Abstract
Objectives
The aim of this study was to define the prevalence and significance of myocardial edema in patients with non–ST-segment elevation acute coronary syndrome (NSTE-ACS).
Background
Most patients with NSTE-ACS undergo angiography, yet not all have obstructive coronary artery disease (CAD) requiring revascularization. Identifying patients with myocardium at risk could enhance the effectiveness of an early invasive strategy. Cardiac magnetic resonance (CMR) can demonstrate edematous myocardium subjected to ischemia but has not been used to evaluate NSTE-ACS patients.
Methods
One hundred consecutive patients with NSTE-ACS were prospectively enrolled to undergo 30-min CMR, including T2-weighted edema imaging and late gadolinium enhancement before coronary angiography. Clinical management including revascularization decision-making was performed without CMR results.
Results
Of 88 adequate CMR studies, 57 (64.8%) showed myocardial edema. Obstructive CAD requiring revascularization was present in 87.7% of edema-positive patients versus 25.8% of edema-negative patients (p < 0.001). By multiple logistic regression analysis after adjusting for late gadolinium enhancement, perfusion, and wall motion scores, TIMI risk score was not predictive of obstructive CAD. Conversely, an increase in T2 score by 1 U increased the odds of subsequent coronary revascularization by 5.70 times (95% confidence interval: 2.38 to 13.62, p < 0.001). Adjusting for peak troponin-I, patients with edema showed a higher hazard of a cardiovascular event or death within 6 months after NSTE-ACS compared with those without edema (hazard ratio: 4.47, 95% confidence interval: 1.00 to 20.03; p = 0.050).
Conclusions
In NSTE-ACS patients, rapid CMR identifies reversibly injured myocardium due to obstructive CAD and predicts worse outcomes. Identifying myocardium at risk may help direct appropriate patients toward early invasive management. (J Am Coll Cardiol 2010;55:2480–8)
Keywords: acute coronary syndrome, cardiac magnetic resonance imaging, edema, ischemia, myocardium
Drug therapies and revascularization have greatly reduced mortality and long-term cardiac impairment for survivors of acute coronary syndromes (ACS) (1,2). Non–ST-segment elevation acute coronary syndromes (NSTE-ACS) comprise over two-thirds of all ACS (3). This group may develop irreversible myocyte injury, contractile dysfunction, and arrhythmias; 20% suffer death, myocardial infarction, or recurrent ischemia by 1 year (3–6). However, predicting short-term and long-term risk remains challenging, and selection of initial management strategy varies in this patient population (7,8).
Variable clinical management including delays in NSTE-ACS care may stem from difficulty in identifying myocardium at risk. Clinical history, electrocardiography (ECG), and biomarkers perform well in establishing the diagnosis of NSTE-ACS (5); however, they are not designed to detect myocardium “at risk” but not yet irreversibly injured. Likewise, Thrombolysis In Myocardial Infarction (TIMI) (6) and other global risk scores on the basis of clinical and ECG data provide an average estimate of an individual’s risk of death or major ischemic events but do not specifically guide management decisions regarding timing of angiography. Because myocardium at risk may be salvaged by revascularization, an invasive strategy to identify obstructive coronary artery disease (CAD) is usually pursued once ACS is diagnosed (5). For patients with myocardium at risk, early intervention is invaluable (9); however, for patients without myocardium at risk, a costly invasive strategy confers no benefit and may add unnecessary bleeding and procedural risk (4). A diagnostic approach that identifies myocardium at risk within the heterogeneous NSTE-ACS population could facilitate timely revascularization and concentrate resource use to the most appropriate patients.
Extensive preclinical and human studies have established that T2 signal hyperintensity by cardiac magnetic resonance (CMR) indicates increased myocardial water content (10–14). T2 may increase within 30 min of ischemia onset—before detectable injury by troponin or late gadolinium enhancement (LGE) (15,16). T2-weighted CMR to identify myocardium that has recently suffered ischemia has been employed to distinguish ACS from non-ACS and new from old infarct scar in patients with undifferentiated chest pain (14,17–19). We sought to extend this work to investigate whether CMR with edema imaging could stratify patients admitted with NSTE-ACS to identify higher-risk patients who would warrant an early invasive management strategy.
Methods
Study population
Consecutive patients hospitalized with NSTE-ACS awaiting coronary angiography were prospectively enrolled over a 20-month period. Diagnosis required both suspected cardiac chest pain or anginal equivalent and either abnormal serum troponin-I (TnI) level or ischemic ECG changes (20). Patients under age 30 years were excluded to minimize coronary events mediated by nonatherosclerotic processes. Contraindication to magnetic resonance such as pacemaker or evidence of illicit drug ingestion constituted additional exclusion criteria. Clinical decision-making was performed by providers blinded to CMR results.
Medical history, clinical and ECG findings, and serological markers were recorded at entry. All patients provided written informed consent to participate in this Institutional Review Board-approved protocol.
CMR examination
Examinations were performed with a 1.5-T CMR system and 12-element phased-array cardiac coil (MAG-NETOM Avanto, Siemens Medical Solutions, Inc., Erlangen, Germany). A physician provided monitoring throughout the study. The CMR protocol (Table 1) included 4 acquisition types in the following order (the first 2 acquired pre-contrast): real-time multi-plane cine imaging suitable for wall motion assessment, T2-weighted imaging, resting first-pass perfusion imaging, and LGE. Cine images were obtained in horizontal long-axis (HLA), vertical long-axis (VLA), 3-chamber, and contiguous short-axis (SAX) planes with non-breathhold, real-time acquisition (21). T2-weighted breath-hold (12 to 15 s) turbo spin echo short tau inversion recovery images of the myocardium were obtained in 10-mm basal, mid, and apical SAX, VLA, 3-chamber, and HLA planes (12). Myocardial perfusion acquisition used an echo-planar first-pass imaging technique in four 10-mm planes: basal/mid/apical SAX, and HLA (22). Perfusion images were obtained at rest during intravenous infusion of 0.1 mmol/kg gadolinium diethylenetriamine penta-acetic acid. Ten minutes after additional 0.1 mmol/kg gadolinium diethylenetriamine penta-acetic acid administration, multi-plane nonbreathhold single-shot LGE images were obtained in the same planes as cine imaging, with appropriate inversion time selection to null normal myocardium (23).
Table 1.
Cardiac Magnetic Resonance Scan Parameters
| Sequence Type | Parallel Acceleration |
TR/TE (ms) |
Spatial Resolution | Temporal Resolution (ms) |
Acquisition Planes | |
|---|---|---|---|---|---|---|
| Function | Real-time SSFP | TSENSE rate 3 | 2.3/1.0 | 3.75 mm × 2.1 mm 8-mm slice thickness |
62 | HLA, VLA, contiguous SAX (10-12 slices), 3CH |
| Edema | T2-weighted triple-inversion STIR segmented turbo spin echo |
GRAPPA rate 2 | 2 × RR/80 | 1.6 mm × 1.6 mm 8-mm slice thickness |
156 | HLA, VLA, 3 SAX (base, mid, and apical), 3CH |
| Perfusion | Single-shot saturation recovery GRE-EPI | TSENSE rate 2 | 5.8/1.2 | 3.1 mm × 2.5 mm 10-mm slice thickness |
70 | 3 SAX (base, mid, and apical), HLA |
| Necrosis | Single-shot inversion recovery steady-state free-precession |
GRAPPA rate 2 | 2.8/1.3 | 2.8 mm × 2.1 mm 8-mm slice thickness |
285 | HLA, VLA, contiguous SAX (10-12 slices), 3CH |
3CH = 3-chamber; GRAPPA = generalized autocalibrating partially parallel acquisitions; GRE-EPI = gradient-echo, echo-planar hybrid imaging; HLA = horizontal long-axis; SAX = short-axis; SSFP = steady-state free-precession; STIR = short tau inversion recovery; TR/TE = repetition time/echo time; TSENSE = Time-adaptive SENSitivity Encoding; VLA = vertical long-axis.
Image analysis
Two CMR experts (S.V.R., O.P.S.) blinded to clinical information rated by consensus, after independent review, each patient’s CMR images by recording 17-segment (24) scores for each of the following: left ventricular (LV) myocardial T2 signal intensity, wall motion, perfusion, and LGE. Each variable was scored: T2: 0-normal, 1-intramyocardial hyperintensity; wall motion: 0-normal, 1-hypokinetic, 2-akinetic, 3-dyskinetic; perfusion: 0-normal, 1-abnormal; and LGE: 0-none, 1-hyperenhancement, 2-microvascular obstruction. Consensus scores were obtained for each study by 2 CMR experts. Segmental T2, perfusion, wall motion, and LGE scores were summed to yield patient-level aggregate scores.
The LV volumes and ejection fraction were estimated with the formula: volume = 0.85 × area^2/length, ml, and ejection fraction = (stroke volume)/end-diastolic volume, % (25).
Subsequent clinical care and outcomes
Invasive coronary angiography was done according to standard techniques. Significant coronary artery stenosis (≥70%) was identified by an independent interventional cardiologist (ELM) by visual assessment. Subsequent management decisions were made by the clinical team on the basis of clinically available data, including findings at coronary angiography but without knowledge of CMR results.
Follow-up at 6 months by phone interview and chart review documented the occurrence of death, heart failure, major arrhythmia, or hospital stay for an acute coronary event.
Statistical analysis
The mean values of continuous variables were compared with the 2-sample t test, given the normal distribution of the variables, and correlation between continuous variables was computed with the Pearson coefficient. Otherwise, comparison of median values was done with the 2-sample Wilcoxon rank-sum test. Prevalence variables were compared with the 2-sample test for proportion. To test the relationship between presence/absence of edema and normal/abnormal initial TnI, a tetrachoric correlation coefficient was calculated. Logistic regression was used to model the relationship between edema-positivity and peak TnI as well as LGE score. Logistic regression of intervention on T2 score was performed by adjusting for LGE score, wall motion score, and myocardial perfusion score. Cox proportional hazards regression model was used to evaluate the composite occurrence of events within 6-month follow-up with a group predictor on the basis of presence/absence of edema.
Results
Study population
Six patients screened were not enrolled due to respiratory or hemodynamic instability (n = 5) or ventricular tachyarrhythmia (n = 1). Of 100 patients enrolled, 5 could not complete CMR examination due to claustrophobia; T2 image quality was inadequate in 7 patients. Patient characteristics for the remaining study population (n = 88) are summarized in Table 2; all were in sinus rhythm at the time of CMR examination.
Table 2.
Baseline Characteristics of Study Population
| All Patients (n = 88) |
Patients Managed Medically (n = 30) |
Patients Receiving Revascularization (n = 58) |
p Value | |
|---|---|---|---|---|
| Age, yrs | 59.1 ± 12.1 | 58.6 ± 11.3 | 59.4 ± 12.5 | 0.78 |
| Male | 57 (64.8) | 16 (53.3) | 41 (69.4) | 0.11 |
| Body mass index, kg/m2 | 29.4 (25.8-33.1) | 27.5 (23.4-31.8) | 30.2 (27.4-34.2) | 0.02* |
| Diabetes | 38 (43.2) | 12 (40.0) | 26 (44.8) | 0.66 |
| Smoker | 45 (51.1) | 12 (40.0) | 33 (56.9) | 0.13 |
| Hypertension | 69 (78.4) | 26 (86.7) | 43 (74.1) | 0.18 |
| Prior CAD | 47 (53.4) | 18 (60.0) | 29 (50.0) | 0.37 |
| Initial troponin-I, mg/dl | 0.11 (0.01-0.99) | 0.05 (0.01-0.30) | 0.14 (0.02-1.13) | 0.17 |
| Peak troponin-I, mg/dl | 8.2 ± 16.2 | 8.3 ± 14.7 | 7.9 ± 18.9 | 0.89 |
| TIMI risk score | 4 (3-5) | 4 (3-5) | 4.5 (3-5) | 0.47 |
Values are mean ± SD, n (%), or median (interquartile range).
p < 0.05 considered significant.
CAD = coronary artery disease; IQR = interquartile range; TIMI = Thrombolysis In Myocardial Infarction.
Subject race was African-American in 11 (13%) and Caucasian in the remainder. The majority of patients were overweight (median body mass index 30.1 kg/m2, interquartile range [IQR] 25.8 to 33.0 kg/m2). Thirty-three (38%) patients in this study group were transferred to our institution after initial presentation to another hospital. Peak TnI for patients who were troponin-positive (n = 77) occurred at admission in 9 (12%), after admission but before catheterization in 49 (64%), and after catheterization in 19 (25%).
CMR findings
Global LV systolic function was preserved in this population: ejection fraction averaged 61 ± 14%. Median wall motion score was 4 (IQR 1 to 10), with 80% of patients having at least 1 dysfunctional LV segment. Median perfusion score was 1 (IQR 0 to 3). Similarly, median LGE score was 1 (IQR 0 to 3); LGE score was non-zero in 53 patients (60.2%). As expected, LGE score increased with increasing peak TnI (R = 0.28, p < 0.05).
T2-weighted and LGE imaging allowed assessment of presence and extent of myocardial edema and irreversible injury (Fig. 1). Edema was detected in 57 patients (64.8%), with a median of 2 (IQR 0 to 3) segments per patient showing edema. Clinical characteristics, including TIMI risk score, were similar between patients with versus those without edema (Table 3). Time from initial admission to CMR was also similar in patients with versus those without edema (45.9 ± 36.1 h vs. 53.9 ± 52.3 h, p = 0.45). There was greater prevalence of myocardial edema in patients transferred in from an outside hospital versus patients presenting directly to Ohio State University (41 of 55 or 74% vs. 16 of 33 or 48%, p = 0.01).
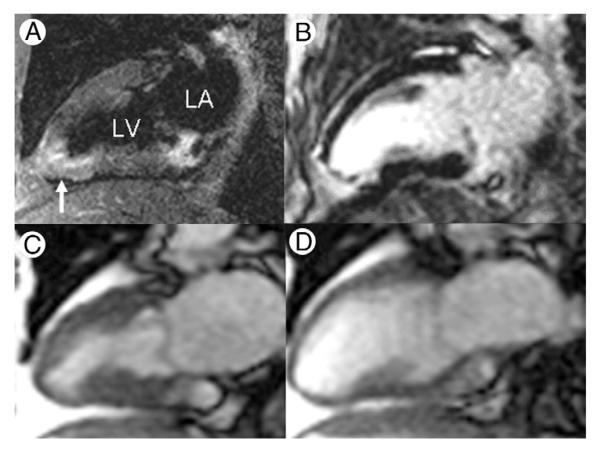
Figure 1. Myocardial Edema at Initial Presentation With NSTE-ACS.
Magnetic resonance images obtained in a 63-year-old female nonsmoker with chest pain, nonspecific electrocardiographic abnormalities, and troponin-I that increased from 0.04 to 2.36 mg/dl over the initial hours of hospital stay. T2-weighted imaging (A; vertical long-axis plane) shows infero-apical edema (arrow), and late postgadolinium enhancement (B) indicates irreversible injury. There is corresponding wall motion abnormality indicated by abnormal myocardial thickening at end-systole (C) compared with end-diastole (D) of a vertical long-axis cine. NSTE-ACS = non–ST-segment elevation acute coronary syndrome.
Table 3.
Patient Characteristics by Myocardial Edema on T2-Weighted Imaging
| Edema Absent (n = 31) |
Edema Present (n = 57) |
p Value | |
|---|---|---|---|
| Age, yrs | 58.2 ± 10.3 | 59.6 ± 13.0 | 0.59 |
| Male | 18 (58.1) | 39 (68.4) | 0.33 |
| Body mass index, kg/m2 | 29.3 (24.5-32.8) | 29.5 (27.2-33.1) | 0.86 |
| Diabetes | 12 (38.7) | 26 (45.6) | 0.53 |
| Smoker | 12 (38.7) | 33 (57.9) | 0.20 |
| Hypertension | 27 (87.1) | 42 (73.7) | 0.14 |
| TIMI risk score | 4 (3-5) | 4 (3-5) | 0.59 |
| History of CAD | 18 (58.1) | 29 (50.9) | 0.52 |
| Baseline ECG, ST-segment depression |
13 (41.9) | 28 (49.1) | 0.52 |
| Peak troponin-I, mg/dl | 0.43 (0.05-1.48) | 4.66 (0.92-12.65) | <0.01 |
Values are mean ± SD, n (%), or median (interquartile range). *p < 0.05 considered significant. ECG = electrocardiography; other abbreviations as in Table 2.
Patients with edema were more likely to have evidence of myocardial injury. Median LGE score was 2 (IQR 0 to 4) in the T2-positive compared with 0 (IQR 0 to 2) in T2-negative patients (p = 0.01). Similarly, patients with edema also had higher peak TnI values by the end of their hospital stay compared with patients without myocardial edema: 11.15 ± 18.4 mg/dl versus 2.72 ± 8.98 mg/dl, respectively (p < 0.05).
Initial TnI positivity was not significantly related to edema positivity (tetrachoric correlation coefficient = 0.31); that is, patients with negative initial TnI could have edema. Indeed, among 34 patients with negative initial TnI, 18 patients were T2-positive; of note, subsequent TnI elevation developed in 17 of 18 patients. We also identified patients with areas of edema in the absence of gross irreversible injury by LGE: of 34 LGE-negative patients, 17 (48.6%) showed edema on T2-weighted imaging (Fig. 2). Initial TnI values did not discriminate within this LGE-negative subgroup (0.37 ± 0.45 mg/dl if edema-positive vs. 0.28 ± 0.47 mg/dl if edema-negative, p = 0.57). Twenty-eight patients demonstrated an area subtended by edema that was larger than that showing irreversible injury by an average of 2.5 LV segments, consistent with edema as marker of area-at-risk.
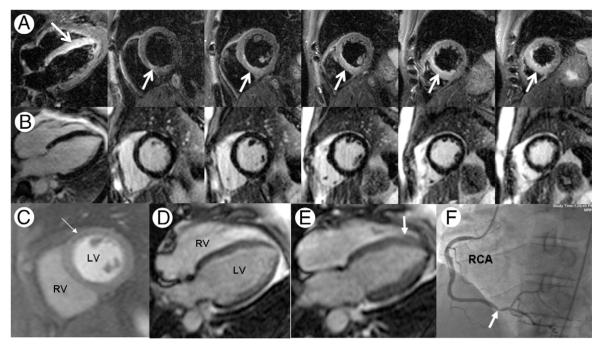
Figure 2. Myocardial Edema Without Necrosis in Unstable Angina.
Magnetic resonance images were obtained in a 41-year-old male smoker with non–ST-segment elevation acute coronary syndrome and serially negative biomarkers including troponin-I and creatine kinase-myocardial band. T2-weighted imaging (A; horizontal long-axis and serial short axis planes) showed edema (arrows) involving the inferoseptum from base to apex. Edema was present without infarction, on the basis of lack of late gadolinium enhancement at the same slice locations (B). Contrast-to-noise in the edematous versus remote myocardial regions averaged 18.8 ± 5.1, consistent with prior reports using this technique. Resting perfusion showed a mild subendocardial abnormality (C, arrow). End-diastolic (D) and end-systolic (E) frames from a horizontal long-axis cine showed abnormal thickening of the septum (E, arrow) compared with the lateral wall. Overall left ventricular (LV) ejection fraction was 40%. Invasive angiography (F) confirmed high-grade right coronary artery (RCA) stenosis (F, arrow) supplying an occluded left anterior descending coronary artery, prompting surgical revascularization.
Angiographic findings
Adding a 30-min CMR protocol to the patients’ initial evaluation process did not impact timing of usual care, with median door-to-catheterization time of 37 (IQR 22 to 63) h. Identification of ≥70% stenosis by angiography resulted in percutaneous coronary intervention alone in 45 patients, coronary artery bypass graft surgery alone in 10, and both percutaneous coronary intervention and coronary artery bypass graft surgery in 3 for a total of 58 (66%) patients being revascularized versus 30 (34%) without significant coronary stenosis treated with medical management alone.
Clinical and functional variables were largely nondiscriminating between patients with versus those without CAD requiring revascularization (Table 2). Median TIMI risk score was 4.5 in patients who were revascularized and 4.0 in those treated medically (p = 0.47). In contrast, presence of edema was an extremely powerful discriminator: 50 of 57 (87.7%) patients showing T2-positivity had obstructive CAD requiring revascularization, compared with 8 of 31 (25.8%) T2-negative patients (p < 0.001) (Fig. 3). After controlling for LGE score, perfusion score, and wall motion score, TIMI risk score was not predictive, whereas T2 score was predictive of obstructive CAD requiring revascularization; an increase in T2 score by 1 U increased the odds of revascularization by 5.70 times (95% confidence interval: 2.38 to 13.62, p < 0.001).
Figure 3. Edema at NSTE-ACS Presentation Portends Need for Coronary Revascularization.
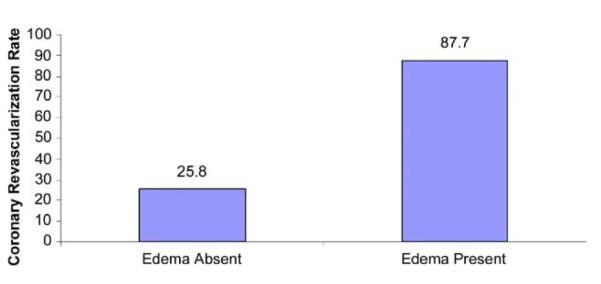
Presence of ≥70% coronary stenosis requiring revascularization was considerably higher in non–ST-segment elevation acute coronary syndrome (NSTE-ACS) patients with myocardial edema by magnetic resonance imaging compared with those without edema.
There were 8 instances where CMR showed no edema, but revascularization was performed. In 2, other CMR findings indicated significant coronary artery stenosis—1 patient had dense segmental wall motion abnormalities, and the second had segmental wall motion and corresponding dense perfusion abnormalities. In the remaining 6, anatomic stenosis by angiography that prompted revascularization produced no corresponding abnormality in any CMR measure (i.e., wall motion, perfusion, T2 imaging, and LGE were all normal). Further evaluation of coronary lesion significance with techniques such as fractional flow reserve was not done.
In 7 instances, CMR showed edema, but coronary revascularization was not performed. All 7 patients had significant coronary atherosclerosis; however, 3 had no technically suitable anatomic targets for revascularization. A fourth had angiographic findings suggestive of plaque that likely produced distal embolization and myocardial sequelae without residual stenosis.
Clinical outcomes
Five patients had disconnected phone numbers without recurrent hospital stays at our institution or the facility where they had initially presented. In the remaining patients, 16 events occurred during the 6 months after initial NSTE-ACS admission: 12 (14%) had recurrent NSTE-ACS (7 unstable angina, 5 subendocardial MI) requiring hospital stay, 2 (2%) were hospitalized for heart failure, and 2 died during the follow-up period—1 due to presumed cardiac arrest at home, and another due to progressive renal failure in the setting of multiple myeloma; of note, all but 2 events occurred in edema-positive patients (Fig. 4).
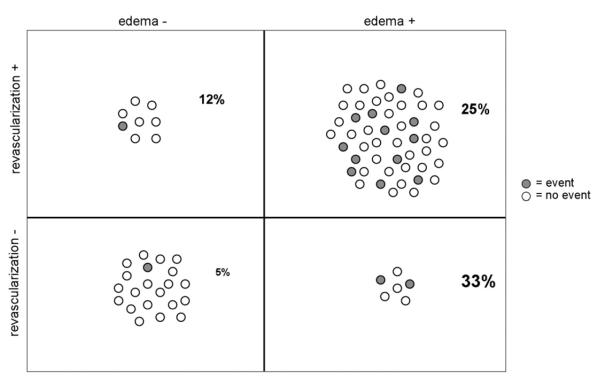
Figure 4. Distribution of Patients by Edema and Revascularization Status.
Shaded circles indicate adverse events at 60-day follow-up; all but 2 occurred in the non–ST-segment elevation acute coronary syndrome patients who were edema-positive at baseline.
Figure 5 shows the Kaplan-Meier survival function estimates in patients by edema status. Applying Cox modeling after adjusting for peak troponin-I demonstrated that, regardless of revascularization, patients with edema showed a higher hazard of a cardiovascular event or death within 6 months after NSTE-ACS compared with those without edema (hazard ratio: 4.47, 95% confidence interval: 1.00 to 20.03; p = 0.050).
Figure 5. Kaplan-Meier Survival Function Estimates in Patients by Edema Status.
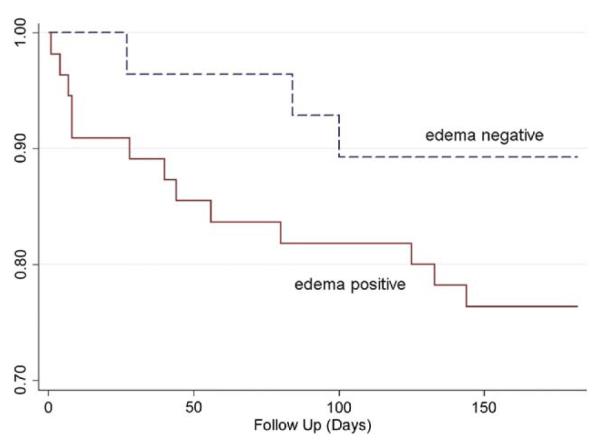
Estimated survival functions for the time to major adverse cardiac event or death in non–ST-segment elevation acute coronary syndrome patients indicates a greater cumulative hazard of subsequent events in patients with edema at presentation compared with those without edema.
Discussion
In this prospective study of patients with NSTE-ACS undergoing invasive coronary angiography, myocardial edema identified by rapid CMR examination distinguished patients with significant CAD requiring coronary revascularization. In contrast, clinical variables that included TIMI risk score, cardiac enzyme levels, and ECG changes—all of which are nonetheless essential to diagnose NSTE-ACS—did not discriminate among patients with established NSTE-ACS as to who would go on to require subsequent revascularization after coronary angiography versus those that would be treated with medical management alone.
Our findings indicate that demonstrating myocardial edema in vivo in patients with NSTE-ACS can be a powerful tool with major clinical implications. It has long been known that myocardial ischemia of sufficient duration leaves behind an area of edema (26,27) that can be visualized by CMR (14,28,29). This can establish the occurrence of ischemic episodes in patients presenting with undifferentiated chest pain (14,17). However, in this work we did not exploit T2-positivity to reveal a “history” of recent myocardial ischemia, because our study enrolled patients in whom the diagnosis of NSTE-ACS was already established. Instead, we used edema imaging to identify myocardium not irreversibly injured but at risk of further injury and hence most likely to benefit from an early invasive strategy. Furthermore, we found a sizable number of patients with NSTE-ACS with only T2-positivity, negative initial troponin-I, and no LGE, consistent with areas of myocardium where transient flow reduction with sufficient duration or severity has produced edema without irreversible injury. The implication in NSTE-ACS is that visualization of edematous but not-yet-irreversibly injured myocardium signals the presence of a coronary artery lesion susceptible to repeated episodes of transient coronary artery thrombus formation, which requires aggressive treatment to minimize irreversible injury.
Review of revascularization strategy used in our series of patients showed consistency with American Heart Association/American College of Cardiology guidelines that advocate percutaneous coronary intervention or coronary artery bypass graft surgery for NSTE-ACS patients identified with significant coronary artery stenosis (5); type of revascularization strategy was not distinguished in our study. Because of the reliance on coronary anatomic findings and current practice that emphasizes an early invasive strategy in management of NSTE-ACS, we maintained this paradigm in our study by adding CMR with T2-weighted imaging of edema in all subjects without affecting time to angiography. Our finding of the ability of CMR to predict flow-limiting CAD suggests that edema imaging may, by demonstrating myocardium in jeopardy, demonstrate a myocardial signature of diverse pathophysiological processes and clinical events with relevance to management. Although our study was not powered to assess outcomes, we identified a trend to worse prognosis in edema-positive patients that was present despite the presumed benefits of greater frequency of revascularization in this cohort.
Study limitations
We did not enroll low-risk patients without a definite plan to pursue invasive coronary angiography. This population represents a subset of all NSTE-ACS patients where the risk/benefit profile of an early invasive strategy is less certain. Lack of randomization is another limitation of our study, as is the relatively small number of outcome events limiting the ability to define the unique prognostic utility of identifying edema. Given the promising results of this work, a prospective randomized trial to assess the merits of CMR in guiding therapeutic strategy in this population is warranted.
Limitations of the short-TI inversion recovery technique for T2-weighted myocardial imaging include sensitivity to motion that may result in the false appearance of relative signal enhancement. Although we used the surface coil normalization algorithm provided by the vendor, we recognize use of a surface coil for T2-weighted imaging as a limitation. Quantitative T2-mapping should offer a more robust method of identifying regional myocardial edema, compared with visual assessment of T2-weighted images (30).
Clinical implications
Potential discrepancies between CMR-derived myocardium at risk and angiography illuminate distinctions between the information provided by the 2 modalities. In patients without edema who were felt to require coronary revascularization after angiography, most actually had no CMR abnormalities by cine, perfusion, or LGE imaging, that is, anatomic disease without myocardial sequelae. The long-term benefit of revascularization in such cases remains uncertain, particularly in light of recent studies that suggest inconsistent improvement in outcomes when adjusting for treatment selection bias (31,32). Conversely, CMR findings of edema without subsequent revascularization need not indicate “false-positives.” Our results indicated a substantial coronary atherosclerosis burden in all such patients, albeit without suitable anatomic targets for revascularization and possible resolution of thrombus before CMR because of intensive anticoagulant therapy.
In this prospective study, we did not randomize patients to early invasive versus selectively invasive strategy on the basis of CMR findings, nor were patients’ revascularization or other management decisions affected by CMR results. Further randomized studies are warranted to study the impact of CMR with edema imaging on selection of management strategies in patients with NSTE-ACS to identify approaches that reduce adverse events (20,32–35). If such studies confirm the predictive value suggested by our work, CMR at regional medical centers could help distinguish patients who would benefit from accelerated transfer to facilities with interventional capabilities from those in whom invasive angiography is not likely to identify need for revascularization. Cost-effectiveness analyses are also required to evaluate adding CMR into the initial evaluation of NSTE-ACS versus current practice, which in many centers routinely deploys even costlier invasive angiography in these patients (36).
Conclusions
Rapid CMR examination including imaging of edema, perfusion, wall motion, and irreversible injury identifies myocardium at risk in NSTE-ACS patients. This diagnostic strategy does not require stress, and may define a myocardial signature predictive of obstructive CAD requiring revascularization. Use of rapid CMR upon admission for NSTE-ACS warrants further evaluation through randomized trials as a tool to optimize selection of an early invasive strategy in these patients.
Acknowledgments
The authors are indebted to Beth McCarthy, RT, Michelle Ballinger, RN, and Tam Tran, BS for their assistance in coordinating all aspects of this study. They are also grateful to Dr. David Verhaert for his editorial feedback and the faculty and staff of Ohio State University CMR for their cooperation with implementing this study.
This work was supported by an Ohio State University DHLRI Research Development Grant, Ischemia and Metabolism Thematic Program. Drs. Raman and Simonetti receive research grant support from Siemens.
Abbreviations and Acronyms
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- ECG
electrocardiography
- HLA
horizontal long-axis
- IQR
interquartile range
- LGE
late gadolinium enhancement
- LV
left ventricular
- NSTE-ACS
non–ST-segment elevation acute coronary syndrome
- SAX
short axis
- TIMI
Thrombolysis In Myocardial Infarction
- TnI
troponin-I
- VLA
vertical long-axis
REFERENCES
- 1.Faxon DP. Early reerfusion strategies after acute ST-segment elevation myocardial infarction: the imortance of timing. Nat Clin Pract Cardiovasc Med. 2005;2:22–8. doi: 10.1038/ncpcardio0065. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Alication of current guidelines to the management of unstable angina and non-ST-elevation myocardial infarction. Circulation. 2003;108:III28–37. doi: 10.1161/01.CIR.0000086952.14979.32. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics 2008 Udate. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Hoenig MR, Doust JA, Aroney CN, Scott IA. Early invasive versus conservative strategies for unstable angina & non-ST-elevation myocardial infarction in the stent era. Cochrane Database Syst Rev. 2006;3:CD004815. doi: 10.1002/14651858.CD004815.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of atients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) J Am Coll Cardiol. 2007;50:e1–157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch A, Windhausen F, Tijssen JG, Verheugt FW, Cornel JH, de Winter RJ. Long-term outcome after an early invasive versus selective invasive treatment strategy in patients with non-ST-elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial): a follow-up study. Lancet. 2007;369:827–35. doi: 10.1016/S0140-6736(07)60410-3. [DOI] [PubMed] [Google Scholar]
- 8.Giugliano RP, White JA, Bode C, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med. 2009;360:2176–90. doi: 10.1056/NEJMoa0901316. [DOI] [PubMed] [Google Scholar]
- 9.Terkelsen CJ, Lassen JF, Norgaard BL, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J. 2005;26:18–26. doi: 10.1093/eurheartj/ehi002. [DOI] [PubMed] [Google Scholar]
- 10.Scholz TD, Martins JB, Skorton DJ. NMR relaxation times in acute myocardial infarction: relative influence of changes in tissue water and fat content. Magn Reson Med. 1992;23:89–95. doi: 10.1002/mrm.1910230110. [DOI] [PubMed] [Google Scholar]
- 11.Karolle BL, Carlson RE, Aisen AM, Buda AJ. Transmural distribution of myocardial edema by NMR relaxometry following myocardial ischemia and reperfusion. Am Heart J. 1991;122:655–64. doi: 10.1016/0002-8703(91)90508-f. [DOI] [PubMed] [Google Scholar]
- 12.Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 13.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Aty H, Simonetti O, Friedrich M. T2-weighted cardiovascular magnetic resonance imaging. J Magn Reson Imaging. 2007;26:452–9. doi: 10.1002/jmri.21028. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson JC, Nielsen G, Groenning BA, et al. Sustained postinfarction myocardial oedema in humans visualised by magnetic resonance imaging. Heart. 2001;85:639–42. doi: 10.1136/heart.85.6.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cury RC, Shash K, Nagurney JT, et al. Cardiac magnetic resonance With T2-weighted imaging imrpoves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–44. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 18.Kwong RY, Arai AE. Detecting patients with acute coronary syndrome in the chest pain center of the emergency department with cardiac magnetic resonance imaging. Crit pathw Cardiol. 2004;3:25–31. doi: 10.1097/01.hpc.0000116584.57152.06. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Aty H, Zagrosek A, Schulz-Menger J, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) J Am Coll Cardiol. 2007;50:652–726. [Google Scholar]
- 21.Simonetti OP, Cook SC, Bello G, Raman SV. Detection of myocardial wall motion abnormalities using real-time TSENSE. Proceedings of the International Society for Magnetic Resonance in Medicine 14th Scientific Meeting. 2006:3598. [Google Scholar]
- 22.Kellman P, Estein FH, McVeigh ER. Adative sensitivity encoding incororating temporal filtering (TSENSE) Magn Reson Med. 2001;45:846–52. doi: 10.1002/mrm.1113. [DOI] [PubMed] [Google Scholar]
- 23.Sievers B, Elliott MD, Hurwitz LM, et al. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007;115:236–44. doi: 10.1161/CIRCULATIONAHA.106.635409. [DOI] [PubMed] [Google Scholar]
- 24.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomogrpahic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 25.Dell’Italia LJ, Blackwell GG, Pearce DJ, Thorn B, Pohost GM. Assessment of ventricular volumes using cine magnetic resonance in the intact dog. A comparison of measurement methods. Invest Radiol. 1994;29:162–7. doi: 10.1097/00004424-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction. Underestimation of myocardial collateral blood flow and overestimation of exerimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation. 1979;60:866–76. doi: 10.1161/01.cir.60.4.866. [DOI] [PubMed] [Google Scholar]
- 27.Desai KV, Laine GA, Stewart RH, et al. Mechanics of the left ventricular myocardial interstitium: effects of acute and chronic myocardial edema. Am J Physiol Heart Circ Physiol. 2008;294:H2428–34. doi: 10.1152/ajpheart.00860.2007. [DOI] [PubMed] [Google Scholar]
- 28.Williams ES, Kalan JI, Thatcher F, Zimmerman G, Knoebel SB. Prolongation of proton sin lattice relaxation times in regionally ischemic tissue from dog hearts. J Nucl Med. 1980;21:449–53. [PubMed] [Google Scholar]
- 29.Foltz WD, Yang Y, Graham JJ, Detsky JS, Dick AJ, Wright GA. T2 fluctuations in ischemic and post-ischemic viable porcine myocardium in vivo. J Cardiovasc Magn Reson. 2006;8:469–74. doi: 10.1080/10976640600572897. [DOI] [PubMed] [Google Scholar]
- 30.Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Res. 2009;30:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirsch A, Windhausen F, Tijssen JG, et al. Diverging associations of an intended early invasive strategy comared with actual revascularization, and outcome in patients with non-ST-segment elevation acute coronary syndrome: the problem of treatment selection bias. Eur Heart J. 2009;30:645–54. doi: 10.1093/eurheartj/ehn438. [DOI] [PubMed] [Google Scholar]
- 32.O’Donoghue M, Boden WE, Braunwald E, et al. Early invasive vs conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction: a meta-analysis. JAMA. 2008;300:71–80. doi: 10.1001/jama.300.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira-Gonzalez I, Permanyer-Miralda G, Heras M, et al. Patterns of use and effectiveness of early invasive strategy in non-ST-segment elevation acute coronary syndromes: an assessment by roensity score. Am Heart J. 2008;156:946–53. 953.e2. doi: 10.1016/j.ahj.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 34.Qayyum R, Khalid MR, Adomaityte J, Papdakos S, Messineo FC. Systematic review: comaring routine and selective invasive strategies for the acute coronary syndrome. Ann Intern Med. 2008;148:186–96. doi: 10.7326/0003-4819-148-3-200802050-00005. [DOI] [PubMed] [Google Scholar]
- 35.Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165–75. doi: 10.1056/NEJMoa0807986. [DOI] [PubMed] [Google Scholar]
- 36.Dijksman LM, Hirsch A, Windhausen F, et al. Cost-effectiveness of early versus selectively invasive strategy in patients with acute coronary syndromes without ST-segment elevation. Int J Cardiol. 2009;131:204–11. doi: 10.1016/j.ijcard.2007.10.019. [DOI] [PubMed] [Google Scholar]