Abstract
The pharmacodynamics of an antimicrobial drug relates its pharmacokinetics to the time course of the antimicrobial effects at the site of the infection. Knowledge of the drug's antimicrobial pharmacodynamic effects (eg, rate and extent of bactericidal action and postantibiotic effect) provides a more rational basis for determination of optimal dosing regimens in terms of the dose and the dosing interval than do the minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) determined in vitro. This article reviews pharmacokinetics, antimicrobial pharmacodynamics, the effect of pharmacodynamics on the emergence of resistant bacterial subpopulations, and the development of pharmacodynamic breakpoints for use in the design of trials of these drugs and in the treatment of infected patients.
Keywords: Pharmacokinetics, Pharmacodynamics, Antimicrobial susceptibility, Concentration-dependent antimicrobial activity, Time-dependent antimicrobial activity
IN VITRO ANTIMICROBIAL ACTIVITY OF DRUGS
Minimal Inhibitory Concentrations and Minimal Bactericidal Concentrations
Despite acknowledged exceptions with certain drug–bacteria combinations, antibacterial drugs are usually divided into two groups: those that are primarily bacteriostatic (ie, that inhibit growth of the organism) and those that are primarily bactericidal (ie, that kill the organism). Bacteriostatic drugs require the aid of host defenses to clear tissues of the infecting microorganism; if the host defenses are systemically inadequate (eg, agranulocytosis) or the host defenses are impaired locally at the site of infection (eg, cardiac vegetation in left-sided endocarditis, cerebrospinal fluid in meningitis), the pathogen will resume growth after stopping the bacteriostatic drug, and the infection will relapse. Bacterial infection in these circumstances will require the use of bactericidal drugs. Bacteriostatic drugs are sufficient for most other infections.
The in vitro antimicrobial activity of drugs is usually assessed by determining of the MIC and MBC after overnight aerobic incubation of a standard and size inoculum of bacteria in a low protein liquid medium at pH 7.2. These in vitro conditions are likely very different from those expected at the site of infection, where the milieu is frequently acidic and anaerobic, and tissue protein may bind a variable amount of the drug. The MIC and MBC, which are determined at a fixed point in time after exposure to drug concentrations that remain constant throughout an overnight incubation period, do not provide information on the time course of the antimicrobial effect of the fluctuating antimicrobial drug levels that are present in a treated patient. In addition, the MIC and MBC are measured against a standard bacterial inoculum (about 105 colony-forming units/mL) that does not necessarily correspond to bacterial densities at the site of infection (108–10 colony-forming units/g of tissue or pus). The in vitro inoculum is also in the exponential phase of growth, unlike the majority of organisms in an established infection, which are nongrowing.
The MIC is defined as the minimal concentration of antibiotic that prevents a clear suspension of 105 colony-forming units (CFUs) of bacteria/mL from becoming turbid after overnight incubation; turbidity usually connotes at least a 10-fold increase in bacterial density. Because clear bacterial suspensions may have bacterial densities that are 105 CFU/mL or less, the MIC may actually be bactericidal to some extent.
If the minimal concentration of the antibiotic that prevented turbidity lowered the bacterial density from 105 to at least 102 CFU/mL, that is, a 99.9% (3-log10) reduction in bacterial inoculum, the minimal concentration that prevented turbidity (ie, the MIC) is also the MBC. For bactericidal drugs, the MBC is usually the same as, and generally not more than fourfold greater than, the MIC. In contrast, the MBCs of bacteriostatic drugs are many-fold greater than their MICs. Bacteriostatic drugs include the macrolides, clindamycin, the tetracyclines, the sulfonamides, linezolid, and chloramphenicol. Bactericidal drugs include the beta-lactams, vancomycin, the aminoglycosides, the fluoroquinolones, daptomycin, and metronidazole.
Time-kill studies, which are used to determine the rate of bactericidal activity, involve sampling a bacterial suspension of 105 CFU/mL in broth at various time intervals, (eg, at 2, 4, 6, and 24 hours of incubation) after addition to a particular concentration of the antibiotic. This method is also used to assess the interaction of two antimicrobial drugs for synergy or antagonism.
The MIC is a measure of the potency of an antimicrobial drug. Isolates of a particular species will have varying MICs; sensitive strains will have relatively low MICs, and resistant strains will have relatively high MICs. The breakpoint MIC is the MIC that separates sensitive and resistant strains, and it was traditionally selected on its ability to distinguish two disparate populations: one population with MICs at less than the breakpoint (ie, susceptible) and one with MICs at more than the breakpoint (ie, resistant). Another attribute of the breakpoint MIC is correspondence to achievable serum drug levels using standard dosing. However, concentrations may be much higher than serum levels for drugs that concentrate at intracellular sites or at excretory sites, such as in urine or bile, or may be considerably lower than serum levels at secluded foci, such as the cerebrospinal fluid, the eye, the prostate, or centers of abscesses.
For example, the breakpoint concentration for susceptibility to azithromycin is 0.5 μg/mL or less, which may be barely higher than the usual peak serum level of 0.4 μg/mL. Because azithromycin in sequestered within phagocytes, this serum concentration may be fine for predicting its effectiveness against intracellular pathogens, such as legionella, mycoplasma, or chlamydia, but may be problematic for extracellular pathogens such as Streptococcus pneumoniae. In addition, drugs that are highly bound to serum protein may have reduced antibacterial activity in serum and will not penetrate tissues as well as drugs that are less protein bound. In these cases, the results of in vitro testing may not predict the in vivo effect.
PHARMACOKINETICS
Pharmacokinetics describes the time course of drug levels in body fluids as a result of absorption, distribution, and elimination of a drug after administration.
Absorption
Most antimicrobial drugs are administered either by the intravenous (IV) or oral administration (PO) routes. Absorption is best described by the drug's bioavailability, which is defined as the percentage of a drug's dose that reaches the systemic circulation.
Intravenous Administration
When the entire dose is administered by the IV route, 100% of that dose is bioavailable. Rates of IV administration can vary from a bolus infusion (in which the total IV dose is given over a very short interval of time, eg, a minute or less) to a very slow infusion over many hours. Delivery of drugs by the IV route is complete by the end of the infusion, when a peak plasma level is achieved. The height of the peak plasma drug level is determined by the rate of IV infusion, the size of the dose, the size of the drug's volume of distribution, and its rate of elimination. Peak plasma drug levels will be the highest after bolus administration because the duration of infusion is too short for significant distribution or elimination of the drug to occur. For these reasons, relatively rapid IV administration is most often chosen for antimicrobial therapy of a patient who has severe infection, when an antimicrobial effect is sought as soon as possible. Slowing the rate of IV infusion allows distribution of the drug in the body and drug elimination to take place, and consequently, lower peak plasma drug levels occur.
Use of bolus administration, however, may be limited by concentration-dependent drug toxicities (eg, red-person's syndrome, which is related to rapid infusion of vancomycin). Also, because the drug will have to be diluted in a relative small volume, bolus infusion will expose the vein through which the drug is infused to high drug concentrations that may limit the use of bolus infusion because of venous irritation and pain. For example, because of its propensity for causing phlebitis when infused by way of a peripheral vein, quinupristin/dalfopristin must be infused using a central venous catheter, in which greater dilution of the drug will occur.
Oral Administration
A few antibacterial agents have excellent bioavailability after oral administration. For example, the fluoroquinolones, metronidazole, tetracycline, minocycline, doxycycline, linezolid, and trimethoprim-sulfamethoxazole are well-absorbed drugs, for which PO and IV doses are similar. Because absorption and distribution is taking place while a drug is being absorbed after oral administration, peak plasma levels can be delayed and usually are not as high as those achieved by IV infusion.
After oral administration, the bioavailability of penicillin G, which is destroyed by gastric acid, is low (< 30%). Penicillin V is more acid-stable, and its bioavailability (60%–70%) is better than that of penicillin G. Amoxicillin offers an advantage over penicillin V in that it has greater oral bioavailability (74%–92%). Only 30% to 55% of an oral ampicillin dose is absorbed.
Many of the oral cephalosporins, such as cefaclor, cefadroxil, cefprozil, cephalexin, ceftibuten, and loracarbef (technically a carbacephem) are acid-stable and have high bioavailability (80%–95%). The bioavailability of cefixime, however, is lower (40%–50%).
Effect of Food on Absorption
Generally, drugs are better absorbed in the small intestine (because of the larger surface area) than in the stomach; therefore, the quicker the stomach emptying, the earlier and higher are the plasma drug concentrations. Food, especially fatty food, delays gastric emptying, delays and lowers peak plasma levels, and may or may not lower a drug's bioavailability. Because eating stimulates production of gastric acid, penicillin G, which is unstable in gastric acid, is best administered in the fasting state (ie, ½ hour before or 2 hours after eating). Penicillin V is also better absorbed in the fasting state. Amoxicillin is equally well absorbed with food or in the fasting state. However, when amoxicillin is combined with clavulanate, absorption of clavulanate potassium is enhanced when it is administered at the start of a meal.
The bioavailability of erythromycin and azithromycin is low (about 40%), and because their bioavailability is furthered lowered in the presence of food, these drugs should be administered in the fasting state, whereas clarithromycin has better bioavailability (50%) and can be administered with or without food.
Food has no effect on the bioavailability of the fluoroquinolones, metronidazole, minocycline, doxycycline, linezolid, and trimethoprim-sulfamethoxazole. Food lowers the bioavailability of the first-generation cephalosporin cefaclor, second-generation loracarbef, and third-generation ceftibuten, but not that of the first-generation cephalosporins cephalexin and cefadroxil, second-generation cefprozil, and third-generation cefixime.
Because most drugs are absorbed from the intestinal mucosa by passive diffusion, absorption across the intestinal epithelium is enhanced if the drug is lipophilic. To this end, some oral cephalosporins are esterified (eg, cefuroxime axetil, cefpodoxime proxetil, and cefditoren pivoxil) to increase lipid solubility and enhance absorption. These prodrugs are hydrolyzed after intestinal absorption by esterases in the intestinal epithelium to their active metabolites. Nevertheless, their bioavailability is relatively low (25%–50%) and is enhanced by concomitant food intake.
Drug interactions can also alter absorption after oral administration. For example, multivalent cations such as aluminum, magnesium, and calcium in antacids can chelate the fluoroquinolones and tetracyclines, which may decrease the intestinal absorption of these antimicrobials after concurrent oral administration.
Oral absorption, of even highly bioavailable agents, may be impaired by poor circulation associated with hypotension. Gastrointestinal absorption also may be altered by ileus, colitis, bowel ischemia, and changes in gastric pH. Many of these conditions may be present in sepsis.
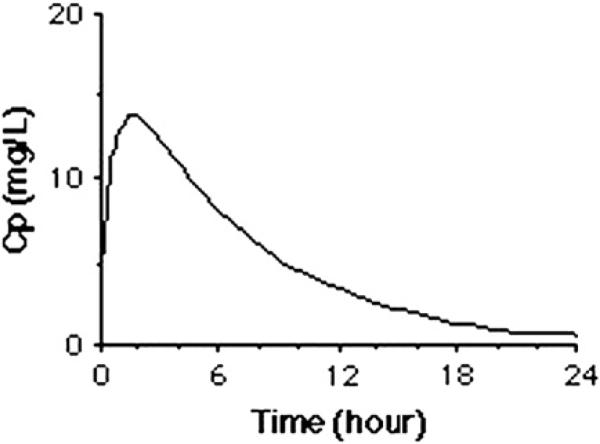
After oral administration, at the time when the rate of the drug entering the plasma (through absorption) and the rate of the drug disappearing from the plasma (through distribution and elimination) are equal, or at completion of IV infusion, the maximal concentration (Cmax) is reached (Fig. 1). Thereafter, the rate of distribution or elimination of the drug exceeds the rate of drug absorption, and the plasma concentration starts to decline to a minimal concentration. The area under the plasma concentration versus time curve (AUC, for “area under the curve”) is a pharmacokinetic measure that indicates the exposure to a drug during the full dosing interval.
Fig.1.
A typical example of a plot of the log plasma concentration (Cp) curve over time that may be measured in plasma after administration of a single IV infusion or oral administration of an antimicrobial drug.
Distribution
Distribution is the process by which a drug diffuses from the intravascular fluid space to extravascular fluid spaces, and it is best described by the drug's volume of distribution. The volume of distribution, which is the volume of body fluid into which a drug's dose is dissolved, is an important determinant of drug concentration.
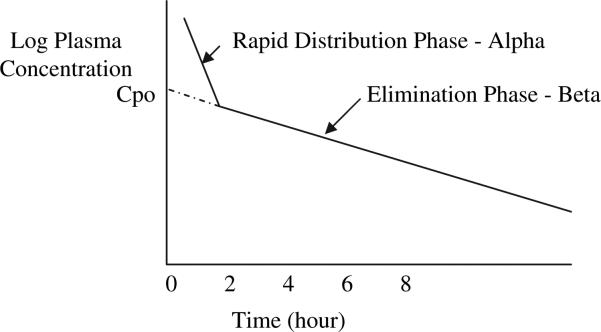
The central volume of distribution (Vc) is a hypothetical volume into which a drug initially distributes on administration. This compartment can be thought of as the plasma in blood vessels and the fluid in tissues that are highly perfused by blood. The Vc is defined mathematically as the dose administered divided by the peak plasma concentration at the end of a bolus IV infusion (Vc = dose/peak serum level). All drugs initially distribute into the smaller Vc before distributing into the more peripheral volume, referred to as the tissue volume of distribution (Vt). Together, the Vc and the Vt, in a two-compartment model, create the apparent volume of distribution (Vd). The Vd is a hypothetical parameter used to describe the volume of fluid that would be required to account for all of the drug in the body; its value may correspond to an anatomic body fluid compartment, but it does not actually represent a discrete anatomic compartment. Because the Vd is hypothetical in nature, it is referred to as an apparent volume. If the linear beta-phase elimination curve is extrapolated to the y-intercept, then the Vd equals the dose divided by this hypothetical zero-time drug concentration (see the Elimination section and Fig. 2).
Fig. 2.
A plot of the log plasma concentration (Cp) over time after rapid IV infusion. The linear beta-phase elimination curve has been extrapolated to the y-intercept to obtain the Cpo, which is the hypothetical zero-time drug concentration.
The largest body fluid compartment is the intracellular compartment. The extracellular compartment is mainly divided into an interstitial compartment (ie, the spaces between the cells) and the plasma. The percentage of body weight in each of these fluid compartments varies by age, sex, and adiposity. Total body water constitutes about 60% of the lean body weight (0.6 L/kg) in adult men and 50% (0.5 L/kg) in adult women. Fatty tissue contains proportionately less water than muscle tissue, so a more adipose person has proportionately less body water than a leaner person. A Vd of about 0.06 L/kg of body weight corresponds to the plasma compartment; a Vd of about 0.2 L/kg, or 1/3 of total body water, corresponds to the extracellular fluid compartment; and a Vd of about 0.4 L/kg, or 2/3 of total body water, corresponds to the intracellular fluid compartment. If the Vd exceeds the total body water (>0.6 L/kg), the drug is likely sequestered in the intracellular fluid of certain tissues.
For example, the Vd of daptomycin and the beta-lactam ceftriaxone, which are highly bound to plasma proteins, approximates the plasma volume because the drugs are part of a large molecular complex that does not diffuse easily out of capillaries. The Vd of most other beta-lactam antibiotics and aminoglycosides, which have lower plasma protein-binding, corresponds to the extracellular fluid compartment. Because the Vd of vancomycin varies widely (0.4–1 L/kg) as a result of highly variable distribution in the body, standard dosing of vancomycin is likely to be associated with a significant degree of variability in serum concentrations.
The Vd of quinupristin/dalfopristin, tigecycline,1 rifampin, clindamycin, metronidazole, trimethoprim, erythromycin, clarithromycin, the tetracyclines, linezolid, and the fluoroquinolones is equal to or greater than that of total body water (≥0.6 L/kg), which suggests wide distribution of these drugs throughout the body. The Vd of azithromycin is greater than 32 L/kg, (ie, >50 times that of total body water), which suggests its sequestration within tissues.
In patients who have high total body water (eg, those who have cirrhosis or congestive heart failure, or are pregnant), the volume of distribution for a given drug may be larger than expected, and the plasma drug levels correspondingly low.2 Sepsis and fever alone may increase the Vd of a drug.3,4
Most sites of infection are extravascular, and treatment of infections in these sites depends on movement of the antimicrobial agent out of the bloodstream and into interstitial and sometimes intracellular fluid. The ability of a drug to do so depends on tissue-related factors (such as perfusion to the tissues, the surface area of the tissue's vascular bed, and specialized vascular bed features, such as tight junctions or capillary pores) and drug-related factors (such as lipid solubility, molecular size, the drug's pKa, and plasma protein binding).
The perfusion rate is greatest for the brain, kidney, liver, and heart. It would be expected that drug concentrations would increase most rapidly in these organs. As the surface area of the capillary bed increases, the rate of diffusion also increases. Poor vascularity as a result of comorbid conditions (eg, large or small vessel peripheral vascular disease) results in impaired drug delivery and difficulty in achieving effective drug concentrations in the infected tissue.
Most drugs cross biologic membranes by passive diffusion. Diffusion occurs when the drug concentration on one side of the membrane is higher than that on the other side, in an attempt to equalize the drug concentration on both sides of the membrane. Capillaries in most parts of the body are fenestrated (ie, have pores between the endothelial cells lining the capillaries). These pores allow rapid diffusion of most drugs into the interstitial space. In some tissues, however, the endothelial cells are connected by “tight junctions,” without the presence of capillary pores between the endothelial cells. The capillary membranes between the blood and the eye, prostate, and brain have effectively no pores. In these areas, drugs must pass through, rather than between, endothelial cells. Because biologic membranes are mainly lipid in nature, the ability of an antimicrobial drug to traverse nonfenestrated capillaries depends on its lipid solubility.
Lipid-soluble drugs, such as metronidazole and rifampin, penetrate nonfenestrated capillary beds better than drugs that are more water soluble, such as beta-lactams, aminoglycosides, and glycopeptides. In some circumstances, for example, in the use of beta-lactams to treat bacterial meningitis, this disadvantage can be overcome by increasing the dose of the drug. In other situations, such as the treatment of intraocular infections, topical or direct instillation is necessary to convey the drug to the site of infection.5,6 Penetration of the antimicrobial agent into the eye and the central nervous system is further complicated by the presence of efflux pumps that actively transport some drugs, notably beta-lactams and the fluoroquinolones out of cerebrospinal fluid and beta-lactams out of vitreous humor.7,8 However, inflammation can partially overcome the exclusion of hydrophilic drugs into tissues that have nonfenestrated capillary beds.
Drug levels in the interstitial fluid relate to the concentration of that portion of a drug that is not bound to plasma protein (ie, free). As an example, beta-lactams that have different percentages of plasma protein binding, when dosed to achieve similar free-plasma drug levels, will have similar interstitial fluid levels, despite having very different total plasma levels.9 However, the penetration of vancomycin, which has serum protein binding levels of 50% or less, into epithelial lining fluid is variable, ranging from 0.4 to 8.1 mg/L after several hours, with an overall blood-to-epithelial-lining fluid penetration ratio of 6:1, and penetration is higher in the presence of lung inflammation.10
Only an unbound drug is considered active against microorganisms. Therefore, despite apparently adequate total plasma levels of highly protein-bound drugs, the concentration of free (ie, active) drug might be less than the MIC of the pathogen, which will necessitate the use of higher doses.11 The clinical significance of this phenomenon was shown by the failure of cefonicid, an agent that is highly active against Staphylococcus aureus in vitro but is highly protein bound in vivo, to cure endocarditis caused by S aureus.12
When the drug ultimately reaches the site of infection, local factors may play a role in the effectiveness of its antibacterial activity. For example, the aminoglycosides and erythromycin have decreased activity at an acid pH, such as occurs in an abscess. The aminoglycosides are also less active against facultative organisms in an anaerobic environment because the penetration of aminoglycosides into bacterial cells depends on an oxygen-dependent reaction.13 Substances that inactivate or lessen the antibacterial activity of antimicrobial agents, such as beta-lactamases and other deactivating enzymes, may be present at the site.14 As another example, aminoglycosides become less active as the concentration of calcium ions increases. Additionally, dense populations of organisms, such as occur in an abscess, tend to be slow growing, and antibiotics that are active against dividing cells, such as beta-lactams, may therefore be less effective in that setting.15 Bacterial meningitis is another infection in which bacterial growth rates tend to be slow, decreasing the effectiveness of beta-lactams.16 The presence of a foreign body may also adversely influence the effectiveness of an antimicrobial agent. The foreign body acts as a nidus on which microorganisms may grow as a biofilm. A biofilm is a community of microorganisms embedded in a matrix secreted by the microorganisms, which helps them attach to other bacteria, host cells, or foreign objects and which shields them against host defenses and penetration by many antimicrobial drugs.17,18
For some microorganisms that are preferentially intracellular pathogens (eg, Salmonella, Listeria, Chlamydia, Mycobacteria, and Mycoplasma), the antimicrobial drugs that are effective against them must reach and be active in the intracellular space occupied by these pathogens. Clindamycin, the macrolides, and linezolid may be actively transported into cells.8,19,20 However, drugs may be also actively transported out of cells, so the intracellular concentration reflects a balance between ingoing and outgoing processes.21,22 Just as with interstitial fluid, local factors within the cell may affect the activity of a drug (eg, pH, enzymatic activity).
Drugs that are weak bases are un-ionized at the pH of extracellular fluid. The relatively lipid-soluble, un-ionized moiety is able to diffuse easily across the cell membrane into the cytoplasm and then into the lysosome. Within the lysosome, where the pH is relatively low, the weak base becomes ionized. The hydrophilic, ionized moiety is unable to diffuse out (ie, is “trapped” within the lysosome). This scenario is believed to explain the intracellular accumulation of azithromycin, the concentration of which can be 100-fold greater within the lysosome than within plasma. Ion trapping is also believed to explain the accumulation of drugs that are weak bases in prostatic fluid, which has a lower pH (pH 6.3) than plasma (pH 7.4). These drugs include clindamycin, erythromycin, and trimethoprim.
Elimination
Fig. 1 represents a typical example of a pharmacokinetic curve that may be seen in plasma after administration of a single IV infusion or oral administration of an antimicrobial drug. After a peak plasma drug level is attained, the plasma level declines as a consequence of drug distribution and elimination. Drugs may be eliminated by being converted to metabolites (mainly in the liver); unchanged drugs or their metabolites may be eliminated in feces or urine by the excretory organs, mainly the kidneys, liver, and gut. Some drugs or their metabolites that are excreted in bile may be reabsorbed into the bloodstream and recycled by a process called enterohepatic circulation.
Renal excretion of drugs and their metabolites is determined by three processes: glomerular filtration, tubular secretion, and passive tubular reabsorption. Most beta-lactams, the aminoglycosides, tetracycline, vancomycin, daptomycin, and the sulfonamides are excreted by the kidneys, either by glomerular filtration, tubular secretion, or both. The aminoglycosides, tetracycline, and vancomycin are excreted primarily by glomerular filtration. More than 80% to 90% of vancomycin is recovered unchanged in urine within 24 hours after administration of a single dose.23 Only that portion of the drug that is not protein bound (ie, free) in the plasma can pass through the glomerular filter, so a high degree of protein binding can prolong the duration of highly protein-bound drugs, such as ceftriaxone, in the body. Most fluoroquinolones are eliminated primarily by renal mechanisms (eg, glomerular filtration and tubular secretion) and, to a lesser extent, by nonrenal mechanisms, such as hepatic metabolism and transepithelial intestinal elimination; the fluoroquinolone moxifloxacin, however, is eliminated mainly by nonrenal mechanisms. Tubular secretion occurs by way of two active transport mechanisms: one for anions (weak organic acids) and one for cations (weak organic bases). Competition between drugs for the carriers can occur within each transport system. The organic acid transport mechanism contributes to the elimination of many beta-lactam antibiotics, fluoroquinolones, and some sulfonamides. Competition between probenecid and these beta-lactams, fluoroquinolones, and sulfonamides for the organic acid transport carriers can prolong the duration that these antimicrobial drugs are in the body.
After filtration, polar substances are eliminated efficiently by the kidneys because they are not freely diffusible across the tubular membrane and so remain in the urine despite the concentration gradient that favors back-diffusion into interstitial fluid. Renal elimination of nonpolar drugs usually depends on metabolic conversion of the drugs in the liver to more polar metabolites, which cannot diffuse out of the tubular lumen and are then excreted in the urine. Erythromycin, azithromycin, moxifloxacin, clindamycin, rifampin, nafcillin, and cefoperazone are excreted mainly by the liver into bile; about 40% of a dose of ceftriaxone is eliminated by the liver in bile, but in the presence of renal failure, hepatic excretion increases. Doxycycline is eliminated by the gut. The streptogramin combination quinupristin/dalfopristin, the oxazolidinone linezolid, and the glycylcycline tigecycline are eliminated mainly by nonrenal mechanisms.
Colistin (also called polymyxin E) belongs to the polymyxin group of antibiotics. It is available for IV administration as colistin methanesulfonate (CMS), which is less toxic but also has less antimicrobial activity than colistin. CMS undergoes rapid hydrolysis in vivo to colistin, the bioactive drug. Notwithstanding that CMS has been used for more than 40 years, understanding of its pharmacokinetics has been problematic because previous pharmacokinetic data on CMS were obtained by using microbiological assays, which are unable to differentiate CMS from colistin. High-pressure liquid chromatography, which can distinguish both components, has shown rapid conversion of CMS to colistin in vivo. CMS is mainly eliminated in urine. The high urinary recovery of colistin after dosing with CMS, despite that fact that colistin is not itself excreted in urine, is likely the result conversion of CMS into colistin within the kidney or bladder, with the majority of the colistin formed in that way being excreted directly into urine.24
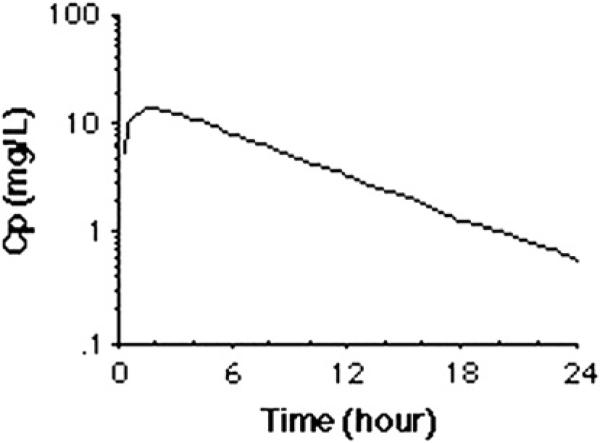
For most drugs, a plot of the log plasma concentration over time results in a straight line, the slope of which equals the elimination rate constant (–Kel) (Fig. 3). The half-life (T1/2) is the time it takes for the plasma drug concentration to decrease by half, and is equal to 0.693/Kel.
Fig. 3.
A plot of the log plasma concentration (Cp) over time.
After rapid IV administration, the decline in plasma drug levels may follow a biphasic curve (see Fig. 2). The T1/2 of the initial phase (alpha-phase T1/2) mainly represents distribution of the drug, and the T1/2 of the second phase (beta-phase T1/2) mainly represents elimination of the drug from the body. For example, in patients who have normal creatinine clearance, vancomycin has an alpha-distribution phase of ~30 minutes to 1 hour and a beta-elimination T1/2 of 6 to 12 hours. The T1/2 that is usually reported is the beta-phase (bp) T1/2. Ninety-four percent of any drug's dose will have been eliminated after four bpT1/2s, and about 99% of the drug will have been eliminated after 6.6 bpT1/2s.
If the drug is dosed more than every 4 to 5 bpT1/2s, the drug concentration will have fallen to almost zero before the next dose, and there will then be little accumulation of drug in the body. However, if the dosing interval is less than 4 bpT1/2s, the drug will start to accumulate, and steadily higher concentrations will occur with each subsequent dose until a steady state is achieved at about 4 bpT1/2s, when the amount of drug administered during each dosing interval exactly replaces the amount of the drug excreted. Similarly, during continuous IV infusion, plasma levels gradually increase until the steady state is achieved at about 4 bpT1/2s. When using continuous IV infusion, to ensure rapid onset of antimicrobial action, a loading dose is given. The loading dose equals the desired therapeutic plasma concentration multiplied by the Vd.
In people whose kidney or liver function has declined, the “normal” dosage of a drug may result in accumulation of the drug if the dosage or the dosing interval is not altered. Toxic side effects may occur as plasma and tissue drug concentrations increase. For example, high levels of imipenem, the penicillins, or the fluoroquinolones may cause seizures; high aminoglycoside levels may exacerbate renal failure or cause hearing impairment or vestibular damage; and high levels of vancomycin, especially in combination with aminoglycosides, may exacerbate renal failure. Therefore, the drug dosage must be adjusted based on the amount of decline in the person's kidney or liver function.
A reduction in the creatinine clearance to 30% of normal or less results in an exponential increase in the bpT1/2 of those drugs that are eliminated by the kidneys. The creatinine clearance can serve as a useful indicator of renal function. A quick estimate of the creatinine clearance can be made using this equation: creatinine clearance = [(140 – age) × ideal body weight in kg]/(serum creatinine × 72) (0.85 for females).
Males: ideal body weight = 50 kg + 2.3 kg for each inch more than 5 ft.
Females: ideal body weight = 45.5 kg + 2.3 kg for each inch more than 5 ft.
The initial dose should be the usual dose given to people with normal renal function. Subsequent doses may be reduced by a percentage based on the estimated creatinine clearance. (See “Use of Antimicrobial Agents in Renal Failure” on page 899 of this issue for details on the use of antibiotics in patients who have renal insufficiency.) An alternative measure is to lengthen the dosing interval. Lengthening the dosing interval results in a concentration versus time curve that approximates the situation in normal renal function, and therefore is preferred for drugs that exhibit concentration-dependent pharmacodynamics (see later discussion). On the other hand, using a longer dosing interval runs the risk for incurring longer periods during which the plasma level has dropped to less than the MIC of the organism, and for that reason, administration of smaller doses given at the regular interval would be preferred for drugs that exhibit time-dependent pharmacodynamics (see later discussion).25,26
The use of hemodialysis, peritoneal dialysis, and continuous arteriovenous hemofiltration further confounds calculations of dose modification. Guidelines for dosage modification in dialysis patients can usually be obtained from the manufacturer's product literature, and they are based on the degree to which the drug is removed by dialysis. (See “Use of Antimicrobial Agents in Renal Failure” on page 899 of this issue for more information.)
Unfortunately, there is no clinical measure of hepatic dysfunction that is easily adaptable for use in modifying doses of antibiotics that are excreted or metabolized by the liver.27,28 In patients who have severe liver disease, it may be prudent to reduce doses of erythromycin, metronidazole, chloramphenicol, and clindamycin, but there are no specific guidelines for most antimicrobial agents.
Under ideal circumstances, dosing is adjusted most accurately by using a combination of calculated estimates followed by periodic monitoring of measured plasma concentrations. Changes in the dosage or dosing interval can be made in response to the measured levels, and follow-up plasma levels can be obtained at the appropriate time (4–5 dosing intervals), whereupon new adjustments can be made. This procedure is particularly helpful in patients whose renal (or hepatic) functioning is fluctuating. Although levels of almost any antimicrobial agent can be measured by using bioassays, radioimmunoassays, or high-pressure liquid chromatography, such laboratory studies are available only for the aminoglycosides and vancomycin, at least within a time-frame that is clinically useful.
PHARMACODYNAMICS
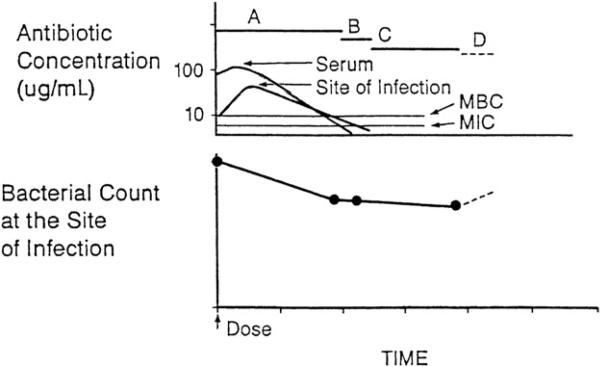
After a dose of a bactericidal drug, the bacterial count may decline in the early portion of the dosing interval, when levels of the portion of the drug not bound to protein exceed the MBC as a result of drug effects and host defenses (Fig. 4). When unbound drug levels decrease to less than the MBC but still exceed the MIC, the bacterial count may remain stable or continue to decline as a result of host defenses.29 For a bacteriostatic drug, when drug levels are in excess of the MIC, the bacterial count declines as a result of host defenses alone. Eventually, unbound drug levels decrease to less than the MIC, at which point any persistent antibacterial effect can be due to several causes. First, persistent suppression of bacterial growth after a brief exposure of bacteria to an antibacterial agent may occur, even in the absence of host defenses; this is the postantibiotic effect (PAE). Second, after antibiotic exposure, organisms may be more susceptible than untreated bacteria to the antibacterial activity of phagocytes; this is called postantibiotic leukocyte enhancement (PALE). Third, drug concentrations that are less than the MIC have been shown to alter bacterial morphology, slow the rate of bacterial growth, and prolong the PAE. The minimal drug concentration that alters bacterial cell morphology has been termed the minimal antibacterial concentration (MAC).
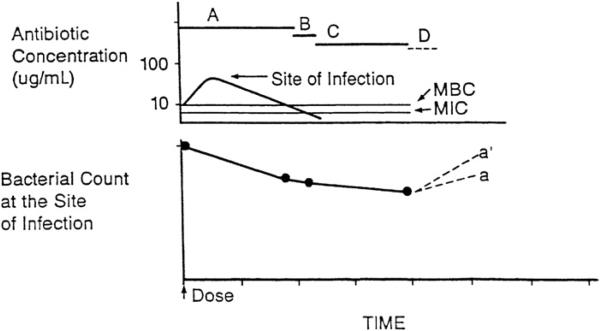
Fig. 4.
Antibiotic pharmacodynamics. A, time during which free-drug levels at the site of infection exceed the MBC; B, time during which free-drug levels at the site of infection are less than the MBC but exceed the MIC; C, persistent antimicrobial effects (postantibiotic effect, postantibiotic leukocyte enhancement, and minimal antibacterial concentration) when free-drug levels at the site of infection are less than the MIC; D, regrowth of residual bacteria. (From Levison ME. Pharmacodynamics of antimicrobial agents: bactericidal and postantibiotic affects. Infect Dis Clin N Am 1995;9:483–95.)
Eventually, residual drug effects wane, and the remaining bacteria will begin to resume growth.29 The extent of regrowth before the next dose is given will depend in part on the inherent doubling time of the organism, on available nutrients being present in the infected tissues, and on the adequacy of host defenses. For example, in the absence of host defenses, such as occurs in early cardiac vegetation cases and in cerebrospinal fluid in cases of early meningitis, the number of microorganisms can double every 20 minutes, which is similar to the doubling time during the logarithmic phase of growth under optimal in vitro conditions (Fig. 5). In contrast, the numbers of Mycobacterium tuberculosis and Treponema pallidum double every 36 hours. Some regrowth may, in fact, restore susceptibility to beta-lactam antibiotics.30
Fig. 5.
A, time during which free-drug levels at the site of infection exceed the MBC; B, time during which free-drug levels at the site of infection are less than the MBC but exceed the MIC; C, persistent antimicrobial effects (PAE, MAC, and PALE) when free-drug levels at the site of infection are less than the MIC; D, regrowth of residual bacteria, with (a) and without (a’) adequate host defenses. Inadequate host defenses at the site of infection may result in a higher residual bacteria population at the time the next dose is given (a’). (From Levison ME. Pharmacodynamics of antimicrobial agents: bactericidal and postantibiotic affects. Infect Dis Clin N Am 1995;9:483–95.)
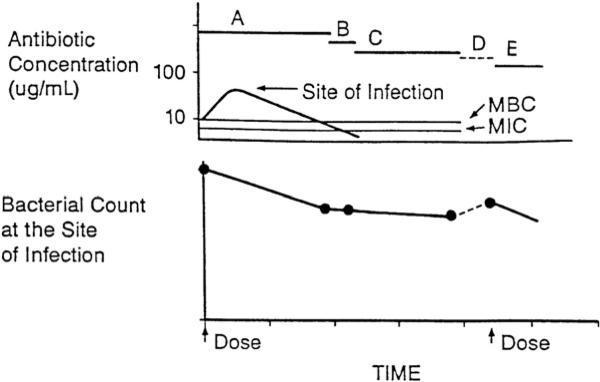
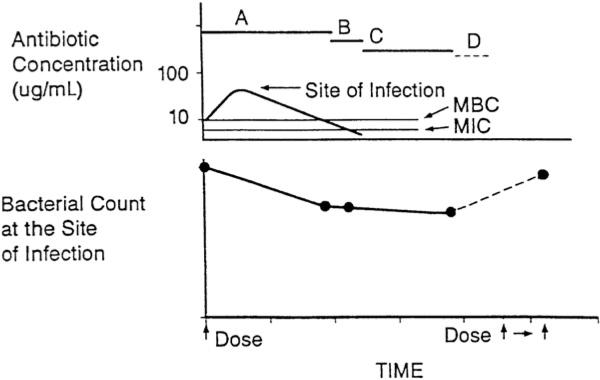
The next dose ideally is given before clinically significant regrowth has occurred (Fig. 6), so that after multiple doses, the tissues are cleared of the pathogen. However, if the doses are spaced too far apart, the residual bacteria may resume growth in the later portion of each dosing interval, and the bacterial count may become equal to, or perhaps exceed, the count at the beginning of the dosing interval, which can compromise drug efficacy (Fig. 7).29 The size of the residual bacterial population at the end of each dosing interval, and ultimately the efficacy of the antimicrobial regimen, thus will depend on the interplay of a variety of bacterial, drug, and host factors that includes (1) the size of the initial bacterial population, (2) the potency (MIC and MBC) and pharmacokinetic characteristics of the antimicrobial agent, (3) the rate and extent of any bactericidal effect, (4) the presence of a PAE, (5) the rate of regrowth of persistent organisms, and (6) the presence of host defenses.
Fig. 6.
A, time during which free-drug levels at the site of infection exceed the MBC; B, time during which free-drug levels at the site of infection are less than the MBC but exceed the MIC; C, persistent antimicrobial effects (PAE, MAC, and PALE) when free-drug levels at the site of infection are less than the MIC; D, regrowth of residual bacteria; E, bactericidal effect following the next dose. If the next dose is given before significant regrowth occurs, multiple doses can eventually clear bacteria from the site of infection. (From Levison ME. Pharmacodynamics of antimicrobial agents: bactericidal and postantibiotic affects. Infect Dis Clin N Am 1995;9:483–95.)
Fig. 7.
A, time during which free drug levels at the site of infection exceed the MBC; B, time during which free drug levels at the site of infection are less then the MBC, but exceed the MIC; C, persistent antimicrobial effects (PAE, MAC, and PALE) when free drug levels at the site of infection are less than the MIC; D, regrowth of residual bacteria. Regrowth as a result of a longer dosing interval, which compromises drug efficacy. (From Levison ME. Pharmacodynamics of antimicrobial agents: bactericidal and postantibiotic affects. Infect Dis Clin N Am 1995;9:483–95.)
Antimicrobial drugs can be divided into three main groups based on pharmacodynamic characteristics that affect bacterial clearance.31 The first group consists of drugs that exhibit mainly time-dependent bactericidal action that has only a minimal relationship to drug concentrations that are greater than the MIC (eg, beta-lactam antibiotics and vancomycin). These drugs have relatively slow bactericidal action, and little increase in bactericidal activity is seen when concentrations are increased to more than a point of maximal killing action, which is approximately four times the MIC. These drugs have short PAEs for gram-positive cocci and no or short PAEs for gram-negative bacilli; the duration that drug levels exceed the MIC relative to the dosing interval and, consequently, the frequency of drug administration are important determinants of outcome for these drugs. A shorter dosing interval will increase the time that concentrations remain greater than the MIC of the infecting microorganism. (The only exceptions among the beta-lactam class are the carbapenems, which possess a PAE against a variety of gram-negative bacilli). Even a paradoxic pattern (the “Eagle effect”) of bactericidal activity, which is characterized by a decreasing rate of killing at higher concentrations, has been reported. Consequently, there is no advantage to achieving high antibiotic concentrations, except that an increase in the dose will increase the duration that levels exceed the MIC and the maximal level (Cmax) in serum, which frequently results in a covariance of time and drug concentrations.
The second group includes drugs that exhibit concentration-dependent bactericidal action and prolonged PAEs (eg, the aminoglycosides, the fluoroquinolones, daptomycin, colistin, metronidazole, possibly the azalide azithromycin, and the ketolides). Both the rate of cidal action and the duration of the PAE for these drugs are concentration-dependent over a wide range of concentrations; consequently the amount of drug (based on the Cmax and AUC relative to the MIC) rather than the dosing frequency determines the efficacy for these drugs.
The third group includes drugs that are predominantly bacteriostatic and that produce moderate to prolonged PAEs (eg, the macrolides, clindamycin, the streptogramins such as quinupristin/dalfopristin, the tetracyclines, tigecycline, and linezolid). Because of their prolonged PAE, their efficacy is determined less by time and more by the AUC that is greater than the MIC.
Usually, drug concentrations in the blood are used to determine pharmacodynamic parameters because of the relative accessibility of this body fluid and the correlation of pharmacodynamic parameters that are based on serum levels. However, the use of serum levels to determine pharmacodynamic parameters may not always be appropriate.31 Because infection usually occurs at extravascular sites, the use of drug concentrations in the blood will only be satisfactory if the blood levels are an adequate surrogate for levels at the site of infection. Theoretically, at equilibrium, free-drug levels in plasma and extracellular tissue fluid should be equal.32 However, depending on the ratio of surface area of the capillary bed to the volume of the tissue compartment, the physico-chemical characteristics of the drug, and special anatomic barriers (eg, those in the brain, eye and prostate), drug levels at the site of certain infections can be much lower than free-drug levels in plasma. For cases of meningitis, the use of cerebrospinal fluid levels is appropriate for determination of pharmacodynamic parameters.33 Recent studies also suggest that concentrations of epithelial lining fluid are important determinants of the efficacy of treatment of bacterial pneumonia, such that concentrations in epithelial lining fluid can be better predictors of outcome than serum levels for certain antibiotics (eg, vancomycin).10 Serum drug levels are also poor predictors of intracellular concentrations, which is of major importance for the treatment of intracellular pathogens. Beta-lactams and vancomycin penetrate cells poorly, whereas other drugs, such as azithromycin, achieve intracellular concentrations many-fold greater than serum levels.
In vitro and animal model studies have documented that the magnitude of the pharmacodynamic parameters required to achieve a specific target (eg, bacteriostasis or various degrees of cidal action) is similar for different drugs within the same class.31 For those drugs that are highly protein bound, efficacy is predicted when serum concentrations of the drug that are not bound to protein, rather than the total drug levels, are used.31 Pharmacodynamics that are derived from in vitro and animal data are concordant with those derived from human data, with some exceptions, as detailed in a later section. Consequently, pharmacodynamics can be used to predict drug efficacy in patients and can provide a rational basis for establishing optimal dosing regimens.34
Time Course of Time-Dependent Bactericidal Action
Because the bactericidal action of beta-lactams is relatively slow,29,31 there will be a relatively large residual population of microorganisms that remain when drug levels decrease to less than the MBC. After drug levels at the site of infection decrease to less than the MIC, the residual population can resume growth quickly because there are either no or short-lived PAEs for most beta-lactams.29,31
Beta-lactams exhibit an inoculum effect; that is, the lower the bacterial density, the lower the concentration of the beta-lactam that is required to inhibit growth.35 The minimal concentration of these drugs that inhibits growth can progressively decrease to less than the standard MIC (determined by using an inoculum of 105 CFU/mL) because the bacterial count progressively falls during the time course of antimicrobial therapy, and thus the time during the dosing interval that levels exceed the MIC may progressively lengthen.
The efficacy for drugs such as the beta-lactams can be optimized by using dosing strategies that maximize the duration of drug exposure (ie, time-dependent bactericidal activity), such as using smaller fractions of the total daily dose given at frequent intervals, larger doses, beta-lactams with long serum T1/2s, such as ceftriaxone with a T1/2 of 6 to 8 hours, or longer IV infusions or even continuous IV infusion. However, there have been few trials of continuous versus intermittent infusions.36–38
Effective dosing regimens for time-dependent antibiotics require that serum drug concentrations exceed the MIC of the causative pathogen for at least 40% to 50% of the dosing interval. For beta-lactam drugs that have high serum protein binding (eg, ceftriaxone and ertapenem), when drug concentrations that are not bound to serum protein are used, the percentage of the dosing interval during which drug concentrations are greater than the MIC, can be used to predict efficacy; these percentages are similar for all beta-lactams within a class. The percentage of time that concentrations are greater than the MIC, which correlates with efficacy, varies among classes within the beta-lactams, and is greater for the cephalosporins and aztreonam than the penicillins, and greater for the penicillins than the carbapenems. Among bacterial species, the percentage is less for staphylococci, for which beta-lactams have a PAE, than for streptococci and gram-negative bacilli, for which beta-lactams do not have a PAE.31 The percent of time that concentrations are greater than the MIC for a dosing interval can be used to compare the effectiveness of different time-dependent antibiotics within a class, and as a corollary, those drugs having the greater potency (ie, a lower MIC) can be anticipated to have a longer percentage of the dosing interval at which concentrations will be greater than the MIC, and therefore to have greater effectiveness.
For susceptible pathogens with MICs that are close to a particular beta-lactam's breakpoint, serum levels of the beta-lactam will be in excess of the MIC for a smaller percentage of the dosing interval than for strains that have lower MICs. For example, patients infected with borderline cephalosporin-sensitive ESBL-producing strains of gram-negative bacilli with MICs from 4 to 8 μg/mL did much worse when treated using cephalosporin monotherapy than did patients infected with strains having lower MICs.39 Similarly, free-drug levels of ceftriaxone can not remain at concentrations greater than the MICs for more than 50% of the 24-hour dosing interval for strains of S aureus, which have MICs close to the breakpoint, especially with a 1-g dose. Also, extending the dosing interval of cefoperazone (which may be no longer available in the United States), which has relatively high serum-protein binding and a short bpT1/2 (2 hours), from 6 or 8 hours to 12 hours for susceptible strains of Pseudomonas aeruginosa that have MICs close to the breakpoint of 16 μg/mL may be similarly problematic.
The glycopeptides vancomycin and teicoplanin demonstrate concentration-independent (ie, time-dependent), slow bactericidal activity and a short PAE in vitro. However, there is conflicting data about which pharmacodynamic parameter best predicts bacterial eradication and clinical outcome. For example, studies that used an in vivo rabbit model of S aureus aortic valve endocarditis40 and clinical studies of S aureus septicemia41 and endocarditis42 showed that the glycopeptides exhibit time-dependent action, with the trough plasma concentration of protein free vancomycin that is greater than the MIC being the therapeutically relevant pharmacodynamic parameter. In contrast, in a study of patients who had a S aureus lower respiratory tract infection, clinical and bacteriologic response to vancomycin therapy was correlated with the 24 hour AUC/MIC value, but no relationship was identified between outcome and vancomycin the percentage of time that concentrations were greater than the MIC.43 In another study of patients who had S aureus bacteremia (MIC range 0.25–1.0 mg/L), no relationship was found between successful outcomes and a specific AUC/MIC value.44 In clinical practice, maintenance of trough serum levels of free drug that are greater than the MIC is most commonly recommended.45
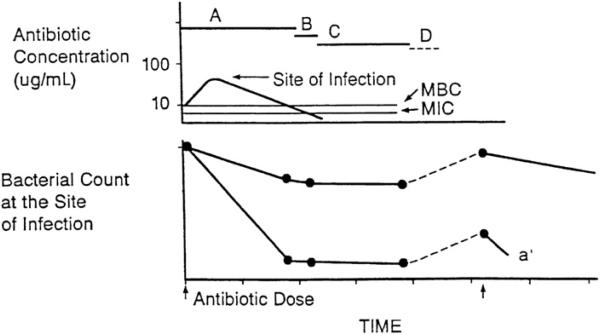
If the rate of cidal action of beta-lactams or vancomycin were increased, lower residual bacterial counts would occur during the dosing interval, when drug levels decrease to less than the MIC; this would prolong the intervals before significant re-growth occurs and either allow for more extended dosing intervals or allow for shorter durations of therapy as a consequence of accelerated clearance of bacteria from sites of infection (Fig. 8). Indeed, combinations of these antibiotics with aminoglycosides can enhance the relatively slow rate of bactericidal activity of beta-lactams and vancomycin. For example, a bacterial cell-wall-active agent alone, such as penicillin, ampicillin, or vancomycin, is at best only slowly bactericidal against enterococci, and an aminoglycoside alone at concentrations achieved in serum after standard dosing exhibits only inhibitory activity, but the combination of the cell-wall-active agent with an aminoglycoside results in rapid bactericidal activity. The synergy achieved by the combination has been shown to be due to the enhanced bacterial penetration of the aminoglycoside in the presence of the cell-wall-active agent.
Fig. 8.
A, time during which free drug levels at the site of infection exceed the MBC; B, time during which free drug levels at the site of infection are less than the MBC, but exceed the MIC; C, persistant antimicrobial effects (PAE, MAC, and PALE) when free drug levels at the site of infection are less than the MIC; D, regrowth of residual bacteria. The effect of more rapid and extensive bactericidal action (a’) on the residual bacterial population despite prolongation of the dosing interval. (From Levison ME. Pharmacodynamics of antimicrobial agents: bactericidal and postantibiotic affects. Infect Dis Clin N Am 1995;9:483–95.)
Synergistic bactericidal activity, usually defined as achieving a 2log10 or greater (ie, ≥99%) reduction in bacterial count after overnight incubation using a combination of antibiotics versus the outcome using each of the agents alone. Synergism has also been shown when using combinations of cell-wall-active agents and aminoglycosides against viridans streptococci, S aureus, and many gram-negative bacilli. Synergistic combinations that more rapidly clear the tissues of the infecting microorganism have been used to shorten the course of antimicrobial therapy for viridans streptococcal endocarditis (penicillin or ceftriaxone plus gentamicin for 2 weeks versus penicillin or ceftriaxone alone for 4 weeks) and for uncomplicated, methicillin-sensitive, S aureus, right-sided endocarditis (nafcillin plus gentamicin for 2 weeks versus nafcillin alone for 4 weeks).
Some combinations of antimicrobial agents have been found to be antagonistic (eg, penicillin plus a tetracycline). In such cases, the penicillin's bactericidal effect, which requires the presence of growing organisms, may be converted to a bacteriostatic effect when combined with a tetracycline that prevents microbial growth. This has been the explanation for the findings of Lepper and Dowling,46 in which patients who had pneumococcal meningitis and were treated using penicillin combined with a tetracycline had 2.6-fold greater mortality than those patients treated using penicillin alone.
Time Course of Concentration-Dependent Bactericidal Action
For drugs that have concentration-dependent bactericidal action, such as aminoglycosides and fluoroquinolones, the rate of bactericidal activity will be maximum at the peak concentration (Cmax) in serum.47–49 As the drug concentration decreases, the rate of bactericidal activity will decrease. Higher doses of the drug will increase not only the rate of reduction of bacteria but also the length of time of drug exposure to bactericidal concentrations. This dependence on the magnitude and the duration of exposure of bactericidal concentrations implies that concentration-dependent drugs are influenced by the Cmax and the area under the serum concentration curve (AUC), whereas for drugs with time-dependent activity, the extent of bactericidal activity will depend mainly on the duration of drug exposure at concentrations great than the MIC.
After drug levels at the site of infection decrease to concentrations that are less than the MIC, there may be persistent suppression of growth that is due to a PAE, the duration of which is also concentration-dependent for aminoglycosides and fluoroquinolones; the higher the drug concentration, the longer the duration of the PAE for these drugs, and the smaller the residual bacterial population at the time of the next dose.
Indeed, effective dosing regimens for concentration-dependent antibiotics require that either the 24-hour protein-free drug AUC/MIC value be at least 100 to 125 for aminoglycosides or fluoroquinolones against gram-negative bacilli3,49,50 and from 25 to 30 for fluoroquinolones against S pneumoniae,51,52 or that the Cmax/MIC value of the causative pathogen be more than 10.53,54 For concentration-dependent drugs, dosing strategies that maximize the intensity of drug exposure, such as giving the total daily dose as a single dose every 24 hours rather than giving smaller divided doses, would maximize the Cmax and possibly allow for comparable efficacy at greater convenience and lower cost.55
The AUC/MIC or the Cmax/MIC ratios also can be used to compare the effectiveness of different concentration-dependent antibiotics. Drugs within a class having the greater potency (ie, lower MICs) will have higher AUC/MIC or Cmax/MIC ratios and therefore can be anticipated to have greater effectiveness. It is clear that an infection caused by susceptible pathogens that have relatively high MICs may not be adequately treated using the standard dosage of a concentration-dependent antimicrobial agent. For example, gentamicin-susceptible strains of P aeruginosa that have MICs close to the breakpoint for gentamicin of 4 μg/mL may respond suboptimally to standard dosing regimens that provide peak serum levels of gentamicin of 6 μg/mL. Similarly, ciprofloxacin-susceptible strains of P aeruginosa that have MICs close to the breakpoint of 2 μg/mL may respond suboptimally to standard dosing regimens that provide peak plasma levels of ciprofloxacin of about 3 to 4 μg/mL, and levofloxacin-susceptible strains of S pneumoniae with MICs close to the breakpoint of 2 μg/mL may respond suboptimally to 500-mg dosing regimens that provide peak plasma levels of levofloxacin of about 5 to 6 μg/mL and an AUC of 55. A 750-mg dose of levofloxacin doubles the peak level and the AUC.56 The higher Cmax and AUC achieved using the higher dosage allow greater confidence in treating patients who may be infected with organisms for which levofloxacin MICs are high.
Higher rates of bactericidal action result in lower residual bacterial counts and longer intervals before significant regrowth occurs (see Fig. 8). Maximizing serum concentrations of drugs that exhibit concentration-dependent bactericidal activity by increasing the dose will maximize the rate and extent of bactericidal activity, if adverse effects are not also concentration-dependent. Dose-dependent toxicity was once believed to limit the ability to administer the total daily dose of an aminoglycoside as a single dose every 24 hours, but data from animal models of infection and human clinical trials suggest that dosing regimens that provide very high peak aminoglycoside concentrations relative to the MIC and prolonged periods of subinhibitory aminoglycoside concentrations have not resulted in greater nephrotoxicity than regimens that provide lower peaks but more persistent inhibitory concentrations,57 although the relationship between the pharmacodynamic parameters and auditory and vestibular toxicity is unclear. Giving the total 24-hour dose as a single dose, rather than in smaller divided doses, and using extended dosing intervals has now become the standard in most clinical settings.3 This strategy may be especially appropriate for treatment of many susceptible pathogens, (eg, P aeruginosa) that have MICs that are close to the breakpoint.58 However, this same strategy may not be appropriate for fluoroquinolones that likely have concentration-dependent toxicity.
All aminoglycosides have similar pharmacokinetics, but there is significant variation in pharmacokinetics in normal individuals and certain patient populations. For example, volume of distribution tends to be elevated in critically ill patients, and clearance is elevated in children, in patients who have cystic fibrosis, and during pregnancy and the early postpartum period, and it is depressed in cases of renal insufficiency. The Cmax is primarily affected by the volume of distribution, and the AUC by the volume of distribution and clearance. Consequently, measurement of aminoglycoside levels is especially important early in the course of treatment, and doses should be adjusted to achieve therapeutic levels.59
Colistin, which is available as CMS, has been increasingly used intravenously for otherwise pan-resistant, nosocomial, gram-negative bacillary infections, especially those caused by P aeruginosa, Klebsiella pneumoniae, and Acinetobacter spp. Colistin demonstrates very rapid concentration-dependent killing action.60–63 Although the concentration-dependent killing action of colistin suggests that single daily dosing rather than divided dosing may be necessary, its lack of a significant PAE at clinical achievable concentrations indicates that infrequent dosing regimens may be problematic.62 Furthermore, the frequent emergence of colistin resistance after initial rapid killing of susceptible P aeruginosa and the emergence of colistin-heteroresistant strains of K pneumoniae and Acinetobacter spp suggest that colistin monotherapy may be inadequate.61,63–67
Bacteriostatic Activity
Erythromycin has exhibited time-dependent efficacy in various models; in contrast, it has been stated that the efficacy of azithromycin and the ketolide antibiotic telithromycin is correlated best with the AUC/MIC ratio.68 It has been theorized that the prolonged PAE observed with azithromycin reduces the drug's dependency on the extent of time for which it should remain at concentrations greater than the MIC. However, the persistence of resistant subpopulations of S pneumoniae and their subsequent emergence may be encouraged by the presence of prolonged periods of sub-MIC concentrations of azithromycin in epithelial lining fluid.
A variety of in vitro and in vivo studies have demonstrated that the streptogramins exhibit concentration-independent killing action and produce prolonged PAEs in gram-positive organisms.69 The efficacy of the streptogramins, which are characterized by this pattern of activity, is best correlated with the 24-hour AUC/MIC ratio. The 24-hour AUC/MIC ratio is also the pharmacodynamic parameter that can best be used to predict the in-vivo activity of clindamycin in a pneumococcal, neutropenic, murine thigh-infection model70 The 24-hour AUC/MIC ratio is also the pharmacodynamic parameter that can best be used to predict the in-vivo activity of tigecycline.1,71,72
A review of the pharmacodynamics of linezolid was published.31 In animal models, the 24-hour AUC/MIC ratio correlated best with the efficacy of a drug,73 although the percentage of time that concentrations were greater than the MIC and the 24-hour AUC/MIC ratio was found to correlate with efficacy in clinical trials.74 The chance of success in treating bacteremia, lower respiratory tract infection, and skin and skin structure infections was greater when linezolid plasma concentrations remained in excess of the MIC for the entire dosing interval. Although linezolid shows bactericidal action against S pneumoniae,75 it is mainly bacteriostatic against S aureus. In a rabbit S aureus endocarditis model, linezolid was bactericidal if the levels were maintained constantly at concentrations that were greater than the MIC by using continuous infusion, and it was only bacteriostatic when administered by using intermittent infusion.76 Linezolid penetrates epithelial lining fluid better than vancomycin, at levels threefold greater than simultaneous serum levels.77
PREVENTION OF RESISTANCE
Subpopulations that have reduced susceptibility to antibiotics are a normal feature of dense populations of some bacterial species, especially P aeruginosa, A baumanii, K pneumoniae, and S aureus. The likelihood that resistant subpopulations will emerge when using antimicrobial therapy will depend on the propensity for resistance within the population, that is, on the spontaneous mutation rate for antibiotic resistance, the ability of host defenses to control the growth of the resistant subpopulation, and the magnitude of the antimicrobial drug levels at the site of infection.78 It is believed that drug levels should exceed at least 8 to 10 times the MIC to prevent emergence of resistant subpopulations, which could be accomplished by using a single daily dosing of an aminoglycoside, using the most potent fluoroquinolone, or using high doses of a beta-lactam. In vitro and animal models of infection have identified the peak MIC ratios and free-drug 24-hour AUC/MIC ratios for fluoroquinolones that are required to prevent the emergence of resistant subpopulations. The minimal prevention dose, which has been shown to vary among bacterial species, is higher for denser bacterial populations and is often higher than the ratios required for efficacy.34,79 The clinical utility of high-dose antimicrobial therapy to prevent the emergence of resistance remains to be proved.80
USE OF PHARMACODYNAMIC BREAKPOINTS FOR ANTIMICROBIAL SUSCEPTIBILITY TESTING
For time-dependent drugs, the minimal serum concentration of free drug that is present for 40% to 50% of the dosing interval is the important parameter for predicting efficacy and can be determined if the peak serum level of free drug after a particular dose regimen and the serum T1/2 of the drug are known. This concentration is the pharmacodynamic breakpoint for time-dependent drugs. If the MIC of the drug that is effective against a particular pathogen or the MIC90 strains of the drug that is effective against a group of clinical isolates of a particular pathogen are at concentrations that are less than this breakpoint, the drug is likely to be clinically useful, and if concentrations are greater than this breakpoint, the drug may not be useful. For example, a 500-mg dose of amoxicillin given every 8 hours or an 875-mg dose given every 12 hours yields a concentration of at least 2 μg/mL for 40% to 50% of the dosing interval, and the MIC90 for amoxicillin against S pneumoniae in the United States is currently less than this breakpoint, allowing for the prediction of clinical success when using these dosing regimens of amoxicillin. The same calculations for defined dosing regimens can be done for other time-dependent drugs to determine their pharmacodynamic breakpoints.81
For concentration-dependent drugs, successful treatment of pneumococcal infections using the fluoroquinolones or azithromycin requires a 24-hour protein-free drug AUC/MIC ratio that is greater than 25 to 35, and successful treatment of gram-negative bacillary infections requires a 24-hour protein-free drug AUC/MIC ratio that is greater than 100–125 or a peak serum level that has a free-drug/MIC ratio that is greater than 8 to 10.82 The pharmacodynamic clinical breakpoint MIC can be calculated by using the following formula: for S pneumoniae, AUC/25, and for gram-negative bacilli, AUC/100, when the average AUC from the dosing regimen is known.81 Similarly, the pharmacodynamic clinical breakpoint MIC can be calculated by using the following formula: peak serum level of free drug/10. For ciprofloxacin, the peak serum level after a 400-mg IV dose is about 4 μg/mL of the total drug and 2.8 μg/mL of the free drug; the respective pharmacodynamic breakpoint MIC would be 0.4 and 0.28 μg/mL for the total drug and free drug; strains of S pneumoniae that have an MIC that is greater than 0.28 μg/mL would be considered to be resistant, those with an MIC of 0.28 μg/mL or less would be considered to be sensitive.
SUMMARY
The importance of pharmacodynamic factors in developing optimal treatment strategies has been confirmed in many studies of in vitro models, in models of infection in animals that attempt to simulate human infections, and in clinical studies. The requirements for bactericidal therapy for endocarditis and meningitis, for synergistic combinations to treat enterococcal endocarditis or to shorten the course of antimicrobial therapy, for obtaining Cmax/MIC ratios that are greater than 10 or 24 hour protein-free drug AUC/MIC ratios that are greater than 100–125 for concentration-dependent agents against gram-negative bacilli and 25 to 35 against S pneumoniae, and for the percentage of time that concentrations are greater than an MIC that is at least 40% to 50% of the dosing interval for time-dependent agents are a few important pharmacodynamic concepts that have been demonstrated in animal models and that that have successfully guided therapy of human infections. Pharmacodynamic concepts can also be used to optimize dosing to prevent the emergence of resistance and to rationalize the determination of antimicrobial susceptibility.
REFERENCES
- 1.Meagher AK, Ambrose PG, Grasela TH, et al. Pharmacokinetic/pharmacodynamic profile for tigecycline—a new glycylcycline antimicrobial agent. Diagn Microbiol Infect Dis. 2005;52(3):165–71. doi: 10.1016/j.diagmicrobio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am. 2003;17(3):503–28. doi: 10.1016/s0891-5520(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 3.Buijk SE, Mouton JW, Gyssens IC, et al. Experience with a once-daily dosing program of aminoglycosides in critically ill patients. Intensive Care Med. 2002;28(7):936–42. doi: 10.1007/s00134-002-1313-7. [DOI] [PubMed] [Google Scholar]
- 4.Tod M, Lortholary O, Seytre D, et al. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. Antimicrob Agents Chemother. 1998;42(4):849–56. doi: 10.1128/aac.42.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leeming JP. Treatment of ocular infections with topical antibacterials. Clin Pharmacokinet. 1999;37(5):351–60. doi: 10.2165/00003088-199937050-00001. [DOI] [PubMed] [Google Scholar]
- 6.Smith A, Pennefather PM, Kaye SB, et al. Fluoroquinolones: place in ocular therapy. Drugs. 2001;61(6):747–61. doi: 10.2165/00003495-200161060-00004. [DOI] [PubMed] [Google Scholar]
- 7.Tamai I, Yamashita J, Kido Y, et al. Limited distribution of new quinolone antibacterial agents into brain caused by multiple efflux transporters at the blood–brain barrier. J Pharmacol Exp Ther. 2000;295(1):146–52. [PubMed] [Google Scholar]
- 8.Barza M. Pharmacologic principles. In: Gorbach S, Bartlett JG, Blacklow NR, editors. Infectious diseases. Vol. 3. Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 172–9. [Google Scholar]
- 9.Tan JS, Salstrom SJ. Levels of carbenicillin, ticrcillin, cephalothin, cefazolin, cefamandole, gentamicin, tobramycin, and amikacin in human serum and interstitial fluid. Antimicrob Agents Chemother. 1977;11:698–700. doi: 10.1128/aac.11.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamer C, de Beco V, Soler P, et al. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob Agents Chemother. 1993;37:281–6. doi: 10.1128/aac.37.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrison MW, Vance-Bryan K, Larson TA, et al. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1990;34:1925–31. doi: 10.1128/aac.34.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers HF, Mills J, Drake TA, et al. Failure of a once-daily regimen of cefonicid for treatment of endocarditis due to Staphylococcus aureus. Rev Infect Dis. 1984;6(Suppl 4):s870–4. doi: 10.1093/clinids/6.supplement_4.s870. [DOI] [PubMed] [Google Scholar]
- 13.Bryan LE, Van den Elzen HM. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976;9(6):928–38. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamberger DM, Herndon BL, Suvarna PR. The effect of zinc on microbial growth and bacterial killing by cefazolin in a Staphylococcus aureus abscess milieu. J Infect Dis. 1993;168(4):893–6. doi: 10.1093/infdis/168.4.893. [DOI] [PubMed] [Google Scholar]
- 15.Neu HC. General concepts on the chemotherapy of infectious diseases. Med Clin North Am. 1987;71(6):1051–64. doi: 10.1016/s0025-7125(16)30796-9. [DOI] [PubMed] [Google Scholar]
- 16.Plorde JJ, Hovland D, Garcia M, et al. Studies on the pathogenesis of meningitis. V. Action of penicillin in experimental pneumococcal meningitis. J Lab Clin Med. 1965;65:71–80. [PubMed] [Google Scholar]
- 17.Quie PG, Belani KK. Coagulase-negative staphylococcal adherence and persistence. J Infect Dis. 1987;156(4):543–7. doi: 10.1093/infdis/156.4.543. [DOI] [PubMed] [Google Scholar]
- 18.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358(9276):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 19.Carryn S, Van Bambeke F, Mingeot-Leclercq MP, et al. Comparative intracellular (THP-1 macrophage) and extracellular activities of beta-lactams, azithromycin, gentamicin, and fluoroquinolones against Listeria monocytogenes at clinically relevant concentrations. Antimicrob Agents Chemother. 2002;46(7):2095–103. doi: 10.1128/AAC.46.7.2095-2103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual A, Ballesta S, Garcia I, et al. Uptake and intracellular activity of linezolid in human phagocytes and nonphagocytic cells. Antimicrob Agents Chemother. 2002;46(12):4013–5. doi: 10.1128/AAC.46.12.4013-4015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carryn S, Chanteux H, Seral C, et al. Intracellular pharmacodynamics of antibiotics. Infect Dis Clin North Am. 2003;17(3):615–34. doi: 10.1016/s0891-5520(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 22.Van Bambeke F, Michot JM, Tulkens PM. Antibiotic efflux pumps in eukaryotic cells: occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J Antimicrob Chemother. 2003;51(5):1067–77. doi: 10.1093/jac/dkg225. [DOI] [PubMed] [Google Scholar]
- 23.Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet. 1986;11:257–82. doi: 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 24.Milne RW, Nation RL, Turnidge JD, et al. Pharmacokinetics of colistin methane-sulphonate and colistin in rats following an intravenous dose of colistin methane-sulphonate. J Antimicrob Chemother. 2004;53:837–40. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- 25.Bush LM, Levison ME. Antibiotic selection and pharmacokinetics in the critically ill. Crit Care Clin. 1988;4(2):299–324. [PubMed] [Google Scholar]
- 26.Beringer PM, Vinks AA, Jelliffe RW, et al. Pharmacokinetics of tobramycin in adults with cystic fibrosis: implications for once-daily administration. Antimicrob Agents Chemother. 2000;44(4):809–13. doi: 10.1128/aac.44.4.809-813.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbeeck RK, Horsmans Y. Effect of hepatic insufficiency on pharmacokinetics and drug dosing. Pharm World Sci. 1998;20(5):183–92. doi: 10.1023/a:1008656930082. [DOI] [PubMed] [Google Scholar]
- 28.Westphal JF, Brogard JM. Drug administration in chronic liver disease. Drug Saf. 1997;17(1):47–73. doi: 10.2165/00002018-199717010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ingerman MJ, Pitsakis PG, Rosenberg AF, et al. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental Pseudomonas endocarditis in the rat. J Infect Dis. 1986;153:707–14. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- 30.Stevens DL, Yan S, Bryant AE. Penicillin-binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J Infect Dis. 1993;167:1401–5. doi: 10.1093/infdis/167.6.1401. [DOI] [PubMed] [Google Scholar]
- 31.Craig WA. Basic pharmacodynamics of antimicrobials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am. 2003;17:479–502. doi: 10.1016/s0891-5520(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 32.Barza M, Cuchural G. General principles of antibiotic tissue penetration. J Antimicrob Chemother. 1985;15(Suppl A):59–75. doi: 10.1093/jac/15.suppl_a.59. [DOI] [PubMed] [Google Scholar]
- 33.Lutsar I, McCracken GH, Friedland IA. Antibiotic pharmacodynamics in cerebrospinal fluid. Clin Infect Dis. 1998;42:2650–9. doi: 10.1086/515003. [DOI] [PubMed] [Google Scholar]
- 34.Ambrose PG, Bhavnani SM, Owens RC. Clinical pharmacodynamics of quinolones. Infect Dis Clin North Am. 2003;17:529–43. doi: 10.1016/s0891-5520(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 35.Brook I. Inoculum effect. Rev Infect Dis. 1989;11:361–8. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- 36.Kasiakou SK, Lawrence KR, Choulis N, et al. Continuous versus intermittent administration of antibacterials with time-dependent action: a systematic review of pharmacokinetic and pharmacodynamic parameters. Drugs. 2005;65(17):2499–511. doi: 10.2165/00003495-200565170-00006. [DOI] [PubMed] [Google Scholar]
- 37.Drusano GL, Preston SL, Fowler C, et al. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis. 2004;189(9):1590–7. doi: 10.1086/383320. [DOI] [PubMed] [Google Scholar]
- 38.Roberts JA, Paratz J, Paratz E, et al. Continuous infusion of beta-lactam antibiotics in severe infections: a review of its role. Int J Antimicrob Agents. 2007;30(1):11–8. doi: 10.1016/j.ijantimicag.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39:2206–12. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers HF, Kennedy S. Effects of dosage peak and trough concentrations in serum, protein binding, and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob Agents Chemother. 1990;34:510–4. doi: 10.1128/aac.34.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harding I, MacGowan AP, White LO, et al. Teicoplanin therapy for Staphylococcus aureus septicaemia: relationship between pre-dose serum concentrations and outcome. J Antimicrob Chemother. 2000;45:835–41. doi: 10.1093/jac/45.6.835. [DOI] [PubMed] [Google Scholar]
- 42.Wilson APR, Gruneberg RN, Neu H. A critical review of the dosage teicoplanin in Europe and the USA. Int J Antimicrob Agents. 1991;4(Suppl 1):s1–30. doi: 10.1016/0924-8579(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 43.Moise-Broder PA, Forrest A, Birmingham MC, et al. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43(13):925–42. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 44.Drew RH, Lu I, Joyce M, et al. Program and abstracts of the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy (Oct 30–Nov 2, 2004; Washington, DC) American Society for Microbiology; Washington, DC: Lack of relationship between predicted area under the time-concentration curve/minimum inhibitory concentration and outcome in vancomycin-treated patients with Staphylococcus aureus bacteremia, [abstract A-1493]. [Google Scholar]
- 45.Rybak MJ, Lomaestro BM, Rotscahfer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–7. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 46.Lepper M, Dowling H. Treatment of pneumococcal meningitis with penicillin compared with penicillin plus aureomycin. Arch Intern Med. 1951;88:489–94. doi: 10.1001/archinte.1951.03810100073006. [DOI] [PubMed] [Google Scholar]
- 47.Drusano GL. Human pharmacodynamics of beta-lactams, aminoglycosides and their combination. Scand J Infect Dis Suppl. 1991;74:235–48. [PubMed] [Google Scholar]
- 48.Lacy MK, Nicolau DP, Nightingale CH, et al. The pharmacodynamics of aminoglycosides. Clin Infect Dis. 1998;27:23–7. doi: 10.1086/514620. [DOI] [PubMed] [Google Scholar]
- 49.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–9. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 50.Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–81. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodnut G. Pharmacodynamics to combat resistance. J Antimicrob Chemother. 2000;46:25–31. doi: 10.1093/oxfordjournals.jac.a020890. [DOI] [PubMed] [Google Scholar]
- 52.Lister PD, Sanders CC. Pharmacodynamics of moxifloxacin, levofloxacin, and sparfloxacin against Streptococcus pneumoniae in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2001;47:811–8. doi: 10.1093/jac/47.6.811. [DOI] [PubMed] [Google Scholar]
- 53.Preston SL, Dursano GL, Berman AL, et al. Pharmacodynamics of levofloxacin. A new paradigm for early clinical trials. JAMA. 1998;279:125–9. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 54.Moore RD, Leitman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ration of peak concentration to minimum inhibitory concentration. J Infect Dis. 1987;155:93–9. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 55.Drusano GL, Ambrose PG, Bhavnani SM, et al. Back to the future: using aminoglycosides again and how to dose them optimally. Clin Infect Dis. 2007;45(6):753–60. doi: 10.1086/520991. [DOI] [PubMed] [Google Scholar]
- 56.Chow AT, Fowler C, Williams RR, et al. Safety and pharmacokinetics of multiple 750-milligram doses of intravenous levofloxacin in healthy volunteers. Antimicrob Agents Chemother. 2001;45(7):2122–5. doi: 10.1128/AAC.45.7.2122-2125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rybak MJ, Abate BJ, Kang SL, et al. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43:1549–65. doi: 10.1128/aac.43.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicolau DP, Freeman CD, Belliveau, et al. Experience with once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother. 1995;39:650–5. doi: 10.1128/AAC.39.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watling SM, Dasta JF. Aminoglycoside dosing considerations in intensive care unit patients. Ann Pharmacother. 1993;27:351–6. doi: 10.1177/106002809302700319. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Turnidge J, Milne R, et al. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45(3):781–5. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Owen RJ, Li J, Nation RL, et al. In vitro pharmacodynamics of colistin against Acinetobacter baumanii clinical isolates. J Antimicrob Chemother. 2007;59:473–7. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- 62.Bergen PJ, Li J, Nation RL, et al. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61(3):636–42. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 63.Poudyal A, Howden BP, Bell JM, et al. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62(6):1311–8. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]
- 64.Rahal JJ. Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(Suppl 2):S95–9. doi: 10.1086/504486. [DOI] [PubMed] [Google Scholar]
- 65.Chitnis S, Chitnis V, Chitnis DS. In vitro synergistic activity of colistin with aminoglycosides, beta-lactams and rifampin against multidrug-resistant gram-negative bacteria. J Chemother. 2007;19(2):226–9. doi: 10.1179/joc.2007.19.2.226. [DOI] [PubMed] [Google Scholar]
- 66.Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clin Microbiol Infect. 2008;14(9):816–27. doi: 10.1111/j.1469-0691.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 67.Song JY, Lee J, Heo JY, et al. Colistin and rifampicin combination in the treatment of ventilator-associated pneumonia caused by carbapenem-resistant Acinetobacter baumanii. Int J Antimicrob Agents. 2008;32(3):281–4. doi: 10.1016/j.ijantimicag.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Maglio D, Nicolau DP, Nightingale CH. Impact of pharmacodynamics on dosing of macrolides, azalides and ketolides. Infect Dis Clin North Am. 2003;17:563–77. doi: 10.1016/s0891-5520(03)00059-x. [DOI] [PubMed] [Google Scholar]
- 69.Carbon C. Pharmacodynamics of macrolides, azalides, and streptogramins: effect on extracellular pathogens. Clin Infect Dis. 1998;27:28–32. doi: 10.1086/514619. [DOI] [PubMed] [Google Scholar]
- 70.Christianson J, Andes DR, Craig WA. Program and abstracts of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy (2001: Chicago, IL). Abstr Intersci Conf Antimicrob Agents Chemother. 2001, Dec 16–19; 41: abstract no. A-1100. American Society for Microbiology; Washington, DC: Pharmacodynamic characteristics of clindamycin against Streptococcus pneumoniae in a murine thigh-infection model. [Google Scholar]
- 71.Passarell JA, Meagher AK, Liolios K, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob Agents Chemother. 2008;52(1):204–10. doi: 10.1128/AAC.00813-07. [Epub 2007 Oct 22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meagher AK, Passarell JA, Cirincione BB, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob Agents Chemother. 2007;51(6):1939–45. doi: 10.1128/AAC.01084-06. [Epub 2007 Mar 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andes D, van Ogtrop ML, Peng J, et al. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother. 2002;46:3484–9. doi: 10.1128/AAC.46.11.3484-3489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rayner CR, Forrest A, Meagher AK, et al. Clinical pharmacodynamics of linezolid in seriously ill patients treated in a compassionate use programme. Clin Pharmacokinet. 2003;42:1411–23. doi: 10.2165/00003088-200342150-00007. [DOI] [PubMed] [Google Scholar]
- 75.Cha R, Akins RL, Ryback MJ. Linezolid, levofloxacin and vancomycin against vancomycin-tolerant and fluoroquinolone-resistant Streptococcus pneumoniae in an in vitro pharmacodynamic model. Pharmacotherapy. 2003;23:1531–7. doi: 10.1592/phco.23.15.1531.31964. [DOI] [PubMed] [Google Scholar]
- 76.Jacqueline C, Batard E, Perez L, et al. In vivo efficacy of continuous infusion versus intermittent dosing of linezolid compared to vancomycin in a methicillin-resistant Staphylococcus aureus rabbit endocarditis model. Antimicrob Agents Chemother. 2002;46:3706–11. doi: 10.1128/AAC.46.12.3706-3711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conte JE, Golden JA, Kipps J, et al. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;26:1475–80. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rybak MJ. Pharmacodynamics: relation to antimicrobial resistance. Am J Infect Control. 2006;34(5 Suppl 1):s38–45. doi: 10.1016/j.ajic.2006.05.227. [discussion: s64–73] [DOI] [PubMed] [Google Scholar]
- 79.Wispelwey B. Clinical implications of pharmacokinetics and pharmacodynamics of fluoroquinolones. Clin Infect Dis. 2005;41(Suppl 2):s127–35. doi: 10.1086/428053. [DOI] [PubMed] [Google Scholar]
- 80.Falagas ME, Bliziotis IA, Rafailidis PI. Do high doses of quinolones decrease the emergence of antibacterial resistance? A systematic review of data from comparative clinical trials. J Infect. 2007;55(2):97–105. doi: 10.1016/j.jinf.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Jacobs MR. Optimization of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin Microbiol Infect. 2001;7:589–96. doi: 10.1046/j.1198-743x.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- 82.Craig WA. Does the dose matter? Clin Infect Dis. 2001;33:s233–7. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]