Summary
Background and objectives
Organized deposits are present in amyloidosis, fibrillary GN, and immunotactoid glomerulopathy. However, the constituents of the deposits are not known.
Design, setting, participants, & measurements
Laser microdissection of glomeruli followed by mass spectrometry was performed to determine the composition of the deposits. The results were compared with cryoglobulinemic GN.
Results
The results are divided into four major groups: amyloidogenic proteins, structural/other proteins, complement proteins, and Igs. With regards to amyloidogenic proteins, large spectra numbers of apolipoprotein E are noted in amyloidosis (41.8±20.9) compared with fibrillary (15.6±12.5) and immunotactoid (12.3±12) glomerulopathy. Apolipoprotein E was absent in cryoglobulinemic GN. Serum amyloid P component is present in large spectra numbers in amyloidosis (14.1±6.7) and small spectra numbers in immunotactoid glomerulopathy, but it is absent in fibrillary and cryoglobulinemic GN. However, large spectra numbers of Ig γ-1 chain C region are present in immunotactoid glomerulopathy (47.3±34.6) compared with fibrillary (16.25±19.7) and cryoglobulinemic (13.3±4.9) GN. All cases of Ig light chain-associated amyloidosis showed spectra for the respective Ig light-chain C region (mean=10±1.7).
Conclusions
Based on the spectra numbers, the study shows that the relative amount of apolipoprotein E to Ig light-chain C region/amyloidogenic proteins or Ig γ-1 chain C region is associated with the organization of the deposits in amyloidosis, fibrillary GN, and immunotactoid glomerulopathy. However, the absence of apolipoprotein E correlates with the lack of fibrillar deposits in cryoglobulinemic GN.
Introduction
The most common renal diseases with organized deposits are amyloidosis, fibrillary GN, and immunotactoid glomerulopathy. The conditions often present with nephrotic syndrome. Amyloid deposits are Congo red-positive, whereas the deposits in fibrillary GN and immunotactoid glomerulopathy are Congo red-negative. Most cases of amyloidosis are Ig light chain-associated (AL), although many forms of amyloid can be nonlight chain (or less commonly, heavy chain) -associated, such as amyloidosis associated with serum amyloid A protein, leukocyte cell-derived chemotaxin-2 (LECT2), and fibrinogen α-chain. Amyloid fibrils are randomly arranged and measure 8–12 nm in diameter, whereas the fibrils in fibrillary GN are also randomly arranged and measure 10–30 nm in diameter. The microtubules in immunotactoid glomerulopathy are often arranged in parallel arrays and measure 10–90 nm in diameter (1,2). Electron microscopy is often needed to make the definitive diagnosis. Most investigators favor separating fibrillary GN from immunotactoid glomerulopathy, whereas others combine fibrillary GN with immunotactoid glomerulopathy into a single group (3–8). Patients with immunotactoid glomerulopathy are more likely to have low complement titers, dysproteinemia, hematologic malignancy, and monoclonal glomerular deposits compared with patients with fibrillary GN, but significant overlap exists between the two pathologic entities (3,4,6,7). The pathogenesis of the fibrillar and microtubular deposits in amyloidosis, fibrillary GN, and immunotactoid glomerulopathy is not understood.
We performed laser microdissection (LMD) of the glomeruli followed by tandem mass spectrometry (MS) -based proteomics analysis to better understand the composition of the deposits and what might lead to the organization of the deposits in these conditions (9). The proteomic analysis of renal amyloidosis was recently described in detail by our group (9,10). In this study, we compare the glomerular protein profile of amyloidosis with the profile cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy.
Materials and Methods
Case Selection
A retrospective review of renal biopsies from seven cases of renal amyloidosis, eight cases of fibrillary GN, four cases of immunotactoid glomerulopathy, and four cases of cryoglobulinemic GN was performed. The biopsy material was sent to the Mayo Clinic Renal Pathology Laboratory for diagnosis and interpretation over a 5-year period (2007–2012). In all cases, routine evaluation, including light microscopy, immunofluorescence microscopy, and electron microscopy, was reviewed. For normal controls, we used day 0 protocol biopsy material from kidney transplant recipients. Clinical information was obtained from the charts. The Institutional Review Board at the Mayo Clinic approved the study.
Specimen, Specimen Preparation, LMD, and MS-Based Proteomic Analysis
The methods have previously been published (9–11). The licensed patent rights to perform protein extraction from paraffin-embedded tissue for the MS-based amyloid testing have been granted by OncoPlex Diagnostics (formerly Expression Pathology Inc.). Briefly, for each case, glomeruli were identified in 6-μm-thick sections of formalin-fixed paraffin-embedded tissues under bright field light. Glomeruli were identified using hematoxylin and eosin-stained sections for nonamyloidosis cases and Congo red-stained sections for amyloidosis. The glomeruli are laser microdissected using the Leica dissector (Leica DM 600 B). Each microdissection is called a sample, and each microdissection contained an area of 50,000–60,000 μm2; this area may involve two to four glomeruli, depending on size. Typically, two to four samples were analyzed for each case. The microdissected material was collected into 0.5-ml microcentrifuge tube caps containing 35 μL Tris/EDTA/0.002% Zwittergent buffer. Microdissected fragments were digested into tryptic peptides overnight and analyzed by liquid chromatography electrospray tandem MS. MS raw data files were queried using three different algorithms (Sequest, Mascot, and X!Tandem), and the results were combined and assigned peptide and protein probability scores in Scaffold (Proteome Software Inc., Portland, OR). For each case, a list of proteins based on peptides identified by MS was generated. Peptide identifications were accepted if they could be established at greater than 90.0% probability as specified by the Peptide Prophet algorithm (12–14). The MS data show spectra that match to a particular protein based on the amino acid sequence available in the database. Some of the peptides from different proteins can be common and shared depending on the homology of their amino acid sequence. However, unique peptides and spectra are distinctive to the particular protein. The Spectra value indicates the total number of mass spectra collected on the mass spectrometer and matched to the protein using the proteomics software. A higher number of mass spectra is indicative of greater abundance and will typically yield greater amino acid sequence coverage. A higher mass spectra value also indicates a higher confidence in the protein identification. Our clinical amyloid testing requires a minimum number of four spectra in all samples before the protein identification will be deemed clinically valid. However, the semiquantitative nature of the technique and high variability preclude statistical comparisons. Results are presented as mean ± SD.
Results
Clinical and Pathologic Findings
The clinical characteristics at the time of presentation are shown in Table 1.
Table 1.
Clinical characteristics of patients
| Case Number | Disease | Age (yr)/Sex | Serum Creatinine (mg/dl) | 24-h Urine Protein (g/d) | Hematuria |
|---|---|---|---|---|---|
| 1 | AA | 66/woman | 3.2 | 9.2 | No |
| 2 | AL | 63/man | NA | NA | No |
| 3 | AL | 55/woman | 9.7 | Nephrotic range | No |
| 4 | AL | 64/woman | NA | Nephrotic range | NA |
| 5 | ALECT2 | 69/man | 1.4 | 12.6 | No |
| 6 | Cryoglobulinemic GN | 72/man | 1.5 | 6.1 | Yes |
| 7 | Cryoglobulinemic GN | 70/woman | 1.8 | 0.7 | Yes |
| 8 | Cryoglobulinemic GN | 51/man | 2.4 | 1.4 | Yes |
| 9 | Cryoglobulinemic GN | 59/man | 3.5 | >300 mg/dl on UA | Yes |
| 10 | Fibrillary GN | 37/man | 0.9 | 3 | No |
| 11 | Fibrillary GN | 63/woman | 1.4 | NA | Yes |
| 12 | Fibrillary GN | 68/man | 0.8 | 5.2 | Yes |
| 13 | Fibrillary GN | 64/man | 1.2 | 1.7 | Yes |
| 14 | Fibrillary GN | 26/woman | NA | NA | NA |
| 15 | Fibrillary GN | 66/man | 1.0 | 3.7 | Yes |
| 16 | Fibrillary GN | 50/woman | 0.9 | 0.7 | Yes |
| 17 | Fibrillary GN | 66/woman | 1.6 | 8 | Yes |
| 18 | Immunotactoid GN | 56/man | 1.2 | 13.4 | No |
| 19 | Immunotactoid GN | 26/woman | 2.7 | 6.4 | Yes |
| 20 | Immunotactoid GN | 52/woman | 3.2 | 7 | Yes |
| 21 | Immunotactoid GN | 56/woman | 4 | 12 | NA |
AA, serum amyloid A protein/reactive secondary amyloidosis; AL, Ig light-chain amyloidosis; NA, not available; ALECT2, leukocyte cell-derived chemotaxin-2 amyloidosis; UA, urinalysis.
Amyloidosis.
We selected seven representative cases of amyloidosis for LMD and MS (cases 1–7). These cases included two cases of serum amyloid A protein/reactive secondary amyloidosis (AA; cases 1 and 2), three cases of AL amyloidosis (cases 3–5), and two cases of LECT2 amyloidosis (cases 6 and 7). Of three AL amyloidosis cases, two cases were AL λ-light chain type (cases 3 and 4), and one case was AL κ-light chain type (case 5).
Cryoglobulinemic GN.
We selected four representative cases of cryoglobulinemic GN (cases 8–11). Electron microscopy studies did not show tubular substructure in any of the cases. Three patients had hepatitis C (cases 8, 9, and 11), whereas one patient had rheumatoid arthritis and vasculitis (case 10). All cases showed positive serology for cryoglobulins.
Fibrillary GN.
We selected eight representative cases of fibrillary GN (cases 12–19) (2). All cases showed glomerular fibrillary deposits that were Congo red-negative. All eight cases showed glomerular mesangial and capillary wall staining for IgG (2–3+), C3 (1–2+), κ-light chains (1–3+), and/or λ-light chains (trace to 2+) on immunofluorescence microscopy. One case also showed small amounts of mesangial IgA (1+; case 8). Five cases were evaluated for paraproteinemia, of which four cases were negative and one case showed a monoclonal IgG κ-paraprotein (case 12).
Immunotactoid Glomerulopathy.
We selected four representative cases of immunotactoid glomerulopathy (cases 20–23). All cases showed glomerular deposits with a microtubular substructure. Immunofluorescence studies in case 20 showed IgG (2+), IgM (1–2+), C3 (2+), and λ-light chains (2+), with trace staining for κ-light chains. Case 21 showed IgG and λ-light chains (with negative κ-light chains). Case 22 showed IgG (3+) and κ-light chains (1+; with negative λ-light chains), whereas case 23 showed IgA (1–2+), IgG (2+), IgM (2+), C3 (2+), κ-light chains (2+), and λ-light chains (1+). Case 20 had multiple myeloma with IgG-λ on electrophoresis studies, case 22 had chronic lymphocytic leukemia with IgG-λ on electrophoresis studies, and case 23 did not have any hematologic malignancy but did have IgA-κ on electrophoresis studies; in case 21, hematologic evaluation was not available. All cases were negative for cryoglobulins, and serological evaluation for autoimmune diseases was negative.
Normal.
We used two cases (cases 24 and 25) of day 0 protocol biopsies of kidney transplant as normal controls. Light microscopy showed normal-appearing glomeruli in both cases.
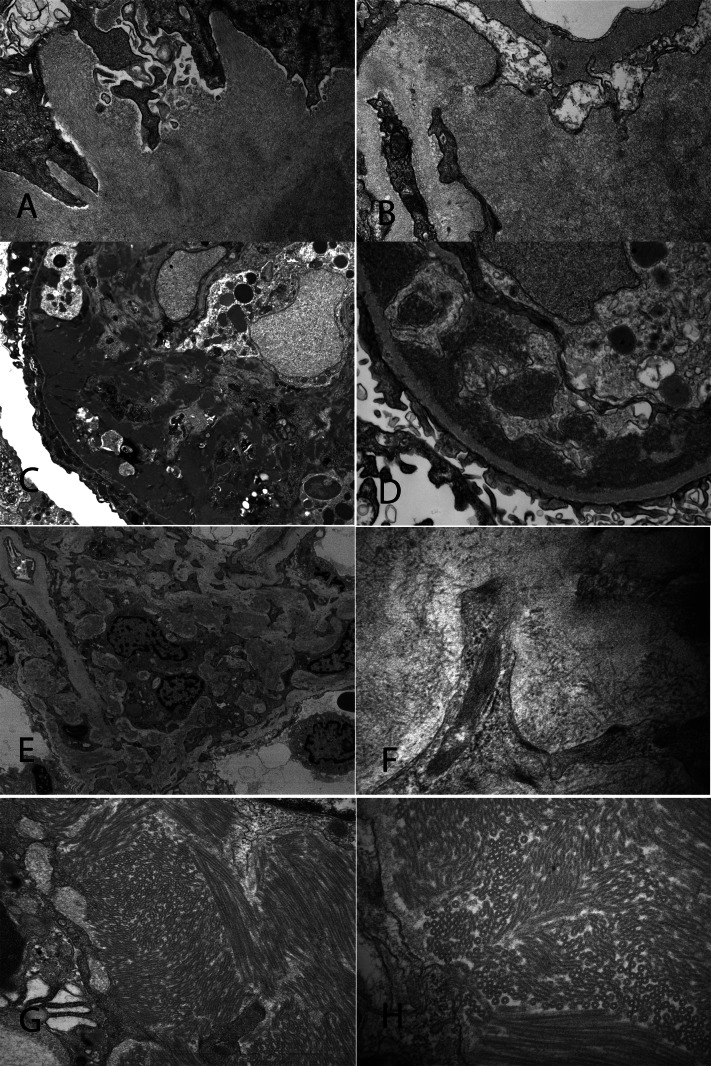
Representative electron microscopy findings from cases of amyloidosis, cryoglobulinemic GN (case 11), fibrillary GN (case 13), and immunotactoid glomerulopathy (case 22) are shown in Figure 1.
Figure 1.
Representative electron microscopy from a case of Ig light-chain amyloidosis, cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy. (A and B) Amyloid fibrils in a case of Ig light-chain amyloidosis. Fibrils measure 9.9 nm in thickness (×25,000 in A; ×30,000 in B). (C and D) Subendothelial deposits in a case of cryoglobulinemic GN (×7830 in C; ×46,000 in D). (E and F) Fibrillary deposits in a case of fibrillary glomerulopathy. Fibrils measure 16.20 nm in diameter (×42,000 in E; ×46,000 in F). (G and H) Deposits with a microtubular substructure in a case immunotactoid glomerulopathy. The microtubules measure 52 nm in diameter (×24,500 in G; ×46,000 in H).
MS
We divide the results into four major groups: amyloidogenic proteins, structural proteins, complement proteins, and Ig proteins.
Amyloidogenic Proteins.
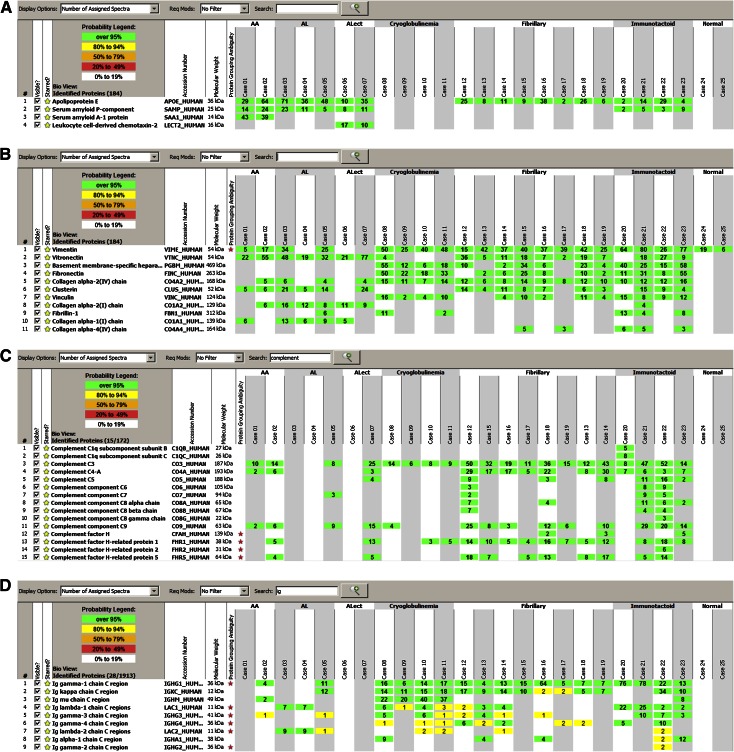
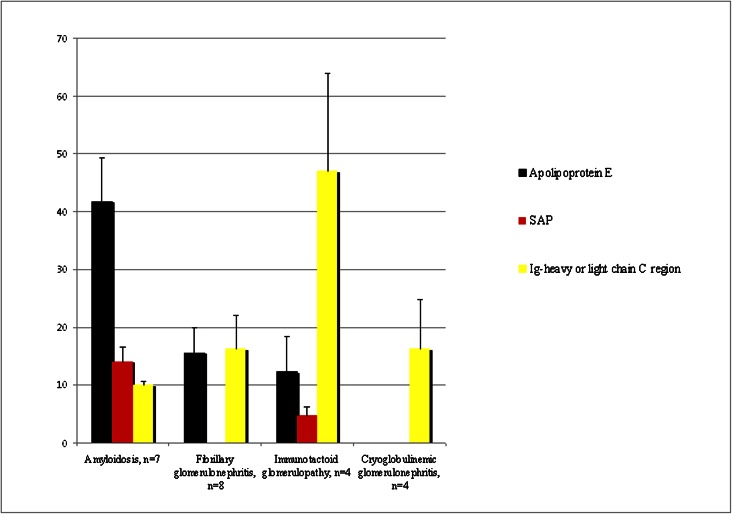
Apolipoprotein E is a major constituent of amyloid (9) (Figure 2A). Apolipoprotein E was also present in fibrillary GN and immunotactoid glomerulopathy, but the spectra numbers were smaller in comparison, whereas glomeruli of cryoglobulinemic GN did not contain apolipoprotein E (Figure 3). Thus, we find that glomeruli from amyloidosis contain larger spectra numbers of apolipoprotein E (mean=41.8±20.9, range=10–71, median=36) compared with glomeruli from fibrillary GN and immunotactoid glomerulopathy (mean=15.6±12.5, range=2–38, median=11 for fibrillary GN; mean=12.3±12, range=2–29, median=9 for immunotactoid glomerulopathy). Glomeruli from amyloidosis contain large spectra numbers for serum amyloid P component (SAP; mean=14.1±6.7, range=5–24, median=11). Surprisingly, small spectra numbers of SAP were present in immunotactoid glomerulopathy (mean=4.8±3, range=2–9, median=4), whereas SAP was negative in cryoglobulinemic and fibrillary GN (Figure 3). As expected, large spectra numbers of serum AA protein and LECT2 were present exclusively in AA and LECT2 amyloidosis, respectively.
Figure 2.
Mass spectrometry results. Scaffold 2 display of proteomic data showing the (A) amyloidogenic proteins, (B) structural proteins, (C) complement proteins, and (D) Igs in amyloidosis, cryoglobulinemic GN, fibrillary GN, immunotactoid glomerulopathy, and normal (control) glomeruli. The probability number (>95% is highlighted by green; 8%–94% by yellow) indicates essentially the percent homology between peptides detected in the specimens and the published amino acid sequences of their corresponding proteins. AA, amyloid A protein/reactive secondary amyloidosis; AL, Ig light-chain amyloidosis; ALECT2, amyloidosis associated with leukocyte cell-derived chemotaxin-2.
Figure 3.
Average spectra numbers of apolipoprotein E, serum amyloid P component (SAP), and Ig heavy- (γ-1) or light-chain C region detected in amyloidosis, fibrillary GN, immunotactoid glomerulopathy, and cryoglobulinemic GN (±SEM).
Structural/Other Proteins.
Important differences were noted in spectra numbers of vitronectin, basement membrane-specific heparan sulfate, fibronectin, clusterin, vinculin, collagen chains, and fibrillin between amyloidosis, cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy (Figure 2B).
Glomeruli from amyloidosis contain large spectra numbers of vitronectin (mean=39.1±21.7, range=19–77, median=32) compared with glomeruli from fibrillary GN (mean=13.1±11.0, range=2–36, median=14.5) and immunotactoid glomerulopathy (mean=13.5±11.6, range=9–27, median=18). Glomeruli from cryoglobulinemic GN did not contain significant spectra numbers of vitronectin.
Large spectra numbers of basement membrane heparan sulfate and fibronectin are noted in cryoglobulinemic GN (mean=22.8±22, range=6–55, median=15 and mean=30.8±14.3, range=18–50, median=27.5, respectively) and immunotactoid GN (mean=34.5±18.7, range=15–58, median=32.5 and mean=26.3±21.7, range=8–55, median=21, respectively) compared with fibrillary GN (mean=9.8±12.3, range=2–34, median=10 and mean=6.6±8.2, range=6–14, median=9.5). Vinculin is also present in cryoglobulinemic GN (mean=8±6.3, range=2–16, median=7), fibrillary GN (mean=3.8±4.4, range=2–11, median=4), and immunotactoid glomerulopathy (mean=11±3.1, range=8–15, median=10.5). Glomeruli from amyloidosis did not contain basement membrane heparan sulfate, fibronectin, or vinculin.
Clusterin is present in amyloidosis (mean=10.7±9.0, range=5–24, median=10), fibrillary GN (mean=6.6±4.4, range=3–14, median=7), and immunotactoid glomerulopathy (mean=11±3.1, range=4–15, media=9), whereas it is absent in cryoglobulinemic GN. Fibrillin was noted in three of four cases of immunotactoid glomerulopathy, two of four cases of cryoglobulinemic GN, and one case of light- and heavy-chain amyloidosis (case 5), whereas it was absent in fibrillary GN.
Collagen α-2(I) chain is present in amyloidosis (mean=8.8±5.0, range=6–16, median=10), whereas it was absent in cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy (except one case that showed low spectra numbers).
Complement Proteins.
Large spectra numbers of C3 are noted in fibrillary (mean=28.4±15.8, range=12–50, median=32), immunotactoid glomerulopathy (mean=30.2±22.4, range=8–52, median=37.5), and cryoglobulinemic GN (mean=9.3±3.4, range=6–14, median=7) (Figure 2C). C4 and C9 are also detected in fibrillary GN (C4: mean=16±10.9, range=5–30, median=17) and immunotactoid glomerulopathy (C4: mean=5.8±1.9, range=3–7, median=5), indicating activation of the classic and terminal pathway of complement.
Glomeruli involved by amyloidosis showed small spectra numbers of C3 and C9 in one of four cases of AL amyloidosis. This case also contained large spectra for Ig γ-1 chain C region, indicating a heavy-chain component. Both cases of AA amyloidosis contained spectra for C3 and C4, and one of two cases of LECT2 amyloidosis also contained C3 and C4.
Ig/Amyloidogenic Proteins.
Large spectra numbers for Ig γ-1 chain C region are present in glomeruli from cryoglobulinemic GN (mean=13.3±4.9, range=6–17, median=15), fibrillary GN (mean=16.3±19.7, range=4–64, median=10), and immunotactoid glomerulopathy (mean=47.3±34.6, range=13–78, median=49) (Figure 2D). Spectra numbers were highest in immunotactoid glomerulopathy (Figure 3). One case (case 5) of AL amyloidosis also contained significant spectra numbers for Ig γ-1 chain C region that likely represented a heavy-chain component. Small spectra numbers were also noted in one case of AA amyloidosis that likely represented entrapped plasma proteins. All three cases of AL showed specific spectra for the respective light chains (mean=10±1.7, range=7–12, median=9): λ-light chains in cases 3 and 4 and κ-light chains in case 5. Two cases of immunotactoid glomerulopathy (cases 20 and 21) showed large spectra numbers for Ig λ-1 C region, and two cases (cases 22 and 23) showed large spectra numbers for Ig κ-1 C region. Glomeruli from cryoglobulinemic GN contained large spectra for Ig μ-C region in addition to Ig γ-1 chain C region.
Normal.
Glomeruli from normal glomeruli showed large spectra for actin, vimentin, actinin, and hemoglobin and smaller spectra for albumin, histones, etc.
Discussion
We compare the glomerular proteomics of amyloidosis, cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy using the techniques of LMD and tandem MS. We divide the findings into four groups of proteins: amyloidogenic proteins, structural/other proteins, complement factors, and Ig.
We find that the basic amyloidogenic protein apolipoprotein E is present in all organized deposits, with relatively large spectra numbers in amyloidosis and in comparison, lower spectra numbers in fibrillary GN and immunotactoid glomerulopathy (amyloidosis > fibrillary GN > immunotactoid glomerulopathy). It is absent in nonorganized deposits of cryoglobulinemic GN. Large spectra numbers of SAP are always noted in amyloidosis. However, SAP is not present or present in very low spectra numbers in fibrillary GN and immunotactoid glomerulopathy, indicating that SAP is not required for fibrillar or microtubular substructure.
Glomeruli from cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy contain large spectra numbers for Ig γ-1 chain C region. However, spectra numbers are highest in immunotactoid glomerulopathy followed by fibrillary GN and cryoglobulinemic GN.
Taken together, these findings suggest that Ig light- or heavy-chain C region along with apolipoprotein E is key to organization of the deposits: high spectra numbers of Ig γ-1 chain region and low spectra numbers of apolipoprotein E are found in immunotactoid glomerulopathy, mid-level spectra numbers of Ig γ-1 chain region and mid levels of apolipoprotein E are found in fibrillary glomerulopathy, whereas Ig light-chain region and high spectra numbers of apolipoprotein E are found in amyloidosis (Figure 3).
We also noted differences in the spectra numbers of structural proteins between the four conditions. Vitronectin mirrors the spectra numbers of apolipoprotein E, with high spectra numbers in amyloidosis and lower spectra numbers in fibrillary GN and immunotactoid glomerulopathy. In general, other than vitronectin and clusterin, we noted higher spectra numbers of structural proteins, such as basement membrane heparan sulfate and fibronectin, in cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy compared with amyloidosis. However, collagen α-2(I) chain is present in amyloidosis, whereas it is absent in cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy (except in low numbers in one case). The significance of these finding is not clearly understood at this time, although the proteins likely contribute to the organization of the deposits.
With regards to complement proteins, we find proteins of the classic and terminal pathways in both fibrillary GN and immunotactoid glomerulopathy. We detected high spectra numbers of C3 in all cases, C4 in 11 of 12 cases, and C9 in 9 of 12 cases of fibrillary GN and immunotactoid glomerulopathy. Surprisingly, we noted C3 in all cases of cryoglobulinemic GN but failed to detect components of the terminal pathway. Complement proteins were absent in AL amyloidosis, except one case that had a heavy-chain component.
Limitations of the study include the relatively small sample size as well as the recognition that the spectra number is not absolutely quantitative, although a higher spectra number is indicative of greater abundance of the protein. Another limitation of the study is that not all proteins digest into the proper size fragments with trypsin and the peptides do not all ionize similarly.
In conclusion, our studies compare the proteomic profile of the deposits in amyloidosis with the profiles of cryoglobulinemic GN, fibrillary GN, and immunotactoid glomerulopathy. Based on the spectra numbers, we find that the relative amount of apolipoprotein E compared with Ig heavy- and/or light-chain C regions determines the type of deposits present in amyloidosis, fibrillary GN, and immunotactoid glomerulopathy. It is tempting to speculate that future therapy could target proteins such as apolipoprotein E and interfere with the formation and tissue deposition of organized deposits.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Rosenmann E, Eliakim M: Nephrotic syndrome associated with amyloid-like glomerular deposits. Nephron 18: 301–308, 1977 [DOI] [PubMed] [Google Scholar]
- 2.Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, Leung N, Fervenza FC: Fibrillary glomerulonephritis: A report of 66 cases from a single institution. Clin J Am Soc Nephrol 6: 775–784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogo A, Qureshi N, Horn RG: Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy. Am J Kidney Dis 22: 367–377, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D’Agati VD: Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 63: 1450–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MM, Korbet SM, Lewis EJ: Immunotactoid glomerulopathy. J Am Soc Nephrol 13: 1390–1397, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bridoux F, Hugue V, Coldefy O, Goujon JM, Bauwens M, Sechet A, Preud’Homme JL, Touchard G: Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int 62: 1764–1775, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Alpers CE, Kowalewska J: Fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol 19: 34–37, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Herrera GA, Turbat-Herrera EA: Renal diseases with organized deposits: An algorithmic approach to classification and clinicopathologic diagnosis. Arch Pathol Lab Med 134: 512–531, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Vrana JA, Theis JD, Leung N, Sethi A, Nasr SH, Fervenza FC, Cornell LD, Fidler ME, Dogan A: Laser microdissection and mass spectrometry-based proteomics aids the diagnosis and typing of renal amyloidosis. Kidney Int 82: 226–234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi S, Theis JD, Leung N, Dispenzieri A, Nasr SH, Fidler ME, Cornell LD, Gamez JD, Vrana JA, Dogan A: Mass spectrometry-based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin J Am Soc Nephrol 5: 2180–2187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, 3rd, Zipfel PF, Dogan A, Smith RJ: Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int 75: 952–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi NHNY, Nakano Y, Tobe T, Mazda T, Tomita M: Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol 2: 413–417, 1990 [DOI] [PubMed] [Google Scholar]
- 13.Nesvizhskii AI, Keller A, Kolker E, Aebersold R: A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Keller A, Nesvizhskii AI, Kolker E, Aebersold R: Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392, 2002 [DOI] [PubMed] [Google Scholar]