Summary
Background and objectives
The therapy and outcome of HIV infection have dramatically changed over the last 15 years, resulting in a change in renal complications. This study analyzed the characteristics of HIV-infected patients and biopsy-proven tubulointerstitial nephropathies to define disease patterns and therapeutic implications.
Design, setting, participants, & measurements
A clinico-pathologic retrospective study of 59 consecutive renal biopsies showing predominant tubular and/or interstitial lesions in HIV-infected patients referred to the nephrology department between 1995 and 2011 was performed. HIV-associated nephropathy and vascular diseases were excluded from the study.
Results
Tubulointerstitial nephropathies accounted for 26.6% of 222 native renal biopsies performed in HIV-infected patients. Two pathologic groups were analyzed, tubulopathy and interstitial nephritis, which represented 49% and 51% of tubulointerstitial nephropathies, respectively. Most patients presented with AKI (76.3%) and high-grade proteinuria (57.7%). Drug-related nephrotoxicity was the leading cause (52.5%). Alternative etiologies included infections (15.2%), dysimmune disorders (8.5%), malignancies (3.4%), and chronic (10.2%) and acute (10.2%) tubulointerstitial nephropathies of undetermined origin. Tubulopathy was strongly associated with antiretroviral drug toxicity (75.9%) and mostly caused by tenofovir (55.2%), which was associated with proximal tubular dysfunction (87.5%), overt Fanconi’s syndrome (37.5%), and nephrogenic diabetes insipidus (12.5%). Interstitial nephritis was associated with a broader spectrum of pathologic lesions and etiologies.
Conclusions
In this series, tubulointerstitial nephropathies accounted for 26.6% of renal diseases in HIV-infected patients. Considering the therapeutic implications of diagnoses of drug toxicity, infection, and dysimmune syndromes, this study underscores the importance of monitoring renal parameters in HIV-infected patients and points to the relevance of kidney biopsy to allow an accurate diagnosis.
Introduction
Interstitial nephritis (IN) and tubulopathy are two main causes of renal disease, representing between 15% and 27% of causes of AKI in Western countries (1,2). Tubulointerstitial nephropathies (TINs) are related to a wide range of causes, including drug toxicity, infections, and autoimmune disorders (1–3). Only a few studies have investigated the characteristics of TIN in HIV-infected patients (4). Indeed, HIV-associated renal diseases have been dominated by glomerular diseases, particularly HIV-1–associated nephropathy (HIVAN) (5–7). In the mid-1990s, combinational use of antiretroviral (ARV) agents in highly active antiretroviral therapy regimen was associated with a dramatic decrease in the mortality of HIV patients and incidence of HIVAN (8–10). In the meantime, nephrotoxicity of new ARVs and renal complications of dysimmune disorders, including immune reconstitution inflammatory syndrome (IRIS) and diffuse infiltrative lymphocytosis syndrome (DILS), were reported (4,11–14). In a North American series of 262 HIV-infected patients who underwent a kidney biopsy, diagnosis of acute IN was established in 11% of renal biopsies between 1995 and 2008 (15). To determine if these findings were similar to European practice, we conducted a retrospective study of the characteristics and clinical course of HIV-infected patients with biopsy-proven TIN.
Materials and Methods
We included all consecutive HIV-infected adults who underwent a renal biopsy at Tenon Hospital (Paris, France) from January of 1995 to April of 2011 in whom the histopathological diagnosis of TIN was retained. The indications for renal biopsies were acute or chronic renal failure and/or proteinuria and/or hematuria. Patients with prominent glomerular disease or vascular nephropathy were excluded. Approval of the study was obtained from the Comité de Protection des Personnes, Ile de France.
Demographics data and comorbid conditions included age, sex, ethnicity, hypertension, and hepatitis B and hepatitis C coinfections. The following clinical data on HIV infection were collected: time since HIV infection diagnosis, Centers for Disease Control and Prevention staging, prior and current opportunistic infections and malignancies, and prior and current ARV therapy. Laboratory measurements at the time of the biopsy included baseline and peak serum creatinine, electrolytes, proteinuria-to-creatininuria ratio (UP/C), hematuria, leukocyturia, glycosuria, CD4 cell count, and plasma HIV RNA load. Estimated GFR (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease equation. CKD was defined by eGFR less than 60 ml/min per 1.73 m2 for at least 3 months. Acute renal injury was classified according to the Risk, Assessment, Failure, Loss, and End Stage Renal Disease classification (16). Proximal tubular dysfunction was defined as two signs among glycosuria, low-molecular weight proteinuria, and low phosphate serum level, one sign plus low serum potassium or uric acid level, or metabolic acidosis. Follow-up data were assessed in the 6-month period after the renal biopsy and at patients’ consultations when available. Complete recovery was defined as full recovery of baseline renal function. Patients were considered to have partial recovery if renal function improved but did not recover to its baseline value. Patients with renal function that remained stable or worsened were considered as having stable or worsening renal outcome, respectively.
Renal biopsies were blindly reviewed by three pathologists specialized in kidney pathology according to standard protocols. Causes of TIN were identified on the basis of the clinician’s diagnosis and the review of follow-up data. Causal relationship between drug and renal damage was based on (1) drug exposure at the onset of renal symptoms, (2) consistent pathologic findings, (3) absence of alternative causes, and (4) complete or partial recovery after drug withdrawal. The first three criteria had to be met to consider a causal relationship, whereas the fourth criterion was optional.
Continuous variables were compared using the Mann–Whitney test, and categorical variables were compared using the chi-squared or Fisher exact test. A value of P<0.05 was considered significant.
Results
Baseline Characteristics and Renal Parameters of 59 Patients
Among 222 renal biopsies analyzed in HIV-infected patients during the study period, 59 (26.6%) patients who showed a TIN (tubulopathy, n=29; IN, n=30) were included in the study. All patients had HIV-1 infection. Four patients were biopsied before 1997, and they all had IN. Baseline characteristics are detailed in Table 1. Median age was 45 years (range=23–85), with a men-to-women ratio of 3.2. Sixteen (27.1%) patients were black. Hepatitis B and hepatitis C coinfections were reported in 12 (20.3%) and 8 (13.6%) patients, respectively. The median time between HIV diagnosis and renal biopsy was 10 years (range=0–22); 29 (58%) patients had a CD4 cell count<200/mm3, and 27 (52.9%) patients had detectable viral replication (median=15,000 copies/ml, range=94–1×107). Thirty-seven (62.7%) patients had reached the AIDS stage. About 90% of patients received a combination of ARVs at the time of biopsy. A past history of kidney disease was reported in 19 (32.2%) patients, including stage III/IV CKD in 13 (22%) patients, pyelonephritis in 3 (5.1%) patients, and urolithiasis in 4 (6.8%; 3 related to indinavir and 1 related to atazanavir) patients. At the time of biopsy median, eGFR was 26 ml/min per 1.73 m2 (range=3–146); 40 (76.3%) patients had AKI or acute kidney failure (AKF), with 11 acute-on-chronic cases. Median UP/C was 160 mg/g (range=0–10,696), with 12 (23.1%) patients showing UP/C>2652 mg/g (Table 2). Analysis of composition of proteinuria was available for 12 of 30 (40%) patients with UP/C>1326 mg/g. LWM proteins predominantly composed proteinuria, and median albuminuria-to-proteinuria ratio was 7.3% (range=0%–33%). Hematuria and leukocyturia were detected in 16 (30.8%) and 9 (20.9%) patients, respectively.
Table 1.
Baseline characteristics of 59 HIV-infected patients and biopsy-proven tubulointerstitial nephropathies
| All Patients (n=59) | Tubulopathy (n=29, 49%) | Interstitial Nephritis (n=30, 51%) | |
|---|---|---|---|
| Median age, yr (range) | 45 (23–85) | 44 (23–85) | 45.5 (29–67) |
| Men, n (%) | 45 (76.3) | 22 (75.8) | 23 (76.7) |
| Black, n (%) | 16 (27.1) | 6 (20.7) | 10 (33.3) |
| AIDS stage, n (%) | 37 (62.7) | 20 (69) | 17 (56.7) |
| Median CD4 count, cell/µL (range) | 178.5 (1–974) | 169 (1–729) | 205 (6–974) |
| CD4 count<200/mm3, n (%) | 29 (58) | 18 (64.3) | 11 (50) |
| Detectable HIV viral load, n (%) | 27 (52.9) | 15 (55.6) | 12 (50) |
| Median, cp/ml (range) | 15.103 (94–10.106) | 8.103 (200–10.106) | 21.103 (94–39.104) |
| Opportunistic infections, n (%) | 37 (62.7) | 20 (69) | 17 (56.7) |
| Tuberculosis | 11 (29.7) | 4 (20) | 7 (41.2) |
| Atypical mycobacterial infection | 7 (18.9) | 3 (15) | 4 (23.5) |
| CMV disease | 17 (45.9) | 11 (55) | 6 (35.3) |
| Pneumocystosis | 8 (21.6) | 6 (30) | 2 (11.8) |
| Toxoplasmosis | 3 (8.1) | 3 (15) | — |
| Esophageal candidiasis | 3 (8.1) | 2 (10) | 1 (5.9) |
| Other | 7 (18.9) | 6 (30) | 1 (5.9) |
| Malignancies, n (%) | |||
| Kaposi sarcoma | 5 (8.5) | 3 (10.3) | 2 (6.7) |
| Multicentric Castleman disease | 1 (1.7) | — | 1 (3.3) |
| Lymphoma | 2 (3.4) | 1 (3.4) | 1 (3.3) |
| Coinfection, n (%) | |||
| Hepatitis B | 12 (20.3) | 7 (24.1) | 5 (16.7) |
| Hepatitis C | 8 (13.6) | 4 (13.8) | 4 (13.3) |
| Exposure to ARV therapy, n (%) | 50 (89.3) | 27 (93.1) | 23 (85.2) |
| Ritonavir | 37 (75.5) | 24 (88.9) | 13 (59.1) |
| Lamivudine | 33 (67.3) | 17 (63) | 16 (72.7) |
| Tenofovir | 29 (59.2) | 21 (77.8) | 8 (36.4) |
| Abacavir | 18 (36.7) | 9 (33.3) | 9 (40.9) |
| Lopinavir | 15 (30.6) | 7 (25.9) | 8 (36.4) |
| Zidovudine | 11 (22.4) | 6 (22.2) | 5 (22.7) |
| Atazanavir | 9 (18.4) | 7 (25.9) | 2 (9.1) |
| Indinavir | 8 (16.3) | 3 (11.1) | 5 (22.7) |
| Stavudine | 5 (10.2) | 3 (11.1) | 2 (9.1) |
| Didanosine | 5 (10.2) | 4 (14.8) | 1 (4.5) |
CMV, cytomegalovirus; ARV, antiretroviral.
Table 2.
Renal parameters at presentation and outcome at last follow-up
| All Patients (n=59) | Tubulopathy (n=29) | Interstitial Nephritis (n=30) | |
|---|---|---|---|
| Renal parameters at kidney biopsy | |||
| Hypertension, n (%) | 12 (24.5) | 6 (24) | 6 (25) |
| Median peak sCr, mg/dl (range) | 2.56 (0.57–16.33) | 2.71 (0.84–16.33) | 2.49 (0.57–13.8) |
| Median estimated GFR, ml/min per 1.73 m2 (range) | 26 (3–146) | 25 (3–94) | 29 (4–146) |
| Acute renal dysfunction, n (%) | |||
| Risk | 7 (11.8) | 3 (10.3) | 4 (13.3) |
| AKI | 26 (44) | 9 (31) | 17 (56.7) |
| AKF | 19 (32.2) | 14 (48.3) | 5 (16.7) |
| Acute-on-chronic renal failure, n (%) | 11 (18.6) | 6 (20.7) | 5 (16.7) |
| Renal replacement therapy, n (%) | 12 (20.3) | 9 (31) | 3 (10) |
| Median Pu/Cu, mg/g (range) | 1414 (0–10,696) | 1630 (0–10,696) | 1330 (0–6541) |
| Pu/Cu ratio, mg/g, n (%) | |||
| <442 | 9 (17.3) | 4 (16.7) | 5 (17.9) |
| 442–1326 | 13 (25) | 5 (20.8) | 8 (28.5) |
| 1326–2652 | 18 (34.6) | 8 (33.3) | 10 (35.7) |
| ≥2652 | 12 (23.1) | 7 (29.2) | 5 (17.9) |
| Hematuria, n (%) | 16 (30.8) | 5 (20) | 11 (40.7) |
| Leukocyturia, n (%) | 9 (20.9) | 2 (9.1) | 7 (33.3) |
| Renal outcome at last follow-up | |||
| Available renal outcome, n (%) | 44 (74.6) | 21 (72.4) | 23 (76.7) |
| Median follow-up time, mo (range) | 9.5 (0.5–156) | 8 (1–132) | 12 (0.5–156) |
| Complete recovery, n (%) | 19 (43.2) | 6 (28.6) | 13 (56.5) |
| Stage III–V CKD, n (%) | 24 (54.5) | 15 (71.4) | 9 (39.1) |
| ESRD | 3 (6.8) | 3 (14.3) | 0 (0) |
| Death, n (%) | 5 (8.5) | 4 (13.7) | 1 (3.3) |
sCr, serum creatinine level; AKF, acute kidney failure; Pu/Cu, proteinuria-to-creatininuria ratio.
Renal Biopsy Findings
Kidney biopsies were divided into two groups: 29 (49%) patients showed tubulopathy, and 30 (51%) patients displayed acute or chronic IN. Baseline characteristics did not differ between the two groups, except that tenofovir and ritonavir exposures were more frequently observed in the tubulopathy group (P=0.005 and P=0.02, respectively). Drug-related nephrotoxicity accounted for 52.5% of overall cases of renal injury. For the remaining 47.5% cases, etiologies included infections (15.2%), dysimmune disorders (8.5%), malignancies (3.4%), chronic TIN (10.2%), and acute TIN of undetermined origin (10.2%) (Table 3).
Table 3.
Etiologies of tubular and interstitial nephropathies in 59 HIV-infected patients
| All Patients (n=59) | Tubulopathy (n=29) | Interstitial Nephritis (n=30) | |
|---|---|---|---|
| Drugs, n (%) | 31 (52.5) | 23 (79.3): tenofovir, 16; cidofovir, 2; didanosine, 1; vancomycin, 1; rifampicin/IoCM, 1; amphotericin b, 1; metformin/tenofovir, 1 | 8 (26.7): indinavir, 2; foscarnet, 2; abacavir, 1; NSAIDs, 2; cotrimoxazol, 1 |
| Infections, n (%) | 9 (15.2) | 4 (13.8): sepsis shock (2 with tenofovir) | 5 (16.7): MAC, 3; tuberculosis, 1; hantavirus, 1 |
| Dysimmune syndromes, n (%) | 5 (8.5) | — | 5 (16.7): DILS, 3; IRIS, 2 |
| Malignancies, n (%) | 2 (3.4) | 1 (3.4) IPL | 1 (3.3) MCD |
| ATIN of undetermined origin, n (%) | 6 (10.2) | 1 (3.4) | 5 (16.7) |
| CTIN, n (%) | 6 (10.2) | — | 6 (20) |
IoCM, iodinated contrast media; NSAIDs, nonsteroidal anti-inflammatory drugs; MAC, Mycobacterium avium Complex; DILS, diffuse infiltrative lymphocytosis syndrome; IRIS, immune reconstitution inflammatory syndrome; IPL, immunoblastic plasmacytoid lymphoma; MCD, multicentric Castleman disease; ATIN, acute tubulointerstitial nephropathy; CTIN, chronic tubulointerstitial nephropathy.
Tubulopathy
Baseline Characteristics.
Summary and detailed characteristics of 29 patients with tubulopathy are shown in Table 2 and Supplemental Table 1, respectively. Twenty-three (79.3%) patients had AKI or AKF, and nine (31%) patients required renal replacement therapy (RRT). Fourteen patients with acute tubular necrosis (ATN) lesions had a lower median GFR (11.5 versus 44 ml/min per 1.73 m2, P=0.001) at presentation and required more frequent RRT (50% versus 13.3%, P=0.05) compared with patients without ATN (n=15). Although median eGFR was comparable with patients with IN, a higher proportion of patients with tubulopathy showed AKF and required RRT at presentation (P=0.02 and P=0.09, respectively). Fifteen (62.5%) patients had a UP/C>1326 mg/g. Proximal tubular dysfunction was recorded in 21 (72%) patients, with 7 patients presenting with complete Fanconi’s syndrome. Three patients displayed nephrogenic diabetes insipidus.
Biopsy Findings.
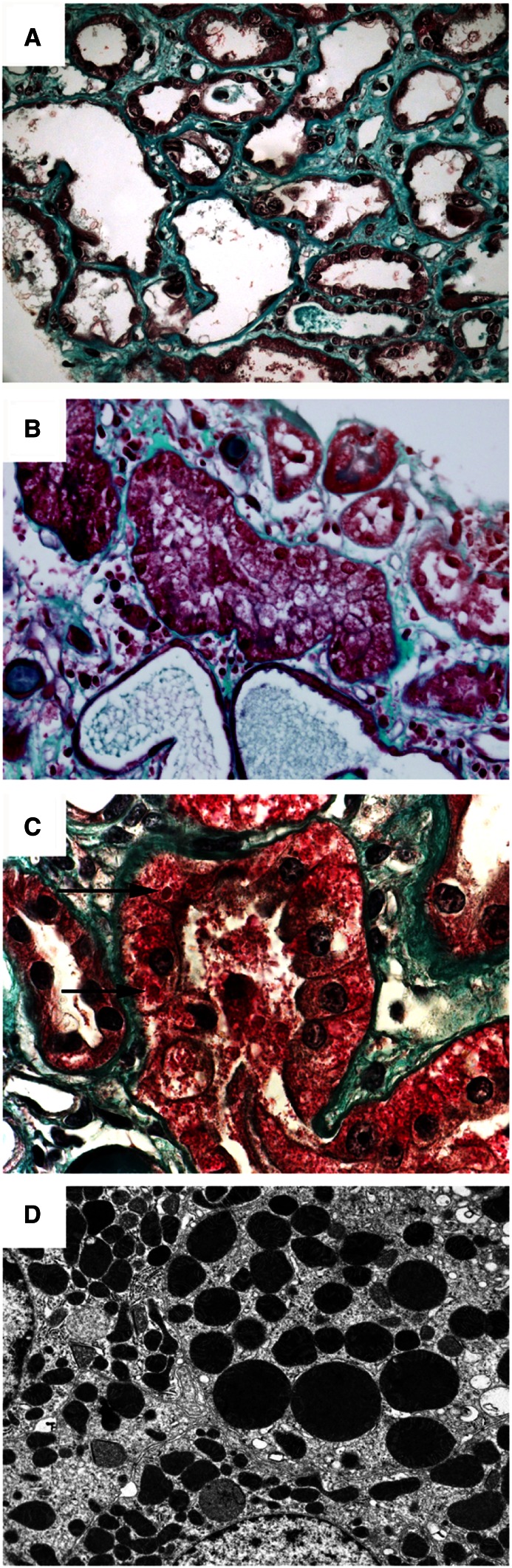
Renal biopsy specimens showed acute tubular injury of varying intensity. Fifteen patients displayed mild to moderate tubular damage that predominated on proximal tubules without ATN. These lesions included cytoplasmic vacuolization and focal loss of brush border but no tubular basement membrane denudation. Fourteen patients had ATN consisting of flattened and ragged tubules with luminal ectasia, cellular casts, diffuse loss of brush border, and tubular basement membrane denudation. Nuclear atypia (dystrophic nuclei and prominent nucleoli) were seen, mostly in patients with ATN. No tubular microcystic dilation, epithelial cell hypertrophy or hyperplasia, or mitotic figures, as usually seen in HIVAN, were observed. The interstitium was focally edematous, with only rare inflammatory cells. Interstitial fibrosis was unremarkable or mild. Vessels and glomeruli were normal, except for four patients with mild to moderate nephroangiosclerosis lesions and one patient with mild diabetic nephropathy. In three patients exposed to tenofovir, eosinophilic cytoplasmic inclusions of proximal tubular cells were associated with ATN (Figure 1, A–C). Electron microscopy was performed in three other tenofovir-treated patients but without tubular cytoplasmic inclusions by light microscopy, and it disclosed dysmorphic, and sometimes abnormally enlarged, mitochondria in proximal tubular cells (Figure 1D).
Figure 1.
Light microscopic and ultrastructural findings in patients with tubulopathy related to tenofovir nephrotoxicity. (A) Toxic acute tubular necrosis affecting the proximal tubules that associated loss of brush border, cytoplasmic reduction, luminal enlargement of tubular sections, epithelial desquamation, and interstitial edema and fibrosis (trichrome stain, ×400). (B) A higher-power view showing swelling and vacuolization of proximal tubular cell cytosol (trichrome stain, ×600). (C) Presence of red intracytoplasmic inclusions (arrows) in proximal tubular epithelial cells (trichrome stain, ×1000). (D) Ultrastructural examination showing abnormally enlarged and dysmorphic mitochondria within a proximal tubular epithelial cell. Original magnification, ×8000.
Etiologic Diagnosis.
Drug-related nephrotoxicity accounted for 79.3% of tubulopathy cases, whereas it represented 26.7% of IN cases (P<0.001) (Table 3). A single culprit agent was identified in 23 cases. Tenofovir accounted for the main offending agent (16 patients, 69.5%). The remaining seven cases were caused by other antiviral drugs (two cidofovir cases and one didanosine case), antimicrobial agents (one vancomycin case, one rifampicin case, and one amphotericin B case), or metformin in addition to tenofovir (one case). ATN caused by septic shock was identified in four patients. Two of them concomitantly received tenofovir and showed proximal tubular dysfunction. One patient presented with duodenal immunoblastic plasmacytoid lymphoma responsible for myeloma cast nephropathy.
Outcome.
Renal follow-up data were available in 21 (72.4%) patients, with a median follow-up of 8 months (range=1–132). Complete recovery of renal function was observed in 6 (28.6%) patients, and stage III–V CKD was seen in 15 (71.4%) patients, with 3 (14.3%) patients requiring long-term dialysis. Four (16%) patients, all presenting with ATN, died within the first 1 month after kidney biopsy (three patients from sepsis and one patient from hematologic malignancy).
Interstitial Nephritis
Baseline Characteristics.
Summary and detailed characteristics of 30 patients with IN are shown in Table 2 and Supplemental Table 2, respectively. Six patients showed acute fever at presentation without skin rash or eosinophilia. Twenty-two (73.3%) patients presented with AKI or AKF. Three (10%) patients required RRT at presentation. Fifteen (53.6%) patients had a UP/C>1326 mg/g. Hematuria and leukocyturia were observed in 40.7% and 33.3% of patients, respectively.
Biopsy Findings and Etiologic Diagnosis.
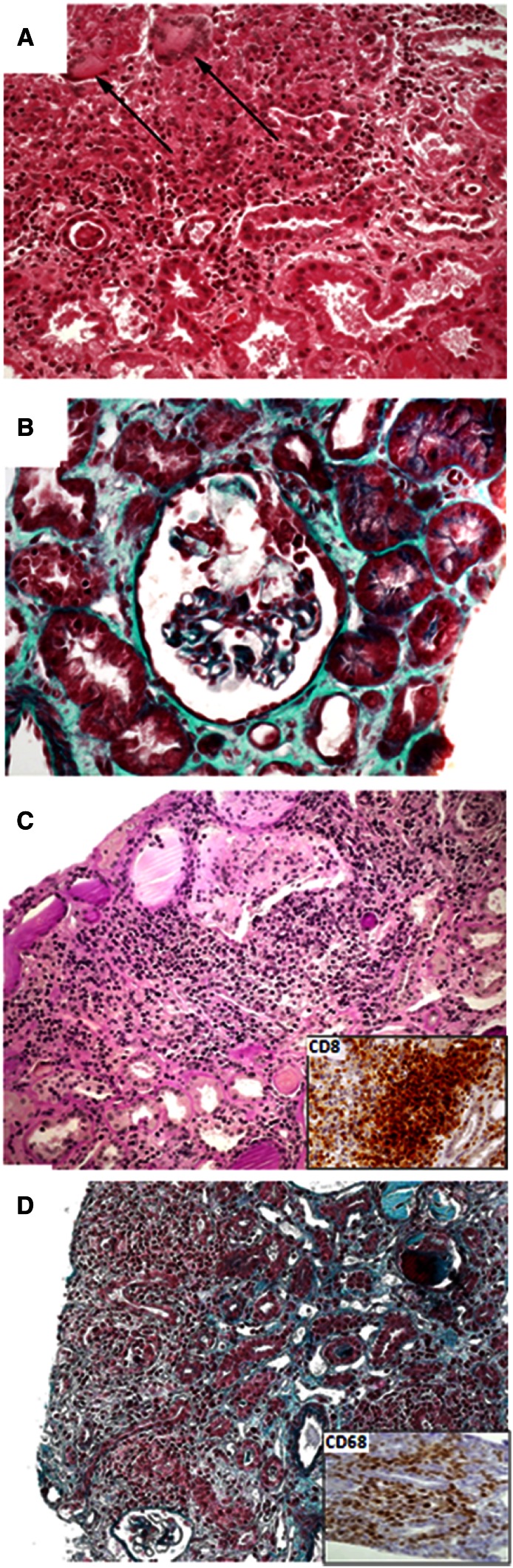
Six (20%) patients presented with granulomatous IN related to Mycobacterium avium complex (three patients), M. tuberculosis (one patient), and undetermined origin (two patients) (Figure 2A). Twenty-four (80%) patients had nongranulomatous IN. Drug-related IN was associated with crystal nephropathy and intratubular precipitation in four patients caused by indinavir or foscarnet (Figure 2B), whereas immunoallergic IN was observed in four patients related to nonsteroidal anti-inflammatory drugs (NSAIDs), abacavir, or cotrimoxazol. DILS was diagnosed in three patients. The kidney biopsy specimen showed marked infiltration by mononuclear cells, essentially CD8+ lymphocytes, with tubulitis (Figure 2C). Two patients with active tuberculosis infection displayed IRIS. They presented with systemic inflammatory symptoms, AKI, bilateral kidney enlargement, and dense interstitial infiltrate composed predominantly of CD68+ macrophages (Figure 2D). In both cases, IRIS occurred a few weeks after antituberculosis and ARV initiation. The patients were treated with steroids and antimicrobial therapy, leading to an improvement of the renal function. Two patients presented with IN related to multicentric Castleman disease and Hantavirus infection, respectively. Finally, three patients presented with acute IN of undetermined origin, and six patients displayed chronic TIN. In these cases, no etiologic diagnosis was established. Particularly, we did not observe prominent plasma cell interstitial infiltrates as recognized in the interstitial disease related to HIVAN. However, causative factors could be suspected on retrospective chart review, including a past history of sepsis shock-related ATN, recurrent indinavir-related renal colic, administration of foscarnet a few months before kidney biopsy, and tenofovir-induced AKI.
Figure 2.
Light microscopic findings in patients with interstitial nephritis. (A) Granulomatous interstitial nephritis associated with tuberculosis characterized by epithelioid granulomas with multinucleated giant cells (arrows) and necrosis (hematoxylin and eosin, ×200). (B) Foscarnet-related crystal nephropathy. The glomerular tuft is partially obliterated by clear foscarnet crystals (trichrome stain, ×400). Some crystals were also seen within tubular lumens, and the renal interstitium showed mild to moderate interstitial fibrosis and tubular atrophy. (C) Interstitial nephritis associated with diffuse infiltrative lymphocytosis syndrome characterized by dense cellular infiltrates composed of lymphocytes, monocytes, and plasma cells (hematoxylin and eosin, ×200). Immunophenotyping analysis shows that lymphocytes were mainly CD3+ and CD8+ T cells (Inset, ×200). (D) Interstitial nephritis related to immune reconstitution inflammatory syndrome (trichrome stain, ×200). The interstitial inflammatory infiltrate was mainly composed by CD68+ macrophages (Inset, ×400).
Outcome.
Renal follow-up data were available in 23 (80%) patients, with a median follow-up of 12 months (range=0.5–156). Renal function returned to baseline in 13 (56.5%) patients. Dialysis was discontinued in three patients who initially required RRT. Nine (39.1%) patients developed stage III–V CKD. One additional patient died shortly after kidney biopsy.
Discussion
During the last 15 years, 59 of 222 (26.6%) HIV-infected patients who underwent a kidney biopsy at our institution showed either prominent tubular (13.1%) or interstitial (13.5%) lesions. These data are consistent with the previously reported frequencies of IN (11%–20%) and biopsy-proven ATN (10%–16.7%) in this population, respectively (4,11,15,17–19). Compared with HIV-infected patients with histopathological diagnosis of glomerular disease established between 1995 and 2007 (10), TIN patients were less frequently of black origin (27% versus 63%), had a better virological control (undetectable viral load: 47% versus 28%), and had a higher percentage of received ARV therapy (89.3% versus 73%). None of the patients with drug-induced IN displayed the classic triad of fever, skin rash, and eosinophilia, which was previously underlined in the series of Parkhie et al. (15). Strikingly, the high-grade proteinuria observed in some patients is unusual for TIN. However, this finding was also pointed out in studies by Parkhie et al. (15) and Herlitz et al. (20), which reported 29 HIV-infected patients with acute IN and 13 HIV patients with tenofovir-induced nephropathy, respectively (15,20). In the series by Parkhie et al. (15), median proteinuria was 2.2 g/d (range=0.1–12.9), with 65.5% of patients presenting with proteinuria>1.5 g/d and 31% of patients presenting with proteinuria>3.5 g/d (15). Median level of proteinuria was 1.5 g/d (range=1.2–2.1) in patients with tenofovir-induced nephropathy (20).
Drug toxicity accounted for nearly 80% of tubulopathy cases. ARV-related tubular toxicity was usually observed with a nucleotide analog reverse transcription inhibitor, mainly tenofovir. Tenofovir may have contributed to ATN in three additional patients presenting with septic shock (two patients) and in conjunction with metformin (one patient), because they all displayed proximal tubular dysfunction. Tenofovir has gained widespread use for the treatment of HIV-1 and hepatitis B infections on the basis of its efficacy and tolerability (21–24). Phase III studies showed excellent renal safety, but cases of tenofovir-related nephrotoxicity were first reported in 2002 (12,25). Proximal tubular dysfunction was then further characterized, and sometimes, overt Fanconi syndrome with renal failure was characterized (19,25–32). In our study, almost 90% and 40% of patients with tenofovir nephrotoxicity displayed proximal tubular dysfunction and overt Fanconi’s syndrome, respectively. Similar findings were noted in patients with cidofovir- and didanosine-related tubulopathy. Most of the patients had at least one risk factor for tenofovir nephrotoxicity: 50% of patients were more than 50 years old, 50% of patients had baseline eGFR under 90 ml/min per 1.73 m2, 25% of patients had stage III/IV CKD, and all but one patients also received ritonavir-boosted ARV regimen (31). Recently, the Columbia group reported pathologic findings in 13 HIV-infected patients with tenofovir nephrotoxicity (20,30). Histologic pattern associated toxic ATN and eosinophilic intracytoplasmic inclusions that corresponded with giant mitochondria within proximal cells (20). Dysmorphic mitochondria were also observed by electron microscopy (20). Similar intracytoplasmic inclusions were observed in 18.8% of our cases of tenofovir-related tubulopathy (n=3), and ultrastructural analysis performed in three biopsy specimens disclosed dysmorphic and giant mitochondria. Proximal tubular dysfunction usually improved after tenofovir withdrawal. However, only 40% of patients achieved complete renal recovery as previously reported (20,31,33). The IN group was associated with a broader spectrum of pathologic lesions and causes. Compared with the tubulopathy group, IN patients tended to have a better renal and overall outcome, maybe because of a less severe renal dysfunction at presentation, although differences in eGFR were not statistically significant. Drug toxicity was a significant cause of IN. Antiviral therapy, including indinavir and foscarnet, was associated with crystal nephropathy (34–37). No case of atazanavir-related IN was identified in contrast to recent reports (38,39). Classic forms of acute IN were also observed with NSAIDs and cotrimoxazol. Nevertheless, drug-related IN only accounted for 26.7% of cases compared with 72% of acute IN cases in the Johns Hopkins’ series (15). Antiviral agents were involved in 62.5% of drug-induced IN in our series, whereas NSAIDs were the main culprits in the Johns Hopkins’ series (15). Such discrepancy may be explained by over-the-counter availability of NSAIDs in the United States and specific demographic characteristics, with predominance of black race (79%), high prevalence of hepatitis C coinfection (62%), and low rate of ARV therapy (approximately 50%) in North American patients (15). In our study, only 27.1% of patients were of black origin, the rate of hepatitis C virus infection was 13.6%, and about 90% of patients received ARV.
We found that mycobacterial infections remained an important cause of granulomatous IN. We also identified IRIS and DILS as specific causes of acute IN. IRIS is related to rapid immune restoration after ARV initiation in patients with a previously treated or subclinical opportunistic infection (39–43). It manifests as systemic inflammation and various organ involvement, including kidneys (40–43). Tissue infiltrates are predominantly composed of CD68+ macrophages. DILS usually associates CD8+ lymphocytosis and CD8+ lymphocytic visceral infiltration, predominantly affecting the salivary glands, lungs, and less frequently, other organs, including the kidney in less than 10% of cases (44–48). HIV patients with IRIS or DILS respond to corticosteroid therapy (49,50). In about one third of cases of acute or chronic IN, no definitive etiology could be established, although some of the patients presented past exposition to cumulative risk factors for developing chronic interstitial lesions. Of note, recent studies suggested that HIVAN might present with subtle glomerular changes (i.e., podocyte hypertrophy and hyperplasia) and prominent tubulointerstitial lesions with tubular microcysts and plasma cell interstitial infiltration (51–53). Such findings were not observed in our series.
Our study has some limitations. First, the prevalence of TIN in the whole HIV population is underestimated, because the study was only based on biopsy-proven cases. Although the indications for kidney biopsy included acute or chronic renal failure and/or persistent proteinuria and/or hematuria, patients with mild deterioration of renal function and/or mild proteinuria were unlikely to be biopsied. Additionally, only patients with a main pathologic diagnosis of TIN were included, whereas patients with TIN lesions secondary to glomerular or vascular disease were not analyzed. The high percentage of proximal tubular dysfunction among patients with tenofovir toxicity may also represent a referral bias. Finally, patients were referred from two Infectious Disease Units that are not representative of the European HIV cohorts because of a higher proportion of black patients.
In summary, we report a series of French HIV-infected patients, focusing on biopsy-proven tubulopathy and IN over the past 15 years. Both pathologic entities are closely associated with specific etiologies. Drug-related nephrotoxicity, mostly caused by ARV therapy, is the leading cause of TIN, underscoring the importance of a careful renal monitoring in HIV-infected patients under ARV therapy. Tenofovir accounts for the main cause of tubulopathy. Because proximal tubular dysfunction is usually observed in cases of tenofovir nephrotoxicity, renal biopsy may be valuable in patients with atypical presentation, particular clinical characteristics (i.e., hepatitis B coinfection and limited ARV alternatives), or lack of recovery after tenofovir withdrawal. In IN, renal pathologic analysis and immunophenotyping of inflammatory infiltrates are useful to recognize granulomatous forms and kidney involvement related to DILS or IRIS. Given the wide range of renal lesions and atypical presentation, kidney biopsy remains a key step in the accurate diagnosis of TIN in HIV-infected patients.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10051012/-/DCSupplemental.
References
- 1.Baker RJ, Pusey CD: The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 19: 8–11, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Praga M, González E: Acute interstitial nephritis. Kidney Int 77: 956–961, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Davison AM, Jones CH: Acute interstitial nephritis in the elderly: A report from the UK MRC Glomerulonephritis Register and a review of the literature. Nephrol Dial Transplant 13[Suppl 7]: 12–16, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Wyatt CM, Morgello S, Katz-Malamed R, Wei C, Klotman ME, Klotman PE, D’Agati VD: The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int 75: 428–434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, Friedman EA: Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 310: 669–673, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Gardenswartz MH, Lerner CW, Seligson GR, Zabetakis PM, Rotterdam H, Tapper ML, Michelis MF, Bruno MS: Renal disease in patients with AIDS: A clinicopathologic study. Clin Nephrol 21: 197–204, 1984 [PubMed] [Google Scholar]
- 7.Pardo V, Aldana M, Colton RM, Fischl MA, Jaffe D, Moskowitz L, Hensley GT, Bourgoignie JJ: Glomerular lesions in the acquired immunodeficiency syndrome. Ann Intern Med 101: 429–434, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Rachakonda AK, Kimmel PL: CKD in HIV-infected patients other than HIV-associated nephropathy. Adv Chronic Kidney Dis 17: 83–93, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD: Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: A 12-year cohort study. AIDS 18: 541–546, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Lescure FX, Flateau C, Pacanowski J, Brocheriou I, Rondeau E, Girard PM, Ronco P, Pialoux G, Plaisier E: HIV-associated kidney glomerular diseases: Changes with time and HAART. Nephrol Dial Transplant 27: 2349–2355, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Berliner AR, Fine DM, Lucas GM, Rahman MH, Racusen LC, Scheel PJ, Atta MG: Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol 28: 478–486, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Coca S, Perazella MA: Rapid communication: Acute renal failure associated with tenofovir: Evidence of drug-induced nephrotoxicity. Am J Med Sci 324: 342–344, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Izzedine H, Harris M, Perazella MA: The nephrotoxic effects of HAART. Nat Rev Nephrol 5: 563–573, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Atta MG, Deray G, Lucas GM: Antiretroviral nephrotoxicities. Semin Nephrol 28: 563–575, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Parkhie SM, Fine DM, Lucas GM, Atta MG: Characteristics of patients with HIV and biopsy-proven acute interstitial nephritis. Clin J Am Soc Nephrol 5: 798–804, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute Dialysis Quality Initiative Workgroup: Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs. Presented at The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed]
- 17.Nochy D, Glotz D, Dosquet P, Pruna A, Guettier C, Weiss L, Hinglais N, Idatte JM, Méry JP, Kazatchkine M. Druet P, Bariéty J: Renal disease associated with HIV infection: A multicentric study of 60 patients from Paris hospitals. Nephrol Dial Transplant 8: 11–19, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Peraldi MN, Maslo C, Akposso K, Mougenot B, Rondeau E, Sraer JD: Acute renal failure in the course of HIV infection: A single-institution retrospective study of ninety-two patients anad sixty renal biopsies. Nephrol Dial Transplant 14: 1578–1585, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, Appel R, Fields TA, Svetkey LP, Flanagan KH, Klotman PE, Winston JA: The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 66: 1145–1152, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS: Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 78: 1171–1177, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Jiménez-Nácher I, García B, Barreiro P, Rodriguez-Novoa S, Morello J, González-Lahoz J, de Mendoza C, Soriano V: Trends in the prescription of antiretroviral drugs and impact on plasma HIV-RNA measurements. J Antimicrob Chemother 62: 816–822, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, Lazzarin A, Schewe K, Lange J, Wyatt C, Curtis S, Chen SS, Smith S, Bischofberger N, Rooney JF: The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: The first 4 years. AIDS 21: 1273–1281, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M: Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 51: 496–505, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Izzedine H, Hulot JS, Vittecoq D, Gallant JE, Staszewski S, Launay-Vacher V, Cheng A, Deray G, Study 903 Team : Long-term renal safety of tenofovir disoproxil fumarate in antiretroviral-naive HIV-1-infected patients. Data from a double-blind randomized active-controlled multicentre study. Nephrol Dial Transplant 20: 743–746, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, Ronco P, Rossert J: Fanconi syndrome and renal failure induced by tenofovir: A first case report. Am J Kidney Dis 40: 1331–1333, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Rifkin BS, Perazella MA: Tenofovir-associated nephrotoxicity: Fanconi syndrome and renal failure. Am J Med 117: 282–284, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Izzedine H, Isnard-Bagnis C, Hulot JS, Vittecoq D, Cheng A, Jais CK, Launay-Vacher V, Deray G: Renal safety of tenofovir in HIV treatment-experienced patients. AIDS 18: 1074–1076, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Créput C, Gonzalez-Canali G, Hill G, Piketty C, Kazatchkine M, Nochy D: Renal lesions in HIV-1-positive patient treated with tenofovir. AIDS 17: 935–937, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Karras A, Lafaurie M, Furco A, Bourgarit A, Droz D, Sereni D, Legendre C, Martinez F, Molina JM: Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: Three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis 36: 1070–1073, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Perazella MA: Tenofovir-induced kidney disease: An acquired renal tubular mitochondriopathy. Kidney Int 78: 1060–1063, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Hall AM, Hendry BM, Nitsch D, Connolly JO: Tenofovir-associated kidney toxicity in HIV-infected patients: A review of the evidence. Am J Kidney Dis 57: 773–780, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Woodward CL, Hall AM, Williams IG, Madge S, Copas A, Nair D, Edwards SG, Johnson MA, Connolly JO: Tenofovir-associated renal and bone toxicity. HIV Med 10: 482–487, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G: Tenofovir-associated acute and chronic kidney disease: A case of multiple drug interactions. Clin Infect Dis 42: 283–290, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Said SM, Nasr SH, Samsa R, Markowitz GS, D’Agati VD: Nephrotoxicity of antiretroviral therapy in an HIV-infected patient. Kidney Int 71: 1071–1075, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Beaufils H, Deray G, Katlama C, Dohin E, Henin D, Sazdovitch V, Jouanneau C: Foscarnet and crystals in glomerular capillary lumens. Lancet 336: 755, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Maurice-Estepa L, Daudon M, Katlama C, Jouanneau C, Sazdovitch V, Lacour B, Beaufils H: Identification of crystals in kidneys of AIDS patients treated with foscarnet. Am J Kidney Dis 32: 392–400, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Daudon M, Estépa L, Viard JP, Joly D, Jungers P: Urinary stones in HIV-1-positive patients treated with indinavir. Lancet 349: 1294–1295, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Brewster UC, Perazella MA: Acute interstitial nephritis associated with atazanavir, a new protease inhibitor. Am J Kidney Dis 44: e81–e84, 2004 [PubMed] [Google Scholar]
- 39.Izzedine H, M’rad MB, Bardier A, Daudon M, Salmon D: Atazanavir crystal nephropathy. AIDS 21: 2357–2358, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, Musher DW, Gathe JC, Jr, Visnegarwala F, Trautner BW: Immune reconstitution inflammatory syndrome: Emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 81: 213–227, 2002 [DOI] [PubMed] [Google Scholar]
- 41.French MA, Price P, Stone SF: Immune restoration disease after antiretroviral therapy. AIDS 18: 1615–1627, 2004 [DOI] [PubMed] [Google Scholar]
- 42.French MA: HIV/AIDS: Immune reconstitution inflammatory syndrome: A reappraisal. Clin Infect Dis 48: 101–107, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, IeDEA Southern and Central Africa : Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: A systematic review and meta-analysis. Lancet Infect Dis 10: 251–261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solal-Celigny P, Couderc LJ, Herman D, Herve P, Schaffar-Deshayes L, Brun-Vezinet F, Tricot G, Clauvel JP: Lymphoid interstitial pneumonitis in acquired immunodeficiency syndrome-related complex. Am Rev Respir Dis 131: 956–960, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Itescu S, Brancato LJ, Winchester R: A sicca syndrome in HIV infection: Association with HLA-DR5 and CD8 lymphocytosis. Lancet 2: 466–468, 1989 [DOI] [PubMed] [Google Scholar]
- 46.Itescu S, Winchester R: Diffuse infiltrative lymphocytosis syndrome: A disorder occurring in human immunodeficiency virus-1 infection that may present as a sicca syndrome. Rheum Dis Clin North Am 18: 683–697, 1992 [PubMed] [Google Scholar]
- 47.Williams FM, Cohen PR, Jumshyd J, Reveille JD: Prevalence of the diffuse infiltrative lymphocytosis syndrome among human immunodeficiency virus type 1-positive outpatients. Arthritis Rheum 41: 863–868, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Basu D, Williams FM, Ahn CW, Reveille JD: Changing spectrum of the diffuse infiltrative lymphocytosis syndrome. Arthritis Rheum 55: 466–472, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q: Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 362: 697–706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, Gengiah T, Gengiah S, Naidoo A, Jithoo N, Nair G, El-Sadr WM, Friedland G, Abdool Karim Q: Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 365: 1492–1501, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wearne N, Swanepoel CR, Boulle A, Duffield MS, Rayner BL: The spectrum of renal histologies seen in HIV with outcomes, prognostic indicators and clinical correlations. Nephrol Dial Transplant 27: 4109–4118, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Arendse C, Okpechi I, Swanepoel C: Acute dialysis in HIV-positive patients in Cape Town, South Africa. Nephrology (Carlton) 16: 39–44, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Swanepoel CR, Wearne N, Duffield MS, Okpechi IG: The evolution of our knowledge of HIV-associated kidney disease in Africa. Am J Kidney Dis 60: 668–678, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.