GN (46%), IgA nephropathy (18%), amyloidosis (15%), tubulointerstitial nephritis (14%), or chronic pyelonephritis (8%).
Table 2.
Medication, vascular access, and preoperative dialysis in 219 patients with ESRD
| Characteristic | Value, n (%) |
|---|---|
| Medication | |
| Aspirina | 164 (75) |
| LMWH | 27 (12) |
| Warfarin | 23 (11) |
| Clopidogrel | 14 (6) |
| Dipyridamole | 5 (2) |
| No antithrombotic medication | 27 (12) |
| Statin | 121 (55) |
| No dialysis | 121 (55) |
| Ongoing preoperative dialysis | 98 (45) |
| HD [duration >3 mo] | 93 (43) [30, 32%] |
| Peritoneal dialysis [duration >3 mo] | 5 (2) [5, 100%] |
| First access operation | 198 (90) |
| Anatomic location | |
| Radiocephalic (wrist) | 199 (91) |
| Brachiocephalic (elbow) | 20 (9) |
| Median access flow (range) (ml/min)b (n=209) | 180 (10–855) |
LMWH, low molecular-weight heparin; HD, hemodialysis.
For 30 patients, aspirin was initiated in association with the access surgery.
According to transient-time ultrasonography immediately after surgery.
Table 3.
Thrombophilia and coagulation abnormalities in all patients, those with patent fistula, and those with failure of primary fistula patency
| Thrombophilia and Coagulation Abnormalities | All Patients (n=219) | Patients with Patent Fistula (n=137) | Patients with Patency Failure (n=82) | P Valuea |
|---|---|---|---|---|
| Thrombophilia | ||||
| Factor V (R506Q) or prothrombin G20210A mutation | 8 (4) | 3 (2) | 5 (6) | 0.16 |
| Low protein S activity among 146 males (<58%)bc | 6 (4) | 3 (3) | 3 (7.5) | 0.35 |
| Low protein C activity (<74%)b | 7 (3) | 3 (2) | 4 (5) | 0.43 |
| Low antithrombin activity (≤60%) | 4 (2) | 0 | 4 (5) | 0.02d |
| Patients with above-mentioned thrombophiliae | 19 (9) | 8 (6) | 11 (13) | 0.05 |
| Positivity for antiphospholipid antibodiesf | 23 (11) | 15 (11) | 8 (10) | 0.78 |
| Coagulation abnormalities | ||||
| Short thrombin time (<17 s, 25th percentile) | 54 (26) | 36 (27) | 18 (23) | 0.49 |
| High factor VIII:c activity (>206%, 75th percentile) | 52 (24) | 32 (24) | 20 (25) | 0.92 |
| Low antithrombin activity (≤79%, 10th percentile) | 23 (11) | 13 (10) | 10 (12) | 0.53 |
| High fibrinogen level (>5.9 g/L, 75th percentile) | 47 (23) | 27 (21) | 20 (26) | 0.43 |
| High d-dimer level (>2.0 mg/L, 75th percentile) | 49 (23) | 33 (26) | 16 (20) | 0.36 |
| High homocysteine level (≥35 µmol/L, 75th percentile) | 51 (24) | 31 (24) | 20 (25) | 0.87 |
Values are expressed as number (percentage) of patients.
By chi-squared or Fisher exact test.
Warfarin users excluded from the analysis.
All female patients had normal protein S activity.
Statistically significant.
More than one type of thrombophilia was found in six patients.
Positivity for lupus anticoagulant, cardiolipin, or β2-glycoprotein I antibodies.
In addition, 11% of patients were positive for antiphospholipid antibodies (lupus anticoagulant, cardiolipin, or β2-glycoprotein I antibodies), and positivity occurred in more men than women (14% versus 3%; P=0.008). Other coagulation abnormalities were present in most (77%) patients (Table 3). Coagulation factor VIII:C (median, 171%; interquartile range [IQR], 59%), fibrinogen (median, 5.2 g/L; IQR, 1.6 g/L), d-dimer (median, 0.9 mg/L; IQR 1.5 mg/L), and homocysteine (median, 27 µmol/L; IQR, 15 µmol/L) levels were all markedly above the normal reference ranges. Moreover, thrombin time was short (<17 seconds) in 25% of patients.
AVF Outcome and Primary Patency
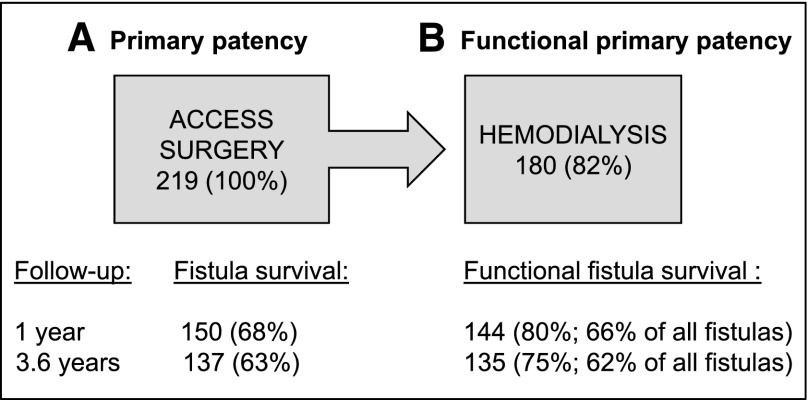
Overall, 46% of AVF failures (i.e., insufficient maturation, thrombosis, or stenosis) had already occurred before HD was initiated. Eleven patients had maturation failure without specific manageable vascular lesions. Most of these were women (73%) and had both diabetes and lower-extremity atherosclerosis (73%); 36% had an upper-arm fistula. Three patients died before the initiation of HD. The 1-year primary patency rate was 68%, and thrombosis or stenosis led to AVF failure in 82 patients (37%) during the follow-up period (mean, 3.6 years; range, 2.3–5.8 years) (Figure 1).
Figure 1.
Primary and functional primary patency of arteriovenous fistula (AVF). (A) AVF survival after the placement of the fistula in 219 patients. (B) Functional AVF survival after the initial fistula cannulation among the 180 patients in whom hemodialysis could be initiated. Success rates are shown at 1 year and at the end of follow-up (mean, 3.6 years).
According to transient-time ultrasonography, the immediate postoperative blood flow through the fistula was not associated with AVF patency (data not shown). Patient groups with and without AVF failure were similar with regard to clinical characteristics, comorbid conditions, mortality, and access details (Tables 1 and 2). The only exception was the overrepresentation of women among patients with AVF failure (51% versus 23%; P<0.001). Kidney transplantation was performed equally in patients with or without AVF failure during the follow-up. Mortality was 11% at 1 year.
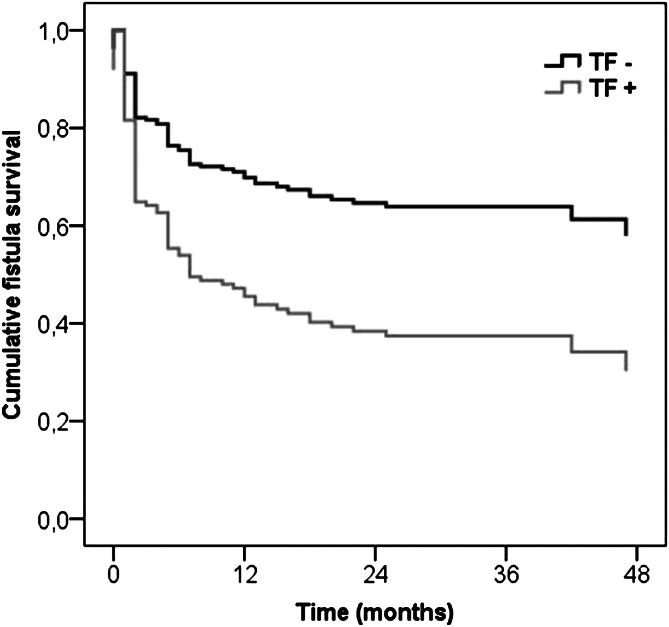
According to Kaplan-Meier analysis, AVF patency was significantly lower in association with three clinical factors. First, in women compared with men, the AVF survival was shortened to 25 months (95% confidence interval [CI], 18–32 months) versus 45 months (95% CI, 41–49 months). Second, patients with thrombophilia had shorter AVF survival than patients without thrombophilia: 19 months (95% CI, 10–27 months) versus 40 months (95% CI, 36–44 months), respectively. Finally, AVF survival was 25 months in patients with a history of vascular access (95% CI, 14–35 months) versus 40 months (95% CI, 36–44 months) in patients having their first vascular access. Positivity for antiphospholipid antibodies was not associated with primary patency of AVF. In the multivariate Cox analysis, female sex (hazard ratio [HR], 2.6; 95% CI, 1.7–4.1) and the presence of thrombophilia (HR, 2.2; 95% CI, 1.2–4.2) were independent risk factors for shortened primary patency of AVF (Figure 2).
Figure 2.
Cumulative arteriovenous fistula survival among patients with (TF+) and without (TF-) thrombophilia. Female sex (P<0.001) and the presence of thrombophilia (P=0.02) were associated with significantly shortened primary patency of arteriovenous fistula. Thrombophilia included factor V Leiden and prothrombin mutations and low levels of natural anticoagulants (antithrombin, protein C, and protein S) (see Materials and Methods).
Functional Primary Patency of Arteriovenous Fistula
In 180 patients (82%), AVF could be used for HD. Among these patients, the 1-year rate of primary functional patency for AVF was 80% (66% of all created AVFs) (Figure 1). Percutaneous transluminal angioplasty was performed in eight patients to enhance patency before initiation of dialysis. Median duration of functional primary patency for radial and brachial AVFs were similar: 18 (IQR, 26) months versus 11 (IQR, 31) months, respectively.
One third of the patients with thrombophilia developed AVF failure before HD. Yet the presence of thrombophilic gene mutations or low antithrombin levels (<60%) (n=9) was associated with a shortened functional primary patency: 19 months (95% CI, 7–31 months) versus 47 months (95% CI, 43–51 months) (P=0.01). In women, the functional patency was shortened: 34 months (95% CI, 25–42 months) versus 50 months (95% CI, 45–54 months) in men (P=0.003). In the multivariate analysis, thrombophilic gene mutations or low antithrombin levels (HR, 3.8; 95% CI, 1.5–9.9), female sex (HR, 2.5; 95% CI, 1.4–4.5), and diabetes (HR, 1.9; 95% CI, 1.1–3.5) independently shortened the functional primary patency.
Discussion
AVF failure, mainly due to thrombosis or stenosis, occurred in 37% of the 219 patients in this study within the mean follow-up of 3.6 years. Almost half of the events had already occurred before the initiation of dialysis, and up to 84% occurred during the first year. After successful initiation of HD, only a few AVFs were abandoned, as previously reported (12). The most significant risk factors for primary patency failure of AVF were female sex, history of previous vascular access, and thrombophilia. Similarly, female sex, the presence of thrombophilic gene mutation, and low antithrombin and diabetes were associated with the loss of functional primary patency.
In this study, thrombophilia prevalence (9%) was equal to that in general population (13) and was similarly distributed between men and women. We followed a conservative strategy and analyzed acquired coagulation abnormalities separately because they may be transient and influenced by dialysis. Acquired coagulation abnormalities were highly prevalent, occurring in 77% of the patients. Antiphospholipid antibodies were more common in men but did not seem to affect the AVF outcome. High incidence of thrombophilia is reportedly associated with risk of thrombosis after access surgery or revascularization among patients with ESRD and those with other vascular conditions (2,11,14). The difference in thrombophilia prevalence among the studies reflects various definitions of thrombophilia.
Thrombosis and stenosis of AVF both compromise the primary patency and coincide, but they cannot always be distinguished. The clinical consequences are also rather similar: insufficient maturation or a need for intervention or access abandonment. In fact, thrombosis, atherosclerosis, and inflammation form a pathogenic continuum in cardiovascular disease (15,16). Inflammatory mechanisms upregulate procoagulants, downregulate anticoagulants, and inhibit fibrinolysis, resulting in prothrombotic states (15–17). Furthermore, acquired coagulation abnormalities (i.e., elevated factor VIII:C level, short thrombin time, and high fibrinogen and d-dimer levels) were frequent in our patients with ESRD (Table 3) and failed to differentiate problems in access thrombosis or stenosis. Remarkably, these acquired abnormalities have been reported to correlate with cardiovascular mortality and other thrombotic events in ESRD (18–20). In this study, in addition to high prevalence of cardiovascular disease (49%), a history of venous thromboembolism (6%) was relatively frequent (Table 1). Further studies are needed to assess the effect and management of the coagulation abnormalities on high cardiovascular mortality and morbidity in ESRD.
The 1-year rate of primary patency of AVF was 68%, and the rate of initial functional primary patency for HD was 86%. Our relatively high success rate may reflect careful patient selection for HD, large patient volume, duplex mapping of suitable veins for vascular access, scrutinized vascular technique, and frequent use of antithrombotics (88%). Additionally, 90% of the patients were having their first access operation. In fact, AVF is the most common access type in Finland (79%) compared with grafts (3%) and a permanent (tunneled) catheter (16%) (Finnish Registry for Kidney Diseases. Report 2008. Helsinki, Finland, 2009; http://www.musili.fi/smtr/english).
General risk factors for access failure include female sex, diabetes, and history of access dysfunction (21–26). In this study, diabetes was associated only with the impaired functional primary patency of AVF. Among women, the underlying reasons for access failure are unclear. One hypothesis is the subcutaneous adipose tissue and small vessel size; in fact, in women, upper-arm fistulas seem to succeed better than forearm fistulas (21,22). Women are also subjected to increased risk for arterial thrombosis, such as myocardial infarction, despite taking aspirin (27).
Some patients with ESRD carry a particularly high risk for recurring access thrombosis or stenosis (5,28,29). These patients with hypercoagulable states or combined thrombophilia should be preoperatively identified to tailor antithrombotic therapy and intensify surveillance, especially during the first, most vulnerable months after surgery. However, the optimal prophylaxis for access thrombosis and stenosis is not settled despite extensive efforts (29–35).
Limitations of this study include moderate sample size and descriptive data on medications that could not be compared. Furthermore, the results represent a single center experience of a racially homogeneous cohort and may not be generalizable to other populations. The magnitude of risk of undiagnosed thrombophilia is difficult to interpret because of the frequent use of antithrombotic treatment in this study.
Routine thrombophilia screening is not recommended before access surgery. However, certain risk factors, such as prior access failure, history of thrombosis at a young age, or unprovoked venous and arterial thrombosis, should raise the suspicion of thrombophilia and necessitate laboratory testing. Thrombophilia appears as an additional risk indicator for AVF failures. Known thrombophilia should alert clinician to enhance and target antithrombotic therapy and surveillance of the access. However, the optimal clinical management requires further studies. We suggest that the surrogate markers of coagulation (e.g., fibrinogen, factor VIII, and d-dimer may be valuable in designing antithrombotic medication, as reported in cardiovascular disease and venous thromboembolism (36,37). Against the background of common acquired coagulation abnormalities, thrombophilia and female sex predispose patients with ESRD to problems with patency of the vascular access. Optimization of the antithrombotic therapy, particularly in association with the initial access surgery and vascular interventions, may improve the outcome. Overall, antithrombotic treatment among HD patients demands more attention. Multicenter randomized trials should assess whether access survival can be improved with tailored antithrombotic therapies while maintaining the balance with bleeding risks.
Disclosures
None.
Acknowledgments
The authors thank Professor Seppo Sarna (Department of Biometry, University of Helsinki) for his assistance in the statistical analysis of the data and Dr. Petri Koskinen (Department of Nephrology, Helsinki University Central Hospital) for valuable comments. Anita Mäkelä, Julio Resendiz, and Marja Lemponen are acknowledged for their contribution to the database.
Study funding was provided by Helsinki University Central Hospital’s Research Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Knoll GA, Wells PS, Young D, Perkins SL, Pilkey RM, Clinch JJ, Rodger MA: Thrombophilia and the risk for hemodialysis vascular access thrombosis. J Am Soc Nephrol 16: 1108–1114, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Sukhatme VP, Cheung AK: Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol 17: 1112–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Födinger M, Mannhalter C, Pabinger I, Koizar D, Rintelen C, Hörl WH, Sunder-Plassmann G: Resistance to activated protein C (APC): Mutation at Arg506 of coagulation factor V and vascular access thrombosis in haemodialysis patients. Nephrol Dial Transplant 11: 668–672, 1996 [DOI] [PubMed] [Google Scholar]
- 5.LeSar CJ, Merrick HW, Smith MR: Thrombotic complications resulting from hypercoagulable states in chronic hemodialysis vascular access. J Am Coll Surg 189: 73–79, discussion 79–81, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Manns BJ, Burgess ED, Parsons HG, Schaefer JP, Hyndman ME, Scott-Douglas NW: Hyperhomocysteinemia, anticardiolipin antibody status, and risk for vascular access thrombosis in hemodialysis patients. Kidney Int 55: 315–320, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Valeri A, Joseph R, Radhakrishnan J: A large prospective survey of anti-cardiolipin antibodies in chronic hemodialysis patients. Clin Nephrol 51: 116–121, 1999 [PubMed] [Google Scholar]
- 8.Adler S, Szczech L, Qureshi A, Bollu R, Thomas-John R: IgM anticardiolipin antibodies are associated with stenosis of vascular access in hemodialysis patients but do not predict thrombosis. Clin Nephrol 56: 428–434, 2001 [PubMed] [Google Scholar]
- 9.Ataç B, Yakupoğlu U, Ozbek N, Ozdemir FN, Bilgin N: Role of genetic mutations in vascular access thrombosis among hemodialysis patients waiting for renal transplantation. Transplant Proc 34: 2030–2032, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Mallamaci F, Bonanno G, Seminara G, Rapisarda F, Fatuzzo P, Candela V, Scudo P, Spoto B, Testa A, Tripepi G, Tech S, Zoccali C: Hyperhomocysteinemia and arteriovenous fistula thrombosis in hemodialysis patients. Am J Kidney Dis 45: 702–707, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ghisdal L, Broeders N, Wissing KM, Mena JM, Lemy A, Wijns W, Pradier O, Donckier V, Racapé J, Vereerstraeten P, Abramowicz D: Thrombophilic factors in stage V chronic kidney disease patients are largely corrected by renal transplantation. Nephrol Dial Transplant 26: 2700–2705, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ, CIMINO study group : Hemodialysis arteriovenous fistula patency revisited: Results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 3: 714–719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosendaal FR: Venous thrombosis: a multicausal disease. Lancet 353: 1167–1173, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Vig S, Chitolie A, Sleight S, Bevan D, Dormandy J, Thompson MM, Halliday A: Prevalence and risk of thrombophilia defects in vascular patients. Eur J Vasc Endovasc Surg 28: 124–131, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Corti R, Hutter R, Badimon JJ, Fuster V: Evolving concepts in the triad of atherosclerosis, inflammation and thrombosis. J Thromb Thrombolysis 17: 35–44, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Esmon CT: Inflammation and thrombosis. J Thromb Haemost 1: 1343–1348, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Esmon CT: Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost 32[Suppl 1]: 49–60, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B: Cardiovascular mortality risk in chronic kidney disease: Comparison of traditional and novel risk factors. JAMA 293: 1737–1745, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Wannamethee SG, Shaper AG, Lowe GD, Lennon L, Rumley A, Whincup PH: Renal function and cardiovascular mortality in elderly men: The role of inflammatory, procoagulant, and endothelial biomarkers. Eur Heart J 27: 2975–2981, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kirmizis D, Tsiandoulas A, Pangalou M, Koutoupa E, Rozi P, Protopappa M, Barboutis K: Validity of plasma fibrinogen, D-dimer, and the von Willebrand factor as markers of cardiovascular morbidity in patients on chronic hemodialysis. Med Sci Monit 12: CR55–CR62, 2006 [PubMed] [Google Scholar]
- 21.Miller PE, Tolwani A, Luscy CP, Deierhoi MH, Bailey R, Redden DT, Allon M: Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 56: 275–280, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Yevzlin AS, Conley EL, Sanchez RJ, Young HN, Becker BN: Vascular access outcomes and medication use: a USRDS study. Semin Dial 19: 535–539, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO: Vascular access survival and incidence of revisions: A comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg 34: 694–700, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Diehm N, van den Berg JC, Schnyder V, Bühler J, Willenberg T, Widmer M, Mohaupt MG, Baumgartner I: Determinants of haemodialysis access survival. Vasa 39: 133–139, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Monroy-Cuadros M, Yilmaz S, Salazar-Bañuelos A, Doig C: Risk factors associated with patency loss of hemodialysis vascular access within 6 months. Clin J Am Soc Nephrol 5: 1787–1792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL: Aspirin for the primary prevention of cardiovascular events in women and men: A sex-specific meta-analysis of randomized controlled trials. JAMA 295: 306–313, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mitsiev I, Reinhold S, Ziemer S, Neumayer HH, Hocher B: Combination of APC resistance and acquired protein S deficiency in a haemodialysis patient with recurrent A-V shunt thrombosis. Nephrol Dial Transplant 14: 2474–2477, 1999 [DOI] [PubMed] [Google Scholar]
- 29.O’Shea SI, Lawson JH, Reddan D, Murphy M, Ortel TL: Hypercoagulable states and antithrombotic strategies in recurrent vascular access site thrombosis. J Vasc Surg 38: 541–548, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kaufman JS: Antithrombotic agents and the prevention of access thrombosis. Semin Dial 13: 40–46, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Kaufman JS, O’Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, Goldfarb DS, Peduzzi PN, Veterans Affairs Cooperative Study Group on Hemodialysis Access Graft Thrombosis : Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol 14: 2313–2321, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI, Dialysis Access Consortium Study Group : Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr, Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW, Feldman HI, DAC Study Group : Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon BS, Beck GJ, Dember LM, Vazquez MA, Greenberg A, Delmez JA, Allon M, Himmelfarb J, Hu B, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Lawson JH, Cotton JR, Jr, Kusek JW, Feldman HI, Dialysis Access Consortium (DAC) Study Group : Use of aspirin associates with longer primary patency of hemodialysis grafts. J Am Soc Nephrol 22: 773–781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasegawa T, Elder SJ, Bragg-Gresham JL, Pisoni RL, Yamazaki S, Akizawa T, Jadoul M, Hugh RC, Port FK, Fukuhara S: Consistent aspirin use associated with improved arteriovenous fistula survival among incident hemodialysis patients in the dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol 3: 1373–1378, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, Folsom AR, Wu K, Benderly M, Goldbourt U, Willeit J, Kiechl S, Yarnell JW, Sweetnam PM, Elwood PC, Cushman M, Psaty BM, Tracy RP, Tybjaerg-Hansen A, Haverkate F, de Maat MP, Fowkes FG, Lee AJ, Smith FB, Salomaa V, Harald K, Rasi R, Vahtera E, Jousilahti P, Pekkanen J, D'Agostino R, Kannel WB, Wilson PW, Tofler G, Arocha-Piñango CL, Rodriguez-Larralde A, Nagy E, Mijares M, Espinosa R, Rodriquez-Roa E, Ryder E, Diez-Ewald MP, Campos G, Fernandez V, Torres E, Marchioli R, Valagussa F, Rosengren A, Wilhelmsen L, Lappas G, Eriksson H, Cremer P, Nagel D, Curb JD, Rodriguez B, Yano K, Salonen JT, Nyyssönen K, Tuomainen TP, Hedblad B, Lind P, Loewel H, Koenig W, Meade TW, Cooper JA, De Stavola B, Knottenbelt C, Miller GJ, Cooper JA, Bauer KA, Rosenberg RD, Sato S, Kitamura A, Naito Y, Palosuo T, Ducimetiere P, Amouyel P, Arveiler D, Evans AE, Ferrieres J, Juhan-Vague I, Bingham A, Schulte H, Assmann G, Cantin B, Lamarche B, Després JP, Dagenais GR, Tunstall-Pedoe H, Woodward M, Ben-Shlomo Y, Davey Smith G, Palmieri V, Yeh JL, Rudnicka A, Ridker P, Rodeghiero F, Tosetto A, Shepherd J, Ford I, Robertson M, Brunner E, Shipley M, Feskens EJ, Kromhout D, Dickinson A, Ireland B, Juzwishin K, Kaptoge S, Lewington S, Memon A, Sarwar N, Walker M, Wheeler J, White I, Wood A; Fibrinogen Studies Collaboration: Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 294: 1799–1809, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Jenkins PV, Rawley O, Smith OP, O’Donnell JS: Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol 157: 653–663, 2012 [DOI] [PubMed] [Google Scholar]