Abstract
Some of the different currently applied approaches that correct presbyopia may reduce stereovision. In this work, stereo-acuity was measured for two methods: (1) monovision and (2) small aperture inlay in one eye. When performing the experiment, a prototype of a binocular adaptive optics vision analyzer was employed. The system allowed simultaneous measurement and manipulation of the optics in both eyes of a subject. The apparatus incorporated two programmable spatial light modulators: one phase-only device using liquid crystal on silicon technology for wavefront manipulation and one intensity modulator for controlling the exit pupils. The prototype was also equipped with a stimulus generator for creating retinal disparity based on two micro-displays. The three-needle test was programmed for characterizing stereo-acuity. Subjects underwent a two-alternative forced-choice test. The following cases were tested for the stimulus placed at distance: (a) natural vision; (b) 1.5 D monovision; (c) 0.75 D monovision; (d) natural vision and small pupil; (e) 0.75 D monovision and small pupil. In all cases the standard pupil diameter was 4 mm and the small pupil diameter was 1.6 mm. The use of a small aperture significantly reduced the negative impact of monovision on stereopsis. The results of the experiment suggest that combining micro-monovision with a small aperture, which is currently being implemented as a corneal inlay, can yield values of stereoacuity close to those attained under normal binocular vision.
OCIS codes: (010.1080) Active or adaptive optics, (330.1400) Vision - binocular and stereopsis
1. Introduction
Presbyopia, causing the loss of accommodation, is a significant degradation of vision associated to the aging eye. The relevance of its correction is enormous from different perspectives. There are many approaches for correcting presbyopia or reducing its effects on the quality of vision. A possible option is monovision, which consists of setting the refraction of one eye for far vision while the other eye is corrected for near vision [1]. The technique can be incorporated by using dedicated contact lenses of different power, using intraocular lenses calculated for different distances, or by appropriately shaping each cornea in laser refractive surgery. A novel solution is based on the combination of micro-monovision with a small aperture in the eye selected for near vision. The practical implementation of the small aperture consists in the implantation of an intracorneal inlay [2,3]. The latter is essentially an opaque ring of 3.8 mm of outer diameter with a central circular pupil of 1.6 mm of diameter (Kamra, Acufocus Inc., Irvine, USA) and 0.01 mm of thickness. The principle of operation of the implant consists in the effective increase of the depth of focus in the eye, therefore allowing the perception of reasonably sharp images for different distances with no need of a change in power. The inlay is made of biocompatible material and remains steady after implantation, which has been reported in long-term studies [4].
A possible drawback associated with the use of the corneal inlay is the impairment of vision under low-light-level conditions. The implanted eye’s natural pupil is expected to dilate in response to low levels of illumination, producing an annular-shaped aperture in the nondominant eye when the pupil size exceeds the opaque ring outer diameter. In spite of these expected problems when using the implant out of photopic conditions, some studies report good performance under mesopic illumination [5]. The binocular nature of vision is surely responsible for the results in moderate and low levels of illumination. In addition, a significant improvement in all tested reading performance parameters has been systematically reported in presbyopic patients implanted with the inlay [6].
The optical quality of eyes implanted with the corneal inlay has been recently studied in detail [7] using biometric data from real eyes. The impact and tolerance to misalignment of the small aperture corneal inlay once implanted were studied [7]. It was obtained that the correct positioning of the inlay is critical to achieve a good vision. In clinical practice, some patients must even undergo re-centering the implant in a second surgery to improve their vision [6]. In the work of Tabernero and Artal [7], the theoretical quality of the retinal images when the corneal inlay was placed in combination with different values of residual myopia was also studied in detail. The optimum compromise is a defocus of 0.75 D myopic in the eye implanted with the corneal inlay [7].
In spite of the detailed knowledge about the optical principles of the corneal inlay and the increasing number of clinical studies, the potential visual quality of subjects implanted with the inlay has not been sufficiently covered in the existing literature. In particular, the potential influence of the corneal inlay on stereopsis has not been studied yet. Stereopsis plays a fundamental role in many everyday tasks [8,9], and it is particularly important for some professionals whose work involves manual skills [10,11]. Monovision could be taken as a limit case for the brain to create the perception of stereopsis [12,13]. The way in which stereopsis is affected when the retinal images formed on each eye differ has been widely studied in the past from different perspectives [14–16]. Most of the previous literature characterized the degradation of stereopsis connected with asymmetric changes in the contrast of the two retinal images [17–20]. More recent studies have explored the role of aberrations, beyond defocus and astigmatism, over stereopsis when they happen to be unequal in the two eyes [21]. Summation has been also investigated in this context [22]. The possibility of using adaptive optics instruments for visual testing [23] has recently allowed tackling this question from a wider perspective [24,25]. In addition to this primary goal, the current experiment should provide information about a potential binocular suppression in the small aperture inlay scheme. This question arose in a previous study [26] involving the use of adaptive optics [24] for studying the effects of the small aperture on visual acuity. Monocular vision with the small aperture exhibited the somehow expected increase in the depth of focus, which manifested on an extended visual acuity across a range of distances. The results showed a significant extension of the distances where visual acuity remained high when the small aperture was applied, compared with the situation of vision through a normal 4 mm of diameter pupil. When evaluating these findings under binocular conditions (leaving one of the eyes natural and the other with the small aperture), the through-focus visual acuity exhibited similar values. The authors hypothesized about the possibility of suppression during the binocular measurements, owing to the large differences in the optical quality of the retinal images projected on each eye [27]. In that case, stereopsis based on retinal disparity is difficult to happen, although other mechanisms could be involved.
In the current work we investigate stereopsis, covering some questions associated to the simultaneous use of monovision and the corneal inlay. The corneal inlay was generated optically by an adaptive optics system. Subjects performed visual testing through a virtual corneal inlay reproducing the implanted eye. Target values of defocus of 0 D, 0.75 D, and 1.5 D myopic were selected for the implanted and the natural eye. The values correspond to the emmetropic eye, the value recommended by the manufacturer to combine with the inlay, and a typical value used in pure monovision, respectively.
2. Methods
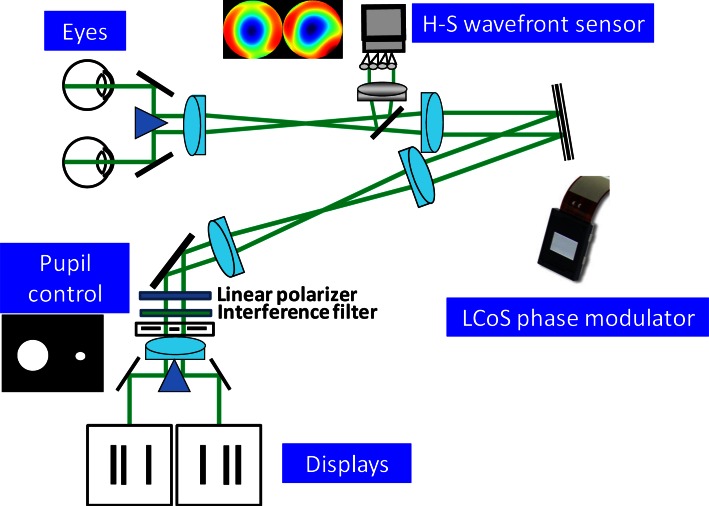
A binocular adaptive optics visual analyzer [24] was developed to perform the experiment. The apparatus was a modified version of one described elsewhere [28]. The system was able to measure and manipulate ocular aberrations from the two eyes simultaneously, while the subject performed visual testing. A schematic diagram is presented in Fig. 1. A single laser beam of 780 nm was divided into two separate beams and sent to each eye. The setup used a double periscope so that light backreflected from each retina was redirected into the system. Those beams entered the system spatially resolved but still close enough to allow the use of single optics for the two beams along the apparatus. A single Hartmann-Shack wavefront sensor was optically conjugated with the exit pupils from both eyes. The two pupils were projected onto the sensor. Custom-developed algorithms allowed video-rate operation for the retrieval of both ocular aberrations [24]. The pupils of both eyes were also conjugated on the surface of a phase modulator, keeping them spatially resolved. The correcting device was a reflective Liquid Crystal on Silicon (LCoS) spatial light modulator (Pluto, Holoeye Photonics AG, Germany). The use of such technology for visual testing purposes has been demonstrated in the past [29,30]. The modulator is a parallel-aligned nematic liquid-crystal device exhibiting full high-definition resolution (1920 × 1080 pixels) and 60 Hz frame rate. The fill factor was over 85%, and the control was performed digitally from a personal computer. Once calibrated these devices have a high fidelity, so it is possible to use them in open loop configuration [29]. Each pupil was associated to an area of 900 × 900 independent pixels on the LCoS for manipulating the aberrations.
Fig. 1.
Schematic diagram of the binocular adaptive optics visual simulator showing the main components. The setup includes a single Hartmann-Shack wavefront sensor and a single correcting device for measurement and manipulation of aberrations from the two eyes. The system incorporates two microdisplays for producing retinal disparity, therefore creating stereopsis.
An additional twisted-liquid-crystal device for intensity modulation (LC2002, Holoeye Photonics AG, Germany) was placed at the exit pupil plane of the apparatus for pupil diameter control. For accomplishing such modulation, appropriate polarization of the incoming beam must be guaranteed. This device has 800 × 600 translucent independent square pixels of 32 µm and operated in transmission. The pupils were generated setting a binary mask with two apertures corresponding to each eye’s pupil. The measured maximum contrast for the used wavelength was 93%. Pupil position was periodically checked using an additional camera and double-pupil tracking software [28]. Since infrared illumination was employed for measuring the eye’s aberrations, it was necessary to correct for the existing chromatic aberration [31,32]. A pair of mirrors redirected the beams into separate optical relays, allowing the operation of two independent microdisplays for showing the stimuli. Having one screen for each eye allowed the generation of three-dimensional stimuli. Two micro-projectors (MPro 120, 3M, USA) were modified for using the internal liquid-crystal display (LCD). An interference filter centered at 550 nm and 40 nm bandwidth was incorporated to the system, closed to the pupil control plane, assuring quasi-monochromatic operation of the displays. The effective pixel size of the microdisplays was 11.75 µm, operating with 800 × 600 resolution. Control of the micro-projectors was accomplished from a computer employing a software package specifically developed for the task. An anti-aliasing filter was used for subpixel resolution.
The two microdisplays allowed the showing of different stimuli to each eye of the subject. Retinal disparity is the change in the relative position of a retinal image produced by a single object due to the parallax of the line of sight of each eye. Retinal disparity creates stereopsis, which is the natural ability for perceiving depth in the scene. The stimulus relative positions were changed in the two microdisplays for inducing local stereopsis in the subjects participating in the experiment [33]. An option for characterizing stereopsis is the measurement of stereo-acuity, defined as the minimum horizontal disparity that allows the observer to perceive relative depth. In our case, it is effectively measured as the minimum disparity that produces an impression of depth. It is strongly dependent on many factors, such as the stimuli shape and exposure time. In this work, a three-needle test was employed. This is a classical stereopsis test consisting of two outer bars, which remain steady and at the same plane at any moment, and a central bar presented at a different position to each eye. The retinal disparity of the central bar was changed for generating the perception of depth. Depending on the type of programmed disparity, the central bar appeared closer (crossed disparity) or farther (uncrossed disparity) than the virtual plane defined by the two other steady bars. A two-alternative forced-choice test was programmed for the experiment. The observer answered “in front” or “behind” after a 3 s exposure of the test, describing the position of the central bar regarding the two lateral ones. When considering the objective lens of 300 mm of focal length in combination with the optics of the experimental system, one pixel of the microdisplay subtended 0.13 arcmin at the entrance pupil of the eye. The perceived dimensions of each bar were 19.5 × 3.9 arcmin. The separation across adjacent lines was 13 arcmin.
The experiment was conducted following the Declaration of Helsinki. Four subjects of ages ranging from 35 to 49 years participated in the experiment. All of them were familiar with the instrument, presented normal refraction, and had no retinal conditions. Before the experiment, the periscope in front of the eyes was modified to align both pupils while allowing subjects to reach binocular image fusion, avoiding double-image perception. This operation assured that lines of sight were natural for the observers, preventing visual discomfort and correcting hypothetical subclinical phorias. To achieve visual fusion, a circumference with a diametric line was shown in both microdisplays with a 90° rotation between them. Therefore, the target for visual fusion was perceived as a cross surrounded by a circle. The linewidth of both figures subtended 3.9 arcmin. All subjects were able to properly accomplish fusion. During the runs and between each exposure of the three-needle test, the fusion target was displayed.
Paralyzed accommodation was induced by instilling cycloplegic drugs (tropicamide 0.5%, a drop every 30 min). Subjects were stabilized to the system by using their dental impression molds. After performing the alignment of the pupils, the ocular aberrations were measured by the wavefront sensor. Astigmatism and defocus were systematically corrected with the LCoS spatial phase modulator in both eyes. The rest of the higher-order aberrations were not corrected. The values of the remaining root mean square (RMS) obtained from high-order aberrations in a pupil of 4 mm of diameter were 0.10, 0.12, 0.13, and 0.14 µm for each subject. The high-order RMS for a 1.6 mm pupil was virtually 0 in every subject. Objective correction of defocus was also refined subjectively. The subjects were given control of the defocus induced by the system and further corrected defocus on each eye sequentially and finally binocularly. The procedure guaranteed that the subjects performed stereopsis tests with their best possible retinal contrast. In order to avoid misalignments during the runs, the subjects answered and performed the two-alternative forced-choice test by pressing a keypad to indicate the perception of the central bar of the test (i.e., beyond or in front). Monovision and different pupil sizes were generated during the experiment using both the LCoS phase modulator and the intensity modulator, respectively. Five different conditions were considered during the experiment: (a) natural vision; (b) 1.5 D monovision; (c) 0.75 D monovision; (d) natural vision and small pupil; (e) 0.75 D monovision and small pupil. In all cases the standard pupil diameter was 4 mm and the small pupil diameter was 1.6 mm. Both the additional defocus for monovision and the small pupil were applied in the nondominant eye when required. Ocular dominance was established by asking the subjects to fixate a distant stimulus with a single eye [34]. None of the subjects participating in the study obtained better visual acuity for far vision associated to the use of one or the other eye. Subjects were not able to perform the task for condition (b), owing to the large difference between retinal images causing suppression. Therefore, no results can be shown.
Stereopsis was generated maintaining the line of sight of the eyes approximately parallel to each other, corresponding to a situation of far vision. The subjects were unaware of which condition was tested at any time. For each condition, different values of induced retinal disparity were programmed. The minimum step was 0.13 arcmin. For each value of retinal disparity the subjects performed 15 trials, divided in 5 sets of measurements to prevent a subject’s fatigue. Disparity was created by either moving the central line symmetrically in each eye by half the programmed disparity, or by moving only the central bar for one of the eyes, with random distribution of situations. The subjects were allowed to rest whenever required. Before the experiment, the subjects were allowed some trial runs to gain some training in the setup, observing stereoscopic tests with different disparities. From the measurements, average values for every condition and retinal disparity were calculated.
3. Results
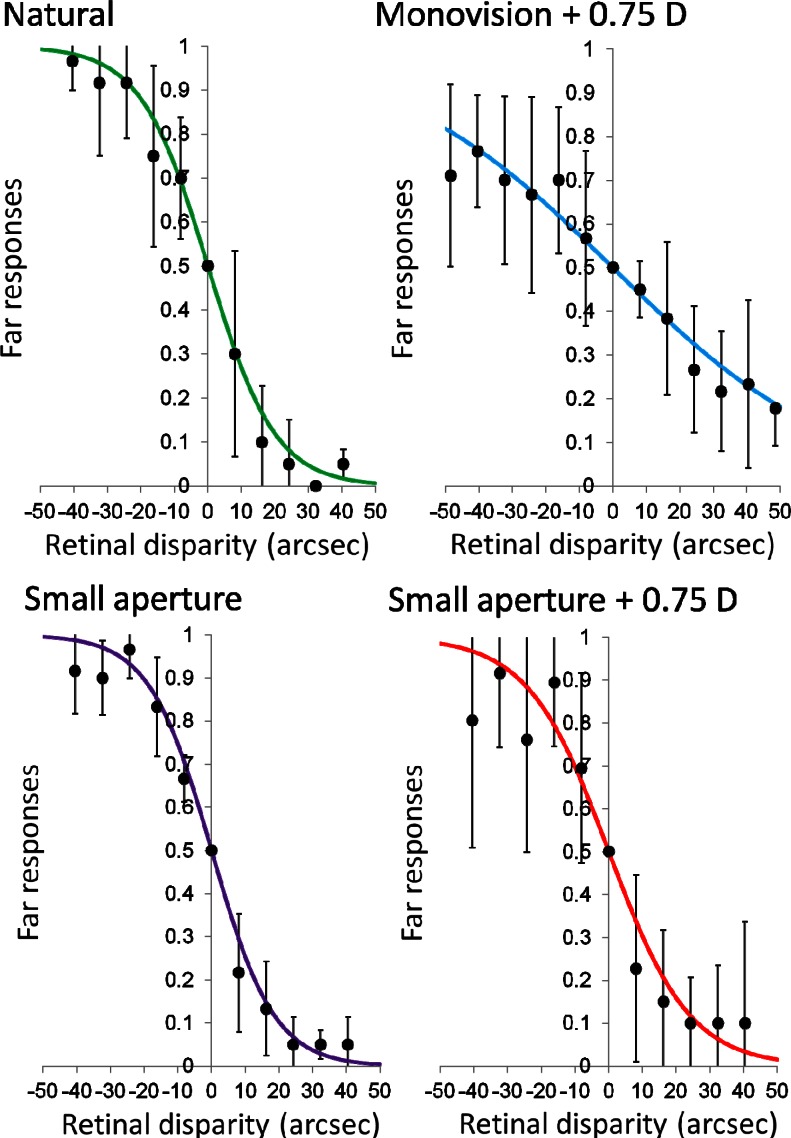
The normalized distribution of the responses as a function of the retinal disparity is presented in Fig. 2. Negative values correspond to uncrossed retinal disparity, while positive values are crossed disparity. For large negative values, the subject should always answer “far” in the two-alternative forced-choice test, and the ratio would tend to 1. On the contrary, for large positive values the subject’s response would tend to be “near,” and the far response ratio would approach to 0. The points in Fig. 2 represent the average results obtained from the four subjects. Error bars are the standard deviation in each case. The experimental points for each condition were fitted to a sigmoid function for determining the detection thresholds. This function is a special case of the Weibull probability distribution, widely employed as psychometric function in many visual tests [12,13]. The mathematical expression of the fitting function was
Fig. 2.
The solid points represent the mean value of far responses from 4 subjects for the three-needle test using a two-alternative forced-choice test. Error bars are the standard deviation. The solid lines are the least squares fitted psychometric curves. The right panels correspond to natural viewing, while the left ones present the results obtained under monovision with 0.75 D. At the bottom, the right panel corresponds to the case of vision with a small aperture in one of the eyes. The left panel presents the results obtained with a small aperture in combination with a monovision of 0.75 D in the same eye.
| (1) |
A routine was developed for performing the curve fitting in Matlab (Matlab, MathWorks, USA), with a being the single free parameter of the Eq. (1) to be found. Ordinary least squares routines were employed for the task. Solid lines in Fig. 2 represent the fitted curve.
The left and right panels in Fig. 2 correspond to both eyes set to best focus for far distance and to 0.75 D monovision, respectively. Top panels correspond to 4-mm pupils in both eyes (i.e., top-left panel shows the results for natural viewing conditions and top-right panel shows the results for standard mild monovision). The squared correlation coefficient R2 was 0.99 and 0.96, respectively, indicating an accurate fitting in both cases. The decrease in the slope of the monovision case is evident as compared with the natural situation, indicating a reduction of stereopsis. The error bars were also larger in the former case, indicating an increase in the intersubject variability of the answers in the two-alternative forced-choice test.
The bottom panels in Fig. 2 show results corresponding to vision through a 4-mm pupil in the dominant eye and a small 1.6-mm pupil in the nondominant eye, with both eyes set to best focus (left panel), or in combination with 0.75 D monovision. The squared correlation coefficient R2 was 0.99 and 0.94, respectively. As in the equal-pupil case, the case involving 0.75 D monovision exhibited wider error bars. However, there was not an evident change in the slope of the fitted curve, suggesting that stereopsis seemed relatively similar in both cases and also comparable to the natural vision case (Fig. 2, top left panel).
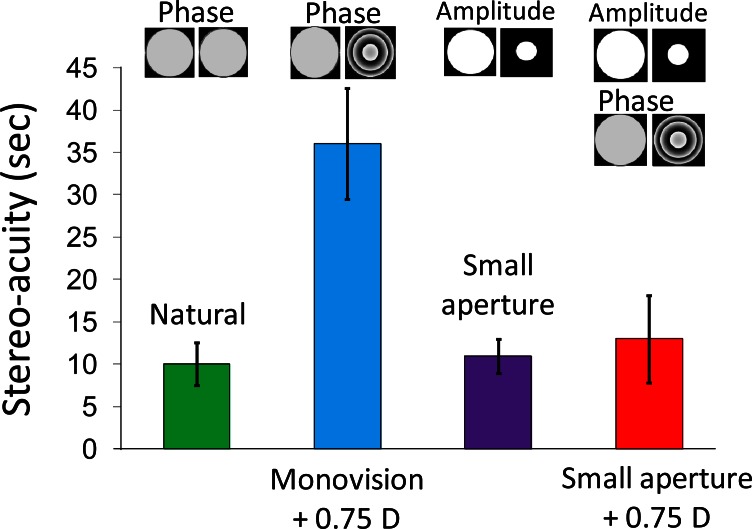
In order to obtain the stereoacuity under each studied condition, the value of 75% of right answers was selected in negative retinal disparities to determine the threshold of detection. This procedure was systematically applied on the fitted sigmoid curves obtained from the average data. The results are depicted in Fig. 3. Error bars correspond to the stereoacuity estimates for the 95% confidence limits of fitted parameter a in Eq. (1). The values obtained for parameter a for the natural conditions, 0.75 D standard monovision, small aperture, and small aperture combined with 0.75 D monovision, were 0.108, 0.030, 0.100, and 0.084 respectively. The average values were 10”, 36”, 11” and 13”, respectively. The larger error bars were found for 0.75 D monovision with 4-mm pupils in both eyes. These values are represented in Fig. 3. It can be seen that monovision with 0.75 D added, which is an atypically small amount, produced a degradation in the stereoacuity values by a factor close to 4; however, the use of a small aperture in one of the eyes significantly reduced the negative impact of monovision on stereopsis to a value close to that achieved under natural vision conditions.
Fig. 3.
Stereoacuity obtained as the average across subjects of the retinal disparity producing 75% of far responses, calculated from the psychometric fitted curve for each subject and condition.
4. Discussion
Liquid-crystal spatial phase modulators are a convenient tool for visual testing applications. In particular, owing to their high resolution, they allow the manipulation of two pupils with a single device, making the system cost effective and reducing the complexity of the control. The requirement of using linearly polarized light is not an actual limitation for this type of experiment, since human vision is insensitive to the plane of vibration of the light and, furthermore, the use of polarized light does not affect ocular aberrations [35]. The phase-wrapped representation of the wavefront inherently enforces the selection of a given wavelength, producing small deviations at different wavelengths. Parallel works were performed by the authors characterizing these effects on vision. We have not found any difference in the results obtained under monochromatic and white light illumination. One of the reasons can be the almost 2 D of chromatic aberration of the eye in the visible spectrum, significantly superior to the modest deviations produced by incorrect phase wrapping at adjacent wavelengths when employing the liquid crystal in combination with white light.
Stereopsis was generated with the lines of sight of the two eyes approximately parallel. In this situation, defocus and pupil sizes were manipulated for studying their influence on stereoacuity. In normal viewing conditions, stereopsis is inherently associated to intermediate and near vision. The lines of sight are then no longer parallel, but they exhibit some convergence. The amount of convergence is proportional to the distance to the object. However, lines of sight parallel are for far vision. In the experiment, stereopsis was induced by generating retinal disparity, so the direction of the lines of sight should not affect the obtained results [36]. All the subjects were able to perceive depth. The reason for performing the measurements in far-vision conditions was twofold. On the one hand, prolonged convergence causes visual fatigue and discomfort. Reducing the subjects’ distress was important, since the programmed tests took typically over 2 h. The other reason was to reduce technical complexities. Convergence would cause the projections of the pupils to be elliptical on the wavefront sensor, LCoS, and amplitude modulator planes, introducing some additional difficulties in the analysis and processing of the obtained data.
The three-needle test leaves open the possibility of accurate responses with just one eye, as reported by Westheimer et al. in 1979 [37]. It could have been possible that some of the subjects performed the task without actually using stereopsis. The subjects were however aware of the nature of the experiment, and they reported true stereopsis based on binocular vision. The fact that the subjects could not perform the task with 1.5 D of blur in the nondominant eye also supports the fact that they were not using the monocular cue. Otherwise monocular cues from the dominant eye would have produced some results.
The sigmoid function chosen for the fitting of the average data obtained in the experiment was possibly the simplest alternative across the different existing probability functions used in psychophysics. Other functions might achieve a higher match in the fitting to the experimental data (e.g., a polynomial curve fitting). The sigmoid function was employed in order to provide a systematic and objective method of evaluating the threshold in stereoacuity. Also, selecting the value of 75% of right answers in the psychometric curve for inferring the threshold was somehow arbitrary. Therefore, employing a more elaborate probability function for describing the data could render a slightly different value for the stereoacuity. However, even if they cannot be taken as exact values for the stereoacuity, the results presented in Fig. 3 can be used to compare different alternatives for correcting presbyopia and suggest that the introduction of a small pupil can be useful to maintain stereopsis, which is severely degraded by monovision even for small add values.
The alleviation of presbyopia by using monovision is a simple and effective approach for many patients. Stereopsis is one of the functions of binocular vision that can be seriously compromised by applying monovision. The results of the experiment showed that a small aperture, implemented, e.g., as an intrastromal corneal inlay, can yield values of stereoacuity similar to those attained under normal binocular vision in photopic conditions. Mesopic and scotopic illumination conditions have not been studied in this work. The use of the small aperture approach under low illumination could cause additional losses of stereoacuity owing to the larger difference across pupils’ diameters [38]. Future works should address these other conditions.
Acknowledgments
This work has been supported by Ministerio de Educación y Ciencia, Spain (grants no. FIS2010-14926 and Consolider Program SAUUL CSD2007-00013) and Fundación Séneca, Murcia, Spain (grant 04524/GERM/06).
References and links
- 1.Evans B. J. W., “Monovision: a review,” Ophthalmic Physiol. Opt. 27(5), 417–439 (2007). 10.1111/j.1475-1313.2007.00488.x [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz O. F., Bayraktar S., Agca A., Yilmaz B., McDonald M. B., van de Pol C., “Intracorneal inlay for the surgical correction of presbyopia,” J. Cataract Refract. Surg. 34(11), 1921–1927 (2008). 10.1016/j.jcrs.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 3.Seyeddain O., Riha W., Hohensinn M., Nix G., Dexl A. K., Grabner G., “Refractive surgical correction of presbyopia with the AcuFocus small aperture corneal inlay: two-year follow-up,” J. Refract. Surg. 26(10), 707–715 (2010). 10.3928/1081597X-20100408-01 [DOI] [PubMed] [Google Scholar]
- 4.Yılmaz O. F., Alagöz N., Pekel G., Azman E., Aksoy E. F., Cakır H., Bozkurt E., Demirok A., “Intracorneal inlay to correct presbyopia: Long-term results,” J. Cataract Refract. Surg. 37(7), 1275–1281 (2011). 10.1016/j.jcrs.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 5.Waring G. O., 4th, “Correction of presbyopia with a small aperture corneal inlay,” J. Refract. Surg. 27(11), 842–845 (2011). 10.3928/1081597X-20111005-04 [DOI] [PubMed] [Google Scholar]
- 6.Dexl A. K., Seyeddain O., Riha W., Hohensinn M., Rückl T., Hitzl W., Grabner G., “Reading performance after implantation of a modified corneal inlay design for the surgical correction of presbyopia: 1-year follow-up,” Am. J. Ophthalmol. 153(5), 994–1001 (2012). 10.1016/j.ajo.2011.08.044 [DOI] [PubMed] [Google Scholar]
- 7.Tabernero J., Artal P., “Optical modeling of a corneal inlay in real eyes to increase depth of focus: optimum centration and residual defocus,” J. Cataract Refract. Surg. 38(2), 270–277 (2012). 10.1016/j.jcrs.2011.07.040 [DOI] [PubMed] [Google Scholar]
- 8.Fielder A. R., Moseley M. J., “Does stereopsis matter in humans?” Eye (Lond.) 10(2), 233–238 (1996). 10.1038/eye.1996.51 [DOI] [PubMed] [Google Scholar]
- 9.O’Connor A. R., Birch E. E., Anderson S., Draper H., FSOS Research Group , “The functional significance of stereopsis,” Invest. Ophthalmol. Vis. Sci. 51(4), 2019–2023 (2010). 10.1167/iovs.09-4434 [DOI] [PubMed] [Google Scholar]
- 10.Mazyn L. I. N., Lenoir M., Montagne G., Savelsbergh G. J. P., “The contribution of stereo vision to one-handed catching,” Exp. Brain Res. 157(3), 383–390 (2004). 10.1007/s00221-004-1926-x [DOI] [PubMed] [Google Scholar]
- 11.Mrotek L. A., Gielen C. C., Flanders M., “Manual tracking in three dimensions,” Exp. Brain Res. 171(1), 99–115 (2006). 10.1007/s00221-005-0282-9 [DOI] [PubMed] [Google Scholar]
- 12.R. W. Reading, Binocular Vision: Foundations and Applications (Butterworths, 1983). [Google Scholar]
- 13.I. P. Howard and B. J. Rogers, Binocular Vision and Stereopsis, Oxford Psychology Series No. 29 (Oxford University Press, 1995). [Google Scholar]
- 14.Lovasik J. V., Szymkiw M., “Effects of aniseikonia, anisometropia, accommodation, retinal illuminance, and pupil size on stereopsis,” Invest. Ophthalmol. Vis. Sci. 26(5), 741–750 (1985). [PubMed] [Google Scholar]
- 15.Schor C., Heckmann T., “Interocular differences in contrast and spatial frequency: effects on stereopsis and fusion,” Vision Res. 29(7), 837–847 (1989). 10.1016/0042-6989(89)90095-3 [DOI] [PubMed] [Google Scholar]
- 16.Cormack L. K., Stevenson S. B., Landers D. D., “Interactions of spatial frequency and unequal monocular contrasts in stereopsis,” Perception 26(9), 1121–1136 (1997). 10.1068/p261121 [DOI] [PubMed] [Google Scholar]
- 17.Halpern D. L., Blake R. R., “How contrast affects stereoacuity,” Perception 17(4), 483–495 (1988). 10.1068/p170483 [DOI] [PubMed] [Google Scholar]
- 18.Legge G. E., Yuanchao G., “Stereopsis and contrast,” Vision Res. 29(8), 989–1004 (1989). 10.1016/0042-6989(89)90114-4 [DOI] [PubMed] [Google Scholar]
- 19.Wood I. C., “Stereopsis with spatially-degraded images,” Ophthalmic Physiol. Opt. 3(3), 337–340 (1983). 10.1111/j.1475-1313.1983.tb00622.x [DOI] [PubMed] [Google Scholar]
- 20.Geib T., Baumann C., “Effect of luminance and contrast on stereoscopic acuity,” Graefes Arch. Clin. Exp. Ophthalmol. 228(4), 310–315 (1990). 10.1007/BF00920053 [DOI] [PubMed] [Google Scholar]
- 21.Jiménez J. R., Castro J. J., Hita E., Anera R. G., “Upper disparity limit after LASIK,” J. Opt. Soc. Am. A 25(6), 1227–1231 (2008). 10.1364/JOSAA.25.001227 [DOI] [PubMed] [Google Scholar]
- 22.Castro J. J., Jiménez J. R., Hita E., Ortiz C., “Influence of interocular differences in the Strehl ratio on binocular summation,” Ophthalmic Physiol. Opt. 29(3), 370–374 (2009). 10.1111/j.1475-1313.2009.00643.x [DOI] [PubMed] [Google Scholar]
- 23.Fernández E. J., Manzanera S., Piers P., Artal P., “Adaptive optics visual simulator,” J. Refract. Surg. 18(5), S634–S638 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Fernández E. J., Prieto P. M., Artal P., “Binocular adaptive optics visual simulator,” Opt. Lett. 34(17), 2628–2630 (2009). 10.1364/OL.34.002628 [DOI] [PubMed] [Google Scholar]
- 25.Fernández E. J., Prieto P. M., Artal P., “Adaptive optics binocular visual simulator to study stereopsis in the presence of aberrations,” J. Opt. Soc. Am. A 27(11), A48–A55 (2010). 10.1364/JOSAA.27.000A48 [DOI] [PubMed] [Google Scholar]
- 26.Tabernero J., Schwarz C., Fernández E. J., Artal P., “Binocular visual simulation of a corneal inlay to increase depth of focus,” Invest. Ophthalmol. Vis. Sci. 52(8), 5273–5277 (2011). 10.1167/iovs.10-6436 [DOI] [PubMed] [Google Scholar]
- 27.Rose D., Blake R., Halpern D. L., “Disparity range for binocular summation,” Invest. Ophthalmol. Vis. Sci. 29(2), 283–290 (1988). [PubMed] [Google Scholar]
- 28.Schwarz C., Prieto P. M., Fernández E. J., Artal P., “Binocular adaptive optics vision analyzer with full control over the complex pupil functions,” Opt. Lett. 36(24), 4779–4781 (2011). 10.1364/OL.36.004779 [DOI] [PubMed] [Google Scholar]
- 29.Prieto P. M., Fernández E. J., Manzanera S., Artal P., “Adaptive optics with a programmable phase modulator: applications in the human eye,” Opt. Express 12(17), 4059–4071 (2004). 10.1364/OPEX.12.004059 [DOI] [PubMed] [Google Scholar]
- 30.Fernández E. J., Prieto P. M., Artal P., “Wave-aberration control with a liquid crystal on silicon (LCOS) spatial phase modulator,” Opt. Express 17(13), 11013–11025 (2009). 10.1364/OE.17.011013 [DOI] [PubMed] [Google Scholar]
- 31.Fernández E. J., Unterhuber A., Prieto P. M., Hermann B., Drexler W., Artal P., “Ocular aberrations as a function of wavelength in the near infrared measured with a femtosecond laser,” Opt. Express 13(2), 400–409 (2005). 10.1364/OPEX.13.000400 [DOI] [PubMed] [Google Scholar]
- 32.Fernández E. J., Artal P., “Ocular aberrations up to the infrared range: from 632.8 to 1070 nm,” Opt. Express 16(26), 21199–21208 (2008). 10.1364/OE.16.021199 [DOI] [PubMed] [Google Scholar]
- 33.Westheimer G., McKee S. P., “Stereogram design for testing local stereopsis,” Invest. Ophthalmol. Vis. Sci. 19(7), 802–809 (1980). [PubMed] [Google Scholar]
- 34.McMonnies C. W., “Monocular fogging in contact lens practice,” Aust. J. Optom. 57, 28–32 (1974). [Google Scholar]
- 35.Prieto P. M., Vargas-Martín F., McLellan J. S., Burns S. A., “Effect of the polarization on ocular wave aberration measurements,” J. Opt. Soc. Am. A 19(4), 809–814 (2002). 10.1364/JOSAA.19.000809 [DOI] [PubMed] [Google Scholar]
- 36.Wong B. P., Woods R. L., Peli E., “Stereoacuity at distance and near,” Optom. Vis. Sci. 79(12), 771–778 (2002). 10.1097/00006324-200212000-00009 [DOI] [PubMed] [Google Scholar]
- 37.Westheimer G., McKee S. P., “What prior uniocular processing is necessary for stereopsis?” Invest. Ophthalmol. Vis. Sci. 18(6), 614–621 (1979). [PubMed] [Google Scholar]
- 38.Westheimer G., McKee S. P., “Stereoscopic acuity with defocused and spatially filtered retinal images,” J. Opt. Soc. Am. A 70(7), 772–778 (1980). 10.1364/JOSA.70.000772 [DOI] [Google Scholar]