Abstract
We report the discovery of a facile transformation between perfluoroaromatic molecules and a cysteine thiolate, which is arylated at room temperature. This new approach enabled us to selectively modify cysteine residues in unprotected peptides, providing access to variants containing rigid perfluoroaromatic staples. This stapling modification performed on a peptide sequence designed to bind the C-terminal domain of an HIV-1 capsid assembly polyprotein (C-CA), showed enhancement in binding, cell permeability, and proteolytic stability properties, as compared to the unstapled analog. Importantly, chemical stability of the formed staples allowed us to use this motif in the native chemical ligation mediated synthesis of a small protein affibody that is capable of binding the Human Epidermal Growth Factor 2 (HER-2) receptor.
Cysteine derivatization of peptides and proteins has evolved into a powerful research and technological tool for post-synthetic modification of these biomolecules.1 There are now several methods available to modify cysteine, which include alkylation, conjugate addition, oxidation, and reduction.2 With the majority of reports on alkylation chemistry (vide supra), methods for mild arylation of cysteine are extremely rare.1,2 This is surprising, since it has been known for a long time that naturally occurring enzymes (e.g., glutathione S-transferase), are capable of selectively arylating glutathione via nucleophilic aromatic substitution (SNAr).3
Recently, Davis and co-workers4 utilized Mukaiyama’s reagent to form an arylated cysteine intermediate that underwent conversion to dehydroalanine. This suggests that it is possible to arylate cysteine in the context of bioconjugation using sufficiently activated reagents.1 To probe arylation of cysteines under mild conditions we evaluated perfluoroaromatic molecules. These species are known to exhibit high reactivity for SNAr reactions under non-forcing conditions,5 are chemically robust, commercially available, and possess a convenient 19F NMR handle that can enable characterization of the products screened in situ up to mid-micromolar concentrations. Schubert recently suggested this system satisfies many of the requirements of a “click” reaction, making it potentially attractive for bioconjugations (Figure 1A).5c–d Here we report the discovery of a cysteine perfluoroarylation, which can be used for mild functionalization of cysteine thiolate moieties in unprotected peptides. We further show how this chemistry can be applied towards peptide stapling rendering a new class of cell-permeable and bioactive peptides and small proteins.
Figure 1.
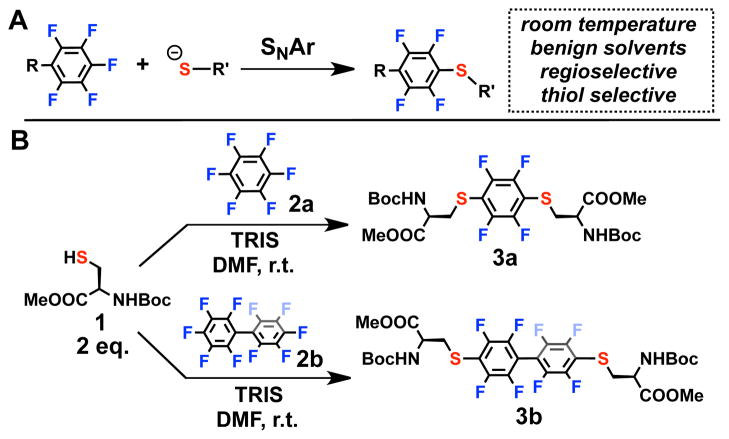
(A) SNAr reaction between thiolate and a perfluroaromatic species. (B) Model reactions carried out between protected cysteine derivative 1 and two commercially available perfluoroaromatic reagents 2a and 2b. In both cases the reaction yields are > 95% as determined by in situ 19F NMR spectroscopy – (see SI).
We focused on determining optimal conditions for coupling cysteine thiols and perfluorinated substrates using a model system featuring protected amino acid precursors. Reacting protected cysteine 1 with hexafluorobenzene 2a in the presence of base in polar organic solvents (see SI) for 4.5 hours led to substantial formation of the disubstituted product 3a (Figure 1, see SI for details on characterization).
Disubstitution (1,4) was observed exclusively in this process, even when hexafluorobenzene was used in excess (10-fold) with 1. Progress of this reaction was monitored by the in situ 19F NMR spectroscopy, where the starting material and product exhibited two distinct resonances (δ -138 and - 167 respectively, see Figure 1 and SI). Importantly, no substitution other than 1,4 was observed under these conditions, highlighting the unique regioselectivity of this process. The observed reactivity pattern was previously rationalized by the steric hindrance of the 2,5- (ortho) C–F sites5a and simultaneous activation of the 4- (para) C–F moiety5bof the aromatic ring by the thioether moiety formed in the monosubstituted species. The ability of the sulfur to stabilize the negative charge in the intermediate species after the first substitution, ultimately leads to increased rate of a second thiolate substitution thus favoring disubstituted species. Similar reactivity was observed when decafluorobiphenyl species 2b was used instead of 2a (see SI and Figure 1). We evaluated chemoselectivity of this SNAr process by screening several amino acids, which could potentially compete with the thiolate as a nucleophile (see SI). We observed no reactivity when competing reactions between carboxylate and amine unprotected cysteine analogs of 1, as well as lysine and histidine were used under similar reaction conditions in DMF at room temperature. The unique regio- and chemoselectivity observed in the reactions between perfluoroaromatic molecules 2a and 2b and cysteine 1 thus prompted us to evaluate this chemistry for stapling of unprotected peptides.
Previous studies established chemical transformations for side-chain crosslinking in a polypeptide for residues positioned in an i, i+4 fashion. The resulting peptide structure is commonly referred to as “stapled” motif.6 Researchers previously showed that “stapled” peptides exhibit different chemical and biological properties than their parent-unstapled surrogates.6 While a number of well-established approaches for peptide stapling currently exist,6 we envisioned that cysteine perfluoroarylation may lead to a new class of these species with new properties owing to the rigidity and lipophilicity of the perfluoroaromatic linkers. To test the developed protocol (vide supra) with unprotected peptides, we designed and synthesized two short peptide sequences 4 and 5 (for 5 see SI) that contained cysteine residues separated by 3 amino acids (i, i+4).
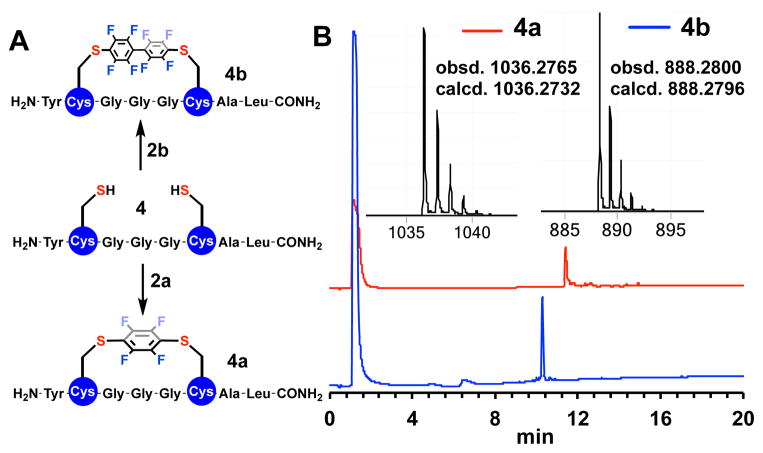
By simply incubating unprotected peptide (0.5–2 mM) 4 in the presence of 50 mM solution of TRIS (tris(hydroxymethyl)aminomethane) base in DMF for 4.5 hours with either 2a or 2b substrates, significant conversion of the starting material was observed by LC-MS (Figure 2 and SI) yielding stapled peptides 4a, b. These same experiments were carried out with peptide 5 (see SI for details on 5 series) and similar outcomes were observed. Progress of the reaction and the identity of the products were monitored by LC-MS and in situ 19F NMR spectroscopy (Figure 2 and SI). In both cases, the observed conversions of the starting peptide were greater than 90%.
Figure 2.
(A) Perfluoroarylation of cysteine residues in an unprotected peptide 4. (B) LC-MS traces of the crude reaction mixtures (214 nm, UV) and mass spectra (inset) of the stapled peptides 4a (bottom) and 4b (top).
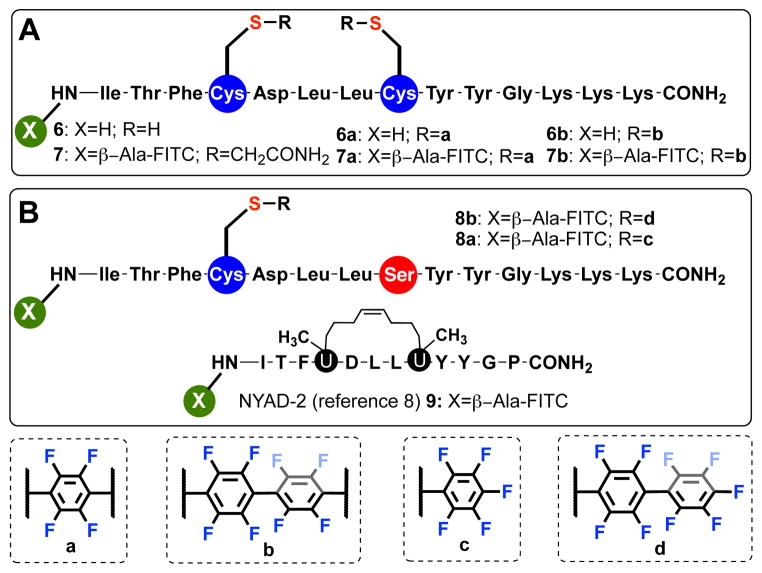
We then decided to evaluate our stapling protocol on more sophisticated peptide sequences to understand how perfluoroaromatic bridges affect the chemical and biological properties of these variants. We thus synthesized a model peptide 6 (Scheme 1) that is capable of binding the C-terminal domain of an HIV-1 capsid assembly polyprotein (C-CA).7 In particular, Cowburn et al. previously showed that stapling this sequence via olefin metathesis (approach originally developed by Grubbs and co-workers6g, later elaborated by Verdine et al.6c) could efficiently stabilize its α-helical conformation, rendering it more efficient in intra-cellular uptake and binding (Kd ~ 1 μM) to C-CA (Scheme 1, peptide 9).8
Scheme 1.
Peptides studied in the context of the C-CA model.
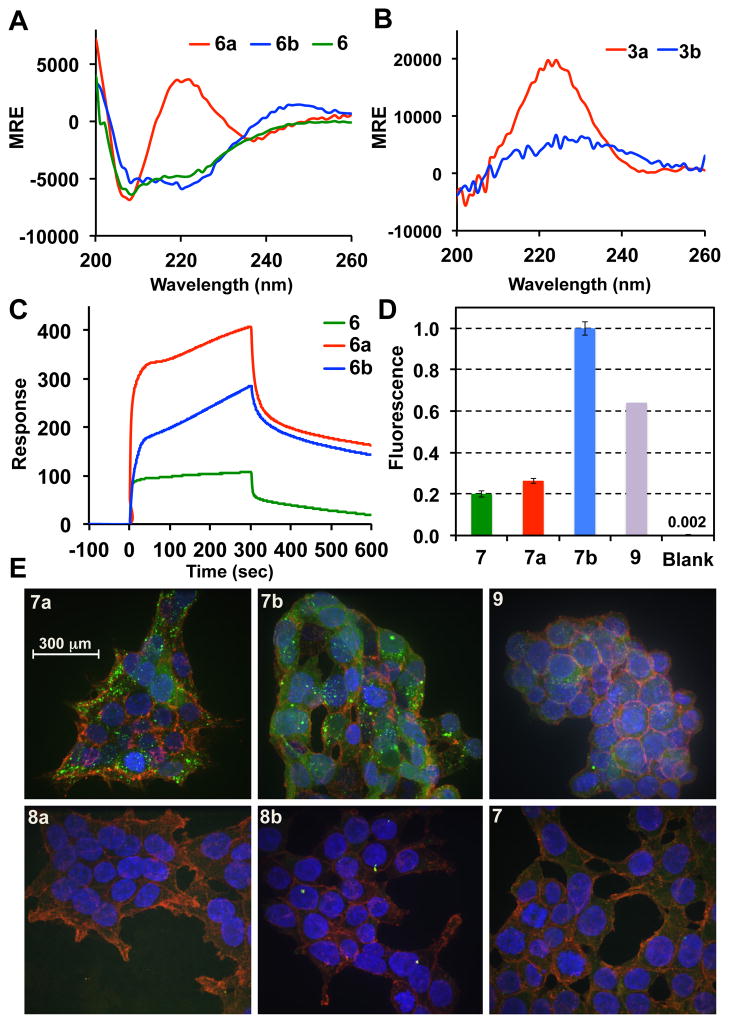
Peptide 6 (Scheme 1A) featuring 14 amino-acid residues with two cysteines positioned in an i, i+4 fashion, was successfully synthesized via Boc/Benzyl in situ neutralization solid-phase peptide synthesis (SPPS).9 Peptide 6 was further stapled with 2a and 2b through the developed protocol on a multi-milligram scale, affording the desired peptides in ~70% yield after RP-HPLC purification. Circular dichroism (CD) spectroscopy was used to probe the effect of stapling on the conformational features of the peptides (Figure 3A and SI). While the CD spectra of 6 and 6b (minima at 208 and 222 nm) showed an α-helical motif, the spectrum of 6a revealed an unusual behavior featuring a maxima at 222 nm and a minima at 208 nm (Figure 3A).6a We hypothesized that the C6F4 linker may be responsible for this behavior and thus performed control CD spectroscopic measurements on 3a and 3b. Indeed, in the case of 3a we observed a well-defined CD spectrum exhibiting absorbance maxima at 222 nm. For 3b, a similar spectrum, but with a decreased molar residual ellipticity (MRE) value centered at ~ 226 nm was observed (Figure 3B). While the nature of the signals in the CD spectra of 3a and 3b is yet to be determined, we were able to semi-quantitatively estimate the helical content of 6, 6a–b, assuming that MRE values arising from the introduction of the perfluorinated staple do not change significantly between 3a/3b and 6a/6b, respectively. We observed that while species 6 exhibited approximately 16% α-helical structure, stapling with 2a significantly enhances the α-helical content of the stapled peptide 6a up to 53%. For 6b, stapling was done with a longer linker (2b) and the α-helical content changed to 36%. This modest change is not surprising since the length of 2b is significantly longer than the distance between two Cys residues positioned at i, i+4 in a model α-helix. These observations were further corroborated from the molecular dynamics (MD) simulations of peptides 6 and 6a, b (see SI).
Figure 3.
(A) CD spectra of 6 and 6a, b and (B) of 3a, b; (C) Biacore binding sensograms of 6 and 6a, b with immobilized C-CA (adsorption/desorption time – 300 sec); (D) Flow cytometry data for FITC-based peptides incubated with HEK293T cells (25 μM; fluorescence response is averaged for each sample and normalized to 7b); (E) Z-stack accumulated fluorescent confocal microscopy images (DNA – blue; cell membrane – red; peptides – green (FITC)) of the HEK293T cells treated with peptides 7, 7a–b (5 μM), unstapled controls 8a and 8b and NYAD-2 – 9.
To test whether the perfluoroaryl crosslinks affects the proteolytic stability of the modified variants, we compared the distributions of cleaved products of 6 and 6a, b by LC-MS after treatment with protease. From these experiments we observed enhanced proteolitic stabilities of stapled 6a, b compared to unstapled peptide 6, when these species were incubated with trypsin or chymotrypsin (see SI). This is consistent with the observed conformational changes in stapled peptides 6a, b rendering these structures less prone to amide-bond cleavage by proteases. A drastic difference was observed when proteinase K was incubated with these peptides. As such, no Leu-Leu cleavage was observed for 6a, b (in contrast to 6) upon prolonged incubation of these peptides in the presence of proteinase K (3 hours, see SI), suggesting that the perfluoroaryl staple is capable of protecting the amide bonds positioned between the two Cys residues (see SI). We then evaluated and compared the binding affinities of peptides 6 and 6a, b using surface-plasmon resonance (SPR) assay enabled by the Biacore system. C-CA protein domain was immobilized on the Au-based chip via covalent attachment chemistry, and peptide analytes were evaluated at 5 μM concentrations (Figure 3C and SI). From these experiments we observed binding affinity differences, where the stapled peptides 6a, b were identified as better binders than 6. Significantly, observed binding trend correlates with the α-helicity of the peptides, which is consistent with the work of Debnath and coworkers.8 We further investigated whether these stapled peptides possess enhanced cellular interaction properties due to the combination of altered secondary structure and enhanced hydrophobicity provided by a perfluorinated staple moiety.10 To evaluate these properties we synthesized FITC-based dye-labeled variants of 6a, b referred to as 7a, b (Scheme 1). Stapling of the unprotected FITC-labeled peptide 7′ occurred smoothly and was not inhibited by the presence of a thiourea moiety (in contrast to the case of the olefin metathesis approach).6c Upon incubation of these peptides at 5μM concentrations with HEK293T cells for 4 hours, we observed significant uptake of the stapled peptides 7a, b (Figure 3E) localized in the endosomes (green punctate fluorescence) and in the cytosol (green fluorescent haze). Importantly, no fluorescent positive cells were observed for the unstapled variant 7 via confocal microscopy, and control experiment with 9 (peptide analog stapled via olefin metathesis, Scheme 1) suggested that 7b qualitatively exhibits similar uptake properties. Additional experiments with flow cytometry revealed similar trend in peptide uptakes (Figure 3D and SI).
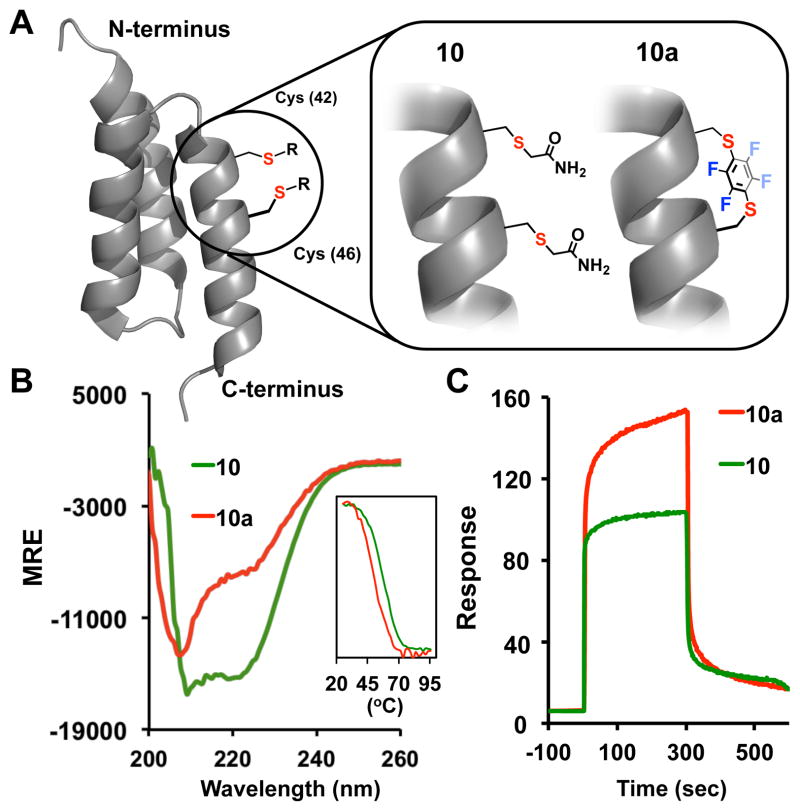
Further control experiments with non-stapled peptide variants 8a and 8b (Scheme 1B) featuring perfluoraromatic units installed only on one cysteine residue suggest that stapling is an essential requirement rendering these peptides cell-permeable. Finally, we evaluated whether the developed stapling chemistry is compatible with the conditions used for the total chemical synthesis of proteins. We chose to install a staple on the C-terminal helix of a previously engineered affibody that has ~ 1 nM affinity for the HER-2 protein receptor.11 Affibodies 10 and 10a were prepared via consecutive native chemical ligation (NCL)12 from three fragments, where the C-terminal peptide was stapled using the developed protocol (Figure 4A and SI). Upon examination of the CD spectra of the synthesized affibodies 10 and 10a we observed a well-defined α-helical motif (Figure 4B), suggesting the introduction of the perfluoroaryl staple did not alter the overall protein topology (note, that the contributions of the perfluoroaryl moieties in these CD spectra still persists, vide supra). Notably, temperature dependent melting properties of 10a were not compromised from the introduction of the staple. Furthermore, upon evaluating binding properties of these affibodies (Figure 4C) we observed similar binding of the stapled affibody as compared to the alkylated congener 10. While the concept of utilizing stapled peptides in the protein total synthesis was originally described by Verdine and Kennedy in their recent patent application,13 to the best of our knowledge, this is the first example demonstrating incorporation of stapled peptide motif within the structure of a larger protein scaffold without significantly altering its functional properties.
Figure 4.
(A) Representation of the HER-2 affibodies (PDB code: 2B89) with chemically modified Cys positions [Cys42, Cys46] via stapling (10a) and alkylation (10); (B) CD spectra of 10 and 10a, and the corresponding temperature-dependent melting curves measured from the CD spectra at 222 nm (inset); (C) Biacore binding sensograms of 10, 10a with immobilized HER-2 extra-celluar binding region (adsorption/desorption time - 300 sec).
In conclusion, we demonstrated a new and mild synthetic platform for cysteine arylation. The developed method operates at room temperature in polar organic solvents and shows excellent selectivity and functional group tolerance. As a result, we were able to utilize this approach towards stapling unprotected peptides, thereby positively altering their biological properties. We further demonstrated incorporation of a perfluoroaryl staple within the small tri-helical affibody protein. We believe this approach may expand the toolkit of available chemical transformations to alter the properties of biomolecules that contain a thiol. In addition, our approach is complementary in several aspects to the olefin metathesis mediated peptide stapling. The chemistry reported here, operates on unprotected peptides, does not necessitate the use of a metal-based catalyst, and poses no requirement for expensive non-natural amino-acid precursors.6c Furthermore, in contrast to bromoalkyl/benzyl chemistry that can produce over-alkylated species, this platform is selective towards cysteines and provides staples featuring lipophilic perfluorinated moieties.6f Efforts to establish in-depth structural understanding of the described stapled system, elucidate precise mechanism for the observed cellular uptake and broaden the synthetic scope of the cysteine arylation are currently under active investigation in our laboratories.
Supplementary Material
Acknowledgments
We thank Prof. Stephen L. Buchwald (S. L. B.) for his encouragement and support. This work was partially funded by the National Institutes of Health (GM46059 for S. L. B.), (GM101762 for A. M. S), MIT start-up fund for B. L. P and Tufts start-up fund for Y.-S. L. J. J. L. is a recipient of a National Science Scholarship (PhD) from A*STAR, Singapore. FACS core facility (MIT) is supported by the National Cancer Institute (P30-CA14051). The authors thank Ms. Amy Rabideau, Dr. Xiaoli Liao, Mr. Mark Simon, and Mr. Rocco Policarpo for technical assistance, Ms. Wendy Salmon (Whitehead Institute) for help with confocal microscopy and Prof. R. John Collier (Harvard) for contributing select laboratory equipment used in this work.
Footnotes
ASSOCIATED CONTENT
Full experimental and characterization details. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests. Patent application covering the content of this manuscript was filed by the MIT-TLO on Sept 23, 2012.
References
- 1.Hermanson GT. Bioconjugate Teachniques. Academic Press; London: 2008. [Google Scholar]
- 2.Chalker JM, Bernardes GJL, Lin YA, Davis BG. Chem Asian J. 2009;4:630. doi: 10.1002/asia.200800427. [DOI] [PubMed] [Google Scholar]
- 3.Ji X, Armstrong RN, Gilliland GL. Biochemistry. 1993;32:12949. doi: 10.1021/bi00211a001. [DOI] [PubMed] [Google Scholar]
- 4.Chalker JM, Gunnoo SB, Boutureira O, Gerstberger SC, Fernández-Gonzáles M, Bernardes GJL, Griffin L, Hailu H, Schofield CJ, Davis BG. Chem Sci. 2011;2:1666. [Google Scholar]
- 5.(a) Langille KR, Peach ME. J Fluor Chem. 1971–72:407. [Google Scholar]; (b) Birchal JM, Green M, Haszeldine RN, Pitts AD. Chem Commun. 1967:338. [Google Scholar]; (c) Becer CR, Babiuch K, Pilz D, Hornig S, Heinze T, Gottschaldt M, Schubert US. Macromolecules. 2009;42:2387. [Google Scholar]; (d) Becer CR, Hoogenboom R, Schubert US. Angew Chem, Int Ed. 2009;27:4900. doi: 10.1002/anie.200900755. [DOI] [PubMed] [Google Scholar]
- 6.Select examples and references within: Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191.Muppidi A, Doi K, Edwardraja S, Drake EJ, Gulick AM, Wang HG, Lin Q. J Am Chem Soc. 2012;134:14734. doi: 10.1021/ja306864v.Verdine GL, Hilinski GJ. Methods in Enzymology. Academic Press; London: 2012. Bautista AD, Appelbaum JS, Craig CJ, Michel J, Shepartz A. J Am Chem Soc. 2010;132:2904. doi: 10.1021/ja910715u.Kumita JR, Smart OS, Woolley GA. Proc Natl Acad Sci (USA) 2000;97:3803. doi: 10.1073/pnas.97.8.3803.Jo H, Meinhardt N, Wu Y, Kulkarni S, Hu Z, Low KE, Davies PL, DeGrado WF, Greenbaum DC. J Am Chem Soc. 2012;134:17704. doi: 10.1021/ja307599z.Blackwell HE, Grubbs RH. Angew Chem, Int Ed. 1998;23:3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V.
- 7.Sticht J, Humbert M, Findlow S, Bodem J, Müller B, Dietrich U, Werner J, Kräusslich HG. Nat Struct Mol Biol. 2005;12:671. doi: 10.1038/nsmb964. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhao Q, Bhattacharya S, Waheed AA, Tong X, Hong A, Heck S, Curreli F, Goger M, Cowburn D, Freed EO, Debnath AK. J Mol Biol. 2008;378:565. doi: 10.1016/j.jmb.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnölzer M, Alewood P, Jones A, Alewood D, Kent SBH. Int J Peptide Protein Res. 1992;40:180. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 10.Müller K, Fäh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 11.Orlova A, Magnusson M, Eriksson TLJ, Nilsson M, Larsson B, Höidén-Guthenberg I, Widström C, Carlsson J, Tolmachev V, Ståhl S, Nilsson FY. Cancer Res. 2006;66:4339. doi: 10.1158/0008-5472.CAN-05-3521. [DOI] [PubMed] [Google Scholar]
- 12.Kent SBH. Chem Soc Rev. 2009;38:338. doi: 10.1039/b700141j. [DOI] [PubMed] [Google Scholar]
- 13.Verdine GL, Kennedy EJ. U. S. Patent Application 20110144306. Filed. 2009 Jul 23;
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.