Abstract
The lymphatic system provides an initial route for cancer cell dissemination in many cancers including melanoma. However, it is largely unknown how the lymphatic system changes during tumor progression due in part to the lack of imaging techniques currently available. In this study, we non-invasively imaged changes of lymphatic function and drainage patterns using near-infrared fluorescence (NIRF) imaging. Dynamic NIRF imaging following intradermal injection of indocyanine green (ICG) was conducted in C57BL/6 mice prior to inoculation of B16F10 murine melanoma cells to the dorsal aspect of the left hindpaw for baseline data or directly to the popliteal lymph node (PLN) and until 21 days post-implantation (p.i.). A series of acquired fluorescent images were quantified to measure lymphatic contractile function. Computed tomography (CT) was also performed to measure the volume of tumor-draining lymph nodes (LNs). We observed significant reduction of lymphatic contractility from 7 days p.i. until 21 days p.i.. Altered lymphatic drainage patterns were also detected at 21 days p.i. in mice with tumor in the paw and at 11 days p.i. in mice with tumor in the PLN, due to lymphatic obstruction of normal lymphatic drainages caused by extensive tumor invasion of draining LNs. Since lymphatic function and architecture were progressively altered during tumor growth and metastasis, non-invasive NIRF imaging may provide a new method to stage disease. In addition, this novel technique can be used as a diagnostic method to non-invasively assess lymphatic response as mechanism of therapeutic action.
OCIS codes: (170.0170) Medical optics and biotechnology, (170.2655) Functional monitoring and imaging, (170.3880) Medical and biological imaging, (170.4580) Optical diagnostics for medicine
1. Introduction
Melanoma is the most lethal skin cancer in large part due to its propensity to metastasize to distant sites. Because the lymphatic system provides an initial transport route for cancer cell dissemination for melanoma, regional lymph node (LN) metastases are regarded as the first site of metastasis in most cutaneous melanoma patients with tumor progression [1,2]. Therefore, sentinel LN (SLN) biopsy after lymphatic mapping with radiocolloid and/or blue dye is considered standard care for staging melanoma. When metastasis is observed within the SLN, complete (elective) LN dissection is performed for regional disease control and cure. However, it has been postulated that massive LN metastasis can obstruct normal lymph flow and change normal lymphatic drainage pathways. Indeed, it has been reported that metastatic melanoma in patients can block normal lymph flow to the SLN [3,4]. In preclinical studies in mice bearing C6 rat glioma, we also demonstrated that extensive tumor invasion of draining LNs can alter or completely block normal lymphatic fluid passage, leading to changes of lymphatic drainage pathways [5].
Recent evidence suggests that tumor-induced lymphangiogenesis promotes LN metastasis and thus can be detected as a novel prognostic predictor of melanoma progression and metastasis [2,6–9]. Since it is associated with metastatic potential, pharmacological inhibition of tumor lymphangiogenesis has also become an emerging therapeutic strategy to arrest metastasis in cancer, including melanoma [10–14]. In preclinical studies, investigators have demonstrated that LN lymphangiogenesis can occur prior to the onset of metastasis and is accompanied by an increase in lymph flow to tumor-draining LN [15–20], thus facilitating tumor cell entry into the lymphatics. These studies suggest that functional imaging of tumor-associated lymphatics could not only provide a possible early diagnostic for tumor-node-metastasis (TNM) staging, but could also be used to direct therapies that seek to disrupt tumor lymphangiogenesis.
The lymphatic system consists of two major types of vessels, initial and collecting lymphatic vessels. Unlike the blood circulatory system, the lymphatic system does not have a central pump, but mainly depends upon muscle contraction. The collecting lymphatics have functional units, called lymphangions, which have spontaneous contractile subunits bound by the secondary valves that prevent backflow and ensure unidirectional flow. Recently, we developed a novel near-infrared fluorescence (NIRF) imaging technique and demonstrated for the first time, the ability to non-invasively image dynamic lymphatic contractile function and architecture in mice with spatio-temporal resolution and sensitivity following intradermal (i.d.) injection of indocyanine green (ICG) [21]. ICG is rapidly taken up by the initial lymphatics and upon surface illumination with tissue-penetrating NIR excitation light, the fluorescence generated by the ICG-laden lymph is collected with high temporal resolution to visualize transport and lymphatic “pumping” activity. Using this approach, we demonstrated altered lymphatic function and drainage patterns in transgenic mice with lymphatic disorders/dysfunction [22,23]. Non-invasive NIRF imaging using microdose amounts of ICG has also been employed to image functional and architectural changes of lymphatics in healthy subjects as well as in patients with breast cancer, head and neck cancer, melanoma, and lymphedema [24–29]. In melanoma patients, we observed that tumor-associated draining lymphatics on the diseased limb were tortuous and dilated as compared to those on the contralateral, disease-free limbs [27].
Yet despite the important role of lymphatics as a critical pathway for cancer metastasis, relatively little is known about how the lymphatics change during tumor progression and metastasis. Prior preclinical imaging studies have not interrogated how the lymphatics alter in structure and function during melanoma progression and metastasis. More importantly, the association between cancer metastasis and tumor lymphangiogenesis has been largely indirect, owing to the inability to directly image the lymphatics in vivo. In this study, we performed non-invasive and longitudinal NIRF lymphatic imaging in mice bearing murine B16F10 melanoma and showed reduced lymphatic contractile function and altered lymphatic drainage pathways in mice with LN metastasis of B16F10 tumor.
2. Materials and methods
2.1 Animals
Six to eight week old female C57BL/6 mice (n = 16, Charles River Laboratories, Inc., Wilmington, MA) were maintained in a pathogen-free mouse colony at the Brown Institute of Molecular Medicine, University of Texas Health Science Center at Houston (UTHSC-H), which is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All animal experimentation was performed after approval by the Institutional Animal Care and Use Committee.
2.2 Tumor model and cell line
B16F10 murine melanoma cells were obtained from American Type Culture Collection (ATCC, Mannassas, VA) and cultured in DMEM-F12 (GIBCO) supplemented with 10% FBS. Cells were maintained at 37 °C in a 5% CO2, 95% air humidity incubator. B16F10 cells (0.5 x 106 cells per mouse in 10 μl sterile PBS) were then implanted intradermally in the dorsal aspect of the left hindpaw (n = 12). Tumor volume was computed from diameters measured with a digital caliper, using the formula V = 0.52 x D12 x D2, where D1 and D2 are short and long tumor diameters, respectively. Three weeks after inoculation, mice were euthanized and tissues were collected for histology.
B16F10 cells (0.5 x 106 cells per mouse in 10 μl sterile PBS) were also inoculated directly into the left PLN of animals (n = 4). Briefly, mice were anesthetized and the skin over the subcutaneous injection site and surrounding area were cleaned with iodine three times, followed by 70% isopropyl alcohol. Two µl of Evans blue dye was intradermally injected on the dorsal aspect of the left hindpaw to delineate the PLN. One minute later, a small (~5 mm) incision was made with scissors through the skin medial to the PLNs in the ventral view of mice without interrupting collecting lymphatic vessels. B16F10 cells (0.5 x 106 tumor cells were injected in 10 µL PBS to the PLN. The skin was closed with surgical glue (Vetbond, 3M Animal Care Products, St. Paul, MN) and the mice were allowed to recover. Mice were monitored daily for signs of pain.
2.3 NIRF lymphatic imaging and CT
NIRF imaging occurred 3 days prior to implantation and again at up to 21 days post-implantation (p.i.). Since optical imaging is impacted by fur, mice were clipped and a depilatory agent (Nair, Church & Dwight Co., Inc., Princeton, NJ) was used to remove residual hair 24 hr before NIRF imaging. At the time of imaging, animals were placed under isofluorane anesthesia and maintained on a 37 °C heating pad. Two µl of 645 µM of ICG (Akorn, Inc., Buffalo Grove, IL) dissolved in mixture of distilled water and 0.9% sodium chloride in a volume ratio of 1:9 and was injected intradermally into the dorsal hindpaw using 34 gauge needle (Nanofil, World Precision Instruments, Inc, Sarasota, FL). Fluorescence images were acquired immediately after injection and for up to 20 min following injection using a custom-built NIRF imaging system described elsewhere [5,22]. To achieve a greater magnification, a macrolens was introduced between the detector and optical filters.
After each NIRF imaging session, computational tomography (CT) imaging was also performed using an INVEON multimodality CT (Siemens Medical Solutions, Knoxville, TN, USA). The CT imaging parameters were x-ray voltage of 80kV with an anode current of 500 µA, and an exposure time of 260 ms of each of the 120 rotation steps over the total rotation of 220◦ at low system magnification. CT images were reconstructed using a Feldkamp cone-beam algorithm with a ramp filter cut off at the Nyquist frequency. The volume of tumor draining PLN was measured using INVEON Research Workplace (Siemens Preclinical Solutions, Knoxville, TN).
2.4 Intravital imaging and tissue immunohistochemistry
After the last imaging session, the left PLN was exposed after a skin incision and intravital color images were acquired using a stereomicroscope (MZ16 A, Leica Microsystems, Inc., Bannockburn, IL). Then, the PLNs and inguinal LNs (ILNs) were harvested for ex vivo NIRF and intravital color imaging. For further histological analysis, tissue samples were fixed in 10% formalin overnight before transfer into 70% ethanol and sent to the Center for Comparative Medicine Pathology Core (Baylor College of Medicine, TX).
Tissue samples were embedded in paraffin and 4 μm sections used in all staining procedures. Following paraffin removal and antigen retrieval using citrate buffer, tissues were blocked with 5% bovine serum albumin (BSA) and stained with rabbit anti-mouse LYVE-1 antibody (AngioBio) and biotin-anti rabbit secondary antibody (Vector Labs). Vectastain Elite ABC system for peroxidase and either DAB or ImmPACT NovaRed as chromagens were used before tissues were counter-stained with hematoxylin (Vector Labs). LYVE-1 expression in three different fields in each section was examined at x40 magnification (Leica Microsystems Inc, Buffalo Grove, IL). Lymphatic vessel number and relative lymphatic vessel area, which is defined as the percentage of positively stained lymphatic vessel area were determined as described previously [30] using Image-Pro Plus (Media Cybernetics, Inc., Bethesda, MD).
2.5 In vivo imaging data analysis
Matlab (The MathWorks, Inc., Natick, MA) and ImageJ (National Institutes of Health, Washington, DC) were used to analyze the fluorescence imaging data to quantify lymphatic function. Fixed regions of interest (ROIs) of equivalent areas were selected along the entire length of the fluorescent lymphatic vessels on fluorescence images as was similarly done for human lymphatic imaging [28] on sequential frames of fluorescence images. The averaged fluorescent intensity within each selected ROIs was plotted as a function of imaging time and length of the lymphatic vessels to generate a three-dimensional (3D) spatio-temporal map. Thus, the frequency of contractile waves of ICG-laden lymph propelled along the lymphatic vessels was measured by assessing the number of fluorescent pulses or “packets” arriving at a ROI.
2.6 Statistical analysis
Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). Values are presented as means ± standard error (SE). The repeated data were analyzed using linear mixed model and pairwise comparisons were conducted by the contrast and an approximate t-test. The non-repeated data were compared by either two-sample t-test or Wilcoxon rank sum test, depending on normality of the data. The significance level was set at p < 0.05.
3. Results and discussion
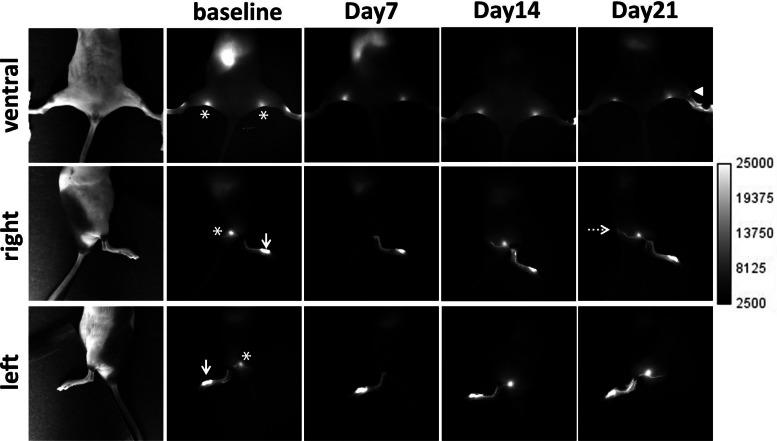
Figure 1 shows an example of in vivo fluorescent images in the ventral, right, and left view of a mouse displaying the lymphatic trafficking of ICG prior to and at 7, 14, and 21 days p.i. of B16F10 cells in the left hindpaw. Non-invasive NIRF images from all mice prior to tumor cell inoculation as baseline data showed consistent, normal lymphatic drainage patterns from the foot, where ICG was intradermally injected, to the PLN (denoted by asterisk), and subsequently to the ischial LN (IsLN) (denoted by broken arrow). We observed the same lymphatic drainage pathways from the foot to the IsLN until 14 days p.i., whereas at 21 days p.i., additional lymphatic drainage from the left hindpaw to the ILN as indicated by an arrowhead in the ventral view in Fig. 1 was visualized, due to mechanical lymphatic obstruction caused by gross LN metastasis.
Fig. 1.
Representative NIRF images in the ventral, right, and left view of a mouse prior to and 7, 14, and 21 days after inoculation of murine B16F10 cells in the left hindpaw. An arrowhead indicates altered lymphatic drainage. Arrows, ICG injection site. Asterisk, PLN. Broken arrow, IsLN.
Magnified fluorescent images of an animal shown in Fig. 1 showed dilated lymphatic vessels around proximal margins of the tumor on the left foot at 21 days p.i., whereas changes of lymphatic vessel architecture were not observed on the tumor-free, contralateral right foot (Fig. 2).
Fig. 2.
Magnified fluorescent images showing lymphatic vessel dilation in the dorsal aspect of the left paw of a mouse at 7, 14, and 21 days p.i. (tumor periphery denoted by red dotted circles) as compared to contralateral right foot. Scale, 1 mm.
In vivo imaging data indicating apparent dilation of lymphatic vessels were confirmed by immunohistochemical stain for the lymphatic vessel marker LYVE-1. Figure 3 shows that although comparable numbers of lymphatic vessels in tumor and contralateral control foot were observed, we found a significant increase of relative peritumoral area covered by lymphatic vessels in the left B16F10 tumor-bearing paw as compared to the tumor-free, contralateral paw.
Fig. 3.
Immunohistochemical stain for LYVE-1 (brown) showing dilated lymphatic vessels in the peritumoral region on the left foot (B) as compared to the contralateral region on the right foot (A). Computer-assisted image analysis shows a comparable number of lymphatic vessels per area in tumor and contralateral regions (C), but a significant increase of relative area occupied by lymphatics in tumor as compared to control right foot (D). n = 7 mice. Scale, 50 µm. * p < 0.05 vs. control.
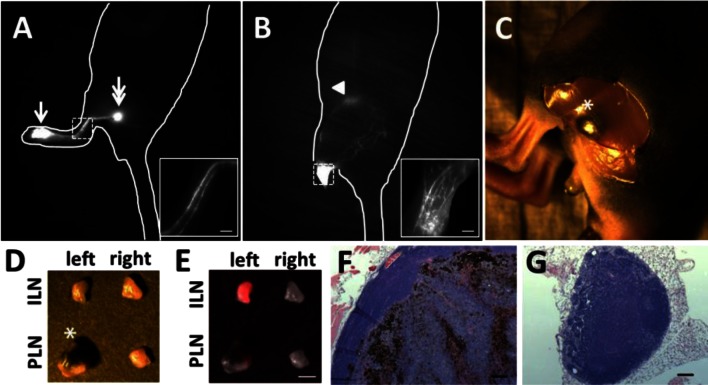
Figure 4 illustrates altered lymphatic drainage to the ILN at 21 days p.i. (Fig. 4B) as compared to the pre-implant, baseline image (Fig. 4A) of the same mouse. Magnified fluorescent images of tumor-draining lymphatic vessels showed numerous fluorescent lymphatic vessels at day 21 p.i. as compared to well-defined two fluorescent vessels from a baseline image (insets in Figs. 4A and 4B). These numerous fluorescent vessels were observed due to obstruction of normal lymph transport and thus rerouting of lymphatic drainage via multiple vessels into the ILN. In order to investigate if changes of normal lymphatic drainage pathways were due to tumor blockage of lymphatic drainage, we performed intravital imaging. Intravital images showed the PLN was enlarged and harbored melanoma cells (Figs. 4C and 4D). Histological analysis also confirmed the enlarged B16F10 tumor cell-infiltrated PLN (Fig. 4F). Overlay of white light and fluorescent images of dissected LNs showed strong ICG fluorescence in the ILN and to lesser extent in the PLN on the tumor bearing side (Fig. 4E), also indicating significantly reduced lymph flow to the PLN due to blockage of normal lymph flow by tumor in the PLN.
Fig. 4.
Fluorescent images of a mouse prior to (A) and 21 days p.i. (B) showing additional lymphatic drainage to the ILN, mainly due to tumor blockage of normal lymphatic drainage to the PLN. Arrow, ICG injection site. Double arrow, PLN. Arrowhead, ILN. The insets show magnified fluorescent images of the rectangles of Figs. 4A and 4B. In Fig. 4B, the injection site was covered to prevent image oversaturation. Scale, 1 mm. An intravital color image (C) after a skin incision above the PLN shows the melanoma-filled PLN. Asterisk, PLN. Color (D) and fluorescent (E) images of dissected PLNs and ILNs on the tumor and contralateral sides of a mouse. Note that the melanoma-filled PLN as indicated by an asterisk in Fig. 4D has low ICG fluorescence as compared to the left ILN due to rerouting of normal lymphatic drainage to the left ILN. Scale, 1 mm. H&E images (x10 objective) confirm extensive PLN metastasis (F) and no metastasis in contralateral PLN (G). Scale, 100 µm.
In a previous study, we similarly observed altered lymphatic function and drainage patterns in C6 rat glioma mice with extensive LN metastasis [5]. Previous reports also showed that metastatic tumor involvement in the SLN can block local lymphatic drainage and thereby change lymphatic drainage pathway of mapping agents to regional non-SLNs which may not yet contain tumor cells, resulting in the increased false-negative SLN rate [3,4,31,32]. It has been reported that lymphatic drainage patterns in the skin are highly variable among patients with identical lesion sites [33], possibly due to the increasing number of lymphatic vessels by tumor lymphangiogenesis, which are not yet functional.
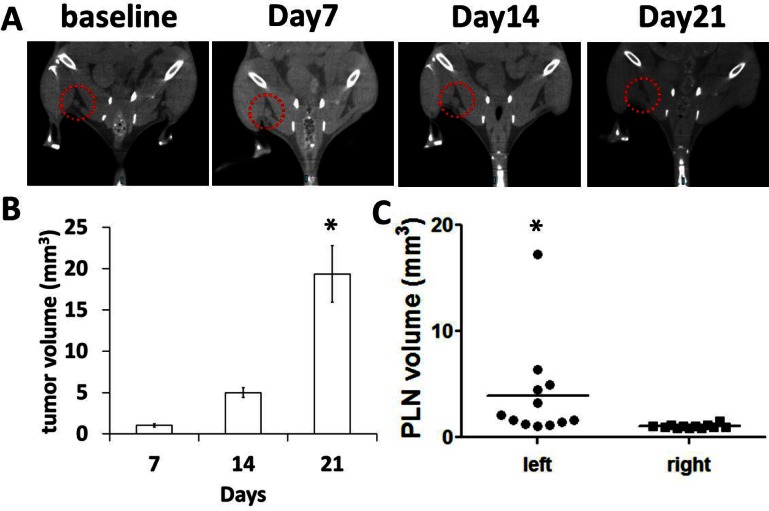
Although CT cannot accurately assess the cancer status of enlarged LNs and differentiate LN tumor volume from overall LN volume, CT was conducted to measure the volume of tumor draining LNs. Our CT data showed significantly enlarged tumor-draining PLNs at 21 days p.i. (Figs. 5A and 5C). The tumor volume on the paw at day 21 was significantly larger than that at day 14 (Fig. 5B).
Fig. 5.
Representative CT images (A) of a mouse prior to and at 7, 14, and 21 days p.i. showing a gradually enlarged tumor-draining PLN as shown in red dotted circles. The primary tumor volume on the left paw (B) is significantly increased at 21 days p.i. as compared to that at 14 days p.i.. * p < 0.05 vs. days 7. The volume of tumor-draining PLNs at 21 days p.i. (C) is significantly larger than that in contralateral PLNs. * p < 0.05 vs. contralateral right PLN.
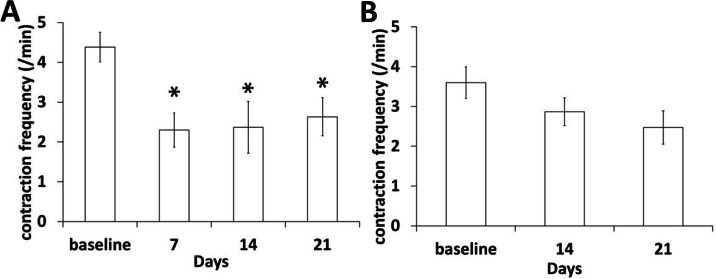
Non-invasive, time-lapse NIRF imaging with 200 ms exposure time showed dynamic movement of fluorescent ICG-laden lymph. We quantified lymphatic contractile function to investigate how tumor growth and metastasis affect lymphatic vessel function in mice with a foot tumor. As shown in Fig. 6, contraction frequency in the afferent lymphatic vessels (Fig. 6A) draining from the tumor to the PLN was significantly reduced from 7 days p.i.. However, we observed no significant changes in the efferent lymphatic vessels from the PLN to the IsLN, although there is a trend toward reduction in contractility (Fig. 6B).
Fig. 6.
Significant reduction of lymphatic contractile function in an afferent popliteal lymphatic vessel during tumor growth and LN metastasis (A) was observed, whereas lymphatic contraction frequency in an efferent popliteal lymphatic vessel was not changed (B). n = 12 mice. * p < 0.05 vs. baseline.
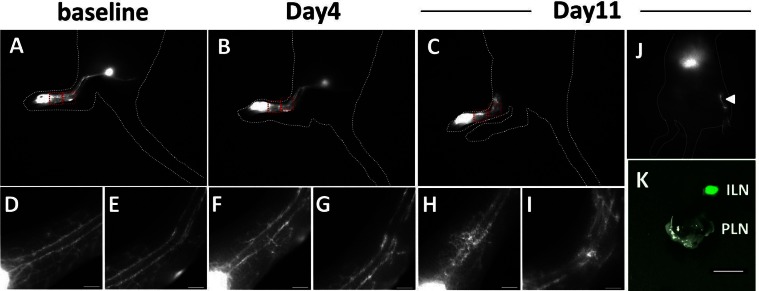
We observed PLN metastasis in 5 out of 12 mice (Table 1) consistent with the previously reported ~40% rate of regional LN metastasis in the B16F10 model [34,35]. Three of 5 mice with LN metastasis showed altered lymphatic drainage to the ILN (Table 1). We observed no ILN metastasis in those three mice. In addition, no evidence of LN metastasis was observed in the other 6 tumor-draining PLNs examined. In order to test our hypothesis that altered lymphatic drainage is due to tumor blockage of normal lymphatic drainage, we also used another model in which the PLN of a mouse was directly injected with B16F10 cells. As shown in magnified images in Figs. 7D–7G, lymphatic drainage patterns did not change at 4 days p.i. as compared to baseline, indicating that surgery did not disrupt normal lymphatic drainage. However, we observed abnormal lymphatic drainage to the ILN in all 4 mice at 11 days p.i. as shown in Fig. 7J. Fluorescent images of a dissected ILN showed strong fluorescence as compared to the PLN with extensive tumor, which had no ICG fluorescence, demonstrating that gross tumor in the PLN blocked and rerouting normal lymphatic drainage to the ILN.
Table 1. Metastatic Frequency of B16F10 to PLNs and the Status of Altered Lymphatic Drainage Pattern*.
| Inoculation | No. of metastatic LNs | No. of altered lymphatic drainage patterns |
|---|---|---|
| B16F10, foot | 5/12 (41.7) | 3/5 (60) |
| B16F10, PLN | 4/4 (100) | 4/4 (100) |
*Percentage in parentheses.
Fig. 7.
Representative fluorescent images of a mouse prior (A, D, E) to and at 4 (B, F, G) and 11 days p.i. (C, H, I). Scale, 1 mm. Magnified fluorescent images (D, E, F, G, H, I) of two rectangles in A, B, and C showed changes of lymphatic drainage during tumor growth in the PLN. Altered lymphatic drainage to the ILN was observed at 11 days P.I. as indicated by an arrowhead (J). The injection site was covered with black paper to prevent camera saturation. Fluorescent image of dissected ILN and PLN (K). Scale, 1 mm.
Our data showing tumor blockage and rerouting of normal lymphatic drainage in three mice at 21 days p.i. mimic advanced-stage melanoma with clinically palpable metastasis. Although altered lymphatic drainage patterns were observed at 21 days p.i., non-invasive functional NIRF imaging demonstrated a significant reduction of lymphatic contractility in tumor-draining lymphatic vessels from 7 days p.i.. Although not statistically significant, our data also showed less lymphatic contractility in an afferent popliteal lymphatic vessel in mice with metastatic PLNs as compared to mice with no LN metastasis (p = 0.25; 2.0 ± 0.34 vs. 3.3 ± 0.71, respectively) at 21 days p.i.. Further investigation is needed to elucidate what specific factors draining from B16F10 tumor to the PLN cause a reduction in lymphatic function. Previous ex vivo studies showed that superfusion of murine melanoma B16BL6 supernatants, but not another murine melanoma K1735 M2, to isolated iliac lymphatic microvessels of ddY mice caused dilation of the vessels and significant inhibition of lymph pump activity in a dose-dependent manner [36]. These authors suggested B16BL6 cells release nonpeptide substances, which cause production and release of endogenous nitric oxide from lymphatic vessels. However, more experimentation is needed to better recapitulate tumor microenvironment and to account for the influence of the lymphatic system on tumor growth and metastasis. For example, altered lymphatic function may cause recurrent lymphatic metastasis possibly arising from cancer cells arrested within the lymphatic system after surgical dissection of primary tumor and SLNs [37]. Moreover, abnormal lymph flow may also lead to breakage of the lymphatic vessel walls and formation of in-transit metastasis [37]. Indeed, it is known that primary melanoma tumors often develop in-transit metastasis in the direction of lymph flow. Previously we observed in-transit metastasis of B16F10 melanoma tumor in the dorsal aspect of the left hindpaw, where we observed tortuous and leaky lymphatic vessels [27].
4. Conclusion
In this study we demonstrated changes of lymphatic function and drainage pathways in response to tumor growth and LN metastasis using NIRF imaging with sufficient spatial and temporal resolution and sensitivity. In addition, we have previously shown non-invasive NIRF imaging of altered lymphatic function and architecture on the diseased foot in melanoma patients using microdose amounts of ICG due to the sensitivity of our device [27]. There are new emerging therapeutic strategies including pharmacological agents to molecularly target the lymphatics and tumor lymphangiogenesis in order to inhibit cancer metastasis and progression. While these new therapies are assessed with indirect measures of efficacy, a diagnostic method to non-invasively assess lymphatic response as mechanism of therapeutic action could accelerate the translation of novel therapies into the clinic.
Acknowledgments
The authors would like to thank Holly Robinson and Gabriel Dickinson for technical assistance with the mice used in these experiments. This work was supported in part by the National Institutes of Health R21 CA159193 (SK) and R01 CA128919 (EMS).
References and links
- 1.Stacker S. A., Achen M. G., Jussila L., Baldwin M. E., Alitalo K., “Lymphangiogenesis and cancer metastasis,” Nat. Rev. Cancer 2(8), 573–583 (2002). 10.1038/nrc863 [DOI] [PubMed] [Google Scholar]
- 2.Rinderknecht M., Detmar M., “Tumor lymphangiogenesis and melanoma metastasis,” J. Cell. Physiol. 216(2), 347–354 (2008). 10.1002/jcp.21494 [DOI] [PubMed] [Google Scholar]
- 3.Uren R. F., Howman-Giles R., Chung D. K., Thompson J. F., “Metastatic occlusion of a lymphatic collecting vessel in a patient with cutaneous melanoma and clinically normal lymph nodes,” Clin. Nucl. Med. 32(4), 312–313 (2007). 10.1097/01.rlu.0000257178.54240.90 [DOI] [PubMed] [Google Scholar]
- 4.Lam T. K., Uren R. F., Scolyer R. A., Quinn M. J., Shannon K. F., Thompson J. F., “False-negative sentinel node biopsy because of obstruction of lymphatics by metastatic melanoma: the value of ultrasound in conjunction with preoperative lymphoscintigraphy,” Melanoma Res. 19(2), 94–99 (2009). 10.1097/CMR.0b013e32832166b7 [DOI] [PubMed] [Google Scholar]
- 5.Kwon S., Sevick-Muraca E. M., “Functional lymphatic imaging in tumor-bearing mice,” J. Immunol. Methods 360(1-2), 167–172 (2010). 10.1016/j.jim.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dadras S. S., Lange-Asschenfeldt B., Velasco P., Nguyen L., Vora A., Muzikansky A., Jahnke K., Hauschild A., Hirakawa S., Mihm M. C., Detmar M., “Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes,” Mod. Pathol. 18(9), 1232–1242 (2005). 10.1038/modpathol.3800410 [DOI] [PubMed] [Google Scholar]
- 7.Alitalo A., Detmar M., “Interaction of tumor cells and lymphatic vessels in cancer progression,” Oncogene 31(42), 4499–4508 (2012). 10.1038/onc.2011.602 [DOI] [PubMed] [Google Scholar]
- 8.Skobe M., Hamberg L. M., Hawighorst T., Schirner M., Wolf G. L., Alitalo K., Detmar M., “Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma,” Am. J. Pathol. 159(3), 893–903 (2001). 10.1016/S0002-9440(10)61765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skobe M., Hawighorst T., Jackson D. G., Prevo R., Janes L., Velasco P., Riccardi L., Alitalo K., Claffey K., Detmar M., “Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis,” Nat. Med. 7(2), 192–198 (2001). 10.1038/84643 [DOI] [PubMed] [Google Scholar]
- 10.Streit M., Detmar M., “Angiogenesis, lymphangiogenesis, and melanoma metastasis,” Oncogene 22(20), 3172–3179 (2003). 10.1038/sj.onc.1206457 [DOI] [PubMed] [Google Scholar]
- 11.Lin J., Lalani A. S., Harding T. C., Gonzalez M., Wu W. W., Luan B., Tu G. H., Koprivnikar K., VanRoey M. J., He Y., Alitalo K., Jooss K., “Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor,” Cancer Res. 65(15), 6901–6909 (2005). 10.1158/0008-5472.CAN-05-0408 [DOI] [PubMed] [Google Scholar]
- 12.Roberts N., Kloos B., Cassella M., Podgrabinska S., Persaud K., Wu Y., Pytowski B., Skobe M., “Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2,” Cancer Res. 66(5), 2650–2657 (2006). 10.1158/0008-5472.CAN-05-1843 [DOI] [PubMed] [Google Scholar]
- 13.Hoshida T., Isaka N., Hagendoorn J., di Tomaso E., Chen Y. L., Pytowski B., Fukumura D., Padera T. P., Jain R. K., “Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications,” Cancer Res. 66(16), 8065–8075 (2006). 10.1158/0008-5472.CAN-06-1392 [DOI] [PubMed] [Google Scholar]
- 14.He Y., Kozaki K., Karpanen T., Koshikawa K., Yla-Herttuala S., Takahashi T., Alitalo K., “Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling,” J. Natl. Cancer Inst. 94(11), 819–825 (2002). 10.1093/jnci/94.11.819 [DOI] [PubMed] [Google Scholar]
- 15.Harrell M. I., Iritani B. M., Ruddell A., “Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis,” Am. J. Pathol. 170(2), 774–786 (2007). 10.2353/ajpath.2007.060761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirakawa S., Kodama S., Kunstfeld R., Kajiya K., Brown L. F., Detmar M., “VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis,” J. Exp. Med. 201(7), 1089–1099 (2005). 10.1084/jem.20041896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji R. C., “Lymph node lymphangiogenesis: a new concept for modulating tumor metastasis and inflammatory process,” Histol. Histopathol. 24(3), 377–384 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Qian C. N., Berghuis B., Tsarfaty G., Bruch M., Kort E. J., Ditlev J., Tsarfaty I., Hudson E., Jackson D. G., Petillo D., Chen J., Resau J. H., Teh B. T., “Preparing the ‘soil’: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells,” Cancer Res. 66(21), 10365–10376 (2006). 10.1158/0008-5472.CAN-06-2977 [DOI] [PubMed] [Google Scholar]
- 19.Van den Eynden G. G., Vandenberghe M. K., van Dam P. J., Colpaert C. G., van Dam P., Dirix L. Y., Vermeulen P. B., Van Marck E. A., “Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node,” Clin. Cancer Res. 13(18), 5391–5397 (2007). 10.1158/1078-0432.CCR-07-1230 [DOI] [PubMed] [Google Scholar]
- 20.Proulx S. T., Luciani P., Derzsi S., Rinderknecht M., Mumprecht V., Leroux J. C., Detmar M., “Quantitative imaging of lymphatic function with liposomal indocyanine green,” Cancer Res. 70(18), 7053–7062 (2010). 10.1158/0008-5472.CAN-10-0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwon S., Sevick-Muraca E. M., “Noninvasive quantitative imaging of lymph function in mice,” Lymphat. Res. Biol. 5(4), 219–232 (2007). 10.1089/lrb.2007.1013 [DOI] [PubMed] [Google Scholar]
- 22.Kwon S., Sevick-Muraca E. M., “Mouse phenotyping with near-infrared fluorescence lymphatic imaging,” Biomed. Opt. Express 2(6), 1403–1411 (2011). 10.1364/BOE.2.001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapinski P. E., Kwon S., Lubeck B. A., Wilkinson J. E., Srinivasan R. S., Sevick-Muraca E., King P. D., “RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice,” J. Clin. Invest. 122(2), 733–747 (2012). 10.1172/JCI46116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevick-Muraca E. M., Sharma R., Rasmussen J. C., Marshall M. V., Wendt J. A., Pham H. Q., Bonefas E., Houston J. P., Sampath L., Adams K. E., Blanchard D. K., Fisher R. E., Chiang S. B., Elledge R., Mawad M. E., “Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study,” Radiology 246(3), 734–741 (2008). 10.1148/radiol.2463070962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sevick-Muraca E. M., “Translation of near-infrared fluorescence imaging technologies: emerging clinical applications,” Annu. Rev. Med. 63(1), 217–231 (2012). 10.1146/annurev-med-070910-083323 [DOI] [PubMed] [Google Scholar]
- 26.Maus E. A., Tan I. C., Rasmussen J. C., Marshall M. V., Fife C. E., Smith L. A., Guilliod R., Sevick-Muraca E. M., “Near-infrared fluorescence imaging of lymphatics in head and neck lymphedema,” Head Neck 34(3), 448–453 (2012). 10.1002/hed.21538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen J. C., Kwon S., Sevick-Muraca E. M., Cormier J. N., “The role of lymphatics in cancer as assessed by near-infrared fluorescence imaging,” Ann. Biomed. Eng. 40(2), 408–421 (2012). 10.1007/s10439-011-0476-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen J. C., Tan I. C., Marshall M. V., Adams K. E., Kwon S., Fife C. E., Maus E. A., Smith L. A., Covington K. R., Sevick-Muraca E. M., “Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence,” Transl. Oncol. 3(6), 362–372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan I. C., Maus E. A., Rasmussen J. C., Marshall M. V., Adams K. E., Fife C. E., Smith L. A., Chan W., Sevick-Muraca E. M., “Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging,” Arch. Phys. Med. Rehabil. 92(5), 756–764, e1 (2011). 10.1016/j.apmr.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dadras S. S., Paul T., Bertoncini J., Brown L. F., Muzikansky A., Jackson D. G., Ellwanger U., Garbe C., Mihm M. C., Detmar M., “Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival,” Am. J. Pathol. 162(6), 1951–1960 (2003). 10.1016/S0002-9440(10)64328-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leijte J. A., van der Ploeg I. M., Valdés Olmos R. A., Nieweg O. E., Horenblas S., “Visualization of tumor blockage and rerouting of lymphatic drainage in penile cancer patients by use of SPECT/CT,” J. Nucl. Med. 50(3), 364–367 (2009). 10.2967/jnumed.108.059733 [DOI] [PubMed] [Google Scholar]
- 32.Norman J., Cruse C. W., Espinosa C., Cox C., Berman C., Clark R., Saba H., Wells K., Reintgen D., “Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma,” Am. J. Surg. 162(5), 432–437 (1991). 10.1016/0002-9610(91)90255-C [DOI] [PubMed] [Google Scholar]
- 33.Uren R. F., Howman-Giles R., Thompson J. F., “Patterns of lymphatic drainage from the skin in patients with melanoma,” J. Nucl. Med. 44(4), 570–582 (2003). [PubMed] [Google Scholar]
- 34.Kawada K., Sonoshita M., Sakashita H., Takabayashi A., Yamaoka Y., Manabe T., Inaba K., Minato N., Oshima M., Taketo M. M., “Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes,” Cancer Res. 64(11), 4010–4017 (2004). 10.1158/0008-5472.CAN-03-1757 [DOI] [PubMed] [Google Scholar]
- 35.Ruddell A., Harrell M. I., Furuya M., Kirschbaum S. B., Iritani B. M., “B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma,” Neoplasia 13(8), 748–757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakaya K., Mizuno R., Ohhashi T., “B16-BL6 melanoma cells release inhibitory factor(s) of active pump activity in isolated lymph vessels,” Am. J. Physiol. Cell Physiol. 281(6), C1812–C1818 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Gershenwald J. E., Fidler I. J., “Cancer. Targeting lymphatic metastasis,” Science 296(5574), 1811–1812 (2002). 10.1126/science.10731318 [DOI] [PubMed] [Google Scholar]