Summary
Background and objectives
Recent understanding of extrarenal production of calcitriol has led to the use of more vitamin D supplementation in CKD populations. This paper reports the effect of cholecalciferol supplementation on calcium absorption.
Design, setting, participants, & measurements
Paired calcium absorption tests were done before and after 12–13 weeks of 20,000 IU weekly cholecalciferol supplementation in 30 participants with stage 5 CKD on hemodialysis. The study was conducted from April to December of 2011. Calcium absorption was tested with a standardized meal containing 300 mg calcium carbonate intrinsically labeled with 45Ca; 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were measured.
Results
25-Hydroxyvitamin D rose from 14.2 ng/ml (11.5–18.5) at baseline to 49.3 ng/ml (42.3–58.1) at the end of the study (P<0.001). 1,25-Dihydroxyvitamin D rose from 15.1 (10.5–18.8) pg/ml at baseline to 20.5 (17.0–24.7) pg/ml at the end of the study (P<0.001). The median baseline calcium absorption was 12% (7%–17%) and 12% (7%–16%) at the end of study.
Conclusions
Patients with stage 5 CKD on hemodialysis had very low calcium absorption values at baseline, and cholecalciferol supplementation that raised 25(OH)D levels to 50 ng/ml had no effect on calcium absorption.
Introduction
It was previously believed that the kidney was the sole site of 1-α hydroxylase activity; therefore, there was no role for supplementing CKD patients on dialysis with vitamin D, because it would not be metabolized to the active form [1,25(OH)2D]. Because the recent extrarenal metabolism and actions of vitamin D have been explored (1), there is a growing interest in using vitamin D supplementation (ergocalciferol or cholecalciferol) in patients with CKD (2,3). In fact, the Kidney Disease: Improving Global Outcomes guidelines recommend using vitamin D in vitamin D-deficient patients in CKD stages 3–5D (2). Although the clinical benefits in these patients have not been defined by many randomized controlled trials, observational studies have found an association between low 25(OH)D levels and mortality (4). Exploratory studies in patients with CKD have found that supplementation with vitamin D has resulted in reduced circulating levels of inflammatory proteins (5), improved glycemic control (6), and reduced need for erythropoiesis-stimulating agents (7,8).
However, despite the potential benefits of vitamin D supplementation, there is also a concern that it may lead to hypercalcemia and ectopic calcification by raising 1,25(OH)2D levels (9) and increasing active intestinal calcium absorption (10).
Previous vitamin D dose response studies in dialysis patients have used average daily doses between 1500 and 3300 IU (11–13) to raise 25(OH)D levels to levels that are acceptable for a healthy person (31–42 ng/ml) (14). Raising 25(OH)D to these levels usually increases calcium absorption in healthy persons ingesting typical calcium intakes (14,15), but there is much less known about the effect on calcium absorption in patients with CKD.
This study examines the effect of cholecalciferol supplementation on calcium absorption in stage 5 CKD patients on hemodialysis.
Materials and Methods
Design
This was an open-label study of oral cholecalciferol with paired calcium absorption tests done at baseline and after 12–13 weeks of weekly doses of oral cholecalciferol in participants with stage 5 CKD requiring maintenance hemodialysis. This trial was registered at clinicaltrials.gov on January 17, 2011 (NCT01325610).
Participants
Forty-seven patients were screened from three hemodialysis centers located in Omaha, NE (latitude 41.2° N). Patients were included if they had been receiving hemodialysis for more than 3 months. The dialysate calcium concentration was kept consistent throughout the study at 2.5 mEq/L or 1.25 mmol/L. Vitamin D status was not a criterion for participation. Patients were excluded if they were pregnant or planned to become pregnant, were hypercalcemic (>10.2 mg/dl) at baseline, had chronic gastrointestinal disease, had liver dysfunction, were taking steroids, had received any investigational drugs within 4 weeks, had an allergy to vitamin D3, or had chronic vitamin D intake >1,000 IU daily. Peritoneal dialysis patients were excluded.
Ten participants declined to enroll. Thirty-seven participants (18 women and 19 men) provided signed written informed consent approved by the Creighton University Institutional Review Board in adherence to the Declaration of Helsinki; 2 participants dropped from the study before visit 1, 2 participants received renal transplants during the study, 2 participants died from causes unrelated to the study, and 1 subject dropped from the study for personal reasons, leaving 30 participants with paired data for analysis (13 women and 17 men).
Intervention
The study was conducted from April to December of 2011. One time per week for 12–13 weeks, at the time of dialysis, participants received an oral dose of 20,000 IU cholecalciferol. Ingestion was observed by study staff, ensuring 100% compliance. The cholecalciferol was an over-the-counter product (oil-based capsule preparation commercially available as Maximum D3, cholecalciferol 10,000 IU per capsule, 0.25 mg; BTR Group, Inc., Pittsfield, IL). The cholecalciferol was analyzed independently by Heartland Assays, Inc. (Ames, IA) and found to contain 9685+391 IU per capsule.
Protocol
At baseline, before starting a hemodialysis session, venous blood was drawn for serum calcium, phosphorus, 25(OH)D, 1,25(OH)2D, and parathyroid hormone (PTH). A calcium absorption test was performed the next day. Oral cholecalciferol was then given weekly for a total of 12–13 doses. Blood was drawn for repeat 25(OH)D, PTH, calcium, and phosphorus on days 7, 21, 35, 55, and 69 and on the day before the second calcium absorption test. Blood was also drawn for repeat 1,25(OH)2D on the day before the second calcium absorption test. The second calcium absorption test was done 1 week after the last cholecalciferol dose was given. All blood was drawn before the next dose of cholecalciferol was given (at the nadir) and directly before the start of a hemodialysis session. Twenty-seven participants were receiving phosphorus binders throughout the study. The six participants who were receiving calcium acetate as a phosphorus binder were switched to sevelamer carbonate for 6 weeks before the start of the protocol.
Calcium Absorption Test
On each day of testing, the fasting participant came to the center and was given a light breakfast consisting of two or three pieces of low calcium bread, toasted and buttered, together with coffee or tea with artificial sweetener (if desired). Halfway through the meal, the participant ingested the calcium test load consisting of 0.75 g calcium carbonate (300 mg elemental calcium) in a capsule intrinsically labeled with 9.1 μCi 45calcium (45Ca). The time of swallowing the test source was counted as time zero, and the subsequent blood sample was taken relative to that time. The subject had exactly the same meal at the second calcium absorption test. The subject was restricted to drink only ionized water provided by our center during the calcium absorption testing. At the first calcium absorption test, blood was drawn at 5 hours after ingestion of the 45Ca for measurement of serum calcium-specific activity. At the second calcium absorption test, blood was drawn at baseline and 5 hours after ingestion of the 45Ca for measurement of serum calcium-specific activity.
Calcium Labeling
The intrinsically labeled calcium carbonate was prepared in our laboratories by adding high-specific activity 45Ca chloride (Perkin Elmer Life Sciences, Waltham, MA) to a solution of Ca chloride hexahydrate dissolved in water, precipitating the carbonate salt with sodium carbonate dissolved in water, washing, filtering, and drying the resultant precipitate, and manually loading the powder into individually weighed gelatin capsules. 45Ca activity and calcium content were verified by analysis.
Analytical Methods
Serum 45Ca-specific activity was measured by liquid scintillation counting (Packard Tri Carb Liquid Scintillation Counter, Model 1900TR; Perkin Elmer Life Sciences). 45Ca was measured against standards prepared from quadruplicate aliquots of the stock tracer solution. Serum 25-hydroxyvitamin D levels were measured by a Liaison instrument (DiaSorin, Inc., Stillwater, MN) using a chemiluminescent assay, which measures total 25(OH)D [i.e., the sum of 25(OH)D2 and 25(OH)D3] with a within-assay coefficient of variation of 2.6%. Our research laboratory facilities participate in the Vitamin D External Quality Assessment Scheme for vitamin D measurement. Intact PTH (hPTH 1–84) was measured by immunoradiometric assay using the DiaSorin N-tact PTH SP IRMA kit (DiaSorin Inc., MN) in our research laboratory. Serum 1,25(OH)2D was measured using competitive radioimmunoassay as previously described (16) at Heartland Assays, Inc. (Ames, IA). The inter- and intra-assay coefficients of variation for this assay are 12.6% and 9.8%, respectively. The laboratory normal reference range is 30–50 pg/ml. Serum calcium and phosphorus were measured by a Beckman Coulter DXC 600-I autoanalyzer (Beckman Coulter, Inc., Brea, CA) in the medical laboratory of Creighton University.
Statistical Analyses
Fractional absorption at 5 hours was calculated using the following method previously reported and validated by Heaney (17). Fractional absorption is estimated from the tracer content of the calcium in the 5-hour blood sample according to the following algorithm:
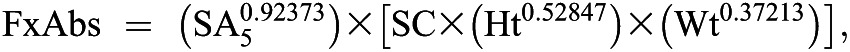 |
in which FxAbs is the calcium absorption fraction, SA5 equals 5-hour serum calcium-specific activity (fraction of oral dose per gram calcium), SC equals the sex coefficient, Ht equals height (meters), and Wt equals weight (kilograms). The sex coefficient (SC) in the foregoing formula varies according to the sex of the participant (i.e., for men, it is 0.3845, and for women, it is 0.3537). At the second test, correction was made for radioactivity in serum calcium present from the earlier dose. Serum calcium was corrected for albumin status using the formula corrected calcium = ((0.8×(4.0−subject’s albumin))+subject’s serum calcium) (18).
Descriptive statistics were generated using the statistics package PASW Statistics 19.0 (SPSS Inc., Chicago, IL) and Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA). All values are expressed in median (interquartile range [IQR]) unless otherwise specified. The within-individual change in laboratory values before and after cholecalciferol supplementation was compared using a paired t test. The participants’ baseline and final 25(OH)D levels, increment 25(OH)D, baseline and final 1,25(OH)2D levels, increment 1,25(OH)2D, and baseline and final calcium absorption fraction correlations were examined using Spearman correlation. Both calcium absorption fractions were compared between those participants who were on vitamin D analogs and those participants who were not, between those participants with and without diabetes, and between those participants on a gastric acid suppressor (proton pump inhibitor or acid blocker) and those participants who were not using a Mann–Whitney U test.
Results
Participants
Thirty participants (seventeen men and thirteen women) completed the cholecalciferol supplementation regimen and both calcium absorption tests. Their median (IQR) age was 53.6 (45.8–65.4) years. Table 1 shows participants’ demographics. Participants had been on maintenance dialysis for an average of 4.5 years. The reasons for renal failure were 8 participants (27%) had diabetic nephropathy, 15 participants (50%) had hypertensive kidney disease, and 7 participants (23%) had other types of kidney disease. Twenty-three participants (77%) were on a calcitriol analog throughout the study (intravenous doxercalciferol). Thirteen participants (43%) were on gastric acid suppressor therapy throughout the study. Eighty percent of the participants reported their race as African American, and one third were diabetic.
Table 1.
Subject demographics
| Variable | Results |
|---|---|
| N | 30 |
| Sex (men/women) | 17/13 |
| Race (African American/Caucasian/other) | 24/5/1 |
| Age (yr) | 53.6 (45.8–65.4) |
| Height (cm) | 170 (161–174) |
| Weight (kg) | 89 (69–106) |
| Body mass index (kg/m2) | 33 (24–37) |
| Years on dialysis | 4.5 (1.7–8.0) |
| On calcitriol analog | 23 (77%) |
| Diabetic | 10 (33%) |
Median (interquartile range).
Laboratory Data
25(OH)D
At baseline, 24 participants (80%) had 25(OH)D levels<20 ng/ml, and 28 participants (93%) had 25(OH)D levels<30 ng/ml (Table 2). Five participants (17%) had 25(OH)D levels less than 10 ng/ml. Median 25(OH)D levels were 14.2 ng/ml (11.5–18.5) at baseline and rose to 49.3 ng/ml (42.3–58.1) at the end of study (P<0.001, Wilcoxon signed ranks test). All participants responded to supplementation with a rise in 25(OH)D, reaching levels>25 ng/ml, and most participants (28) reached 25(OH)D levels>30 ng/ml. There was positive correlation between baseline 25(OH)D levels and final 25(OH)D levels (Spearman-ρ, R=0.592, P=0.001) but no correlation between increment of 25(OH)D and baseline 25(OH)D levels. There was a significant inverse correlation between body mass index and both baseline and final 25(OH)D levels (Spearman-ρ, R=−0.388, P=0.03 and R=−0.441, P=0.02, respectively).
Table 2.
Participants’ laboratory results
| N | 30 | |||
|---|---|---|---|---|
| Baseline | Post-treatment | Change | Pa | |
| FxAbs (%)b | 12 (7–17) | 12 (7–16) | 0.01 (−0.05 to 0.03) | 0.50 |
| 25(OH)D (ng/ml) | 14.2 (11.5–18.5) | 49.3 (42.3–58.1) | 32.0 (27.5–40.6) | <0.001 |
| Calcium (mg/dl) | 9.0 (8.5–9.5) | 9.0 (8.5–9.2) | −0.0 (−0.3 to 0.1) | 0.82 |
| Albumin (g/dl) | 3.6 (3.4–3.8) | 3.6 (3.4–3.9) | 0.1 (−0.0 to 0.2) | 0.48 |
| Corrected calciumc | 9.3 (8.7–9.9) | 9.2 (8.8–9.6) | −0.2 (−0.3 to 0.4) | 0.53 |
| Phosphorus (mg/dl) | 5.9 (4.7–7.1) | 5.8 (4.7–6.6) | −0.5 (−1.3 to 0.7) | 0.25 |
| Parathyroid hormone (pg/ml) | 325 (218–552) | 376 (269–611) | 42 (−127 to 218) | 0.28 |
| 1,25(OH)2D (pg/ml) | 15.1 (10.5–18.8) | 20.5 (17.0–24.7) | 5.6 (1.9–11.1) | 0.002 |
Median (interquartile range).
P value for individual change from baseline to end of study (paired t test).
Calcium absorption fraction.
Corrected calcium=((0.8×(4.0−subject’s albumin))+subject’s serum calcium).
1,25(OH)2D
1,25(OH)2D levels rose with cholecalciferol supplementation from 15.1 (10.5–18.8) pg/ml at baseline to 20.5 (17.0–24.7) pg/ml at the end of study (P<0.001, Wilcoxon signed ranks test). The median within-subject change was 5.6 (1.9–11.1) pg/ml. There were no correlations between 1,25(OH)2D and 25(OH)D levels at baseline, between 1,25(OH)2D and 25(OH)D levels at the end of study, or between the increment in 1,25(OH)2D and increment in 25(OH)D levels.
Calcium Absorption
The median (IQR) FxAbs was 12% (7–17) at baseline and 12% (7–16) at the end of study (Table 2). The median within-subject change was 0.01% (−0.05–0.03). There were no statistically significant differences in FxAbs at either time point between those participants who were on a vitamin D analog throughout the study and those participants who were not, between those participants with or without diabetes, between those participants on a gastric acid blocker and those participants who were not, or between racial groups. The second FxAbs was positively correlated with the baseline FxAbs (Spearman-ρ, R=0.621, P<0.001). There were no correlations between either FxAbs and 25(OH)D levels at baseline or end of study or between either FxAbs and 1,25(OH)2D levels at baseline or end of study.
Other Laboratory Values
There were no statistically significant changes in serum calcium, albumin, calcium corrected for albumin level, phosphorus, or PTH from baseline to the end of study.
Discussion
This study shows that raising 25(OH)D to levels that are acceptable in healthy participants has no effect on calcium absorption in stage 5 CKD patients on chronic hemodialysis conducted according to current protocols. This result is despite raising 1,25(OH)2D to levels similar to those levels reported in other studies of response to vitamin D in hemodialysis (11,13).
To explain this finding, it may be helpful to review calcium homeostasis in the dialysis patient. The dialysis patient has very few outlets for calcium loss (i.e., they are not regulating calcium balance through their kidney, and many are not very physically active; therefore, little calcium is lost in sweat). A small amount (200 mg) will be lost in gastrointestinal secretions (19) or through efflux during dialysis, although in studies of the dialysate calcium concentrations used in this study (2.5 mEq/L or 1.25 mmol/L), there is either no transfer of calcium (20) or a small transfer of 100–200 mg elemental calcium to the patient per dialysis session (21). As in a healthy person, calcium input will be derived from the gastrointestinal tract and bone. Although the patient cannot respond to high PTH levels with increased 1,25(OH)2D production and increased active calcium absorption, they can respond with an increase in calcium influx from bone resorption (22).
Why did increasing 25(OH)D and 1,25(OH)2D levels have no effect on calcium absorption in this study? There could be several explanations. (1) The doxercalciferol that the majority of the participants takes could have increased calcium absorption to the maximum possible, and adding cholecalciferol had no additional effect on calcium absorption. We find this reason unlikely, because all participants had low calcium absorption at baseline regardless of their use of a calcitriol analog and adding cholecalciferol did not change calcium absorption in either group. (A healthy person will have absorption of about 34% under these conditions [23].) Therefore, this explanation is not satisfactory. (2) The vitamin D receptor expression is downregulated by low 1,25(OH)2D, high PTH, or both (24,25); therefore, much higher 1,25(OH)2D concentrations are required to achieve calcium absorption than could be achieved by reasonable cholecalciferol supplementation. (3) There is inhibition of calcium absorption because of something specific to uremia (metabolic acidosis for example) (26). The second and third explanations are the most plausible. A similar explanation could explain the lack of effect on PTH (i.e., much higher levels of 1,25(OH)2D or its analog are needed to decrease PTH secretion).
In interpreting these calcium absorption results, it is important to know that, under these conditions of a physiologic, meal-sized calcium load taken with a meal, the absorption in a healthy person is 25%–40%, with some variability between individuals (23). This method only measures the unidirectional flow of calcium from the gut to the bloodstream, and it makes no provision for flow of calcium from the body through digestive enzymes or sloughed off cells. Net absorption, taking into account both absorption and calcium loss, results in a net absorption of ∼10% in healthy persons. In this study and previous studies of dialysis patients (27), we see percent calcium absorption values that are very low, and we see no response to vitamin D or doxercalciferol. The most probable interpretation is a defect in the mucosal response to vitamin D.
Historically, renal patients on hemodialysis have been reported to have poor calcium absorption, similar to the levels that we observed (28–31). These absorption levels (∼15%) are in the range usually attributed to passive calcium absorption alone. To put this finding into perspective, healthy persons absorb ∼34% of the calcium load in these conditions (23). In one older study of renal disease, calcitriol increased calcium absorption in patients with chronic renal disease on hemodialysis (9), but this effect was not reproduced in a recent study comparing calcitriol with paricalcitol (27). Although calcitriol is clearly associated with hypercalcemia in dialysis patients, it cannot be assumed this result is a result of increased calcium absorption. The calcium may also be coming from bone as 1,25(OH)2D stimulates osteoclast bone resorption in vitro (32,33). It is also possible that 1,25(OH)2D changes the physical–chemical equilibrium at the bone surface by acting on the bone lining cells.
An older study of calcifediol [25(OH)D] in pharmacologic doses that increased 25(OH)D levels to >250 ng/ml, increased calcium absorption significantly, but 1,25(OH)2D measurements were not available, and it is unknown how much of the effect on calcium absorption was caused by a direct effect of 25(OH)D versus the presumed increase in 1,25(OH)2D levels (30,34). Interestingly, another study from the same period comparing 25(OH)D and calcitriol found that, although both increased serum calcium and phosphorus levels, only the 25(OH)D improved bone mineralization on histomorphometric examination of bone biopsies (35). These differences in effects of the vitamin D metabolites should be explored further.
The strengths of this study are that we had a population of CKD patients who were clearly vitamin D-deficient at baseline, we had 100% compliance with the vitamin D supplementation, we had clear vitamin D repletion in all participants, the calcium dose used was representative of the calcium load that would be eaten during a meal, and we raised 1,25(OH)2D levels as well as 25(OH)D levels. The main limitation of this study was that we did not collect dietary information during the study. Although we did limit calcium supplementation and the participants were instructed to limit dairy foods, the participants may have been noncompliant. We had limited power to detect differences in absorption between various subgroups of patients. A larger study would be needed to find if any differences exist between subgroups of patients. Although this study was done in patients typical of our population of dialysis patients, it does not apply universally to patients on different calcium binding regimens, calcitriol, or other calcitriol analogs. Although we showed that concurrent use of doxercalciferol had no effect on the change in calcium absorption, the effects of additional vitamin D supplementation in a person receiving calcitriol may be entirely different. It has been hypothesized that both 1,25(OH)2D and 25(OH)D are needed for calcium absorption (36). Additional studies should be done on calcium absorption in the setting of calcitriol, other analogs, and other dialysate calcium concentrations. Also, our participants did not use calcium-based phosphorus binders. Although using calcium-based binders would have had a null effect on our study by eliminating the need for additional calcium and calcium absorption, the long-term effects of combining high doses of calcium based phosphorus binders and cholecalciferol supplementation are unknown.
In conclusion, patients with stage 5 CKD on hemodialysis had very low calcium absorption values at baseline, and cholecalciferol supplementation that raised 25(OH)D levels to 50 ng/ml had no effect on calcium absorption. Long-term studies on the effects of vitamin D supplementation on clinical outcomes in the dialysis population are needed.
Disclosures
None.
Acknowledgments
We thank Susan M. Dowell and Lisa Moffat for their hard work in recruiting and retaining participants and Bethanie West for editorial assistance.
This trial was registered on clinicaltrials.gov on January 17, 2011 (NCT01325610).
This work was supported by a grant from Dialysis Clinics, Inc. L.A.G.A. was supported by National Institutes of Health Grant K23 AR055542.
Dialysis Clinics, Inc. had no involvement in study design, data analysis, or interpretation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 2.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 3.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 4.Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S: Vitamin D status and mortality risk in CKD: A meta-analysis of prospective studies. Am J Kidney Dis 58: 374–382, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A: Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 5: 905–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blair D, Byham-Gray L, Lewis E, McCaffrey S: Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr 18: 375–382, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kumar VA, Kujubu DA, Sim JJ, Rasgon SA, Yang PS: Vitamin D supplementation and recombinant human erythropoietin utilization in vitamin D-deficient hemodialysis patients. J Nephrol 24: 98–105, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW: Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract 105: c132–c138, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Vergne-Marini P, Parker TF, Pak CY, Hull AR, DeLuca HF, Fordtran JS: Jejunal and ileal absorption in patients with chronic renal disease. Effect of 1alpha-hydroxycholecalciferol. J Clin Invest 57: 861–866, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peacock M: Calcium metabolism in health and disease. Clin J Am Soc Nephrol 5[Suppl 1]: S23–S30, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Armas LAG, Andukuri R, Barger-Lux J, Heaney RP, Lund R: 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol 7: 1428–1434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokmak F, Quack I, Schieren G, Sellin L, Rattensperger D, Holland-Letz T, Weiner SM, Rump LC: High-dose cholecalciferol to correct vitamin D deficiency in haemodialysis patients. Nephrol Dial Transplant 23: 4016–4020, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Jean G, Souberbielle JC, Chazot C: Monthly cholecalciferol administration in haemodialysis patients: A simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant 24: 3799–3805, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Heaney RP, Dowell MS, Hale CA, Bendich A: Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22: 142–146, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP: The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol 97: 13–19, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL: Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem 42: 586–592, 1996 [PubMed] [Google Scholar]
- 17.Heaney RP: Quantifying human calcium absorption using pharmacokinetic methods. J Nutr 133: 1224–1226, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Correcting the calcium. BMJ 1: 598, 1977 [PMC free article] [PubMed] [Google Scholar]
- 19.Heaney RP, Skillman TG: Secretion and excretion of calcium by the human gastrointestinal tract. J Lab Clin Med 64: 29–41, 1964 [PubMed] [Google Scholar]
- 20.Hou SH, Zhao J, Ellman CF, Hu J, Griffin Z, Spiegel DM, Bourdeau JE: Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 18: 217–224, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Andress DL: Bone and mineral guidelines for patients with chronic kidney disease: A call for revision. Clin J Am Soc Nephrol 3: 179–183, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Brown E, Juppner H: Parathyroid hormone: Synthesis, secretion, and action. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 6th Ed., edited by Christakos S, Holick M, Washington, DC, The American Society for Bone and Mineral Research, 2006, pp 90–98 [Google Scholar]
- 23.Heaney RP, Dowell MS, Barger-Lux MJ: Absorption of calcium as the carbonate and citrate salts, with some observations on method. Osteoporos Int 9: 19–23, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Meyer J, Fullmer CS, Wasserman RH, Komm BS, Haussler MR: Dietary restriction of calcium, phosphorus, and vitamin D elicits differential regulation of the mRNAs for avian intestinal calbindin-D28k and the 1,25-dihydroxyvitamin D3 receptor. J Bone Miner Res 7: 441–448, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Reinhardt TA, Horst RL: Parathyroid hormone down-regulates 1,25-dihydroxyvitamin D receptors (VDR) and VDR messenger ribonucleic acid in vitro and blocks homologous up-regulation of VDR in vivo. Endocrinology 127: 942–948, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Hoenderop JGJ, Nilius B, Bindels RJM: Calcium absorption across epithelia. Physiol Rev 85: 373–422, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Lund RJ, Andress DL, Amdahl M, Williams LA, Heaney RP: Differential effects of paricalcitol and calcitriol on intestinal calcium absorption in hemodialysis patients. Am J Nephrol 31: 165–170, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Coburn JW, Koppel MH, Brickman AS, Massry SG: Study of intestinal absorption of calcium in patients with renal failure. Kidney Int 3: 264–272, 1973 [DOI] [PubMed] [Google Scholar]
- 29.Kurz P, Monier-Faugere MC, Bognar B, Werner E, Roth P, Vlachojannis J, Malluche HH: Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int 46: 855–861, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Recker R, Schoenfeld P, Letteri J, Slatopolsky E, Goldsmith R, Brickman A. The efficacy of calcifediol in renal osteodystrophy. Arch Intern Med 138: 857–863, 1978 [PubMed] [Google Scholar]
- 31.Malluche H, Werner E, Ritz E: Intestinal absorption of calcium and whole-body calcium retention in incipient and advanced renal failure. Miner Electrolyte Metab 1: 263–270, 1978 [Google Scholar]
- 32.Raisz LG, Trummel CL, Holick MF, DeLuca HF: 1,25-dihydroxycholecalciferol: A potent stimulator of bone resorption in tissue culture. Science 175: 768–769, 1972 [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Garabedian M, DeLuca HF: 1,25-dihydroxycholecalciferol: Metabolite of vitamin D3 active on bone in anephric rats. Science 176: 1146–1147, 1972 [DOI] [PubMed] [Google Scholar]
- 34.Recker R, Schoenfeld P, Letteri J, Goldsmith R, Brickman AS: 25 hydroxyvitamin D (calcifediol) in renal osteodystrophy: Long term results of a six-center trial. Presented at the Fourth Workshop on Vitamin D, Berlin, West Germany, February 18–22, 1979 [Google Scholar]
- 35.Fournier A, Bordier P, Gueris J, Sebert JL, Marie P, Ferrière C, Bedrossian J, DeLuca HF: Comparison of 1 alpha-hydroxycholecalciferol and 25-hydroxycholecalciferol in the treatment of renal osteodystrophy: Greater effect of 25-hydroxycholecalciferol on bone mineralization. Kidney Int 15: 196–204, 1979 [DOI] [PubMed] [Google Scholar]
- 36.Heaney RP, Barger-Lux MJ, Dowell MS, Chen TC, Holick MF: Calcium absorptive effects of vitamin D and its major metabolites. J Clin Endocrinol Metab 82: 4111–4116, 1997 [DOI] [PubMed] [Google Scholar]


