Abstract
Background & Aims
The Notch signaling pathway is required for the expansion of undifferentiated pancreatic progenitor cells during embryonic development and has been implicated in the progression of pancreatic ductal adenocarcinoma (PDAC). The interaction of Notch ligands with their receptors promotes a γ-secretase-dependent cleavage of the Notch receptor and release of the Notch intracellular domain, which translocates to the nucleus and activates transcription. We investigated the role of this pathway in PDAC progression.
Methods
We tested the effects of a γ-secretase inhibitor (GSI) that blocks Notch signaling in PDAC cell lines and a genetically engineered mouse model of PDAC (Kras p53 L/+ mice).
Results
Notch signaling was activated in PDAC precursors and advanced tumors. The GSI inhibited the growth of premalignant pancreatic duct-derived cells in a Notchdependent manner. Additionally, in a panel of over 400 human solid tumor-derived cell lines, PDAC cells, as a group, were more sensitive to the GSI than any other tumor type. Finally, the GSI completely inhibited tumor development in the genetically engineered model of invasive PDAC (p<0.005 χ2 test; compared with mice exposed to vehicle).
Conclusions
These results suggest that Notch signaling is required for PDAC progression. Pharmacologic targeting of this pathway offers therapeutic potential in this treatment-refractory malignancy.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancerrelated mortality in the United States with over 34,000 deaths predicted in 2008.1 It is characterized by its treatment-refractory behavior and dismal 5-year survival. The current standard of care for advanced disease, gemcitabine, results in modest clinical benefit, with a median increase in overall patient survival of 5 weeks.2 To date, clinical trials using a range of targeted therapies have failed to appreciably extend survival.
The cell of origin and molecular pathogenesis of PDAC have yet to be fully defined. PDAC evolves from duct-like tubular complexes (metaplasias) and ductal precursor lesions known as Pancreatic Intraepithelial Neoplasias (PanINs) that occur in association with Kras mutations.3 It is not clear in which cells the Kras mutations arise, leading to PDAC,4–6 although recent evidence supports the notion that acinar cells can serve as a cell of origin.7, 8 Furthermore, while a number of developmental signaling pathways have been proposed to contribute to PanIN initiation and progression, most have yet to be functionally tested. Defining the processes that are required for progression from PanIN precursor lesions to PDAC could lead to the design of improved treatment approaches to this disease. The validation of new targets is of particular significance in PDAC since progress in the therapeutic targeting of RAS has lagged compared to advances in targeting other commonly detected oncoproteins (e.g. EGFR, KIT, etc)9.
The Notch signaling pathway has been implicated in the pathogenesis of a number of malignancies, including PDAC10. Notch signaling is activated by interaction of Notch ligands with their receptors, promoting a γ-secretase-dependent cleavage of the Notch receptor and release of the Notch intracellular domain (Notch-IC)10, 11. Notch-IC translocates to the nucleus where it serves to activate transcription by binding to the CSL transcription factor and Mastermind-like transcriptional co-activator MAML. This complex activates the transcription of a number of target genes, including members of the Hairy enhancer of split (Hes) family.
During pancreatic development, the Notch signaling pathway promotes expansion of pancreatic progenitors as reflected by gain- and loss-of-function studies12–16. In the adult pancreas the Notch target gene Hes1 is expressed in centroacinar cells and some ductal cells indicating likely Notch signaling activity in these cells17. The centroacinar localization is notable as these cells have been proposed to have progenitor-like properties and are candidates for the PDAC cell-of-origin6. In response to pancreatic damage or oncogene expression, Hes1 is upregulated in association with the development of duct-like tubular complexes7, 17–21, and multiple components of the Notch signaling pathway are expressed or upregulated in advanced PDAC7, 17, 22. In addition, Notch signaling is required for normal pancreatic exocrine regeneration in response to pancreatic injury provoked by cholecystokinin analogue, cerulein23, and may contribute to the proliferation of PDAC cell lines24. In sum, these data point to a potential contribution of Notch signaling in PDAC pathogenesis from its earliest to latest stages.
The proteolytic cleavage by γ-secretase required to activate Notch provides a therapeutically vulnerable point in the pathway25, and a number of γ-secretase inhibitors (GSIs) have been developed that effectively block Notch activity and can inhibit growth of some tumor cell lines. In this study, we have directly monitored Notch activity in the normal and neoplastic pancreas and studied the capacity of a GSI to block PDAC growth in a large panel of human cell lines and a genetically engineered mouse model (GEMM) that recapitulates the genetics and histopathogenesis of the human disease.
Methods
Cell culture
Human PDAC cell lines were obtained from ATTC, from Anirban Maitra, and from Christine Iacobuzio-Donahue. Derivation and cultivation of murine pancreatic duct cells and PanIN cells from the pancreata of wild type or Pdx1-Cre LSL-KRASG12D mice, respectively, were as described previously26. PDAC cell lines from Pdx-Cre p53 Kras mice were established and cultivated as described27.
Animals
Therapeutic studies were performed with Pdx1-Cre; LSL-KrasG12D; p53lox/+ mice28. To examine activation of Notch signaling in vivo, these mice were crossed to a transgenic GFP reporter strain29. qRT-PCR analysis of Notch pathway components was performed with Pdx1-Cre; LSL-KrasG12D; Ink4a/Arflox/++ mice, which develop PanIN lesions and PDAC with similar kinetics28.
Compound
MRK-003 was synthesized according to standard medicinal chemistry procedures and was provided by Merck Research Laboratories, Boston, MA. For the in vitro experiments, stocks were prepared at 10mM in DMSO and dilutions were made directly before use. For the in vivo experiments, MRK-003 was dosed as a suspension in 0.5% methylcellulose (Sigma) made fresh daily. Animals were treated by gavage with 100mg/kg once daily, using a 3-days on and 4-days off intermittent dose schedule. Control animals were treated with vehicle alone using an equivalent dosing schedule.
Histology and immunohistochemistry
Tissues were processed as reported previously28. Antibodies and conditions for immunohistochemistry are presented in the Supplementary Methods. The histology was reviewed by a pancreatic pathologist. For the quantitation of the PanIN to total pancreas tissue ratio we counted the percentage of cells in 5–10 100× microsope fields / sample. In each field the different tissues (normal or diseased) were marked using Photoshop and finally compared with each other. One total microscope field was calculated as 100%.
Cellular, molecular, and statistical analyses
Detailed descriptions of procedures are provided in Supplementary Methods.
Results
Activation of the Notch pathway in Murine PDAC
Previous studies have suggested that Notch signaling is activated throughout the course of PDAC progression based on the expression of Notch pathway components17. To directly assess the activity of the pathway in PDAC pathogenesis, and to validate the use of Hes1 expression as a surrogate for Notch activity, we crossed a transgenic Notch-responsive GFP reporter strain29 onto the Pdx1-Cre LSL-KrasG12D p53Lox/+ mouse model of PDAC (from hereon, designated Kras p53 L/+ mice)28. In the normal pancreas, strong Notch signaling, as visualized by immunohistochemical (IHC) staining for the GFP reporter, was most pronounced in cells occupying a centroacinar position (Fig. 1A), consistent with the reported centroacinar staining pattern of Hes117, 20. The majority of premalignant PanIN lesions, previously reported to exhibit strong Hes1 staining, also showed activation of the Notch reporter (Fig. 1B). Hence, as assessed by an independent measure of pathway function, Notch signaling is active in centroacinar cells, PanIN, and PDAC.
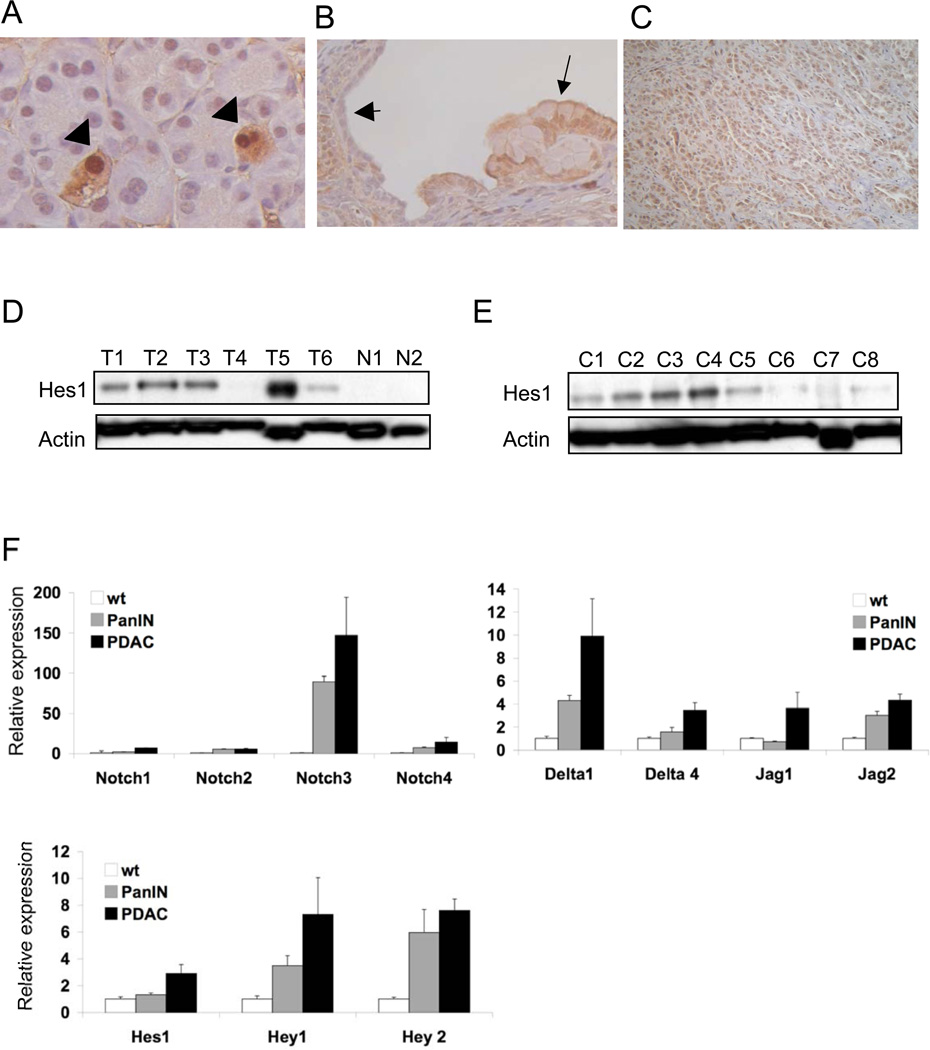
Figure 1. Notch pathway activation accompanies PDAC progression.
The Notch reporter strain [24] confirms pathway activation in normal pancreatic centroacinar cells A, arrowheads) and PanIN lesions of Pdx1-Cre LSL-KrasG12D p53 L/+ mice (B). Note staining in PanIN epithelium (arrows) but not in adjacent normal ductal epithelium arrowhead). (C) Hes1 is widely expressed in the tumor cells of advanced mouse PDAC. D) Western blot analysis demonstrates a significant increase in Hes1 expression in primary mouse PDAC tumors (T1–T6) and (E) PDAC cell lines (C1–C8) compared to normal pancreatic tissue (N1, N2). (F) Quantitative reverse transcription-PCR (qRTPCR) analysis of Notch pathway components in cultured wildtype duct cells, duct cells derived from PanIN-bearing pancreata, and PDAC cells (mean +/− SEM of 3 independent lines for each group). A,B and C, original magnification 400×.
Next we wished to further define Notch pathway activity in the mouse model of advanced PDAC. By immunoblot analysis 8/9 primary PDACs were found to overexpress Hes1, and IHC confirmed that the tumor cells were the primary source of expression (Figure 1C and D). Notably, the only tumor lacking Hes1 expression (T4) displayed undifferentiated histopathology (data not shown). In accord with previous findings of Notch activation in early human pancreatic lesions and PDAC17, expression profiling and quantitative reverse-transcriptase PCR (qRT-PCR) revealed that the Notch receptors, their ligands (Jagged and Delta families), and known target genes (Hes1, Hey1) were broadly upregulated in murine PanIN and PDAC relative to normal murine pancreas (Supplementary Figure 1).
To confirm that the Notch pathway was activated in the neoplastic epithelium, we performed immunoblot and qRT-PCR analysis of primary pancreatic ductal cells derived from Pdx1-Cre LSL-KrasG12D mice27, 30 (PanIN cell lines) and PDAC cell lines from the Kras p53 L/+ model. All PanIN cell lines tested exhibited readily detectable Hes1 levels (Fig. 2A and data not shown), and 7/8 PDAC cell lines also overexpressed Hes1 (Fig. 1E). Moreover, induction of multiple Notch pathway components was observed in cultured PanIN and PDAC cells relative to cultured primary duct cells (Fig. 1F). Notch3 showed the most pronounced induction of all pathway components tested, consistent with prior observations from human pancreatic tumors17. The induction of several Notch ligands during PanIN and PDAC progression suggests that paracrine or autocrine signaling by tumor cells may be at least partially responsible for pathway activation in vivo.
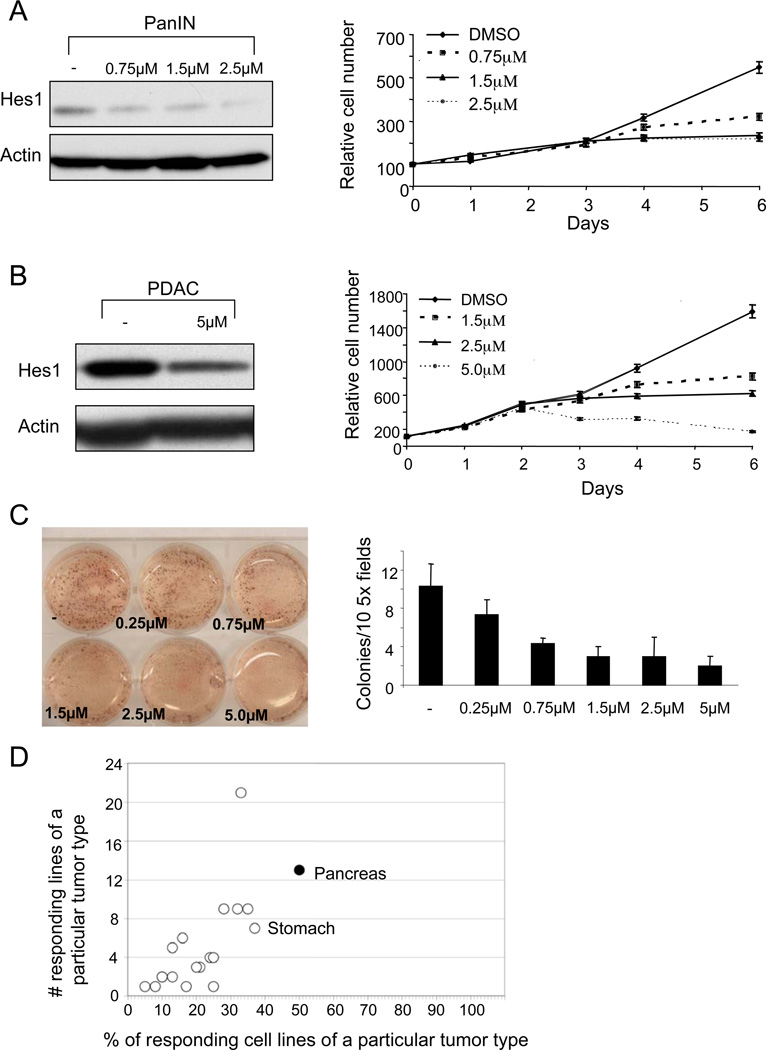
Figure 2. PanIN and PDAC cell lines are sensitive to GSI treatment.
(A) PanIN cells treated with the indicated concentrations of the GSI exhibit decreased levels of Hes1 protein by Western blot analysis (left) and a dose-dependent shift in the growth curve right). (B) Treatment of the mouse PDAC cell line (N490) with the GSI results in a similar decrease in Hes1 protein levels (left) and growth inhibition (right). Note that most treatment conditions led to a plateau in the growth curves, suggesting that the compound exerts static rather than toxic effects. (C) Soft agar assay of a GSI-treated human PDAC cell line (Panc1), with quantitation on right. Note significant difference between colony numbers in cells treated with ≥1.5µM GSI compared to vehicle-treated cells (p<0.005; Student’s T test). (D) A set of 434 human cancer-derived cell lines comprising 23 different solid tumor types were assessed for growth inhibition following 3 days of exposure to 5 µM MRK-003. The proportion of cell lines from each tumor type that showed >25% decreased growth is plotted on the X-axis (% responders) and the total number of responders is plotted on the y-axis. In addition to pancreatic cancer-derived cell lines (50%; 13/26), only stomach cancer-derived cell lines (37%; 7/19) also showed a response rate of >35%.
Murine and human PDAC cell lines are sensitive to GSI
Based on the observed activation of the Notch pathway throughout the course of PDAC progression, we sought to assess the sensitivity to Notch inhibition of PanIN and PDAC cell lines by treatment with the cyclic sulfamide GSI, MRK-00331. MRK-003 is a potent (0.72 nM for NICD cleavage in vitro) and specific GSI that is orally bioavailable, with a plasma half-life of 12hr at 100mg/kg. MRK-003 treatment led to a rapid reduction in Hes1 expression in PanIN cell lines at concentrations as low as 0.75 µM, consistent with an inhibition of Notch activity (Fig. 2A left panel). Treatment of PanIN cells with 0.75–2.5 µM MRK-003 effectively blocked proliferation after 3–4 days exposure (Fig. 2A right panel). Next we selected a set of human PDAC-derived cell lines (N=3) and those derived from the Kras p53 mouse model (N=4) that showed robust Hes1 expression. These cells lines also demonstrated substantially reduced cell proliferation upon MRK-003 treatment, accompanied by loss of Hes1 expression (Fig. 2B, Supplementary Fig. 2, and data not shown). Furthermore, MRK-003 strongly inhibited the ability of murine and human PDAC cells to form clones in soft agar compared to vehicle treated cells, indicating that active Notch signaling is necessary for anchorage-independent clonogenic growth of these cells (Fig. 2C and data not shown).
We next examined whether the effects of MRK-003 seen in these assays were specific for pancreatic cancer, or reflected a more general ability to inhibit cell growth. We employed a high-throughput platform32 to assess the sensitivity of 434 human cell lines derived from a variety of solid tumor types to MRK-003. Notably, we found that, as a group, PDAC-derived cell lines exhibit the greatest sensitivity to Notch inhibition compared to the other tumor types, with half of the lines (13/26) showing at least a 25% decrease in cell number after 3 days treatment with 5 µM MRK-003 relative to vehicle (Fig. 2D). In contrast, cell lines from other cancer types, including those reported to be sensitive to GSI, showed considerably lower response rates, including breast (28%), kidney (24%), and non-small cell lung cancer (33%)10, 33, 34. These data suggest that γ-secretase inhibition may be particularly effective in the context of PDAC.
MRK-003 effectively inhibits Notch signaling in vivo
Given the strong induction of Notch signaling in PanIN and the sensitivity of PanIN cell lines to MRK-003, we wished to test the possibility that GSI treatment could block the development of PanIN and/or PDAC in vivo. Previous work evaluating GSIs for the treatment of Alzheimer's disease and recent findings in renal cell carcinoma, demonstrated that rapid weight loss is dose-limiting, in part due to the role of Notch signaling in the control of intestinal progenitor cell fate33, 35–37. We developed an intermittent dosing regimen consisting of three daily oral doses of 100mg/kg followed by four days recovery. Intermittent treatment maintains target inhibition associated with in vivo efficacy while achieving tolerable intestinal side effects (manuscript in preparation). This dose and schedule effectively attenuated Notch signaling as indicated by a decrease in Hes1 expression in subcutaneously implanted PDACs (Fig. 3A and Supplementary Fig. 3A).
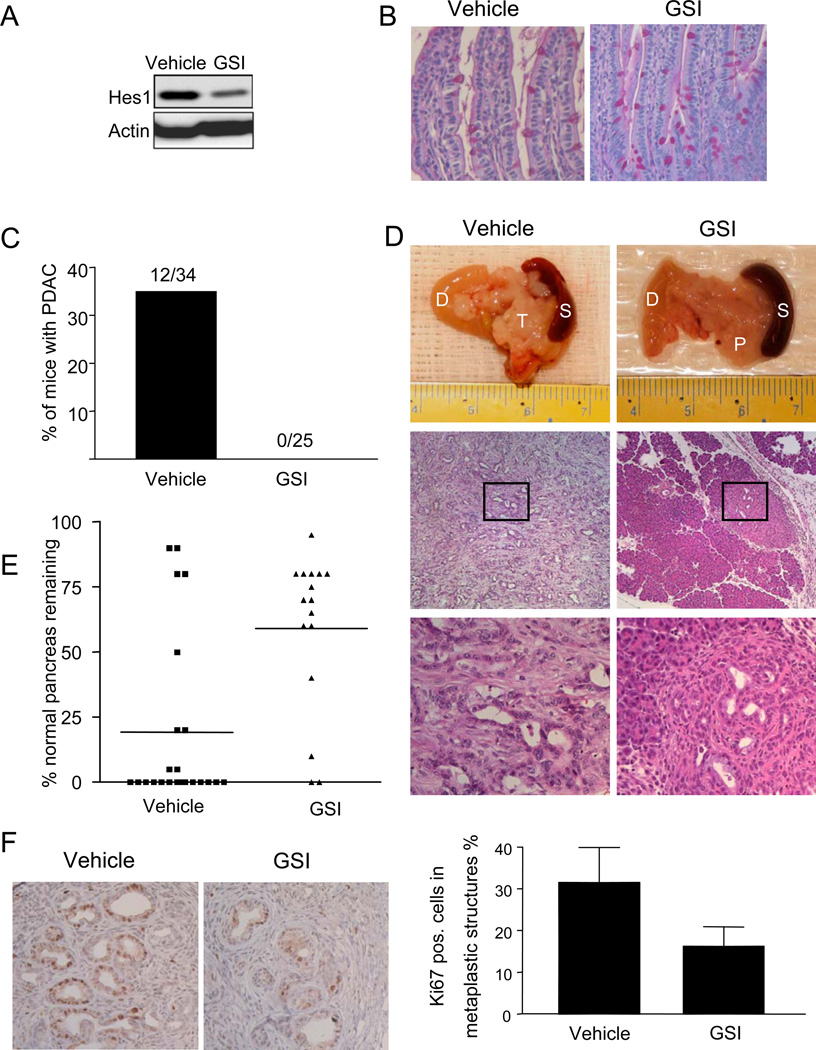
Figure 3. GSI attenuates PDAC development in Kras p53 mice.
(A) Tumor cells were transplanted as xenografts into SCID mice, and the mice were treated systemically with GSI or vehicle, resulting in a decrease in Hes1 protein levels. (B) PAS staining of small intestine from control and GSI-treated mice, showing an increase in goblet cells in the GSI-treated animal. (C) PDAC incidence in GSI-treated and control mice at ages 11–13 weeks. (D) Macroscopic and histological images of representative pancreas (P), tumor (T), and surrounded duodenum (D) and spleen (S) from vehicle-treated and GSI-treated mice. The boxed region in the middle panels is shown at higher magnification in the lower panels. The vehicle-treated mouse has a PDAC, while the GSI-treated mouse has focal PanIN lesions. (E) The proportion of pancreatic tissue occupied by PanIN lesions was quantified in vehicle- (N=23) and GSI-treated (N=16) pancreata. GSI treatment resulted in a significant (p<0.005; chi squared test) reduction in the abundance of PanINs. (F) Left panel. Ki67 staining of metaplastic ducts. Right panel. Quantitation of Ki67 staining in the neoplastic epithelium, showing significant (p<0.005; Student’s T test) reduction in Ki67+ cells following GSI treatment. C, magnification 100× and 400×, E, magnification 400×.
To further establish the in vivo efficacy of this dosing regimen, we assessed two biomarkers of γ-secretase activity: processing of the Amyloid Precursor Protein (APP) and intestinal goblet cell differentiation38. APP is processed by a two-step proteolytic pathway with γ-secretase serving as the last step that releases the Aβ40 peptide39. MRK-003-treated animals showed a significant reduction in the serum levels of Aβ40 compared to vehicle-treated controls, indicating effective inhibition of γ-secretase (Supplementary Fig. 3B). γ-secretase activity is also required for normal intestinal homeostasis, and inhibition of the Notch pathway within the intestinal crypt cells promotes goblet cell differentiation35, 38. Consistent with an inhibitory effect on Notch signaling, MRK-003 provoked goblet cell differentiation (Fig 3B). Nevertheless, the intestinal epithelium returned to normal after the four-day recovery period and both wildtype and Kras p53 mice treated with GSI showed normal weight gain and overall health through multiple cycles of this dosing regimen (data not shown). Furthermore, thymus and spleen were grossly normal with no detectable changes in organ size. Hence, MRK-003 can effectively block GSI activity and inhibit Notch signaling in vivo without overtly deleterious side effects.
γ-secretase activity is required for PDAC progression
To determine whether γ-secretase activity is required for the growth of premalignant and malignant cells in vivo, we used a highly faithful GEMM of PDAC: the Kras p53 L/+ model28. We initiated a treatment trial in which Kras p53 L/+ mice and control animals were treated with MRK-003 or vehicle starting at age 6 weeks. At this time point, Kras p53 L/+ mice exhibit isolated PanIN lesions but do not harbor established PDAC (unpublished data). In untreated mice, PanIN lesions progress to advanced PDAC requiring euthanasia with a mean latency of ~ 18 weeks. Necropsy at earlier ages reveals focal PDAC as early as ~ 11 weeks (mean ~ 15 weeks; unpublished data). Mice were maintained on the dosing regimen until they were euthanized either at 11 or 13 weeks, enabling analysis of the impact of MRK-003 on PanIN-to-PDAC progression.
Autopsy revealed that 12 of 34 of the vehicle-treated mice had gross pancreatic tumors whereas none of 25 MRK-003 treated animals exhibited pancreatic tumors (Fig. 3C and D; p<0.005). There was also a marked effect of MRK-003 treatment on PanIN formation. Most untreated mice (65%) lacked any detectable normal pancreatic tissue, and rather had a mixture of PanIN, ductal metaplasia and fibrosis. In GSI-treated animals, by contrast, diseased areas were focal in nearly all cases. Overall, the area occupied by normal pancreas comprised the majority of pancreatic tissue in only 20% of untreated mice while 75% of treated mice had predominantly normal pancreas (Fig. 3E; p<0.005). GSI-treatment suppressed abundance of PanINs (p<0.005) and but not metaplastic ducts (Supplementary Fig. 3C). This increase in the regions of diseased pancreas in untreated mice was also reflected in an increase in the mean pancreatic-to-body mass ratio (Supplementary Fig. 3D; p<0.005, see Methods). GSI treatment of wild type control animals had no effect on pancreatic mass or histology (data not shown), indicating a specific effect of MRK-003 on Kras-driven tumorigenesis. These results suggest that sustained Notch pathway activity is required for the development of premalignant lesions and their progression to advanced PDAC in mice.
MRK-003 blocks epithelial proliferation
Next, we assessed whether MRK-003 affected cell proliferation or apoptosis in evolving PanINs in specimens analyzed after 4 weeks of treatment. In vehicle-treated mice, Ki-67 staining was abundant in regions of ductal metaplasia and PanIN, relative to normal pancreatic ducts (N=6 mice in each group, data not shown). MRK-003-treatment resulted in a significant decrease in Ki-67 staining in these lesions compared to the vehicle-treated animals (Fig. 3F; p<0.05). By contrast, comparable proportions of apoptotic nuclei were noted by TUNEL staining in PanIN lesions and tubular complexes from MRK-003- and vehicle-treated animals (Supplementary Fig. 4A). These results suggest that γ–secretase inhibition reduces the proliferative rate of premalignant cells but does not strongly influence apoptosis during PDAC progression in vivo.
MRK-003 exerts its growth effects through Notch signaling
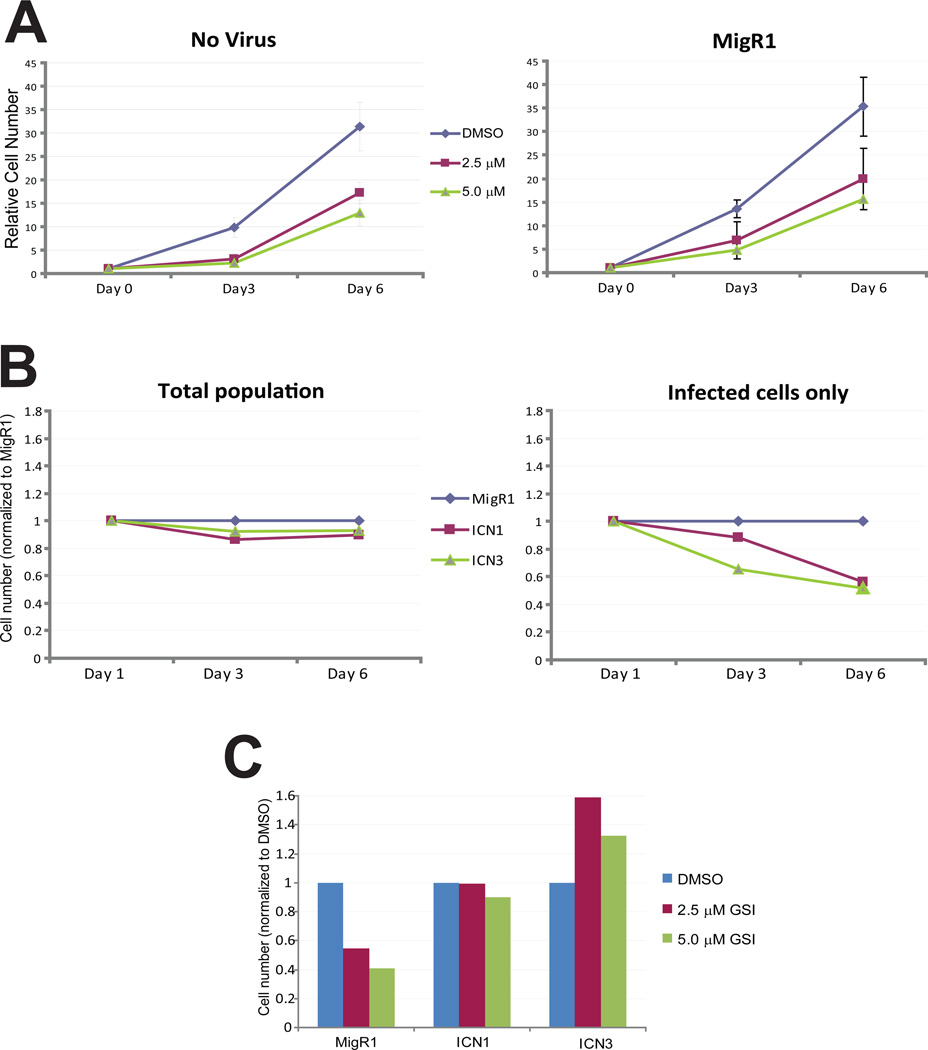
Although these results indicate that MRK-003 effectively inhibits the Notch pathway, it is possible that its effects on cell proliferation are independent of this activity. To determine whether inhibition of Notch signaling mediates the growth inhibitory activity of the compound, we used MigR1 retroviral infection40 to reactivate Notch signaling in PDAC cells exposed to MRK-003. Infectivity was monitored with an IRES-GFP contained within the viral vector, and infection rates reproducibly achieved 40–50%. Viral infection with the parental vector had no effect on the dose-responsiveness of NB507 PDAC cells to MRK-003 (Fig. 4A), and constitutively activive Notch1 (ICN1) or Notch3 (ICN3) did not augment cell growth on their own (Fig. 4B). By contrast, infected cells were rescued from the growth-inhibitory effects of MRK-003 upon expression of ICN1 and ICN3 (Fig. 4C). These results suggest that MRK-003 inhibits PDAC cell growth through its effects on Notch signaling. Notably, we found that all five GSI-sensitive PDAC cell lines tested had robust expression of Hes1, while Hes1 was reduced or absent in a set of five GSI-resistant lines (Supplementary Fig. 5). Hence, relative Hes1 expression appears to be associated with GSI-sensitivity in PDAC cell lines. Infection of PDAC cells with retroviruses expressing the Notch target genes, c-Myc- or Hes1, did not prevent GSI-induced growth inhibition (Supp. Fig. 6), indicating that these genes are not sufficient to mediate the rescue effects of ICN.
Figure 4. Notch signaling rescues cells from the growth inhibitory effects of GSI.
(A) Retroviral infection has no effect on growth inhibition of NB507 PDAC cells by MRK-003. Cell number was determined 3 days or 6 days following treatment with the GSI (left panel); infection with MigR1 retrovirus did not alter the dose-response curve. (B) Cell number was determined following infection with control (empty) MigR1 virus, or virus encoding a constitutively active form of Notch1 (ICN1) or Notch3 (ICN3). ICN1 and ICN3 expression had minimal effects on NB507 cell number compared to MigR1 when the total population was examined (left panel); when only infected cells were measured, ICN1and ICN3 had a mild inhibitory effect on growth (right panel). (C) ICN1 and ICN3 prevented GSI-induced growth inhibition after 6 days of treatment (only infected cells are shown). These results are representative of two independent experiments.
Discussion
In this study, we used Kras p53 L/+ mice – which recapitulate most features of human pancreatic cancer, including progression from PanIN to PDAC, distant metastases, and reproducible genomic changes – to determine whether Notch signaling is required for the development of advanced lesions. We have confirmed that the Notch pathway is activated from the earliest stages of murine PDAC and that human and murine premalignant and malignant cells depend upon Notch signaling for growth. Indeed, human PDAC-derived cell lines – which commonly exhibit resistance to chemotherapeutic agents – demonstrate increased sensitivity to blockade of the pathway in comparison with other solid tumors. Most significantly, treatment of Kras p53 L/+ mice with the GSI, MRK-003, attenuates the progression of PanIN to PDAC. Therefore, the Notch signaling pathway appears to be required for initiation of murine PDAC and may be a viable therapeutic target in this treatment-refractory malignancy in humans.
Since we treated mice early in the PanIN-to-PDAC sequence, it is possible that Notch signaling is required at multiple stages of disease progression. For example, MRK-003 might block the initial development of PanIN lesions and/or tubular complexes. Previous work has implicated Notch signaling in PanIN formation and ductal metaplasia, a process by which tubular complexes arise from either acinar or Hes1-expressing centroacinar cells17, 20, 41. Alternatively, enhanced Notch signaling may allow established tubular complexes or PanIN lesions to proliferate and acquire more aggressive characteristics. Consistent with this notion, Ki67 staining of similarly-graded PanIN lesions from GSI- and vehicle-treated pancreata revealed that MRK-003 constrains the proliferation of these cells. Future studies will be needed to define the relative importance of effects on the initiation of tubular complexes and PanIN versus the progression of PanIN to PDAC.
Although the observed activity of MRK-003 in PDAC-derived cell lines is consistent with a cell autonomous role for Notch signaling, it is possible that GSI blocks tumor progression through non cell-autonomous effects. Recent studies have shown that Notch signaling can contribute to tumorigenesis by restraining non-productive angiogenesis42, 43. Correspondingly, inactivation of Notch signaling can promote the formation of non-functional vessels resulting in poor tumor perfusion and decreased tumorigenesis. Similarly, immune surveillance is thought to play an important role in tumor progression. GSI could conceivably mediate an anti-tumor effect by changing the balance of growth-promoting tumor associated macrophages or immunosuppressive regulatory T-cells44, 45. In limited studies of tumor vasculature and lymphocyte infiltration, we did not observe an effect of GSI treatment on the tumor vasculature or stroma (Supplementary Fig. 4B and data not shown). Finally, we cannot rule out the possibility that inhibition of a γ-secretase sensitive pathway other than Notch is responsible for blocking tumor progression.
Molecular “crosstalk” between Ras and Notch signaling is well known to occur during development46. The two pathways also exhibit cooperativity in tumor cells, where Notch appears to be a common and critical downstream effector of oncogenic Ras signaling47. Consistent with the notion that Notch signaling plays an important role in the progression of murine PDAC, recent work has shown that ectopic activation of Notch signaling promotes PanIN initiation and progression8. The mechanism of Notch activation in evolving PDAC is not yet defined. Oncogenic Kras could contribute to Notch induction in since, as we have shown, KrasG12D expressing PanIN-derived cells exhibit increased expression of Notch signaling components relative to wildtype duct cells. Alternatively, previous studies have shown that EGFR signaling can activate Notch in cultured acinar cells17. EGFR is markedly upregulated in metaplastic regions and in PanINs from Pdx1-Cre KrasG12D mice and is a potential inducer of Notch in this setting.
Despite the γ-secretase-sensitive role that Notch signaling plays in controlling cell differentiation in multiple adult tissues, including the intestine35, 48, 49, mice treated with a 3-day on 4-day off dosing regimen for over 5 months showed no overt ill effects. Hence, intermittent treatment provides a potent anti-tumor effect with minimal toxicity. Future studies with MRK-003, used as either a single agent or in combination with other drugs, will further delineate the mechanism and efficacy of this and similar compounds. Faithful GEMMs that recapitulate the molecular pathogenesis of human cancer provide a stringent platform for testing the efficacy of candidate therapeutic agents. Such models have the potential to play an especially crucial role in the preclinical evaluation of therapeutics for cancers that have no effective treatment. While the effects we observed on tumor progression were dramatic, additional investigation of potential efficacy in the setting of more advanced pancreatic malignancy is clearly warranted.
Supplementary Material
Acknowledgements
The authors would like to thank Patricia Zadnik for technical assistance, Billy Kim, Ned Sharpless and Kwok-Kin Wong for critical reading of the manuscript, Tyler Jacks, David Tuveson, Anton Berns, Anil Rustgi, Melanie Westcott, Doug Melton, Nicholas Gaiano, Chris Wright, Mark Chiang, and Warren Pear for generously providing reagents, and Jamie Freedman for initial support of this project.
Grant Support: This work was supported by grants to N.B. from the Waxman Foundation, Harvard Stem Cell Institute, the Linda Verville Foundation and the NIH (5K01CA104647 and 5P01CA117969), and from funds provided by Merck & Co. R.P. was supported by the Deutsche Forschungsgemeinschaft. A.D.R. was supported by the NIH (T32-DK007066). B.Z.S. was supported by an AACR Career Development Award from the Pancreatic Cancer Action Network.
Abbreviations
- GSI
γ-secretase inhibitor
- PDAC
pancreatic ductal adenocarcinoma
- PanIN
Pancreatic Intraepithelial Neoplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Merck & Co. provided funds to N.B. for these studies.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Hansel DE, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Annu Rev Genomics Hum Genet. 2003;4:237–256. doi: 10.1146/annurev.genom.4.070802.110341. [DOI] [PubMed] [Google Scholar]
- 4.Konieczny SF, Leach SD. Metaplastic metamorphoses in the mammalian pancreas. Gastroenterology. 2007;133:2056–2059. doi: 10.1053/j.gastro.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Murtaugh LC, Leach SD. A case of mistaken identity? Nonductal origins of pancreatic "ductal" cancers. Cancer Cell. 2007;11:211–213. doi: 10.1016/j.ccr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Maitra A, Hruban RH. A new mouse model of pancreatic cancer: PTEN gets its Akt together. Cancer Cell. 2005;8:171–172. doi: 10.1016/j.ccr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De La OJ, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2008;105:18907–18912. doi: 10.1073/pnas.0810111105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox AD, Der CJ. Ras family signaling: therapeutic targeting. Cancer Biol Ther. 2002;1:599–606. doi: 10.4161/cbt.306. [DOI] [PubMed] [Google Scholar]
- 10.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–2762. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 12.Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, Hrabe de Angelis M, Lendahl U, Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 13.Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 15.Hald J, Hjorth JP, German MS, Madsen OD, Serup P, Jensen J. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev Biol. 2003;260:426–437. doi: 10.1016/s0012-1606(03)00326-9. [DOI] [PubMed] [Google Scholar]
- 16.Esni F, Ghosh B, Biankin AV, Lin JW, Albert MA, Yu X, MacDonald RJ, Civin CI, Real FX, Pack MA, Ball DW, Leach SD. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 18.Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, Depinho RA, Wu H, Melton DA, Dor Y. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Gomez G, Englander EW, Wang G, Greeley GH., Jr Increased expression of hypoxia-inducible factor-1alpha, p48, and the Notch signaling cascade during acute pancreatitis in mice. Pancreas. 2004;28:58–64. doi: 10.1097/00006676-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Buchler P, Gazdhar A, Schubert M, Giese N, Reber HA, Hines OJ, Giese T, Ceyhan GO, Muller M, Buchler MW, Friess H. The Notch signaling pathway is related to neurovascular progression of pancreatic cancer. Ann Surg. 2005;242:791–800. doi: 10.1097/01.sla.0000189115.94847.f1. discussion 800–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5:483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]
- 25.Shih Ie M, Wang TL. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Res. 2007;67:1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber FS, Deramaudt TB, Brunner TB, Boretti MI, Gooch KJ, Stoffers DA, Bernhard EJ, Rustgi AK. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004;127:250–260. doi: 10.1053/j.gastro.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, Hanahan D, Redston MS, Chin L, Depinho RA. From the Cover: Both p16Ink4a and the p19Arf-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 30.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 31.Sparey T, Beher D, Best J, Biba M, Castro JL, Clarke E, Hannam J, Harrison T, Lewis H, Madin A, Shearman M, Sohal B, Tsou N, Welch C, Wrigley J. Cyclic sulfamide gamma-secretase inhibitors. Bioorg Med Chem Lett. 2005;15:4212–4216. doi: 10.1016/j.bmcl.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 32.McDermott U, Sharma SV, Settleman J. High-throughput lung cancer cell line screening for genotype-correlated sensitivity to an EGFR kinase inhibitor. Methods Enzymol. 2008;438:331–341. doi: 10.1016/S0076-6879(07)38023-3. [DOI] [PubMed] [Google Scholar]
- 33.Sjolund J, Johansson M, Manna S, Norin C, Pietras A, Beckman S, Nilsson E, Ljungberg B, Axelson H. Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J Clin Invest. 2008;118:217–228. doi: 10.1172/JCI32086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konishi J, Kawaguchi KS, Vo H, Haruki N, Gonzalez A, Carbone DP, Dang TP. Gamma-secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 2007;67:8051–8057. doi: 10.1158/0008-5472.CAN-07-1022. [DOI] [PubMed] [Google Scholar]
- 35.Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 36.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 37.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 39.Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer's disease Abeta amyloid peptides. Peptides. 2002;23:1285–1297. doi: 10.1016/s0196-9781(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 40.Pear WS, Miller JP, Xu L, Pui JC, Soffer B, Quackenbush RC, Pendergast AM, Bronson R, Aster JC, Scott ML, Baltimore D. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 41.Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 42.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 43.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 44.Kared H, Adle-Biassette H, Fois E, Masson A, Bach JF, Chatenoud L, Schneider E, Zavala F. Jagged2-expressing hematopoietic progenitors promote regulatory T cell expansion in the periphery through notch signaling. Immunity. 2006;25:823–834. doi: 10.1016/j.immuni.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Ostroukhova M, Qi Z, Oriss TB, Dixon-McCarthy B, Ray P, Ray A. Treg-mediated immunosuppression involves activation of the Notch-HES1 axis by membrane-bound TGF-beta. J Clin Invest. 2006;116:996–1004. doi: 10.1172/JCI26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundaram MV. The love-hate relationship between Ras and Notch. Genes Dev. 2005;19:1825–1839. doi: 10.1101/gad.1330605. [DOI] [PubMed] [Google Scholar]
- 47.Weijzen S, Rizzo P, Braid M, Vaishnav R, Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC, Rudolf M, Siziopikou K, Kast WM, Miele L. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 48.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, Zhang L, Higgins GA, Parker EM. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 49.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.