Summary
Background and objectives
A 1-year multicenter prospective randomized controlled study was conducted on the effects of vitamin E-bonded polysulfone dialyzers on erythropoiesis-stimulating agent response in hemodialysis patients.
Design, setting, participants, & measurements
Major inclusion criteria were use of high-flux polysulfone dialyzers with 50–70 ml/min β2-microglobulin clearance over 3 months, transferrin saturation over 20%, same erythropoiesis-stimulating agent for over 3 months, and hemoglobin at 10–12 g/dl. Hemodialysis patients were placed in four interventional groups: two hemoglobin ranges (10.0–10.9 or 11.0–11.9 g/dl) and two dialyzers. Patients were randomly assigned by central registration to a vitamin E-bonded polysulfone dialyzers or polysulfone control group. Primary end point was relative erythropoiesis resistance index at baseline between groups at 12 months. Erythropoiesis resistance index was defined as total weekly erythropoiesis-stimulating agent dose divided by hemoglobin.
Results
There were no statistically significant differences in age or sex. There was no significant difference in relative erythropoiesis resistance index between vitamin E-bonded polysulfone dialyzers and control groups at 12 months (vitamin E-bonded polysulfone dialyzers: 1.1, control: 1.3). The vitamin E-bonded polysulfone dialyzers group showed better relative erythropoiesis resistance index than the control group at 11.0–11.9 g/dl hemoglobin (vitamin E-bonded polysulfone dialyzers: 1.0, control: 1.4 at 12 months, significant difference) but no difference at 10.0–10.9 g/dl hemoglobin.
Conclusions
The overall relative erythropoiesis resistance index showed no difference between the vitamin E-bonded polysulfone dialyzers and control groups, although the change in relative erythropoiesis resistance index differed according to hemoglobin level.
Introduction
Patients on maintenance hemodialysis are constantly exposed to a state of excessive oxidative stress, in part because of contact with the dialyzers during extracorporeal circulation (1–4). Excessive oxidative stress in hemodialysis patients leads to cardiovascular events and affects the erythrocytes. High-flux polysulfone dialyzers are the most commonly used type in Japan. These dialyzers provide good biocompatibility; however, if a switch is made to similar high-flux dialyzers, improved biocompatibility is a priority (5,6).
Cellulose dialyzers bonded with vitamin E, which confers antioxidant action, are reported to maintain or increase hemoglobin (Hb) levels, even at reduced doses of human recombinant erythropoietin (rHuEPO) (7,8). A study on the link between erythrocyte creatinine content and erythrocyte lifespan has reported that vitamin E-bonded dialyzers prolong erythrocyte lifespan (9). Floridi et al. (10) have confirmed and quantified in vitro the antioxidant capacity of vitamin E-bonded polysulfone dialyzers. However, clinical research reports on the effects of vitamin E-bonded dialyzers have hitherto been mostly single-center studies (11,12), with no multicenter prospective studies yet reported.
In the present study, a multicenter prospective randomized controlled trial, we compared erythropoiesis-stimulating agent (ESA) response in hemodialysis patients using vitamin E-bonded high-flux polysulfone dialyzers (VPS-HA) with conventional high-flux polysulfone dialyzers as controls.
Materials and Methods
This multicenter prospective randomized controlled trial was conducted at 48 centers in Japan from August of 2008 to March of 2011. It complied with the ethical principles laid out in the Declaration of Helsinki (amended in 2000) and Good Clinical Practice.
The protocol and ethical considerations were initially approved by the Kameda Medical Center’s Ethics Committee and then, the Ethics Committees at the individual centers. Each patient gave their written informed consent to participate after receiving a full explanation of the details of the research. The clinical trial was registered with the Clinical Trial Registry of the University Hospital Medical Information Network (UMIN-CTR ID UMIN000001285).
Each patient was allocated to a target Hb range depending on one's prestudy level of Hb measured for 3 months, and the target Hb level was kept during this trial.
We decided beforehand that two successive deviations from the ESA medication standard or a C-reactive protein (CRP) test result of more than 2 mg/dl would lead to the patient being dropped from the study.
Participants
Patients on hemodialysis therapy for CKD (excluding polycystic kidney disease) who satisfied the following entry criteria were recruited to the study. (1) Patient had been on hemodialysis therapy for at least 1 year using polysulfone (PS) high-performance dialyzers (13) with a β2-microglobulin clearance of 50–70 ml/min for 3 months before study initiation. (2) Patient had iron status above the target level in the National Kidney Foundation–Kidney Disease Outcomes Quality Initiative Practice Guidelines (14) for anemia caused by CKD, maintained a plasma ferritin level above 100 ng/mg and/or transferrin saturation (TSAT) above 20%, had a Hb level of 10.0–12.0 g/dl, and had used the same ESA (rHuEPO or darbepoetin alfa [DA]) for 3 months before the start of the study.
Methods
The protocol is outlined in Figure 1. PS dialyzers of the same functional class (type 4) as the VPS-HA were used as controls (PS-con group). The study was managed as a multicenter, open-label, randomized trial. The patients were randomly allocated using the central University Hospital Medical Information Network Internet Data and Information Center for Medical Research (INDICE) registration system to the VPS-HA group (conventional dialyzer switched to VPS-HA) or the PS-con group (continued use of the same conventional PS dialyzer). The study period was 12 months. We defined erythropoiesis resistance index (ERI) as the total weekly ESA dose divided by the Hb value.
Figure 1.
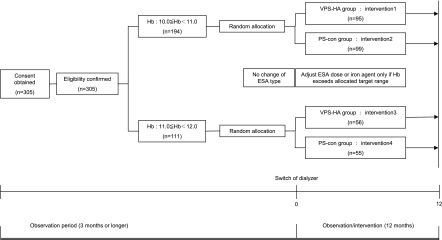
Outline of protocol. Each patient was allocated to target hemoglobin range depending on one’s prestudy level of hemoglobin measured for 3 months, and the target hemoglobin level was kept during this trial. ESA, erythropoiesis-stimulating agent; Hb, hemoglobin level, PS-con, type 4 polysulfone membrane; VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane.
Criteria for Anemia Management.
All patients had their Hb and TSAT measured precisely after checking that the minimum dosage of intravenous iron and/or ESA was the same at all the facilities.
The dose of ESA was adjusted and administered intravenously to maintain the Hb level within the target range corresponding to the level at study initiation (10.0≤Hb≤10.9 or 11.0≤Hb≤11.9 g/dl). If the Hb level exceeded the target range or there was no iron deficiency (TSAT 20% or above), no iron agent was used, and the dose of ESA (rHuEPO or DA) was adjusted accordingly. If iron deficiency was present (TSAT below 20%), an iron agent (type and administration method decided at individual centers) was supplemented until TSAT reached 20%, at which point iron treatment was discontinued. Administration of ESA was changed to 3000±1500 U/wk for rHuEPO or 15±5 µg/2 wk for DA. Each patient received the same rHuEPO or DA agent throughout the study period. Minimal dosages at all facilities for ESA were virtually the same, and therefore, we assumed no difference between groups A and B within or between the facilities taking part in the study.
End Points.
The primary end point was relative ERI, an index of response to ESA at baseline. We designated ERI as the total weekly dose of ESA divided by the Hb level. The dose of ESA was the dose needed to maintain the target Hb level in each group (10.0–10.9 or 11.0–11.9 g/dl) using two kinds of dialyzer (VPS-HA or PS-con). We, thus, set up the following four intervention groups.
Intervention 1 (object group): VPS-HA, Hb range matched 10.0–10.9 g/dl as Hb at the initial point.
Intervention 2 (control group): type 4 polysulfone dialyzers, Hb range matched 10.0–10.9 g/dl as Hb at the initial point.
Intervention 3 (object group): VPS-HA, Hb range matched 11.0–11.9 g/dl as Hb at the initial point.
Intervention 4 (control group): type 4 polysulfone dialyzers, Hb range matched 11.0–11.9 g/dl as Hb at the initial point.
We used random assignment for dialyzer type using the University Hospital Medical Information Network Internet Data and Information Center for Medical Research (INDICE) central allocation system and nonrandom assignment for the Hb target. The Hb target was set at the value seen in the 3 months preceding the study. We set several allocation factors (facility, age [above and below 65 years], sex, Hb range, ferritin, presence or absence of diabetes, and type and dosage of ESA) and kept the Hb ranges as closely matching as possible between groups 1 and 2 and groups 3 and 4.
We defined, as the primary outcome, a significant difference in ERI between groups in the same Hb range at 12 months.
The secondary end points were peripheral blood hematology, TSAT, ferritin, transferrin, indirect bilirubin, and high-sensitivity CRP. Other parameters observed during the study period included dosage of iron agent, type and dosage of antihypertensive agent, dialysate endotoxin level, and cardiovascular events.
Statistical Analyses.
Statistical analyses were performed using XLSTAT (version 2010; Addinsoft SARL, Paris, France).
Data are expressed as mean ± SD. Baseline characteristics were compared using t and chi-squared tests. Primary and secondary outcome measures were compared using t test, Wilcoxon signed-ranks test, or repeated-measures ANOVA for relative values and analysis of covariance for absolute values followed by Scheffé posthoc test. A two-tailed P<0.05 was regarded as statistically significant. The absolute analysis of covariance values for each month were compared among the paired groups based on the value at the start of the study, which was then set at one to provide a comparative index.
Results
Forty-eight institutions in Japan participated in the clinical trial. A total of 305 patients satisfying the entry criteria was initially registered and randomly allocated using a central registration method (Table 1). Figure 2 summarizes the patients’ dispositions. On completion of the 1-year study period, 213 patients had adhered to the protocol, and 92 patients had dropped out of or been excluded from the study. The reasons for dropping out were similar between the two groups: cardiovascular events, cerebrovascular events, malignant tumors, infections, hemorrhage (gastrointestinal), death, anemia management, high inflammatory response, renal transplantation, dialyzer-related problems, withdrawal of consent, and missing data (data not shown). Consequently, 213 patients were analyzed in the present study. The characteristics of our study population are shown in Table 2.
Table 1.
Results of patient allocation
| Allocation Factor | 10≤Hb<11 | 11≤Hb<12 | ||
|---|---|---|---|---|
| VPS-HA Group | PS-Con Group | VPS-HA Group | PS-Con Group | |
| Age (yr) | ||||
| ≤65 | 55 | 46 | 19 | 30 |
| >65 | 40 | 53 | 37 | 25 |
| Sex | ||||
| Men | 61 | 54 | 36 | 45 |
| Women | 34 | 45 | 20 | 10 |
| Serum ferritin (ng/ml) | ||||
| ≤100 | 70 | 65 | 28 | 30 |
| >100 | 25 | 34 | 28 | 25 |
| Diabetes | ||||
| With | 28 | 40 | 14 | 16 |
| Without | 67 | 59 | 42 | 39 |
| Type of erythropoiesis-stimulating agent | ||||
| DA | 38 | 44 | 27 | 25 |
| rHuEPO | 57 | 55 | 29 | 30 |
| Total | 95 | 99 | 56 | 55 |
Each patient was allocated to target hemoglobin range depending on one's prestudy level of hemoglobin measured for 3 months, and the target hemoglobin level was kept during this trial. Hb, hemoglobin; VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane; PS-con, type 4 polysulfone dialysis membrane; DA, darbepoetin alfa; rHuEPO, human recombinant erythropoietin.
Figure 2.
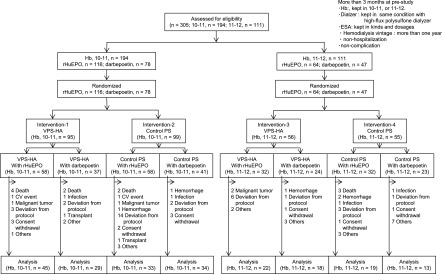
Patient disposition. Each patient was allocated to target hemoglobin range depending on one’s prestudy level of hemoglobin measured for 3 months, and the target hemoglobin level was kept during this trial. Death, cardiovascular, cerebrovascular, malignant tumor, and others as content; deviation from protocol, changing the type of erythropoiesis-stimulating agent (ESA), inadequate ESA dose, and dialyzer related as content; Hb, hemoglobin; hemorrhage, gastrointestinal hemorrhage; PS-con, type 4 polysulfone membrane; rHuEPO, human recombinant erythropoietin; transplant, renal transplantation, others, or change of hospital (patient factor); VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane; 10.0–10.9, hemoglobin range of 10.0–10.9 g/dl as target hemoglobin; 11.0–11.9, hemoglobin range of 11.0–11.9 g/dl as target hemoglobin.
Table 2.
Profile of patients analyzed
| Item | 10.0≤Hb<11.0 | 11.0≤Hb<12.0 | ||||
|---|---|---|---|---|---|---|
| VPS-HA | PS-Con | P Value | VPS-HA | PS-Con | P Value | |
| No. of patients analyzed | 74 | 67 | 40 | 32 | ||
| Men (%) | 63.5 | 58.2 | 0.52 | 67.5 | 68.8 | 0.91 |
| Age (yr; mean ± SD) | 64.6±11.4 | 61.5±12.2 | 0.14 | 57.4±13.7 | 62.8±12.5 | 0.12 |
| Body mass index | 21.2±3.4 | 21.2±3.5 | 0.56 | 20.9±2.6 | 21.4±3.0 | 0.59 |
| Dialysis period (yr) | 80.4±67.3 | 73.5±74.1 | 0.38 | 92.3±79.8 | 84.1±72.5 | 0.87 |
| ESA dose | ||||||
| rHuEPO (U/wk) | 4500 (2250, 4500) | 4500 (3000, 6000) | 1.00 | 3000 (1500, 5625) | 3000 (2625, 4500) | 0.74 |
| DA (μg/wk) | 20 (15, 30) | 20 (15, 30) | 0.81 | 20 (15, 20) | 10 (10, 15) | <0.001a |
| Iron agent treatment (%) | 27.0 | 17.9 | 0.20 | 7.5 | 25.0 | 0.04a |
| Antihypertensive (ARB and/or ACE; %) | 56.8 | 52.2 | 0.59 | 55.0 | 46.9 | 0.49 |
| Laboratory tests | ||||||
| White cell count (/μl) | 5590±1653 | 5770±1909 | 0.74 | 5879±1608 | 5728±1680 | 0.67 |
| Red cell count (×104/μl) | 331.7±27.5 | 332.6±31.5 | 0.85 | 350.9±37.3 | 343.6±34.8 | 0.20 |
| Hb (g/dl) | 10.7±0.8 | 10.7±0.8 | 0.98 | 11.2±0.9 | 11.1±0.9 | 0.24 |
| Hematocrit (%) | 32.5±2.5 | 32.4±2.5 | 0.80 | 34.4±3.1 | 33.4±2.9 | 0.18 |
| Platelet count (×104/μl) | 18.0±6.1 | 17.4±6.3 | 0.48 | 18.9±5.6 | 18.0±5.7 | 0.59 |
| Reticulocyte count (‰) | 13.6±7.4 | 14.8±8.3 | 0.35 | 12.9±6.7 | 12.6±5.4 | 0.94 |
| Iron (μg/dl) | 68.7±23.2 | 75.4±48.5 | 0.90 | 74.0±23.8 | 72.6±21.0 | 0.89 |
| Ferritin (ng/ml) | 180.9 (85.7, 321.8) ±171.8 | 105.0 (55.9, 216.0) | 0.04a | 93.7 (48.8, 124.5) | 132.3 (88.0, 195.6) | 0.01a |
| Transferrin (mg/dl) | 172.1±40.4 | 176.0±35.8 | 0.30 | 187.2±35.3 | 169.9±26.3 | 0.04a |
| TSAT (%) | 29.3±10.3 | 32.5±22.1 | 0.61 | 29.5±8.5 | 31.9±9.2 | 0.34 |
| Indirect bilirubin (mg/dl) | 0.22±0.10 | 0.20±0.10 | 0.20 | 0.20±0.11 | 0.25±0.12 | 0.03a |
| hs-CRP (mg/dl) | 0.14±0.28 | 0.13±0.16 | 0.29 | 0.14±0.23 | 0.18±0.27 | 0.18 |
| Dialysate endotoxin (EU/ml) | ||||||
| <0.001 | 75.0% | 77.4% | >0.99 | 84.6% | 77.4% | 0.99 |
| 0.001–0.05 | 13.2% | 11.3% | 10.3% | 12.9% | ||
| 0.05–0.5 | 11.8% | 8.1% | 5.1% | 9.7% | ||
| <0.5 | 0.0% | 3.2% | 0.0% | 0.0% | ||
Values are mean ± SD or median (25th, 75th for ESA dose and ferritin). Statistical testing of baseline characteristics between the VPS-HA and PS-con groups was performed using the Mann–Whitney U and chi-squared tests. Each patient was allocated to target Hb range depending on one’s prestudy level of Hb measured for 3 months, and the target Hb level was kept during this trial. Hb, hemoglobin; VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane; PS-con, type 4 polysulfone dialysis membrane; ESA, erythropoiesis-stimulating agent; rHuEPO, human recombinant erythropoietin; DA, darbepoetin alfa; ARB, angiotensin II receptor blocker; ACE, angiotensin-converting enzyme inhibitor; TSAT, transferrin saturation; hs-CRP, high-sensitivity C-reactive protein.
P<0.05.
Changes in Hb Level
In patients with target Hb of 10.0–10.9 g/dl, Hb levels were mostly maintained within the target range (10.7±0.9). In patients with target Hb of 11.0–11.9 g/dl, the Hb levels fluctuated around 11.0 g/dl (11.1±0.9).
Changes in ERI
We analyzed the ERI as a function of ESA. Table 3 shows the changes in ERI (weekly ESA dose/Hb) from study initiation to study completion. In Table 3, ERI is expressed as a ratio relative to the baseline level (time 0).
Table 3.
Changes in relative erythropoietic resistance index and hemoglobin from study initiation to study completion
| Group | Observation Period (mo) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Relative ERI(10.0–11.9) | |||||||||||||
| VPS-HA | 1.0 | 1.1 | 1.0 | 1.0 | 1.1 | 1.0 | 1.0 | 1.1 | 1.1 | 1.1 | 1.2 | 1.2 | 1.1 |
| P (versus 0) | — | 0.91 | 0.15 | 0.15 | 0.54 | 0.37 | 0.36 | 0.89 | 0.58 | 0.70 | 0.79 | 0.11 | 0.20 |
| PS-con | 1.0 | 1.1 | 1.2 | 1.2 | 1.2 | 1.3 | 1.2 | 1.2 | 1.2 | 1.3 | 1.4 | 1.4 | 1.3 |
| P (versus 0) | — | 0.95 | 0.64 | 0.92 | 0.42 | 0.29 | 0.67 | 0.45 | 0.40 | 0.42 | 0.15 | 0.13 | 0.56 |
| P (VPS-HA versus PS-con) | — | 0.36 | 0.47 | 0.34 | 0.58 | 0.05 | 0.22 | 0.43 | 0.28 | 0.53 | 0.14 | 0.04a | 0.09 |
| Hb (g/dl,10.0–11.9) | |||||||||||||
| VPS-HA | 10.8 | 10.8 | 10.8 | 10.8 | 10.8 | 10.7 | 10.7 | 10.6 | 10.6 | 10.7 | 10.7 | 10.8 | 10.7 |
| PS-con | 10.7 | 10.7 | 10.7 | 10.8 | 10.8 | 10.7 | 10.6 | 10.6 | 10.6 | 10.6 | 10.6 | 10.8 | 10.8 |
| Relative ERI (10.0–10.9) | |||||||||||||
| VPS-HA | 1.0 | 1.0±0.9 | 1.0±0.6 | 1.0±0.6 | 1.1±0.9 | 1.1±0.8 | 1.0±0.7 | 1.0±0.7 | 1.1±0.7 | 1.1±1.0 | 1.2±1.3 | 1.3±1.8 | 1.2±1.2 |
| P (versus 0) | — | 0.47 | 0.08 | 0.11 | 0.92 | 0.54 | 0.48 | 0.97 | 0.82 | 0.95 | 0.98 | 0.58 | 0.71 |
| PS-con | 1.0 | 1.1±0.7 | 1.2±0.8 | 1.2±0.9 | 1.2±1.0 | 1.2±0.9 | 1.1±0.8 | 1.2±0.9 | 1.2±0.9 | 1.3±1.2 | 1.4±1.3 | 1.3±1.2 | 1.2±1.1 |
| P (versus 0) | — | 0.78 | 0.71 | 0.86 | 0.13 | 0.59 | 0.32 | 0.91 | 0.88 | 0.84 | 0.98 | 0.96 | 0.78 |
| P (VPS-HA versus PS-con) | — | 0.27 | 0.26 | 0.18 | 0.77 | 0.67 | 0.72 | 0.52 | 0.85 | 0.95 | 0.67 | 0.74 | 0.58 |
| Hb (g/dl, 10.0–10.9) | |||||||||||||
| VPS-HA | 10.7±0.8 | 10.7±0.8 | 10.7±0.8 | 10.6±0.8 | 10.6±0.7 | 10.6±0.7 | 10.6±0.8 | 10.5±0.8 | 10.4±0.8 | 10.5±0.9 | 10.6±0.8 | 10.7±0.9 | 10.6±0.9 |
| PS-con | 10.7±0.8 | 10.6±0.8 | 10.5±0.9 | 10.7±0.9 | 10.8±1.0 | 10.6±0.9 | 10.5±1.0 | 10.5±0.8 | 10.6±1.0 | 10.5±1.0 | 10.6±1.0 | 10.7±1.0 | 10.7±0.9 |
| Relative ERI (11.0–11.9) | |||||||||||||
| VPS-HA | 1.0 | 1.1±0.5 | 1.1±0.7 | 1.1±0.7 | 1.2±1.0 | 1.0±0.6 | 1.0±0.5 | 1.1±0.6 | 1.1±0.7 | 1.1±0.9 | 1.2±0.8 | 1.0±0.7 | 1.0±0.7 |
| P (versus 0) | — | 0.41 | 0.93 | 0.82 | 0.36 | 0.50 | 0.55 | 0.86 | 0.53 | 0.57 | 0.63 | <0.05a | 0.09 |
| PS-con | 1.0 | 1.1±0.4 | 1.1±0.9 | 1.1±0.9 | 1.2±0.8 | 1.6±0.9 | 1.4±0.9 | 1.3±0.9 | 1.4±0.8 | 1.3±0.9 | 1.6±0.8 | 1.6±1.1 | 1.4±0.8 |
| P (versus 0) | — | 0.74 | 0.79 | 0.93 | 0.39 | <0.01a | 0.02a | 0.11 | 0.07 | 0.07 | <0.01a | <0.01a | 0.13 |
| P (VPS-HA versus PS-con) | — | 0.98 | 0.79 | 0.88 | 0.41 | <0.001a | 0.04a | 0.43 | 0.08 | 0.24 | 0.03a | <0.001a | 0.01a |
| Hb (g/dl, 11.0–11.9) | |||||||||||||
| VPS-HA | 11.2±0.9 | 11.0±0.6 | 11.1±0.8 | 11.3±0.7 | 11.3±0.8 | 11.1±0.7 | 11.1±0.8 | 10.9±0.9 | 11.1±0.8 | 11.0±0.9 | 11.0±0.7 | 11.0±0.8 | 11.0±1.1 |
| PS-con | 11.1±0.9 | 10.9±0.9 | 11.2±0.9 | 11.2±0.9 | 10.9±0.8 | 10.8±0.8 | 10.8±0.8 | 10.7±1.0 | 10.8±1.1 | 10.9±0.9 | 10.7±0.8 | 11.0±0.7 | 11.1±0.8 |
Each patient was allocated to target hemoglobin range depending on one's prestudy level of hemoglobin measured for 3 months, and the target hemoglobin level was kept during this trial. Erythropoietic resistance index (ERI) was calculated by dividing total weekly dose of erythropoiesis-stimulating agent by hemoglobin level. ERI is expressed as a ratio relative to the baseline level (time 0). Values are mean ± SD. Statistical testing between the VPS-HA and PS-con groups was performed using the Mann–Whitney U test. Statistical testing of 0 months versus each month was performed using repeated measures ANOVA followed by a posthoc test. VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane; PS-con, type 4 polysulfone dialysis membrane; 10.0–10.9, target hemoglobin range from 10.0 to 10.9 g/dl; 11.0–11.9, target hemoglobin range from 11.0 to 11.9 g/dl.
P<0.05.
There was no significant difference in ERI between the VPS-HA and PS-con groups at 12 months (1.1±0.2 for VPS-HA, 1.3±0.6 for PS-con, P=0.09). In patients with target Hb of 10.0–10.9 g/dl, there was no statistically significant difference in relative ERI between the VPS-HA and PS-con groups (1.2±1.2 for VPS-HA, 1.2±1.1 for PS-con, P=0.58). However, in patients with target Hb of 11.0–11.9 g/dl, there proved to be a statistically significant difference in relative ERI between the VPS-HA and PS-con groups (P=0.01): the ERI of the VPS-HA group remained at almost the same as the baseline or significantly lower (P<0.05), whereas the ERI of the PS-con group significantly increased (P<0.01) at 11 months and was significantly higher than baseline (1.0±0.7 for VPS-HA, 1.4±0.8 for PS-con at 12 months, P=0.01).
For subgroup analysis of each type of ESA in Table 4, in consideration of covariance, the absolute values for every month were compared among the paired groups based on the value at the start of the study. The value at the start of the study was then set at one to provide a comparative index. When there were significant differences between groups, the data of the group with lower ERI were underlined in Table 4. In patients with target Hb of 10.0–10.9 g/dl, there was a significant increase with rHuEPO at 4 months in the PS-con group (P=0.02), but in patients with target Hb of 10.0–10.9 g/dl, there was not a significant increase with rHuEPO in the VPS-HA group (P=0.39). There were no statistically significant differences in patients with target Hb of 10.0–10.9 g/dl with DA in either group (1.3±1.1 for VPS-HA, P=0.39; 1.3±0.9 for PS-con, P=0.94 at 4 months). Intergroup analysis also showed the relative value of the PS-HA group to be lower than the relative value of the PS-con group in most months.
Table 4.
Changes in relative erythropoietic resistance index and hemoglobin by type of erythropoiesis-stimulating agent from study initiation to study completion
| Group | Observation Period (mo) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Relative ERI (10.0–10.9) rHuEPO | |||||||||||||
| VPS-HA | 1.0 | 1.0±0.4 | 1.0±0.6 | 0.9±0.7 | 1.0±0.7 | 1.0±0.8 | 1.1±0.8 | 1.0±0.7 | 1.0±0.7 | 1.1±0.7 | 1.4±1.6 | 1.5±2.2 | 1.2±1.4 |
| P (versus 0) | — | 0.82 | 0.11 | 0.06 | 0.39 | 0.48 | 0.74 | 0.90 | 0.67 | 0.69 | 0.28 | 0.88 | 0.62 |
| PS-con | 1.0 | 1.2±0.9 | 1.3±0.9 | 1.2±1.0 | 1.2±1.2 | 1.1±1.0 | 1.1±0.9 | 1.2±1.0 | 1.1±1.0 | 1.4±1.4 | 1.5±1.5 | 1.4±1.5 | 1.2±1.3 |
| P (versus 0) | — | 0.64 | 0.83 | 0.15 | 0.02a | 0.27 | 0.13 | 0.18 | 0.34 | 0.39 | 0.45 | 0.36 | 0.20 |
| P (VPS-HA versus PS-con) | — | <0.001b | <0.001b | <0.001b | <0.001b | <0.01b | <0.001b | <0.01b | <0.01b | <0.01b | <0.01b | <0.01b | <0.01b |
| Hb (g/dl, 10.0–10.9) rHuEPO | |||||||||||||
| VPS-HA | 10.6±0.8 | 10.6±0.9 | 10.7±1.0 | 10.7±0.9 | 10.7±0.7 | 10.6±0.7 | 10.6±0.9 | 10.5±0.8 | 10.4±0.8 | 10.5±0.9 | 10.6±0.8 | 10.8±0.8 | 10.8±0.9 |
| PS-con | 10.7±0.8 | 10.6±0.9 | 10.6±1.0 | 10.8±0.9 | 10.7±0.8 | 10.5±0.9 | 10.5±0.8 | 10.6±0.8 | 10.7±0.8 | 10.6±1.0 | 10.7±1.0 | 10.9±1.0 | 10.9±0.9 |
| Relative ERI (10.0–10.9) DA | |||||||||||||
| VPS-HA | 1.0 | 1.0±1.3 | 1.0±0.6 | 1.0±0.5 | 1.3±1.1 | 1.1±0.7 | 1.0±0.5 | 1.1±0.7 | 1.1±0.7 | 1.2±1.2 | 0.9±0.6 | 1.1±0.9 | 1.1±0.8 |
| P (versus 0) | — | 0.38 | 0.45 | 0.85 | 0.36 | 0.92 | 0.48 | 0.82 | 0.88 | 0.69 | 0.20 | 0.49 | 0.99 |
| PS-con | 1.0 | 1.1±0.4 | 1.1±0.7 | 1.3±0.7 | 1.3±0.9 | 1.2±0.8 | 1.1±0.8 | 1.3±0.9 | 1.2±0.9 | 1.2±1.0 | 1.2±1.0 | 1.2±0.9 | 1.2±0.9 |
| P (versus 0) | — | 0.96 | 0.75 | 0.27 | 0.94 | 0.75 | 0.94 | 0.26 | 0.48 | 0.59 | 0.49 | 0.35 | 0.39 |
| P (VPS-HA versus PS-con) | — | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b | <0.001b |
| Hb (g/dl, 10.0–10.9) DA | |||||||||||||
| VPS-HA | 10.8±0.8 | 10.7±0.7 | 10.7±0.6 | 10.5±0.8 | 10.4±0.7 | 10.5±0.6 | 10.6±0.8 | 10.4±0.7 | 10.4±0.9 | 10.6±0.9 | 10.7±0.8 | 10.5±1.0 | 10.3±0.7 |
| PS-con | 10.7±0.8 | 10.7±0.7 | 10.5±0.9 | 10.6±0.9 | 10.8±1.1 | 10.8±0.8 | 10.7±1.1 | 10.6±0.8 | 10.5±1.2 | 10.6±1.1 | 10.6±0.9 | 10.6±1.0 | 10.4±0.8 |
| Relative ERI (11.0–11.9) rHuEPO | |||||||||||||
| VPS-HA | 1.0 | 1.2±0.6 | 1.3±0.8 | 1.2±0.8 | 1.4±1.3 | 1.1±0.8 | 1.1±0.6 | 1.4±0.7 | 1.4±0.8 | 1.5±1.0 | 1.5±0.9 | 1.3±0.9 | 1.4±0.9 |
| P (versus 0) | — | 0.11 | 0.20 | 0.72 | 0.72 | 0.67 | 0.73 | 0.13 | 0.13 | 0.04a | 0.19 | 0.51 | 0.29 |
| PS-con | 1.0 | 1.0±0.3 | 1.0±0.9 | 0.9±0.9 | 1.1±0.8 | 1.5±0.8 | 1.4±0.9 | 1.4±0.9 | 1.3±0.8 | 1.3±0.9 | 1.6±0.9 | 1.5±1.3 | 1.3±0.7 |
| P (versus 0) | — | 0.82 | 0.30 | 0.21 | 0.78 | 0.13 | 0.23 | 0.14 | 0.30 | 0.40 | 0.05 | 0.31 | 0.43 |
| P (VPS-HA versus PS-con) | — | <0.001b | <0.01b | 0.16 | <0.05a | <0.01b | <0.05a | <0.01b | <0.01b | <0.05a | <0.01b | <0.01b | <0.001b |
| Hb (g/dl, 11.0–11.9) rHuEPO | |||||||||||||
| VPS-HA | 11.1±1.0 | 11.3±1.0 | 11.3±1.2 | 11.5±0.9 | 11.4±0.7 | 11.3±0.7 | 11.1±0.7 | 10.9±0.8 | 11.1±0.9 | 11.0±0.8 | 11.2±0.6 | 11.3±0.9 | 11.2±1.2 |
| PS-con | 11.0±1.0 | 10.9±0.9 | 11.0±1.2 | 11.3±1.0 | 10.8±0.7 | 10.6±0.7 | 10.5±0.9 | 10.6±0.8 | 10.9±0.9 | 11.1±1.0 | 11.0±0.9 | 11.2±0.8 | 11.1±0.8 |
| Relative ERI (11.0–11.9) DA | |||||||||||||
| VPS-HA | 1.0 | 0.9±0.2 | 0.8±0.4 | 1.0±0.5 | 0.9±0.5 | 0.9±0.5 | 0.9±0.3 | 0.9±0.4 | 0.7±0.3 | 0.7±0.3 | 0.8±0.3 | 0.6±0.3 | 0.7±0.3 |
| P (versus 0) | — | 0.65 | 0.16 | 0.49 | 0.10 | 0.16 | 0.23 | 0.22 | 0.02a | <0.001b | 0.04a | <0.001b | <0.001b |
| PS-con | 1.0 | 1.3±0.5 | 1.2±0.8 | 1.4±0.9 | 1.3±0.9 | 1.9±0.9 | 1.5±1.0 | 1.3±0.9 | 1.5±0.8 | 1.4±1.0 | 1.5±0.9 | 1.8±0.8 | 1.4±1.0 |
| P (versus 0) | — | 0.43 | 0.43 | 0.11 | 0.10 | <0.01b | 0.03a | 0.48 | 0.12 | 0.08 | 0.06 | <0.001b | 0.16 |
| P (VPS-HA versus PS-con) | — | <0.001b | <0.01b | <0.01b | <0.001b | <0.001b | <0.001b | <0.01b | <0.01b | <0.01b | <0.01b | <0.001b | <0.01b |
| Hb (g/dl, 11.0–11.9) DA | |||||||||||||
| VPS-HA | 11.3±0.9 | 11.1±0.6 | 11.2±0.6 | 11.2±0.7 | 11.3±0.9 | 11.0±0.8 | 11.1±0.8 | 11.1±0.9 | 11.3±0.8 | 11.2±1.0 | 11.0±0.8 | 11.0±0.8 | 11.0±0.9 |
| PS-con | 11.2±0.9 | 11.0±1.0 | 11.1±0.8 | 11.0±0.7 | 11.3±0.9 | 11.6±1.0 | 11.5±1.1 | 11.4±1.6 | 11.0±1.7 | 11.2±1.2 | 10.9±1.0 | 11.1±0.7 | 11.4±0.8 |
Each patient was allocated to target hemoglobin range depending on one's prestudy level of hemoglobin measured for 3 months, and the target hemoglobin level was kept during this trial. Erythropoietic resistance index (ERI) was calculated by dividing total weekly dose of erythropoiesis stimulating-agent (rHuEPO or DA) by hemoglobin level. ERI is expressed as a ratio relative to the baseline level (time 0). Values are mean ± SD. Statistical testing between the VPS-HA and PS-con groups was performed using the Mann–Whitney U test. Statistical testing was performed using repeated measures analysis of covariance followed by Scheffé posthoc test. When there were significant differences between groups, the data of the group with lower ERI were underlined. VPS-HA, vitamin E-bonded high-flux polysulfone dialysis membrane; rHuEPO, human recombinant erythropoietin; PS-con, type 4 polysulfone dialysis membrane; DA, darbepoetin alfa; 10.0–10.9, target hemoglobin range from 10.0 to 10.9 g/dl; 11.0–11.9, target hemoglobin range from 11.0 to 11.9 g/dl.
P<0.05.
P<0.01.
Next, we analyzed the changes in ERI over the 12-month period of observation. ERI change of VPS-HA (P=0.11) and PS-con (P=0.13) groups was less than 20% and more than 40%, respectively, at 11 months to baseline, and no significant difference was found.
In the PS-con group, in patients with target Hb of 11.0–11.9 g/dl treated with rHuEPO, the change in ERI at 10 months was significantly higher than at 1, 2, 3, and 4 months (P=0.02, P<0.001, P<0.001, and P=0.02, respectively), with differences of around 60%. In patients with target Hb of 11.0–11.9 g/dl treated with rHuEPO, a transient but significant increase in ERI was observed at 9 months in the VPS-HA group, whereas a pattern of increase was found transiently at 5 and 10 months in the PS-con group. In patients treated with DA, ERI decreased significantly from 8 months until the end of the study in the VPS-HA group, whereas in the PS-con group, ERI increased significantly at 5, 6, and 11 months. In these patients, significant differences in ERI between the VPS-HA and PS-con groups were detected: the ERI values of the VPS-HA group were lower than the values of the PS-con group over the whole period.
A comparison of ERI between the two target Hb ranges showed ERI to be lower and variability to be smaller in patients with target Hb of 11.0–11.9 g/dl compared with patients with target Hb of 10.0–10.9 g/dl (rHuEPO: 269.9±232.0 versus 401.4±271.4, P<0.001; DA: 1.48±0.79 versus 1.94±1.25, P=0.09). Each ESA was administered in the following way: at 4357±2530 U/wk, giving an rHuEPO dosage of 21.1±10.6 mcg/wk to the Hb 10.0–10.9 g/dl VPS-HA group; at 4475±2526 U/wk, giving an rHuEPO dosage of 21.5±14.3 mcg/wk to the Hb 10.0–10.9 g/dl PS-con group; at 3500±2440 U/wk, giving an rHuEPO dosage of 20.6±8.4 mcg/wk to the Hb 11.0–11.9 g/dl VPS-HA group; and at 3700±1932 U/wk, giving an rHuEPO dosage of 13.6±6.7 mcg/wk to the Hb 11.0–11.9 g/dl PS-con group.
Selected Secondary End Points
During the study period, high-sensitivity CRP did not change significantly in either group, and there were no statistically significant differences between the two groups. However, indirect bilirubin decreased significantly compared with the baseline at 4 and 7 months in the VPS-HA group (0.20±0.11 and 0.16±0.08 versus 0.22 at baseline, P=0.01 and P=0.02, respectively) but did not change during the study period in the PS-con group.
Changes in Iron-Related Indices
Ferritin decreased significantly and progressively in both the VPS-HA and PS-con groups. There were no statistically significant differences between the two groups (P=0.64). TSAT showed no changes. There were also no statistically significant differences in transferrin level between the two groups. For TSAT, ferritin, and transferrin, there were no differences between VPS-HA and PS-con or between both Hb ranges.
Discussion
We defined, as the primary outcome, a significantly different ERI between groups at 12 months.
The results of our study revealed no difference between the VPS-HA and PS-con groups. The ERI in the Hb range of 10.0–10.9 g/dl was not statistically significantly different between the two groups; however, for the ERI in the Hb 11.0–11.9 g/dl range, the VPS-HA group showed significantly lower values than the PS-con group.
ESA dose levels of Japanese patients in this study were lower than those ESA dose levels administered to North American patients. We surmise the reasons to be that Japanese patients with an arteriovenous fistula for blood access suffer lower oxidative stress, that Japanese target Hb is lower than North American target Hb (15), and that there are differences in blood test timing between Japan and North America.
Patients with a target Hb of 11.0–11.9 g/dl were able to maintain the target Hb level, even at reduced ESA doses. The present results, thus, suggest that the antioxidant effect of VPS-HA effectively reduces oxidative stress in dialysis patients, which then leads to the ESA dose-reducing effect. Patients in the VPS-HA group treated with DA showed decreased ERI after 8 months, but we regard this finding as an exploratory subgroup analysis only.
Oxidative stress is also implicated in anemia in dialysis patients (16). The chief mechanism is thought to be caused by hemolysis (17). Moreover, patients with vitamin E deficiency have been reported to be more susceptible to hemolysis associated with oxidative stress (18). Reports indicate that vitamin E supplementation attenuates oxidative stress in dialysis patients (19). A study using various stress markers provides good evidence of the efficacy of vitamin E-coated dialyzers in reducing oxidative stress in dialysis patients (20). Based on our existing findings, we speculate the mechanism of the ESA response-improving effect of VPS-HA to be as follows. In dialysis patients, oxidative stress induces increased lipid peroxidation in the erythrocyte membrane, thus weakening it, and the consequent abnormal viscosity and morphology of the erythrocytes shorten erythrocyte lifespan because of hemolysis. Vitamin E is, thus, anticipated to stop the chain reaction of cell membrane lipid peroxidation, stabilize the erythrocyte membrane (21), and prolong erythrocyte lifespan. Use of vitamin E-bonded dialyzers has been reported to improve abnormal erythrocyte morphology, thus narrowing the range of erythrocyte size distribution (8), reduce hemolysis, thus reducing indirect bilirubin (22), and prolong erythrocyte lifespan, leading to lower erythrocyte creatinine levels (9). Use of VPS-HA is, therefore, likely to prolong erythrocyte lifespan, which would explain our observation, several months after switching to this dialyzers, of data evidencing its anemia therapy-favorable properties.
In this study, it proved difficult to make direct measurements of oxidative stress, such as lipid peroxides in the erythrocyte membranes, because of no change in the state of oxidation reduction in this multicenter study.
Conversely, patients in the PS-con group treated with DA showed a wider range of Hb variability for the Hb target ranges of 11.0–11.9 g/dl.
Specifically, the ERI of the VPS-HA group that was administered with DA as ESA significantly decreased compared with the ERI at baseline and was significantly lower than the ERI of PS-con group over the whole period. The results of this trial, thus, suggest a statistically significant difference between DA and rHuEPO. There are known differences, such as molecular structure and half-life, between these two ESAs. DA also shows a long cycle length for Hb variability and has small cyclic amplification, and therefore, variation of ERI was readily induced with our synergistic combination therapy of DA and vitamin E-bonded dialyzers. In a future trial, we plan to examine the differences in joint effects between VPS-HA and ESAs not assessed in this study.
Our comparison of ERI between the two target Hb ranges also showed ERI to be lower, with smaller variability, in patients with target Hb of 11.0–11.9 g/dl than in those patients with a target Hb of 10.0–10.9 g/dl. Notably, patients in the PS-con group treated with DA showed a wider range of Hb variability in the target Hb target ranges of 11.0–11.9 g/dl than the VPS-HA group. We conclude that VPS-HA has the potential to improve response to ESA. Long-term observations may reveal lower mortality.
Interestingly, the results of our multiple comparisons showed ERI to vary according to range of Hb or type of ESA, even when using VPS-HA. However, the design of this study did not permit the results to lead to any clear conclusions, including conclusions as to a mechanism. It was difficult to assess how the dialyzer affected ERI in ERI comparisons between the intervention groups categorized by type of ESA.
No assessment of inflammation markers, such as IL-6, was performed in this study. However, assessment of inflammation markers will help to elucidate the details of the mechanism. We plan a future comparison of each kind of ESA with a control group in a large-scale population.
The overall relative ERI showed no difference between VPS-HA and conventional polysulfone membranes. A secondary analysis showed an ESA dose-reducing effect in patients with Hb of 11.0–11.9 g/dl.
Disclosures
Asahi Kasei Kuraray Medical Co. Ltd. provided funds for laboratory diagnostic tests only in our present study. E.N. has received grants from Asahi Kasei Kuraray Medical Co. Ltd. for another study. The other authors have not received any consultants’ fees or grants from Asahi Kasei Kuraray Medical Co. Ltd.
Supplementary Material
Acknowledgments
We would like to thank the following participating physicians from various parts of Japan (institutional affiliations are provided in the Supplemental Material): Ken Kihara, Hiroshi Kita, Minoru Chimata, Jin Kusano, Makoto Hiramatsu, Keisuke Maruyama, Naofumi Yamamoto, Masanori Mukai, Nobuyuki Suzuki, Takekazu Shimazu, Teruhiko Maeba, Yasushi Kanno, Takayasu Ohtake, Hiroshi Kataoka, Nobuyuki Amemiya, Mamiko Ohara, Yoshihiko Watanabe, Kenji Yamaguchi, Tamaki Imai, Kazushi Takahashi, Yoshiko Shimizu, Hiroyuki Takemura, Taketoshi Inokami, Isao Kumagai, Shinji Fukuda, Kumiko Hirokoshi, Hiroko Kawamoto, Nobuo Kajitani, Naoko Yamashita, Yoichi Akai, Takashi Ishizu, Isao Tadomega, Junichiro Mera, Koichi Hamaguchi, Maho Ohmori, Fumiyoshi Nakayama, Atsumi Harada, Yuzuru Sato, Hidetoshi Ito, Hiroaki Ogata, Masahiro Yamamoto, Noriyo Yoshida, Akiko Takejima, Yukihiro Wada, Toshio Takahashi, Yuji Koyama, Emi Fujikura, Masatsugu Sato, Shiro Oguma, Jun Urae, Iehisa Matsuyama, Mitsuko Iwasaki, Mikio Wakasa, Ken Tanaka, Makoto Matsuyama, Shoko Mizuno, Norio Miyabe, Daisuke Nagasaku, Nobuhiro Sasaki, Masayuki Nagahara, Yoshihiko Nishian, Takashi Miyamoto, Takahiro Kuragano, and Masakazu Murayama.
The clinical trial was registered with Clinical Trial Registry of University Hospital Medical Information Network (UMIN-CTR ID: UMIN000001285) at 2008/08/07 (http://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000001567&language=E).
Footnotes
Present address: Dr. Tsutomu Sanaka, Lifestyle Disease and CKD Center, Edogawa Hospital, Tokyo, Japan.
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04680512/-/DCSupplemental.
References
- 1.Himmelfarb J, Lazarus JM, Hakim R: Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during dialysis. Am J Kidney Dis 17: 271–276, 1991 [DOI] [PubMed] [Google Scholar]
- 2.Sanaka T: Reactive oxygen hypothesis [in Japanese]. Toseki-kai-shi 14: 283–287, 1991 [Google Scholar]
- 3.Usberti M, Gerardi GM, Gazzotti RM, Benedini S, Archetti S, Sugherini L, Valentini M, Tira P, Bufano G, Albertini A, Di Lorenzo D: Oxidative stress and cardiovascular disease in dialyzed patients. Nephron 91: 25–33, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Epperlein MM, Nourooz-Zadeh J, Jayasena SD, Hothersall JS, Noronha-Dutra A, Neild GH: Nature and biological significance of free radicals generated during bicarbonate hemodialysis. J Am Soc Nephrol 9: 457–463, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Koremoto M, Takahara N, Takahashi M, Okada Y, Satoh K, Kimura T, Hirai T, Ebihara I, Nagasaku D, Miyata S, Maniwa S, Kouzuma T, Arimura T, Kamei J: Improvement of intradialytic hypotension in diabetic hemodialysis patients using vitamin E-bonded polysulfone membrane dialyzers. Artif Organs 36: 901–910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura Y, Kamimura K, Yoshioka N, Hosotani Y, Tsuchida K, Koremoto M, Minakuchi J: The effect of vitamin E-bonded polysulfone membrane dialyzer on a new oxidative lipid marker [published online ahead of print February 10, 2013]. J Artif Organs doi: 10.1007/s10047-013-0689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huraib S, Tanimu D, Shaheen F, Hejaili F, Giles C, Pagayon V: Effect of vitamin-E-modified dialysers on dialyser clotting, erythropoietin and heparin dosage: A comparative crossover study. Am J Nephrol 20: 364–368, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi S, Moriya H, Aso K, Ohtake T: Vitamin E-bonded hemodialyzer improves atherosclerosis associated with a rheological improvement of circulating red blood cells. Kidney Int 63: 1881–1887, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Nakatan T, Takemoto Y, Tsuchida AK: The effect of vitamin E-bonded dialyzer membrane on red blood cell survival in hemodialyzed patients. Artif Organs 27: 214–217, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Floridi A, Piroddi M, Pilolli F, Matsumoto Y, Aritomi M, Galli F: Analysis method and characterization of the antioxidant capacity of vitamin E-interactive polysulfone hemodialyzers. Acta Biomater 5: 2974–2982, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Usberti M, Gerardi GM, Micheli AM, Tira P, Bufano G, Gaggia P, Movilli E, Cancarini GC, De Marinis S, D’Avolio G, Broccoli R, Manganoni A, Albertin A, Di Lorenzo D: Effects of a vitamin E-bonded membrane and of glutathione on anemia and erythropoietin requirements in hemodialysis patients. J Nephrol 15: 558–564, 2002 [PubMed] [Google Scholar]
- 12.Usberti M, Gerardi GM, Bufano G, Tira P, Micheli A, Albertini A, Floridi A, Di Lorenzo D, Galli F: Effects of erythropoietin and vitamin E-modified membrane on plasma oxidative stress markers and anemia of hemodialyzed patients. Am J Kidney Dis 40: 590–599, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Saito A: Definition of high-performance membranes—from the clinical point of view. Contrib Nephrol 173: 1–10, 2011 [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation : IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: Update 2000. Am J Kidney Dis 37[Suppl 1]: S182–S238, 2001 [DOI] [PubMed] [Google Scholar]
- 15.McFarlane PA, Pisoni RL, Eichleay MA, Wald R, Port FK, Mendelssohn D: International trends in erythropoietin use and hemoglobin levels in hemodialysis patients. Kidney Int 78: 215–223, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Usberti M, Lima G, Arisi M, Bufano G, D’Avanzo L, Gazzotti RM: Effect of exogenous reduced glutathione on the survival of red blood cells in hemodialyzed patients. J Nephrol 10: 261–265, 1997 [PubMed] [Google Scholar]
- 17.Ogihara H, Ogihara T, Miki M, Yasuda H, Mino M: Plasma copper and antioxidant status in Wilson’s disease. Pediatr Res 37: 219–226, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Mino M: Clinical uses and abuses of vitamin E in children. Proc Soc Exp Biol Med 200: 266–270, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Islam KN, O’Byrne D, Devaraj S, Palmer B, Grundy SM, Jialal I: Alpha-tocopherol supplementation decreases the oxidative susceptibility of LDL in renal failure patients on dialysis therapy. Atherosclerosis 150: 217–224, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Calò LA, Naso A, Pagnin E, Davis PA, Castoro M, Corradin R, Riegler P, Cascone C, Huber W, Piccoli A: Vitamin E-coated dialyzers reduce oxidative stress related proteins and markers in hemodialysis—a molecular biological approach. Clin Nephrol 62: 355–361, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Peuchant E, Carbonneau MA, Dubourg L, Thomas MJ, Perromat A, Vallot C, Clerc M: Lipoperoxidation in plasma and red blood cells of patients undergoing haemodialysis: Vitamins A, E, and iron status. Free Radic Biol Med 16: 339–346, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Kataoka H, Amemiya N, Mochizuki T: Anemia-improving effect of vitamin E-bonded membrane [in Japanese]. Vitamembrane 8: 16–19, 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


