Summary
Background and objectives
CKD is a well known poor prognostic factor in myocardial infarction (MI). This study evaluated the prognostic significance of CKD, particularly in association with increasing age, in MI patients.
Design, setting, participants, & measurements
This study was based on a retrospective cohort, the Korean Acute Myocardial Infarction Registry. Patients with a discharge diagnosis of MI were analyzed to investigate the association of CKD with mortality risk according to age. A total of 11,268 patients (mean age 63.0±12.6 years) were included and followed for 1 year.
Results
In the full cohort, 26% of patients had CKD (n=2929). The prevalence of CKD was higher with advancing age. Eight hundred sixty-one patients (7.6%) died and the interaction for 1-year mortality between age strata and estimated GFR (eGFR) strata was significant (P<0.001). Within each age category, the absolute 1-year mortality was higher in patients with a low eGFR. However, the adjusted relative mortality risk for a low eGFR was lower with increasing age (adjusted hazard ratio [95% confidence interval] for 1-year mortality at eGFR <30 ml/min per 1.73 m2: 4.84 [1.93−12.15], 4.53 [2.42−8.47], 3.51 [2.42−5.09], and 3.30 [2.41−4.52] for patients aged <55, 55−64, 65−74, and ≥75 years compared with those with eGFR ≥60 ml/min per 1.73 m2, respectively).
Conclusions
For all age categories, the overall mortality was significantly higher as eGFR declined. The association of a lower eGFR with mortality was weaker with increasing age, indicating that the prognostic significance of CKD in MI patients is age dependent.
Introduction
CKD is well known as a poor prognostic factor in myocardial infarction (MI) (1,2), and the prevalence of CKD, defined as an estimated GFR (eGFR) <60 ml/min per 1.72 m2, is markedly higher in the elderly population (3,4). The high incidence of CKD in elderly individuals may be attributed to several comorbidities associated with age, and to age-related changes in kidney function and anatomy (5,6). Recently, it has been suggested that the clinical significance of a patient’s CKD stage might vary with age (7,8). O’Hare et al. reported that, in the general population, the effect of CKD on mortality declined with advancing age, and that mortality risk stratification in an elderly population should not be based on the same eGFR cutoff points as for a young population (7).
Increased life expectancy is contributing to a steady growth in the elderly population; consequently, more elderly patients are presenting to hospitals with MI (9). Although many elderly MI patients have CKD in the clinical setting, little is known about the prognostic significance of a low eGFR in this population. Given the powerful prognostic effect of CKD in MI patients, it needs to be demonstrated whether advancing age influences the effect of CKD on the prognosis of MI, before CKD staging is implemented in MI patients regardless of age.
In this study, we compared the prevalence and clinical outcome of CKD according to age, and assessed whether the association of CKD and survival in patients with MI was age dependent.
Materials and Methods
Korean Acute Myocardial Infarction Registry
The study population was derived from the Korean Acute Myocardial Infarction Registry (KAMIR). KAMIR is a Korean prospective, open, observational, multicenter online registry that has been investigating the risk factors for mortality in MI patients since November 2005. The 52 hospitals with facilities for primary percutaneous coronary intervention (PCI) participated and enrolled patients who agreed to participate in this registry. Before the initiation of KAMIR, several investigator meetings were held and a practical steering committee was selected from the major hospitals enrolled. The committee’s task was to standardize the care given in clinical practice, as well as the study protocol, in order to minimize the differences in medical care among the different hospitals and across the different time periods. Data were collected at each site by a trained study coordinator using a standardized case report form. Data were registered and submitted from individual institutions via password-protected Internet-based electronic case report forms. The study protocol was approved by the ethics committee at each participating institution. Clinical follow-up was performed for 12 months. Patients were required to visit the outpatient clinic at the end of the first month, 6 months and 12 months after discharge, and when angina-like symptoms occurred.
Patient Population and Renal Function Assessment
We assessed a cohort of 13,898 consecutive patients who were admitted to the hospital between November 1, 2005 and July 31, 2008, and had a discharge diagnosis of MI. MI was diagnosed on the basis of a characteristic clinical presentation, serial changes on an electrocardiogram suggesting infarction or injury, and an increase in cardiac enzyme levels (10). From this cohort, 1229 patients (8.8%) for whom GFR could not be estimated and 1401 patients (10.0%) whose 12-month survival was unknown were excluded. A final population of 11,268 patients was analyzed in this study. The level of creatinine was measured at the time of presentation to the hospital, and renal function was assessed based on the estimation of GFR. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation as follows: ml/min per 1.73 m2 = 141× minimum (creatinine/κ, 1)α × maximum (creatinine/κ, 1)–1.209 × 0.993age × 1.018 (if female) × 1.159 (if black), where κ is 0.7 for women and 0.9 for men and α is –0.329 for women and –0.411 for men (11). We used a modified National Kidney Foundation classification of CKD to divide eGFR into the following ranges: at least 60 ml/min per 1.73 m2, 45–59 ml/min per 1.73 m2, 30–44 ml/min per 1.73 m2, and <30 ml/min per 1.73 m2 (12). Individuals corresponding to CKD stages 4 and 5 were combined because there were relatively few patients in this category. CKD was defined as an eGFR <60 ml/min per 1.73 m2.
Variables
Baseline variables included age, sex, body mass index (BMI), several coronary risk factors—hypertension (HTN), diabetes mellitus (DM), ischemic heart disease (IHD), hyperlipidemia, and current smoking—and Killip class. Left ventricular ejection fraction (LVEF) was checked by two-dimensional echocardiography. Use of certain medications at any time during the in-hospital period was recorded: aspirin, clopidogrel, anticoagulation, β-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and statins.
Statistical Analyses
We divided the patients into four groups according to their age: <55, 55–64, 65–74, and ≥75 years. Continuous variables with normal distributions are presented as means ± SDs and were compared using one-way ANOVA. Pearson’s chi-squared test or Fisher’s exact test was used to evaluate differences among categorical variables. Cox proportional hazards regression analyses were performed to evaluate the prognostic significance of CKD for mortality in MI patients. The confounders analyzed in this study include age, sex, BMI, comorbidities (HTN, DM, IHD, hyperlipidemia, and smoking status), Killip class, diagnosis of ST-elevation MI (STEMI) versus non-STEMI (NSTEMI), PCI, and medical treatments during hospitalization. The interaction between age strata and eGFR strata was assessed by entering the interaction term in a Cox proportional hazards model. The covariates were the same as above and age and eGFR were analyzed as strata. All statistical tests were two tailed, and a P value <0.05 was considered significant. Statistical analysis was performed using the Statistical Package for Social Sciences software (version 18.0; SPSS, an IBM Company, Armonk, NY).
Results
A total of 11,268 patients (mean age 63.0±12.6 years; men, 71.0%) were included in this study. Of these, 2929 patients (26%) had CKD, defined as eGFR <60 ml/min per 1.73 m2. Table 1 describes the demographics and clinical features according to the age category. With increasing age, patients had a lower BMI and were more likely to be women; the incidence of HTN, DM, and history of IHD was higher, whereas that of hyperlipidemia and smoking history was lower. On arrival at the hospital, older age was associated with lower BP, lower LVEF, and higher Killip class. The proportion of patients with NSTEMI was higher, and PCI was less frequently performed with increasing age. With regard to in-hospital medications, older patients were less likely to receive antiplatelet agents, β-blockers, ACE inhibitors or ARBs, and statins.
Table 1.
Baseline characteristics and clinical features of patients according to age category
| <55 yr (n=3034) | 55−64 yr (n=2699) | 65−74 yr (n=3329) | ≥75 yr (n=2206) | P Value | |
|---|---|---|---|---|---|
| Age (yr) | 46.7±6.0 | 59.7±2.9 | 69.5±2.9 | 79.7±4.0 | <0.001 |
| Men (%) | 2771 (91.4) | 2134 (79.2) | 2061 (62.0) | 1032 (46.8) | <0.001 |
| BMI (kg/m2) | 25.1±3.5 | 24.3±3.1 | 23.6±3.2 | 22.5±3.6 | <0.001 |
| Past History | |||||
| HTN | 1041 (34.4) | 1282 (47.6) | 1869 (56.4) | 1259 (57.3) | <0.001 |
| DM | 601 (19.9) | 800 (29.7) | 1111 (33.5) | 631 (28.7) | <0.001 |
| IHD | 325 (10.7) | 424 (15.7) | 617 (18.6) | 441 (20.1) | <0.001 |
| Hyperlipidemia | 357 (11.8) | 304 (11.3) | 282 (8.5) | 142 (6.5) | <0.001 |
| Smoking | 2429 (80.5) | 1678 (62.5) | 1605 (48.5) | 823 (37.6) | <0.001 |
| Initial presentation | |||||
| SBP | 130±28 | 129±29 | 128±29 | 126±31 | <0.001 |
| DBP | 82±25 | 79±17 | 77±16 | 75±18 | <0.001 |
| HR | 78±22 | 77±23 | 77±21 | 79±23 | 0.03 |
| Killip class | 1.3±0.7 | 1.4±0.8 | 1.5±0.9 | 1.7±1.0 | <0.001 |
| LVEF (%) | 53±11 | 52±12 | 51±13 | 49±13 | <0.001 |
| Diagnosis | <0.001 | ||||
| STEMI | 1949 (64.2) | 1617 (59.9) | 1864 (56.0) | 1198 (54.3) | |
| NSTEMI | 1085 (35.8) | 1082 (40.1) | 1465 (44.0) | 1008 (45.7) | |
| PCI | 2619 (86.5) | 2309 (85.7) | 2774 (83.5) | 1664 (75.6) | <0.001 |
| In-hospital mediation | |||||
| Aspirin | 2990 (99.1) | 2650 (98.7) | 3268 (98.7) | 2157 (98.1) | 0.03 |
| Clopidogrel | 2923 (96.9) | 2583 (96.5) | 3193 (96.4) | 2091 (95.1) | 0.01 |
| Anticoagulation | 2294 (76.0) | 2095 (78.0) | 2628 (79.4) | 1718 (78.2) | 0.01 |
| β-blocker | 2303 (76.3) | 1984 (73.9) | 2435 (73.5) | 1489 (67.7) | <0.001 |
| ACE inhibitor or ARB | 2467 (81.7) | 2152 (80.1) | 2666 (80.5) | 1710 (77.8) | 0.01 |
| Statin | 2298 (76.1) | 1996 (74.3) | 2411 (72.8) | 1512 (68.8) | <0.001 |
| Prevalence of CKD (ml/min per 1.73 m2) | |||||
| mean eGFR | 88.3±22.2 | 77.8±21.1 | 67.7±22.3 | 57.4±21.0 | <0.001 |
| <0.001 | |||||
| eGFR ≥60 | 2798 (92.2) | 2241 (83.0) | 2255 (67.7) | 1045 (47.4) | |
| eGFR 45−59 | 129 (4.3) | 284 (10.5) | 597 (17.9) | 526 (23.8) | |
| eGFR 30−44 | 32 (1.1) | 76 (2.8) | 249 (7.5) | 402 (18.2) | |
| eGFR <30 | 75 (2.5) | 98 (3.6) | 228 (6.8) | 233 (10.6) |
Data are presented as n (%) or mean ± SD. BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; IHD, ischemic heart disease; SBP, systolic BP; DBP, diastolic BP; HR, heart rate; LVEF, left ventricular ejection fraction; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated GFR.
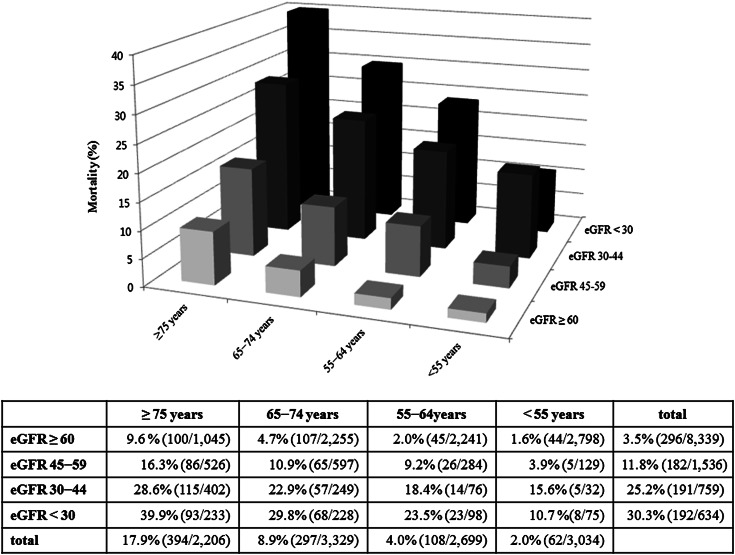
As expected, eGFR tended to decrease and the prevalence of CKD increased with advancing age (Table 1). Figure 1 shows the 1-year mortality according to age and eGFR category. Eight hundred sixty-one patients (7.6%) died and the overall mortality was higher with increasing age (2.0%, 4.0%, 8.9%, and 17.9% for patients aged <55 years, 55−64 years, 65−74 years, and ≥75 years, respectively; P<0.001). In all age categories, the overall mortality increased significantly as eGFR declined (P<0.001 for all age categories).
Figure 1.
One-year mortality according to age and eGFR category. eGFR, estimated GFR.
We performed an interaction test to investigate whether increasing age was associated with the risk of death in MI patients in each eGFR category. The interaction between age strata and eGFR strata was significant in adjusted Cox proportional models for 1-year death (P<0.001). In unadjusted Cox proportional analysis, the relative risk for death increased proportionally as eGFR decreased regardless of age category (Table 2). After adjustment for baseline characteristics, clinical features, and hospital management, the relative risk for death was still significantly associated with decreasing eGFR in all age categories. Although the absolute 1-year mortality was higher at lower eGFR with each age category, the adjusted relative mortality risk for lower eGFR decreased with increasing age (Figure 1 and Table 2). For instance, compared with people with an eGFR >60 ml/min per 1.73 m2, the relative risk for death among people with an eGFR <30 ml/min per 1.73 m2 decreased with increasing age (hazard ratio [95% confidence interval]: 4.84 [1.93−12.15], 4.53 [2.42−8.47], 3.51 [2.42−5.09], and 3.30 [2.41−4.52] for patients aged <55, 55−64, 65−74, and ≥75 years, respectively). With the exception of eGFR 45−59 ml/min per 1.73 m2 in patients aged <55 years, the relative risk for death with CKD tended to decrease with increasing age.
Table 2.
Cox proportional analysis for 1-year mortality by eGFR in each age category
| eGFR (ml/min per 1.73 m2) | <55 yr (n=3034) | 55−64 yr (n=2699) | 65−74 yr (n=3329) | ≥75 yr (n=2206) | P for Interaction |
|---|---|---|---|---|---|
| Unadjusted model | <0.001 | ||||
| ≥60 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| 45−59 | 2.51 (1.00−6.33) | 4.72 (2.91−7.65) | 2.37 (1.74−3.23) | 1.76 (1.32−2.36) | |
| 30−44 | 10.73 (4.25−27.05) | 9.93 (5.45−18.10) | 5.47 (3.97−7.54) | 3.36 (2.57−4.39) | |
| <30 | 6.90 (3.25−14.65) | 12.79 (7.74−21.14) | 7.14 (5.27−9.68) | 5.15 (3.88−6.83) | |
| Adjusted modela | <0.001 | ||||
| ≥60 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| 45−59 | 1.87 (0.70−5.00) | 2.49 (1.47−4.21) | 1.92 (1.39−2.65) | 1.62 (1.20−2.19) | |
| 30−44 | 5.04 (1.64−15.51) | 3.76 (1.89−7.49) | 2.85 (1.98−4.11) | 2.53 (1.90−3.37) | |
| <30 | 4.84 (1.93−12.15) | 4.53 (2.42−8.47) | 3.51 (2.42−5.09) | 3.30 (2.41−4.52) | |
| Death | 62 (2.0) | 108 (4.0) | 297 (8.9) | 394 (17.9) |
Data are presented as hazard ratios (95% confidence intervals) or n (%). eGFR, estimated GFR; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction.
Analyzed confounders included age, sex, body mass index, comorbidities (hypertension, diabetes mellitus, ischemic heart disease, hyperlipidemia, smoking status), Killip class, diagnosis (STEMI versus NSTEMI), percutaneous coronary intervention, and medical treatments during hospitalization.
Discussion
In this study, in all age categories, the overall mortality increased significantly as eGFR declined. However, the relative mortality risk associated with a low eGFR decreased with increasing age, suggesting that the association of low eGFR with mortality was weaker in older patients. In contrast to most previous studies that focused on the association of CKD with mortality in MI patients (1,2), our data revealed that the prognostic significance of CKD in MI patients is age dependent.
Current guidelines apply the same CKD criteria without taking age into account, and consider that CKD is present when the patient’s eGFR is <60 ml/min per 1.73 m2 (12). However, the aging kidney undergoes structural and functional changes that lead to a decline of kidney function (5,6), and a decreased eGFR is a common finding in the elderly population (13). Our study also showed that the prevalence of CKD, defined as eGFR <60 ml/min per 1.72 m2, is higher in older patients; this raises questions about current risk stratification based on the same CKD criteria regardless of a patient’s age.
In this study, which was conducted in a cohort of 11,268 MI patients, comorbid conditions such as HTN, DM, and IHD were more prevalent among older patients. Indeed, older patients were less likely to receive evidence-based medications and reperfusion therapy, even though there is convincing evidence, obtained mainly through randomized controlled trials, to suggest that those treatments improve survival after MI. These age-related patterns are concordant with previous reports and may explain the high mortality in older patients (14–16).
The lower relative risk of death attributable to CKD in older patients might reflect a higher overall mortality and a higher prevalence of comorbid conditions in older patients, which could minimize the effect of a single variable, such as CKD, on mortality. Previous studies have reported the association of eGFR with survival according to age groups in the general population (7,8). Raymond et al. reported that, within CKD stages, the relative risk of death decreased as age increased, and that people aged ≥75 years, with an eGFR of 45−59 ml/min per 1.73 m2—who represent 41% of this age group—have no increased relative risk of mortality (8). Similarly, O’Hare et al. reported that the association of eGFR with mortality was weaker in elderly individuals than in younger age groups, whereas a moderate reduction in eGFR (50−59 ml/min per 1.73 m2) was not associated with an increased relative risk of mortality in people aged ≥65 years (7). Our findings are consistent with those of previous studies in terms of the attenuated relative risk for death associated with eGFR with increasing age; however, the statistical significance of the association between a moderate reduction in eGFR category (eGFR 45−59 ml/min per 1.73 m2) and mortality persisted among older patients. In contrast to previous studies, our study analyzed an MI population, not a general population, and features of this high-risk population might influence the effect of CKD on mortality. In addition, the CKD-EPI equation was used to estimate GFR in this study, in contrast with previous studies, which used the Modified Diet in Renal Disease (MDRD) study equation. It has been well recognized that the MDRD study equation underestimates eGFR for participants with a measured GFR approximately 60 ml/min per 1.73 m2 (17,18). This might attenuate the clinical significance of a moderate reduction in eGFR (eGFR 45−59 ml/min per 1.73 m2) by including more patients in this category compared with when using the CKD-EPI equation.
Although the relative risk of death for decreased eGFR is lower in older patients than in younger patients, the absolute difference in mortality risk among each eGFR categories is greater in the older age patients. In addition, the adjusted hazard ratio is still >3.0 for eGFR <30 ml/min per 1.73 m2 in the oldest group. Therefore, CKD is still an important risk factor to consider even in the oldest patients.
This study has several limitations. Although we adjusted for multiple confounding factors, it is possible that some unmeasured confounders remained. Second, assessment of kidney function was based on a single serum creatinine value obtained at the time of presentation to the hospital. This value could have been affected by the hemodynamic or metabolic status and may have included individuals with AKI whose eGFR was not estimated at equilibrium. Third, only 62 deaths in 12 months were observed in patients aged <55 years; hence, the large confidence interval and the lack of precision of point estimation should be allowed for in any interpretation.
In conclusion, the prevalence of CKD increased dramatically in older patients, whereas overall mortality increased with advancing age. Although the relative risk for death increased in proportion to the decrease in eGFR, within each CKD category, the association of decreasing eGFR with the relative risk for death was weaker in older patients.
Disclosures
None.
Acknowledgments
The KAMIR investigators are as follows: Myung Ho Jeong, MD; Young Keun Ahn, MD; Sung Chull Chae, MD; Jong Hyun Kim, MD; Seung Ho Hur, MD; Young Jo Kim, MD; In Whan Seong, MD; Dong Hoon Choi, MD; Jei Keon Chae, MD; Taek Jong Hong, MD; Jae Young Rhew, MD; Doo Il Kim, MD; In Ho Chae, MD; Jung Han Yoon, MD; Bon Kwon Koo, MD; Byung Ok Kim, MD; Myoung Yong Lee, MD; Kee Sik Kim, MD; Jin Yong Hwang, MD; Myeong Chan Cho, MD; Seok Kyu Oh, MD; Nae Hee Lee, MD; Kyoung Tae Jeong, MD; Seung Jea Tahk, MD; Jang Ho Bae, MD; Seung Woon Rha, MD; Keum Soo Park, MD; Chong Jin Kim, MD; Kyoo Rok Han, MD; Tae Hoon Ahn, MD; Moo Hyun Kim, MD; Ki Bae Seung, MD; Wook Sung Chung, MD; Ju Young Yang, MD; Chong Yun Rhim, MD; Hyeon Cheol Gwon, MD; Seong Wook Park, MD; Young Youp Koh, MD; Seung Jae Joo, MD; Soo Joong Kim, MD; Dong Kyu Jin, MD; Jin Man Cho, MD; Byung Ok Kim, MD; Sang-Wook Kim, MD; Jeong Kyung Kim, MD; Tae Ik Kim, MD; Deug Young Nah, MD; Si Hoon Park, MD; Sang Hyun Lee, MD; Seung Uk Lee, MD; Hang-Jae Chung, MD; Jang Hyun Cho, MD; Seung Won Jin, MD; Yang Soo Jang, MD; Jeong Gwan Cho, MD; and Seung Jung Park, MD.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (2011-0009743), and by the Korea Science and Engineering Foundation through the Medical Research Center for Gene Regulation (2012-0009448) at Chonnam National University.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Bae EH, Lim SY, Cho KH, Choi JS, Kim CS, Park JW, Ma SK, Jeong MH, Kim SW: GFR and cardiovascular outcomes after acute myocardial infarction: Results from the Korea Acute Myocardial Infarction Registry. Am J Kidney Dis 59: 795–802, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA: Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 351: 1285–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, Kusek JW, Nayfield SG, Schmader K, Tian Y, Ashworth JR, Clayton CP, Parker RP, Tarver ED, Woolard NF, High KP, workshop participants : Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol 20: 1199–1209, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Prakash S, O’Hare AM: Interaction of aging and chronic kidney disease. Semin Nephrol 29: 497–503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindeman RD: Overview: Renal physiology and pathophysiology of aging. Am J Kidney Dis 16: 275–282, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Lindeman RD, Goldman R: Anatomic and physiologic age changes in the kidney. Exp Gerontol 21: 379–406, 1986 [DOI] [PubMed] [Google Scholar]
- 7.O’Hare AM, Bertenthal D, Covinsky KE, Landefeld CS, Sen S, Mehta K, Steinman MA, Borzecki A, Walter LC: Mortality risk stratification in chronic kidney disease: One size for all ages? J Am Soc Nephrol 17: 846–853, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Raymond NT, Zehnder D, Smith SC, Stinson JA, Lehnert H, Higgins RM: Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial Transplant 22: 3214–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS: Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 362: 2155–2165, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Alpert JS, Thygesen K, Antman E, Bassand JP: Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 36: 959–969, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 13.Garg AX, Papaioannou A, Ferko N, Campbell G, Clarke JA, Ray JG: Estimating the prevalence of renal insufficiency in seniors requiring long-term care. Kidney Int 65: 649–653, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Nauta ST, Deckers JW, Akkerhuis KM, van Domburg RT: Age-dependent care and long-term (20year) mortality of 14,434 myocardial infarction patients: Changes from 1985 to 2008 [published online ahead of print March 31, 2012]. Int J Cardiol doi:10.1016/j.ijcard.2012.03.064 [DOI] [PubMed] [Google Scholar]
- 15.Rosengren A, Wallentin L, Simoons M, Gitt AK, Behar S, Battler A, Hasdai D: Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J 27: 789–795, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Devlin G, Gore JM, Elliott J, Wijesinghe N, Eagle KA, Avezum A, Huang W, Brieger D, GRACE Investigators : Management and 6-month outcomes in elderly and very elderly patients with high-risk non-ST-elevation acute coronary syndromes: The Global Registry of Acute Coronary Events. Eur Heart J 29: 1275–1282, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS: Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43: 112–119, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]